Abstract
Despite calls to ensure proportionate representation of both sexes in biomedical research, women continue to be underrepresented in Cardiovascular Disease (CVD) clinical trials. A comprehensive analysis of seven large suspected Ischemic Heart Disease/Coronary Artery Disease (HD/CAD) clinical trials (PROMISE, ISCHEMIA, CIAO-ISCHEMIA, ORBITA, FAME, FAME 2 and COURAGE trial) provides understanding of contributions to barriers to enrollment of women and leads to strategies to address these barriers. Specifically, in the seven trials, enrollment of women did not exceed 27%, while numerous barriers are evident. Proposed strategies to improve womeńs inclusion in clinical trials, include adding reproductive stage/estrogen status, attention to study design inclusion/exclusion criteria using female thresholds, consideration of diagnostic and intervention study design to be inclusive, increasing women and minorities in leadership positions, including sex as a biological variable (SABV) in study design and statistical analysis, and addressing social and race/ethnicity barriers. Dedicated action to actualizing these steps are needed at this time to developing diagnostic and therapeutic strategies resulting in better care and improved outcomes for CVD in women.
Introduction
Cardiovascular disease (CVD) is the leading cause of death in American women, counting for up to 50% of mortality in disease populations.1, 2 Although large clinical studies conducted exclusively in women, such as the Women’s Health Initiative,3–5 Women’s Health Study,6–8 and the Womeńs Ischemia Syndrome Evaluation (WISE) studies9–11 have contributed to representation of female-specific biomedical research, women continue to be underrepresented in enrolled cohorts of CVD clinical trials,12–15 as well as scientific leadership in CVD investigation.12, 16, 17
Women with CVD represent 45% of the target population, compared to 30% of enrollment in CVD trials.18 Overall, in CVD trials, the lowest enrollment of women is in heart failure (24%) and the highest in pulmonary arterial hypertension (77%).15 In suspected ischemic heart disease (IHD)/coronary artery disease (CAD) trials, enrollment of women has been slow, ranging from 24% to 28%.15 Current guideline-directed CAD therapies are based on data that predominantly includes male patients. The reasons why CAD trials populations are not female-representative are unclear, but sex-based differences in clinical presentation of IHD often exclude women from trials for not meeting inclusion criteria. Compared to men, IHD in women is identified less often,19–21 judged to be non-cardiac more often,22, 23 diagnosed at a more advanced age,24 and is treated less aggressively.21, 25 Furthermore, normal coronaries or non-obstructive coronary artery disease (CAD) is more prevalent in women with suspected IHD (up to 65%) compared to men (up to 32%).26, 27
Improving equality for women and men in CAD trials would improve outcomes for both.28, 29 The objectives of this review are to perform a comprehensive analysis of seven large clinical trials in CAD and barriers reducing female enrollment, and to outline strategies to improve womeńs trial enrollment to reflect the real-world patient population, incorporating sex-stratified analysis and collection of sex-specific data.
Sex Distribution of Suspected IHD/CAD Clinical Trials and Contributing Factors
We performed an extensive literature search on articles that addressed challenges in women enrollment in CVD trials focusing in IHD/CAD trials, and we analyzed the variables in seven large clinical trials in IHD/CAD potentially contributing to enrollment of women. We did not perform a systematic review due to paucity of data to conduct an extensive review. Table 1 summarizes data from these seven IHD/CAD trials (PROMISE,30 ISCHEMIA,31 CIAO-ISCHEMIA,32 ORBITA,33 FAME,34 FAME 2,35 and COURAGE36).
Table 1:
Summary of 7 CVD trials focused on suspected IHD/CAD, according to inclusion/exclusion criteria that contributed to enrollment of women
| TRIAL | PROMISE | ISCHEMIA | CIAO-ISCHEMIA | ORBITA | FAME | FAME 2 | COURAGE |
|---|---|---|---|---|---|---|---|
| First Author |

|
|

|

|

|
|
|
| Senior Author |

|
|

|
|

|

|
|
| No. of patients | 10,006 | 5,179 | 208 | 200 | 1,005 | 1,220 | 2,287 |
| Mean age (years) | 60 | 64 | 63 | 66 | 64 | 63 | 61 |
| Women Inclusion | 52.7% | 23% | 66% | 27% | 26% | 23% | 15% |
| Primary intent of the trial intervention | Diagnostic | Therapeutic (procedure) | Diagnostic | Therapeutic (procedure) | Therapeutic (procedure) | Therapeutic (procedure) | Therapeutic (procedure) |
| Type of intervention | CTA vs. Functional testing. | Invasive strategy of MT, angiography, and revascularization when feasible vs. Initial conservative strategy MT alone, with angiography reserved for failure of MT. | SAQ Angina Frequency score and stress echocardiography over one year in ISCHEMIA trial screen failures with INOCA. | PCI vs. Placebo procedure for angina relief | Angiography-guided PCI vs. FFR-guided PCI. | FFR-guided PCI + best available MT (PCI group) vs. Best available MT alone (medical-therapy group). | PCI and optimal MT (PCI group) vs. Optimal MT alone (medical-therapy group). |
| Inclusion Criteria |
|
- Obstructive CAD (Stable CAD, after clinically indicated stress testing showed moderate or severe reversible ischemia on imaging tests or severe ischemia on exercise tests without imaging). |
- Non-obstructive CAD (Patients with moderate or severe ischemia on stress echocardiography and no obstructive CAD by CTA). |
- Obstructive CAD (18–85 years with angina or equivalent symptoms and at least one angiographically significant lesion ≥70% in a single vessel that was clinically appropriate for PCI). |
- Obstructive CAD (Multivessel CAD, defined as stenoses of at least 50% of the vessel diameter in at least 2–3 of the major epicardial coronary arteries, and if PCI was indicated) |
- Obstructive CAD Referred to PCI for:
|
- Obstructive CAD
|
| Exclusion Criteria |
|
|
|
|
|
|
(Abb.: PROMISE, “Prospective Multicenter Imaging Study for Evaluation of Chest Pain” (ClinicalTrials.gov number, NCT01174550); ISCHEMIA, “International Study of Comparative Health Effectiveness With Medical and Invasive Approaches” (ClinicalTrials.gov number, NCT01471522); CIAO-ISCHEMIA, “Changes in Ischemia and Angina Over 1 Year Among ISCHEMIA Trial Screen Failures With no Obstructive CAD on Coronary CT Angiography” (ClinicalTrials.gov number, NCT02347215); ORBITA, “Objective Randomised Blinded Investigation With Optimal Medical Therapy of Angioplasty in Stable Angina” (ClinicalTrials.gov number, NCT02062593); FAME, “Fractional Flow Reserve Versus Angiography for Multivessel Evaluation” (ClinicalTrials.gov number, NCT00267774); FAME 2, “Fractional Flow Reserve (FFR) Guided Percutaneous Coronary Intervention (PCI) Plus Optimal Medical Treatment (OMT) Verses OMT” (ClinicalTrials.gov number, NCT01132495); COURAGE, “Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation” (ClinicalTrials.gov number, NCT00007657; CTA=coronary computed tomography angiogram; CAD=coronary artery disease; RF=risk factor; PAS=peripheral artery disease; CVD=cerebrovascular disease; HTN=hypertension; ACS=acute coronary syndrome; MT=medical therapy; GFR=glomerular filtration rate; LM=left main; LVEF=left ventricular ejection fraction; SAQ=Seattle Angina Questionnaire; INOCA=ischemia with non obstructive coronary arteries; PCI=percutaneous coronary intervention; CABG=coronary artery bypass graft; DES=drug eluting stent; CTO=chronic total occlusion; PTHN=pulmonary hypertension; FFR=fractional flow reserve; HF=heart failure)
Except for the PROMISE and CIAO-ISCHEMIA trials which focused on suspected IHD rather than obstructive CAD as an inclusion criteria, enrollment of women did not exceed 27%. (Figure 1).
Figure 1:
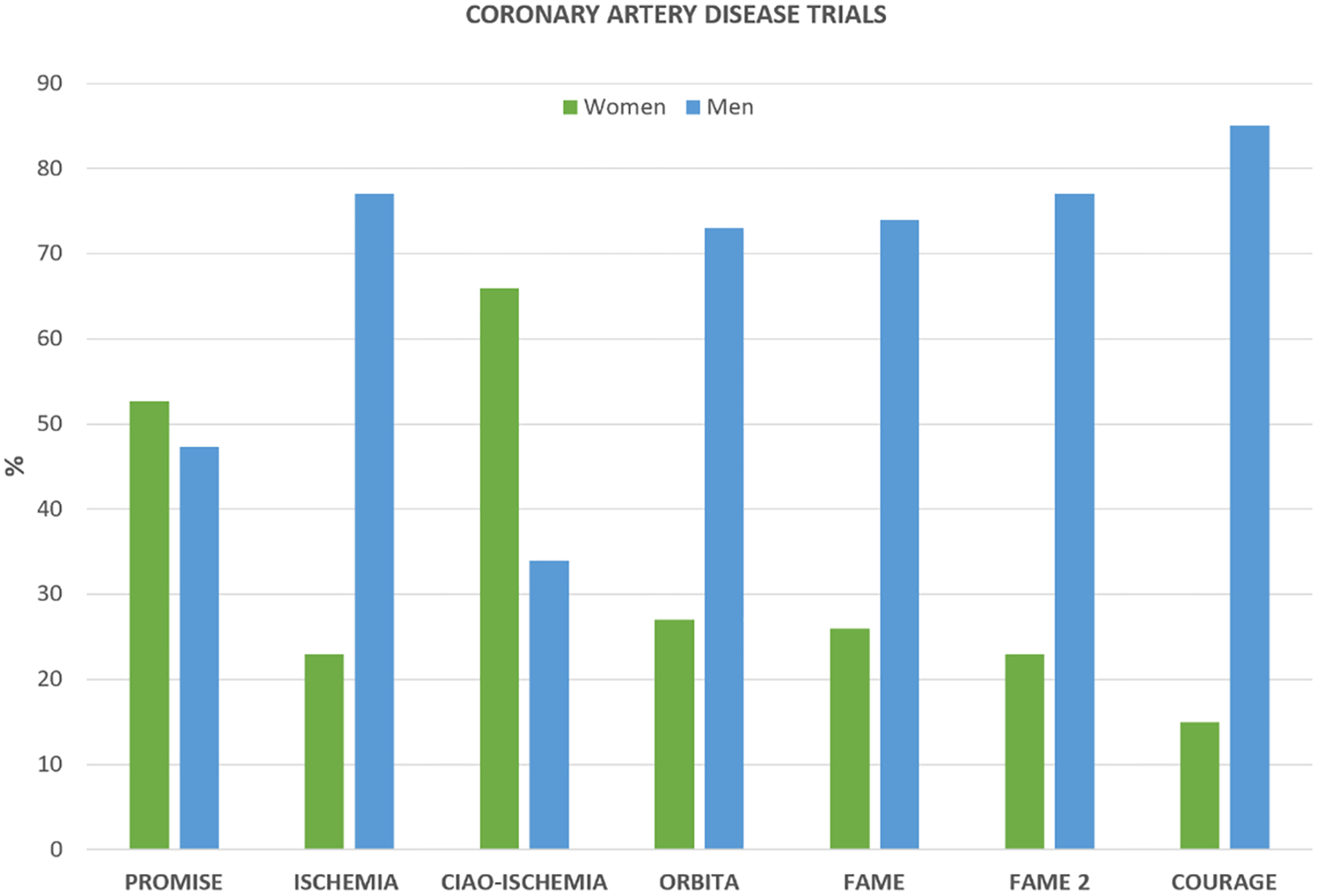
Gender Distribution of Coronary Artery Disease Trials
When the primary intent of the trial intervention was therapeutic, including procedures, there was lower enrollment of women. By contrast, in the two trials where the primary intent of the intervention was diagnostic and not including an invasive procedure, women were well represented. Although contemporary ACC/AHA guidelines recommend invasive management of acute coronary syndrome, irrespective of gender, studies have demonstrated that women are less likely to undergo an invasive procedure such as coronary angiography or coronary intervention.37–39 Thus, choice of intervention in randomized clinical trials may contribute to female participant under-representation.
Symptoms such as angina, multivessel CAD or angiographically significant coronary lesions were the inclusion criteria in most of these trials. Thus, lower enrollment of women in these trials may be explained by lower number of women referred for screening, as they are less likely to meet the inclusion criteria and may be less willing to participate in a study that involves an invasive procedure.
Failure to meet an angina inclusion criteria may be attributed to sex differences in clinical presentation, as women are more often judged as non-cardiac clinical presentation including the lack of chest pain as the main symptom of CAD.40 In CIAO-ISCHEMIA, where the enrollment was two-thirds women, the inclusion criteria was ischemia in the setting of non-obstructive CAD, a condition that is more reported in women compared to men.26
Notably, the first author of the primary trial publication was a woman in 3/7 trials (PROMISE, CIAO-ISCHEMIA, ORBITA), of which 2/3 (PROMISE, CIAO-ISCHEMIA) had high female enrollment. The senior author was a woman only in one trial (CIAO-ISCHEMIA). Further, we note that study of INOCA is predominantly been led by female investigators.
As we discuss later, the under-representation of women in leadership positions could be another factor contributing to lower women enrollment in clinical trials.
Barriers to IHD/CAD Trial Enrollment of Women
Reproductive stages/Role of estrogen
Historically, women of childbearing potential were banned from participating in clinical research, a policy set forth in 1977 by the Food and Drug Administration (FDA) due to the potential for medication causing serious birth defect.41 It was not until 1993 when the NIH Revitalization Act passed into law this policy was rescinded. However, a review of clinical trial publications from 2000 through 2020 identified that women of childbearing potential remain underrepresented in many disease categories and have a negative association with enrollment in clinical research.42
Another barrier to enrolling women in clinical research is the complex role of endogenous and exogenous estrogen over a woman’s lifespan. The historical misconception was that fluctuating hormones would not only confound trial results but also be more costly due to the added measurements.43 Over the past several decades the role of hormones and cardiovascular health has been extensively studied in both randomized controlled studies such as the Women’s Health Initiative (WHI),44 and observational cohort studies such as the Study of Women Across the Nation (SWAN),45 the WISE studies,9–11 and the Nurses’ Health Studies (NHS).46 These studies all have contributed to the understanding that different reproductive stages have positive or negative implications on cardiovascular health. For example, the early loss of endogenous estrogen in women who enter premature menopause (<40 years), whether surgically or natural, increases future risk of CVD. While women with longer reproductive lifespan have lower rates of CVD events.47 Therefore, when enrolling women into clinical trials reproductive stages should include reproductive, menopausal transition and the age and type of menopause. Several menopause status algorithms have been published such as those used hormones and bleeding patterns in the WISE cohort.48
Study design: Inclusion/Exclusion Criteria
While there is an increased awareness of the impact of CAD in women, there remain disparities between men and women in CAD diagnostic assessment. Sex-related differences in clinical presentation and biomarkers may explain under-diagnosis of IHD and low screening and/or enrollment of women in trials (Figure 2). Cardiac biomarkers (troponin and brain natriuretic peptide) and clinical presentation play an important role in diagnosis and risk assessment of CAD. Use of inappropriate diagnostic thresholds in troponin and atypical presentation of myocardial infarction (MI) in women likely contribute to under-diagnosis.
Figure 2:
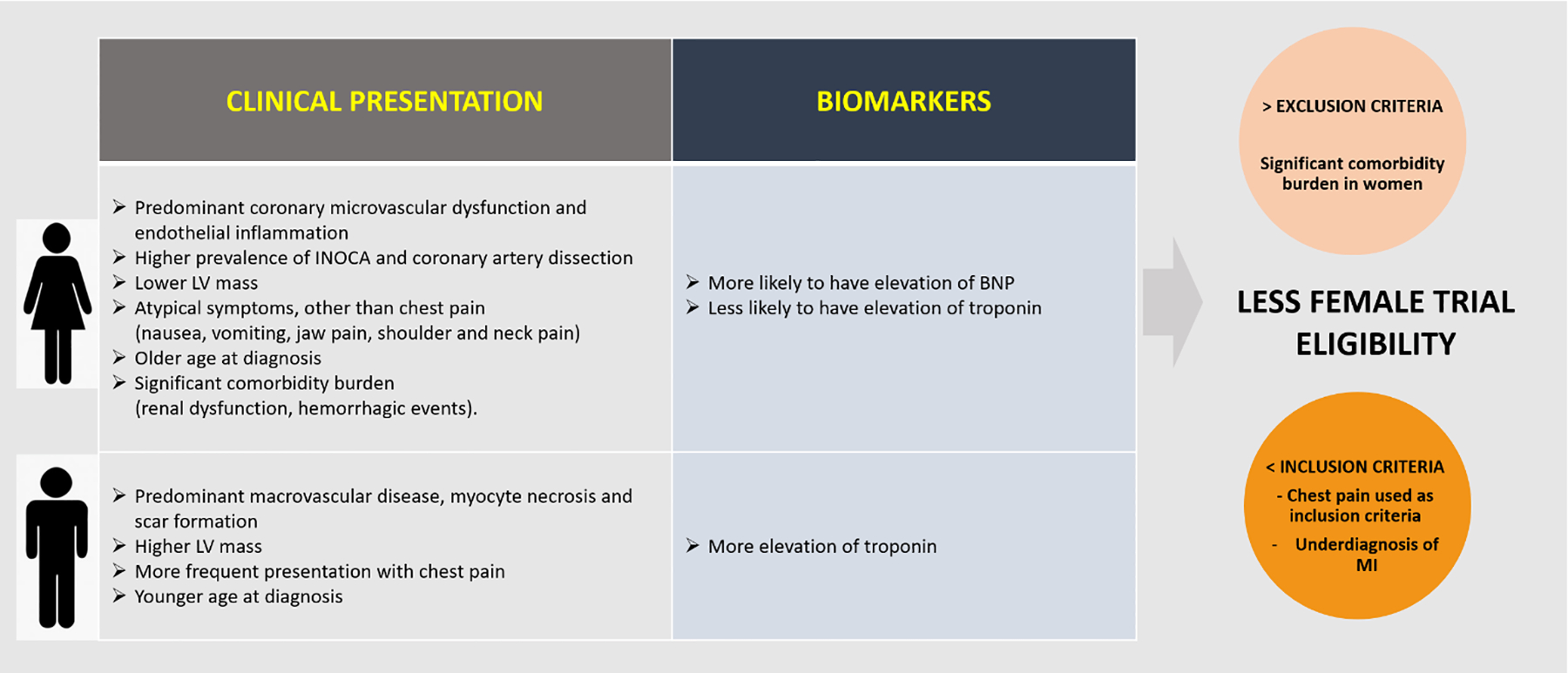
Sex Distribution in Clinical Presentation and Biomarkers in Coronary Artery Disease
(Abb.: INOCA=ischemia with non-obstructive coronary arteries; LV=left ventricular; BNP=brain natriuretic peptide; MI: myocardial infarction)
Clinical presentation.
There are some clinical characteristics in presentation that explain why in some studies enrollment of women is lower, such as the fact that women have higher prevalence of ischemia with non-obstructive coronary arteries (INOCA) and spontaneous coronary artery dissection (SCAD).22, 49–51 Obviously these patients cannot be included in trials where the inclusion criteria is obstructive CAD, such as ISCHEMIA, FAME, and COURAGE. However, some sex-related differences in clinical characteristics and ischemic symptoms may drive under-enrollment of women in CAD trials. For example, women more commonly present with CAD symptoms other than chest pain, such as jaw pain, neck and shoulder pain, fatigue, nausea, vomiting or atypical angina that more often is ascribed to digestive rather than cardiac etiology,23, 52 contributing to low enrollment of women in CAD trials focused on angina. Women may also have more comorbidities (given their older age at disease presentation),53, 54 such as renal dysfunction or history of a hemorrhagic event, resulting in meeting exclusion criteria. Similarly, since women tend to develop CAD about 10 years later than men,55 underrepresentation of women in CAD trials could be explained by lack of success in recruiting older women compared to older men.
Cardiac biomarkers.
Sex differences in cardiac biomarkers of myocardial injury may contribute to lower rate of MI diagnosis and subsequent IHD diagnosis in women with chest pain.56 Compared to men, women are less likely to have biomarker evidence of myocyte necrosis, elevated CK-MB or troponin, but more likely to have elevation of brain natriuretic peptide (BNP), a marker used in risk stratification of acute coronary syndromes.57 In 2007, the National Academy of Clinical Biochemistry (NACB) and International Federation of Clinical Chemistry (IFCC) committee recommended use of sex-specific reference ranges for some cardiac biomarkers.58
Despite the widespread use of cardiac troponin as a marker of MI and that some studies have shown higher circulating troponin levels in males compared to females,56, 57, 59, 60 there is no sex-specific reference value reported, with consequent under-diagnosis of MI in women. Thus, women at risk may be missed when using male sex-specific thresholds with standard troponin criteria. High-sensitivity troponin assay using sex specific diagnostic thresholds help closing this gender bias.56 High-sensitivity troponins have demonstrated that the 99th percentile reference limits are nearly two-fold higher in men than in women.61, 62 In a prospective cohort study of 1126 patients with suspected ACS (46% women), a high sensitivity troponin assay with sex-specific troponin threshold (women 16 ng/L, men 35 ng/L) doubled the diagnosis of MI in women compared to a contemporary assay with single threshold (50 ng/L).56 Thus, trial inclusion criteria that do not use sex specific diagnostic thresholds for MI may underrepresent women, who remain at risk for significant morbidity/mortality despite lower troponin or CK-MB levels.63
Putative mechanisms of sex differences in biomarker levels include that men present with predominant obstructive CAD with atherosclerotic plaque rupture and myocyte necrosis, while women more often have non-obstructive CAD with coronary microvascular dysfunction (CMD) leading to ischemic myocyte injury.64 Other potential mechanisms include sex differences in left ventricular mass and remodeling,65 troponin release and clearance kinetics, blood pressure, and sympathetic nervous system activity.62, 66 When biomarkers are used as inclusion/exclusion criteria, sex-related differences can reduce female trial eligibility. A broader, multi marker approach would be appropriate for CAD studies, and a possible solution to enroll more women in trials.
Study Design: Intervention/Outcome
Pharmacologic interventions.
Enrollment of women in CAD clinical trials have been low (<30%) in both pharmacologic (antithrombotic,67–69 lipid-lowering,70–72 anti-inflammatory73–76) and procedural trials.33–36, 77–79 Clinical trials of statins, which are currently recommended for primary and secondary prevention of CAD for both women and men, have historically enrolled lower women, including only 20–27% women.70, 71 In meta-analyses, the effect size of prevention with statin therapy was smaller or nonsignificant in women compared with men, leading the American Heart Association (AHA) to state that “evidence-based data supporting a statistically significant reduction of CVD events and all-cause mortality in primary prevention in women are lacking for statins”.80 Not unexpectedly, guideline-recommended statin use, persistence and adherence remain lower in women compared to men,81, 82 and women are less likely to believe statins are effective or safe compared with men.82 A recent systematic review of 60 lipid-lowering randomized clinical trials (N=485,409) conducted between 1990 and 2018 demonstrated an increase in the enrollment of women over time (from 19.5% to 33.6%) but found that a common limiting factor was inclusion of only postmenopausal women or surgically sterile women.83 Indeed, concern for reproductive/fetal safety is a common reason for excluding pregnant and lactating patients in clinical trials.84 However, given the rising trend of MI hospitalizations and mortality in young women85 and recent reports that CVD is the leading cause of death in pregnant and postpartum women in the United States,85, 86 trial criteria that exclude young, pregnant, or postpartum women should be reconsidered. Legislative efforts to improve pregnancy category labelling of drugs resulted in the 2015 “Pregnancy and Lactation Labelling Rule”, which was more descriptive about the risks of using a drug during pregnancy and lactation; however, the challenges associated with unknown drug safety continue to limit the study and the inclusion in clinical trials of this subpopulation. Recently the U.S. Food and Drug Administration (FDA) released a Drug Safety Communication requesting to remove the contraindication against using statins in all pregnant patients, stating that statins may be beneficial in preventing serious events in very high CVD risk pregnant patients.87
Clinical trials to specifically study pharmacologic interventions in women should be developed with special considerations for high CVD risk pregnant or lactating women. Although ACE-I/ARB clinical trials should continue to exclude pregnant women, protocols can be designed to enroll women of reproductive age who are willing to avoid pregnancy during the study and undergo a negative urine pregnancy test.88, 89
Procedural interventions.
Because most procedural trials include obstructive CAD as inclusion criteria fewer women are often eligible.90–94 Indeed, percutaneous coronary intervention and coronary artery bypass graft trial participants have been predominantly male, being 70–85% of the total of participants.33–36, 77–79 However, registry data indicate that among patients with stable obstructive CAD, cardiovascular outcomes are not lower among women compared to men.95 For example, in an analysis of the CONFIRM registry of patients undergoing coronary computed tomography angiography (CCTA) for suspected CAD, women with non-obstructive left main CAD had a nearly 80% higher risk for adverse cardiovascular events than men, and the relative hazard for 3-vessel CAD was higher for women compared to men.96, 97 Thus, efforts to improve representation of women should remain a goal in procedural CAD intervention trials.
Choice of primary outcomes.
Primary outcomes of CAD trials are often composite outcomes of death from cardiovascular causes and myocardial infarction and should include sufficient sex-stratified analysis and reporting.98 Patient reported outcomes, such as angina-related symptoms, function, and quality of life are often not selected as primary outcomes, but may help identify important sex-specific long-term prognosis.99 For example the recent ISCHEMIA trial found that an initial invasive strategy, as compared to an initial conservative strategy, did not reduce the primary endpoint of all-cause mortality or ischemic cardiovascular events among patients with stable obstructive CAD and moderate or severe ischemia.100 However, patients randomized to the invasive strategy had greater improvement in angina-related health status (as measured by the Seattle Angina Questionnaire [SAQ]) compared to those randomized to the conservative strategy, and differences were larger for patients with more frequent angina at baseline.101 Sex-differences in improvement in SAQ have not yet been reported in the ISCHEMIA trial, although women had more frequent angina compared to men at baseline.102 Thus, it is unknown whether the invasive strategy improved angina-related health study in women. Since sex-specific biological and gender-related socio-cultural differences in angina exist, sex-stratified analysis of SAQ should be considered as a pre-specified key outcome in CAD clinical trials.103
Choice of primary outcome should be carefully considered in the setting of ischemia with non- obstructive CAD. Indeed, in a secondary analysis of ISCHEMIA, women were more likely to have non-obstructive CAD, less extensive CAD on CCTA, and less severe ischemia on stress imaging.102 The PROMISE trial similarly demonstrated that women with suspected CAD were less likely to have a positive stress test or obstructive CAD on CCTA and had lower event rates compared to men.104, 105 The WISE study demonstrated a high prevalence of CMD in women with chest pain and without obstructive CAD,106 and CMD in the absence of obstructive CAD can result in abnormal stress tests.107 Taken together, these studies suggest that higher prevalence of CMD in the setting of non-obstructive CAD in women likely contribute to observed sex differences in test results and event rates. The WISE study also demonstrated that although women without obstructive CAD have better event-free survival than women with obstructive CAD,108 CMD, persistent chest pain, and evidence of inflammation in women with non-obstructive CAD predicted adverse outcomes.26, 109
In the ORBITA trial, the primary endpoint was the difference in exercise time increment between the groups, determined via serial cardiopulmonary exercise testing.33 Since elderly women have higher prevalence of physical disability compared to men,110 inclusion of exercise-based procedures could have contributed to lower female enrollment, although sex-differences in participant age was not reported in ORBITA.
Women in Leadership Positions
The proportion of women in clinical trial leadership positions and their contribution as first and senior author in publications has increased over time.17 However, this up trend has been slow and remains low, as it was demonstrated in different studies (Figure 3). Specifically, Jagsi et al. found that from 1970 to 2004, the proportion of women among U.S. physician authors of original research, published in 6 top tier journals, increased from 5.9% to 29.3% of first authors and from 3.7%t to 19.3% of senior authors.111 Ouyang et al. evaluated CVD trials published in 3 prominent journals from 1980 to 2017 and found an increase of 9.5% to 26% for female first author and 5.9% to 17.4% for female senior author.112 Labinaz et al. evaluated preclinical studies between 2006 and 2016 in top CVD journals and found that the proportion of women as first author increased from 32.3% to 46.3%, and for senior author from 11.8% to 26%.113 According to a recent report, women represent only 10.1% of cardiovascular clinical trial leadership committees.16
Figure 3:

Women as First and Senior Author in publications over the last decades according to different studies
The most cited factors identified as barriers to the academic advancement of women are the constraints of traditional sex roles, manifestations of sexism in the medical environment and lack of effective mentors.113, 114 Carr et al. reported that women who had children published less and had less institutional support than their male colleagues.115 These barriers have been accentuated during the Covid-19 pandemic, likely due to the shift to on-line teaching/curriculum adjustments, and extraprofessional caregiving responsibilities of children and family members which disproportionately affect women.116, 117
Trials with women as first or senior author tended to enroll higher proportions of female participants and stimulate female investigators to take part in cardiovascular clinical research. In fact, a recent analysis of heart failure trials confirmed this finding and found the proportion of women authors per trial was the only independent predictor of enrollment of female participants, possibly due to 1) women authors studying female predominant conditions, 2) higher likelihood of female participants to enroll in trials conducted by women investigators, or 3) study design that is more accommodating to female participants.12 Thus, one way to increase the participatory rate of women in trials would be to ensure a diverse research team. Womeńs involvement in a research team is associated with higher quality research,118 considering sex as a biological variable (SABV), and with higher rates of reporting sex-specific outcomes.113
Statistical considerations
SABV is a research strategy to recognize sex as an important variable to consider when designing studies and assessing results. SABV should be incorporated in the study design and statistical analysis of clinical trials. In the study design, the proportion of women enrolled should be viewed relative to a priori expectancies. Sex can be considered as a stratification variable yielding a factorial design, which allows researchers to estimate more representative treatment effects, maximizes power to test the interaction between treatment and sex, and perform sub-group analyses. In the statistical analysis, SABV can be considered at three different levels: (i) adjusting analyses for sex in order to obtain a more accurate estimate of the treatment effect; (ii) testing interaction between sex and treatment when sex differences in the treatment effect are of interest and (iii) performing sub-group (or stratified) analyses by sex yielding treatment effects separated by sex that are not necessarily different when the interaction between sex and treatment is not statistically significant, but can be informative for a future study. Multivariate analysis to identify significant correlates of the primary end-point should include both sex and age. Furthermore, automatic designation of end-points according to patient sex should be performed, as mandated by several journals.119 Some journals do not mandate the reporting of sex considerations but encourage authors to follow the “Sex and Gender Equity in Research (SAGER) guidelines”.120
Among the seven listed trials (Table 1), only three of them adjusted their analyses by sex as a covariate even though adjusting for covariates has been shown to improve power in the statistical analysis of clinical trials.121, 122 Sub-group analyses were performed in all seven trials and did not find statistically significant interaction effects, and the subgroup results agreed with the main results.
Notably, where differences in the treatment effects for sex are observed such that a p-value indicating the presence of treatment effect for a given sex is statistically significant but not significant for the other sex, claims of sex differences should not be made.123 Researchers often incorrectly conclude there are sex differences solely based on stratified analyses instead of considering those results to plan future studies when the interaction is not statistically significant.124
Social Barriers to Study Access/Participations
Although sex may affect subject’s consent to participate in the study and also their completion of a study, literature regarding the determinants is lacking. Some studies show that women are more likely to participate in health research than, while others do not.125–128 Women are less likely than men to agree to participate in randomized controlled trials, which may be driven by a general discomfort with the randomization process.127, 128 Many factors may influence the decision to participate in a clinical trial. We summarize in Table 2 contributors to womeńs decisions to participate in clinical trials. Thus, to ensure eligibility of women in CAD clinical trials, it is essential to increase awareness about the risk of heart disease in women, among women themselves and in health care providers, take into account the interpersonal relationships and social norms of the community where the study is conducted, and recognize the impact of study visit hours, travel time, and scheduling child or older adult care.
Table 2.
Contributors Influencing Womeńs Participation in CVD Clinical Studies and Trials
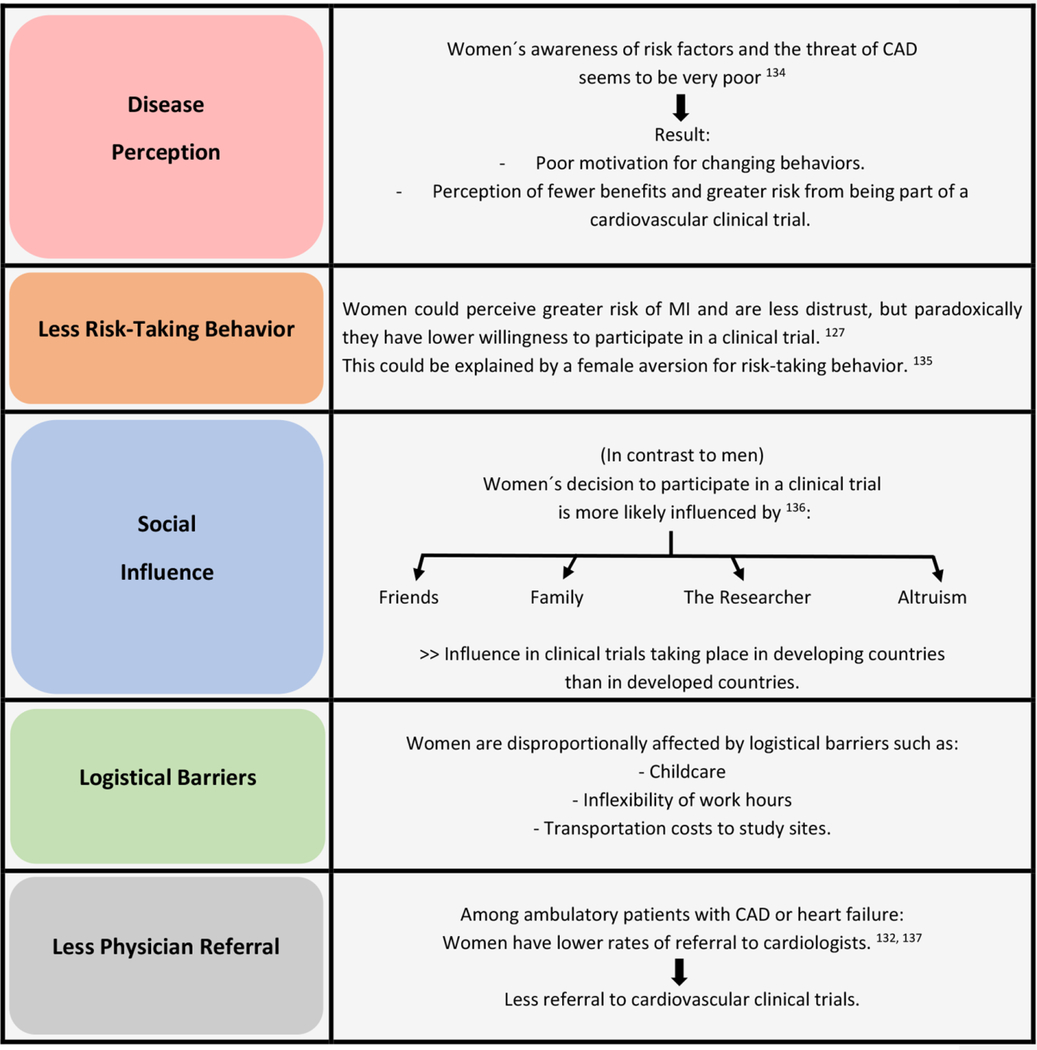
|
The Role of Race/Ethnicity
There are additional barriers to racial and ethnic minority groups to clinical trial participation. Racial and ethnicity health inequities exist, including structural inequities, less access to care and less utilization of evidence-based medicines, resulting in minority women have higher mortality/morbidity from IHD.129, 130 Racial/ethnic minorities who either are uninsured or underinsured, often seek their medical attention in community health centers and are less likely to receive a specialty referral.131 Also, women from community health centers were less likely to obtain initial and follow-up consultations than men.132 According to the 2019 AHA Survey of Womeńs Cardiovascular Disease Awareness, awareness of the leading cause of death in women – heart disease – declined from 65% in 2009 to 44% in 2019, particularly in non-Hispanic Black and Hispanic Women.133 The role of race and ethnicity in female participants and in leadership positions, likely contributes adversely to under-enrollment of minority groups in CVD clinical trials.
Strategies to Improve Enrollment of Women
Overcoming barriers to enrollment of women success will involve joint work between research team, health care system, community, and governmental authorities. In Table 3 we summarize strategies to improve enrollment of women in CVD studies and trials.
Table 3.
Strategies to improve womeńs enrollment in CVD focused on suspected IHD/CAD Trials
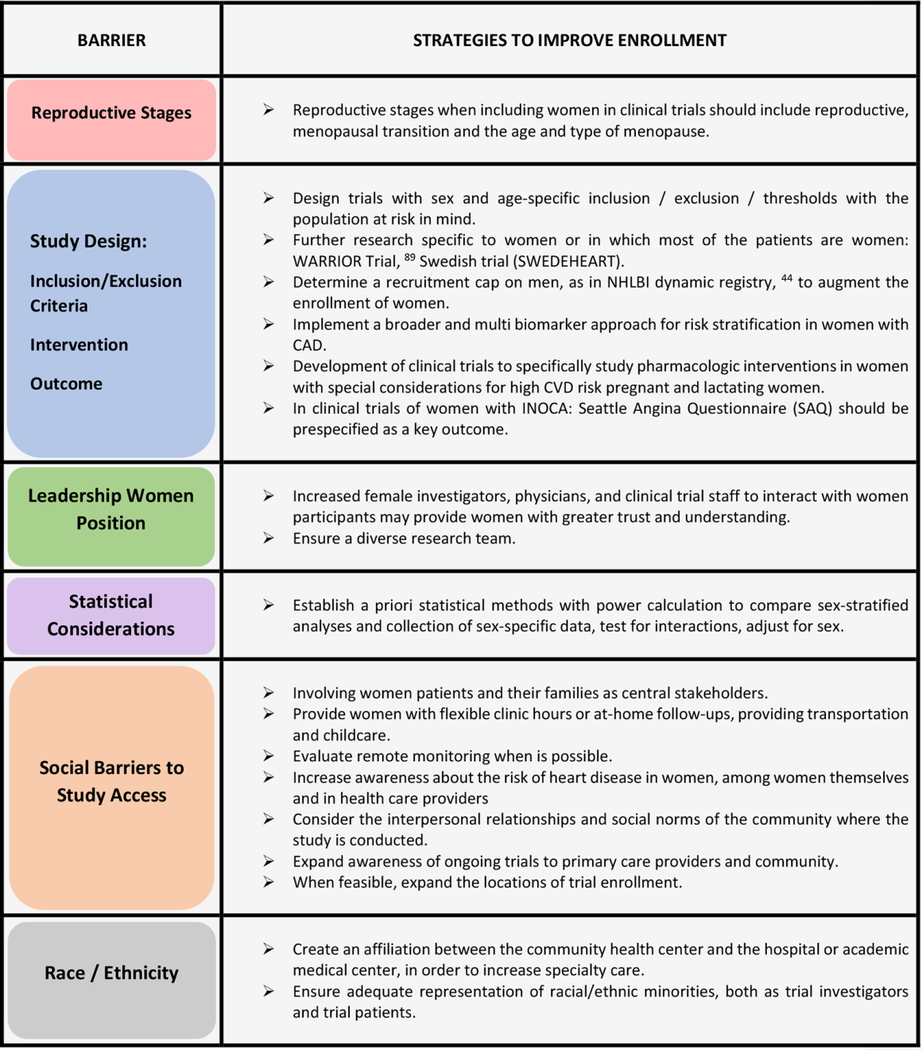
|
Summary and Conclusions
Despite efforts to ensure proportionate representation of both sexes in biomedical research, women continue to be underrepresented in the CAD clinical trials. Comprehensive analysis of seven large suspected IHD/CAD clinical trials (PROMISE, ISCHEMIA, CIAO-ISCHEMIA, ORBITA, FAME, FAME 2, and COURAGE trial) provide understanding of contributions to barriers to enrollment of women and lead to strategies to address these barriers. Specifically, in the seven trials we analyzed, enrollment of women did not exceed 27%. Numerous barriers are evident including sex differences obstructive CAD prevalence, clinical presentations, biomarkers, CVD outcomes, representation of women in leadership positions, statistical design and power, and social/racial/ethnic representation. Proposed strategies to improve womeńs inclusion in clinical trials, include adding reproductive stage/estrogen status, attention to study design inclusion/exclusion criteria using female thresholds, consideration of diagnostic and intervention study design to be inclusive, increasing women and minorities in leadership positions, including SABV in study design and statistical analysis, and addressing social and race/ethnicity barriers. Dedicated action to actualizing these steps are needed at this time to developing diagnostic and therapeutic strategies resulting in better care and improved outcomes for CVD in women.
Sources of Funding:
This work was supported by research funding from NIH R01HL146158 (C.N. Bairey Merz, J. Wei), R01HL124649 (C.N. Bairey Merz), R01HL153500 (J. Wei), U54AG065141 (C.N. Bairey Merz), the Barbra Streisand Women’s Cardiovascular Research and Education Program (C.N. Bairey Merz), the Linda Joy Pollin Women’s Heart Health Program (C.N. Bairey Merz), the Erika Glazer Women’s Heart Health Project (C.N. Bairey Merz), and the Adelson Family Foundation (C.N. Bairey Merz).
Footnotes
Conflicts of interest: C.N. Bairey Merz has served as consultant for Sanofi, Abbott Diagnostics, and iRhythm. Dr. Wei has served as consultant and advisory board member for Abbott Vascular. The other authors report no conflicts.
References
- 1.Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP and Winston M. Cardiovascular disease in women. Circulation. 1993;88:1999–2009. [DOI] [PubMed] [Google Scholar]
- 2.Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2015;385:117–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manson JE, Hsia J, Johnson KC, Rossouw JE, Assaf AR, Lasser NL, Trevisan M, Black HR, Heckbert SR, Detrano R, Strickland OL, Wong ND, Crouse JR, Stein E and Cushman M. Estrogen plus Progestin and the Risk of Coronary Heart Disease. New England Journal of Medicine. 2003;349:523–534. [DOI] [PubMed] [Google Scholar]
- 4.Anderson G, Limacher M, Assaf AR and al. e. Effects of Conjugated Equine Estrogen in Postmenopausal Women With Hysterectomy: the Womeńs Health Initiative randomized controlled trial. . JAMA. 2004;291:1701. [DOI] [PubMed] [Google Scholar]
- 5.Hsia J, Langer RD, Manson JE, Kuller L, Johnson KC, Hendrix SL, Pettinger M, Heckbert SR, Greep N, Crawford S, Eaton CB, Kostis JB, Caralis P and Prentice R. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–65. [DOI] [PubMed] [Google Scholar]
- 6.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH and Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. Jama. 2005;294:47–55. [DOI] [PubMed] [Google Scholar]
- 7.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH and Buring JE. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women’s Health Study: a randomized controlled trial. Jama. 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 8.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH and Buring JE. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed B, Merz CNB and Sopko G. Are we ‘WISE’r? Findings from the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation Study. Women’s Health. 2006;2:57–64. [DOI] [PubMed] [Google Scholar]
- 10.Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL and Sopko G. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology and feasibility report. J Am Coll Cardiol. 1999;33:1453–61. [DOI] [PubMed] [Google Scholar]
- 11.Barsky L, Merz CNB, Wei J, Shufelt C, Handberg E, Pepine C, Rutledge T, Reis S, Doyle M, Rogers W, Shaw L and Sopko G. Even “WISE-R?”-an Update on the NHLBI-Sponsored Women’s Ischemia Syndrome Evaluation. Curr Atheroscler Rep. 2020;22:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reza N, Tahhan AS, Mahmud N, DeFilippis EM, Alrohaibani A, Vaduganathan M, Greene SJ, Ho AH, Fonarow GC, Butler J, O’Connor C, Fiuzat M, Vardeny O, Piña IL, Lindenfeld J and Jessup M. Representation of Women Authors in International Heart Failure Guidelines and Contemporary Clinical Trials. Circ Heart Fail. 2020;13:e006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PY, Alexander KP, Hammill BG, Pasquali SK and Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. Jama. 2001;286:708–13. [DOI] [PubMed] [Google Scholar]
- 14.Gurwitz JH, Col NF and Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. Jama. 1992;268:1417–22. [PubMed] [Google Scholar]
- 15.Scott PE, Unger EF, Jenkins MR, Southworth MR, McDowell TY, Geller RJ, Elahi M, Temple RJ and Woodcock J. Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs. J Am Coll Cardiol. 2018;71:1960–1969. [DOI] [PubMed] [Google Scholar]
- 16.Denby KJ, Szpakowski N, Silver J, Walsh MN, Nissen S and Cho L. Representation of Women in Cardiovascular Clinical Trial Leadership. JAMA Intern Med. 2020;180:1382–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang TY and DesJardin JT. Time to End “Manels” in Clinical Trial Leadership. JAMA Intern Med. 2020;180:1383–1384. [DOI] [PubMed] [Google Scholar]
- 18.Gong IY, Tan NS, Ali SH, Lebovic G, Mamdani M, Goodman SG, Ko DT, Laupacis A and Yan AT. Temporal Trends of Women Enrollment in Major Cardiovascular Randomized Clinical Trials. Can J Cardiol. 2019;35:653–660. [DOI] [PubMed] [Google Scholar]
- 19.DeStefano F, Merritt RK, Anda RF, Casper ML and Eaker ED. Trends in nonfatal coronary heart disease in the United States, 1980 through 1989. Arch Intern Med. 1993;153:2489–94. [PubMed] [Google Scholar]
- 20.Tobin JN, Wassertheil-Smoller S, Wexler JP, Steingart RM, Budner N, Lense L and Wachspress J. Sex bias in considering coronary bypass surgery. Ann Intern Med. 1987;107:19–25. [DOI] [PubMed] [Google Scholar]
- 21.Shaw LJ, Miller DD, Romeis JC, Kargl D, Younis LT and Chaitman BR. Gender differences in the noninvasive evaluation and management of patients with suspected coronary artery disease. Ann Intern Med. 1994;120:559–66. [DOI] [PubMed] [Google Scholar]
- 22.Sarma AA, Braunwald E, Cannon CP, Guo J, Im K, Antman EM, Gibson CM, Newby LK, Giugliano RP, Morrow DA, Wiviott SD, Sabatine MS and O’Donoghue ML. Outcomes of Women Compared With Men After Non-ST-Segment Elevation Acute Coronary Syndromes. J Am Coll Cardiol. 2019;74:3013–3022. [DOI] [PubMed] [Google Scholar]
- 23.van Oosterhout REM, de Boer AR, Maas A, Rutten FH, Bots ML and Peters SAE. Sex Differences in Symptom Presentation in Acute Coronary Syndromes: A Systematic Review and Meta-analysis. J Am Heart Assoc. 2020;9:e014733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan SS, Nessim S, Gray R, Czer LS, Chaux A and Matloff J. Increased mortality of women in coronary artery bypass surgery: evidence for referral bias. Ann Intern Med. 1990;112:561–7. [DOI] [PubMed] [Google Scholar]
- 25.Krumholz HM, Douglas PS, Lauer MS and Pasternak RC. Selection of patients for coronary angiography and coronary revascularization early after myocardial infarction: is there evidence for a gender bias? Ann Intern Med. 1992;116:785–90. [DOI] [PubMed] [Google Scholar]
- 26.Bairey Merz CN, Pepine CJ, Walsh MN and Fleg JL. Ischemia and No Obstructive Coronary Artery Disease (INOCA): Developing Evidence-Based Therapies and Research Agenda for the Next Decade. Circulation. 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jespersen L, Hvelplund A, Abildstrøm SZ, Pedersen F, Galatius S, Madsen JK, Jørgensen E, Kelbæk H and Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J. 2012;33:734–44. [DOI] [PubMed] [Google Scholar]
- 28.Mirin AA. Gender Disparity in the Funding of Diseases by the U.S. National Institutes of Health. J Womens Health (Larchmt). 2021;30:956–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baird MD, Zaber MA, Chen A, Dick AW, Bird CE, Waymouth M, Gahlon G, Quigley DD, Al-Ibrahim H and Frank L. Research Funding for Women’s Health: Modeling Societal Impact. Santa Monica, CA: RAND Corporation; 2021. [Google Scholar]
- 30.Douglas PS, Hoffmann U, Lee KL, Mark DB, Al-Khalidi HR, Anstrom K, Dolor RJ, Kosinski A, Krucoff MW, Mudrick DW, Patel MR, Picard MH, Udelson JE, Velazquez EJ and Cooper L. PROspective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J. 2014;167:796–803.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maron DJ, Hochman JS, O’Brien SM, Reynolds HR, Boden WE, Stone GW, Bangalore S, Spertus JA, Mark DB, Alexander KP, Shaw L, Berger JS, Ferguson TB Jr., Williams DO, Harrington RA and Rosenberg Y. International Study of Comparative Health Effectiveness with Medical and Invasive Approaches (ISCHEMIA) trial: Rationale and design. Am Heart J. 2018;201:124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds HR, Picard MH, Spertus JA, Peteiro J, Lopez Sendon JL, Senior R, El-Hajjar MC, Celutkiene J, Shapiro MD, Pellikka PA, Kunichoff DF, Anthopolos R, Alfakih K, Abdul-Nour K, Khouri M, Bershtein L, De Belder M, Poh KK, Beltrame JF, Min JK, Fleg JL, Li Y, Maron DJ and Hochman JS. Natural History of Patients With Ischemia and No Obstructive Coronary Artery Disease: The CIAO-ISCHEMIA Study. Circulation. 2021;144:1008–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Lamee R, Thompson D, Dehbi HM, Sen S, Tang K, Davies J, Keeble T, Mielewczik M, Kaprielian R, Malik IS, Nijjer SS, Petraco R, Cook C, Ahmad Y, Howard J, Baker C, Sharp A, Gerber R, Talwar S, Assomull R, Mayet J, Wensel R, Collier D, Shun-Shin M, Thom SA, Davies JE and Francis DP. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet. 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
- 34.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’ t Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA and Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 35.De Bruyne B, Pijls NH, Kalesan B, Barbato E, Tonino PA, Piroth Z, Jagic N, Möbius-Winkler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd KG, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Jüni P and Fearon WF. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. [DOI] [PubMed] [Google Scholar]
- 36.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB and Weintraub WS. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. [DOI] [PubMed] [Google Scholar]
- 37.Singh JA, Lu X, Ibrahim S and Cram P. Trends in and disparities for acute myocardial infarction: an analysis of Medicare claims data from 1992 to 2010. BMC Med. 2014;12:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Z, Fang J, Gillespie C, Wang G, Hong Y and Yoon PW. Age-specific gender differences in in-hospital mortality by type of acute myocardial infarction. Am J Cardiol. 2012;109:1097–103. [DOI] [PubMed] [Google Scholar]
- 39.Murphy AC, Dinh D, Koshy AN, Lefkovits J, Clark DJ, Zaman S, Duffy SJ, Brennan A, Reid C and Yudi MB. Comparison of Long-Term Outcomes in Men versus Women Undergoing Percutaneous Coronary Intervention. Am J Cardiol. 2021;153:1–8. [DOI] [PubMed] [Google Scholar]
- 40.Khan NA, Daskalopoulou SS, Karp I, Eisenberg MJ, Pelletier R, Tsadok MA, Dasgupta K, Norris CM and Pilote L. Sex differences in acute coronary syndrome symptom presentation in young patients. JAMA Intern Med. 2013;173:1863–71. [DOI] [PubMed] [Google Scholar]
- 41.McCarthy CR. Historical background of clinical trials involving women and minorities. Acad Med. 1994;69:695–8. [DOI] [PubMed] [Google Scholar]
- 42.Steinberg JR, Turner BE, Weeks BT, Magnani CJ, Wong BO, Rodriguez F, Yee LM and Cullen MR. Analysis of Female Enrollment and Participant Sex by Burden of Disease in US Clinical Trials Between 2000 and 2020. JAMA Network Open. 2021;4:e2113749–e2113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu KA and Dipietro Mager NA. Women’s involvement in clinical trials: historical perspective and future implications. Pharmacy Practice. 2016;14:708–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Investigators WGftWsHI. Risks and Benefits of Estrogen Plus Progestin in Healthy Postmenopausal WomenPrincipal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 45.El Khoudary SR, Greendale G, Crawford SL, Avis NE, Brooks MM, Thurston RC, Karvonen-Gutierrez C, Waetjen LE and Matthews K. The menopause transition and women’s health at midlife: a progress report from the Study of Women’s Health Across the Nation (SWAN). Menopause. 2019;26:1213–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colditz GA, Philpott SE and Hankinson SE. The Impact of the Nurses’ Health Study on Population Health: Prevention, Translation, and Control. Am J Public Health. 2016;106:1540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, Giles GG, Bruinsma F, Simonsen MK, Hardy R, Kuh D, Gold EB, Crawford SL, Derby CA, Matthews KA, Demakakos P, Lee JS, Mizunuma H, Hayashi K, Sievert LL, Brown DE, Sandin S, Weiderpass E and Mishra GD. Association Between Reproductive Life Span and Incident Nonfatal Cardiovascular Disease: A Pooled Analysis of Individual Patient Data From 12 Studies. JAMA Cardiol. 2020;5:1410–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson BD, Merz CN, Braunstein GD, Berga SL, Bittner V, Hodgson TK, Gierach GL, Reis SE, Vido DA, Sharaf BL, Smith KM, Sopko G and Kelsey SF. Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt). 2004;13:872–87. [DOI] [PubMed] [Google Scholar]
- 49.Safdar B, Spatz ES, Dreyer RP, Beltrame JF, Lichtman JH, Spertus JA, Reynolds HR, Geda M, Bueno H, Dziura JD, Krumholz HM and D’Onofrio G. Presentation, Clinical Profile, and Prognosis of Young Patients With Myocardial Infarction With Nonobstructive Coronary Arteries (MINOCA): Results From the VIRGO Study. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs AK, Johnston JM, Haviland A, Brooks MM, Kelsey SF, Holmes DR Jr., Faxon DP, Williams DO and Detre KM. Improved outcomes for women undergoing contemporary percutaneous coronary intervention: a report from the National Heart, Lung, and Blood Institute Dynamic registry. J Am Coll Cardiol. 2002;39:1608–14. [DOI] [PubMed] [Google Scholar]
- 51.Herscovici R, Sedlak T, Wei J, Pepine CJ, Handberg E and Bairey Merz CN. Ischemia and No Obstructive Coronary Artery Disease (INOCA): What Is the Risk? Journal of the American Heart Association. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eastwood JA, Johnson BD, Rutledge T, Bittner V, Whittaker KS, Krantz DS, Cornell CE, Eteiba W, Handberg E, Vido D and Bairey Merz CN. Anginal symptoms, coronary artery disease, and adverse outcomes in Black and White women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. J Womens Health (Larchmt). 2013;22:724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G and Zheng ZJ. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. Jama. 2012;307:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldberg RJ, O’Donnell C, Yarzebski J, Bigelow C, Savageau J and Gore JM. Sex differences in symptom presentation associated with acute myocardial infarction: a population-based perspective. Am Heart J. 1998;136:189–95. [DOI] [PubMed] [Google Scholar]
- 55.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, Bairey Merz CN, Al-Mallah MH, Budoff MJ and Blaha MJ. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah AS, Griffiths M, Lee KK, McAllister DA, Hunter AL, Ferry AV, Cruikshank A, Reid A, Stoddart M, Strachan F, Walker S, Collinson PO, Apple FS, Gray AJ, Fox KA, Newby DE and Mills NL. High sensitivity cardiac troponin and the under-diagnosis of myocardial infarction in women: prospective cohort study. Bmj. 2015;350:g7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiviott SD, Cannon CP, Morrow DA, Murphy SA, Gibson CM, McCabe CH, Sabatine MS, Rifai N, Giugliano RP, DiBattiste PM, Demopoulos LA, Antman EM and Braunwald E. Differential expression of cardiac biomarkers by gender in patients with unstable angina/non-ST-elevation myocardial infarction: a TACTICS-TIMI 18 (Treat Angina with Aggrastat and determine Cost of Therapy with an Invasive or Conservative Strategy-Thrombolysis In Myocardial Infarction 18) substudy. Circulation. 2004;109:580–6. [DOI] [PubMed] [Google Scholar]
- 58.Apple FS, Jesse RL, Newby LK, Wu AH, Christenson RH, Cannon CP, Francis G, Morrow DA, Ravkilde J, Storrow AB, Tang W, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, Panteghini M and Tate J. National Academy of Clinical Biochemistry and IFCC Committee for Standardization of Markers of Cardiac Damage Laboratory Medicine Practice Guidelines: analytical issues for biochemical markers of acute coronary syndromes. Clin Chem. 2007;53:547–51. [DOI] [PubMed] [Google Scholar]
- 59.Herman E, Knapton A, Rosen E, Zhang J, Estis J, Agee SJ, Lu QA, Todd JA and Lipshultz SE. Baseline serum cardiac troponin I concentrations in Sprague-Dawley, spontaneous hypertensive, Wistar, Wistar-Kyoto, and Fisher rats as determined with an ultrasensitive immunoassay. Toxicol Pathol. 2011;39:653–63. [DOI] [PubMed] [Google Scholar]
- 60.Säfström K, Lindahl B and Swahn E. Risk stratification in unstable coronary artery disease--exercise test and troponin T from a gender perspective. FRISC-Study Group. Fragmin during InStability in Coronary artery disease. J Am Coll Cardiol. 2000;35:1791–800. [DOI] [PubMed] [Google Scholar]
- 61.Apple FS, Ler R and Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–81. [DOI] [PubMed] [Google Scholar]
- 62.Shah ASV, Ferry AV and Mills NL. Cardiac Biomarkers and the Diagnosis of Myocardial Infarction in Women. Curr Cardiol Rep. 2017;19:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei J, Mehta PK, Grey E, Garberich RF, Hauser R, Bairey Merz CN and Henry TD. Sex-based differences in quality of care and outcomes in a health system using a standardized STEMI protocol. Am Heart J. 2017;191:30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.AlBadri A, Wei J, Quesada O, Mehta PK, Xiao Y, Ko YA, Anderson RD, Petersen J, Azarbal B, Samuels B, Henry TD, Cook-Wiens G, Handberg EM, Van Eyk J, Pepine CJ and Bairey Merz CN. Coronary Vascular Function and Cardiomyocyte Injury: A Report From the WISE-CVD. Arterioscler Thromb Vasc Biol. 2020;40:3015–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA and McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. Jama. 2010;304:2503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Motiwala SR, Sarma A, Januzzi JL and O’Donoghue ML. Biomarkers in ACS and heart failure: should men and women be interpreted differently? Clin Chem. 2014;60:35–43. [DOI] [PubMed] [Google Scholar]
- 67.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF and Harrington RA. Ticagrelor versus Clopidogrel in Patients with Acute Coronary Syndromes. New England Journal of Medicine. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 68.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM and Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. [DOI] [PubMed] [Google Scholar]
- 69.Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, Džavík V, Escaned J, Gil R, Gurbel P, Hamm CW, Henry T, Huber K, Kastrati A, Kaul U, Kornowski R, Krucoff M, Kunadian V, Marx SO, Mehta SR, Moliterno D, Ohman EM, Oldroyd K, Sardella G, Sartori S, Shlofmitz R, Steg PG, Weisz G, Witzenbichler B, Han YL, Pocock S and Gibson CM. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. 2019;381:2032–2042. [DOI] [PubMed] [Google Scholar]
- 70.Gutierrez J, Ramirez G, Rundek T and Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med. 2012;172:909–19. [DOI] [PubMed] [Google Scholar]
- 71.Fulcher J, O’Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C and Keech A. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–405. [DOI] [PubMed] [Google Scholar]
- 72.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT Jr., Juliano RA, Jiao L, Granowitz C, Tardif JC and Ballantyne CM. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 73.Tardif J-C, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, Berry C, López-Sendón J, Ostadal P, Koenig W, Angoulvant D, Grégoire JC, Lavoie M-A, Dubé M-P, Rhainds D, Provencher M, Blondeau L, Orfanos A, L’Allier PL, Guertin M-C and Roubille F. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. New England Journal of Medicine. 2019;381:2497–2505. [DOI] [PubMed] [Google Scholar]
- 74.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P and Glynn RJ. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. New England Journal of Medicine. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
- 75.Ridker PM, Everett BM, Pradhan A, MacFadyen JG, Solomon DH, Zaharris E, Mam V, Hasan A, Rosenberg Y, Iturriaga E, Gupta M, Tsigoulis M, Verma S, Clearfield M, Libby P, Goldhaber SZ, Seagle R, Ofori C, Saklayen M, Butman S, Singh N, Le May M, Bertrand O, Johnston J, Paynter NP and Glynn RJ. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. New England Journal of Medicine. 2018;380:752–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nidorf SM, Fiolet ATL, Mosterd A, Eikelboom JW, Schut A, Opstal TSJ, The SHK, Xu XF, Ireland MA, Lenderink T, Latchem D, Hoogslag P, Jerzewski A, Nierop P, Whelan A, Hendriks R, Swart H, Schaap J, Kuijper AFM, van Hessen MWJ, Saklani P, Tan I, Thompson AG, Morton A, Judkins C, Bax WA, Dirksen M, Alings M, Hankey GJ, Budgeon CA, Tijssen JGP, Cornel JH and Thompson PL. Colchicine in Patients with Chronic Coronary Disease. N Engl J Med. 2020;383:1838–1847. [DOI] [PubMed] [Google Scholar]
- 77.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Ståhle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD and Mohr FW. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–72. [DOI] [PubMed] [Google Scholar]
- 78.Stone GW, Sabik JF, Serruys PW, Simonton CA, Généreux P, Puskas J, Kandzari DE, Morice M-C, Lembo N, Brown WM, Taggart DP, Banning A, Merkely B, Horkay F, Boonstra PW, van Boven AJ, Ungi I, Bogáts G, Mansour S, Noiseux N, Sabaté M, Pomar J, Hickey M, Gershlick A, Buszman P, Bochenek A, Schampaert E, Pagé P, Dressler O, Kosmidou I, Mehran R, Pocock SJ and Kappetein AP. Everolimus-Eluting Stents or Bypass Surgery for Left Main Coronary Artery Disease. New England Journal of Medicine. 2016;375:2223–2235. [DOI] [PubMed] [Google Scholar]
- 79.Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, López-Sendón J, Faxon DP, Mauri L, Rao SV, Feldman L, Steg PG, Avezum Á, Sheth T, Pinilla-Echeverri N, Moreno R, Campo G, Wrigley B, Kedev S, Sutton A, Oliver R, Rodés-Cabau J, Stanković G, Welsh R, Lavi S, Cantor WJ, Wang J, Nakamya J, Bangdiwala SI and Cairns JA. Complete Revascularization with Multivessel PCI for Myocardial Infarction. New England Journal of Medicine. 2019;381:1411–1421. [DOI] [PubMed] [Google Scholar]
- 80.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML and Allison MA. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142:e506–e532. [DOI] [PubMed] [Google Scholar]
- 81.Colantonio LD, Rosenson RS, Deng L, Monda KL, Dai Y, Farkouh ME, Safford MM, Philip K, Mues KE and Muntner P. Adherence to Statin Therapy Among US Adults Between 2007 and 2014. J Am Heart Assoc. 2019;8:e010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nanna MG, Wang TY, Xiang Q, Goldberg AC, Robinson JG, Roger VL, Virani SS, Wilson PWF, Louie MJ, Koren A, Li Z, Peterson ED and Navar AM. Sex Differences in the Use of Statins in Community Practice. Circ Cardiovasc Qual Outcomes. 2019;12:e005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Khan SU, Khan MZ, Raghu Subramanian C, Riaz H, Khan MU, Lone AN, Khan MS, Benson EM, Alkhouli M, Blaha MJ, Blumenthal RS, Gulati M and Michos ED. Participation of Women and Older Participants in Randomized Clinical Trials of Lipid-Lowering Therapies: A Systematic Review. JAMA Netw Open. 2020;3:e205202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Illamola SM, Bucci-Rechtweg C, Costantine MM, Tsilou E, Sherwin CM and Zajicek A. Inclusion of pregnant and breastfeeding women in research - efforts and initiatives. Br J Clin Pharmacol. 2018;84:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khan SU, Yedlapati SH, Lone AN, Khan MS, Wenger NK, Watson KE, Gulati M, Hays AG and Michos ED. A comparative analysis of premature heart disease- and cancer-related mortality in women in the USA, 1999–2018. Eur Heart J Qual Care Clin Outcomes. 2021. [DOI] [PubMed] [Google Scholar]
- 86.Petersen EE, Davis NL, Goodman D, Cox S, Mayes N, Johnston E, Syverson C, Seed K, Shapiro-Mendoza CK, Callaghan WM and Barfield W. Vital Signs: Pregnancy-Related Deaths, United States, 2011–2015, and Strategies for Prevention, 13 States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;68:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.www.FDA.gov. Statins: Drug Safety Communication - FDA Requests Removal of Strongest Warning Against Using Cholesterol-lowering Statins During Pregnancy. 2021. [Google Scholar]
- 88.Pauly DF, Johnson BD, Anderson RD, Handberg EM, Smith KM, Cooper-DeHoff RM, Sopko G, Sharaf BM, Kelsey SF, Merz CNB and Pepine CJ. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). American heart journal. 2011;162:678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Handberg EM, Merz CNB, Cooper-Dehoff RM, Wei J, Conlon M, Lo MC, Boden W, Frayne SM, Villines T, Spertus JA, Weintraub W, O’Malley P, Chaitman B, Shaw LJ, Budoff M, Rogatko A and Pepine CJ. Rationale and design of the Women’s Ischemia Trial to Reduce Events in Nonobstructive CAD (WARRIOR) trial. Am Heart J. 2021;237:90–103. [DOI] [PubMed] [Google Scholar]
- 90.Yan AT, Yan RT, Tan M, Fung A, Cohen EA, Fitchett DH, Langer A and Goodman SG. Management patterns in relation to risk stratification among patients with non-ST elevation acute coronary syndromes. Arch Intern Med. 2007;167:1009–16. [DOI] [PubMed] [Google Scholar]
- 91.Akhter N, Milford-Beland S, Roe MT, Piana RN, Kao J and Shroff A. Gender differences among patients with acute coronary syndromes undergoing percutaneous coronary intervention in the American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR). Am Heart J. 2009;157:141–8. [DOI] [PubMed] [Google Scholar]
- 92.Bugiardini R, Yan AT, Yan RT, Fitchett D, Langer A, Manfrini O and Goodman SG. Factors influencing underutilization of evidence-based therapies in women. Eur Heart J. 2011;32:1337–44. [DOI] [PubMed] [Google Scholar]
- 93.Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, Fitzgerald G, Jackson EA and Eagle KA. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart. 2009;95:20–6. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen HL, Goldberg RJ, Gore JM, Fox KA, Eagle KA, Gurfinkel EP, Spencer FA, Reed G, Quill A and Anderson FA Jr. Age and sex differences, and changing trends, in the use of evidence-based therapies in acute coronary syndromes: perspectives from a multinational registry. Coron Artery Dis. 2010;21:336–44. [DOI] [PubMed] [Google Scholar]
- 95.Steg PG, Greenlaw N, Tardif JC, Tendera M, Ford I, Kääb S, Abergel H, Fox KM and Ferrari R. Women and men with stable coronary artery disease have similar clinical outcomes: insights from the international prospective CLARIFY registry. Eur Heart J. 2012;33:2831–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T and Berman DS. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 97.Xie JX, Eshtehardi P, Varghese T, Goyal A, Mehta PK, Kang W, Leipsic J, ÓH B, Bairey Merz CN, Berman DS, Gransar H, Budoff MJ, Achenbach S, Callister TQ, Marques H, Rubinshtein R, Al-Mallah MH, Andreini D, Pontone G, Cademartiri F, Maffei E, Chinnaiyan K, Raff G, Hadamitzky M, Hausleiter J, Feuchtner G, Kaufmann PA, Villines TC, Chow BJW, Min JK and Shaw LJ. Prognostic Significance of Nonobstructive Left Main Coronary Artery Disease in Women Versus Men: Long-Term Outcomes From the CONFIRM (Coronary CT Angiography Evaluation For Clinical Outcomes: An International Multicenter) Registry. Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wenger NK. Women and coronary heart disease: a century after Herrick: understudied, underdiagnosed, and undertreated. Circulation. 2012;126:604–11. [DOI] [PubMed] [Google Scholar]
- 99.Norekvål TM, Fridlund B, Rokne B, Segadal L, Wentzel-Larsen T and Nordrehaug JE. Patient-reported outcomes as predictors of 10-year survival in women after acute myocardial infarction. Health Qual Life Outcomes. 2010;8:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, Chaitman BR, Senior R, López-Sendón J, Alexander KP, Lopes RD, Shaw LJ, Berger JS, Newman JD, Sidhu MS, Goodman SG, Ruzyllo W, Gosselin G, Maggioni AP, White HD, Bhargava B, Min JK, Mancini GBJ, Berman DS, Picard MH, Kwong RY, Ali ZA, Mark DB, Spertus JA, Krishnan MN, Elghamaz A, Moorthy N, Hueb WA, Demkow M, Mavromatis K, Bockeria O, Peteiro J, Miller TD, Szwed H, Doerr R, Keltai M, Selvanayagam JB, Steg PG, Held C, Kohsaka S, Mavromichalis S, Kirby R, Jeffries NO, Harrell FE Jr., Rockhold FW, Broderick S, Ferguson TB Jr., Williams DO, Harrington RA, Stone GW and Rosenberg Y. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N Engl J Med. 2020;382:1395–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Spertus JA, Jones PG, Maron DJ, O’Brien SM, Reynolds HR, Rosenberg Y, Stone GW, Harrell FE, Boden WE, Weintraub WS, Baloch K, Mavromatis K, Diaz A, Gosselin G, Newman JD, Mavromichalis S, Alexander KP, Cohen DJ, Bangalore S, Hochman JS and Mark DB. Health-Status Outcomes with Invasive or Conservative Care in Coronary Disease. New England Journal of Medicine. 2020;382:1408–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reynolds HR, Shaw LJ, Min JK, Spertus JA, Chaitman BR, Berman DS, Picard MH, Kwong RY, Bairey-Merz CN, Cyr DD, Lopes RD, Lopez-Sendon JL, Held C, Szwed H, Senior R, Gosselin G, Nair RG, Elghamaz A, Bockeria O, Chen J, Chernyavskiy AM, Bhargava B, Newman JD, Hinic SB, Jaroch J, Hoye A, Berger J, Boden WE, O’Brien SM, Maron DJ and Hochman JS. Association of Sex With Severity of Coronary Artery Disease, Ischemia, and Symptom Burden in Patients With Moderate or Severe Ischemia: Secondary Analysis of the ISCHEMIA Randomized Clinical Trial. JAMA Cardiol. 2020;5:773–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mehta PK, Wei J, Shufelt C, Quesada O, Shaw L and Bairey Merz CN. Gender-Related Differences in Chest Pain Syndromes in the Frontiers in CV Medicine Special Issue: Sex & Gender in CV Medicine. Front Cardiovasc Med. 2021;8:744788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hemal K, Pagidipati NJ, Coles A, Dolor RJ, Mark DB, Pellikka PA, Hoffmann U, Litwin SE, Daubert MA, Shah SH, Ariani K, Bullock-Palmer RP, Martinez B, Lee KL and Douglas PS. Sex Differences in Demographics, Risk Factors, Presentation, and Noninvasive Testing in Stable Outpatients With Suspected Coronary Artery Disease: Insights From the PROMISE Trial. JACC Cardiovasc Imaging. 2016;9:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pagidipati NJ, Hemal K, Coles A, Mark DB, Dolor RJ, Pellikka PA, Hoffmann U, Litwin SE, Udelson J, Daubert MA, Shah SH, Martinez B, Lee KL and Douglas PS. Sex Differences in Functional and CT Angiography Testing in Patients With Suspected Coronary Artery Disease. J Am Coll Cardiol. 2016;67:2607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.AlBadri A, Bairey Merz CN, Johnson BD, Wei J, Mehta PK, Cook-Wiens G, Reis SE, Kelsey SF, Bittner V, Sopko G, Shaw LJ, Pepine CJ and Ahmed B. Impact of Abnormal Coronary Reactivity on Long-Term Clinical Outcomes in Women. J Am Coll Cardiol. 2019;73:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cassar A, Chareonthaitawee P, Rihal CS, Prasad A, Lennon RJ, Lerman LO and Lerman A. Lack of correlation between noninvasive stress tests and invasive coronary vasomotor dysfunction in patients with nonobstructive coronary artery disease. Circ Cardiovasc Interv. 2009;2:237–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharaf B, Wood T, Shaw L, Johnson BD, Kelsey S, Anderson RD, Pepine CJ and Bairey Merz CN. Adverse outcomes among women presenting with signs and symptoms of ischemia and no obstructive coronary artery disease: findings from the National Heart, Lung, and Blood Institute-sponsored Women’s Ischemia Syndrome Evaluation (WISE) angiographic core laboratory. Am Heart J. 2013;166:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Johnson BD, Shaw LJ, Pepine CJ, Reis SE, Kelsey SF, Sopko G, Rogers WJ, Mankad S, Sharaf BL, Bittner V and Bairey Merz CN. Persistent chest pain predicts cardiovascular events in women without obstructive coronary artery disease: results from the NIH-NHLBI-sponsored Women’s Ischaemia Syndrome Evaluation (WISE) study. Eur Heart J. 2006;27:1408–15. [DOI] [PubMed] [Google Scholar]
- 110.Oman D, Reed D and Ferrara A. Do elderly women have more physical disability than men do? Am J Epidemiol. 1999;150:834–42. [DOI] [PubMed] [Google Scholar]
- 111.Jagsi R, Guancial EA, Worobey CC, Henault LE, Chang Y, Starr R, Tarbell NJ and Hylek EM. The “gender gap” in authorship of academic medical literature--a 35-year perspective. N Engl J Med. 2006;355:281–7. [DOI] [PubMed] [Google Scholar]
- 112.Ouyang D, Sing D, Shah S, Hu J, Duvernoy C, Harrington RA and Rodriguez F. Sex Disparities in Authorship Order of Cardiology Scientific Publications. Circ Cardiovasc Qual Outcomes. 2018;11:e005040. [DOI] [PubMed] [Google Scholar]
- 113.Labinaz A, Marbach JA, Jung RG, Moreland R, Motazedian P, Di Santo P, Clancy AA, MacDonald Z, Simard T, Hibbert B and Ramirez FD. Female Authorship in Preclinical Cardiovascular Research: Temporal Trends and Influence on Experimental Design. JACC Basic Transl Sci. 2019;4:471–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yedidia MJ and Bickel J. Why aren’t there more women leaders in academic medicine? the views of clinical department chairs. Acad Med. 2001;76:453–65. [DOI] [PubMed] [Google Scholar]
- 115.Carr PL, Ash AS, Friedman RH, Scaramucci A, Barnett RC, Szalacha L, Palepu A and Moskowitz MA. Relation of family responsibilities and gender to the productivity and career satisfaction of medical faculty. Ann Intern Med. 1998;129:532–8. [DOI] [PubMed] [Google Scholar]
- 116.Viglione G Are women publishing less during the pandemic? Here’s what the data say. Nature. 2020;581:365–366. [DOI] [PubMed] [Google Scholar]
- 117.Andersen JP, Nielsen MW, Simone NL, Lewiss RE and Jagsi R. COVID-19 medical papers have fewer women first authors than expected. Elife. 2020;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Campbell LG, Mehtani S, Dozier ME and Rinehart J. Gender-heterogeneous working groups produce higher quality science. PLoS One. 2013;8:e79147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Docherty JR, Stanford SC, Panattieri RA, Alexander SPH, Cirino G, George CH, Hoyer D, Izzo AA, Ji Y, Lilley E, Sobey CG, Stanley P, Stefanska B, Stephens G, Teixeira M and Ahluwalia A. Sex: A change in our guidelines to authors to ensure that this is no longer an ignored experimental variable. Br J Pharmacol. 2019;176:4081–4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heidari S, Babor TF, De Castro P, Tort S and Curno M. Sex and Gender Equity in Research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Steyerberg EW, Bossuyt PM and Lee KL. Clinical trials in acute myocardial infarction: should we adjust for baseline characteristics? Am Heart J. 2000;139:745–51. [DOI] [PubMed] [Google Scholar]
- 122.Lingsma H, Roozenbeek B and Steyerberg E. Covariate adjustment increases statistical power in randomized controlled trials. J Clin Epidemiol. 2010;63:1391; author reply 1392–3. [DOI] [PubMed] [Google Scholar]
- 123.Gelman A and Stern H. The Difference between ‘Significant’ and ‘Not Significant’ Is Not Itself Statistically Significant. The American Statistician 2006;60:328–31. [Google Scholar]
- 124.Garcia-Sifuentes Y and Maney DL. Reporting and misreporting of sex differences in the biological sciences. Elife. 2021;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Glass D, Kelsall H, Slegers C, Forbes AB, Loff B, Zion D and Fritschi L. A telephone survey of factors affecting willingness to participate in health research surveys. BMC Public Health. 2015;15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen SC, Sinaii N, Bedarida G, Gregorio MA, Emanuel E and Grady C. Phase 1 healthy volunteer willingness to participate and enrollment preferences. Clin Trials. 2017;14:537–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ding EL, Powe NR, Manson JE, Sherber NS and Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. 2007;167:905–12. [DOI] [PubMed] [Google Scholar]
- 128.Peterson ED, Lytle BL, Biswas MS and Coombs L. Willingness to participate in cardiac trials. Am J Geriatr Cardiol. 2004;13:11–5. [DOI] [PubMed] [Google Scholar]
- 129.Kurian AK and Cardarelli KM. Racial and ethnic differences in cardiovascular disease risk factors: a systematic review. Ethn Dis. 2007;17:143–52. [PubMed] [Google Scholar]
- 130.Bonow RO, Grant AO and Jacobs AK. The cardiovascular state of the union: confronting healthcare disparities. Circulation. 2005;111:1205–7. [DOI] [PubMed] [Google Scholar]
- 131.Cook NL, Hicks LS, O’Malley AJ, Keegan T, Guadagnoli E and Landon BE. Access to specialty care and medical services in community health centers. Health Aff (Millwood). 2007;26:1459–68. [DOI] [PubMed] [Google Scholar]
- 132.Cook NL, Ayanian JZ, Orav EJ and Hicks LS. Differences in specialist consultations for cardiovascular disease by race, ethnicity, gender, insurance status, and site of primary care. Circulation. 2009;119:2463–70. [DOI] [PubMed] [Google Scholar]
- 133.Cushman M, Shay CM, Howard VJ, Jiménez MC, Lewey J, McSweeney JC, Newby LK, Poudel R, Reynolds HR, Rexrode KM, Sims M and Mosca LJ. Ten-Year Differences in Women’s Awareness Related to Coronary Heart Disease: Results of the 2019 American Heart Association National Survey: A Special Report From the American Heart Association. Circulation. 2021;143:e239–e248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Biswas MS, Calhoun PS, Bosworth HB and Bastian LA. Are women worrying about heart disease? Womens Health Issues. 2002;12:204–11. [DOI] [PubMed] [Google Scholar]
- 135.Doyal L. Sex, gender, and health: the need for a new approach. Bmj. 2001;323:1061–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lobato L, Bethony JM, Pereira FB, Grahek SL, Diemert D and Gazzinelli MF. Impact of gender on the decision to participate in a clinical trial: a cross-sectional study. BMC Public Health. 2014;14:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ehrmann Feldman D, Xiao Y, Bernatsky S, Haggerty J, Leffondré K, Tousignant P, Roy Y and Abrahamowicz M. Consultation with cardiologists for persons with new-onset chronic heart failure: a population-based study. Can J Cardiol. 2009;25:690–4. [DOI] [PMC free article] [PubMed] [Google Scholar]


