Abstract
Objective:
To examine whether patterns of body mass index (BMI) percentile gains across childhood predict BMI percentile, overweight and obesity, waist circumference, and elevated or prehypertensive blood pressure at age 15.
Methods:
Trained technicians in the Study of Early Child Care and Youth Development assessed children’s weight and height from birth to 15 years and waist circumference and blood pressure at age 15 (n = 1,132). Children’s BMI percentile trajectories from age 2 to age 13 along with 28 demographic and social covariates were used to predict BMI percentile, waist circumference, overweight, obesity, and elevated or prehypertensive blood pressure. Linear and logistic regressions were used to predict BMI percentile, overweight, obesity, waist circumference, and elevated or prehypertensive blood pressure.
Results:
Children were classified into one of four BMI percentile trajectories: “low stable” (28.4%), “low-to-high” (11.8%), “median stable” (29.0%), and “high rising” (30.7%). Children in trajectory classes characterized by persistent above average BMI percentile or by periods of rapid BMI percentile gains were more likely than their peers to experience poor weight and elevated or prehypertensive outcomes in adolescence. Trajectory class membership explained substantially more variance in adolescent health outcomes than demographic covariates alone. Estimated maternal BMI was a key independent predictor of adolescent outcomes.
Conclusions:
Different patterns of BMI percentile gains, namely those with rapid gains or persistently above average BMI percentile, from ages 2–13 predicted weight, waist circumference, and elevated or prehypertensive blood pressure at age 15, above and beyond demographic and social characteristics.
Keywords: childhood obesity, body mass index, weight gain, trajectories, adolescents
Introduction
Over the past several decades, childhood obesity in the United States (U.S.) has increased nearly threefold; one-third of all children and adolescents in the U.S. are currently considered overweight or obese.1 Childhood overweight and obesity have been identified as risk factors for obesity, heart disease, and metabolic syndrome in adulthood.2–4 Yet population-level rates of overweight and obesity can obscure distinct patterns of change over time,5–9 and the rate and timing of weight gain may affect later health. Identifying which patterns of weight gain in childhood forecast health outcomes in adolescence could be key in determining which children are most in need of intervention.
Evidence that early or rapid childhood weight gain leads to health problems in adolescence is accumulating.5–8 However, there is considerable variation in the methodologies used to reach these conclusions, including the modeling technique employed (i.e., growth mixture modeling vs. latent growth curve modeling), the type of BMI metric used (i.e., raw BMI vs. standardized estimates), the timing of assessments (i.e., few timepoints, trajectories overlap with outcome), the outcomes examined, and the use of early risk and family-level covariates. We sought to expand upon the existing literature in several ways. First, we used BMI percentile as a standardized estimate to model trajectories from data with 9 time points (2–13 years of age).9 Four trajectories emerged and that were distinct from those found in previous literature: low stable, low-to-high, median stable, and high rising (Figure 1). Second, unlike three studies we identified,6–8 this study’s trajectory classes end at age 13 and do not overlap with the age of the outcome (age 15), yielding a separation of predictor and outcome that meets a minimum criterion for prediction. Third, in contrast to the previous studies that included few covariates, we included 28 covariates to capture aspects of the home environment and the mother-child relationship that may influence both BMI across childhood and adolescent health outcomes.10–12 We included child and family demographics, breastfeeding, estimated maternal BMI, maternal depressive symptoms, maternal smoking, maternal education, family income-to-needs ratio, family stressful life events, mothers’ observed sensitivity, and parent-child attachment. Each of these improvements upon the past literature allowed us to address our key question: Do patterns of BMI percentile gains across childhood predict adolescent health outcomes?
Figure 1.
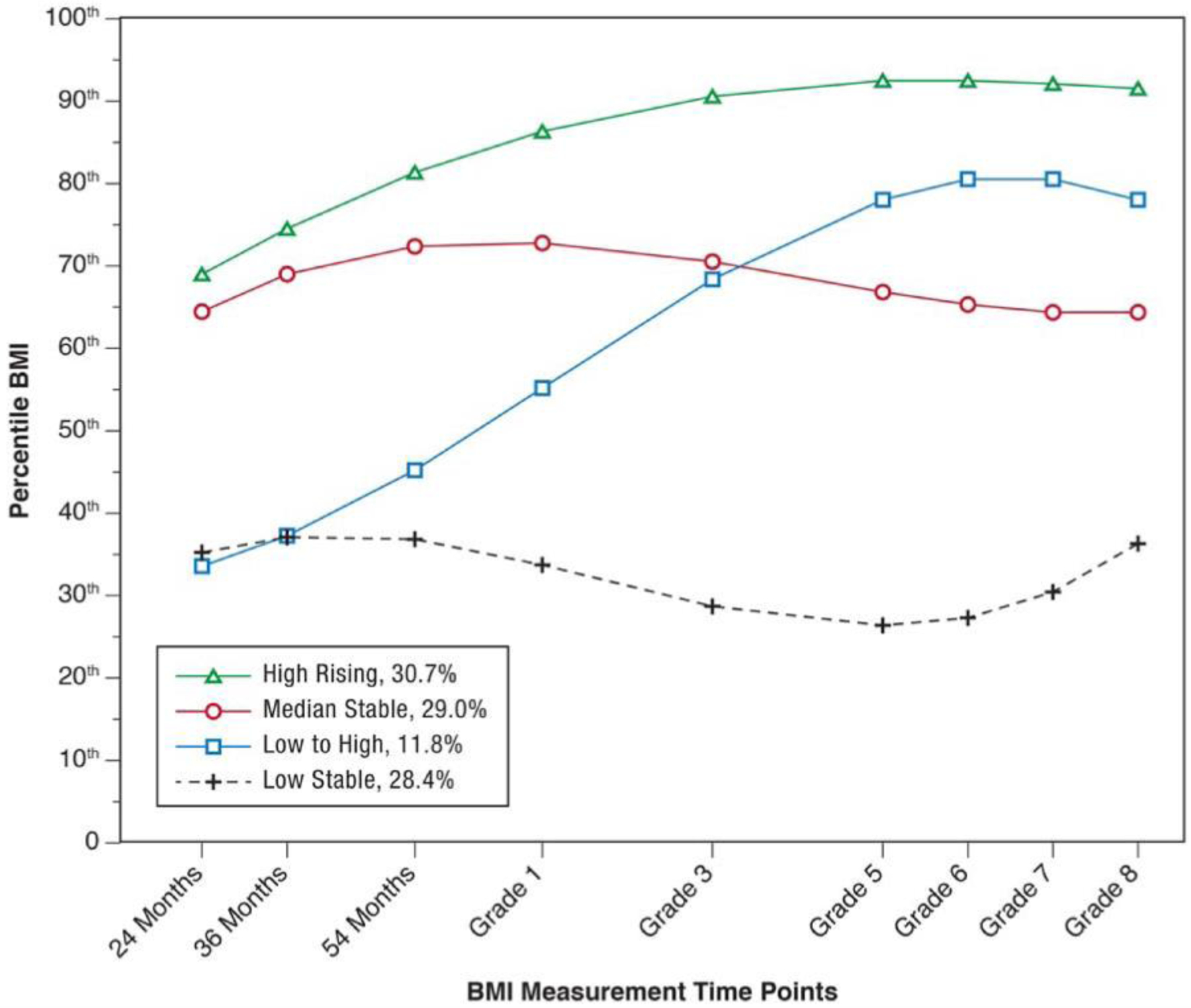
Classes of BMI Percentile Trajectories from 24 Months through Age 13 (Grade 8). Figure reprinted from “Identification of children’s BMI trajectories and prediction from weight gain in infancy,” by A. Bichteler and E T. Gershoff, Obesity 2018;26:1053. (doi:10.1002/oby.22177).
Method
Data Source and Sample
Data were drawn from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Study of Early Child Care and Youth Development (SECCYD).13 The SECCYD was designed to study the how experiences of childcare predicted later development. In 1991, participants were recruited after giving birth at one of 24 hospitals at 10 sites across the U.S. Participants were ineligible if mothers were less than 18 years of age, had plans to move within 3 years, did not speak English, had given birth to a child with a disability, or had a child who remained in the hospital for more than 7 days after birth. This study employed conditional random sampling to ensure socioeconomic and ethnic diversity. A total of 1,364 children were recruited and followed until 2007. The 10 sites involved in the SECCYD received approval from their respective Institutional Review Boards (IRBs) prior to data collection, and the University of Texas at Austin IRB approved these secondary analyses. Additional details on the study are available elsewhere.13,14
The present study used data from 14 waves of the SECCYD: birth, months 1, 6, 15, 24, 36, and 54; and years 6, 8, 10, 11, 12, 13, and 15. Inclusion criteria were that children must have been born between 37- and 41-weeks’ gestation (which resulted in 56 children omitted from our sample) and have at least 2 time points of data (which resulted in 172 children omitted from our sample). An additional 4 children were dropped based on anomalous data identified in the initial identification of BMI trajectories.9 These criteria resulted in 232 children omitted from the sample and a final analytic sample size of 1,132 children.
Measures
BMI Percentile.
Children’s height and weight were assessed by medical professionals or trained technicians when they were 2, 3, 4.5, 6, 8, 10, 11, 12, 13, and 15 years old using a calibrated measuring stick for height (measured twice to the nearest 1/8th of an inch) and a calibrated 2-beam scale for weight (measured twice and to the nearest 0.25 pound). The average of two measurements for weight and height were used as the final variables. Weight (kg) was divided by height squared (m2) to create raw BMI scores that were then compared to the Centers for Disease Control and Prevention growth charts to determine BMI percentiles specific to age and sex (M = 0.66, SD = 0.27).15
BMI Percentile Trajectory Classes from Age 2 to 13.
Children were classified into one of four BMI percentile trajectory groups: “low stable” (28.4%), “low-to-high” (11.8%), “median stable” (29.0%), and “high rising” (30.7%) trajectory classes (Figure 1). Details on the modeling procedures used to estimate the trajectory classes are available elsewhere.9
Overweight and Obesity Statuses at Age 15.
Overweight status was determined if BMI > 85th percentile, while having obesity was determined if BMI > 95th. 15,16 Nearly a third (31.1%) of the sample met the criteria for being overweight and 15.6% met the criteria for having obesity.
Waist Circumference at Age 15.
Waist circumference (cm) was measured by certified personnel using a 1.5-meter flexible plastic anthropometric tape while the adolescent was standing with their feet together. The average of the two closest of three total measurements was retained as a continuous measure of waist circumference (M = 75.78, SD = 12.23).
Elevated or Prehypertensive Blood Pressure at Age 15.
Adolescents’ systolic (SBP) and diastolic (DBP) blood pressure was measured by certified personnel. Five readings were taken at 1-minute intervals from the non-dominant arm while the adolescent was seated using a DINAMAP Pro 100 model from GE Healthcare (Critikon, Milwaukee, WI). The average of the last three readings was retained as the measure of blood pressure. Elevated or prehypertensive blood pressure (EPH-BP) was determined by comparing these average values to age-, sex-, and height-specific tables from the National Heart, Lung, and Blood Institute17 and the 2017 American Academy of Pediatrics Clinical Practice Guideline.18 EPH-BP was indicated if the adolescent’s average SBP ≥ 120 mm Hg or their DBP ≥ 80 mm Hg. A total of 28.7% of the sample was identified as having EPH-BP.
Covariates.
Child birth weight for gestational age was calculated as birth weight in grams divided by gestational age in weeks. Their percent weight increase from 0–15 months was calculated as the ratio of the difference in weight from 0–15 months to birth weight for gestational age x 100. Child BMI at 15 months was calculated from children’s length and weight using the WHO Growth Standard Charts.19 Whether the child was ever breastfed was assessed at the 1-month wave. Birth certificate information was used to create binary indicators of child sex (1 = female), race (1 = Black), and ethnicity (1 = Hispanic).
Lumeng et al. (2012) estimated maternal BMI by coding videotapes of mothers when their children were 15 and 24 months old.20 Two independent observers (ICCs > .80) were trained to code maternal figures using the Stunkard Figure Rating Scale from 1–9,21 where higher scores represent higher BMI. Mothers self-reported their depressive symptoms (Center for Epidemiological Studies Depression Scale)22 at 1, 6, 15, and 24 months. At the 24-month wave, mothers reported prenatal smoking on a 5-point scale (1 = did not smoke, 2 = smoked/quit before pregnancy, 3 = smoked/stopped in first trimester, 4 = smoked during first trimester/stopped before birth, and 5 = smoked throughout year). Maternal sensitivity to the child was coded from structured home observations and included mothers’ sensitivity to their children, their positive regard toward them, and their level of intrusiveness (reverse-scored). Scores were averaged within and across the 6-, 15-, and 24-month waves to create a single measure of maternal sensitivity. Parent-child attachment was assessed in a laboratory setting and was coded as secure, avoidant, or anxious-resistant.23 Mothers also reported their years of education when their children were 1 month of age.
Families’ income-to-needs ratio was calculated by dividing annual income by the poverty threshold set for their family size by the U.S. Census at birth and age 24 months and averaged.24 Mothers reported the number of stressful life events (e.g., job loss, death of loved one) since the child’s birth that the family had experienced at 15 months and at 54 months. Indicators for nine of the data collection sites were included in all initial models (10th site used as the reference) to test for site-specific shared variance.
Statistical Analysis
Linear regressions modeled childhood BMI percentile trajectory class as a predictor of the continuous outcomes of BMI percentile and waist circumference; logistic regressions were used to model the dichotomous outcomes of overweight, obesity, and EPH-BP. Initial models included child BMI percentile trajectory, and all child, maternal and family covariates. We identified the most parsimonious model for each outcome using backward stepwise reduction in the Akaike Information Criterion (AIC), a method to compare models with differing numbers of variables. For each outcome, a series of models was run that systematically omitted one covariate at a time; if the model without that variable was superior (indicated by a statistically significantly lower AIC), the variable was dropped from the final model. A final model for each outcome was run with the variables identified through the AIC reduction process. Covariates that did not significantly contribute to explaining variance in the outcome were omitted. The analyses were conducted using R.25 Aiken & West’s26 simple slopes strategy was used to disentangle significant two-way interactions among covariates.
To quantify the impact of the childhood BMI percentile trajectories, the fit of the final models with and without the BMI trajectories was compared using R2 and AIC. For the logistic regressions, we calculated Tjur’s R2,27 which is the difference between the means of the predicted value for each category of the variable and has been shown to be equivalent to values based on squared residuals.
Results
Participant Characteristics
Nearly half of the sample of children was female (49.1%). Most parents indicated their race as White (81.1%), with 12.1% Black or African American, 1.5% Asian or Pacific Islander, less than 1% American Indian, Eskimo, or Aleutian, and 4.9% of another race. Parents reported that 5.9% of the children were of Hispanic ethnicity.
BMI-Based Outcomes
Children’s BMI percentile trajectory classes emerged as significant predictors of BMI percentile at age 15. Compared to children in the low stable class, children in the high rising, median stable, and low-to-high trajectory classes had higher BMI percentiles at age 15 (Bs = .43, .22, & .25 respectively, all SEs = .02, all p < .001; see Table 1). Mothers’ BMI when children were 15 months predicted higher adolescent BMI percentiles, while maternal sensitivity and family income-to-needs ratio were each inversely associated with BMI percentiles. There was also a significant interaction between children’s birthweight for age and their percent weight increase from 0–15 months (Table 1), such that the relationship between birthweight and age 15 BMI percentile was strongest for those children who gained weight faster than average.
Table 1.
Best-Fitting Linear and Logistic Regression Models Predicting BMI-Based Outcomes at Age 15
| Outcome: BMI Percentile | Beta | SE | t-value | P |
|---|---|---|---|---|
| BMI percentile trajectory classes, 2 to 13 (reference: low stable class) | ||||
| High rising | 0.43 | 0.02 | 22.55 | *** |
| Median stable | 0.22 | 0.02 | 11.75 | *** |
| Low-to-high | 0.25 | 0.02 | 10.38 | *** |
| Child is female | 0.03 | 0.01 | 1.83 | |
| Child’s birth weight for age | < −0.01 | < 0.01 | −1.41 | |
| Child’s percent weight increase, 0–15 mos. | −0.16 | 0.09 | −1.77 | |
| Estimated maternal BMI, 15 mos. | 0.01 | < 0.01 | 2.88 | ** |
| Maternal average sensitivity, 6–24 mos. | −0.02 | < 0.01 | −2.75 | ** |
| Child has avoidant attachment style | −0.01 | 0.02 | −0.78 | |
| Child has anxious-resistant attachment style | −0.04 | 0.02 | −2.03 | * |
| Income-to-needs ratio, 0–24 mos. | −0.01 | < 0.01 | −2.32 | * |
| Child’s birth weight for age X Child’s percent weight increase, 0–15 mos. | < 0.01 | < 0.01 | 2.05 | * |
| Outcome: Overweight | OR | 95% CI | P | |
|
| ||||
| BMI percentile trajectory classes, 2 to 13 (reference: low stable class) | ||||
| High rising | 59.72 | 27.77 | 128.43 | *** |
| Median stable | 4.90 | 2.20 | 10.91 | *** |
| Low-to-high | 8.94 | 3.75 | 21.30 | *** |
| Child is female | 0.61 | 0.41 | 0.91 | * |
| Estimated maternal BMI, 24 mos. | 1.24 | 1.09 | 1.41 | *** |
| Maternal years of education | 0.92 | 0.83 | 1.02 | |
| Income-to-needs ratio, 0–24 mos. | 0.90 | 0.82 | 1.00 | * |
| Outcome: Obesity | OR | 95% CI | p | |
|
| ||||
| BMI percentile trajectory classes, 2 to 13 (reference: high rising class)1 | ||||
| Median stable | 0.08 | 0.04 | 0.17 | *** |
| Low-to-high | 0.22 | 0.10 | 0.45 | *** |
| Estimated maternal BMI, 15 mos. | 1.48 | 1.28 | 1.71 | *** |
| Maternal years of education | 0.88 | 0.79 | 0.97 | * |
Note. SE = standard error; BMI = body mass index; mos. = months. Hg = weight measured in hectograms; OR = odds ratio; CI = confidence interval. Variables with at least marginal significance were retained per stepwise AIC reduction method.
No children with obesity were classified in the low stable class and so it was dropped from the analysis and the high rising group was used as the reference.
p < .05
p <.01
p <.001.
Whether children were classified as overweight at age 15 was also strongly predicted by their BMI percentile trajectory (Table 1). Children in the low-to-high rising class were almost twice as likely to be overweight at 15 as those in the median stable class (OR = 8.94 vs OR = 4.90), despite having much lower BMI on average through Grade 3 (Figure 1). Greater estimated maternal BMI at 24 months and family income-to-needs ratio (inverse) also predicted overweight status.
In the models predicting adolescents’ obesity status, the low stable class could not be used as the basis for comparison because no adolescents were categorized as obese in that class; instead, the high rising class was used as the reference category. Children in the median stable BMI percentile trajectory class were 92% less likely to be obese than children in the high rising class, whereas children in the low-to-high trajectory class were 78% less likely to be obese (see Table 1); in other words, children in the high rising class had a very high likelihood of being obese at age 15. Maternal estimated BMI when children were 15 months independently increased the odds of the adolescent being obese at age 15, while mother’s years of education lowered them.
To quantify the extent to which the BMI percentile trajectory classes added to the prediction of BMI-based outcomes at age 15, we compared models without the trajectory classes (with only the retained covariates) to the models with the trajectory classes. The child, mother, and family covariates explained 12% to 18% of the variability in the adolescent BMI-based outcomes (Table 2). Adding the child’s BMI trajectory to each model increased explanatory power 1.5–2.5 times, also reflected in considerably lower AICs.
TABLE 2.
Comparison of Models With and Without BMI Trajectory Classes Predicting Age 15 Health Outcomes
| Model Fit by Age 15 Health Outcome | Without Trajectory Classes | With Trajectory Classes | Improvement |
|---|---|---|---|
| BMI Percentile | |||
| R2 | 0.18 | 0.50 | 170% |
| AIC | 12.06 | −373.70 | −361.64 |
| Overweight | |||
| Tjur’s R2 | 0.12 | 0.42 | 250% |
| AIC | 908.73 | 648.57 | −260.16 |
| Obesity | |||
| Tjur’s R2 | 0.12 | 0.31 | 158% |
| AIC | 611.92 | 463.22 | −148.70 |
| Waist Circumference | |||
| R2 | 0.22 | 0.44 | 100% |
| AIC | 5988.50 | 5746.70 | −241.80 |
| Elevated or Prehypertensive Blood Pressure | |||
| Tjur’s R2 | 0.12 | 0.18 | 50% |
| AIC | 876.55 | 822.81 | −53.74 |
Note. Increases in R2 indicate increase in percent variance explained; decreases in AIC indicate improvement in fit. Tjur’s R2, the Coefficient of Discrimination, is read as a pseudo-R2 for use with binary outcomes.
Waist Circumference
Children’s BMI percentile trajectory classes likewise emerged as significant predictors of waist circumference at age 15 (Table 3). Adolescents who had had high rising (B = 15.22, SE = 0.95, p < .001), low-to-high (B = 4.88, SE = 1.17, p < .001), and median stable (B = 2.97, SE = 0.91, p < .01) trajectories had significantly larger waist circumferences than those who had been in the low stable class. Higher birth weight, percent weight increase from 0–15 months, and mothers’ estimated BMI at 15 months also predicted greater waist circumference. Being female, being Black, and greater maternal sensitivity were associated with lower waist circumference. The child, maternal, and family covariates explained 22% of the variability in waist circumference; BMI trajectory explained an additional 22%, with substantial improvement in the model’s AIC (Table 2).
Table 3.
Best-Fitting Linear and Logistic Regression Models Predicting Waist Circumference and Elevated or Prehypertensive Blood Pressure at Age 15
| Outcome: Waist Circumference | B | SE | t-value | p |
|---|---|---|---|---|
| BMI percentile trajectory classes, 2 to 12 (reference: low stable class) | ||||
| High rising | 15.22 | 0.95 | 15.98 | *** |
| Median stable | 2.97 | 0.91 | 3.27 | ** |
| Low-to-high | 4.88 | 1.17 | 4.18 | *** |
| Child is female | −4.20 | 0.69 | −6.08 | *** |
| Child is Black | −2.82 | 1.15 | −2.45 | * |
| Child’s birth weight for age | < 0.01 | < 0.01 | 2.88 | ** |
| Child’s percent weight increase, 0–15 mos. | 3.60 | 1.18 | 3.06 | ** |
| Estimated maternal BMI, 15 mos. | 1.11 | 0.23 | 4.91 | *** |
| Maternal average sensitivity, 6–24 mos. | −0.76 | 0.28 | −2.71 | ** |
| Outcome: Elevated or Prehypertensive Blood Pressure | Odds Ratio | 95% Confidence Interval | p | |
|
| ||||
| BMI percentile trajectory classes, 2 to 12 (reference: low stable class) | ||||
| High rising | 4.49 | 2.82 | 7.14 | *** |
| Median stable | 1.73 | 1.05 | 2.84 | * |
| Low-to-high | 1.28 | 0.64 | 2.54 | |
| Child is female | 0.25 | 0.18 | 0.37 | *** |
| Maternal prenatal smoking | 1.13 | 0.99 | 1.29 | |
Note. SE = standard error; BMI = body mass index; mos. = months. Variables with at least marginal significance were retained per stepwise AIC reduction method.
p < .05
p <.01
p <.001.
Elevated or Prehypertensive Blood Pressure
Blood pressure qualifying as EPH was the least well explained by the modeling. Children with higher average BMI percentiles throughout childhood (high rising and median stable) were more likely than those with lower averages (low-to-high and low stable) to qualify (Table 3). Girls were significantly less likely to have EPH-BP than boys; although maternal smoking was retained through the AIC model comparison process, it was not a statistically significant predictor of EPH-BP at age 15. Adding the BMI trajectory classes to the model explained an additional 6% of the variability in EPH-BP and led to a modest improvement in the AIC (Table 3).
Discussion
Employing BMI percentile trajectory classes from 9 data points collected between ages 2 and 13 and by including a large set of covariates, the current study extends the existing literature on the prediction of adolescent outcomes from patterns of earlier BMI percentile gain. The four trajectories we employed exhibited patterns that were distinct from the existing literature.9 In addition to consistently high and low BMI percentile groups, the trajectory classes used in the current study include a group of children who started with low BMI percentile and ended with the second highest BMI percentile (“low to high”) and a group of children who started with the second highest BMI percentile, remained stable over time, and ended with the third highest BMI percentile (“median stable”; see Figure 1). The two classes characterized by increasing BMI percentile across childhood, rather than stably high BMI percentile across childhood, were most predictive of unhealthy outcomes at age 15. Thus, although the average BMI percentile of the low-to-high class was at the 55th percentile, children in that class had worse health outcomes at age 15 than did children in the median stable class that averaged at the 68th BMI percentile. These results suggest that a steep increase in BMI percentile across childhood may be a key indicator of later health problems, more so than a BMI percentile that is relatively high but stable. A potential clinical implication is that pediatric health care providers should consider providing obesity prevention counseling not only to the parents of children with an elevated BMI, but also to the parents of children with rapidly increasing BMI’s, even when within the normal range.
Previous studies found that rising trajectories in childhood were associated with greater BMI percentile, greater waist circumference, and EPH-BP in adolescence.5–8 Here, BMI percentile rank, being overweight, and waist circumference at age 15 were predicted by BMI percentile trajectory. EPH-BP was the least well-explained outcome by the BMI percentile trajectories or the covariates. Others have reported that children identified in the highest BMI trajectory in childhood and those with early persistent obesity exhibit the highest levels of systolic and diastolic blood pressure.7,8 However, one study found that adolescents in elevated BMI trajectory class displayed greater levels of SBP but not of DBP. 6 This suggests that those in the highest BMI classes have the greatest risk for EPH-BP but that the relation between BMI trajectory class and EPH-BP becomes less clear for those in the lower weight classes. There are other mechanisms, untested here, at work in the development of EPH-BP.28
Estimated maternal BMI was the most consistent covariate predictor of adolescent weight outcomes. Others have reported that maternal weight after delivery is linked to increased odds of preschooler overweight10 and increased risk for obesity in adolescence;11 these associations were stronger for mothers who were overweight or obese pre-pregnancy. Future research should consider also paternal BMI, which has been linked to child BMI,29 further suggesting that child overweight and obesity is the result of epigenetics, familial genetics, the shared environment, and health behaviors (e.g., feeding, physical activity).30,31
Compared with estimated maternal BMI, the remaining early risk and family-level covariates had less consistent relations with the five outcomes. Maternal sensitivity was associated with lower BMI percentile and waist circumference at age 15, which may be explained by maternal feeding behaviors. Lumeng et al.20 found that maternal intrusive feeding behaviors were linked to less maternal sensitivity and greater child adiposity. Mothers who meet their child’s needs promptly and accurately may not use intrusive feeding behaviors, and thus reduce their child’s risk for over-nutrition and obesity. The SES covariates (i.e., maternal education, income-to-needs ratio) were only related to decreased risk for overweight and obesity. McGrath et al.32 reported that neighborhood income, but not family SES, was associated with EPH-BP, and Abreu et al.33 found that mother’s education, but not father’s education, was only linked to adiposity in adolescent boys. Childhood characteristics (i.e., birth weight for gestational age, percent weight increase, Black race) were significantly related to waist circumference at age 15, whereas being female was associated with decreased risk for overweight and EPH-BP. Others have reported inconsistencies using these predictors of weight and blood pressure outcomes.32,34,35 It is important to note, however, that race predicted BMI trajectory class membership when the classes were first identified, with Black children more likely than children of other races to be in the high rising, median stable, and low-to-high trajectories.9 Although retained, maternal prenatal smoking was not significantly related to EPH-BP at age 15. Some important covariates were eliminated during AIC stepwise testing such as child BMI at 15 months, breastfeeding, Hispanic ethnicity, maternal stressful life events, and maternal depression.
This study relied on trajectories estimated purely from BMI percentile, without time-varying covariates throughout the period. A future study including these predictors or other predictors measured across childhood could further refine trajectories and provide insight into their inflection points. Additional predictors that were not available, lacked detailed information (e.g., dietary intake), or were only available for a subset of participants (e.g., pubertal timing) might explain further variance in outcomes and/or yield more explicit information on how these predictors relate to or mediate each other.
The current study has several strengths. The SECCYD dataset contained weight-related variables from birth to adolescence for a relatively large sample well-matched to population averages of race, ethnicity, and SES. Modeling these frequent, repeated measures yielded four distinct patterns of BMI percentile trajectories,9 which in turn provided a parsimonious means of identifying links between patterns of BMI percentile change in childhood and health in adolescence. By retaining the low-to-high pattern in the original trajectory model, we identified important differences seen in children who are gaining considerable weight in middle childhood.
This study demonstrates that the rate at which children gain weight in childhood is predictive of their weight outcomes and EPH-BP in adolescence. This is especially true for steeply increasing BMI percentile across childhood, which was more predictive of adolescent health outcomes than stably high BMI. Tracking BMI change among child patients and their mothers may assist with early identification of who is at greater risk for elevated weight and blood pressure and who could benefit from intervention to reduce adverse outcomes before adolescence.
What’s New.
Patterns of BMI percentile gains characterized by rapid gains or persistently above average BMI percentiles from ages 2 to 13 predicted weight, waist circumference, and prehypertension at age 15. Estimated maternal BMI was also a key predictor of adolescent outcomes.
ACKNOWLEDGEMENTS:
Writing of this study was supported by award P2CHD042849 to the Population Research Center from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: The authors have nothing to disclose.
References
- 1.Fryar CD, Carroll MD, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats 2020.
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7 [DOI] [PubMed] [Google Scholar]
- 3.Llewellyn A, Simmonds M, Owen CG, Woolacott N. Childhood obesity as a predictor of morbidity in adulthood: a systematic review and meta-analysis. Obes Rev 2016;17:56–67. doi: 10.1111/obr.12316 [DOI] [PubMed] [Google Scholar]
- 4.Simmonds M, Llewellyn A, Owen CG, Woolacott N. Predicting adult obesity from childhood obesity: a systematic review and meta‐ analysis. Obes Rev 2016;17:95–107. doi: 10.1111/obr.12334 [DOI] [PubMed] [Google Scholar]
- 5.Kwon S, Janz KF, Letuchy EM, Burns TL, Levy SM. Association between body mass index percentile trajectories in infancy and adiposity in childhood and early adulthood. Obesity (Silver Spring) 2017;25:166–171. doi: 10.1002/oby.21673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barraclough JY, Garden FL, Toelle BG, et al. Weight gain trajectories from birth to adolescence and cardiometabolic status in adolescence. J Pediatr 2019;208:89–95.e4. doi: 10.1016/j.jpeds.2018.12.034 [DOI] [PubMed] [Google Scholar]
- 7.Ventura AK, Loken E, Birch LL. Developmental trajectories of girls’ BMI across childhood and adolescence. Obesity (Silver Spring) 2009;17:2067–2074. doi: 10.1038/oby.2009.123 [DOI] [PubMed] [Google Scholar]
- 8.Ziyab AH, Karmaus W, Kurukulaaratchy RJ, Zhang H, Arshad SH. Developmental trajectories of Body Mass Index from infancy to 18 years of age: prenatal determinants and health consequences. J Epidemiol Community Health 2014;68:934–941. doi: 10.1136/jech-2014-203808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bichteler A, Gershoff ET. Identification of children’s BMI trajectories and prediction from early weight gain. Obesity 2018;26:1050–1056. doi: 10.1002/oby.22177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson CA, Cohen AK, Rehkopf DH, et al. Pregnancy and post-delivery maternal weight changes and overweight in preschool children. Prev Med 2014;60:77–82. doi: 10.1016/j.ypmed.2013.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstet Gynecol 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gundersen C, Mahatmya D, Garasky S, Lohman B. Linking psychosocial stressors and childhood obesity. Obes Rev 2011;12:e54–63. doi: 10.1111/j.1467-789X.2010.00813.x [DOI] [PubMed] [Google Scholar]
- 13.Eunice Kennedy Shriver National Institute of Child Health and Human Development. NICHD Study of Early Child Care and Youth Development (SECCYD) Overview. https://www.nichd.nih.gov/research/supported/seccyd/overview. Updated June 10, 2019. Accessed September 8, 2020.
- 14.Nader PR, O’Brien M, Houts R, et al. Identifying risk for obesity in early childhood. Pediatrics 2006;118:e594–e601. doi: 10.1542/peds.2005-2801 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Clinical Growth Charts. https://www.cdc.gov/growthcharts/clinical_charts.htm. Updated June 16, 2017. Accessed September 8, 2020. [Google Scholar]
- 16.Centers for Disease Control and Prevention. Defining Childhood Obesity. https://www.cdc.gov/obesity/childhood/defining.html. Updated July 3, 2018. Accessed September 8, 2020. [Google Scholar]
- 17.National Heart, Lung, and Blood Institute (NHLBI); National Institutes of Health (NIH); U.S. Department of Health and Human Services. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. https://www.nhlbi.nih.gov/files/docs/resources/heart/hbp_ped.pdf. Revised May 2005. Accessed September 8, 2020.
- 18.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 2017;140; e20171904. doi: 10.1542/peds.2017-1904 [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO child growth standards: Methods and development. Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. https://www.who.int/childgrowth/standards/technical_report/en/. Updated 2006. Accessed September 8, 2020.
- 20.Lumeng JC, Ozbeki TN, Appugliese DP, Kaciroti N, Corwyn RF, Bradley RH. Observed assertive and intrusive maternal feeding behaviors increase child adiposity. Am J Clin Nutr 2012;95:640–647. doi: 10.3945/ajcn.111.024851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stunkard AJ, Sørensen T, Schulsinger F. Use of the Danish Adoption Register for the study of obesity and thinness. Res Publ Assoc Res Nerv Ment Dis 1983;60:115–120. [PubMed] [Google Scholar]
- 22.Radloff L The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977;1:385–401. doi: 10.1177/014662167700100306 [DOI] [Google Scholar]
- 23.Ainsworth MDS, Blehar M, Waters E, Wall S. Patterns of attachment: A psychological study of the strange situation. Erlbaum; 1978. [Google Scholar]
- 24.U.S. Census. How the Census Bureau Measures Poverty. https://www.census.gov/topics/income-poverty/poverty/guidance/poverty-measures.html. Updated August 27, 2019. Accessed September 8, 2020.
- 25.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2015. [Google Scholar]
- 26.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- 27.Tjur T Coefficients of determination in logistic regression models - a new proposal: The coefficient of discrimination. Am Stat 2009;63:366–372. doi: 10.1198/tast.2009.08210 [DOI] [Google Scholar]
- 28.Hamoen M, de Kroon MLA, Welten M, et al. Childhood prediction models for hypertension later in life: a systematic review. J Hypertens 2019;37:865–877. doi: 10.1097/HJH.0000000000001970 [DOI] [PubMed] [Google Scholar]
- 29.Durmuş B, Arends LR, Ay L, et al. Parental anthropometrics, early growth and the risk of overweight in pre-school children: the Generation R Study. Pediatr Obes 2013;8:339–350. doi: 10.1111/j.2047-6310.2012.00114.x [DOI] [PubMed] [Google Scholar]
- 30.Ross MG, Desai M. Developmental programming of offspring obesity, adipogenesis, and appetite. Clin Obstet Gynecol 2013;56:529–536. doi: 10.1097/GRF.0b013e318299c39d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chu L, Retnakaran R, Zinman B, Hanley AJG, Hamilton JK. Impact of maternal physical activity and infant feeding practices on infant weight gain and adiposity. Int J Endocrinol 2012;2012:293821. doi: 10.1155/2012/293821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGrath JJ, Matthews KA, Brady SS. Individual versus neighborhood socioeconomic status and race as predictors of adolescent ambulatory blood pressure and heart rate. Soc Sci Med 2006;63:1442–1453. doi: 10.1016/j.socscimed.2006.03.019 [DOI] [PubMed] [Google Scholar]
- 33.Abreu S, Santos R, Moreira C, Santos PC, Mota J, Moreira P. Food consumption, physical activity and socio-economic status related to BMI, waist circumference and waist-to-height ratio in adolescents. Public Health Nutr 2014;17:1834–1849. doi: 10.1017/S1368980013001948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter MA, Dubois L, Tremblay MS, Taljaard M, Jones BL. Trajectories of childhood weight gain: the relative importance of local environment versus individual social and early life factors. PloS one 2012;7:e47065. doi: 10.1371/journal.pone.0047065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Péneau S, Giudici KV, Gusto G, et al. Growth trajectories of body mass index during childhood: Associated factors and health outcome at adulthood. J Pediatr 2017;186:64. doi: 10.1016/j.jpeds.2017.02.010 [DOI] [PubMed] [Google Scholar]


