Abstract
Tissue engineering, using a combination of living cells, bioactive molecules, and three-dimensional porous scaffolds, is a promising alternative to traditional treatments such as the use of autografts and allografts for bone and cartilage tissue regeneration. Scaffolds, in this combination, can be applied either through surgery by implantation of cell-seeded pre-fabricated scaffolds, or through injection of a solidifying precursor and cell mixture, or as an injectable cell-seeded pre-fabricated scaffold. In situ forming and pre-fabricated injectable scaffolds can be injected directly into the defect site with complex shape and critical size in a minimally invasive manner. Proper and homogeneous distribution of cells, biological factors, and molecular signals in these injectable scaffolds is another advantage over pre-fabricated scaffolds. Due to the importance of injectable scaffolds in tissue engineering, here different types of injectable scaffolds, their design challenges, and applications in bone and cartilage tissue regeneration are reviewed.
Keywords: In situ injectable hydrogels, Injectable microparticles, Injectable shape memory scaffolds, Bone tissue engineering, Cartilage tissue engineering
Introduction
Tissue engineering is a combination of cells, signals, scaffolds, and bioreactors (Khan et al. 2015). This means that the cells are cultured in a three-dimensional scaffold which plays the role of ECM for cell growth (Eftekhari et al. 2020). Signals are used for cell growth and differentiation, resulting in more cellular adhesion. Bioreactors are simulators of body's response kinetics that cause a mass transfer to the tissue and improve the mechanical properties (Cleutjens and Creemers 2002; Langer and Vacanti 1999).
The permanent and inseparable part of tissue engineering is scaffold composed of natural or synthetic polymers (Behtouei et al. 2022; Kiran et al. 2020). These synthetic matrices for tissue engineering induce and guide tissue regeneration and are gradually replaced with new tissue (Pourjavadi et al. 2019). Natural polymers possess qualities such as promoting cell growth, less inflammatory response, immunological reactions, and toxicity and they can mimic the chemical composition of natural ECM. However, they suffer from a lack of reproducibility, control on their rate of biodegradability, and weak mechanical strength.
An ideal scaffold has a three-dimensional structure (Abdollahi Boraei et al. 2021; Ahmadian et al. 2019). It must be biodegradable with a controllable degradation rate, so that not only its rate of degradation has to be well matched with the rate of regeneration of tissue, but also it has to maintain its mechanical properties during tissue regeneration. Its structure should be porous with interconnected pores to allow the entrance of nutrients required for cells and the exit of waste produced by them (Liu et al. 2009; Nemati Hayati et al. 2012) and provide the perfect environment for adhesion, growth, and differentiation of cells. Spatiotemporal control, easy formation, and lack of toxic by-products are the other requirements of an ideal scaffold (Yang et al. 2014; Nikpour et al. 2021).
Bones form the body skeleton, which not only provides structure and protect inner organs, but also produce red and white blood cells and store minerals. Bone can be damaged by arthritis, fractures, infections, osteoporosis and tumors in their lifetime (Kumar Meena et al. 2019). Bone formation is complex and occurs by two mechanisms: intramembranous and endochondral bone formation (Shapiro 2008). Completion of the restoration of a damaged bone requires mechanical stability and the presence of periosteal cells and inflammatory cells in the defect site (Temenoff and Mikos 2000). Three essential factors for bone reconstruction include bone conduction, bone induction, and bone marrow cell. Growth factors are frequently used to differentiate cells into bone tissue. However, unwanted bone formation raises doubts about the application of growth factors. Improved and co-substituted hydroxyapatite is an alternative option for osteoconduction and increasing osteogenesis (Ressler et al. 2021).
Cartilage is an avascular, aneural tissue in the body that is usually associated with bone (Toniato et al. 2019; Eftekhari et al. 2020). Due to its avascular nature, low cell density, and metabolic activity, injured cartilage cannot heal sufficiently for even small defects (Li et al. 2018; Chuang et al. 2018). One of the most popular and challenging cartilage-related diseases is the damages to the meniscus. The meniscus is a shock absorber located between the tibia and femoral cartilage (Kim et al. 2018). Because of its joint-stabilizing role, damaged meniscus might aggravate articular cartilage degeneration and induce the progression of other joint diseases (Kim et al. 2018).
Today, the irregular shape of bone and cartilage defects, their critical size, and the risk of implant migration have led to the use of injectable scaffolds instead of pre-fabricated ones (Kumar Meena et al. 2019). Injectable scaffolds not only have the ability to fill defects of any shape and adhere to the environmental tissue, but also minimize aggressive treatment, are easily manageable, and improve patient compliance (Mohamadnia et al. 2009; Solouk et al. 2014; Makvandi et al. 2021). They can also encapsulate cells easily and then provide a favorable microenvironment for cell survival and growth, elicit specific cellular responses, and direct new tissue formation (Li et al. 2018). They can be used in the drug delivery of bioactive molecules (Temenoff and Mikos 2000; Hunt and Grover 2010).
Because of the importance of injectable systems in both bone and cartilage tissue engineering and meniscus therapy, in this article, we will introduce different types of injectable hydrogels in terms of structure and cross-linking reactions, outline their advantages and disadvantages and compare them in terms of their applications.
Injectable systems based on in situ forming hydrogels
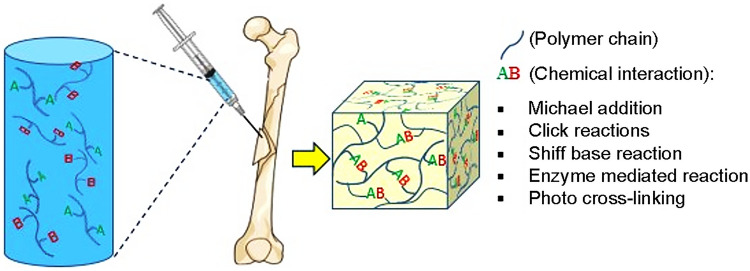
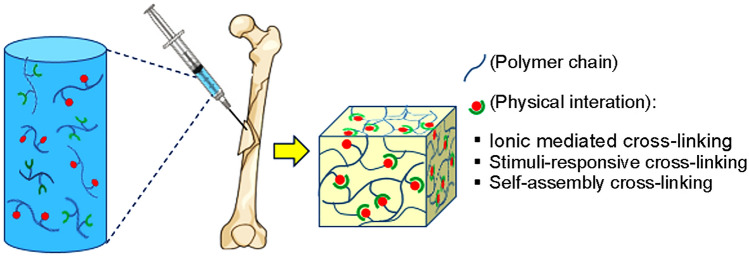
Hydrogels are nanoporous, three-dimensional networks capable of absorbing large amounts of water (Kunkit et al. 2019). Hydrogels are widely used for tissue engineering due to similar structures to the macromolecular-based components in the body (Lee et al. 2001). Hydrogels can be prepared both chemically and physically. Chains of chemical hydrogels form a network using covalent bonding (Kunkit et al. 2019). In situ forming injectable hydrogels fabricated through different chemical mechanisms include click reactions, Michael addition reaction, Schiff base reaction, enzyme-mediated reaction, and photo-cross-linking, whereas physical hydrogels are cross-linked using physical interaction such as ion, hydrophobicity, and interaction between chains or particles (Jin et al. 2009; Singh et al. 2018).
The summary of the formation mechanisms of the chemical and physical hydrogels are shown in Figs. 1 and Fig. 2, respectively.
Fig. 1.
Schematic of chemical cross-linking of in situ injectable hydrogels
Fig. 2.
Schematic of physical cross-linking of in situ injectable hydrogels
Chemically cross-linked hydrogels
Click reactions
Click reactions are efficient, fast, regiospecific, with high selectivity, and without toxic by-products; therefore, they can be used in bioapplications. Previously, the click chemistry was known as a reaction between an azide and terminal acetylene groups in the presence of copper catalysts (Tong et al. 2014; Jiang et al. 2014; Yang et al. 2014). Today, click reactions without copper catalysts and pseudo click reactions have also been developed. These reactions are categorized into three groups: CuAAC click reactions, copper-free click reactions, and pseudo click reactions (Jiang et al. 2014).
Copper-free click hydrogels
Copper-free click hydrogels are usually produced through azide-–alkyne cycloaddition by which cyclooctyne molecules immediately react with azides without copper as catalyst (Laughlin et al. 2008; Jiang et al. 2014). One of the applications of this type of reaction is mentioned in the work of Liu et al. (2016), who used the ability of azide–alkyne cycloaddition between hyPCL32-BCN and hyPCL32-N3 to design a cross-linked dendrimer for bone tissue engineering. As mentioned previously, the main advantage of this reaction for preparing tissue-engineered scaffold is the lack of a toxic cross-linker.
Highly selective cycloaddition between a diene and a dienophile without any catalyst and by-products is also a click reaction called the Diels–Alder reaction (Wei et al. 2009). This reaction is accelerated in water due to the increased hydrophobic effect (Nimmo et al. 2011). In 2009, Nimmo et al. (2011) modified hyaluronic acid by the furan group, and then modified hyaluronic acid was cross-linked through dimaleimide polyethylene glycol through the Diels–Alder reaction. As a result, they could produce a widely used hydrogel for tissue engineering without catalyst, photoinitiator, and other extra materials that may cause toxicity. Bai et al. (2017) functionalized chondroitin sulfate and F127 by furan and maleimide. Besides the ability to cross link by the Diels–Alder reaction, this hydrogel formed a physical network with temperature variations. The chemical network enhanced its strength and its physical network led to a rapid networking process. The scaffold obtained from the combination of these two networks presented good results in bone tissue engineering.
Recently, Ghanian et al. (2018) developed a dual cross-linking strategy to prepare in situ forming tough hydrogels. They functionalized alginate with furan to participate in Diels–Alder click reaction with maleimide end groups of a four-arm poly(ethylene glycol) cross-linker. The derivative of alginate was also cross-linked physically by calcium ion. This hydrogel showed interesting properties including improved toughness, self-healing ability, moldability, injectability, self-recovery and cytocompatibility.
Thiol–ene reaction is another type of copper-free click reaction that involves step-growth reactions and polymer chain growth reactions. Generation of thiyl radicals under light irradiation, formation of the carbon–carbon double bond by thiyl radicals, and finally multiplying the formed carbon-based radical through carbon–carbon double bond are steps of the thiol–ene reaction (Gopinathan and Noh 2018). However, this thiol–ene reaction suffers from the risk of producing harmful radical species. These radical species may cause a cross-reaction of the backbone with the thiol groups of cells, proteins, and some drugs (Koshy et al. 2016). Shih and Lin (2012) made an interesting comparison between the hydrogels prepared by thiol–ene and Michael addition reactions. They realized that the thiol–ene reaction has faster gel points and higher network density. In addition, its hydrolytic degradation rate depends on either the network density or the ester bond. However, due to the high dependence of its swelling rate on the number of macromers, thiol–ene hydrogels have not received much attention.
Pseudo click reaction
Pseudo click reaction includes thiol–-Michael and aldehyde–hydrazide reactions (Jiang et al. 2014).
A thiol-Michael addition consists of adding a thiol across a double bond in acrylate, vinyl sulfone, or maleimide, resulting in thioethers with or without the help of a basic catalyst. This reaction can take place with a wide variety of functional groups (Jiang et al. 2014; Chatani et al. 2013; Li et al. 2010). However, the reaction of aldehyde–hydrazide because of the simplicity, versatility, and lack of toxic by-products is more accepted (Jiang et al. 2014). Dmitri and colleagues (Ossipov et al. 2007) synthesized hydrazide and aldehyde derivatives of PVA and produced an injected hydrogel by using a carbamate bond formation. They observed that by mixing the precursor solutions of these two types of polymers with N2a cells, and the cells only survived for 4 days. In addition, due to the lack of destructive bonds and subsequently the absence of cavity formation around the cells, their growth is prevented physically. Therefore, to eliminate this problem, it is required to use either labile bonds or a degradable polymeric backbone in the system.
Michael addition
Michael addition is a highly selective reaction between α,β-unsaturated carbonyl compounds like unsaturated polyvinyl sulfone and thiols or amines as a nucleus. Throughout the reaction, double bonds of polyvinyl sulfone open and react with the SH groups of thiol to form C–S bonds and, respectively, network (Zheng Shu et al. 2004; Yang et al. 2014; Mather et al. 2006). The advantages of this reaction for biomedical applications include high selectivity, absence of toxic reagent and side products, and applicable for injectable systems. Michael addition reactions by thiols are known as pseudo click reactions too.
Jin et al. (2010) designed an injectable hydrogel using hyaluronic acid and polyethylene glycol through Michael addition reaction. To this end, they covalently conjugated the thiol groups into HA and the vinyl sulfone groups into PEG to form a three-dimensional network in less than 14 min under physiological conditions. The degradation time of this hydrogel was evaluated for a maximum of 21 days and showed good distribution and survival of cells. Besides, the enzymatic tyraminate degradation time of this hydrogel could be controlled by the molecular weight of the used polymers. All of these results testify to the potency of this hydrogel in tissue engineering.
Kim et al. (2010) also used a similar Michael addition reaction between the thiol group of thiolated heparin and acrylate group of diacrylated PEG to prepare heparin-based hydrogel as a carrier of hepatocyte cells. This reaction was achievable at the physiological temperature, at which the prepared hydrogel does not cause any toxicity to the cells and the loaded growth factor was released in a controlled manner.
Schiff base reaction
The tendency of the aldehyde groups to amines present on polymer chains, called Schiff base reaction, leads to the formation of the covalently cross-linked network (Emami et al. 2021; Chang et al. 2017). It is notable that the amine groups of cellular biomolecules can also react with aldehyde and cause toxicity which has to be considered in preparing hydrogels using the Schiff base reaction (Yang et al. 2014). During research conducted by Yan and his colleagues in 2016 (Yan et al. 2016a, b), PLGA derivatives of aldehyde and hydrazide were prepared by activation using EDC and oxidation of NaIO4. Injectable hydrogels were cross-linked through Schiff base reaction in physiological condition. The results indicated that by changing the molar ratio of amine to aldehyde, the rate of degradation and cross-linking, swelling ratio and rheological properties would be controlled. Moreover, chondrocyte loading showed that this hydrogel can maintain the round or oval phenotype of chondrocytes, and cells within the hydrogel have survival and growth potential. In other words, this hydrogel is a good candidate as a synthetic chondral extracellular matrix for cartilage tissue engineering.
Enzyme-mediated reaction
Enzymes can also be used to cross-link tyraminated polymers (Xu et al. 2013). The phenolic groups are oxidized in the presence of H2O2 and HRP and form di- and ter-tyrosine protein conjugations (Kondiah et al. 2016). Enzymatic reactions can occur under body conditions in terms of temperature, pH, and humidity, and because of their specific performance, removal of side reactions is more feasible. The rate of enzymatic reactions which is usually higher than that of other chemical reactions can be controlled by setting the concentration of the polymer and the enzyme. So far, a large number of natural polymers including alginate, carboxymethyl cellulose, gelatin, and tyramine functionalized dextran have been used to prepare in situ forming enzymatic hydrogels (Yang et al. 2014; Jin et al. 2007). Jin et al. (2011) formed an injectable hydrogel through co-cross-linking of tyramine derivatives of heparin and dextran using H2O2 and HRP. Due to the simultaneous networking of two polymers, the hydrogel sooner than any of the two polymers reaches the point of gelation and exhibits a higher modulus. The presence of heparin in the hydrogel structure leads to improved swelling behavior, better transfer of nutrients to cells, and the withdrawal of wastes from it.
Photocross-linking
Free radical polymerization is initiated using a free radical initiator which causes the formation of free radicals in the monomers or oligomers and then follows the propagation stage. The last stage, termination, occurs when two radicals on the polymer chains during propagation are covalently bonded (Bulmus 2011).
If the initiator produces free radicals using visible or UV irradiation, the mechanism of cross-linking is called photopolymerization (Hashemi Doulabi et al. 2015). Photopolymerization not only is able to be spatially and temporally controlled, but also has minimal heat production and fast curing rates (less than a second to a few minutes). It can be carried out in bulk or interfacially, in which bulk polymerization is more common and interfacial polymerization is used for a thin coating of hydrogels for special applications such as cell coverage (Kondiah et al. 2016).
Due to the toxicity of monomers and their limitations in biological applications, macromers are used in the fabrication of hydrogels.
One of the main challenges of photopolymerization is the narrow range of applicable biological temperature and pH (Yang et al. 2014) (Nguyen and West 2002). In addition, photoinitiators must be biocompatible, water-soluble, non-toxic, and stable for biological applications. Depending on the activation mechanism of the initiators, photopolymerization is classified into three groups: radical photopolymerization by photocleavage hydrogen abstraction, and cationic photopolymerization (Nguyen and West 2002). The last one due to protonic acid production is not usable in tissue engineering.
Initiators that produce free radicals using photocleavage include aromatic carbonyl compounds such as acetophenone derivatives, in which their bonds of C–C, C–O, C–Cl, or C–S break by light radiation and generate free radical (Nguyen and West 2002).
Initiators such as aromatic ketones lose hydrogen through UV irradiation to generate a radical donor (Nguyen and West 2002).
Elisseeff and his colleagues (Elisseeff et al. 2000) researched on photo-encapsulating of chondrocytes in a hydrogel. Photopolymerization was used to transform a liquid polymer solution composed of poly(ethylene oxide)-dimethacrylate and PEG to a hydrogel while directly encapsulating the chondrocytes. The results showed that the number of cells inside the hydrogel reduced at the early times, and then reached a plateau finally, but the ECM level with an equilibrium modulus constantly increased.
In 2007, Lee and Tae (2007) suggested that it would be better to irradiate the prepared polymeric solution by UV to initiate cross-linking at the molecular level and inject it before forming the viscose solution. This overcomes the formation of stable hydrogels and normal tissues are not damaged by direct UV radiation. He tested his hypothesis on DA-PF 127, which is either UV cross-linked or temperature sensitive. The final results of the assessment of the sustainability and cytotoxicity of hydrogel were acceptable for tissue engineering.
Sharifi et al. designed a new injectable and in situ forming system based on photo-cross-linked poly(e-caprolactone fumarate). The network was fabricated using PCLF macromers, a photoinitiation system (comprising initiator and accelerator), and the active ingredient N-vinyl-2-pyrrolidone as a cross-linker and reactive diluent. The study of cross-linking revealed that it is facilitated up to a certain concentration of cross-linker (NVP) and most of the NVP remained unreacted above this value. Photo-cross-linked PCLF network with optimum NVP content exhibited no significant cytotoxicity (Sharifi et al. 2007, 2008, 2011; Sharifi et al. 2009a, b).
In aother research, Hashemi Doulabi et al. (2008) used propylene oxide as a new and different proton scavenger to enhance the in situ photo-cross-linking capability of poly(ethylene glycol) and fumaric acid copolymers. Macromers were photocured for 300 s in the presence of a visible light initiator/accelerator couple and a reactive diluent. Results indicated this photocurable copolymer can be used as precursors to prepare scaffolds with controlled hydrophilicity, swelling, and mechanical properties in tissue engineering.
Schematic and examples of chemically cross-linked injectable hydrogels are reported in Fig. 1 and Table 1, respectively.
Table 1.
Chemically cross-linked injectable hydrogels
| Polymer | Mechanism of gelation | Chemical cross-linker | Chemical factors | Biological factor | Year | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Acrylated hyaluronic acid | Michael-type addition reaction | PEG-SH4 |
BMP-2 hMSCs |
2007 | Kim et al. (2007) | |
| 2 | b-CD-g-PNIPAM/G2.5 PAMAM-Ad | Noncovalent host–guest interaction and DA chemical cross-linking | PEG2K-–AMI | 2016 | Bai et al. (2016) | ||
| 3 | F127@ChS and PEG-AMI | DA click chemistry and thermos gelation | BMP-4 | 2017 | Bai et al. (2017) | ||
| 4 | Tetrazine-modified HA and transcyclooctene-modified HA | Click reaction | Cytomodulin-2 (CM) and (hPLSCs) | 2019 | Park et al. (2019) | ||
| 5 | Collagen I and thiolated hyaluronic acid | Self-cross-linking process of disulfide bond derived from two free thiol groups | Chondrocytes | 2018 | Chen et al. (2018) | ||
| 6 | Carboxymethyl chitosan and oxidized alginate | Schiff base reaction | nHAp and calcium carbonate microspheres containing tetracycline hydrochloride | 2018 | Ren et al. (2018) | ||
| 7 | Tyramine derivatives of gelatin and hyaluronic acid | Enzyme-mediated reaction | HRP | 2017 | Sanmartín-Masiá et al. (2017) | ||
| 8 | Tyramine-conjugated hyaluronic acid and gelatin | Enzyme-mediated reaction |
Tyrosinase EDC/sulfo-NHS |
Rabbit meniscus fibrochondrocyte | 2018 | Kim et al. (2018) | |
| 9 | Tyramine-conjugated hyaluronic acid and regenerated silk fibroin (SF) | Enzyme-mediated reaction | HRP | 2021 | Ziadlou et al. (2021) | ||
| 10 | DA-PF 127 | Photo-cross-linking and thermogelation | Irgacure 2959 as an initiator | 2007 | Lee and Tae (2007) | ||
| 11 | (EGAMA-CS)/(PEG-DA)/(DMMA) | Photo-cross-linking | 2010 | Ma et al. (2010) | |||
| 12 | Polydopamine-coated PCL-DA | Photo-cross-linking | 2014 | Zhang et al. (2014) | |||
| 13 | CSMA | Photo-cross-linking | PETMP as an initiator | HAp, MSCs | 2015 | Hadi Derakhshan et al. (2015) | |
| 14 | GelMA | Photo-cross-linking | nHAp | 2016 | Thakur et al. (2016) | ||
| 15 | MeGel,Ac-b-CDs | Host–guest interactions and photo-cross-linking | 2017 | Feng et al. (2017) | |||
| 16 | GelMA-2D nanosilicate | Covalently cross-linking (photopolymerization) | Ciba IRGACURE 2959, Ciba as an photoinitiator | 2015 | Xavier et al. (2015) | ||
| 17 | PAG | Covalent cross-linking | Adipic acid dihydrazide | Calvarial osteoblasts | 2001 | Lee et al. (2001) | |
| 18 | Collagen, chitosan, and lysine-modified hyaluronic acid | Covalently cross-linking | Genipin | 2021 | Gilarska et al. (2020) | ||
| 19 | ADA-GEL | Covalently cross-linking and ionic gelation | CaCl2 |
(nBG) MSCs |
2014 | Rottensteiner et al. (2014) | |
| 20 | Functionalizes chondroitin sulfate and oxidized pullulan | Covalent hydrazone self-cross-linking | 2018 | Li et al. (2018) | |||
| 21 | CryoGelMA | Radical polymerization (shape memory hydrogel) | 2014 | Koshy et al. (2014) | |||
| 22 | OPF | Chemically cross-linking via radical polymerization | PEG-DA | Redox initiators, APS and AA | 2003 | Shin et al. (2003) | |
| 23 | PNiPAAm containing pendant epoxy rings | Chemical and thermogelation | PAMAM | MSCs encapsulated with GMPs | 2014 | Tzouanas et al. (2014) | |
| 24 | P(NiPAAm-co-GMA-co-DBA-co-AA) TGM | Chemical and thermogelation | PAMAM | 2015 | Vo et al. (2015) | ||
| 25 | Alginate, and gelatin | Chemically and thermogelation | EDC/NHS | Nitrogen-doped carbon dots as a reinforcement | 2021 | Ghanbari et al. (2021) | |
| 26 | NiPAAm-AAm-MAEP | Chemically cross-linking after thermogelation | MSCs | 2015 | Watson et al. (2015) | ||
| 27 | star-shaped PLA and aniline trimer (AT) | Chemically cross-linking (electroactive shape memory polymer) | HDI | Sn(Oct)2 as an initiator and co-initiator inositol | 2015 | Xie et al. (2015) | |
| 28 | Covalently cross-linked alginate hydrogels | (Shape memory hydrogel) | Bifunctional cross-linker (AAD) | 2004 | Thornton et al. (2004) | ||
| 29 | PEGMEM, DEM | Chemically cross-linking | 1,4-Butanediol diacrylate | Zn, Sr | 2017 | Tommasi et al. (2016) | |
| 30 | Collagen type I and activated chondroitin sulfate | Physical and chemical cross-linking | chondrocytes | 2018 | Gao et al. (2018a, b) | ||
| 31 | Collagen type II and activated chondroitin sulfate | Chemical cross-linking between Col II and CS-sNHS and physical self-assembly of Col II | chondrocytes | 2018 | Gao et al. (2018a, b) | ||
| 32 | DNA | Chemical cross-linking and electrostatic cross-linking | Oxidized alginate | Silicate nanodisks | simvastatin | 2020 | Basu et al. (2020) |
| 33 | Oligo(poly(ethylene glycol) fumarate), CNT-poly(ethylene glycol)-acrylate | Chemical cross-linking | PEG-DA | Ammonium persulfate as a cross-linking initiator, and l-ascorbic acid as a cross-linking accelerator, and black phosphorus nanosheets | 2020 | Liu et al. (2020) |
Physically cross-linked hydrogels
Injectable hydrogels prepared by ionic gelation
These hydrogels are prepared by divalent or trivalent cations that form ion chain connections (Aalaie et al. 2008; Sivashanmugam et al. 2015; Aalaie and Vasheghani-Farahani 2012). Alginate is one of these polymers which can be cross-linked using Ca2+, Mg2+, and Ba2+ ions to form reversible hydrogels (Nair and Laurencin 2007; Donati et al. 2009; Sivashanmugam et al. 2015). However, due to the migration of ionic molecules of the hydrogel into the body fluid, alginate hydrogels are unstable and by changing the concentration of calcium, alginate, and molecular weight, their mechanical properties can be improved (Yang et al. 2014). The combination of covalent and photo-cross-linking mechanisms with physical systems is another solution to improve the mechanical properties of these hydrogels (Sun et al. 2012a, b). Rottensteiner et al. (2014) prepared an ionic hydrogel through the bonding of alginate aldehydes and gelatin using calcium chloride and distributed bioglass in nanoscale to improve not only mechanical properties, but also survival, adhesion, growth, and differentiation of cells. The behavior of hydrogel in the cellular contact was investigated by loading the mesenchymal stem cells in it. The results were satisfactory for bone tissue engineering, except for the sample with 0.1% of bioglass, which showed slight toxicity compared to the control sample without bioglass. Yan and his colleagues (Yan et al. 2016a, b) in 2016 produced alginate-based hydrogels using CaCO3 as an ionic cross-linker. To improve osteo-regeneration and bioactivity of hydrogel, nanohydroxyapatite and gelatinous microparticles loaded with tetracycline hydrochloride were distributed within the hydrogel. The results indicated that the presence of HAp and gelatin particles inside the hydrogel would improve its mechanical properties and reduce the rate of degradation. Besides, tetracycline hydrochloride caused better osteoblastic behavior, resulting in hydrogel as a suitable candidate for bone tissue engineering.
Stimuli-responsive injectable hydrogels
Injectable hydrogels prepared by thermally induced gelation are networks that the solubility of their constituent chains changes as a result of changing temperature and phase transition to sol–gel state (Ghaeini-Hesaroeiye et al. 2020). LCST is usually the threshold of gelation that can be manipulated and shifted to body temperature through techniques such as copolymerization (Zhang et al. 2019). The solution, once injected into the body, becomes a gel in response to body temperature. Usually, the drug is loaded in thermally injectable hydrogels at ambient temperature. The chemical structure, molecular weight, and concentration of polymers affect the gelation of these hydrogels (Kondiah et al. 2016; Chang et al. 2017).
Poly(NIPAAm) is a well-known polymer with thermal gelation ability (Motlaq et al. 2019; Zajforoushan Moghaddam et al. 2017; Wu et al. 2020). Its temperature of gelation is 32 °C, which can be set to body temperature by copolymerization with hydrophilic polymers such as PEO (Amsden 2015; Yang et al. 2014; Sivashanmugam et al. 2015). Methylcellulose is another good thermoresponsive polymer.
Lack of need for any chemicals to stimulate gelation is the main advantage of these hydrogels. However, their weak mechanical properties and stability limit their applications. To this end, hydrogels are designed to respond simultaneously to two environmental stimuli (such as temperature and pH) or (temperature and light) for phase transition. Consequently, not only the mechanical strength increases, but also the gelation in the needle during injection would be avoided. This can be achieved by copolymerization of a temperature-sensitive polymer with polymers that have pH or photosensitive segments (Kim et al. 2008).
Kondiah et al. (2017) developed a temperature-sensitive hydrogel in 2017 with the blending of Pluronic F127, PPF, and PEG-PCL-PEG, which is a solution at a temperature below 25 °C and gel-like at the physiological temperature. Simvastatin was loaded within the hydrogel and it was examined at the ex vivo and in vitro conditions. The results indicated that its abilities of defect filling, matrix hardening, and flexibility to regenerate small bone were suitable, and the hydrogel was similar to the natural tissue in terms of density.
Vo and colleagues (Vo et al. 2017) in 2017 designed a hydrogel with improved mechanical properties using copolymer P(NiPAAm-co-GMA-co-DBA-co-AA), which was cross-linked both thermally and chemically using PAMAM. One of the interesting results of this study was the ability of self-mineralization of hydrogel (in other words self-precipitation of calcium phosphate) which provided a suitable environment for osteoinduction. They proved that calcium and not phosphate increased during 56 days due to mineral nucleation. This is due to the presence of protein in a culture medium containing serum, which may occur for two reasons. Larger cavities of the swelled hydrogel and hydrogen bonding between the protein and the hydrogel's structure made the entrance and the presenting of protein in hydrogel possible.
Recently, Vasheghani-Farahani et al. (Kazemi-Aghdam et al. 2021), to enhance the mechanical properties of a well-known thermosensitive hydrogel (chitosan/glycerophosphate), used chitosan-modified halloysite nanotubes. The loaded halloysite nanotubes with icariin as an osteogenic inducer improved mechanical strength and differentiation of stem cells to bone tissue. This is because of the stiff nature, tubular structure and the ability for dynamic delivery of the halloysite nanotubes.
Self-assembling injectable hydrogels
Self-assembling hydrogels are prepared through non-covalent bonds in the absence of any chemical agents and initiators. As a result, they have high biocompatibility. On the other hand, these hydrogels suffer from weak mechanical strength and their cross-linking density cannot be adjusted (Hou et al. 2004). Self-assembling hydrogels are classified based on either self-assembly mechanisms or types of their bindings and interactions.
Phase separation and amphiphilic are self-assembly mechanisms of these hydrogels. Phase separation, which was introduced in 1990, involved dissolving a hydrophobic polymer in a water-soluble and non-toxic solvent. Gelation occurs when the water penetrates the matrix resulting in solvent displacement. The most commonly used solvents for phase separation include NMP, DMSO, THF, acetone, polypropylene glycol, and 2-pyrrolidone (Kondiah et al. 2016). Variables such as polymer molecular weight, concentration, and type of solvent affect the rate of deposition (Bakhshi et al. 2006; Mohamadnia et al. 2009).
The amphiphilic mechanism occurs due to the low solubility of hydrophobic moieties in an aqueous media. As soon as amphiphilic polymers are placed in an aqueous media, macromolecules arrange to minimize the interactions between hydrophobic moieties and water. Hydrogels prepared by amphiphilic polymers with charge or electrostatic or van der Waals forces are more stable (Kretlow and Klouda 2007).
One of the most striking examples in this regard is amphiphilic peptides (Kondiah et al. 2016). Amphiphilic peptides have a hydrophobic alkyl tail and a peptide head. The peptide head consists of hydrophobic amino acids and a short sequence of charged amino acids. Self-assembly of these molecules initiates in an aqueous media in the presence of multivalent salts or pH which change as screeners of repulsive intermolecular forces. By alkyl hydrophobic interactions and creating intramolecular beta structures, hydrogels are formed. The results indicate that the concentration of salt ions in the gelation buffer has a great influence on the properties of the final structure, in particular the mechanical properties (Cui et al. 2010; Dehsorkhi et al. 2014).
Self-assembling hydrogels are divided into two groups based on the type of interactions: complementary binding and host–guest interactions. Complementary bindings such as ligand–receptor pairs, antigen–antibody pairs, and base-pairing interactions have a strong tendency to each other which can be used as the injectable hydrogels (Yang et al. 2014). One of the most common examples is the streptavidin–biotin pair.
Host–guest interactions take place by either cyclodextrins or cucurbituril. CDs are natural cyclic oligosaccharides by which they have a hydrophobic inner cavity and form a complex with guest molecules such as PEG. However, they have poor stability in the body. The affinity of cucurbit to guest molecules is stronger than the CDs (Yang et al. 2014; Li 2010). Furthermore, it can interact with two guest molecules simultaneously. Cucurbit is a hollowed symmetrical macromolecule.
In 2008, Manakker and his colleagues (van de Manakker et al. 2008) developed a new hydrogel based on PEG. To this end, they modified star-shaped eight-arm poly(ethylene glycol) using β-CD groups and cholesterol separately. The hydrogel was prepared through a self-assembly mechanism by forming an interaction between β-CDs. The main advantage of this temperature reversible hydrogel was the ability to control properties using polymer concentration, molecular weight, and the ratio of stoichiometry used for β-CD and cholesterol.
In another research, Appel et al. (2010) demonstrated that preparing a hydrogel with a high binding constant is more feasible by functionalization of a supramolecule through cucurbituril as a guest molecule and methyl viologen or naphthoxy derivatives as a host molecule. Its cross-link density can also be controlled by changing the amount of cucurbituril.
Schematic and examples of physically cross-linked injectable hydrogels are reported in Fig. 2 and Table 2, respectively.
Table 2.
Physically cross-linked injectable hydrogels
| Polymer | Mechanism of gelation | Cross-linker agent | Chemical factors | Biological factor | Year | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Alginate | Ionic gelation | Calcium chloride |
β-TCP MSC |
2008 | Matsuno et al. (2008) | |
| 2 | Core–shell structured collagen–alginate hydrogel | Ionic gelation | Ca2+ | MSCs | 2014 | Perez et al. (2014) | |
| 3 | Alginate/GDL (GMs) | Ionic gelation | CaCO3 |
Tetracycline hydrochloride HAp |
2016 | Yan et al. (2016a, b) | |
| 4 | Chitosan biguanidine and carboxymethylcellulose | Ionic gelation | BMP-2 and VEGF | 2021 | Divband et al. (2021) | ||
| 5 | Gellan xanthan gels along with chitosan nanoparticles | Ionic and chemical and thermogelation | DMEM(Ca2+) |
bFGF BMP7 |
2012 | Dyondi et al. (2012) | |
| 6 | ADA-GEL | Covalently cross-linking and Ionic gelation | (CaCl2) |
(nBG) MSCs |
2014 | Rottensteiner et al. (2014) | |
| 7 | SMO-PCLA-PEG-PCLA-SMO block copolymer | pH and thermogelation |
hMSCs rhBMP-2 |
2009 | Kim et al. (2008) | ||
| 8 | Chitosan, ad heparin | pH and thermogelation | HAp | 2020 | Kocak et al. (2020) | ||
| 9 | Poly(propylene fumarate) (PPF) | Thermogelation | 2006 | Shi et al. (2006) | |||
| 10 | PNIPAM−PAA(PEA)−PNIPAM | Thermogelation | 2013 | Lin et al. (2013) | |||
| 11 | PEGePCLePEG | Thermogelation | ABM | 2014 | Ni et al. (2014) | ||
| 12 | MPEG-PCL | Thermogelation | hTMSCs | 2014 | Kwon et al. (2014) | ||
| 13 | Copolymers (PLGA-PEG-PLGA) | Thermogelation | Simvastatin | 2015 | Yan et al. (2015) | ||
| 14 | Xylan/chitosan | Thermogelation | 2016 | Bush et al. (2016) | |||
| 15 | Poly propylene fumerate (PPF), Pluronic F127 and PEG-PCL-PEG | Thermogelation | PEG as an initiator and Sn(Oct)2 as a catalyst | Simvastatin | 2017 | Kondiah et al. (2017) | |
| 16 | CS/CSn-GP | Thermogelation |
pDNA BMP2 |
2017 | Li et al. (2017) | ||
| 17 | CS/GP(CS/CMCS NPs) | Thermogelation | SDF-1α | 2017 | Mi et al. (2017) | ||
| 18 | PLGA–PEG–PLGA | Thermogelation |
nHAp P-15 peptide b |
2017 | Gohil and Kumar (2017) | ||
| 19 | Chitosan and κ-carrageenan and poly(NIPAM) | Thermogelation | Au nanoparticles and MG-63 | 2019 | Pourjavadi et al. (2019) | ||
| 20 | Chitosan | Thermogelation | NaHCO3/α-MEM | HAp | 2018 | Ressler et al. (2018) | |
| 21 | HA | Thermogelation | Ag NPs and | β-TCP | 2020 | Makvandi et al. (2020) | |
| 22 | Methylcellulose | Thermogelation | Trisodium citrate | Bassorin and halloysite nanotubes | 2021 | Varshosaz et al. (2021) | |
| 23 | Methoxy polyethylene glycol–polycaprolactone (MP) and RGD-conjugated MP | Thermogelation | 2020 | Kim et al. (2020) | |||
| 24 | Chitosan | Thermogelation | Sodium-β-glycerol phosphate pentahydrate | Halloysite nanoclay | 2021 | Kazemi-Aghdam et al. (2021) | |
| 25 | DA-PF 127 | Photopolymerization and thermogelation | Irgacure 2959 as an initiator | 2007 | Lee and Tae (2007) | ||
| 26 | Gellan gum, PEGMA, and (2-methacrylamidoethyl dihydrogen phosphate) | Thermogelation and photopolymerization | PEG-DA, and photoinitiator I2959 | 2020 | Li et al. (2020) | ||
| 27 | Chitosan, and polygalacturonic acid | Thermogelation and self-assembling | Sodium-β-glycerol phosphate pentahydrate | HAp | 2020 | Wasupalli and Verma (2020) | |
| 28 | Zn-CS | Chemical and thermogelation | β-GP | 2013 | Niranjan et al. (2013) | ||
| 29 | PNiPAAm containing pendant epoxy rings | Chemical and thermogelation | PAMAM | MSCs encapsulated with GMPs | 2014 | Tzouanas et al. (2014) | |
| 30 | NiPAAm-co-AAm-co-MAEP | Chemical and thermogelation | APS and TEMED as an initiators | 2014 | Watson et al. (2014) | ||
| 31 | NiPAAm-AAm-MAEP | Chemical and thermogelation | MSCs | 2015 | Watson et al. (2015) | ||
| 32 | P(NiPAAm-co-GMA-co-DBA-co-AA) TGM | Chemical and thermogelation | PAMAM | 2015 | Vo et al. (2015) | ||
| 33 | Poly(phosphazene) | Chemical and thermogelation | BMP-2 | 2017 | Seo et al. (2017) | ||
| 34 | P(NiPAAm-co-GMA-co-DBA-co-AA) | Chemical and thermogelation | PAMAM | AIBN as an initiator | 2017 | Vo et al. (2017) | |
| 35 | P(NiPAAm-co-GMA-co-DBA-co-AA) TGM | Chemical and thermogelation | PAMAM | 2017 | Vo et al. (2017) | ||
| 36 | F127@ChS and PEG-AMI | DA click chemistry and thermogelation | BMP-4 | 2017 | Bai et al. (2017) | ||
| 37 | MeGel,Ac-b-CDs | Host–guest interactions and photo-cross-linking | 2017 | Feng et al. (2017) | |||
| 38 | (β-CD)-modified HA and adamantane-modified HA | Host − guest interactions | MSCs | 2020 | Jeong et al. (2020) | ||
| 39 | b-CD-g-PNIPAM/ G2.5 PAMAM-Ad | Noncovalent host–guest interaction and Diels–Alder (DA) chemical cross-linking | PEG2K–AMI | 2016 | Bai et al. (2016) | ||
| 40 | Squid pen chitosan/glycerol phosphate | Electrostatic cross-linking | HA/b-TCP | 2016 | Shavandi et al. (2016) | ||
| 41 | Methacrylate gelatin | Electrostatic and van der waal cross-linking and photopolymerization | Silicate nanoplatelets (Laponite) | Stromal cell-derived factor 1 alpha | 2020 | Shi et al. (2020) | |
| 42 | PSAGE/PPF resins | CaCO3 and citric acid | BPO as an initiator and DMPT as an activator | 2017 | Śmiga-Matuszowicz et al. (2017) | ||
| 43 | HA-HAp | Physically cross-linking (colloidal gel) |
DCC or DBM as ECM VEGF as growth factor |
2017 | Townsend et al. (2017) | ||
| 44 | Collagen type II and activated chondroitin sulfate | Chemical cross-linking between Col II and CS-sNHS and physical self-assembly of Col II | Chondrocytes | 2018 | Gao et al. (2018a, b) | ||
| 45 | Collagen type I and activated chondroitin sulfate | Physical and chemical cross-linking | Chondrocytes | 2018 | Gao et al. (2018a, b) |
Injectable systems based on microparticles and nanoparticles
Micro and nanoparticles have long been used as a carrier for drugs, growth factors, biomolecules, and cells lately (Eshghi Esfahani et al. 2021). In 1964, Chang proposed the idea of using microcapsules using a very thin polymer membrane to protect the cells from the immune system and introduced the term “synthetic cell” to express the concept of biopsy. Biopsy has provided extensive therapies for diseases such as diabetes, hemophilia, cancer, and kidney problems (Baruch and Machluf 2006; Orive et al. 2003). However, lack of cell migration is the main challenge in cell encapsulating for tissue engineering. In other words, when the cells are encapsulated within the microparticles, they collapse and cannot grow, expand or migrate (Orive et al. 2003). For this reason, the cells are cultured on the surface of the microparticles, and then the microparticles and the cells are injected into the defect (Bhatia et al. 2005; Chia et al. 2002; Yeh et al. 2006). Microparticles of gelatin and collage are examples of injectable microparticles to treat bone defects (Wang et al. 2003; Kim et al. 2005).
Compared to solid microparticles, porous microparticles play a more significant role in cell therapy due to more available surface for cell growth and easy migration (Chung and Park 2007; Zhou et al. 2021). Kim et al. (Van Tomme et al. 2008a, b) reported the production of porous microparticles with average size of about 200 μm and porosities of about 30 μm for cartilage tissue engineering. These microparticles are applicable in the preparation of engineered scaffolds through connecting and forming a porous rigid structure. Due to the relatively round shape of microparticles and their microscale diameters, it can be assured that the resulting porosity of hydrogels based on microparticles is completely connected.
So far, few works have been done on the production of hydrogels based on microparticles. Van Tam and colleagues (Van Tomme et al. 2005a, b, 2006, 2008a, b) have worked on in situ cross-linking hydrogels based on microparticles as a result of electrostatic interaction. These microparticles were made of polyhydroxy methacrylate (HEMA) and dextran which have various positive and negative charges at pH of 7. Salem et al. (Krishnamachari et al. 2008; Salem et al. 2003) also reported an injectable system based on PLA microparticles, which were cross-linked through the avidin–biotin system. Their system was a cellular composite and the size of the microparticles was practically smaller than the dimension of the cells. Bagheri Khoulenjani et al. (2010), Bagheri-khoulenjani et al. (2012), and Bagheri-Khoulenjani et al. (2013) fabricated a natural injectable nanocomposite for bone tissue engineering applications based on gelatin/chitosan/nanohydroxyapatite with the ability to be solidified in situ using the benefits of avidin–biotin systems. The steps of this project include fabrication of microspheres using water-in-oil emulsions, biotination of microspheres using NHS-PEG-Biotin spacer via interaction of NH2 groups of microspheres and NHS-ester group of spacers. Results confirmed that the suspension concentration and particle size of microspheres affect the strength and pore size of the hydrogels, respectively.
Schematic and examples of injectable systems based on microparticles are reported in Fig. 3 and Table 3, respectively.
Fig. 3.
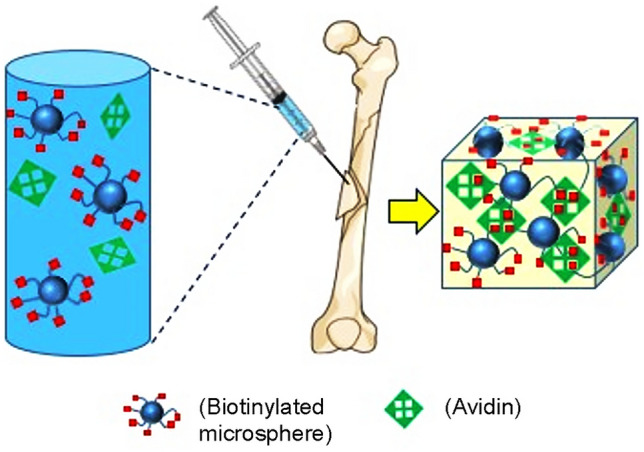
Schematic of injectable systems based on micro particles
Table 3.
Injectable systems based on microparticles and nanoparticles
| Polymer | Mechanism of gelation | Chemical factors | Biological factor | Year | Ref. | |
|---|---|---|---|---|---|---|
| 1 | PLA-PEG-Biotin and PVA | Self-assembly (avidin–biotin systems) | Human osteoblast sarcoma | 2003 | Salem et al. (2003) | |
| 2 |
Hydroxyethyl methacrylate-derivatized dextran–dimethylaminoethyl methacrylate and hydroxyethyl methacrylate-derivatized dextran–methacrylic acid |
Self-assembly |
TEMED KPS |
2005 | Van Tomme et al. (2005a, b) | |
| 3 | Amine-functionalized PLGA and PLGA | Self-assembly | Bovine articular chondrocytes | 2008 | Chung et al. (2008) | |
| 4 | Gelatin/chitosan | Self-assembly (avidin–biotin systems) | Nano hydroxyapatite | 2010 | Bagheri Khoulenjani et al. (2010) | |
| 5 | SS-PLLA | Self-assembly | Chondrocytes | 2011 | Liu et al. (2011) | |
| 6 | PHEMA-g-PLLA-acrylic | Self-assembly |
TGF-β1 BMP-2 Bone marrow-derived mesenchymal stem cells |
2015 | Zhang et al. (2015) | |
| 7 | PLGA/chitosan | Self-assembly | Chondrocytes | 2014 | Fang et al. (2014) | |
| 8 | Sr-HA-graft-poly(γ-benzyl-l-glutamate) | Self-assembly | Hydroxyapatite adipose-derived stem cells | 2017 | Gao et al. (2017) | |
| 9 | Chitosan and dialdehyde bacterial cellulose | Self-assembly | Bone marrow-derived mesenchymal stem cells | 2018 | Wang et al. (2018) | |
| 10 | Strontium-substituted hydroxyapatite-graft-poly(γ-benzyl-l-glutamate) | Self-assembly | Adipose-derived stem cells | 2018 | Yan et al. (2018) |
Injectable systems based on shape memory polymers
There is a group of materials that can change their shape spontaneously and immediately in the presence of suitable environmental triggers, called shape-changing materials. Other stimulus-responsive materials are shape memory materials which can be deformed to temporary shape and then return to the original state through elastic deformation stored in them by applying an appropriate stimulus (Sun et al. 2012a, b).
The shape memory property in polymers is based on two parts: elastic and transition (Khan et al. 2020). The task of the elastic part is maintaining the elasticity of the system and the transition part is responsible for changing the stiffness (Sun and Huang 2010).
Stimulants used to recover the shape memory polymers include temperature, solvent, light, humidity, and pH. These systems have the ability to retrieve by more than one type of stimulus.
Shape memory polymers can form hydrogels which are more flexible than shape memory rubbers and their shape is recovered by heating.
Due to biodegradability, narrow temperature range, rapid stimulating, large deformation, adjustable stiffness, and elastic properties, they can be used in medical applications. One of the commercial products in this field is a self-tightenable biodegradable suture that solved the problem of limited space for tying sutures (Lendlein and Langer 2002).
Shape memory polymers are categorized into four groups (Liu et al. 2007):
Covalently cross-linked glassy thermoset networks.
Covalently cross-linked semi-crystalline networks-
Physically cross-linked glassy copolymers-
Physically cross-linked semi-crystalline block copolymers.
Covalently cross-linked glassy thermoset networks can return to their original shape well due to the rubber elasticity caused by covalent cross-links. The rubber module of these systems is controllable by the amount of covalent cross-linking. However, the performance of secondary processes is difficult due to this covalent cross-linking (Liu et al. 2007).
Shape recovery of semi-crystalline rubbers is faster and secondary form of them is fixed by crystallization, but difficulty in secondary processability is their main issue as well (Liu et al. 2007).
Unlike covalently cross-linked networks, physically cross-linked copolymers showed good processability, so that they can even be electrospun. Physically cross-linked glassy copolymers include homopolymers with low or semi-crystallinity and melt miscible alloys with at least one semi-crystalline part in which crystals play the role of physical cross-links (Liu et al. 2007).
David Mooney is a pioneer in the use of cryogels as an injectable hydrogel with the shape memory property (Koshy et al. 2014; Bencherif et al. 2012; Thornton et al. 2004). In 2012, He et al. prepared nanoporous hydrogels and macroporous cryogels using methacrylated alginate at room temperature and −20 °C, respectively. The results showed that cryogels have the potential to compress up to 90% of strain by force and retrieval of their shape as they enter the body. In addition, cryogels have large, interconnected cavities that are recovered after entering the body and allowed cell growth. To fill large cavity defects, a large number of small cryogels instead of a large piece can be simultaneously used (Bencherif et al. 2012). During another research (Koshy et al. 2014) in 2014, they prepared a cryogel with shape memory property using methacrylated gelatin. They showed that this cryogel allows the adhesion and growth of the cells, and degrades enzymatically in the body.
Recently, Mirzadeh et al. (Goodarzi et al. 2020) developed a preformed injectable scaffold based on methacrylate gelatin for cartilage tissue engineering. They loaded betamethasone sodium phosphate in cellulose nanocrystal (CNC) and polyamidoamine (PAMAM) dendrimers as drug carrier embedded within the scaffold. In their next research, they fabricated an interpenetrating network using gelatin methacrylate and hyaluronic acid (Jonidi Shariatzadeh et al. 2021). So far, high mechanical stability had been achieved by the cryogelation and chemical cross-linking of Gel-MA as well as physical cross-linking of HA and made these cryogels a promising candidate for cartilage tissue application.
Schematic and examples of injectable systems based on shape memory polymers are reported in Fig. 4 and Table 4, respectively.
Fig. 4.
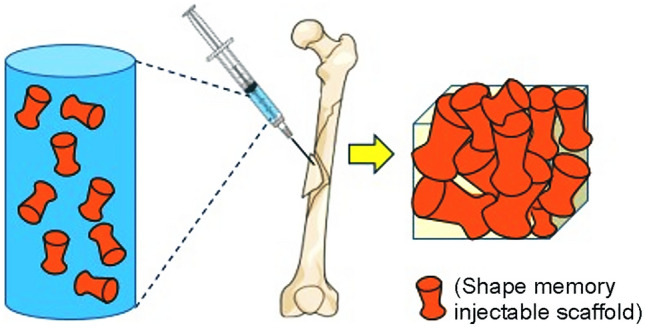
Schematic of injectable systems based on shape memory polymers
Table 4.
Injectable systems based on shape memory polymers
| Polymer | Mechanism of gelation | Cross-linker agent | Chemical factors | Biological factor | Year | Ref. | |
|---|---|---|---|---|---|---|---|
| 1 | Poly(e-caprolactone)dimethacrylate (PCLDMA) | Photo-cross-linking | 2009 | Neuss et al. (2009) | |||
| 2 | Methacrylated alginate | Free-radical polymerization | Ammonium persulfate as initiator and tetramethylethylenediamine as catalyst | 2012 | Bencherif et al. (2012) | ||
| 3 | Poly(3-caprolactone) and poly(ethylene glycol) | Thiol–ene chemistry | tetrathiol | 2,2-dimethoxy-2-phe- nylacetophenone as photoinitiator and NaCl for porogen leaching | TGF-b | 2013 | Baker et al. (2013) |
| 4 | Methacrylated gelatin | Free-radical polymerization | Ammonium persulfate as initiator and tetramethylethylenediamine as catalyst | 2014 | Koshy et al. (2014) | ||
| 5 | Poly(e-caprolactone) diacrylate coated with polydopamine | Photo-cross-linking | 2,2-dimethoxy-2-phenyl acetophenone as photoinitiator | 2014 | Zhang et al. (2014) | ||
| 6 | Methacrylatedhyaluronic acid | Free-radical polymerization | Ammonium persulfate as initiator and tetramethylethylenediamine as catalyst | 2017 | Cheng et al. (2017) | ||
| 7 | Poly(octamethylene maleate (anhydride) citrate) | Photo-cross-linking | Irgacure 2959 as photoinitiator and poly(ethylene glycol) dimethyl ether | 2017 | Montgomery et al. (2017) | ||
| 8 | Methacrylate gelatin | Free-radical polymerization | Ammonium persulfate as initiator and tetramethylethylenediamine as catalyst and cellulose nanocrystal | Betamethasone sodium phosphate | 2020 | Goodarzi et al. (2020) | |
| 9 | Methacrylate gelatin | Free-radical polymerization | FeCl3 | Ammonium persulfate as initiator and tetramethylethylenediamine as catalyst and cellulose nanocrystal | 2021 | Jonidi Shariatzadeh et al. (2021) |
Future perspective
Today, due to the benefits of injectable hydrogels compared to pre-fabricated hydrogels, they have attracted a lot of attention. Non-invasiveness, high performance in filling irregular bone and cartilage defects, low cost and providing more favorable conditions for the patients and the surgeons are the main advantages of injectable hydrogels. However, they have some disadvantages. The major challenges of in situ forming hydrogels for regenerative medicine are the spatiotemporal control of gelation, the integrity, and the porosity of the final gel. Studies showed that chemically cross-linked hydrogels exhibit favorable mechanical properties and stability, but the probability of cytotoxicity caused by chemical reactions limits their applications. Producing the harmful radical species in thiol–ene reactions, the remaining aldehyde in Schiff base reactions, narrow range of applicable biological temperature and pH of photopolymerization, and the lake of labile bonds and appropriate porosity are the challenges of chemically cross-linked injectable hydrogels that should be addressed. On the other hand, physically cross-linked hydrogels show minimized toxicity risks and more easily production methods. However, they are not robust for bone tissue engineering applications as a result of ion migration and show a slow response time. Furthermore, applicable physically injected hydrogels require high solid content, which increases the final cost and viscosity, and some discomfort would be caused to patients as result. In general, physical in situ forming injectable hydrogels is suitable for the engineering of non-load-bearing tissues, and chemical in situ forming injectable hydrogels is more suitable in tissues that require high mechanical strength. This has led to the use of both chemical and physical cross-linking systems simultaneously to achieve optimal cartilage and bone regeneration.
The shape memory injectable hydrogels are a new topic that has been recently interested. These hydrogels are a combination of pre-prepared and injectable hydrogels. They can be condensed due to their flexibility, passed through a conventional needle, and can retrieve their original shape using various stimuli such as temperature, moisture, and pH. This kind of hydrogel does not have disadvantages of in situ forming injectable hydrogels, such as the risk of displacement of the solution and the lack of proper gelation time and porosity. However, the use of several small pieces of shape memory hydrogel instead of one integrated piece to cover the defect and relatively high injection force compared to the in situ forming injectable hydrogels are the main challenges of the shape memory injectable hydrogels.
To see the commercial use of injectable hydrogels in the future, their disadvantages should be eliminated through the advancement of material science and methodology of production to imitate the morphological and functional properties of bone and cartilage tissue. Only then can we expect injectable hydrogels to become the main option in bone and cartilage tissue engineering in the near future.
Conclusion
Hydrogels are widely used three-dimensional platforms for tissue engineering. The presence of water in their structure not only simulates ECM for the cells, but also helps to encapsulate, manipulate, and transfer their contents to the surrounding tissue in a homogenous manner. In the field of orthopedic injuries, the defect with irregular shape and critical size would limit the use of preformed hydrogels. Therefore, injectable hydrogels not only completely cover the defects, but also do not require secondary surgery and are applied in the least invasive manner. Currently, drugs, biomolecules, cells, and their combinations can be encapsulated in injectable hydrogels. The disadvantages of injectable systems now prevent them from being used commercially on a large scale, and only handful are commercially available. It is hoped that by the advancement of science, these disadvantages will be eliminated and the performance of injectable hydrogels will be improved.
Abbreviations
- AA
Ascorbic acid
- AAD
Adipic acid dihydrazide
- AAm
Monomers acrylamide
- ABM
Acellular bone matrix
- Ac-b-CD
Acrylate b-cyclodextrin
- ADA
Alginate dialdehyde
- APS
Ammonium persulfate
- bFGF
Basic fibroblast growth factor
- BMP-2
Bone morphogenic protein-2
- BMP-4
Bone morphogenetic protein-4
- BPO
Benzoyl peroxide
- CD
Cyclodextrins
- CS/GP
Chitosan/β-glycerol phosphate disodium salt
- CSMA
Chondroitin sulfate methacrylate
- DA-PF 127
Diacrylated pluronic F 127
- DBA
Dimethyl-γ-butyrolactone acrylate
- DBM
Demineralized bone matrix
- DCC
Decellularized cartilage
- DEM
(2-(Dimethylamino)ethyl methacrylate)
- DMEM
Dulbecco’s modified Eagle’s medium
- DMF
Dimethylformamide
- DMPT
N,N-Dimethyl-p-toluidine
- DMSO
Dimethyl sulfoxide
- ECM
Extracellular matrix
- EDC
N-(3- Dimethylaminopropyl)-N′-ethylcarbodiimide
- EGAMA-CS
Methacryloyloxy ethyl carboxyethyl chitosan
- F127-AMI
Maleimido-terminated F127
- F127@ChS
F127 cross-linked chondroitin sulfate
- G2.5 PAMAM-Ad
Adamantane-decorated generation 2.5 poly(amido amine)s
- GelMA
Gelatin with pendant methacrylate groups
- GMA
Glycidyl methacrylate
- HA
Hyaluronic acid
- HAp
Hydroxyapatite
- HDI
Hexamethylenediisocyanate
- hMSC
Human mesenchymal stem cells
- hPLSCs
Human periodontal ligament stem cells
- HRP
Horseradish type VI
- LCST
Lower critical solution temperature
- MAEP
Monoacryloxyethyl phosphate
- MeGel
Methacrylated gelatin
- MPEG
Methoxy polyethylene glycol
- nBG
Nanoscaled bioactive glass
- NVP
N-Vinyl-2-pyrrolidone
- OPF
Oligo(poly(ethylene glycol) fumarate)
- p(NiPAAm)
Poly(N-isopropylacrylamide)
- PAG
Poly (aldehyde guluronate)
- PAMAMs
Polyamidoamines
- PCL
Poly(ε-caprolactone)
- PCL-DA
PCL diacrylate
- PCLF
Poly (e-caprolactone fumarate)
- PEG
Polyethylene glycol
- PEG2K-AMI
Maleimido-terminated poly(ethylene glycol)
- PEG-DA
PEG-diacrylate
- PEGMEM
Poly(ethylene glycol) methyl ether methacrylate
- PEO
Poly(ethylene oxide)
- PETMP
Pentaerythritol tetrakis 3-mercaptopropionate
- PLA
Polylactide
- PSAGE
Poly(3-allyloxy-1,2-propylene succinate)
- rhBMP-2
Recombinant human bone morphogenetic protein-2
- SDF-1α
Stromal cell-derived factor-1α
- SMO–PCLA–PEG–PCLA–SMO
Sulfamethazine oligomers–poly(e-caprolactone-co-lactide)–poly(ethylene glycol)–poly(e-caprolactone-co-lactide)–sulfamethazine oligomers
- sulfo-NHS
N-Hydroxysulfosuccinimide
- TEA
Triethylamine
- TEMED
Tetramethylethylenediamine
- TGM
Thermogelling macromer
- VEGF
Vascular endothelial growth factor
Funding
The authors would like to thank the National Institute for Medical Research Development (NIMAD) for their scientific and financial support. This work has been funded by the National Institute for Medical Research Development (NIMAD) (grant No. 942955).
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shadab Bagheri-Khoulenjani, Email: s.bagheri@aut.ac.ir.
Hamid Mirzadeh, Email: mirzadeh@aut.ac.ir.
References
- Aalaie J, Vasheghani-Farahani E. Swelling behavior of sulfonated polyacrylamide nanocomposite hydrogels in electrolyte solutions: comparison of theoretical and experimental results. Iran Polym J. 2012;21(3):175–183. doi: 10.1007/s13726-012-0016-3. [DOI] [Google Scholar]
- Aalaie J, Vasheghani-Farahani E, Rahmatpour A, Semsarzadeh MA. Effect of montmorillonite on gelation and swelling behavior of sulfonated polyacrylamide nanocomposite hydrogels in electrolyte solutions. Eur Polym J. 2008;44(7):2024–2031. doi: 10.1016/j.eurpolymj.2008.04.031. [DOI] [Google Scholar]
- Abdollahi Boraei SB, Nourmohammadi J, Bakhshandeh B, Dehghan MM, Gholami H, Calle Hernández D, Gonzalez Z, Ferrari B. Enhanced osteogenesis of gelatin–halloysite nanocomposite scaffold mediated by loading strontium ranelate. Int J Polym Mater Polym Biomater. 2021;70(6):392–402. doi: 10.1080/00914037.2020.1725754. [DOI] [Google Scholar]
- Ahmadian E, Eftekhari A, Maleki Dizaj S, Sharifi S, Mokhtarpour M, Nasibova AN, Khalilov R, Samiei M. The effect of hyaluronic acid hydrogels on dental pulp stem cells behavior. Int J Biol Macromol. 2019;140:245–254. doi: 10.1016/j.ijbiomac.2019.08.119. [DOI] [PubMed] [Google Scholar]
- Amsden B. Biodegradable injectable in situ forming drug delivery systems. J Control Release. 2015;80:9–28. doi: 10.1016/S0168-3659(02)00008-1. [DOI] [PubMed] [Google Scholar]
- Appel EA, Biedermann F, Rauwald U, Jones ST, Zayed JM, Scherman OA. Supramolecular cross-linked networks via host-guest complexation with cucurbit[8]uril. J Am Chem Soc. 2010;132(40):14251–14260. doi: 10.1021/ja106362w. [DOI] [PubMed] [Google Scholar]
- Bagheri Khoulenjani S, Etrati-Khosroshahi M, Mirzadeh H (2010) Fabrication and characterization of a natural injectable nanocomposite for bone tissue engineering applications. Amirkabir University of Technology
- Bagheri-khoulenjani S, Mirzadeh H, Etrati-khosroshahi M, Shokrgozar MA. A novel injectable bio-nanocomposite for bone tissue engineering applications. Int J Artif Organs. 2012;35(8):557. [Google Scholar]
- Bagheri-Khoulenjani S, Mirzadeh H, Etrati-Khosroshahi M, Shokrgozar MA. Particle size modeling and morphology study of chitosan/gelatin/nanohydroxyapatite nanocomposite microspheres for bone tissue engineering. J Biomed Mater Res A. 2013;101(6):1758–1767. doi: 10.1002/jbm.a.34481. [DOI] [PubMed] [Google Scholar]
- Bai X, Lü S, Cao Z, Gao C, Duan H, Xu X, Sun L, Gao N, Feng C, Liu M. Self-reinforcing injectable hydrogel with both high water content and mechanical strength for bone repair. Chem Eng J. 2016;288:546–556. doi: 10.1016/j.cej.2015.12.021. [DOI] [Google Scholar]
- Bai X, Lü S, Cao Z, Ni B, Wang X, Ning P, Ma D, Wei H, Liu M. Dual crosslinked chondroitin sulfate injectable hydrogel formed via continuous diels-alder (DA) click chemistry for bone repair. Carbohydr Polym. 2017;166:123–130. doi: 10.1016/j.carbpol.2017.02.062. [DOI] [PubMed] [Google Scholar]
- Baker RM, Henderson JH, Mather PT. Shape memory poly(ε-caprolactone)-co-poly(ethylene glycol) foams with body temperature triggering and two-way actuation. J Mater Chem B. 2013;1(38):4916. doi: 10.1039/c3tb20810a. [DOI] [PubMed] [Google Scholar]
- Bakhshi R, Vasheghani-Farahani E, Mobedi H, Jamshidi A, Khakpour M. The effect of additives on naltrexone hydrochloride release and solvent removal rate from an injectable in situ forming PLGA implant. Polym Adv Technol. 2006;17(5):354–359. doi: 10.1002/pat.717. [DOI] [Google Scholar]
- Baruch L, Machluf M. Alginate–chitosan complex coacervation for cell encapsulation: effect on mechanical properties and on long-term viability. Biopolymers. 2006;82(6):570–579. doi: 10.1002/bip.20509. [DOI] [PubMed] [Google Scholar]
- Basu S, Pacelli S, Paul A. Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery. Acta Biomater. 2020;105:159–169. doi: 10.1016/j.actbio.2020.01.021. [DOI] [PubMed] [Google Scholar]
- Behtouei E, Zandi M, Askari F, Daemi H, Zamanlui S, Arabsorkhi-Mishabi AH, Pezeshki-Modaress M. Bead-free and tough electrospun PCL/gelatin/PGS ternary nanofibrous scaffolds for tissue engineering application. J Appl Polym Sci. 2022;139(2):12–14. doi: 10.1002/app.51471. [DOI] [Google Scholar]
- Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, Mooney DJ. Injectable preformed scaffolds with shape-memory properties. PNAS USA. 2012;109(48):19590–19595. doi: 10.1073/pnas.1211516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia SR, Khattak SF, Roberts SC. Polyelectrolytes for cell encapsulation. Curr Opin Colloid Interface Sci. 2005;10(1):45–51. doi: 10.1016/j.cocis.2005.05.004. [DOI] [Google Scholar]
- Bulmus V. RAFT polymerization mediated bioconjugation strategies. Polym Chem. 2011;2(7):1463–1472. doi: 10.1039/C1PY00039J. [DOI] [Google Scholar]
- Bush JR, Liang H, Dickinson M, Botchwey EA. Xylan hemicellulose improves chitosan hydrogel for bone tissue regeneration. Polym Adv Technol. 2016;27(8):1050–1055. doi: 10.1002/pat.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang B, Ahuja N, Ma C, Liu X. Injectable scaffolds: preparation and application in dental and craniofacial regeneration. Mater Sci Eng R Rep. 2017;111:1–26. doi: 10.1016/j.mser.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatani S, Nair DP, Bowman CN. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol-michael addition reaction and polymerization. Polym Chem. 2013;4(4):1048–1055. doi: 10.1039/C2PY20826A. [DOI] [Google Scholar]
- Chen Y, Sui J, Wang Q, Yin Y, Liu J, Wang Q, Han X, Sun Y, Fan Y, Zhang X. Injectable self-crosslinking HA-SH/Col I blend hydrogels for in vitro construction of engineered cartilage. Carbohydr Polym. 2018;190:57–66. doi: 10.1016/j.carbpol.2018.02.057. [DOI] [PubMed] [Google Scholar]
- Cheng L, Ji K, Shih T-Y, Haddad A, Giatsidis G, Mooney DJ, Orgill DP, Nabzdyk CS. Injectable shape-memorizing three-dimensional hyaluronic acid cryogels for skin sculpting and soft tissue reconstruction. Tissue Eng A. 2017;23(5–6):243–251. doi: 10.1089/ten.tea.2016.0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S, Wan AC, Quek C, Mao H, Xu X, Shen L, Ng M, Leong K, Yu H. Multi-layered microcapsules for cell encapsulation. Biomaterials. 2002;23(3):849–856. doi: 10.1016/S0142-9612(01)00191-0. [DOI] [PubMed] [Google Scholar]
- Chuang EY, Chiang CW, Wong PC, Chen CH. Hydrogels for the application of articular cartilage tissue engineering: a review of hydrogels. Adv Mater Sci Eng. 2018 doi: 10.1155/2018/4368910. [DOI] [Google Scholar]
- Chung HJ, Park TG. Surface engineered and drug releasing pre-fabricated scaffolds for tissue engineering. Adv Drug Deliv Rev. 2007;59(4):249–262. doi: 10.1016/j.addr.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Kim IK, Kim TG, Park TG. Highly open porous biodegradable microcarriers: in vitro cultivation of chondrocytes for injectable delivery. Tissue Eng A. 2008;14(5):607–615. doi: 10.1089/tea.2007.0263. [DOI] [PubMed] [Google Scholar]
- Cleutjens JPM, Creemers EEJM. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J Card Fail. 2002;8(6):S344–348. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehsorkhi A, Castelletto V, Hamley IW. Self-assembling amphiphilic peptides. J Pept Sci. 2014;20(7):453–467. doi: 10.1002/psc.2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divband B, Aghazadeh M, Haleem Al-qaim Z, Samiei M, Hussein FH, Shaabani AR, Shahi S, Sedghi R. Bioactive chitosan biguanidine-based injectable hydrogels as a novel BMP-2 and VEGF carrier for osteogenesis of dental pulp stem cells. Carbohydr Polym. 2021;273:118589. doi: 10.1016/j.carbpol.2021.118589. [DOI] [PubMed] [Google Scholar]
- Donati I, Asaro F, Paoletti S. Experimental evidence of counterion affinity in alginates: the case of nongelling ion Mg2+ J Phys Chem B. 2009;113(39):12877–12886. doi: 10.1021/jp902912m. [DOI] [PubMed] [Google Scholar]
- Dyondi W, Banerjee R, Banerjee R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int J Nanomed. 2012;8:47. doi: 10.2147/IJN.S37953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eftekhari A, Maleki Dizaj S, Sharifi S, Salatin S, Rahbar Saadat Y, Zununi Vahed S, Samiei M, Ardalan MR, Rameshrad M, Ahmadian E, Cucchiarini M. The use of nanomaterials in tissue engineering for cartilage regeneration; current approaches and future perspectives. Int J Mol Sci. 2020;21(2):536. doi: 10.3390/ijms21020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, Langer R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J Biomed Mater Res. 2000;51(2):164. doi: 10.1002/(sici)1097-4636(200008)51:2<164::aid-jbm4>3.3.co;2-n. [DOI] [PubMed] [Google Scholar]
- Emami Z, Ehsani M, Zandi M, Daemi H, Ghanian MH, Foudazi R. Modified hydroxyapatite nanoparticles reinforced nanocomposite hydrogels based on gelatin/oxidized alginate via schiff base reaction. Carbohydr Polym Technol Appl. 2021;2:100056. doi: 10.1016/j.carpta.2021.100056. [DOI] [Google Scholar]
- Eshghi Esfahani R, Zahedi P, Zarghami R. 5-Fluorouracil-loaded poly(vinyl alcohol)/chitosan blend nanofibers: morphology, drug release and cell culture studies. Iran Polym J. 2021;30(2):167–177. doi: 10.1007/s13726-020-00882-w. [DOI] [Google Scholar]
- Fang JJ, Zhang Y, Yan S, Liu Z, He S, Cui L, Yin J. Poly(l-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10(1):276–288. doi: 10.1016/j.actbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Feng Q, Wei K, Lin S, Xu Z, Sun Y, Shi P, Li G, Bian L. Corrigendum to ‘mechanically resilient, injectable, and bioadhesive supramolecular gelatin hydrogels crosslinked by weak host–guest interactions assist cell infiltration and in situ tissue regeneration’ [Biomaterials 101C (2016) 217–228] Biomaterials. 2017;112:346–347. doi: 10.1016/j.biomaterials.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Gao L, Huang Z, Yan S, Zhang K, Xu S, Li G, Cui L, Yin J. Sr-HA-graft-poly(γ-benzyl-l-glutamate) nanocomposite microcarriers: controllable Sr2+ release for accelerating osteogenenisis and bony nonunion repair. Biomacromol. 2017;18(11):3742–3752. doi: 10.1021/acs.biomac.7b01101. [DOI] [PubMed] [Google Scholar]
- Gao Y, Kong W, Li B, Ni Y, Yuan T, Guo L, Lin H, Fan H, Fan Y, Zhang X. Fabrication and characterization of collagen-based injectable and self-crosslinkable hydrogels for cell encapsulation. Colloids Surf B. 2018;167:448–456. doi: 10.1016/j.colsurfb.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Gao Y, Li B, Kong W, Yuan L, Guo L, Li C, Fan H, Fan Y, Zhang X. Injectable and self-crosslinkable hydrogels based on collagen type II and activated chondroitin sulfate for cell delivery. Int J Biol Macromol. 2018;118(2018):2014–2020. doi: 10.1016/j.ijbiomac.2018.07.079. [DOI] [PubMed] [Google Scholar]
- Ghaeini-Hesaroeiye S, Boddohi S, Vasheghani-Farahani E. Dual responsive chondroitin sulfate based nanogel for antimicrobial peptide delivery. Int J Biol Macromol. 2020;143:297–304. doi: 10.1016/j.ijbiomac.2019.12.026. [DOI] [PubMed] [Google Scholar]
- Ghanbari M, Salavati-Niasari M, Mohandes F. Thermosensitive alginate-gelatin-nitrogen-doped carbon dots scaffolds as potential injectable hydrogels for cartilage tissue engineering applications. RSC Adv. 2021;11(30):18423–18431. doi: 10.1039/d1ra01496j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanian MH, Mirzadeh H, Baharvand H. In situ forming, cytocompatible, and self-recoverable tough hydrogels based on dual ionic and click cross-linked alginate. Biomacromol. 2018;19(5):1646–1662. doi: 10.1021/acs.biomac.8b00140. [DOI] [PubMed] [Google Scholar]
- Gilarska A, Lewandowska-Łańcucka J, Guzdek-Zając K, Karewicz A, Horak W, Lach R, Wójcik K, Nowakowska M. Bioactive yet antimicrobial structurally stable collagen/chitosan/lysine functionalized hyaluronic acid-based injectable hydrogels for potential bone tissue engineering applications. Int J Biol Macromol. 2020;155:938–950. doi: 10.1016/j.ijbiomac.2019.11.052. [DOI] [PubMed] [Google Scholar]
- Gohil SV, Kumar N. An injectable and biomimetic multi-phase nanocomposite for non-invasive bone tissue engineering: fabrication and mechanistic evaluation. Polym Adv Technol. 2017;28(11):1453–1463. doi: 10.1002/pat.4022. [DOI] [Google Scholar]
- Goodarzi K, Jonidi Shariatzadeh F, Solouk A, Akbari S, Mirzadeh H. Injectable drug loaded gelatin based scaffolds as minimally invasive approach for drug delivery system: CNC/PAMAM nanoparticles. Eur Polym J. 2020;139:109992. doi: 10.1016/J.EURPOLYMJ.2020.109992. [DOI] [Google Scholar]
- Gopinathan J, Noh I. Click chemistry-based injectable hydrogels and bioprinting inks for tissue engineering applications. Tissue Eng Regen Med. 2018;15(5):531–546. doi: 10.1007/s13770-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi Derakhshan Z, Shaghaghi B, Padash Asl M, Majidi M, Ghazizadeh L, Chegini A, Bonakdar S. In situ forming hydrogel based on chondroitin sulfate-hydroxyapatite for bone tissue engineering. Inter J Polym Mater Polym Biomater. 2015;64(17):919–926. doi: 10.1080/00914037.2015.1030662. [DOI] [Google Scholar]
- Hashemi Doulabi AS, Mirzadeh H, Imani M, Sharifi S, Atai M, Mehdipour-Ataei S. Synthesis and preparation of biodegradable and visible light crosslinkable unsaturated fumarate-based networks for biomedical applications. Polym Adv Technol. 2008;19(9):1199–1208. doi: 10.1002/pat.1112. [DOI] [Google Scholar]
- Hashemi Doulabi AS, Mirzadeh H, Samadi N, Bagheri-Khoulenjani S, Atai M, Imani M. Potential application of a visible light-induced photocured hydrogel film as a wound dressing material. J Polym. 2015;2015:1–10. doi: 10.1155/2015/867928. [DOI] [Google Scholar]
- Hou QP, De Bank PA, Shakesheff KM. Injectable scaffolds for tissue regeneration. J Mater Chem. 2004;14(13):1915–1923. doi: 10.1039/b401791a. [DOI] [Google Scholar]
- Hunt NC, Grover LM. Cell encapsulation using biopolymer gels for regenerative medicine. Biotechnol Lett. 2010;32(6):733–742. doi: 10.1007/s10529-010-0221-0. [DOI] [PubMed] [Google Scholar]
- Jeong SH, Kim M, Kim TY, Kim H, Ju JH, Hahn SK. Supramolecular injectable hyaluronate hydrogels for cartilage tissue regeneration. ACS Appl Bio Mater. 2020;3(8):5040–5047. doi: 10.1021/acsabm.0c00537. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Chen J, Deng C, Suuronen EJ, Zhong Z. Click hydrogels, microgels and nanogels: emerging platforms for drug delivery and tissue engineering. Biomaterials. 2014;35(18):4969–4985. doi: 10.1016/j.biomaterials.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Jin R, Hiemstra C, Zhong Z, Feijen J. Enzyme-mediated fast in situ formation of hydrogels from dextran–tyramine conjugates. Biomaterials. 2007;28(18):2791–2800. doi: 10.1016/j.biomaterials.2007.02.032. [DOI] [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Dijkstra PJ, Karperien M, van Blitterswijk CA, Zhong ZY, Feijen J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials. 2009;30(13):2544–2551. doi: 10.1016/j.biomaterials.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Krouwels A, Dijkstra PJ, van Blitterswijk CA, Karperien M, Feijen J. Synthesis and characterization of hyaluronic acid-poly(ethylene glycol) hydrogels via michael addition: an injectable biomaterial for cartilage repair. Acta Biomater. 2010;6(6):1968–1977. doi: 10.1016/j.actbio.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Jin R, Moreira Teixeira LS, Dijkstra PJ, van Blitterswijk CA, Karperien M, Feijen J. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. J Control Release. 2011;152(1):186–195. doi: 10.1016/j.jconrel.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Jonidi Shariatzadeh F, Solouk A, Bagheri Khoulenjani S, Bonakdar S, Mirzadeh H. Injectable and reversible preformed cryogels based on chemically crosslinked gelatin methacrylate (GelMA) and physically crosslinked hyaluronic acid (HA) for soft tissue engineering. Colloids Surf B. 2021;203:111725. doi: 10.1016/j.colsurfb.2021.111725. [DOI] [PubMed] [Google Scholar]
- Kazemi-Aghdam F, Jahed V, Dehghan-Niri M, Ganji F, Vasheghani-Farahani E. Injectable chitosan hydrogel embedding modified halloysite nanotubes for bone tissue engineering. Carbohydr Polym. 2021;269:118311. doi: 10.1016/j.carbpol.2021.118311. [DOI] [PubMed] [Google Scholar]
- Khan F, Tanaka M, Rafi Ahmad S. Fabrication of polymeric biomaterials: a strategy for tissue engineering and medical devices. J Mater Chem B. 2015;3:8224–8249. doi: 10.1039/C5TB01370D. [DOI] [PubMed] [Google Scholar]
- Khan M, Ali Shah L, Rehman T, Khan A, Iqbal A, Ullah M, Alam S. Synthesis of physically cross-linked gum arabic-based polymer hydrogels with enhanced mechanical, load bearing and shape memory behavior. Iran Polym J. 2020;29(4):351–360. doi: 10.1007/s13726-020-00801-z. [DOI] [Google Scholar]
- Kim H-W, Yoon B-H, Kim H-E. Microsphere of apatite-gelatin nanocomposite as bone regenerative filler. J Mater Sci Mater Med. 2005;16(12):1105–1109. doi: 10.1007/s10856-005-4714-7. [DOI] [PubMed] [Google Scholar]
- Kim J, Kim IS, Cho TH, Lee KB, Hwang SJ, Tae G, Noh I, Lee SH, Park Y, Sun K. Bone regeneration using hyaluronic acid-based hydrogel with bone morphogenic protein-2 and human mesenchymal stem cells. Biomaterials. 2007;28(10):1830–1837. doi: 10.1016/j.biomaterials.2006.11.050. [DOI] [PubMed] [Google Scholar]
- Kim HK, Shim WS, Kim SE, Lee S-H, Kang E, Kim J-H, Kim K, Kwon IC, Lee DS. Injectable in situ–forming PH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng A. 2008;15(4):923–933. doi: 10.1089/ten.tea.2007.0407. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31(13):3596–3603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, An YH, Kim HD, Kim K, Lee SH, Yim HG, Kim BG, Hwang NS. Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int J Biol Macromol. 2018;110:479–487. doi: 10.1016/j.ijbiomac.2017.12.053. [DOI] [PubMed] [Google Scholar]
- Kim HJ, You SJ, Yang DH, Eun J, Park HK, Kim MS, Chun HJ. Injectable hydrogels based on MPEG-PCL-RGD and BMSCs for bone tissue engineering. Biomater Sci. 2020;8(15):4334–4345. doi: 10.1039/d0bm00588f. [DOI] [PubMed] [Google Scholar]
- Kiran RT, Singh VK, Singh MK, Krishnamoorthi S, Kumar K. Synthesis, characterization of β-CD based novel hydrogels with dual objectives of drug release and dye removal. Iran Polym J. 2020;29(7):615–623. doi: 10.1007/s13726-020-00826-4. [DOI] [Google Scholar]
- Kocak FZ, Talari ACS, Yar M, Rehman IU. In-situ forming ph and thermosensitive injectable hydrogels to stimulate angiogenesis: potential candidates for fast bone regeneration applications. Int J Mol Sci. 2020 doi: 10.3390/ijms21051633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondiah PJ, Choonara YE, Kondiah PPD, Marimuthu T, Kumar P, Du Toit LC, Pillay V (2016) A review of injectable polymeric hydrogel systems for application in bone tissue engineering. Molecules 21(11) [DOI] [PMC free article] [PubMed]
- Kondiah PJ, Choonara YE, Kondiah PPD, Kumar P, Marimuthu T, du Toit LC, Pillay V. Development of an injectable pseudo-bone thermo-gel for application in small bone fractures. Int J Pharm. 2017;520(1–2):39–48. doi: 10.1016/j.ijpharm.2017.01.039. [DOI] [PubMed] [Google Scholar]
- Koshy ST, Ferrante TC, Lewin SA, Mooney DJ. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials. 2014;35(8):2477–2487. doi: 10.1016/j.biomaterials.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshy ST, Desai RM, Joly P, Li J, Bagrodia RK, Lewin SA, Joshi NS, Mooney DJ. Click-crosslinked injectable gelatin hydrogels. Adv Healthc Mater. 2016;5(5):541–547. doi: 10.1002/adhm.201500757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretlow JD, Klouda L. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007;59(4):263–273. doi: 10.1016/j.addr.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Krishnamachari Y, Pearce ME, Salem AK. Self-assembly of cell–microparticle hybrids. Adv Mater. 2008;20(5):989–993. doi: 10.1002/adma.200701689. [DOI] [Google Scholar]
- Kumar Meena L, Rather H, Kedaria D, Vasita R. Polymeric microgels for bone tissue engineering applications—a review. Int J Polym Mater Polym Biomater. 2019 doi: 10.1080/00914037.2019.1570512. [DOI] [Google Scholar]
- Kunkit N, Deekaikam T, Chaimuang S, Pekkoh J, Manokruang K. Physical hydrogels prepared from cationically modified pectin with tunable sol-gel phase transition behaviors. Int J Polym Mater Polym Biomater. 2019 doi: 10.1080/00914037.2019.1695208. [DOI] [Google Scholar]
- Kwon JS, Kim SW, Kwon DY, Park SH, Son AR, Kim JH, Kim MS. In vivo osteogenic differentiation of human turbinate mesenchymal stem cells in an injectable in situ-forming hydrogel. Biomaterials. 2014;35(20):5337–5346. doi: 10.1016/j.biomaterials.2014.03.045. [DOI] [PubMed] [Google Scholar]
- Langer RS, Vacanti JP. Tissue engineering: the challenges ahead. Sci Am. 1999;280:86–89. doi: 10.1038/scientificamerican0499-86. [DOI] [PubMed] [Google Scholar]
- Laughlin ST, Baskin JM, Amacher SL, Bertozzi CR. In vivo imaging of membrane-associated glycans in developing zebrafish. Science. 2008;320(5876):664–667. doi: 10.1126/science.1155106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Tae G. Formulation and in vitro characterization of an in situ gelable, photo-polymerizable pluronic hydrogel suitable for injection. J Control Release. 2007;119(3):313–319. doi: 10.1016/j.jconrel.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Lee KY, Alsberg E, Mooney DJ. Degradable and injectable poly(aldehyde guluronate) hydrogels for bone tissue engineering. J Biomed Mater Res A. 2001;56(2):228–233. doi: 10.1002/1097-4636(200108)56:2<228::AID-JBM1089>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lendlein A, Langer R. Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science. 2002;296(5573):1673–1676. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- Li J. Self-assembled supramolecular hydrogels based on polymer–cyclodextrin inclusion complexes for drug delivery. NPG Asia Mater. 2010;2:112–118. doi: 10.1038/asiamat.2010.84. [DOI] [Google Scholar]
- Li G-Z, Randev RK, Soeriyadi AH, Rees G, Boyer C, Tong Z, Davis TP, Becer CR, Haddleton DM. Investigation into thiol-(meth)acrylate michael addition reactions using amine and phosphine catalysts. Polym Chem. 2010;1(8):1196–1204. doi: 10.1039/C0PY00100G. [DOI] [Google Scholar]
- Li H, Ji Q, Chen X, Sun Y, Xu Q, Deng P, Hu F, Yang J. Accelerated bony defect healing based on chitosan thermosensitive hydrogel scaffolds embedded with chitosan nanoparticles for the delivery of BMP2 plasmid DNA. J Biomed Mater Res A. 2017;105(1):265–273. doi: 10.1002/jbm.a.35900. [DOI] [PubMed] [Google Scholar]
- Li T, Song X, Weng C, Wang X, Sun L, Gong X, Yang L, Chen C. Self-crosslinking and injectable chondroitin sulfate/pullulan hydrogel for cartilage tissue engineering. Appl Mater Today. 2018;10:173–183. doi: 10.1016/j.apmt.2017.12.002. [DOI] [Google Scholar]
- Li A, Xu H, Yu P, Xing J, Ding C, Yan X, Xie J, Li J. Injectable hydrogels based on gellan gum promotes in situ mineralization and potential osteogenesis. Eur Polym J. 2020;141:110091. doi: 10.1016/j.eurpolymj.2020.110091. [DOI] [Google Scholar]
- Lin Z, Cao S, Chen X, Wu W, Li J. Thermoresponsive hydrogels from phosphorylated ABA triblock copolymers: a potential scaffold for bone tissue engineering. Biomacromol. 2013;14(7):2206–2214. doi: 10.1021/bm4003442. [DOI] [PubMed] [Google Scholar]
- Liu C, Qin H, Mather PT. Review of progress in shape-memory polymers. J Mater Chem. 2007;17(16):1543. doi: 10.1039/b615954k. [DOI] [Google Scholar]
- Liu C, Han Z, Czernuszka JT. Gradient collagen/nanohydroxyapatite composite scaffold: development and characterization. Acta Biomater. 2009;5(2):661–669. doi: 10.1016/j.actbio.2008.09.022. [DOI] [PubMed] [Google Scholar]
- Liu X, Jin X, Ma PX. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat Mater. 2011;10(5):398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Miller AL, Fundora KA, Yaszemski MJ, Lu L. Poly(ε-caprolactone) dendrimer cross-linked via metal-free click chemistry: injectable hydrophobic platform for tissue engineering. ACS Macro Lett. 2016;5(11):1261–1265. doi: 10.1021/acsmacrolett.6b00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, George MN, Li L, Gamble D, Miller AL, Gaihre B, Waletzki BE, Lu L. Injectable electrical conductive and phosphate releasing gel with two-dimensional black phosphorus and carbon nanotubes for bone tissue engineering. ACS Biomater Sci Eng. 2020;6(8):4653–4665. doi: 10.1021/acsbiomaterials.0c00612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Yang D, Li Q, Wang K, Chen B, Kennedy JF, Nie J. Injectable hydrogels based on chitosan derivative/polyethylene glycol dimethacrylate/n, n-dimethylacrylamide as bone tissue engineering matrix. Carbohydr Polym. 2010;79(3):620–627. doi: 10.1016/j.carbpol.2009.09.015. [DOI] [Google Scholar]
- Makvandi P, Ali GW, Sala FD, Abdel-Fattah WI, Borzacchiello A. Hyaluronic acid/corn silk extract based injectable nanocomposite: a biomimetic antibacterial scaffold for bone tissue regeneration. Mater Sci Eng C. 2020;107:110195. doi: 10.1016/j.msec.2019.110195. [DOI] [PubMed] [Google Scholar]
- Makvandi P, Ashrafizadeh M, Ghomi M, Najafi M, Heydari Sheikh Hossein H, Zarrabi A, Mattoli V, Varma RS. Correction to: injectable hyaluronic acid-based antibacterial hydrogel adorned with biogenically synthesized AgNPs-decorated multi-walled carbon nanotubes. Prog Biomater. 2021;10(4):321–322. doi: 10.1007/s40204-021-00170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather BD, Viswanathan K, Miller KM, Long TE. Michael addition reactions in macromolecular design for emerging technologies. Prog Polym Sci. 2006;31(5):487–531. doi: 10.1016/j.progpolymsci.2006.03.001. [DOI] [Google Scholar]
- Matsuno T, Hashimoto Y, Adachi S, Omata K, Yoshitaka Y, Ozeki Y, Umezu Y, Tabata Y, Nakamura M, Satoh T. Preparation of injectable 3D-formed beta-tricalcium phosphate bead/alginate composite for bone tissue engineering. Dent Mater J. 2008;27(6):827–834. doi: 10.4012/dmj.27.827. [DOI] [PubMed] [Google Scholar]
- Mi L, Liu H, Gao Y, Miao H, Ruan J. Injectable nanoparticles/hydrogels composite as sustained release system with stromal cell-derived factor-1α for calvarial bone regeneration. Int J Biol Macromol. 2017 doi: 10.1016/j.ijbiomac.2017.03.098. [DOI] [PubMed] [Google Scholar]
- Mohamadnia Z, Ahmadi E, Rafieni M, Mirzadeh H, Mobedi H. Investigation of drug release and 1H-NMR analysis of the in situ forming systems based on poly(lactide-co-glycolide) Polym Adv Technol. 2009;20(1):48–57. doi: 10.1002/pat.1279. [DOI] [Google Scholar]
- Montgomery M, Ahadian S, Huyer LD, Rito ML, Civitarese RA, Vanderlaan RD, Wu J, Reis LA, Momen A, Akbari S, Pahnke A, Li RK, Caldarone CA, Radisic M. Flexible shape-memory scaffold for minimally invasive delivery of functional tissues. Nat Mater. 2017;16(10):1038–1046. doi: 10.1038/nmat4956. [DOI] [PubMed] [Google Scholar]
- Motlaq VF, Momtazi L, Zhu K, Knudsen KD, Nyström B. Differences in self-assembly features of thermoresponsive anionic triblock copolymers synthesized via one-pot or two-pot by atom transfer radical polymerization. J Polym Sci B Polym Phys. 2019;57(9):524–534. doi: 10.1002/polb.24808. [DOI] [Google Scholar]
- Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8):762–798. doi: 10.1016/j.progpolymsci.2007.05.017. [DOI] [Google Scholar]
- Nemati Hayati A, Hosseinalipour SM, Rezaie HR, Shokrgozar MA. Characterization of poly(3-hydroxybutyrate)/nano-hydroxyapatite composite scaffolds fabricated without the use of organic solvents for bone tissue engineering applications. Mater Sci Eng C. 2012;32(3):416–422. doi: 10.1016/j.msec.2011.11.013. [DOI] [Google Scholar]
- Neuss S, Blomenkamp I, Stainforth R, Boltersdorf D, Jansen M, Butz N, Perez-Bouza A, Knüchel R. The use of a shape-memory poly(e{lunate}-caprolactone)dimethacrylate network as a tissue engineering scaffold. Biomaterials. 2009;30(9):1697–1705. doi: 10.1016/j.biomaterials.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23(22):4307–4314. doi: 10.1016/S0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- Ni PY, Ding QX, Fan M, Liao JF, Qian ZY, Luo JC, Li XQ, Luo F, Yang ZM, Wei YQ. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35(1):236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- Nikpour P, Salimi-Kenari H, Rabiee SM. Biological and bioactivity assessment of dextran nanocomposite hydrogel for bone regeneration. Prog Biomater. 2021;10(4):271–280. doi: 10.1007/s40204-021-00171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmo CM, Owen SC, Shoichet MS. Diels-alder click cross-linked hyaluronic acid hydrogels for tissue engineering. Biomacromol. 2011;12(3):824–830. doi: 10.1021/bm101446k. [DOI] [PubMed] [Google Scholar]
- Niranjan R, Koushik C, Saravanan S, Moorthi A, Vairamani M, Selvamurugan N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int J Biol Macromol. 2013;54(1):24–29. doi: 10.1016/j.ijbiomac.2012.11.026. [DOI] [PubMed] [Google Scholar]
- Orive G, Hernandez RM, Gascon AR, Calafiore R, Chang TMS, Vos PD, Hortelano G, Hunkeler D, Lacik I, James Shapiro AM, Pedraz JL. Cell encapsulation: promise and progress. Nat Med. 2003;9(1):104–107. doi: 10.1038/nm0103-104. [DOI] [PubMed] [Google Scholar]
- Ossipov DA, Brännvall K, Forsberg-Nilsson K, Jöns Hilborn J. Formation of the first injectable poly(vinyl alcohol) hydrogel by mixing of functional PVA precursors. J Appl Polym Sci. 2007;106(1):60–70. doi: 10.1002/app.26455. [DOI] [Google Scholar]
- Park SH, Seo JY, Park JY, Ji YB, Kim K, Choi HS, Choi S, Kim JH, Min BH, Kim MS. An injectable, click-crosslinked, cytomodulin-modified hyaluronic acid hydrogel for cartilage tissue engineering. NPG Asia Mater. 2019;11(1):1–16. doi: 10.1038/s41427-019-0130-1. [DOI] [Google Scholar]
- Perez RA, Kim M, Kim T-H, Kim J-H, Lee JH, Park J-H, Knowles JC, Kim HW. Utilizing core-shell fibrous collagen-alginate hydrogel cell delivery system for bone tissue engineering. Tissue Eng A. 2014;20(1–2):103–114. doi: 10.1089/ten.TEA.2013.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourjavadi A, Doroudian M, Ahadpour A, Azari S. Injectable chitosan/κ-carrageenan hydrogel designed with au nanoparticles: a conductive scaffold for tissue engineering demands. Int J Biol Macromol. 2019;126:310–317. doi: 10.1016/j.ijbiomac.2018.11.256. [DOI] [PubMed] [Google Scholar]
- Ren B, Chen X, Du S, Ma Y, Chen H, Yuan G, Li J, Xiong D, Tan H, Ling Z, Chen Y, Hu X, Niu X. Injectable polysaccharide hydrogel embedded with hydroxyapatite and calcium carbonate for drug delivery and bone tissue engineering. Int J Biol Macromol. 2018;118:1257–1266. doi: 10.1016/j.ijbiomac.2018.06.200. [DOI] [PubMed] [Google Scholar]
- Ressler A, Ródenas-Rochina J, Ivanković M, Ivanković H, Rogina A, Ferrer GG. Injectable chitosan-hydroxyapatite hydrogels promote the osteogenic differentiation of mesenchymal stem cells. Carbohydr Polym. 2018;197:469–477. doi: 10.1016/j.carbpol.2018.06.029. [DOI] [PubMed] [Google Scholar]
- Ressler A, Žužić A, Ivanišević I, Kamboj N, Ivanković H. Ionic substituted hydroxyapatite for bone regeneration applications: a review. Open Ceram. 2021;6:1–16. doi: 10.1016/j.oceram.2021.100122. [DOI] [Google Scholar]
- Rottensteiner U, Sarker B, Heusinger D, Dafinova D, Rath SN, Beier JP, Kneser U, Horch RE, Detsch R, Boccaccini AR, Arkudas A. In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials. 2014;7(3):1957–1974. doi: 10.3390/ma7031957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem AK, Rose FRAJ, Oreffo ROC, Yang X, Davies MC, Mitchell JR, Roberts CJ, Stolnik-Trenkic S, Tendler SJB, Williams PM, Shakesheff KM. Porous polymer and cell composites that self-assemble in situ. Adv Mater. 2003;15(3):210–213. doi: 10.1002/adma.200390047. [DOI] [Google Scholar]
- Sanmartín-Masiá E, Poveda-Reyes S, Ferrer GG. Extracellular matrix-inspired gelatin/hyaluronic acid injectable hydrogels. Int J Polym Mater Polym Biomater. 2017;66(6):280–288. doi: 10.1080/00914037.2016.1201828. [DOI] [Google Scholar]
- Seo B-B, Koh J-T, Song S-C. Tuning physical properties and BMP-2 release rates of injectable hydrogel systems for an optimal bone regeneration effect. Biomaterials. 2017;122:91–104. doi: 10.1016/j.biomaterials.2017.01.016. [DOI] [PubMed] [Google Scholar]
- Shapiro F. Bone development and its relation to fracture repair. The role of mesenchymal osteoblasts and surface osteoblasts. Eur Cells Mater. 2008;15:53–76. doi: 10.22203/eCM.v015a05. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Mirzadeh H, Imani M, Atai M, Ziaee F (2007) Photopolymerization and shrinkage kinetics of in situ crosslinkable n-vinyl-pyrrolidone/poly(e-caprolactone fumarate ) networks. 10.1002/jbm.a [DOI] [PubMed]
- Sharifi S, Mirzadeh H, Imani M, Ziaee F, Tajabadi M, Jamshidi A, Atai M. Synthesis, photocrosslinking characteristics, and biocompatibility evaluation of n-vinyl pyrrolidone/polycaprolactone fumarate biomaterials using a new proton scavenger. Polym Adv Technol. 2008;19(6):1828–1838. doi: 10.1002/pat.1134. [DOI] [Google Scholar]
- Sharifi S, Imani M, Mirzadeh H, Atai M, Ziaee F, Bakhshi R. Synthesis, characterization, and biocompatibility of novel injectable, biodegradable, and in situ crosslinkable polycarbonate-based macromers. J Biomed Mater Res A. 2009;90A(3):830–843. doi: 10.1002/jbm.a.32138. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Mirzadeh H, Imani M, Rong Z. Injectable in situ forming drug delivery system based on poly(e-caprolactone fumarate) for tamoxifen citrate delivery: gelation characteristics, in vitro drug release and anti-cancer evaluation. Acta Biomater. 2009;5(6):1966–1978. doi: 10.1016/j.actbio.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Sharifi S, Shafieyan Y, Mirzadeh H, Bagheri-Khoulenjani S, Rabiee SM, Imani M, Atai M, Shokrgozar MA, Hatampoor A. Hydroxyapatite scaffolds infiltrated with thermally crosslinked polycaprolactone fumarate and polycaprolactone itaconate. J Biomed Mater Res A. 2011;98A(2):257–267. doi: 10.1002/jbm.a.33108. [DOI] [PubMed] [Google Scholar]
- Shavandi A, Bekhit DA, Sun AE, Ali MA. Injectable gel from squid pen chitosan for bone tissue engineering applications. J Sol-Gel Sci Technol. 2016;77(3):675–687. doi: 10.1007/s10971-015-3899-6. [DOI] [Google Scholar]
- Shi X, Hudson JL, Spicer PP, Tour JM, Krishnamoorti R, Mikos AG. Injectable nanocomposites of single-walled carbon nanotubes and biodegradable polymers for bone tissue engineering. Biomacromol. 2006;7(7):2237–2242. doi: 10.1021/bm060391v. [DOI] [PubMed] [Google Scholar]
- Shi Z, Xu Y, Mulatibieke R, Zhong Q, Pan X, Chen Y, Lian Q, Luo X, Shi Z, Zhu Q. Nano-silicate-reinforced and SDF-1α-loaded gelatin-methacryloyl hydrogel for bone tissue engineering. Int J Nanomedicine. 2020;15:9337–9353. doi: 10.2147/IJN.S270681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih H, Lin C-C. Cross-linking and degradation of step-growth hydrogels formed by thiol–ene photoclick chemistry. Biomacromol. 2012;13(7):2003–2012. doi: 10.1021/bm300752j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Ruhé PQ, Mikos AG, Jansen JA. In vivo bone and soft tissue response to injectable, biodegradable oligo(poly(ethylene glycol) fumarate) hydrogels. Biomaterials. 2003;24(19):3201–3211. doi: 10.1016/S0142-9612(03)00168-6. [DOI] [PubMed] [Google Scholar]
- Singh YP, Moses JC, Bhardwaj N, Mandal BB. Injectable hydrogels: a new paradigm for osteochondral tissue engineering. J Mater Chem B. 2018;6(35):5499–5529. doi: 10.1039/c8tb01430b. [DOI] [PubMed] [Google Scholar]
- Sivashanmugam A, Kumar RA, Priya MV, Nair SV, Jayakumar R. An overview of injectable polymeric hydrogels for tissue engineering. Eur Polym J. 2015;72:543–565. doi: 10.1016/j.eurpolymj.2015.05.014. [DOI] [Google Scholar]
- Śmiga-Matuszowicz M, Łukaszczyk J, Pilawka R, Basiaga M, Bilewicz M, Kusz D. Novel crosslinkable polyester resin-based composites as injectable bioactive scaffolds. Int J Polym Mater Polym Biomater. 2017;66(1):1–11. doi: 10.1080/00914037.2016.1180614. [DOI] [Google Scholar]
- Solouk A, Mirzadeh H, Amanpour S. Injectable scaffold as minimally invasive technique for cartilage tissue engineering: in vitro and in vivo preliminary study. Prog Biomater. 2014;3(2–4):143–151. doi: 10.1007/s40204-014-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Huang WM. Mechanisms of the multi-shape memory effect and temperature memory effect in shape memorypolymers. Soft Matter. 2010;6(18):4403–4406. doi: 10.1039/C0SM00236D. [DOI] [Google Scholar]
- Sun J-Y, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z. Highly stretchable and tough hydrogels. Nature. 2012;489(7414):133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Huang WM, Ding Z, Zhao Y, Wang CC, Purnawali H, Tang C. Stimulus-responsive shape memory materials: a review. Mater Des. 2012;33(1):577–640. doi: 10.1016/j.matdes.2011.04.065. [DOI] [Google Scholar]
- Temenoff JS, Mikos AG. Injectable biodegradable materials for orthopedic tissue engineering. Biomaterials. 2000;21(23):2405–2412. doi: 10.1016/S0142-9612(00)00108-3. [DOI] [PubMed] [Google Scholar]
- Thakur T, Xavier JR, Cross L, Jaiswal MK, Mondragon E, Kaunas R, Gaharwar AK. Photocrosslinkable and elastomeric hydrogels for bone regeneration. J Biomed Mater Res A. 2016;104(4):879–888. doi: 10.1002/jbm.a.35621. [DOI] [PubMed] [Google Scholar]
- Thornton AJ, Alsberg E, Hill EE, Mooney DJ. Shape retaining injectable hydrogels for minimally invasive bulking. J Urol. 2004;172(2):763–768. doi: 10.1097/01.ju.0000130466.84214.f7. [DOI] [PubMed] [Google Scholar]
- Tommasi G, Perni S, Prokopovich P. An injectable hydrogel as bone graft material with added antimicrobial properties. Tissue Eng Part A. 2016;22(11–12):862–872. doi: 10.1089/ten.tea.2016.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong R, Tang L, Ma L, Tu C, Baumgartner R, Cheng J. Smart chemistry in polymeric nanomedicine. Chem Soc Rev. 2014;43:6982–7012. doi: 10.1039/c4cs00133h. [DOI] [PubMed] [Google Scholar]
- Toniato TV, Stocco TD, dos Santos MD, Santanna LB, Tim CR, Marciano FR, Silva-Filho EC, Campana-Filho SP, de Oliveira LA. Hybrid chitosan/amniotic membrane-based hydrogels for articular cartilage tissue engineering application. Int J Polym Mater Polym Biomater. 2019 doi: 10.1080/00914037.2019.1636249. [DOI] [Google Scholar]
- Townsend JM, Dennis SC, Whitlow J, Feng Y, Wang J, Andrews B, Nudo RJ, Detamore MS, Berkland CJ. Colloidal gels with extracellular matrix particles and growth factors for bone regeneration in critical size rat calvarial defects. AAPS J. 2017;19(3):703–711. doi: 10.1208/s12248-017-0045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzouanas SN, Ekenseair AK, Kasper FK, Mikos AG. Mesenchymal stem cell and gelatin microparticle encapsulation in thermally and chemically gelling injectable hydrogels for tissue engineering. J Biomed Mater Res A. 2014;102(5):1222–1230. doi: 10.1002/jbm.a.35093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Manakker F, van der Pot M, Vermonden T, van Nostrum CF, Hennink WE. Self-assembling hydrogels based on β-cyclodextrin/cholesterol inclusion complexes. Macromolecules. 2008;41(5):1766–1773. doi: 10.1021/ma702607r. [DOI] [Google Scholar]
- Van Tomme SR, De Geest BG, Braeckmans K, De Smedt SC, Siepmann F, Siepmann J, van Nostrum CF, Hennink WE. Mobility of model proteins in hydrogels composed of oppositely charged dextran microspheres studied by protein release and fluorescence recovery after photobleaching. J Control Release. 2005;110(1):67–78. doi: 10.1016/j.jconrel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, van Steenbergen MJ, De Smedt SC, van Nostrum CF, Hennink WE. Self-gelling hydrogels based on oppositely charged dextran microspheres. Biomaterials. 2005;26(14):2129–2135. doi: 10.1016/j.biomaterials.2004.05.035. [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, van Nostrum CF, de Smedt SC, Hennink WE. Degradation behavior of dextran hydrogels composed of positively and negatively charged microspheres. Biomaterials. 2006;27(22):4141–4148. doi: 10.1016/j.biomaterials.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, Mens A, van Nostrum CF, Hennink WE. Macroscopic hydrogels by self-assembly of oligolactate-grafted dextran microspheres. Biomacromol. 2008;9(1):158–165. doi: 10.1021/bm700931q. [DOI] [PubMed] [Google Scholar]
- Van Tomme SR, van Nostrum CF, Dijkstra M, De Smedt SC, Hennink WE. Effect of particle size and charge on the network properties of microsphere-based hydrogels. Eur J Pharm Biopharm. 2008;70(2):522–530. doi: 10.1016/j.ejpb.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Varshosaz J, Sajadi-Javan ZS, Kouhi M, Mirian M. Effect of bassorin (derived from gum tragacanth) and halloysite nanotubes on physicochemical properties and the osteoconductivity of methylcellulose-based injectable hydrogels. Int J Biol Macromol. 2021;192(June):869–882. doi: 10.1016/j.ijbiomac.2021.10.009. [DOI] [PubMed] [Google Scholar]
- Vo TN, Ekenseair AK, Spicer PP, Watson BM, Tzouanas SN, Roh TT, Mikos AG. In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering. J Control Release. 2015;205:25–34. doi: 10.1016/j.jconrel.2014.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo TN, Tatara AM, Santoro M, van den Beucken JJJP, Leeuwenburgh SCG, Jansen JA, Mikos AG. Acellular mineral deposition within injectable, dual-gelling hydrogels for bone tissue engineering. J Biomed Mater Res A. 2017;105(1):110–117. doi: 10.1002/jbm.a.35875. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Lin FH, Sun JS, Huang YC, Chueh SC, Hsu FY. Collagen-hydroxyapatite microspheres as carriers for bone morphogenic protein-4. Artif Organs. 2003;27(2):162–168. doi: 10.1046/j.1525-1594.2003.06953.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yuan X, Yu K, Meng H, Zheng Y, Peng J, Lu S, Liu X, Xie Y, Qiao K. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials. 2018;171:118–132. doi: 10.1016/j.biomaterials.2018.04.033. [DOI] [PubMed] [Google Scholar]
- Wasupalli GK, Verma D. Injectable and thermosensitive nanofibrous hydrogel for bone tissue engineering. Mater Sci Eng C. 2020;107:110343. doi: 10.1016/j.msec.2019.110343. [DOI] [PubMed] [Google Scholar]
- Watson BM, Kasper FK, Engel PS, Mikos AG. Synthesis and characterization of injectable, biodegradable, phosphate-containing, chemically cross-linkable, thermoresponsive macromers for bone tissue engineering. Biomacromol. 2014;15(5):1788–1796. doi: 10.1021/bm500175e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson BM, Vo TN, Tatara AM, Shah SR, Scott DW, Engel PS, Mikos AG. Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials. 2015;67:286–296. doi: 10.1016/j.biomaterials.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H-L, Yang Z, Zheng L-M, Shen Y-M. Thermosensitive hydrogels synthesized by fast diels–alder reaction in water. Polymer. 2009;50(13):2836–2840. doi: 10.1016/j.polymer.2009.04.032. [DOI] [Google Scholar]
- Wu WX, Huang YC, Lee WF. Effect of poly(ethylene glycol)-derived crosslinkers on the properties of thermosensitive hydrogels. Iran Polym J. 2020;29(8):679–691. doi: 10.1007/s13726-020-00831-7. [DOI] [Google Scholar]
- Xavier JR, Thakur T, Desai P, Jaiswal MK, Sears N, Cosgriff-Hernandez E, Kaunas R, Gaharwar AK. Bioactive nanoengineered hydrogels for bone tissue engineering: a growth-factor-free approach. ACS Nano. 2015;9(3):3109–3118. doi: 10.1021/nn507488s. [DOI] [PubMed] [Google Scholar]
- Xie M, Wang L, Ge J, Guo B, Ma PX. Strong electroactive biodegradable shape memory polymer networks based on star-shaped polylactide and aniline trimer for bone tissue engineering. ACS Appl Mater Interfaces. 2015;7(12):6772–6781. doi: 10.1021/acsami.5b00191. [DOI] [PubMed] [Google Scholar]
- Xu K, Lee F, Gao SJ, Chung JE, Yano H, Kurisawa M. Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-Α2a for liver cancer therapy. J Control Release. 2013;166(3):203–210. doi: 10.1016/j.jconrel.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Yan Q, Xiao LQ, Tan L, Sun W, Wu T, Chen LW, Mei Y, Shi B. Controlled release of simvastatin-loaded thermo-sensitive PLGA-PEG-PLGA hydrogel for bone tissue regeneration: in vitro and in vivo characteristics. J Biomed Mater Res A. 2015;103(11):3580–3589. doi: 10.1002/jbm.a.35499. [DOI] [PubMed] [Google Scholar]
- Yan J, Miao Y, Tan H, Zhou T, Ling Z, Chen Y, Xing X, Hu X. Injectable alginate/hydroxyapatite gel scaffold combined with gelatin microspheres for drug delivery and bone tissue engineering. Mater Sci Eng C. 2016;63:274–284. doi: 10.1016/j.msec.2016.02.071. [DOI] [PubMed] [Google Scholar]
- Yan S, Zhang X, Zhang K, Di H, Feng L, Li G, Fang J, Cui L, Chen X, Yin J. Injectable in situ forming poly(l-glutamic acid) hydrogels for cartilage tissue engineering. J Mater Chem B. 2016;4(5):947–961. doi: 10.1039/C5TB01488C. [DOI] [PubMed] [Google Scholar]
- Yan S, Xia P, Xu S, Zhang K, Li G, Cui L, Yin J. Nanocomposite porous microcarriers based on strontium-substituted HA-g-poly(γ-benzyl-l-glutamate) for bone tissue engineering. ACS Appl Mater Interfaces. 2018;10(19):16270–16281. doi: 10.1021/acsami.8b02448. [DOI] [PubMed] [Google Scholar]
- Yang JA, Yeom J, Hwang BW, Hoffman AS, Hahn SK. In situ-forming injectable hydrogels for regenerative medicine. Prog Polym Sci. 2014;39(12):1973–1986. doi: 10.1016/j.progpolymsci.2014.07.006. [DOI] [Google Scholar]
- Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, III, Langer R, Khademhosseini A. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27(31):5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Zajforoushan Moghaddam S, Zhu K, Nyström B, Thormann E. Thermo-responsive diblock and triblock cationic copolymers at the silica/aqueous interface: a QCM-D and AFM study. J Colloid Interface Sci. 2017;505:546–555. doi: 10.1016/j.jcis.2017.06.044. [DOI] [PubMed] [Google Scholar]
- Zhang D, George OJ, Petersen KM, Jimenez-Vergara AC, Hahn MS, Grunlan MA. A bioactive ‘self-fitting’ shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater. 2014;10(11):4597–4605. doi: 10.1016/j.actbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Gupte MJ, Jin X, Ma PX. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Adv Funct Mater. 2015;25(3):350–360. doi: 10.1002/adfm.201402618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yu J, Ren K, Zuo J, Ding J, Chen X. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromol. 2019;20(4):1478–1492. doi: 10.1021/acs.biomac.9b00043. [DOI] [PubMed] [Google Scholar]
- Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25(7):1339–1348. doi: 10.1016/j.biomaterials.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Wu W, Fang J, Yin J. Polymer-based porous microcarriers as cell delivery systems for applications in bone and cartilage tissue engineering. Int Mater Rev. 2021;66(2):77–113. doi: 10.1080/09506608.2020.1724705. [DOI] [Google Scholar]
- Ziadlou R, Rotman S, Teuschl A, Salzer E, Barbero A, Martin I, Alini M, Eglin D, Grad S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater Sci Eng C. 2021;120:111701. doi: 10.1016/j.msec.2020.111701. [DOI] [PubMed] [Google Scholar]