Abstract
Immune checkpoint inhibitor therapy improves survival in patients with metastatic renal cell carcinoma (RCC) but has not been studied well preoperatively in patients with localized disease undergoing nephrectomy. We conducted a single-center study to evaluate the safety and feasibility of neoadjuvant nivolumab in patients undergoing nephrectomy for localized RCC. Eligible patients had a >20% risk of recurrence, as estimated by a preoperative nomogram. Patients received nivolumab every 2 wk for four treatments prior to surgery. The primary endpoints were feasibility, defined as completing at least three treatments without significant surgical delay, and safety, defined as the rate of surgical complications. Treatment effects were assessed by radiomics and immunohistochemistry. A total of 18 patients (11 men; median age 60 yr) with clear cell RCC were enrolled. All received at least one dose of nivolumab and proceeded to nephrectomy without delay; 16/18 patients completed all four doses. Two patients discontinued nivolumab for immune-related adverse events, and four had surgical complications as per the Clavien-Dindo classification. Integrated pathology plus radiomic analysis demonstrated an association between post-treatment immune infiltration and low entropy apparent diffusion coefficient on magnetic resonance imaging. Nivolumab prior to nephrectomy was safe and feasible, without significant surgical delays and with an expected rate of immune-related adverse events.
Patient summary:
We evaluated the outcomes for patients with localized kidney cancer who received immunotherapy prior to surgery to remove their kidney tumor. In a small group of patients who had cancer confined to the kidney, this approach appeared safe and feasible.
Keywords: Immune checkpoint inhibitors, Immunotherapy, Neoadjuvant, Nephrectomy, Renal cell carcinoma
Approximately 30% of patients with renal cell carcinoma (RCC) are initially diagnosed with localized disease with high-risk features, and their risk of recurrence can be ≥40% [1]. To date, there is no standard perioperative systemic therapy shown to improve overall survival in localized RCC. The use of immune checkpoint inhibitors (ICIs) as adjunct to surgery in localized RCC has garnered significant interest, given their efficacy in advanced disease. Adjuvant pembrolizumab, a programmed cell death protein-1 (PD-1) inhibitor, was recently shown to improve disease-free survival in patients with clear cell RCC at a high risk of recurrence compared with placebo [2]. Neoadjuvant ICIs follow the rationale that the renal primary will provide ample antigen source for an enduring cancer-specific immune response. Few data exist on the safety of ICIs prior to nephrectomy and whether surgical complications or immune-related adverse events (irAEs) lead to worse outcomes.
We completed a single-arm pilot study at Memorial Sloan Kettering Cancer Center (MSKCC; ClinicalTrials.gov identifier NCT02595918) to examine the safety and feasibility of neoadjuvant nivolumab in patients with localized RCC. Eligible patients had clear cell RCC at a high risk of recurrence, defined as a 12-yr probability of metastases of ≥20%, as per a preoperative nomogram [3]. Additional trial design details are included in the Supplementary material. During screening, all patients underwent a biopsy to confirm clear cell histology. Patients received nivolumab every 2 wk for four treatments, with surgery 7–14 d after the last dose. Renal protocol magnetic resonance imaging (MRI) was performed at study entry, and after the final nivolumab dose and prior to surgery, with tumor response assessed by RECIST 1.1. Surveillance with cross-sectional imaging of chest and abdomen was done at 3 mo, and then as per the standard of care. Recurrence status was assessed 24–28 mo after surgery.
The primary endpoints of the study were safety and feasibility of preoperative nivolumab. Feasibility was defined as receiving at least three doses of nivolumab and completing surgery without significant delay. All patients who received at least one dose were evaluable for toxicity and graded according to the Common Terminology Criteria for Adverse Events (version 4.0). Surgical complications were graded according to the Clavien-Dindo classification. Recurrence-free survival was calculated as the time from nephrectomy to recurrence or death, and estimated using the Kaplan-Meier method. Analyses were performed using R version 3.6.0.
For exploratory analysis, pathological and radiomic features were examined before and after nivolumab (Supplementary material). Tumor regression, tumor necrosis, and tumor infiltrating lymphocytes (TILs) were quantified. For radiomics, on apparent diffusion coefficient (ADC) maps, a volumetric radiomic analysis was performed to measure the texture described by Haralick et al [4], which provides information about the spatial distribution of intensity levels in a neighborhood, and entropy, which represents the randomness of gray-level distribution.
From May 2016 to September 2019, 21 patients were screened and 18 (11 men; median age 60 yr) with clear cell RCC were enrolled (Supplementary Table 1). Owing to slow accrual, the trial was closed early. Most patients (56%) presented with localized or systemic symptoms, and the median tumor size at baseline was 8.8 cm (range 6.4–14.2 cm).
Although all 18 patients completed surgery without delay, 17/18 received at least three nivolumab doses, making the feasibility rate as per the predefined criteria 94% (confidence interval [CI] 73–100%). Two patients had discontinued nivolumab early, both for irAEs requiring systemic corticosteroids (grade 3 transaminitis and grade 2 intolerable arthralgias; Supplementary Table 2). One patient had grade 3 colitis and acute kidney injury 6 mo after the last dose of nivolumab; after workup did not yield other causes, he was treated with immunosuppressives with resolution of symptoms. However, beyond the predetermined monitoring period, the colitis was attributed to nivolumab. Perioperative and pathological details are included in Table 1. The median time to nephrectomy after the last dose of nivolumab was 10.5 d (range 9–13 d). The median (range) estimated blood loss, operating room time, and length of stay were 200 (150–363) ml, 174 (158–195) min, and 1.5 (1–2) d, respectively. One patient required intraoperative blood transfusion. Postoperative morbidity was noted in two patients with lymph node dissection who developed a chylous leak requiring paracentesis (Clavien 3b), and one of them further required glue embolization and Denver shunt (Clavien 3b). Although not directly related to nephrectomy, one patient experienced testicular infarction in the context of a concomitant inguinal hernia repair.
Table 1 –
Perioperative outcomes for study participants
| Patient | Sex | Approach | Pathological stage (pT) |
LOS (d) |
30-d readmission |
90-d mortality |
Morbidity (Clavien- Dindo) |
irAE Grade ≥2 |
Detail—irAE or surgical complication(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Robotic | 4 | 1 | No | No | None | Yes | G2 thyroiditis |
| 2 | F | Open | 3A | 3 | No | No | None | No | |
| 3 | M | Robotic | 3A | 2 | No | No | None | No | |
| 4 | F | Robotic | 3A | 1 | No | No | None | No | |
| 5 | F | Robotic | 3A | 1 | No | No | None | Yes | G3 hepatitis |
| 6 | F | Robotic | 3A | 1 | No | No | None | No | |
| 7 | M | Robotic | 3A | 1 | No | No | None | No | |
| 8 | F | Open | 3A | 3 | No | No | None | No | |
| 9 | M | Open | 3A | 2 | No | No | None | No | |
| 10 | M | Robotic | 3A | 1 | No | No | None | No | |
| 11 | F | Open | 3A | 2 | No | No | None | No | |
| 12 | M | Robotic | 3A | 1 | No | No | None | Yes | G3 colitis and AKI a |
| 13 | F | Open | 3A | 2 | No | No | None | No | |
| 14 | M | Robotic | 3A | 2 | No | No | None | No | |
| 15 | M | Robotic | 3A | 1 | No | No | 3b | No | Chylous leak |
| 16 | M | Open | 3A | 4 | No | No | 3b | No | Chylous leak |
| 17 | M | Robotic | 3A | 6 | No | No | 4a b | No | Testicular infarction |
| 18 | M | Robotic | 1B | 1 | No | No | None | No |
AKI = acute kidney injury; F = female; irAE = immune-related adverse event; LOS = length of stay; M = male.
The irAEs occurred >6 mo after the last dose of nivolumab, no other cause was found, and patients responded promptly to immunosuppression.
Testicular infarction secondary to concomitant inguinal hernia repair, deemed unrelated to treatment/nephrectomy.
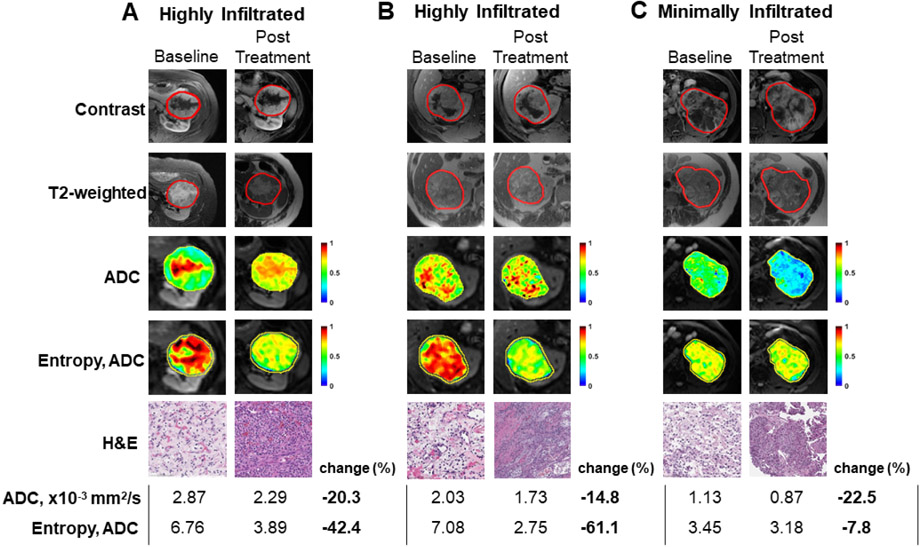
All patients were evaluable for primary tumor response prior to nephrectomy. The best response was stable disease for all patients; the median change in the largest diameter was +0.85% (range −6.2% to +7.9%). With a median follow-up of 22.7 mo (range 4–29.8 mo), the median recurrence-free survival at 1 yr was 82% (95% CI 65–100%; Supplementary Fig. 1 and 2). We compared the prenivolumab biopsy specimen and the postnivolumab nephrectomy specimen. At least 5% tumor regression was observed in ten of 14 evaluable cases (range 0–40%; Supplementary Table 3). No tumor necrosis was seen before nivolumab; only one tumor had associated necrosis after nivolumab. The average percent of TILs in adjacent normal and tumor epithelial and stromal elements was similar before and after nivolumab, although some cases exhibited more notable TIL infiltration after treatment (Supplementary Table 4 and Fig. 1). For all cases, MRI sequences were compared before and after nivolumab. Conventional T1 postcontrast and T2-weighted images did not show discernable differences before and after nivolumab in tumors that became highly and moderately immune infiltrated. On ADC maps, a volumetric radiomic analysis was performed to measure Haralick texture features, used to detect image features that can be invisible to the human eye, including the spatial distribution of intensity levels in a neighborhood [4,5]. After treatment, ADC values were lower for most tumors (Supplementary Table 5), but entropy (randomness of gray-level distribution) ADC values were substantially lower than the baseline in tumors that became highly immune infiltrated.
Fig. 1 –
MRI and pathological responses in patients before and after nivolumab. Examples of tumors that (A and B) became highly immune infiltrated after nivolumab, and (C) had no discernible change in moderate immune infiltration. The top panels for each include MRI T1 postcontrast image, T2-weighted image, apparent diffusion coefficient (ADC), and entropy texture of ADC overlaid on segmented tumor. While conventional T1 postcontrast and T2-weighted images did not show discernable differences between highly immune infiltrated tumors (Fig. 1A and 1B) and moderate immune infiltrated tumor (Fig. 1C), after treatment, the mean ADC values were lower in all tumors, but entropy ADC values were substantially lower in highly infiltrated tumors. ADC = apparent diffusion coefficient; H&E = hematoxylin and eosin; MRI = magnetic resonance imaging.
The treatment of advanced RCC has been revolutionized by ICIs, but their potential for neoadjuvant use remains to be defined. Concerns over preoperative ICI use stem from their mechanism of enhancing T-cell activation: tissue inflammation could add technical difficulty during surgery, or irAEs and need for steroids might complicate the perioperative period [6]. In this pilot study, no related intraoperative complications or 30-d readmissions were observed. Although there were Clavien-Dindo complications graded >2, these were attributed less likely to nivolumab and more likely to more extensive lymph node dissections. The favorable safety profile is similar to that in other reports, including one trial on neoadjuvant nivolumab × 3 and nephrectomy for locally advanced clear cell RCC [7-9]. Nivolumab was well tolerated, and although two patients required systemic corticosteroids for irAEs, both proceeded to surgery without delay. Of note, we did not find significant primary tumor responses to four doses of nivolumab, with all patients having stable disease prior to nephrectomy. This is consistent with the primary renal tumor objective response rate of 6% seen in the NIVOREN phase II trial of nivolumab in metastatic RCC (34103181). Encouragingly, despite this being a high-risk group, we also did not see progressive disease by RECIST during the nivolumab administration period. As exploratory endpoints, we investigated pre-/postnivolumab changes in entropy on MRI with histological changes. In select cases with the highest postnivolumab immune infiltration, we saw no discernable changes in conventional T1- and T2-weighted images but demonstrated lower ADC entropy, suggesting possibly more homogeneous tumors. It will be of interest to investigate radiomics in larger studies, including in the metastatic space.
Limitations to this study include the small number of patients and relatively short follow-up. The trial was closed early due to low accrual. We noted a decrease in accrual once an adjuvant immunotherapy study opened at our institution, speaking of the challenges of neoadjuvant versus adjuvant studies from both the patient and the surgeon standpoint. Long-term follow-up and larger phase III trials, such as the PROSPER RCC trial (NCT03055013) studying perioperative ICIs, will be necessary to assess the benefits of neoadjuvant ICIs.
Supplementary Material
In a study of preoperative nivolumab in patients with localized renal cell carcinoma, the approach appeared to be safe and feasible, with most patients completing therapy without surgical delays. Preliminary data show that radiomics as a predictive biomarker could be explored further.
Acknowledgments:
Investigational product was provided by Bristol Myers Squibb.
Funding/Support and role of the sponsor:
This work was sponsored by National Cancer Institute Division of Cancer Treatment and Diagnosis and funded in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. Maria I. Carlo is supported by The Kidney Cancer Association and Harold Amos Medical Faculty Development Award.
Footnotes
Financial disclosures: Martin H. Voss certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Ritesh Kotecha reports research grants or contracts from Pfizer and Takeda. Chung-Han Lee reports research funding from Eisai, Bristol-Myers Squibb, Exelixis, Pfizer, and Calithera; and consulting or advisory role with Exelixis and Eisai. DF reports research funding from Seattle Genetics and Novartis. Robert J. Motzer reports consulting or advisory role with Pfizer, Novartis, Eisai, Exelixis, Merck, Genentech, Incyte, Lilly, and Roche; institutional research funding from Pfizer, Bristol Myers Squibb, Eisai, Novartis, Genentech, and Roche; and travel/accommodation from Bristol-Myers Squibb. A. Ari Hakimi reports advisory role with Merck. Martin H. Voss reports research grants from Bristol-Myers Squibb and Genentech/Roche; honoraria from Novartis; travel/accommodation from Eisai, Novartis, and Takeda; and consulting or advisory role with Alexion Pharmaceuticals, Bayer, Calithera Biosciences, Corvus Pharmaceuticals, Exelixis, Eisai, GlaxoSmithKline, Natera, Novartis, and Pfizer. The remaining authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Abu-Ghanem Y, Powles T, Capitanio U, et al. The impact of histological subtype on the incidence, timing, and patterns of recurrence in patients with renal cell carcinoma after surgery—results from RECUR Consortium. Eur Urol Oncol 2021;4:473–82. [DOI] [PubMed] [Google Scholar]
- [2].Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021;385:683–94. [DOI] [PubMed] [Google Scholar]
- [3].Mano R, Duzgol C, Ganat M, et al. Preoperative nomogram predicting 12-year probability of metastatic renal cancer—evaluation in a contemporary cohort. Urol Oncol 2020;38:853.e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haralick RM, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern 1973;3:610–21. [Google Scholar]
- [5].Arivazhagan S, Ganesan L, Priyal SP. Texture classification using Gabor wavelets based rotation invariant features. Pattern Recognit Lett 2006;27:1976–82. [Google Scholar]
- [6].Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gorin MA, Patel HD, Rowe SP, et al. Neoadjuvant nivolumab in patients with high-risk nonmetastatic renal cell carcinoma. Eur Urol Oncol. In press. 10.1016/j.euo.2021.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Singla N, Elias R, Ghandour RA, et al. Pathologic response and surgical outcomes in patients undergoing nephrectomy following receipt of immune checkpoint inhibitors for renal cell carcinoma. Urol Oncol 2019;37:924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pignot G, Thiery-Vuillemin A, Walz J, et al. Nephrectomy after complete response to immune checkpoint inhibitors for metastatic renal cell carcinoma: a new surgical challenge? Eur Urol 2020;77:761–3. [DOI] [PubMed] [Google Scholar]
- [10].Courcier J, Dalban C, Laguerre B, et al. Primary renal tumour response in patients treated with nivolumab for metastatic renal cell carcinoma: results from the GETUG-AFU 26 NIVOREN Trial. Eur Urol 2021;80:325–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.