Clinical Challenge
Case:
A 29 year-old woman with a 5-year history of systemic lupus erythematous (SLE), manifested by arthralgia, skin rash, positive anti-double stranded DNA antibodies and low complement components 3 and 4 (C3, C4), on low-dose prednisone and hydroxychloroquine, presented with new onset nephrotic range proteinuria (3.5g/day). Her serum creatinine (SCr) concentration was 0.6mg/dL. A kidney biopsy showed Class IV lupus nephritis (LN) with wire loop lesions, endocapillary hypercellularity, mesangial hypercellularity, 1 glomerulus with karyorrhectic debris and mild interstitial fibrosis (Fig. 1). National Institutes of Health activity and chronicity indices were 7/24 and 1/12, respectively. She was initially treated with three intravenous doses of methylprednisolone totaling 2g followed by prednisone, 0.5 mg/kg/d with a tapering schedule, and intravenous cyclophosphamide, 500 mg every 2 weeks for 6 doses. Cyclophosphamide was to be followed by mycophenolate mofetil (MMF) maintenance (2g/d), but at her 12 week follow-up proteinuria was 3.4 g/day and SCr was 0.8mg/dL. Given this lack of response, MMF was started at 3g/d, tacrolimus 1 mg bid was added, prednisone was recycled to 0.5 mg/kg/d and the taper restarted. Despite these medication changes, the patient had persistent proteinuria (3 g/d) and hypocomplementemia 6 months later. The patient was given two 1g doses of rituximab and MMF was continued. Some progress was noted with improved complement levels, but the patient became leukopenic and MMF had to be reduced to 500 mg/d. Voclosporin became available around this time and was added. The patient’s proteinuria decreased to 500 mg/d.
Figure 1: The kidney biopsy of a patient with refractory lupus nephritis.
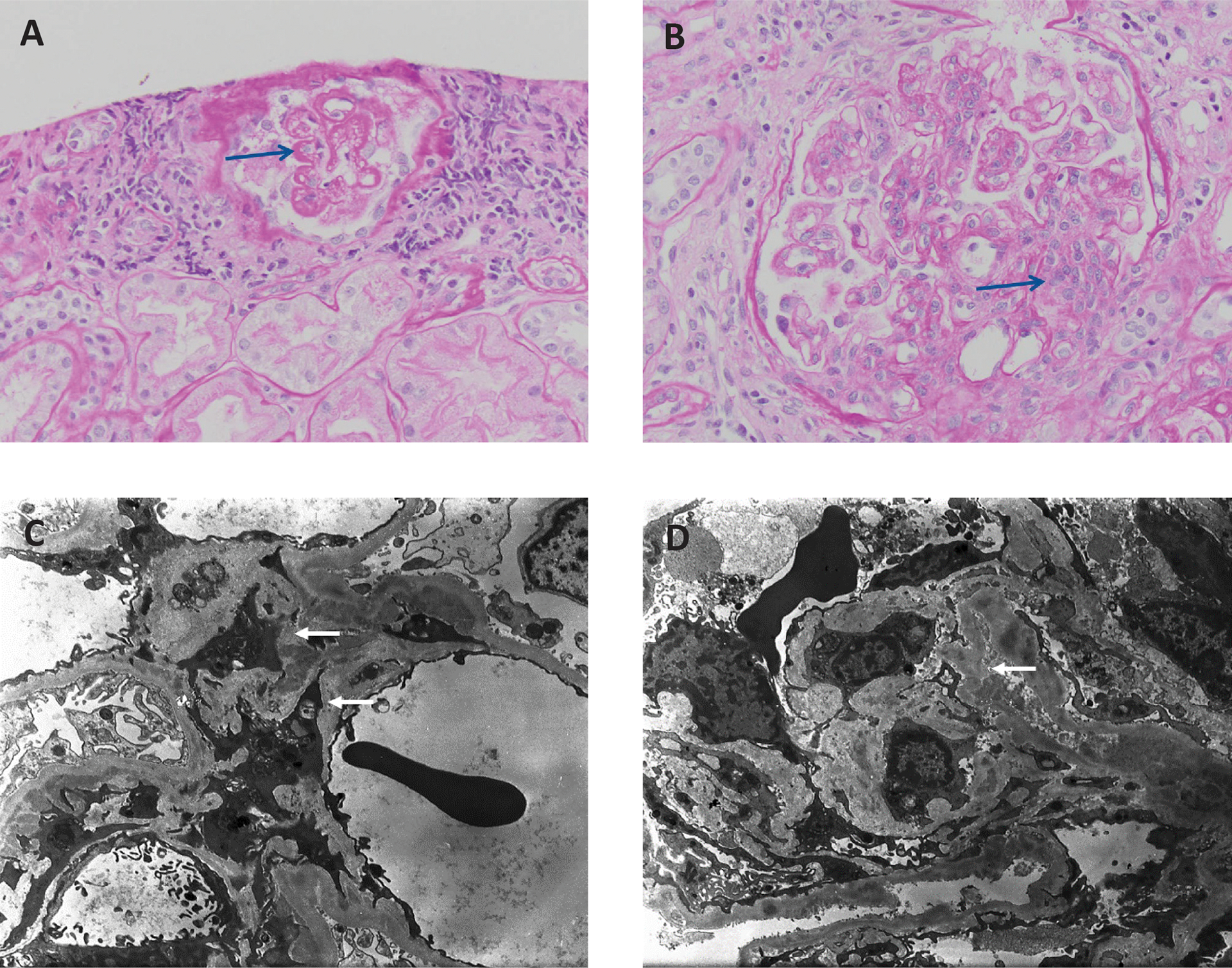
Light microscopy demonstrating (A) wire loop lesions and (B) mesangial and endocapillary hypercellularity. Electron micrographs showing (C) mesangial and D) subendothelial immune complex deposits.
Background
Systemic Lupus Erythematosus (SLE) affects the kidneys in about half of all patients (1). Lupus Nephritis (LN), the most common manifestation of kidney involvement in lupus, typically occurs within the first 6–36 months of diagnosis, and in 25–50% of patients LN is the initial presentation of SLE (2). Despite prompt diagnosis and treatment with aggressive immunosuppression, it has been reported that 14–33% of LN patients fail to respond and are refractory to treatment (3, 4).
If no response to a single course of one standard therapy is taken as refractory disease, then we suspect that the reported or perceived frequency of refractory LN is largely overestimated. Several factors besides drug resistance contribute to a poor response to treatment and should be excluded before labeling a patient as treatment resistant. For example, non-adherence to prescribed treatment is very common among patients with autoimmune diseases. Using a Medicaid Database, adherence of over 4000 patients with SLE was assessed during their first year of therapy using the criterion of medication refilled >80% of time as “adherent”. Fewer than 25% of patients met this metric (5). Female sex, younger age, Black race and Hispanic ethnicity were associated with higher odds of non-compliance. Other important barriers identified were health literacy, financial issues, access to healthcare, perceived treatment inefficacy and side effects (6). It is therefore prudent to assess adherence before changing treatment plans or adding additional immunosuppression. Obtaining pharmacy refill records, doing pill counts and monitoring drug levels may help in ascertaining adherence. Monitoring hydroxychloroquine levels also does seem to improve adherence (7). Hydroxychloroquine has a long, 40–50 day half-life, and serum levels do not fluctuate much, so an undetectable hydroxychloroquine level suggests long-term non-adherence (8). Once non-adherence is unmasked a non-judgmental conference with the patient (and their family) should be pursued, incorporating open discussion to identify and address barriers, improve disease understanding, and if possible, simplify the treatment regimen and decrease pill burden (9).
Although standard-of-care (SOC) regimens for LN are well-established, adequate drug dosing can be challenging. Most patients start treatment within prescribed dose ranges, but how an individual metabolizes a specific drug is generally not known a priori. Additionally, dosing is often decreased in response to reported side effects, and this may affect efficacy. Ideally, treatment adequacy for individual patients would be determined by monitoring drug blood levels, but the therapeutic range for most SOC LN drugs has not been established (10). Furthermore, therapeutic drug monitoring (TDM) can be challenging in the clinical setting. Measurement of the area under the concentration-time curve (AUC) is the most accurate determination of an individual’s exposure to a drug, but involves obtaining blood samples repeatedly over time. Trough concentrations are more convenient, and sometimes do correlate with a drug’s AUC (11). MMF dose adjustment could be considered in non-responsive patients if pre-dose levels are consistently <3–4.5 mg/L (10, 11). Therapeutic drug monitoring of the calcineurin inhibitors (CNIs) cyclosporine and tacrolimus is more helpful for monitoring adherence and toxicity than efficacy (10). Voclosporin levels do not need to be monitored (12), and most clinical laboratories do not offer testing. Pharmacodynamic effects based on a drug’s specific mechanism of action may be used in lieu of blood levels. For example, the efficacy of rituximab (RTX) in LN seems to depend on the achieving complete peripheral B cell depletion (13).
Patients with LN must be given sufficient time to achieve a kidney response. The European League Against Rheumatism (EULAR) guidelines point out that patients with nephrotic-range proteinuria at baseline may need an additional six to twelve months to achieve complete clinical response (14). On the other hand, continuing an ineffective therapy for many months waiting for a complete response will likely result in chronic damage to the kidneys. Determining the balance between sufficient duration and prolonging exposure of the kidneys to inflammatory injury may be guided by the observation that early, albeit partial response to treatment is associated with complete response later. A decline in proteinuria of at least 25% at 8 weeks has been shown to associate with a 50% reduction in proteinuria after 6 months of therapy (15), and a fall in proteinuria of at least 50% after 6 months of therapy predicts good long-term kidney survival (16). Patients who have a reduction in proteinuria of ≥25% after 2–3 months of treatment are expected to continue to show improvement and may not need to switch therapy; on the other hand patients who do not show improvement are candidates for a change in treatment. It is important to emphasize that these thresholds are only intended to provide a starting point for decision making and are not rigid. Some patients who did not meet these thresholds went on to have good long-term responses, and conversely, some patients who did achieve these metrics did not do well (15, 16). Monitoring LN should be a continuous process using real-time data for flexible decision making.
While there is no consensus definition for a complete kidney response, most clinical trials require proteinuria to fall to ≤700–500 mg/d and SCr or estimated glomerular filtration rate (eGFR) to be within 10–25% of baseline (15,17). Ideally, a complete kidney response based on these clinical thresholds would reflect resolution of intrarenal inflammation, but there is considerable discordance between clinical and histologic responses (18–20). Proteinuria and SCr reflect both acute inflammatory kidney injury and chronic kidney damage. Therefore persistent proteinuria and/or an elevated SCr must be correctly interpreted. This is particularly difficult for LN patients who have had their disease for a long time, or who have experienced multiple LN flares, and in whom accumulated chronic damage may result in proteinuria and kidney function that will never meet complete clinical response criteria. Persistence of proteinuria or an abnormal eGFR in patients who have been treated for more than 18–24 months therefore does not always represent ongoing immune-mediated kidney injury that has evaded treatment (21). A kidney biopsy may be needed to differentiate active disease from chronic damage and resolved inflammation in such cases (18, 19). A kidney biopsy may also demonstrate an unanticipated process, such as antiphospholipid nephropathy, that would require a different intervention.
Finally, kidney disease in LN patients may progressively worsen despite adequate therapy because of genetic factors, giving the appearance of non-responsiveness. For example, the genetic variations of apolipoprotein L1 (ApoL1) that predispose patients of African ancestry to end-stage kidney disease (ESKD) (22) are found in patients with LN and are associated with ESKD and a shorter timeline to ESKD (23). Progressive kidney failure in such individuals may not be due to treatment resistance (24). Alternatively, some genetic conditions, like auto-inflammatory disorders characterized by increased type 1 interferon production, may have similar clinical and pathologic findings as LN, but do not respond well to conventional LN treatments (25).
Approach
Once a LN patient is diagnosed as treatment-resistant they often are exposed to more and more potent immunosuppressive treatments, sequentially or in parallel (26, 27), and are at ever increasing risk of serious short and long-term adverse events. After SOC treatments are exhausted, the evidence of efficacy for any of the proposed salvage therapies is very limited. Given the paucity of evidence for alternative therapies, and keeping in mind the previously discussed situations that may mimic treatment-resistance, we consider refractory LN as no response (or worsening) of proteinuria and/or eGFR to two different SOC induction regimens after 4–6 months in patients who are taking their prescribed medications in doses that are generally believed to be therapeutic. This approach is operationalized in Figure 2.
Figure 2:

Approach to the diagnosis of refractory lupus nephritis.
The proposed algorithm takes into account patient adherence, therapeutic drug monitoring, and tries to balance time in treatment against accumulating chronic kidney injury. These attributes are evidence-based, at least to the extent that there is evidence. However, the proposal to sequentially try two different SOC induction regimens is more opinion-based, and finds rationale in the heterogeneity of LN. Not every patient is expected to respond to the same medication. Over half the patients enrolled in all contemporary clinical trials of LN, even those considered successful, did not achieve a clinical complete response by the end of the trial. It seems reasonable to try another established regimen before reaching for rescue therapies. This approach is consistent with the independent LN guidelines from the EULAR, American College of Rheumatology (ACR) and Kidney Disease: Improving Global Outcomes (KDIGO) consortium (14, 28).
The LN guidelines suggest adherent patients who are first treated with MMF + glucocorticoids should be switched to Euro-lupus or NIH dosing of cyclophosphamide (CYC) + glucocorticoids and vice-versa. We suggest that after poor response to the first therapy used, strong consideration be given to switching to a regimen that adds voclosporin or belimumab, one of the newly approved LN therapies, to SOC. The addition of each of these drugs to background SOC significantly improved the number of patients having a good treatment response (29, 30). Voclosporin responses were better in patients who came into the trial already on MMF, and belimumab showed a larger beneficial effect in relapsed patients compared to de novo LN (31, 32). These observations suggest that both of these novel therapies improve response rates in patients who have already been exposed to some immunosuppression. Of note, belimumab may be less effective in patients with proteinuria ≥3g/d, and voclosporin use should be used cautiously or not at all in patients with a significantly impaired GFR of <45 ml/min (29, 30). If voclosporin is not available, tacrolimus could be considered, assuming a CNI class effect (33, 34).
Given that belimumab and voclosporin are new tools for LN management, there may be an inclination to use these medications as rescue therapies for refractory LN. However neither drug has been systematically evaluated in refractory patients, and such patients were excluded from the pivotal trials of voclosporin and belimumab. Interestingly, there are reports that the addition of a CNI (tacrolimus or cyclosporine) to MMF may result in a response in refractory or relapsing LN, but these have been small uncontrolled studies (33, 35).
Once a diagnosis of treatment-resistant LN has been established, the most common next step has been the addition of B-cell targeted therapy, specifically RTX. A fair amount of data on the response of refractory patients to RTX has been published, and while generally of poor to modest quality, provide some evidence for its use. Evidence for any particular therapy in the case of RTX failure is severely limited. The risk-benefit of increasing immunosuppression in these patients must be assessed. In such cases it is prudent to assess the status of the patient’s immune system and bone marrow by measuring quantitative immunoglobulin levels and leukocyte subset levels (36). If uncontrolled LN has been going on for a while, it is often useful to do a kidney biopsy and assess chronic damage. Patients with severe interstitial fibrosis, tubular atrophy and global glomerulosclerosis have irreversible kidney damage and will inevitably need dialysis. The amount of viable kidney parenchyma left to save and the likelihood of need for kidney replacement therapy even if this parenchyma is saved will figure importantly in calculating the risk to benefit of intensifying immunosuppression further.
Of note, although race and ethnicity may associate with an increased likelihood of developing refractory LN, once a patient is declared refractory, as defined above, we do not choose subsequent rescue therapies based on race or ethnicity. Treatment options are too few and evidence for differential efficacy by race or ethnicity too sparse to make this feasible.
Our patient was compliant with therapy. Despite treatment with Eurolupus CYC, MMF, and tacrolimus the patient did not respond. Our approach to rescue therapy for patients such as this is shown in Figure 3. The evidence supporting this approach is discussed below.
Figure 3:

Therapeutic approach to the patient with refractory lupus nephritis.
Evidence
Rituximab
In the development of LN, B cells play a central role in producing pathogenic autoantibodies, initiating release of cytokines like IL-6 and TNF alpha and activating T cells by providing co-stimulatory support. B-cell directed biologics deplete or impair the function of B cells. Although rituximab (RTX) does not deplete plasma cells directly, it prevents repletion of plasma cells by depleting precursor B cells (37).
RTX, which is directed against the B-cell surface molecule CD20, is a chimeric mouse-human monoclonal antibody. Many uncontrolled studies and open-label observational studies have reported efficacy of RTX in patients with refractory LN with response rates of 50% to 80% (38–45). Table 1 summarizes these RTX data. Of note, in these studies there was significant variation in how refractory LN was defined, and relapsing patients were frequently clustered with refractory patients. Concomitant therapies and duration of follow up also varied considerably between studies.
Table 1:
Rituximab for refractory lupus nephritis: Selected studies
| Reference | N | Refractory or relapsing lupus nephritis | Follow up (months) | Rituximab Dose Regimen | Response to rituximab |
|---|---|---|---|---|---|
| Gunnarsson et. al (38) | 7 | CYC resistant LN | 6 | 375 mg/m2/week × 4 | SLEDAI scores improved, decrease in the renal activity index- on repeat biopsy at 6 months |
| Goswami et.al (39) | 14 | Refractory or relapsing LN | 6 | Not specified | CR 71.4% PR 28.6% |
| Davies et. al (40) | 18 | All patients had severe, active disease and had failed conventional therapy including MMF and CYC | 6 | Two 1 g infusions two weeks apart. | CR 61.1% PR 11.1% |
| Melander et. al (13) | 20 | Eighteen patients (90%) had already received at least one conventional therapy including intravenous CYC in 15 patients, with a median cumulative dose of 6 g | 22 | 375 mg/m2/week × 4 | CR 35% PR 25% |
| Contis et.al (41) | 17 | All patients with refractory LN defined as resistant to standard treatment with CYC | 12 | 375 mg/m2/week × 4 (10 patients) or two infusions of 1 g at day 0 and day 15 (seven Patients). | CR or PR 53% |
| Jonsdottir et. al (42) | 25 | 23 patients refractory to conventional therapy including CYC and/or MMF | 12 | 375 mg/m2/week × 4 | CR 64% PR 24% |
| Lindholm et.al (43) | 17 | signs of active kidney inflammation despite ongoing treatment with CYC (n = 14) or MMF (n = 3) | 6–12 | 375 mg/m2/week × 4 | CR 12% PR 53% |
| Iwata et. al (44) | 63 with SLE, 36 with LN | All refractory to high-dose steroids and various conventional therapies including CYC (54%), MMF (16%), CNI (14%) | 12 | 500 mg twice at one-week intervals (days 1 and 8) in 22 patients; 500 mg at one-week intervals (days 1, 8, 15 and 22) in nine patients; 1000 mg infused at two-week intervals (days 1, 15) in seven patients; 1000 mg infused (days 1, 15, 168, 182) in 25 patients |
BILAG score improved in 83.3% UPCR decreased significantly |
| Iaccrino et.al (45) | 145 with SLE, 68 with LN | All had failure of at least one immunosuppressant | 12 | 118 with two infusions (1 g), two weeks apart; 27 with 375 mg/m2/week × 4, followed in 10 cases by two further doses, after 1 and 2 months | CR 30.9% PR 63.2% |
Abbreviations: CYC, cyclophosphamide; MMF, mycophenolate mofetil; CNI, calcineurin inhibitor, CR, complete response; PR, partial response; SLEDAI, Systemic Lupus Erythematosus Disease Activity Index
Based on a systematic analysis of 26 studies that described 300 patients with refractory LN, defined as being un-responsive to previous therapy with one or more immunosuppressive agents, the addition of RTX converted 40% of the refractory patients to complete clinical kidney responders, and 34% to partial responders (46). In the reports selected for this systematic review, a RTX dose of 375 mg/m2 once a week × 4 was most commonly used (49%), followed by 1000 mg x2, two weeks apart in 37% of patients. Thirty percent of cases received CYC along with RTX, 25% received MMF, 7% received azathioprine and 4% received methotrexate (46). Treatment responses were more common in Class III LN and less frequent in Class IV or V LN. Another meta-analysis of 31 studies described 1112 patients with refractory lupus, from which 10 studies with 223 refractory LN patients showed that 46% and 32% of patients achieved a complete and partial kidney response, respectively after RTX was added. Refractory disease was defined as resistance to traditional therapy; use of prior therapies was not described (47). Further large well-designed multicenter randomized controlled trials are warranted to establish the role of rituximab in refractory LN. In the future, obinutuzumab, an anti-CD20 monoclonal antibody like rituximab but more potent may be evaluated in refractory LN, but at present it is in a phase III trial after a successful phase II trial (48).
Several other approaches to refractory LN treatment have been reported (Table 2). In general, the evidence supporting these therapies is minimal and of low quality. Nonetheless, depending on a patient’s specific situation, it may be necessary to consider these alternatives.
Table 2.
Other therapies tried for refractory lupus nephritis
| Reference | Therapy | N | Refractory/Relapsing LN | Follow up (months) | Response |
|---|---|---|---|---|---|
| Choi et. al (33) | MMF plus tacrolimus | 29 | 12 Refractory and 17 relapsing | 12 | CR 25.9% PR 29.6% |
| Jesus et.al (35) | MMF plus tacrolimus | 17 | MMF-resistant patients (30) | 6 | CR 35% PR 35% |
| Segarra et. al (51) | Bortezomib plus dexamethasone | 12 | Refractory | 9–30 | CR 8.3% PR 83.3% |
| Zhang et.al (52) | Bortezomib plus dexamethasone | 6 | Refractory | 6–24 | CR 60% PR 20% |
| Ostendorf et.al (55) | Daratumumab | 1 | Refractory | 12 | Proteinuria improved |
| Tam et. al (58) | Leflunomide | 17 | Refractory or intolerant to conventional treatment | 12 | CR 29% PR 47% |
| Levy et. al (61) | Intravenous immunoglobulin | 7 | Refractory, patients who failed CYC | 6 | Decreased proteinuria in all patients |
| Monova et.al (63) | Intravenous immunoglobulin | 58 | Refractory | 7 years | CR 30% PR 40% |
| Zhang et.al (65) | Interleukin-2 therapy | 10 | Refractory to at least two conventional treatments | 6 | Decreased proteinuria |
| Pickering et. al (67) | Eculizumab | 1 | Refractory to CYC, rituximab, MMF and tacrolimus | 18 | Decreased proteinuria, improved renal function |
| Mougiakakos et.al (68) | CAR-T | 1 | Refractory to CYC, MMF, Tacrolimus, Rituximab, belimumab | 1.5 | CR achieved |
Abbreviations: LN, lupus nephritis; CYC, cyclophosphamide; MMF, mycophenolate mofetil; CR, complete response; PR, partial response
Anti-plasma cell therapy
An underlying pathogenic mediator of refractory LN could be long-lived autoreactive plasma cells that are resistant to commonly used immunosuppressive therapies (49). Bortezomib, a proteasome inhibitor, eliminates plasma cells, thereby reducing autoantibody production, blocks T-cell-dependent inflammatory responses, and decreases interferon-alpha induction by disrupting Toll-like receptor signaling in dendritic cells (50).
A series of 12 patients resistant to induction therapy with CYC, steroids, MMF, and rituximab, were treated with bortezomib plus dexamethasone. A complete clinical kidney response was achieved in one patient and 11 patients had a partial response with improvement in proteinuria, SCr and serological markers after a mean of 6 bortezomib cycles (51). Similarly, a series of five patients with refractory LN also showed reduction of proteinuria and improved kidney function with 4 cycles of bortezomib plus glucocorticoids. Over 6 to 24-months of follow-up, three patients achieved a complete response, one had a partial response, and one patient progressed and required kidney replacement therapy (52). Like other B-cell therapies, bortezomib may cause hypogammaglobinemia requiring intravenous immunoglobulin rescue (51, 53). Also concerning, patients receiving bortezomib may develop a disabling peripheral neuropathy, although the incidence of this adverse event is decreased if subcutaneous dosing is used (54).
Another approach to plasma cell depletion is through the anti-CD38 monoclonal antibody daratumumab, which kills plasma cells and modulates effector T-cell responses (55). A recent case report of two patients with life-threatening, refractory SLE, one of whom had LN showed excellent clinical and serological responses to daratumumab given weekly for 4 weeks followed by longer-term belimumab (55). The LN patient had an improvement in proteinuria from over 6 g/d to around 1 g/d and normalization of SCr levels during the 12-month follow-up period.
While this report, which describes only two patients, provides low-quality evidence within the hierarchy of types of clinical investigation, it offers potential mechanistic insights into refractory LN. Both of these refractory lupus patients had been treated with bortezomib prior to daratumumab, but did not achieve satisfactory responses. This suggests, at least for some patients, that depleting long-lived plasma cells will not be sufficient to control lupus activity. A deeper dive into potential cellular targets for daratumumab showed that in addition to plasma cells, CD38 is expressed on plasma-blasts, mature B cells, and plasmacytoid dendritic cells in lupus patients, and that CD38 expressing T cells are expanded (55). Thus targeting CD38-expressing cells of various types may have contributed to the effect of daratumumab.
An immunoproteasome inhibitor, KZR-616, is current under investigation for LN, but not specifically for refractory (56).
It is important to keep in mind that bortezomib and daratumumab only transiently deplete plasma cells; this effect must be maintained using additional immunosuppression to prevent autoreactive B-cell precursors from developing into autoreactive plasma cells (55). This was the rationale for following up daratumumab with belimumab after lupus came under control (55).
Leflunomide
Leflunomide, an inhibitor of dihydroorotate dehydrogenase, targets lymphocytes and has anti-proliferative and anti-inflammatory actions. A meta-analysis comparing leflunomide to CYC suggested a better safety profile and improved efficacy for leflunomide in LN, but similar effects on the systemic lupus erythematosus disease activity index (SLEDAI) (57). Leflunomide was used to treat 17 refractory or intolerant (to conventional immunosuppression) LN patients (58). Subsequently, 76% of the patients achieved a response (complete in 29%, partial in 47%). More extensive evaluation of leflunomide will be needed before it can be recommended for refractory LN.
Intravenous immunoglobulin
Intravenous immunoglobulin (IVIg) is a biologic therapy comprised of polyclonal antibodies derived from the plasma of a large pool of healthy donors. In addition to being used to treat hypogammaglobulinemia, it has the potential to treat inflammatory diseases, cancer and autoimmune diseases. IVIg tips the balance of activating and inhibitory immune responses by neutralizing auto-antibodies through anti-idiotype binding, up-regulating inhibitory Fc-receptors, and increasing clearance of pathogenic autoantibodies via the reticuloendothelial system (59, 60). Seven patients with biopsy proven class IV or V LN and nephrotic syndrome who had failed therapy with cyclophosphamide and prednisone, showed an improvement in proteinuria with 1 to 6 courses of high-dose IVIg (61). Beneficial effects of IVIg have also been shown in a small Italian cohort (n=12) with refractory SLE, and a Bulgarian cohort (n=58) with treatment refractory chronic glomerulonephritis (62, 63). The main advantage of IVIg is that it is not immunosuppressive, and may therefore be useful in patients who have been over-immunosuppressed and are at risk of infection.
Interleukin-2 therapy
Low-dose interleukin-2 (IL-2) has been used to influence the balance of T cells in SLE patients away from conventional (effector) phenotypes to regulatory phenotypes (64). A small series of 10 patients with refractory or relapsed LN was treated with low-dose, recombinant IL-2 and after 12 weeks, seven patients had a fall in proteinuria of ≥50%, and 2 of these had a complete renal response (65). This was accompanied by a significant expansion of peripheral T regulatory cells (65). IL-2 immunotherapy to restore T-cell regulatory homeostasis in LN may be a novel therapeutic approach for resistant LN, but needs to be tested in larger, randomized-controlled trials.
Anti-complement therapy
The importance of the complement system in the pathogenesis of kidney injury in LN is well-established by experimental models, and is believed to translate to human disease (66). Since refractory LN is characterized by persistent kidney inflammation, anti-complement therapies may be useful in controlling this inflammation, especially if complement-driven. While there are now several complement-targeted therapeutics in various stages of development, eculizumab, a monoclonal antibody that binds to complement component 5 (C5) and prevents formation of C5a and the membrane attack complex (C5b-9) has had the longest clinical exposure. Most of the reports of eculizumab and SLE/LN have been in the context of concomitant thrombotic microangiopathy (TMA). However, a report detailed the successful use of eculizumab for the treatment of a patient with severe LN and no TMA who had failed CYC, MMF, RTX and tacrolimus. During follow-up over 18 months, the patient achieved a sustained and rapid improvement in kidney function and proteinuria (67). While much more evidence will be needed, understanding in whom uncontrolled disease is mediated by complement would facilitate the application of anti-complement therapies in resistant LN.
Chimeric antigen receptor-modified T cell therapy
The principle of CAR-T cell technology is to engineer autologous T cells to express a specific antigen receptor so the modified T cells can recognize and only kill those cells that express the antigen (68). This targeted effect of T-cells is much faster and longer lasting compared to monoclonal antibody therapy. Chimeric antigen receptor (CAR)–modified T cells have mostly been developed to recognize CD19 and other B-cell surface antigens for use in refractory or relapsed B cell malignancies. Harnessing B cell-directed CAR T-cells to treat SLE has garnered attention as a way to deplete autoreactive B cells completely and for a long duration (68).
A recent case report described a 20 year old with refractory SLE/LN treated with CAR-T cells after achieving lymphodepletion with fludarabine and cyclophosphamide. Proteinuria decreased dramatically and the SLEDAI score fell from 16 at baseline to 0 at follow-up (68). Another case report described a 41-year-old with stage IV diffuse large B-cell lymphoma (DLBCL) and a 20-year-history of SLE who was treated with CD19-BCMA compound CAR-T cells with dual targeting of CD19 on B-cells and BCMA (CD269) on plasma cells (69). The patient’s SLE remained stable and DLBCL in remission for over 23 months despite receiving no additional immunosuppressive or chemotherapy.
Anifrolumumab
Anifrolumumab, recently approved by the US Food and Drug Administration for the treatment of SLE, is a fully human, IgG1κ monoclonal antibody to the type 1 interferon receptor subunit 1, and inhibits signaling by all type 1 interferons (70,71). Anifrolumumab is currently being evaluated for LN, and results from a phase II trial will be available later this year (72). While there are no current data regarding the use of anifrolumumab in refractory LN, a transcriptomic analysis of protocol kidney biopsies after induction therapy showed that interferon pathway transcripts remained upregulated in the kidneys of patients who did not respond to therapy, but declined in patients who had a complete clinical response after induction (73). These data raise the possibility that LN patients who do not respond to SOC treatment may have ongoing intra-renal interferon activity that could respond to an interferon antagonist.
Plasma exchange
Like IVIg, plasma exchange (PLEX) can be administered without significantly immunosuppressing patients, if plasma rather than an albumin solution is added back. However, the evidence for PLEX or immunoadsorption in refractory LN is minimal, consisting mostly of single case reports and small observational studies. In contrast, a well-designed randomized controlled trial of PLEX added to SOC therapy in patients with severe (not necessarily refractory) LN did improve clinical outcomes (74).
Hematopoietic stem cell transplantation
Autologous stem cell transplantation temporarily resets the adaptive immune system and depletes autoreactive immunologic memory (75). In two large studies of patients with SLE the probability of 5-year disease-free survival was 50% after autologous stem cell transplantation (76, 77). More recently, 22 patients with refractory/relapsing LN underwent autologous stem cell transplantation. At 72 months median follow-up, 18 patients were in complete clinical remission (78). The relapse rate was 27% and treatment-related mortality was 5%. Given the higher risk of short-term mortality associated with autologous stem cell transplantation, and the risk of recurrence after transplant, we consider this approach only when other options are exhausted.
Mesenchymal stem cell transplantation
Mesenchymal stem cells (MSCs) have immunomodulatory properties and have shown therapeutic benefits when given to patients with autoimmune conditions. In patients with LN and in mouse models of LN, transplantation of MSCs has been shown to suppress autoimmunity and restore kidney function (79). MSC transplantation induces regulatory immune cells and suppresses Th1, Th17, T follicular helper cell, and B-cell responses (79). Although several single-arm studies have shown a therapeutic benefit of MSCs in patients with LN refractory to conventional treatments, when tested in a randomized double blind trial, albeit a small cohort, an additional benefit of MSCs over SOC was not observed (80). Better designed, larger, randomized-controlled trials are needed to evaluate role of MSCs in treatment of LN patients.
Discussion
The management of LN that is truly refractory to SOC is challenging diagnostically and therapeutically. There is no standard definition or specific laboratory test for refractory disease, and because non-response to treatment may have several etiologies, refractory LN is a diagnosis of exclusion. It is critical to understand all of the contributing factors to non-response before labeling a patient as treatment resistant, because the consequence of this diagnosis is generally piling on more immunosuppression and increasing the risk of adverse iatrogenic outcomes. We suggest the most common causes of non-response in the lupus and LN populations are non-adherence to treatment and under-treatment with conventional drugs. While adherence issues can be difficult to address, and titrating SOC therapies is difficult without established therapeutic levels for most drugs, neither requires escalating immunosuppression. For patients who are resistant to SOC treatments, the choice of what should be used next is based on evidence that is of low to modest quality. There have been no randomized clinical trials of rescue therapies for LN. As such, the studies supporting regimens for refractory disease are mainly observational, uncontrolled, and suffer from inclusion of a heterogeneous group of patients previously treated with variable immunosuppression. Of all the therapeutic approaches to refractory disease, treatment with the anti-CD20 biologic rituximab has garnered the most attention. There have been a sufficient number of such studies that several systematic analyses have shown improved outcomes in resistant patients after the addition of rituximab. The fact that there appears to be a favorable signal for anti-CD20 suggests this may be a reasonable first approach to the refractory patient, and eliminating autoreactive B cells that may not have been depleted by other therapies fits into most pathogenic constructs of SLE. Nonetheless, it would be prudent to study anti-CD20 biologics in refractory LN in a well-controlled trial of patients having uniformly defined refractory disease. The role of newly approved LN drugs and drugs that are in development remains to be seen. The possibility that molecular evaluation of the kidneys from refractory patients may provide clues to inflammatory pathways not controlled by conventional treatments is exciting, and suggests that in the future patients with refractory disease may be able to be treated more precisely, thereby avoiding immunosuppressive roulette.
Finally, given the ongoing SARS-COV-2 pandemic, it cannot be overstated that many of the approaches to refractory disease will put these patients at high risk for severe infection, and may prevent adequate protection from vaccination. We suggest that pre-exposure monoclonal antibodies be given to refractory patients, and that all other safety measures including social distancing and masking be maintained.
Acknowledgments
This work was funded by NIH RO1 AR071947 (BHR)
References:
- 1.Parikh SV, Almaani S, Brodsky S, Rovin BH. Update on lupus nephritis: core curriculum 2020. Am J Kidney Dis. 2020;76:265–281. [DOI] [PubMed] [Google Scholar]
- 2.Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. Am J Med Sci. 2013;346:319–323. [DOI] [PubMed] [Google Scholar]
- 3.Anders HJ, Hiepe F. Treatment options for refractory lupus nephritis. Clin J Am Soc Nephrol. 2019;14:653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moroni G, Ponticelli C. The multifaceted aspects of refractory lupus nephritis. Expert Rev Clin Immunol. 2015;11:281–8. [DOI] [PubMed] [Google Scholar]
- 5.Feldman CH, Collins J, Zhang Z, Xu C, Subramanian SV, Kawachi I, et al. Azathioprine and mycophenolate mofetil adherence patterns and predictors among medicaid beneficiaries with systemic lupus erythematosus. Arthritis Care Res (Hoboken). 2019;71:1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia Popa-Lisseanu MG, Greisinger A, Richardson M, O’Malley KJ, Janssen NM, Marcus DM, et al. Determinants of treatment adherence in ethnically diverse, economically disadvantaged patients with rheumatic disease. J Rheumatol. 2005;32:913–9. [PubMed] [Google Scholar]
- 7.Durcan L, Clarke WA, Magder LS, Petri M. Hydroxychloroquine blood levels in systemic lupus erythematosus: clarifying dosing controversies and improving adherence. J Rheumatol. 2015;42:2092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rainsford KD, Parke AL, Clifford-Rashotte M, Kean WF. Therapy and pharmacological properties of hydroxychloroquine and chloroquine in treatment of systemic lupus erythematosus, rheumatoid arthritis and related diseases. Inflammopharmacology. 2015;23:231–69. [DOI] [PubMed] [Google Scholar]
- 9.Costedoat-Chalumeau N, Houssiau FA. Improving medication adherence in patients with lupus nephritis. Kidney Int. 2021;99:285–287. [DOI] [PubMed] [Google Scholar]
- 10.Mok CC. Therapeutic monitoring of the immuno-modulating drugs in systemic lupus erythematosus. Expert Rev Clin Immunol. 2017;13:35–41. [DOI] [PubMed] [Google Scholar]
- 11.Luszczynska P, Pawinski T. Therapeutic drug monitoring of mycophenolic acid in lupus nephritis: a review of current literature. Ther Drug Monit 2015; 37:711–717 [DOI] [PubMed] [Google Scholar]
- 12.Van Gelder T, Huizinga RB, Noukens J, Lisk L, Solomons N. Use of therapeutic drug monitoring does not add clinical value for voclosporin in patients with lupus nephritis [abstract no. PO1918]. J Am Soc Nephrol. 2020;31:594. [Google Scholar]
- 13.Melander C, Sallée M, Trolliet P, Candon S, Belenfant X, Daugas E, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol. 2009;4:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, et al. 2019 Update of the joint european league against rheumatism and european renal association-european dialysis and transplant association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79:713–723. [DOI] [PubMed] [Google Scholar]
- 15.Dall’Era M, Stone D, Levesque V, Cisternas M, Wofsy D. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res (Hoboken). 2011. Mar;63(3):351–7. doi: 10.1002/acr.20397. Epub 2010 Nov 15. [DOI] [PubMed] [Google Scholar]
- 16.Houssiau FA, Vasconcelos C, D’Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term follow-up of patients in the euro-lupus nephritis trial. Arthritis Rheum. 2004;50:3934–40. [DOI] [PubMed] [Google Scholar]
- 17.Mackay M, Dall’Era M, Fishbein J, Kalunian K, Lesser M, Sanchez-Guerrero J, et al. Establishing surrogate kidney end points for lupus nephritis clinical trials: development and validation of a novel approach to predict future kidney outcomes. Arthritis Rheumatol. 2019;71:411–419. [DOI] [PubMed] [Google Scholar]
- 18.Pagni F, Galimberti S, Goffredo P, Basciu M, Malachina S, Pilla D, et al. The value of repeat biopsy in the management of lupus nephritis: an international multicentre study in a large cohort of patients. Nephrol Dial Transplant. 2013;28:3014–23. [DOI] [PubMed] [Google Scholar]
- 19.Narváez J, Ricse M, Gomà M, Mitjavila F, Fulladosa X, Capdevila O, et al. The value of repeat biopsy in lupus nephritis flares. Medicine (Baltimore). 2017;96:e7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alvarado AS, Malvar A, Lococo B, Alberton V, Toniolo F, Nagaraja HN, et al. The value of repeat kidney biopsy in quiescent Argentinian lupus nephritis patients. Lupus. 2014;23:840–7. [DOI] [PubMed] [Google Scholar]
- 21.Malvar A, Pirruccio P, Alberton V, Lococo B, Recalde C, Fazini B, et al. Histologic versus clinical remission in proliferative lupus nephritis. Nephrol Dial Transplant. 2017;32:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman BI, Limou S, Ma L, Kopp JB. APOL1-associated nephropathy: a key contributor to racial disparities in ckd. Am J Kidney Dis. 2018;72:S8–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freedman BI, Langefeld CD, Andringa KK, Croker JA, Williams AH, Garner NE, et al. End-stage renal disease in African Americans with lupus nephritis is associated with APOL1. Arthritis Rheumatol. 2014;66:390–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vajgel G, Lima SC, Santana DJS, Oliveira CBL, Costa DMN, Hicks PJ, et al. Effect of a Single Apolipoprotein L1 Gene Nephropathy Variant on the Risk of Advanced Lupus Nephritis in Brazilians. J Rheumatol. 2020;47:1209–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frémond ML, Nathan N. COPA syndrome, 5 years after: Where are we? Joint Bone Spine. 2021;88:105070. (Epub 2020) [DOI] [PubMed] [Google Scholar]
- 26.Kalloo S, Aggarwal N, Mohan P, Radhakrishnan J. Lupus nephritis: treatment of resistant disease. Clin J Am Soc Nephrol. 2013;8:154–61. [DOI] [PubMed] [Google Scholar]
- 27.Ripoll È, Merino A, Grinyó JM, Torras J. New approaches for the treatment of lupus nephritis in the 21st century: from the laboratory to the clinic. Immunotherapy. 2013;5:1089–101. [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021. Oct;100(4S):S1–S276. [DOI] [PubMed] [Google Scholar]
- 29.Furie R, Rovin BH, Houssiau F, Malvar A, Teng YKO, Contreras G, et al. Two-year, randomized, controlled trial of belimumab in lupus nephritis. N Engl J Med. 2020;383:1117–1128. [DOI] [PubMed] [Google Scholar]
- 30.Rovin BH, Teng YKO, Ginzler EM, Arriens C, Caster DJ, Romero-Diaz J, et al. Efficacy and safety of voclosporin versus placebo for lupus nephritis (AURORA 1): a double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2021;397:2070–2080. [DOI] [PubMed] [Google Scholar]
- 31.Rovin BH, Furie R, Teng YKO, Contreras G, Malvar A, Yu X, et al. A secondary analysis of the belimumab international study in lupus nephritis trial examined effects of belimumab on kidney outcomes and preservation of kidney function in patients with lupus nephritis. Kidney Int. 2021:S0085–2538 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 32.Anders HJ, Rovin B, Zhao MH, Malvar A, Hiromura K, Jones-Leone AR, et al. Effects of belimumab (bel) on renal outcomes in patients (pts) with relapsed and newly diagnosed active lupus nephritis (ln). J Am Soc Nephrol Suppl. 2021; 32: 46–49. (Abstract) [Google Scholar]
- 33.Choi CB, Won S, Bae SC. Outcomes of multitarget therapy using mycophenolate mofetil and tacrolimus for refractory or relapsing lupus nephritis. Lupus. 2018;27:1007–1011. [DOI] [PubMed] [Google Scholar]
- 34.Cortes-Hernandez J, Torres-Salido MT, Medrano AS, Tarres MV, Ordi-Ros J. Long- term outcomes–mycophenolate mofetil treatment for lupus nephritis with addition of tacrolimus for resistant cases. Nephrol Dial Transplant 2010;25:3939–48. [DOI] [PubMed] [Google Scholar]
- 35.Jesus D, Rodrigues M, da Silva JAP, Inês L. Multitarget therapy of mycophenolate mofetil and cyclosporine A for induction treatment of refractory lupus nephritis. Lupus. 2018;27:1358–1362. [DOI] [PubMed] [Google Scholar]
- 36.Barmettler S, Ong MS, Farmer JR, Choi H, Walter J. Association of immunoglobulin levels, infectious risk, and mortality with rituximab and hypogammaglobulinemia. JAMA Netw Open. 2018;1:e184169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leandro MJ. B-cell subpopulations in humans and their differential susceptibility to depletion with anti-CD20 monoclonal antibodies. Arthritis Res Ther. 2013;15:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunnarsson I, Sundelin B, Jonsdottir T, Jacobson SH, Henriksson EW, van Vollenhoven RF. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 2007;56:1263–1272. [DOI] [PubMed] [Google Scholar]
- 39.Goswami RP, Sircar G, Sit H, Ghosh A, Ghosh P. Cyclophosphamide versus mycophenolate versus rituximab in lupus nephritis remission induction: a historical head-to-head comparative study. J Clin Rheumatol. 2019;25:28–35. [DOI] [PubMed] [Google Scholar]
- 40.Davies RJ, Sangle SR, Jordan NP, Aslam L, Lewis MJ, Wedgwood R, et al. Rituximab in the treatment of resistant lupus nephritis: therapy failure in rapidly progressive crescentic lupus nephritis. Lupus. 2013;22:574–82. [DOI] [PubMed] [Google Scholar]
- 41.Contis A, Vanquaethem H, Truchetet ME, Couzi L, Rigothier C, Richez C, et al. Analysis of the effectiveness and safety of rituximab in patients with refractory lupus nephritis: a chart review. Clin Rheumatol. 2016;35:517–22. [DOI] [PubMed] [Google Scholar]
- 42.Jónsdóttir T, Zickert A, Sundelin B, Henriksson EW, van Vollenhoven RF, Gunnarsson I. Long-term follow-up in lupus nephritis patients treated with rituximab--clinical and histopathological response. Rheumatology (Oxford). 2013;52:847–55. [DOI] [PubMed] [Google Scholar]
- 43.Lindholm C, Börjesson-Asp K, Zendjanchi K, Sundqvist AC, Tarkowski A, Bokarewa M. Longterm clinical and immunological effects of anti-CD20 treatment in patients with refractory systemic lupus erythematosus. J Rheumatol. 2008;35:826–33. [PubMed] [Google Scholar]
- 44.Iwata S, Saito K, Hirata S, Ohkubo N, Nakayamada S, Nakano K, et al. Efficacy and safety of anti-CD20 antibody rituximab for patients with refractory systemic lupus erythematosus. Lupus. 2018;27:802–811. [DOI] [PubMed] [Google Scholar]
- 45.Iaccarino L, Bartoloni E, Carli L, Ceccarelli F, Conti F, De Vita S, et al. Efficacy and safety of off-label use of rituximab in refractory lupus: data from the Italian Multicentre Registry. Clin Exp Rheumatol. 2015;33:449–56. [PubMed] [Google Scholar]
- 46.Weidenbusch M, Römmele C, Schröttle A, Anders HJ. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant. 2013;28:106–11. [DOI] [PubMed] [Google Scholar]
- 47.Alshaiki F, Obaid E, Almuallim A, Taha R, El-Haddad H, Almoallim H. Outcomes of rituximab therapy in refractory lupus: A meta-analysis. Eur J Rheumatol. 2018;5:118–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furie RA, Aroca G, Cascino MD, Garg JP, Rovin BH, Alvarez A, et al. B-cell depletion with obinutuzumab for the treatment of proliferative lupus nephritis: a randomised, double-blind, placebo-controlled trial. Ann Rheum Dis. 2021:annrheumdis-220920. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiepe F, Dörner T, Hauser AE, Hoyer BF, Mei H, Radbruch A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol. 2011;7:170–8. [DOI] [PubMed] [Google Scholar]
- 50.Ichikawa HT, Conley T, Muchamuel T, Jiang J, Lee S, Owen T, et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of type I interferon and autoantibody-secreting cells. Arthritis Rheum. 2012;64:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segarra A, Arredondo KV, Jaramillo J, Jatem E, Salcedo MT, Agraz I, et al. Efficacy and safety of bortezomib in refractory lupus nephritis: a single-center experience. Lupus. 2020;29:118–125. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, Liu Z, Huang L, Hou J, Zhou M, Huang X, et al. The short-term efficacy of bortezomib combined with glucocorticoids for the treatment of refractory lupus nephritis. Lupus. 2017;26:952–958. [DOI] [PubMed] [Google Scholar]
- 53.Singh M, Thomas VM, Mulay S. Bortezomib-induced motor neuropathy: A case report. J Oncol Pharm Pract. 2020;26:1549–1552. [DOI] [PubMed] [Google Scholar]
- 54.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol. 2011;12:431–40. [DOI] [PubMed] [Google Scholar]
- 55.Ostendorf L, Burns M, Durek P, Heinz GA, Heinrich F, Garantziotis P, et al. Targeting CD38 with daratumumab in refractory systemic lupus erythematosus. N Engl J Med. 2020;383:1149–1155. [DOI] [PubMed] [Google Scholar]
- 56.Kezar Life Sciences. A Study of KZR-616 in Patients With SLE With and Without Lupus Nephritis (MISSION). ClinicalTrials.gov Identifier: NCT03393013 [Google Scholar]
- 57.Cao H, Rao Y, Liu L, Lin J, Yang H, Zhang X, et al. The efficacy and safety of leflunomide for the treatment of lupus nephritis in chinese patients: systematic review and meta-analysis. PLoS One. 2015;10:e0144548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tam LS, Li EK, Wong CK, Lam CW, Li WC, Szeto CC. Safety and efficacy of leflunomide in the treatment of lupus nephritis refractory or intolerant to traditional immunosuppressive therapy: an open label trial. Ann Rheum Dis. 2006;65:417–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wenderfer SE, Thacker T. Intravenous immunoglobulin in the management of lupus nephritis. Autoimmune Dis. 2012;2012:589359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. [DOI] [PubMed] [Google Scholar]
- 61.Levy Y, Sherer Y, George J, Rovensky J, Lukac J, Rauova L, et al. Intravenous immunoglobulin treatment of lupus nephritis. Semin Arthritis Rheum. 2000;29:321–7. [DOI] [PubMed] [Google Scholar]
- 62.Francioni C, Galeazzi M, Fioravanti A, Gelli R, Megale F, Marcolongo R. Long-term i.v. Ig treatment in systemic lupus erythematosus. Clin Exp Rheumatol. 1994;12:163–8. [PubMed] [Google Scholar]
- 63.Monova D, Belovezhdov N, Altunkova I, Monov S. Intravenous immunoglobulin G in the treatment of patients with chronic glomerulonephritis: clinical experience lasting 15 years. Nephron. 2002;90:262–6. [DOI] [PubMed] [Google Scholar]
- 64.Suárez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC. T cells and autoimmune kidney disease. Nat Rev Nephrol. 2017;13:329–43. [DOI] [PubMed] [Google Scholar]
- 65.Zhang X, Feng R, Shao M, Wang Y, Sun X, He J. Low-Dose Interleukin-2 as an Alternative Therapy for Refractory Lupus Nephritis. Rheumatol Ther. 2021;8:1905–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sekine H, Ruiz P, Gilkeson GS, Tomlinson S. The dual role of complement in the progression of renal disease in NZB/W F(1) mice and alternative pathway inhibition. Mol Immunol. 2011; 49:317–323. [DOI] [PubMed] [Google Scholar]
- 67.Pickering MC, Ismajli M, Condon MB, McKenna N, Hall AE, Lightstone L, et al. Eculizumab as rescue therapy in severe resistant lupus nephritis. Rheumatology (Oxford). 2015;54:2286–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mougiakakos D, Kronke G, Volkl S, Kretschmann S, Aigner M, Kharboutli S, et al. CD19-targeted CAR T cells in refractory systemic lupus erythematosus. N Engl J Med. 2021;385:567–9. [DOI] [PubMed] [Google Scholar]
- 69.Zhang W, Feng J, Cinquina A, Wang Q, Xu H, Zhang Q, et al. treatment of systemic lupus erythematosus using BCMA-CD19 compound CAR. Stem Cell Rev Rep. 2021;17:2120–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morand EF, Furie R, Tanaka Y, Bruce IN, Askanase AD, Richez C, et al. TULIP-2 Trial Investigators. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N Engl J Med. 2020;382:211–221. [DOI] [PubMed] [Google Scholar]
- 71.Furie R, Morand E, Bruce I, Manzi S, Kalunian K, Vital E, et al. Type I interferon inhibitor anifrolumab in active systemic lupus erythematosus (TULIP-1): a randomized, controlled, phase 3 trial. Lancet Rheumatol. 2019;1:e208–e219. [DOI] [PubMed] [Google Scholar]
- 72.Zeneca Astra. A Multicentre, Randomised, Double-blind, Placebo-controlled, Phase 2 Study Evaluating the Efficacy and Safety of Anifrolumab in Adult Subjects With Active Proliferative Lupus Nephritis. clinical trial NCT02547922. https://clinicaltrials.gov/ct2/show/NCT02547922 [Google Scholar]
- 73.Parikh SV, Malvar A, Song H, Alberton V, Lococo B, Vance J, et al. Molecular imaging of the kidney in lupus nephritis to characterize response to treatment. Transl Res. 2017;182:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lewis EJ, Hunsicker LG, Lan SP, Rohde RD, Lachin JM. A controlled trial of plasmapheresis therapy in severe lupus nephritis. The lupus nephritis collaborative study group. N Engl J Med. 1992;326:1373–1379. [DOI] [PubMed] [Google Scholar]
- 75.Alexander T, Thiel A, Rosen O, Massenkeil G, Sattler A, Kohler S, et al. Depletion of autoreactive immunologic memory followed by autologous hematopoietic stem cell transplantation in patients with refractory SLE induces long-term remission through de novo generation of a juvenile and tolerant immune system. Blood. 2009;113:214–23. [DOI] [PubMed] [Google Scholar]
- 76.Jayne D, Passweg J, Marmont A, Farge D, Zhao X, Arnold R,et al. European group for blood and marrow transplantation; european league against rheumatism registry. autologous stem cell transplantation for systemic lupus erythematosus. Lupus. 2004;13:168–76. [DOI] [PubMed] [Google Scholar]
- 77.Burt RK, Traynor A, Statkute L, Barr WG, Rosa R, Schroeder J, et al. Nonmyeloablative hematopoietic stem cell transplantation for systemic lupus erythematosus. JAMA. 2006;295:527–535. [DOI] [PubMed] [Google Scholar]
- 78.Huang X, Chen W, Ren G, Zhao L, Guo J, Gong D, et al. autologous hematopoietic stem cell transplantation for refractory lupus nephritis. Clin J Am Soc Nephrol. 2019;14:719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li W, Chen W, Sun L. An update for mesenchymal stem cell therapy in lupus nephritis. Kidney Dis (Basel). 2021;7:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436–1439. [DOI] [PubMed] [Google Scholar]


