Abstract
Many bacteria respond to changes in environmental conditions by entering the viable-but-nonculturable state. We have determined that copper can induce nutrient-starved Agrobacterium tumefaciens and Rhizobium leguminosarum cells to become viable but nonculturable. This is the first report of a chemical inducer of this condition.
One bacterial response to a change in the environment is a reduced ability to give rise to colonies when plated on solid growth medium. This response can occur when cells undergo cell stress (9), cell wounding (6), or entry into the viable-but-nonculturable (VBNC) condition (8). The VBNC condition differs from the other bacterial responses in that VBNC cells are viable yet do not undergo sufficient cell division to give rise to visible growth when added to nonselective growth medium, and cells can remain VBNC for more than a year (5).
Many gram-negative organisms have been reported to become VBNC (5). Cells that become VBNC usually do so in response to certain environmental stress conditions. Although the specific conditions that result in the VBNC state differ for different organisms, a lack of nutrients is the most common condition. No specific chemical has been reported to cause cells to become VBNC. Manahan and Steck (4) examined Agrobacterium tumefaciens 646 and Rhizobium meliloti Rm1021 and found that cells became VBNC when inoculated in autoclaved, filtered drinking water from one source but not when inoculated into sterile deionized water or autoclaved, filtered drinking water from a second source.
During these tests, it was noticed that samples of VBNC-inducing drinking water stored in acid-washed glass bottles kept for periods exceeding approximately 6 months lost the ability to induce the VBNC condition. Having drinking water originating from the same source yet differing in the ability to allow the VBNC condition suggested that any chemical differences between the fresh and stored drinking water samples might relate to the ability to induce the VBNC condition. These two water samples were compared by using an intercoupled plasma analyzer and mass spectrophotometer (by Gordan Wallace, Perkin-Elmer, Rockville, Md.) that measured the concentrations of 39 elements to a level of parts per million. Only one element, copper, was present at a higher level (60 ppm) in the VBNC-inducing water than in the non-VBNC-inducing water (data not shown).
To determine if copper could induce the VBNC condition, a microcosm was established by using A. tumefaciens At493 (Rfr Cbr) (10) as follows. Cells were grown overnight with aeration at 28°C in YEP broth (10 g of yeast extract per liter, 10 g of peptone per liter, 5 g of NaCl per liter) containing 10 μg of rifampin per ml and 20 μg of carbenicillin per ml. Two milliliters of the overnight culture was inoculated into 10 ml of prewarmed medium and incubated at 28°C with aeration until the optical density at 600 nm reached 0.8 to 1.0. Cells were harvested by centrifugation at room temperature, washed three times in distilled water and then added to establish a 20-ml microcosm containing the indicated concentration of copper in a 125-ml Erlenmeyer flask to a final concentration of 5 × 105 cells per ml. At various times, culturability was measured as follows. Copper was removed from a sample of a microcosm either by washing cells collected onto a 0.2-μm-pore-size cellulose nitrate filter (Whatman, Inc., Clifton, N.J.) or by harvesting cells via centrifugation and washing them three times in microcosm medium lacking copper. Cells were diluted as necessary in microcosm medium and then plated in triplicate onto YEP plates. Colonies were counted after incubation of the plates at 25°C for at least 3 days. Addition of cupric sulfate to a final concentration of 60 ppm (∼0.001 mM) caused a decrease in culturability to an undetectable level within 2 days (data not shown). Similar results were obtained in three independent experiments.
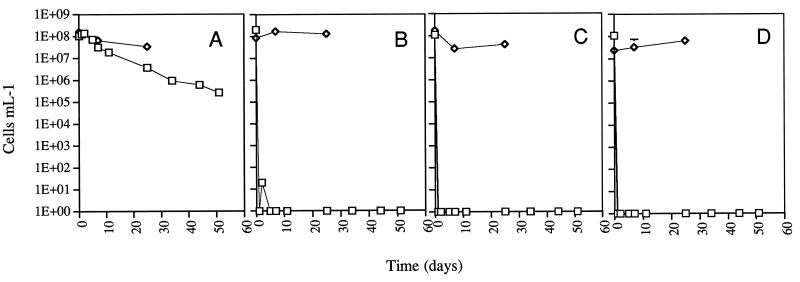
Previous studies had shown that microcosms established in VBNC-inducing drinking water became VBNC only when at a cell concentration of less than 107/ml (4). Examination of cells in a 1× AB salts solution (1 g of NH4Cl per liter, 0.3 g of MgSO4 per liter, 0.15 g of KCl per liter, 0.01 g of CaCl2 per liter, 2.5 mg of FeSO4 per liter), resulted in a decrease in the maximum cell concentration that would allow a microcosm to become VBNC (unpublished results). To determine if copper would induce cells to become nonculturable when in a salts solution, cupric sulfate was added to a final concentration of 0.05, 0.5, or 5 mM to a microcosm containing cells at a concentration of 108/ml established in a 1× AB salts solution. As is shown in Fig. 1, the microcosm became nonculturable within 1 to 2 days at all of the cupric sulfate concentrations. Similar results were obtained in five independent experiments. This result indicates that cells in a salts solution can become nonculturable and that increasing the concentration of copper allows microcosms containing higher cell concentrations to become nonculturable.
FIG. 1.
Culturability and viability of A. tumefaciens. Exponentially growing cells were inoculated into AB salts containing various concentrations of cupric sulfate. At various times after inoculation (time zero), aliquots were removed and the numbers of culturable (□) and viable (◊) cells were determined as described in Materials and Methods. Panels: A, microcosm without cupric sulfate; B, microcosm containing 0.05 mM cupric sulfate; C, microcosm containing 0.5 mM cupric sulfate; D, microcosm containing 5 mM cupric sulfate. The error bars represent 1 standard deviation; in most cases, the error bars are too small to be visible.
A long-term lack of culturability can be due to cell death or entry into the VBNC condition. To differentiate between these possibilities, cells in these microcosms were assayed for viability by using the LIVE/DEAD BacLight Bacterial Viability Kit (Molecular Probes Inc., Eugene, Oreg.). This assay uses two fluorescent nucleic acid stains which differ in the ability to penetrate cell membranes. Bacteria with intact membranes (i.e., viable bacteria) stain fluorescent green, and bacteria with damaged membranes (i.e., dead bacteria) stain fluorescent red. Cells in a sample of a microcosm were harvested by filtration as described above and suspended in 1 ml of sterile water. Three microliters of a 1:2 mixture of Reagent Mix A and Reagent Mix B was added to the bacterial suspension, and it was incubated at room temperature in the dark for 15 min. The cells were collected by filtration onto 0.2-μm-pore-size black polycarbonate filters (Poretics Corp., Livermore, Calif.) and quantitated by epifluorescence microscopy using a Standard 16 model Zeiss light microscope utilizing a 50-W Osram HBO mercury short-arc UV light source and a Zeiss filter cube. At least 100 cells over at least four fields of vision were scored per tested sample.
When cells from each microcosm on days 7 and 25 were examined by this assay, at least 10% of the cells were viable (Fig. 1). Similar results have been obtained with both AB salts and distilled-water microcosms. These results indicate that the decrease in culturability that occurs in response to the addition of cupric sulfate to either distilled water or a salts solution is due, at least in part, to cells becoming VBNC and not due solely to cell death. This is the first published report of a chemical inducer of the VBNC response in bacteria.
To determine if the chemical salt form of copper affects the ability to induce the VBNC condition, cupric acetate was added to microcosms instead of cupric sulfate. No difference in cell response was observed in two independent experiments (data not shown). These results indicate that copper, and not the anion, is responsible for induction of the VBNC condition.
To determine if copper induces the VBNC condition in microbes other than A. tumefaciens, Rhizobium leguminosarum F6 (1) was similarly examined. Growth of cells was as described for A. tumefaciens, except that Luria-Bertani broth containing 2.5 mM MgSO4 and 2.5 mM CaCl2 was used for initial growth. For R. leguminosarum, the culturability of 40-ml microcosms in 125-ml Erlenmeyer flasks containing cells at a concentration of 108/ml dropped to undetectable levels after addition of cupric sulfate to final concentrations ranging from 0.005 to 0.5 mM, while viability, as measured by the LIVE/DEAD BacLight Bacterial Viability Kit, remained at 106 cells per ml or higher (Fig. 2). In this and two subsequent experiments, it was observed that for 0.005 mM cupric sulfate, cells showed only a transient decrease in nonculturability before gradually returning to the initial levels.
FIG. 2.
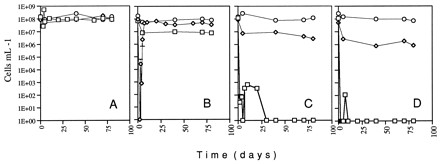
Culturability and viability of R. leguminosarum. Exponentially growing cells were inoculated into AB salts containing various concentrations of cupric sulfate. At various times after inoculation (time zero), aliquots were removed and the total number of cells (○) and the numbers of culturable (□) and viable (◊) cells were determined. Panels: A, microcosm without cupric sulfate; B, microcosm containing 0.005 mM cupric sulfate; C, microcosm containing 0.05 mM cupric sulfate; D, microcosm containing 0.5 mM cupric sulfate. The error bars represent 1 standard deviation; in most cases, the error bars are too small to be visible.
The LIVE/DEAD BacLight Bacterial Viability Kit has only recently been used for examination of the VBNC condition (2, 7). Although it is doubtful that copper-induced killing would change the membrane of dead cells such that they would stain fluorescent green, especially after periods exceeding a month, we wanted to confirm that cells treated with cupric sulfate were viable by using an independent assay. The method chosen for this purpose was the Kogure assay (3), in which a sample of cells was removed from a microcosm, harvested as described above, and suspended in 1 ml of sterile water. A 0.05-ml volume of 5% yeast extract and 0.01 ml of nalidixic acid (Sigma Chemical Co., St. Louis, Mo.) at 10 mg ml−1 was added to the cells, which were then incubated in the dark for 47 h at 30°C. The cells were then stained with 0.1 ml of 0.1% acridine orange (Sigma Chemical Co.) for 50 min at room temperature, collected by filtration onto 0.2-μm-pore-size black polycarbonate filters, and quantitated by epifluorescence microscopy as described above. Elongated cells (at least 2.5 times the normal length) were counted microscopically to yield a direct viable count. At least 100 cells were scored.
R. leguminosarum cells from a 70-day-old microcosm lacking copper and containing 0.05 mM cupric sulfate were examined for viability. In both microcosms, at least 5% of cells were elongated compared to the untreated control (Fig. 3).
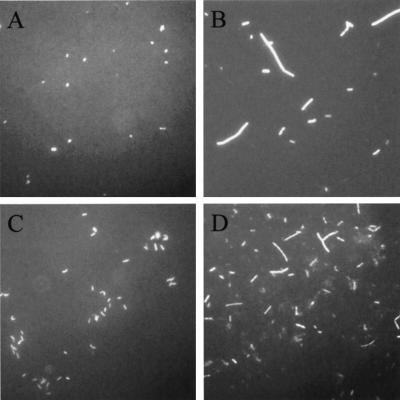
FIG. 3.
Viability of R. leguminosarum. Cells from day 70 (Fig. 2) were examined before and after being subjected to the Kogure viability assay. Panels: A, culturable cells from an AB salts microcosm; B, culturable cells from an AB salts microcosm subjected to the Kogure assay; C, VBNC cells from an AB salts microcosm containing 0.05 mM cupric sulfate; D, VBNC cells from an AB salts microcosm containing 0.05 mM cupric sulfate and subjected to the Kogure assay.
Attempts to characterize the interaction of VBNC bacteria with culturable bacteria (e.g., conjugation) have been complicated by the difficulty in having to not subject the culturable bacteria to the VBNC-inducing conditions. To determine if copper is necessary to maintain the VBNC state, VBNC microcosms were established and the exogenous copper was removed by either filtration or multiple rounds of centrifugation and washing. Cells were then suspended in the original microcosm medium lacking cupric sulfate, and culturability and viability were monitored. No change in culturability or viability was observed for the length of the experiment (1 week) after removal of the copper (data not shown). These results suggest that transient exposure to copper is sufficient to induce and maintain the VBNC condition in these bacteria. We do not know if intracellular pools of copper or membrane-bound copper content changes after the removal of exogenous copper.
The ability of plant-associated microbes to become VBNC suggests that culture-based assays commonly used to monitor for the presence of microbes, including genetically modified microorganisms released into the environment, will underestimate the number of viable organisms. The identification of copper as a VBNC inducer should lead to a better understanding of the VBNC response and suggests that VBNC bacteria are likely to exist in some soils, especially those soils treated with copper as a biocide.
(Some of these results were presented in a poster at the 98th General Meeting of the American Society for Microbiology, Atlanta, Ga., 17 to 21 May 1998.)
Acknowledgments
We thank Edward Menhinick and Gordan Wallace for assistance with water analysis and Rita Colwell and Russell Hill for generously providing water samples.
This work was supported in part by The University of North Carolina at Charlotte.
REFERENCES
- 1.Chaudri A M, McGrath S P, Giller K E, Angle J S, Chaney R L. Screening of isolates and strains of Rhizobium leguminosarum biovar trifolii for heavy metal resistance using buffered media. Environ Toxicol Chem. 1993;12:1643–1651. [Google Scholar]
- 2.Cole S P, Cirillo D, Kagnoff M F, Guiney D G, Eckmann L. Coccoid and spiral Helicobacter pylori differ in their abilities to adhere to gastric epithelial cells and induce interleukin-8 secretion. Infect Immun. 1997;65:843–846. doi: 10.1128/iai.65.2.843-846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kogure K, Simidu U, Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979;25:415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- 4.Manahan S H, Steck T R. The viable but nonculturable state in Agrobacterium tumefaciens and Rhizobium meliloti. FEMS Microbiol Ecol. 1997;22:29–38. [Google Scholar]
- 5.McDougald D, Rice S A, Weichart D, Kjelleberg S. Nonculturability: adaption or debilitation. FEMS Microbiol Ecol. 1998;25:1–9. [Google Scholar]
- 6.McFeters G A, Kippin J S, LeChevallier M W. Injured coliforms in drinking water. Appl Environ Microbiol. 1986;51:1–5. doi: 10.1128/aem.51.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigsbee W, Simpson L M, Oliver J D. Detection of viable but nonculturable state in Escherichia coli O157:H7. J Food Safety. 1997;16:255–262. [Google Scholar]
- 8.Xu H-S, Roberts N C, Singleton F L, Atwell R W, Grimes D J, Colwell R R. Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8:313–323. doi: 10.1007/BF02010671. [DOI] [PubMed] [Google Scholar]
- 9.Yura T, Nagai H, Mori H. Regulation of the heat-shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]
- 10.Yusibov V, Steck T R, Gupta V, Gelvin S B. Association of single-stranded transferred DNA from Agrobacterium tumefaciens with tobacco cells. Proc Natl Acad Sci USA. 1994;91:2994–2998. doi: 10.1073/pnas.91.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]