Abstract
Background
The occurrence of chronic pulmonary aspergillosis (CPA) among drug sensitive pulmonary tuberculosis (PTB) patients on optimal therapy with persistent symptoms was investigated.
Methods
We consecutively enrolled participants with PTB with persistent pulmonary symptoms after 2 months of anti-TB treatment at Mulago Hospital, Kampala, Uganda between July 2020, and June 2021. CPA was defined as a positive Aspergillus-specific IgG/IgM immunochromatographic test (ICT), a cavity with or without a fungal ball on chest x-ray (CXR), and compatible symptoms >3 months.
Results
We enrolled 162 participants (median age 30 years; IQR: 25 — 40), 97 (59.9%) were male, 48 (29.6%) were HIV-infected, and 15 (9.3%) had prior PTB. Thirty-eight (23.4%) sputum samples grew A. niger and 13 (8.0%) A. fumigatus species complexes. Six (3.7%) participants had intra-cavitary fungal balls, and 52 (32.1%) had cavities. Overall, 32 (19.8%) participants had CPA. CPA was associated with prior PTB (adjusted odds ratio (aOR): 6.61, 95% CI: 1.85 — 23.9, p=0.004), and far advanced CXR changes (aOR: 4.26, 95%CI: 1.72 — 10.52, p=0.002). The Aspergillus IgG/IgM ICT was positive in 10 (31.3%) participants with CPA.
Conclusions:
CPA may cause persistent respiratory symptoms in up to one-fifth of patients after intensive treatment for PTB. The Aspergillus IgG/IgM ICT positivity rate was very low and may not be used alone for the diagnosis of CPA in Uganda.
Keywords: Pulmonary Tuberculosis, Persistent symptoms, Chronic Pulmonary Aspergillosis, Uganda
INTRODUCTION
Chronic pulmonary aspergillosis (CPA) is a heterogenous, destructive and debilitating parenchymal lung disease that predominantly occurs in patients with underlying pulmonary diseases or damage 1. Pulmonary tuberculosis (PTB) is an important risk factor for CPA 2,3, with an estimated 1.2 million cases of CPA estimated to complicate treated PTB globally 4.
Patients with CPA frequently present with prominent pulmonary and systemic symptoms, such as chronic productive cough, hemoptysis, chest pain, weight loss, fevers, and fatigue, which are clinically indistinguishable from PTB symptoms leading to delayed and/or misdiagnosis 5. In addition, chest imaging findings in CPA such as fibrosis and cavitation overlap with PTB lesions, and may therefore be unrecognized and left untreated, leading to poor outcomes 5. Radiological phenotypes of CPA include 1) Aspergillus nodule – presenting as an isolated single or multiple pulmonary nodules, 2) simple aspergilloma – with a single well defined cavity with an intracavitary aspergilloma (fungal ball), 3) chronic cavitary pulmonary aspergillosis (CCPA) - a chronic cavitary disease with enlargement of existing cavities and formation of new cavity with or without a fungal ball and no demonstrable hyphal invasion of the parenchyma, and 4) chronic fibrosing pulmonary aspergillosis (CFPA), a late stage disease characterized by marked paracavitary and pleural fibrosis 6.
Uganda is a high TB burden country with an estimated TB prevalence of about 253 per 100,000 population 7. Patients with active TB may have persistent respiratory symptoms during or after successful completion of their treatment. Currently, it is unclear how often persistent symptoms in patients with treated TB are related to CPA. Most of the existing data available is on patients who have completed TB treatment and there is limited data on those still receiving treatment who present with persistent respiratory symptoms. For example, in a laboratory-based study on persons living with HIV in Uganda, Aspergillus-specific IgG antibodies were elevated in 9% of the participants at the end of TB treatment8. However, a full diagnostic work-up for CPA was not performed in them. In a population-based study of previously treated PTB patients in Northern Uganda, about 6.5% of the participants developed CPA within 2 years of TB treatment. 9
The goal of the intensive TB treatment phase (first 2 months) with 4 active anti-TB agents is to sterilize sputum and alleviate clinical symptoms 10. Therefore, we hypothesized that persistent symptoms of PTB after the intensive phase of drug sensitive PTB treatment may be due to co-existent or incident CPA. The aim of this study was 2-fold. First, we determined the prevalence and factors associated with CPA among patients with drug sensitive PTB with persistent symptoms after 2 months of anti-TB therapy, and secondly, we evaluated the diagnostic performance of an Aspergillus-specific IgG/IgM immunochromatographic test (ICT) for the diagnosis of CPA in Uganda.
METHODS
Study design
This was a cross-sectional study conducted at the National TB control center of Mulago National Referral Hospital (MNRH), Kampala, Uganda between 1st July 2020 and 30th June 2021.
Study Setting
The TB Unit at MNRH serves as the national TB treatment center in Uganda. The unit uses a mixed model of care, whereby, 1) very sick patients are hospitalized at the start of their TB treatment until clinically stable, and 2) outpatient care where patients continue treatment from the community under supervision. The unit manages about 1,500 TB patients annually, making it the largest treatment center in the country.
Study population
We enrolled all eligible patients 18 years and older with microbiologically confirmed drug sensitive PTB (DS-PTB) using GeneXpert MTB/RIF and persisting pulmonary and/or systemic symptoms despite 2 months of standard anti-TB treatment. Patients on second line anti-TB regimens, pregnant women, critically ill patients, and those with extra-pulmonary TB were excluded.
Sample size estimation and study procedure
Using previous data on a 12% seroprevalence of Aspergillus IgG among patients with active TB in Uganda 8, at 95% confidence interval (CI), power of 80% and type I error of 5%, a sample size of 162 participants was estimated.
For all eligible participants, a trained medical officer collected data on clinical and demographic characteristics such as age, sex, HIV status, clinical symptoms, duration of TB treatment, prior history of TB diagnosis, occupation, income, and education status using a standardized semi-structured questionnaire. Participants were consecutively recruited until required sample size was reached.
Chest radiograph imaging and interpretation
A chest radiography (CXR) for each participant was performed at enrolment and interpreted by a qualified radiologist. The scale for grading severity of disease on CXR was defined specifically for this study by the radiologist. Minimal, moderate, and far advanced disease was classified based on involvement of one, two or more zones respectively and coupled to unilateral or bilateral lung disease. Two horizontal lines were used to divide the lungs into 3 regions (upper, middle, and lower) giving a total of 6 zones. Severity within each zone was then scored as 0 for no disease, 1 for less than 50% disease and 2 for greater than 50% disease. Unilateral zonal severity was reported as: 0 for no disease, 1–4 for mild disease, 5–8 for moderate disease, and 9–12 for severe disease. Lung zonal predominance was reported as: Upper zone, middle zone, lower zone, upper and middle, upper and lower, middle and lower, and upper, middle, and lower zone predominant. Presence of a cavity elevated the severity classification. Cavity size was recorded as the maximum diameter on planar imaging. A CXR was suggestive of CPA if it showed cavities, pericavitary infiltrates, a fungal ball (intracavitary content), pericavitary fibrosis, or pleural thickening.
Aspergillus IgG/IgM serology and sputum cultures
Blood samples were obtained for Aspergillus-specific IgG/IgM assay. Aspergillus-specific IgG/IgM ICT (LD Bio, Lyon, France) was performed according to the manufacturer’s instruction 11 and interpreted visually by a trained medical mycologist. Sputum samples were collected for high volume sputum (HVS) cultures to isolate Aspergillus species as previously described 12. All isolates were identified phenotypically and reported as species complex.
Diagnosis of chronic pulmonary aspergillosis
CPA was defined as the presence of persistent respiratory symptoms (at least a cough or hemoptysis lasting for 3 months or more), suggestive CXR findings (cavities, pericavitary infiltrates, a fungal ball (intra-cavitary content), pericavitary fibrosis, or pleural thickening), and evidence of Aspergillus infection (a positive HVS culture and/or Aspergillus IgG/IgM ICT) consistent with the Global Fungal Infection Forum II diagnostic criteria for CPA in resource-limited settings 13. The data from each patient was carefully evaluated by the lead investigator (MN) who made a provisional diagnosis of CPA. Two trained medical mycologists (FB and RK) experienced in the diagnosis of CPA independently evaluated MN’s provisional diagnosis and any discrepancy was resolved by an expert in CPA (DWD).
Data analysis
Categorical variables were summarized using proportions and percentages. Means and standard deviation were calculated for normally distributed continuous variables. To compare different variables or different categories, the chi square test, and student t test were applied after testing appropriate assumptions. For the regression analysis, the outcome variable of CPA diagnosis was dichotomized as 1 “yes CPA” 0” No CPA”. Univariate analysis was conducted for each independent variable and the outcome of CPA diagnosis. Factors with a p< 0.25 or previously known in the literature like age, sex of an individual, previous treatment for pulmonary Tuberculosis were considered for the multivariable regression mode. We assessed interaction through forming two-way interaction terms and performing likelihood ratio tests. Variables considered in the initial multivariable model were entered into a backward stepwise regression model. Confounding was assessed by considering a 10% or more change in the odds ratio with a model with the variable and one without. The goodness of fit of the model was assessed using the Hosmer-Lemeshow goodness of fit test. The odds ratios with their 95%CI are presented. A p<0.05 was considered statistically significant. STATA version 14.0 was used for data analysis.
Ethics
The Makerere University School of Biomedical Science Research and Ethics Committee (SBS-795) and the Uganda National Council for Science and Technology (HS739ES) approved the study protocol. All study participants provided informed written consent. The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to.
Results
Baseline clinical and radiological characteristics of the participants
Of the 162 participants enrolled, 97 (60.0 %) were male with a median age for all participants of 30 (IQR: 25 — 40) years. Forty-eight (29.6%) participants were living with HIV and 15 (9.3%) were previously treated for PTB. Fifty-two (32.1%) participants had history of alcohol use and 13 (8.0%) had a history of smoking tobacco. There was only 1 (0.6%) participant with history of COPD, bronchial asthma, and chicken pox each. None of the participants had a known history of sarcoidosis, whooping cough, or lung cancer.
All participants had chronic productive cough. Other frequent symptoms were, chest pain (92.6%, n=150), night sweats (90.7%, n=147), weight loss (51.8%, n=84), fatigue (46.3%, n=75), shortness of breath (41.4%, n = 67), loss of appetite (36.4%, n=59), and fever (19.1%, n=31). Twenty (12.4%) participants had hemoptysis (Table 2).
Table 2.
Table of other clinical characteristics
| Characteristics | All, (%) | CPA positive n (%) n=32 | CPA negative n(%) n=130 | P-value |
|---|---|---|---|---|
|
| ||||
| Shortness of breath | 67 (41.4) | 10 (27.8) | 57 (45.2) | 0.061 |
| Weight loss | 84 (51.8) | 18 (56.3) | 66 (50.8) | 0.900 |
| Fatigue | ||||
| yes | 75 (46.3) | 15 (46.9) | 60 (46.1) | 0.899 |
| Fever | ||||
| yes | 31 (19.1) | 5 (15.6) | 26 (20.0) | 0.669 |
| Loss of appetite | ||||
| yes | 59 (36.4) | 11 (34.4) | 48 (36.9) | 0.663 |
| Shortness of breath | ||||
| yes | 67 (41.4) | 9 (28.1) | 58 (44.6) | 0.061 |
| Hemoptysis | ||||
| Yes | 20 (12.4) | 7 (21.9) | 13 (10.0) | 0.142 |
| Night sweats | ||||
| Yes | 105 (64.8) | 18(56.3) | 87 (66.9) | 0.086 |
| Chest pain | ||||
| Yes | 150 (92.6) | 29 (90.6) | 121 (93.1) | 0.016 |
| Wheezing | ||||
| Yes | 15 (9.3) | 12 (9.2) | 3 (9.4) | 0.828 |
Twenty-nine (17.9%) participants had normal CXR findings. However, 63 (38.9%) participants had far advanced disease, and 70 (52.6%) had pathology in both lungs. Six (3.7%) participants had fungal balls, 52 (32.1%) had cavities, and 58 (35.8%) had pleural thickening (Table 3).
Table 3.
Table showing how X ray findings vary by CPA diagnosis
| Characteristic | All, n(%) | CPA positive n (%) n=32 | CPA negative n(%) n=130 | P value |
|---|---|---|---|---|
|
| ||||
| Disease location, n=133 | ||||
| Right chest | 36 (27.1) | 10 (25.7) | 26 (25.7) | |
| Left chest | 27 (20.3) | 9 (28.1) | 18 (17.8) | |
| both | 70 (52.6) | 13 (40.6) | 57 (56.4) | 0.273 |
| Number of lung zones involved by disease (n=162) | ||||
| 0 | 29 (17.9) | 0 (0.0) | 29 (22.3) | |
| 1 | 19 (11.7) | 2 (6.2) | 17 (13.1) | |
| 2 | 34 (21.0) | 10 (31.2) | 24 (18.5) | |
| 3 | 26 (16.1) | 8 (25.0) | 18 (13.8) | |
| 4 | 19 (11.7) | 5 (15.6) | 14 (10.8) | |
| 5 | 11 (6.8) | 1 (3.0) | 10 (7.7) | |
| 6 | 24 (14.8) | 6 (25.0) | 18 (13.8) | 0.009 |
| Infiltrates in upper lung field, n=100 | ||||
| Present in the right side | 31 (21.8) | 9 (32.1) | 22 (30.5) | |
| Present in the left side | 28 (25.2) | 9 (32.1) | 19 (26.4) | |
| Present in both | 41 (53.0) | 10 (35.7) | 31 (43.1) | 0.771 |
| Fibrosis/volume loss (n=83) | ||||
| Present in the right side | 40 (48.2) | 12 (42.9) | 28 (50.9) | |
| Present in the left side | 29 (34.9) | 11 (39.3) | 18 (32.7) | |
| Present in both | 14 (16.9) | 5 (17.9) | 9 (16.4) | 0.778 |
| Cavity n=52 | ||||
| Present in the right side | 28 (53.8) | 7 (36.8) | 21 (63.6) | |
| Present in the left side | 20 (38.5) | 11 (57.9) | 9 (27.3) | |
| Present in both | 4 (7.7) | 1 (5.3) | 1 (9.0) | 0.096 |
| Milliary disease, n=3 | ||||
| 1 | 1 (33.3) | 0 (0.0) | 1 (33.3) | |
| 3 | 2 (66.7) | 0 (0.0) | 2 (66.7) | |
| Adenopathy n=15 | ||||
| Present in the right side | 1 (6.7) | 1 (33.3) | 0 (0.0) | |
| Present in the left side | 3 (20.0) | 0 (0.0) | 3 (25.0) | |
| Present in both | 11 (73.3) | 2 (66.7) | 9 (75.0) | 0.275 |
| Pleural thickening, n=58 | ||||
| Present in the right side | 26 (44.8) | 9 (40.9) | 17 (47.2) | |
| Present in the left side | 20 (34.5) | 8 (35.4) | 12 (33.3) | |
| Present in both | 12 (20.7) | 5 (22.7) | 7 (19.4) | 0.883 |
| Pleural effusion, n=23 | ||||
| Present in the right side | 12 (52.2) | 4(50.0) | 8 (53.3) | |
| Present in the left side | 9 (39.1) | 4(50.0) | 5 (33.3) | |
| Present in both | 2 (8.7) | 0 (0.0) | 2 (13.3) | 0.695 |
| Fungal ball, n=6 | ||||
| Present in the right side | 5 (83.3) | 2 (66.7) | 3 (100.0) | |
| Present in the left side | 1 (16.7) | 1 (33.3) | 0 (0.0) | 1.00 |
| Nodular disease, n=27 | ||||
| Present in the right side | 2 (7.4) | 0 (0.0) | 2 (9.5) | |
| Present in the left side | 2 (7.4) | 1(16.7) | 1 (4.8) | |
| Present in both | 23 (85.2) | 5 (83.3) | 18 (85.7) | 0.659 |
| Other findings, n=10 | ||||
| Present in the right side | 5 (50.0) | 1 (50.0) | 4 (50.0) | |
| Present in the left side | 2 (20.0) | 0 (0.0) | 2 (25.0) | |
| Present in both | 3 (30.0) | 1 (50.0) | 2 (25.0) | 1.00 |
Prevalence of chronic pulmonary aspergillosis
Overall, 32/162 (19.8 %) participants had CPA (Figure 1). Overall, 11 (6.8%) participants tested positive on the Aspergillus IgG/IgM ICT (Figure 2). Thirty-eight (23.4%) sputum samples grew A. niger and 13 (8.0%) grew A. fumigatus species complexes (Figure 3). Nineteen (50%) of the 38 participants whose sputum samples grew A. niger complex had CPA. Comparatively, 7 (53.8 %) of 13 participants whose sputum samples grew A. fumigatus complex had CPA. Isolation of Aspergillus species was higher in CPA cases compared to non-CPA cases (19/32 (59.4%) versus 19/130 (14.6%), p <0.001). At multivariable analysis, factors statistically significantly associated with CPA were as follows; prior PTB (aOR: 6.61, 95%CI: 1.85 — 23.9, p=0.004), and far advanced CXR changes (aOR: 4.26, 95%CI: 1.72 — 10.52, p<0.002), (Table 1). Figures 4 and 5 illustrates imaging findings in a non-CPA and CPA cases, respectively.
Figure. 1. Study enrollment.
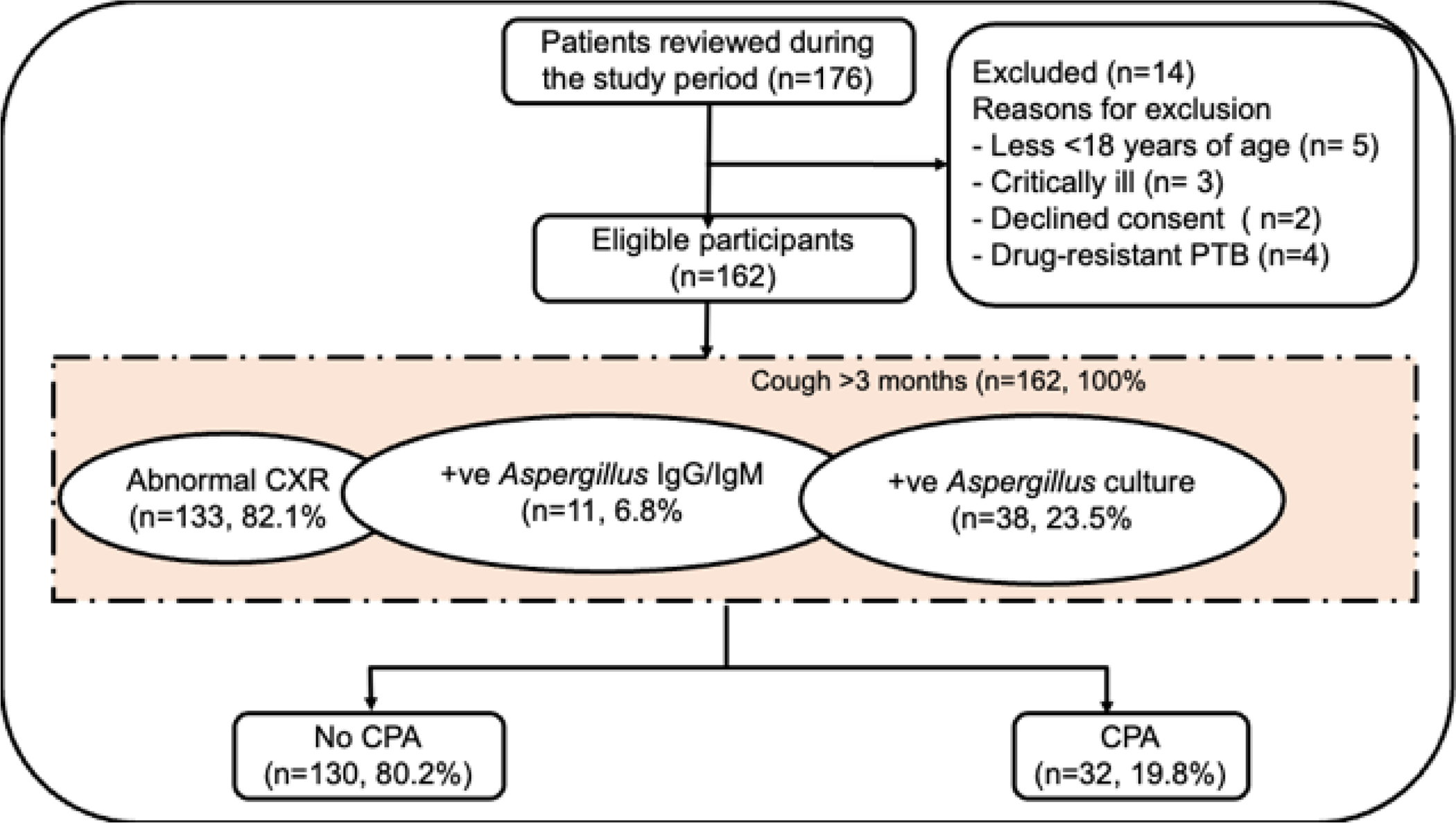
Figure 2. A positive Aspergillus IgG/IgM ICT.

Figure 3. A positive Aspergillus fumigatus fungal culture.
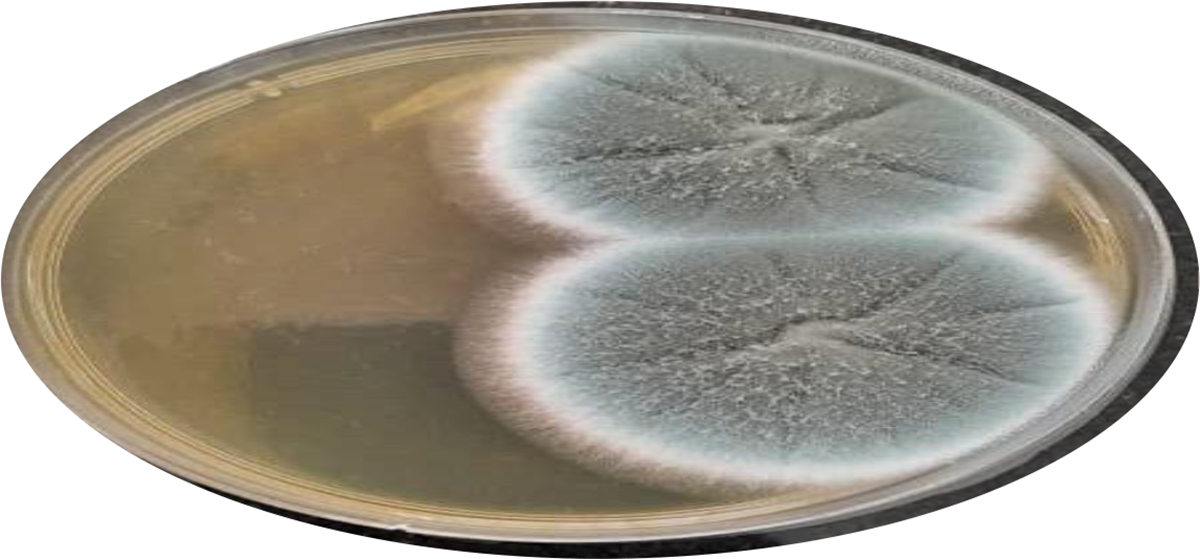
Table 1.
Factors associated to CPA in active PTB
| Characteristics | All, (%) | CPA negative n (%) n=130 | CPA positive n(%) n=32 | Crude OR, (95 %CI) | P value | Adjusted OR, (95 %CI) | P value |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Smoking status | |||||||
| No | 149 (92.0) | 122(93.9) | 27 (84.4) | Reference | |||
| Yes | 13 (8.0) | 8 (6.1) | 5 (15.6) | 2.82 (0.85–9.31) | 0.088 | ||
| Currently working | |||||||
| No | 98 (60.5) | 81 (62.3) | 17(53.1) | Reference | |||
| Yes | 64 (39.5) | 49 (37.7) | 15 (46.9) | 1.45 (0.67–3.18) | 0.343 | ||
| HIV | |||||||
| Negative | 114 (70.4) | 90 (69.2) | 24 (75.0) | Reference | Reference | ||
| Positive | 48 (29.6) | 39 (30.8) | 8 (25.0) | 0.75 (0.31–1.81) | 0.523 | 0.75 (0.28–2.01) | 0.567 |
| Sex | |||||||
| Male | 97 (60.0) | 73 (55.6) | 24(75.0) | Reference | Reference | ||
| Female | 65 (40.0) | 57 (43.8) | 8(25.0) | 0.43 (0.18–1.02) | 0.056 | 0.69 (0.26–1.88) | 0.478 |
| Prior TB | |||||||
| No | 147 (90.7) | 123 (94.6) | 28 (75.0) | Reference | Reference | ||
| Yes | 15 (9.3) | 7 (5.4) | 8 (25.0) | 5.85(1.94–17.67) | 0.002 | 6.66(1.85–23.9) | 0.004 |
| Alcohol intake | |||||||
| No | 110 (58.0) | 92 (70.8) | 18 (56.2) | Reference | |||
| Yes | 52 (32.0) | 38 (29.2) | 14 (43.8) | 1.88 (0. 85–4.17) | 0.075 | ||
| Age categorized (n=161) | |||||||
| <40 | 119 (74.0) | 99 (76.7) | 20(62.5) | Reference | Reference | ||
| 40–49 | 21 (13.0) | 17 (13.2) | 4 (12.5) | 1.16 (0.35–3.83) | 0.802 | 0.72 (0.16–3.23) | 0.670 |
| 50 and above years | 21 (13.0) | 13 (10.1) | 8(25.0) | 3.05 (1.12–8.31) | 0.030 | 2.32 (0.72–7.49) | 0.157 |
| Measles | |||||||
| No | 143 (88.3) | 116(89.2) | 27 (84.4) | Reference | |||
| Yes | 19 (11.7) | 14 (10.8) | 5 (15.6) | 1.53(0.51–4.63) | 0.447 | ||
| Disease extent on Chest X-ray (n=162) | 16 (9.9) | ||||||
| Normal/minimal moderate advanced disease | 99 (69.8) | 88 (67.7) | 11(34.4) | Reference | Reference | ||
| Far advanced disease | 63 (30.2) | 42 (32.3) | 21(65.6) | 4.0 (1.77–9.05) | 0.001 | 4.26(1.72–10.52) | 0.002 |
Figure 4. A CXR of a CPA negative case.
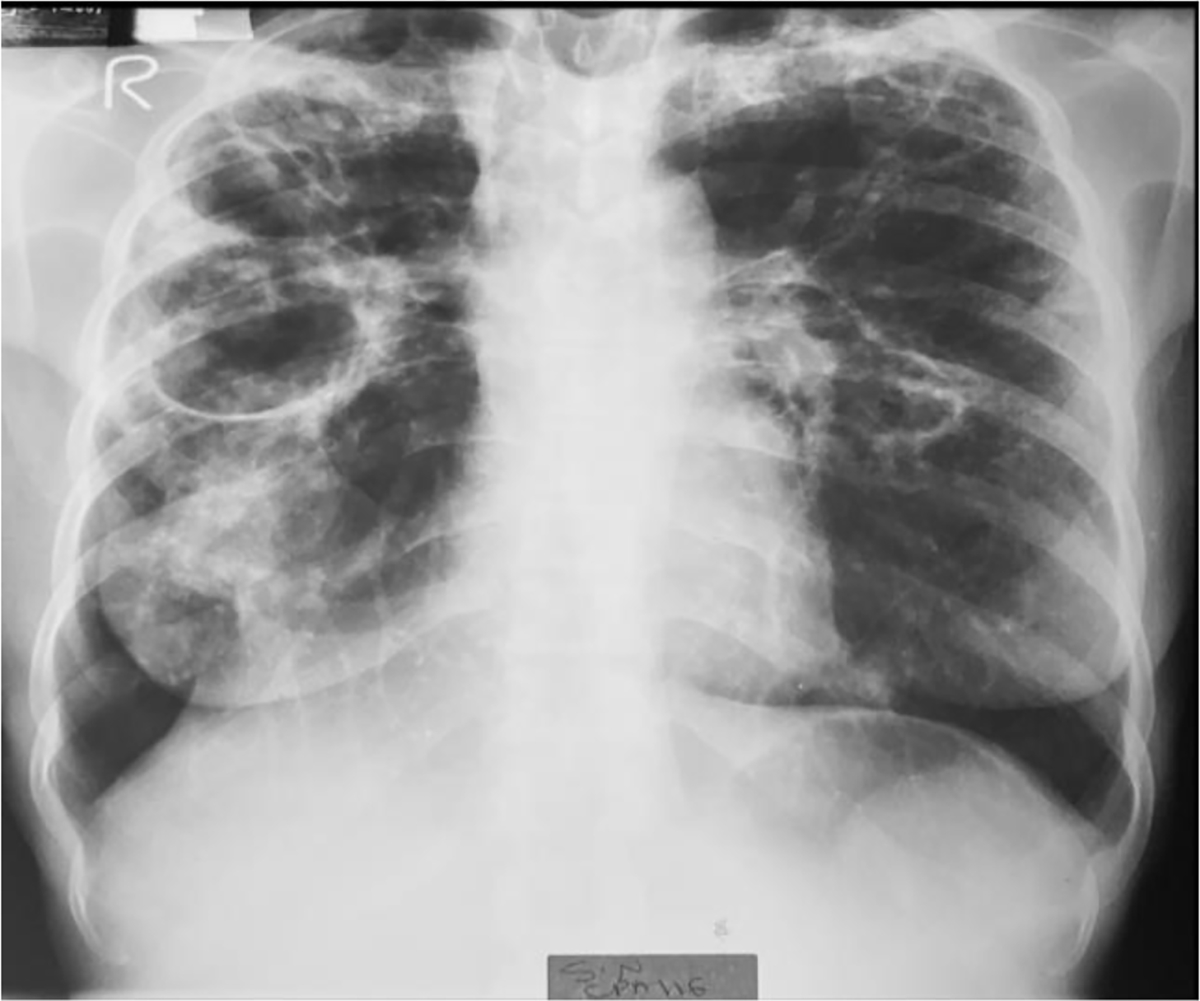
Thirty-six-year-old female who was enrolled with complaints of a persistent cough, fevers, nights sweats, and chest pain after 2 months of PTB therapy. She had no prior TB treatment history. She was HIV negative. CXR showed multiple bilateral cavities, extensive air space infiltrates, and fibrosis. Aspergillus LFD was negative and fungal culture showed no significant growth
Figure 5. A CXR of a confirmed CPA case.
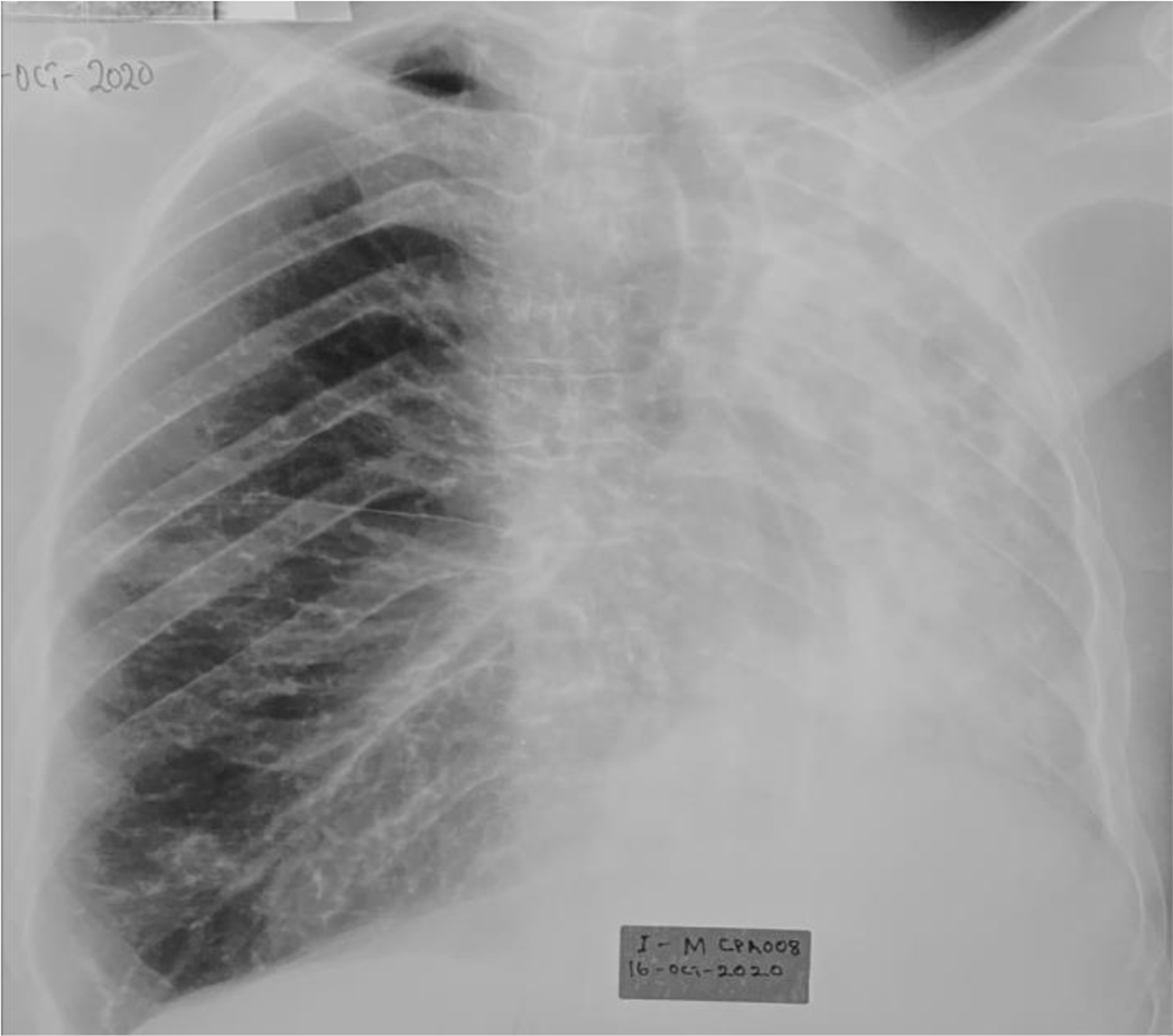
A 43-year male who was enrolled with complaints of a persistent cough, weight loss, and nights sweats after 2 months of PTB therapy. He had previously been treated for PTB 7 years ago and cured. He was HIV positive with a CD4 count of 423 cells/microlitre and a viral load of less than 50 copies/ml. He had history of smoking (1.5 pack years), and history of alcohol abuse. CXR showed far advanced disease with left lung collapse, and right linear opacities. Aspergillus LFD was positive and fungal culture grew A. fumigatus.
Aspergillus IgG/IgM ICT positivity rate
The Aspergillus IgG/IgM ICT was positive in 10 participants with CPA (10/32, 31.3%) and 1 participant without CPA (1/126, 0.8%) (p<0.001).
DISCUSSION
We found CPA in about 20% of the participants with persistent respiratory symptoms after 2 months of standard anti-TB treatment — the intensive phase. The aim of the intensive phase of PTB treatment is to rapidly achieve sputum M. tuberculosis culture conversion and alleviate pulmonary and systemic symptoms by using a combination of 4 anti-mycobacterial agents 14. Our findings suggest that CPA is a possible cause of persistent symptoms in microbiologically confirmed PTB patients on effective anti-TB regimen. In addition, our data showed that the odds of having CPA was about 7-fold higher in subjects with prior treated PTB compared to those receiving PTB treatment for the first time and over 4-fold higher in patients with far advanced CXR changes compared to those with normal or minimal CXR changes. Therefore, these group of patients should be targeted for a full evaluation for CPA. CPA is a treatable disease and early diagnosis and commencement of appropriate antifungal therapy significantly improves health-related quality of life of these patients 15.
A recent study evaluating over 1,200 cases of CPA in Africa identified current active or previous PTB as an underlying structural lung disease in over 70% of the cases 16. A few epidemiological studies from across Africa and other countries in Asia have reported varying CPA prevalence based on the population of TB patients studied. In an earlier study from Northern Uganda among post-TB patients, the prevalence of CPA was about 5% 9. In Nigeria, among patients with predominantly smear-negative PTB, about 9% of the participants had CPA at the end of their TB therapy17. In addition, among GeneXpert/smear-negative participants at their end of TB therapy in Indonesia, CPA was found in 22% of the participants 18. In Iran, among 124 patients with TB (94 with current TB and 30 with previous TB) in Iran, 3 (2.4 %) had simple aspergilloma and 14 (11.3 %) CCPA 19. In a more recent community-based study at Vietnam National Lung Hospital, 38 (54.3%) of 70 post-TB patients had CPA 20. The findings from these studies suggests that CPA prevalence varies depending on the population of TB patients studied. Of concern is the relatively high occurrence of CPA in patients being treated for PTB without microbiological confirmation, which could imply a misdiagnosis of CPA for a smear negative PTB, and the possibility of incident CPA during PTB therapy as suggested by the current study. Therefore, CPA should be considered not only in previously treated patients, but also in those currently receiving therapy for PTB with suggestive clinical features. CPA should also be a differential diagnosis in those with GeneXpert/smear negative sputum and should be investigated especially when symptoms persist. 21
Aspergillus serology is central in the diagnosis of CPA 22. Current clinical practice guidelines recommend it as a key test for the diagnosis of CPA 23. However, in this study, we found only about 31% of CPA patients to have a positive Aspergillus IgG serological test. This is in contrast with a previous report by Denning and colleagues where Aspergillus antibodies were elevated in nearly all patients 1. Recent studies have shown varying prevalence of Aspergillus positivity among CPA patients. In Nigeria, only 8.9% of CPA patients had a positive Aspergillus IgG 17, in Vietnam it was 89% 20, in Iran 44% and in Indonesia 80% 18. These could be due to the varying Aspergillus platforms used, Aspergillus species isolated, the timing of the surveys (during or after PTB treatment) and genetics of the varying study population studied. The LD Bio Aspergillus IgG/IgM has recently been validated for serological diagnosis of CPA at the point of care with excellent sensitivity and specificity 24. In Indonesia, the sensitivity of this ICT assay was 80% with a corresponding specificity of 70% 18. On the other hand, the sensitivity and specificity for the LDBio Aspergillus ICT were 91.6% and 98.0%, respectively in a cohort of CPA patients at the National Aspergillosis Centre in the UK24. There is evidence that Aspergillus IgG cut off values vary with population, and performance on the Aspergillus ICT is not an exception to the rule. We recently showed that Aspergillus IgG/IgM ICT used in this study had a 0% sensitivity in patients with fungal asthma, despite having a 91% sensitivity in a Caucasian population 25. Therefore, standardization of these serology platforms in the Ugandan population is urgent and justified.
Patents with CPA are often heavily symptomatic 26. All the patients in our study had chronic productive cough. Surprisingly, only about 12% of our study participants had hemoptysis. In the largest ever review of CPA patients in Africa, almost 60% reported hemoptysis, significantly contributing to their death 16. Hemoptysis is a common cause of death, even in developed countries, and bronchial artery embolization or surgery may be required to arrest bleeding 27. In a recent systematic review by Bongomin and colleagues 28, among nearly 900 CPA patients managed surgically in Africa, post-operative mortality was as low as 5%, suggesting a possible role of surgery in the management of CPA in Africa were antifungal agents are not widely available. However, when antifungals are available, affordable and accessible to the patients, and there is no indication for surgery, itraconazole or voriconazole are the preferred first line agents for the management of CPA 6. Antifungal agents alleviate symptoms, improve quality of life and overall survival 29,30.
A common post-PTB sequela is traction bronchiectasis, which is associated with Aspergillus bronchitis 31. These patients are usually highly symptomatic, but do not have cavitary changes or pleural thickening on chest imaging. However, they will usually have positive Aspergillus cultures of sputum and may have Aspergillus IgG detectable in serum 31. Patients with Aspergillus bronchitis usually respond to antifungal therapy 31. Some of the patients with probable CPA in this study may have Aspergillus bronchitis, depending on the precise imaging findings. This is an area for further evaluation.
This study has some limitations. This was a single center study, involving mainly patients from the central region of Uganda and may not be representative of the entire Uganda population. All isolates of Aspergillus were identified phenotypically, therefore, there was a possibility of misidentification of phenotypically indistinguishable species. Future multicenter studies recruiting nationally representative participants are recommended. There are no established diagnostic criteria for PTB-associated CPA, as previous criteria do not recognize CPA to occur during active PTB. In addition, there is no known diagnostic cut off for Aspergillus IgG suitable for the diagnosis of CPA in Ugandans and the Aspergillus IgG/IgM has not been previously validated among Ugandans, posing a risk of diagnostic inaccuracy, because diagnostic performance may vary by race and ethnicity. Also, we were unable to do bronchoscopies to further enhance recovery of Aspergillus and for biomarker assays on bronchoalveolar lavage sample. Lastly, we performed culture on a single sputum sample and positive cultures may have been from contaminations, particularly that a high proportion of cultures were positive for A. niger. Radiological phenotypes of CPA are defined using chest CT; therefore, we have missed some important imaging findings by using only CXR.
In conclusion, CPA is highly prevalent among active PTB patients with persistent respiratory symptoms, especially those with history of prior PTB treatment and those with advanced CXR changes in Uganda. CPA screening may be considered among this subset of patients. A. niger was a common species of Aspergillus in our setting. Aspergillus IgG/IgM LFA positivity rate was very low and thus may not be used singly for the diagnosis of CPA in Uganda.
Acknowledgement
We thank the MNRH TB unit for hosting the study. We’re grateful to the study team based there (Betty Namara, Jane Oyeru, Sam Nyole, and Esther Mbabazi) who were key in participant identification, recruitment, and retention. We’re grateful to the Infectious Diseases Institute (IDI) translational laboratory for managing the study samples.
An oral abstract of this work was presented at the 10th Trends in Medical Mycology (TIMM) Conference that happened in October 2021 in Aberdeen, Scotland.
Funding
Research reported in this publication was supported by the Fogarty International Center of the National Institutes of Health under grant #D43TW009345 awarded to the Northern Pacific Global Health Fellows Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure
There is no conflict of interest to disclose.
References
- 1.Denning DW, Riniotis K, Dobrashian R, Sambatakou H. Chronic Cavitary and Fibrosing Pulmonary and Pleural Aspergillosis: Case Series, Proposed Nomenclature Change, and Review. Clin Infect Dis. 2003;37(s3):S265–S280. doi: 10.1086/376526 [DOI] [PubMed] [Google Scholar]
- 2.Bongomin F Post-tuberculosis chronic pulmonary aspergillosis: An emerging public health concern. Chowdhary A, ed. PLOS Pathog. 2020;16(8):e1008742. doi: 10.1371/journal.ppat.1008742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. 2011;37(4):865–872. doi: 10.1183/09031936.00054810 [DOI] [PubMed] [Google Scholar]
- 4.Denning DW, Pleuvry A, Cole DC. Global burden of chronic pulmonary aspergillosis as a sequel to pulmonary tuberculosis. Bull World Health Organ. 2011;89(12):864–872. doi: 10.2471/BLT.11.089441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baluku JB, Nuwagira E, Bongomin F, Denning DW. Pulmonary TB and chronic pulmonary aspergillosis: clinical differences and similarities. Int J Tuberc Lung Dis. 2021;25(7):537–546. doi: 10.5588/ijtld.21.0034 [DOI] [PubMed] [Google Scholar]
- 6.Denning DW, Cadranel J, Beigelman-Aubry C, et al. Chronic pulmonary aspergillosis: Rationale and clinical guidelines for diagnosis and management. Eur Respir J. 2016;47(1):45–68. doi: 10.1183/13993003.00583-2015 [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Uganda Tuberculosis Profile; 2018. www.who.int/tb/data.
- 8.Kwizera R, Parkes-Ratanshi R, Page ID, et al. Elevated Aspergillus-specific antibody levels among HIV infected Ugandans with pulmonary tuberculosis. BMC Pulm Med. 2017;17(1):149. doi: 10.1186/s12890-017-0500-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Page ID, Byanyima R, Hosmane S, et al. Chronic pulmonary aspergillosis commonly complicates treated pulmonary tuberculosis with residual cavitation. Eur Respir J. 2019;53(3). doi: 10.1183/13993003.01184-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horsburgh CR, Barry CE, Lange C. Treatment of Tuberculosis. N Engl J Med. 2015;373(22):2149–2160. doi: 10.1056/NEJMra1413919 [DOI] [PubMed] [Google Scholar]
- 11.Piarroux RP, Romain T, Martin A, et al. Multicenter evaluation of a novel immunochromatographic test for anti-aspergillus IgG detection. Front Cell Infect Microbiol. 2019;9(JAN):1–7. doi: 10.3389/fcimb.2019.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergidis P, Moore CB, Novak-Frazer L, et al. High-volume culture and quantitative real-time PCR for the detection of Aspergillus in sputum. Clin Microbiol Infect. December 2020. doi: 10.1016/j.cmi.2019.11.019 [DOI] [PubMed] [Google Scholar]
- 13.Denning DW, Page ID, Chakaya J, et al. Case definition of chronic pulmonary aspergillosis in resource-constrained settings. Emerg Infect Dis. 2018;24(8):e1–e13. doi: 10.3201/eid2408.171312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grace AG, Mittal A, Jain S, et al. Shortened treatment regimens versus the standard regimen for drug-sensitive pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;2019(12). doi: 10.1002/14651858.CD012918.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Shair K, Atherton GT, Harris C, Ratcliffe L, Newton PJ, Denning DW. Long-term antifungal treatment improves health status in patients with chronic pulmonary aspergillosis: A longitudinal analysis. Clin Infect Dis. 2013;57(6):828–835. doi: 10.1093/cid/cit411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olum R, Osaigbovo II, Baluku JB, Stemler J, Kwizera R, Bongomin F. Mapping of chronic pulmonary aspergillosis in africa. J Fungi. 2021;7(10):790. doi: 10.3390/jof7100790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oladele RO, Irurhe NK, Foden P, et al. Chronic pulmonary aspergillosis as a cause of smear-negative TB and/or TB treatment failure in Nigerians. Int J Tuberc Lung Dis. 2017;21(9):1056–1061. doi: 10.5588/ijtld.17.0060 [DOI] [PubMed] [Google Scholar]
- 18.Rozaliyani A, Rosianawati H, Handayani D, et al. Chronic Pulmonary Aspergillosis in Post Tuberculosis Patients in Indonesia and the Role of LDBio Aspergillus ICT as Part of the Diagnosis Scheme. J Fungi. 2020;6(4):318. doi: 10.3390/jof6040318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedayati MT, Azimi Y, Droudinia A, et al. Prevalence of chronic pulmonary aspergillosis in patients with tuberculosis from Iran. Eur J Clin Microbiol Infect Dis. 2015;34(9):1759–1765. doi: 10.1007/s10096-015-2409-7 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen NTB, Le Ngoc H, Nguyen NV, et al. Chronic Pulmonary Aspergillosis Situation among Post Tuberculosis Patients in Vietnam: An Observational Study. J Fungi. 2021;7(7):532. doi: 10.3390/jof7070532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwizera R, Katende A, Bongomin F, Nakiyingi L, Kirenga BJ. Misdiagnosis of chronic pulmonary aspergillosis as pulmonary tuberculosis at a tertiary care center in Uganda: a case series. J Med Case Rep. 2021;15(1):140. doi: 10.1186/s13256-021-02721-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis - Quo vadis? Med Mycol. 2015;53(5):417–439. doi: 10.1093/mmy/myv020 [DOI] [PubMed] [Google Scholar]
- 23.Bongomin F, Govender NP, Chakrabarti A, et al. Essential in vitro diagnostics for advanced HIV and serious fungal diseases: international experts’ consensus recommendations. Eur J Clin Microbiol Infect Dis. 2019;38(9):1581–1584. doi: 10.1007/s10096-019-03600-4 [DOI] [PubMed] [Google Scholar]
- 24.Stucky Hunter E, Richardson MD, Denning DW. Evaluation of LDBio Aspergillus ICT Lateral Flow Assay for IgG and IgM Antibody Detection in Chronic Pulmonary Aspergillosis. J Clin Microbiol. 2019;57(9). doi: 10.1128/JCM.00538-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter ES, Page ID, Richardson MD, Denning DW. Evaluation of the LDBio aspergillus ICT lateral flow assay for serodiagnosis of allergic bronchopulmonary aspergillosis. Kniemeyer O, ed. PLoS One. 2020;15(9 September 2020):e0238855. doi: 10.1371/journal.pone.0238855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bongomin F, Asio LG, Baluku JB, Kwizera R, Denning DW. Chronic Pulmonary Aspergillosis: Notes for a Clinician in a Resource-Limited Setting Where There Is No Mycologist. J Fungi. 2020;6(2):75. doi: 10.3390/jof6020075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bongomin F, Harris C, Hayes G, Kosmidis C, Denning DW. Twelve-month clinical outcomes of 206 patients with chronic pulmonary aspergillosis. Chotirmall SH, ed. PLoS One. 2018;13(4):e0193732. doi: 10.1371/journal.pone.0193732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bongomin F, Olum R, Kwizera R, Baluku JB. Surgical management of chronic pulmonary aspergillosis in Africa: A systematic review of 891 cases. Mycoses. 2021;64(10):1151–1158. doi: 10.1111/myc.13359 [DOI] [PubMed] [Google Scholar]
- 29.Al-Shair K, Muldoon EG, Morris J, Atherton GT, Kosmidis C, Denning DW. Characterisation of fatigue and its substantial impact on health status in a large cohort of patients with chronic pulmonary aspergillosis (CPA). Respir Med. 2016;114:117–122. doi: 10.1016/j.rmed.2016.03.020 [DOI] [PubMed] [Google Scholar]
- 30.Agarwal R, Vishwanath G, Aggarwal AN, Garg M, Gupta D, Chakrabarti A. Itraconazole in chronic cavitary pulmonary aspergillosis: A randomised controlled trial and systematic review of literature. Mycoses. 2013;56(5):559–570. doi: 10.1111/myc.12075 [DOI] [PubMed] [Google Scholar]
- 31.Chrdle A, Mustakim S, Bright-Thomas RJ, Baxter CG, Felton T, Denning DW. Aspergillus bronchitis without significant immunocompromise. Ann N Y Acad Sci. 2012;1272(1):73–85. doi: 10.1111/j.1749-6632.2012.06816.x [DOI] [PubMed] [Google Scholar]


