Abstract
Soybean (Glycine max L.) is an economically important crop, and is cultivated worldwide, although increasingly long periods of drought have reduced the productivity of this plant. Research has shown that inoculation with arbuscular mycorrhizal fungi (AMF) provides a potential alternative strategy for the mitigation of drought stress. In the present study, we measured the physiological and morphological performance of two soybean cultivars in symbiosis with Rhizophagus clarus that were subjected to drought stress (DS). The soybean cultivars Anta82 and Desafio were grown in pots inoculated with R. clarus. Drought stress was imposed at the V3 development stage and maintained for 7 days. A control group, with well-irrigated plants and no AMF, was established simultaneously in the greenhouse. The mycorrhizal colonization rate, and the physiological, morphological, and nutritional traits of the plants were recorded at days 3 and 7 after drought stress conditions were implemented. The Anta82 cultivar presented the highest percentage of AMF colonization, and N and K in the leaves, whereas the DS group of the Desafio cultivar had the highest water potential and water use efficiency, and the DS + AMF group had thermal dissipation that permitted higher values of Fv/Fm, A, and plant height. The results of the principal components analysis demonstrated that both cultivars inoculated with AMF performed similarly under DS to the well-watered plants. These findings indicate that AMF permitted the plant to reduce the impairment of growth and physiological traits caused by drought conditions.
Subject terms: Biotechnology, Plant sciences
Introduction
Soybean [Glycine max (L.) Merrill] is the world’s most economically important legume1–4. However, increasingly frequent periods of water deficit provoked by climate change may cause significant losses in the productivity of the crop5–7. Ongoing climate change may result in an increase in global surface temperatures of 1.8–3.6 °C by the end of this century, and constitute one of the greatest challenges faced by human society8.
Drought stress interferes directly with the primary metabolism of plants by inducing stomatal closure, which is essential to prevent water loss. This, in turn, limits the input of CO2, which inhibits photosynthesis9–11, compromising growth and yields12,13. Drought also causes an accumulation of reactive oxygen species through an imbalance in the photosystem, which leads to the peroxidation of lipids and the degradation of chlorophyll14,15.
A number of different strategies have been tested to mitigate drought stress, particularly the symbiotic association of arbuscular mycorrhizal fungi (AMF) with plants. These fungi are known to exude a glomalin-related soil protein, which contributes to the stability of the soil aggregate and increases the capture of C from the soil16–19. Plant-AMF symbiosis also promotes the growth of root hairs through its influence on auxin synthesis and transport20. The AMFs are linked to soil properties, which favor the porosity that ensures nutrient uptake21–23 and improves the plant-water relationship24–27.
The formation of arbuscular structures inside the roots of the host plant provides a pathway for nutritional exchange between the fungus and its host28. Hyphae also modulate water transport between the soil and the plant29,30. The activation of the lignin synthesis pathway also improves hydraulic transport between cells15, increasing transport between the source and sink by expanding the expression of the sucrose transporters18.
The accumulation and transport of solutes, in turn, confers osmotic adjustment31,32, and antioxidant defense31,33, which maintain the plant’s metabolism, even in environments with low water availability. Physiological and metabolic responses have been related to the high productivity of plants when associated with AMF in soil under drought stress4,27,32,34,35. Inoculation with AMF may thus provide a potentially valuable strategy for increasing crop productivity and for improving ecosystem sustainability36,37. The AMF of the genus Rhizophagus (= Glomus38) can survive and multiply in semi-arid ecosystems, even under conditions of low water availability39, and Rhizophagus clarus produces larger spores than other species, such as R. irregularis and R. intraradices40. Given this, R. clarus has received considerable attention, especially as it can grow symbiotically, using the myristate as a source of carbon and energy41,42.
The present study assessed the morphophysiological and nutritional performance of two soybean cultivars, Anta82 and Desafio, which have contrasting levels of tolerance to drought stress, following inoculation and symbiosis with R. clarus, which was isolated from soils of the Brazilian Cerrado savanna. The results show that this symbiosis may contribute significantly to the capacity of the plant to tolerate drought stress.
Results
Mycorrhizal colonization
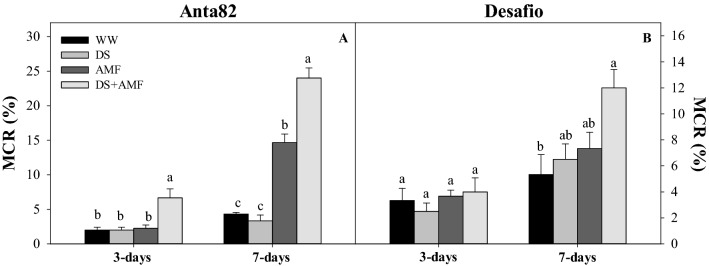
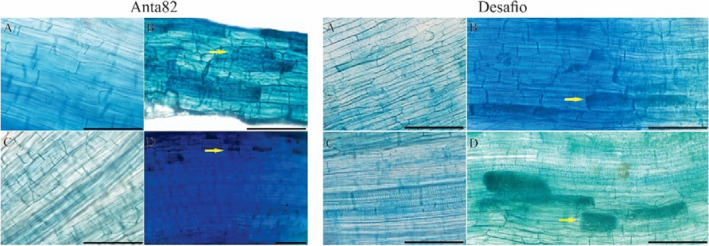
The DS + AMF group of the Anta82 cultivar presented a significantly higher percentage of colonization by R. clarus 3 days after the initiation of the drought stress trial (F3.15 = 9.22, p = 0.0019). On day 7 (F3.15 = 84.25, p < 0.0001), colonization was significantly higher in both the AMF and the DS + AMF groups (Fig. 1A). In the case of the Desafio cultivar, colonization in the DS + AMF group was significantly higher than that in the WW plants at 7 days (F3.15 = 4.64, p = 0.0223) (Fig. 1B). The colonization of the soybean root cortex by R. clarus arbuscules is shown in Fig. 2.
Figure 1.
Mycorrhizal colonization (MCR) on days 3 and 7 in the (A) Anta82 and (B) Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The bars represent the mean ± SEM (n = 4). Pairs of means in the same period (3 or 7 days) with different letters are significantly different (p < 0.05), based on Tukey’s post hoc test.
Figure 2.
Root cortex in the Anta82 and Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The yellow arrows indicate areas of mycorrhizal colonization.
Water relations
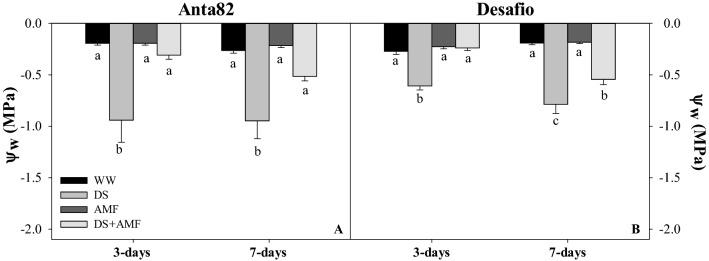
The plants of the DS group of the Anta82 cultivar presented a water potential (Ψw) of − 0.94 MPa after 3 days of treatment (F3.15 = 10.51, p = 0.0011), which was significantly lower than all the other treatments (WW, AMF and DS + AMF), which had Ψw values of no less than − 0.31 MPa (Fig. 3). On day 7, the DS group maintained significantly higher values (Ψw = − 0.95 MPa), while the DS + AMF plants remained similar to the AMF and WW treatments (Fig. 3).
Figure 3.
Water potential (Ψw) on days 3 and 7 in the (A) Anta82 and (B) Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The bars represent the mean ± SEM (n = 4). Pairs of means in the same period (3 or 7 days) with different letters are significantly different (p < 0.05) based on Tukey’s post hoc test.
After 3 days, (F3.15 = 38.85, p = 0.0001) the DS group of the Desafio cultivar had a significantly lower Ψw value, than the WW, AMF, and DS + AMF groups, which were, once again all similar to one another (Fig. 3B). At 7 days (F3.15 = 32.12, p = 0.0001) the DS + AMF had a significantly higher Ψw, of − 0.54 MPa, than the DS group, which had a value of − 0.79 MPa (Fig. 3B).
Photosynthetic traits
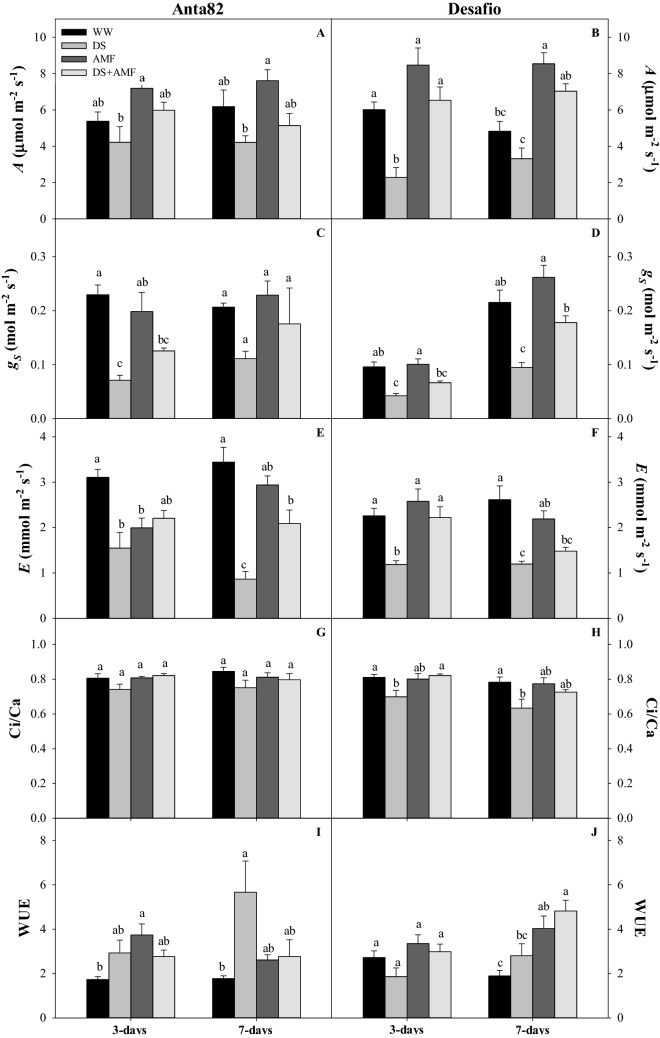
The photosynthetic rate (A) was reduced significantly by drought stress in the Anta82 cultivar on days 3 (F3.15 = 4.214, p = 0.0298) and 7 (F3.15 = 4.80, p = 0.0201) (Fig. 4A). The stomatal conductance (gS) was significantly lower in the DS plants than in the WW plants on day 3 (F3.15 = 11.825, p = 0.0007) (Fig. 4C). The transpiration rate (E) was altered significantly in the DS group on day 7 (F3.15 = 18.93, p = 0.0001) (Fig. 4E). The Ci/Ca ratio was not affected by either treatment (Fig. 4F). Water use efficiency (WUE) increased significantly in the AMF and DS groups on days 3 (F3.15 = 3.988, p = 0.0349) and 7 (F3.15 = 4.42, p = 0.0258), respectively (Fig. 4G). Photosynthetic rates (A) varied significantly in the Desafio cultivar on both days 3 (F3.15 = 14.206, p = 0.0003) and 7 (F3.15 = 18.06, p = 0.0001) (Fig. 4B). On day 7, the inoculated plants (groups AMF and DS + AMF) presented higher photosynthetic rates in comparison with groups WW and DS (Fig. 4B). The stomatal conductance (gS) was higher in the WW and AMF groups on days 3 (F3.15 = 13.59, p = 0.0004) and 7 (F3.15 = 14.97, p = 0.0002) (Fig. 4D), as was the transpiration rate (E) on days 3 (F3.15 = 8.67, p = 0.0025) and 7 (F3.15 = 12.427, p = 0.0005) (Fig. 4F). The Ci/Ca ratio was lower in the DS plants than in the DS + AMF group on day 3 (Fig. 4H). On day 7, the WUE (F3.15 = 7.47, p = 0.0044) was significantly higher in the DS + AMF plants than in the WW and DS groups (Fig. 4J).
Figure 4.
Photosynthetic rate (A), stomatal conductance (gS), transpiration (E), the ratio between internal and external CO2 concentrations (Ci/Ca), and water use efficiency (WUE) on days 3 and 7 in the (A,C,E,G,I) Anta82 and (B,D,F,H,J) Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The bars represent the mean ± SEM (n = 4). Pairs of means in the same period (3 or 7 days) with different letters are significantly different (p < 0.05) based on Tukey’s post hoc test.
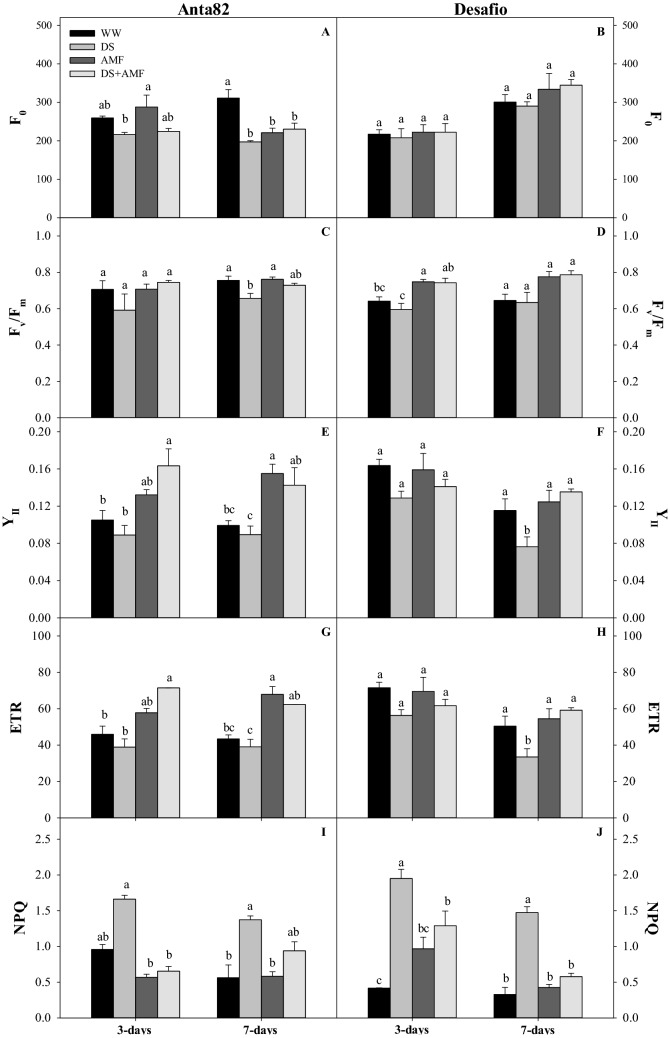
Chlorophyll a fluorescence
The minimum fluorescence (F0) was reduced significantly in the DS, AMF, and DS + AMF groups of the Anta82 cultivar after 7 days (F3.15 = 11.19; p = 0.0008) (Fig. 5A). The maximum quantum yield of PSII (Fv/Fm) decreased significantly in the DS group on day 7 (FAnta3.15 = 11.195; p = 0.0008) (Fig. 5C). Additionally, on day 7, significant reductions were observed in the effective quantum yield of PSII (YII) (F3.15 = 7.818; p = 0.0037) and the electron transport rate (ETR) (F3.15 = 7.341; p = 0.0437) of the WW and DS plants in comparison with the AMF and DS + AMF groups (Fig. 5E,G). Non-photochemical quenching (NPQ) increased significantly in the DS plants on both day 3 (F3.15 = 71.326, p < 0.0001) and day 7 (F3.15 = 10.655, p = 0.0010) after treatment imposition (Fig. 5I).
Figure 5.
Minimal fluorescence (F0), maximum quantum yield of the PSII (Fv/Fm), effective quantum yield of PSII (YII), electron transport rate (ETR), and non-photochemical quenching (NPQ) on days 3 and 7 in the (A,C,E,G,I) Anta82 and (B,D,F,H,J) Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The bars represent the mean ± SEM (n = 4). Pairs of means in the same period (3 or 7 days) with different letters are significantly different (p < 0.05) based on Tukey’s post hoc test.
In the case of the Desafio cultivar, the minimum fluorescence did not change significantly on either day 3 (F3.15 = 0.120; p = 0.9469) or day 7 (F3.15 = 1.107; p = 0.3845) (Fig. 5B). The maximum quantum yield of PSII increased slightly in the inoculated plants on day 3, although no differences were found among the treatments on day 7 (Fig. 5D). Similarly, neither the effective quantum yield of PSII (F3.15 = 2.071, p = 0.1577) nor the electron transport rate (F3.15 = 2.234, p = 0.1368) varied among the treatments after 3 days, although a significant increase was observed in the DS + AMF plants in comparison with the DS group after 7 days (YII: F3.15 = 6.077, p = 0.009; ETR: F3.15 = 6.078, p = 0.0093) (Fig. 5F,H). Non-photochemical quenching (NPQ) was significantly higher in the DS and DS + AMF groups than in the WW group on day 3 (F3.15 = 9.428; p = 0.0018). On day 7, the NPQ of the inoculated plants (AMF and DS + AMF) was no different from that of the WW group (Fig. 5J).
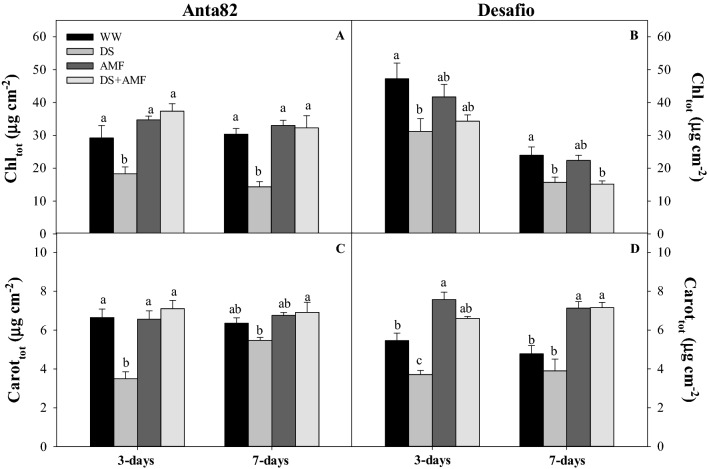
Photosynthetic pigments
The total chlorophyll and carotenoid concentrations were reduced significantly in the DS plants of the Anta82 cultivar after 3 (F3.15 = 11.16, p = 0.0009; F3.15 = 15.84, p = 0.0002, respectively) and 7 days (F3.15 = 14.39, p = 0.0003, F3.15 = 4.257, p = 0.0289, respectively) (Fig. 6A,C). In the cultivar Desafio, total chlorophyll was reduced significantly in the DS and DS + AMF plants in comparison with the WW group on day 7 (F3.15 = 15.05, p = 0.0002) (Fig. 6B). The carotenoid concentration was significantly higher on days 3 (F3.15 = 31.95, p = 0.0002) and 7 (F3.15 = 15.05, p = 0.0002) in the AMF and DW + AMF plants (Fig. 6D).
Figure 6.
Total chlorophyll (Chltot) and total carotenoid (Carottot) contents on days 3 and 7 in the (A,C) Anta82 and (B,D) Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The bars represent the mean ± SEM (n = 4). Pairs of means in the same period (3 or 7 days) with different letters are significantly different (p < 0.05) based on Tukey’s post hoc test.
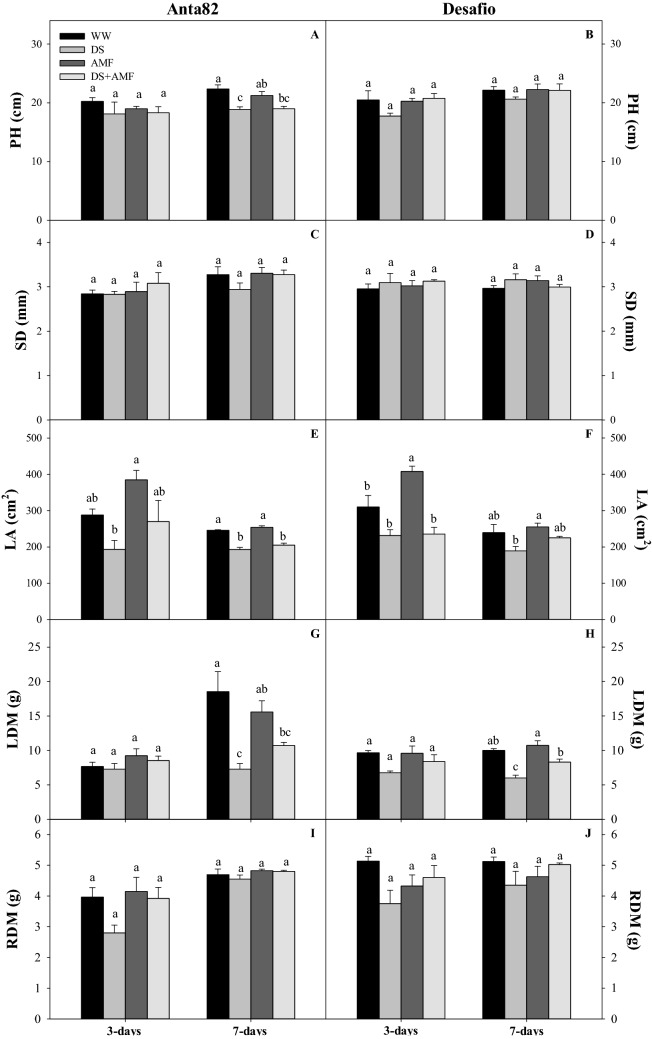
Morphological traits
In the Anta82 cultivar, plant height (F3.15 = 9.421, p = 0.0018), leaf area (F3.15 = 41.140, p < 0.0001), and leaf dry matter (F3.15 = 8.385, p = 0.0028) were all reduced significantly in the DS and DS + AMF plants in comparison with the WW group on day 7 (Fig. 7A,E,G). Stem diameter and root dry matter were not affected by either treatment in Anta82 on either day 3 (F3.15 = 0.475, p = 0.7053; F3.15 = 3.043, p = 0.0704, respectively) or day 7 (F3.15 = 1.463, p = 0.2740; F3.15 = 1.173, p = 0.3609) (Fig. 7C,I).
Figure 7.
Plant height (PH), stem diameter (SD), leaf area (LA), leaf dry matter (LDM) and root dry matter (RDM) on days 3 and 7 in the (A,C,E,G,I) Anta82 and (B,D,F,H,J) Desafio soybean cultivars under different conditions of inoculation and water stress. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated. The bars represent the mean ± SEM (n = 4). Pairs of means in the same period (3 or 7 days) with different letters are significantly different (p < 0.05) based on Tukey’s post hoc test.
In the case of the Desafio cultivar, by contrast, neither plant height (day 3: F3.15 = 2.170, p = 0.1446; day 7: F3.15 = 0. 945, p = 0.4495), stem diameter (day 3: F3.15 = 0.336, p = 0.7998; day 7: F3.15 = 1.124, p = 0.3780) and root dry matter (day 3: F3.15 = 2.675, p = 0.0944; day 7: F3.15 = 1.502, p = 0.2641) were altered significantly by the treatments (Fig. 7B,D,J). However, leaf area (F3.15 = 4.094, p = 0.0324) and leaf dry matter (F3.15 = 20.058, p < 0.0001) increased significantly by day 7 in the inoculated plants (AMF and DS + AMF) in comparison with the WW group (Fig. 7F,H).
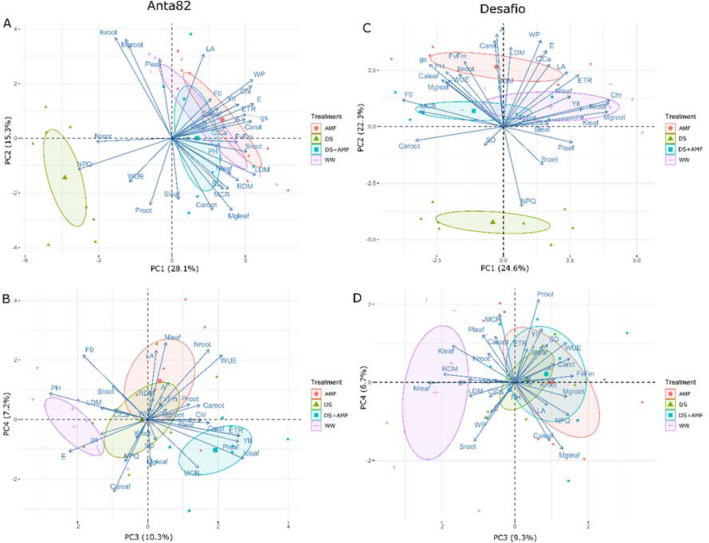
Principal components analyses
The first two components explained 43.4% of the variance in the Anta82 data and 46.9% of that in the Desafio data. The plot of the first two PCA axes clearly differentiated a DS cluster from the other groups in the Anta82 data (Fig. 8A,B).
Figure 8.
PCA biplot of the first four dimensions (first and second at top and third and fourth at bottom) using (A,B) Anta82 and (C,D) Desafio data. and Desafio data at right. WW = well-watered, noninoculated, DS = drought stress, noninoculated, WW + AMF = well-watered and inoculated, DS + AMF = drought stress and inoculated.
The first PCA axis of the Desafio data, which explains 24.6% of the variance, is not systematically related to the different treatments (Fig. 8C,D), while the second axis clustered the DS records to the negative side of the plot, the DS + AMF and WW in the middle, and the AMF records toward the positive side. In the case of the Anta82 cultivar, most of the variables (LDM, E, Ψw, Chl, Carot, Sroot, gS, A, ETR, RDM, YII, Fv/Fm, Mgleaf, Nleaf, MCR, Ci/Ca, SD, PH, Caleaf, and Caroot) were significantly correlated (p < 0.05) with the first PCA axis, which means that they decreased with drought stress. In contrast, WUE, Mgroot, Kroot, Nroot and NPQ correlated negatively with this axis, which means that these variables increased with drought stress in the Anta82 cultivar.
In the case of the Desafio cultivar, Ψw, Carot, LDM, E, Fv/Fm, Ci/Ca, gS, PH, LA, Nroot, ETR, Caleaf, WUE, Mgleaf, and RDM were all correlated positively with PC2, while Sroot and NPQ were correlated negatively with this axis. This means that the former group of variables tends to increase with AMF and decrease with the DS treatments, whereas Sroot and NPQ increase with DS and decrease with AMF in the Desafio cultivar.
The third axis explained 10.3% of the variance in the Anta82 data and 9.3% of that in the Desafio data. PC3 was thus able to differentiate WW from the other treatments in both cultivars. In the case of the Anta82 cultivar, PC3 also clustered the DS + AMF data. The Kleaf, YII, ETR, Pleaf, WUE, Carot, and Nroot variables were positively correlated with the third PCA axis in the Anta82 data, which means that these variables increase with DS + AMF and decrease with WW, while F0, E and PH were negatively correlated with this axis.. These variables thus presented the opposite pattern, increasing with WW and decreasing with DS + AMF. In the case of the Desafio cultivar, Fv/Fm, Carot, NPQ, Mgroot, WUE, and Mgleaf were all positively correlated with PC3, while Kleaf, RDM, and Nleaf were negativelycorrelated . As the WW data were located toward the negative side of the third axis, the former set of variables decreased and the latter increased with this treatment.
Overall, the results of the PCA demonstrated that plants under drought stress colonized with AMF clustered together with the well-watered plants of both cultivars, which clearly indicates that inoculation with AMF contributed to a reduction in the physiological impairment of the plant provoked by drought conditions.
The negative effects of drought also appear to be more pronounced in the Anta82 cultivar than in Desafio. In particular, the third axis of the Anta82 dataset and the second axis of the Desafio data provide insights into the physiological impact of AMF on the plants, independent of their watering regime.
Discussion
The Anta82 and Desafio soybean cultivars presented differential responses to the inoculation and water treatments. The results of the present study support the hypothesis that inoculation with the arbuscular mycorrhizal fungus R. clarus leads to the colonization of the roots of the soybean plant and that, under drought stress, it favors the water status and metabolic activities of the plant, particularly in the case of the more drought-tolerant Desafio cultivar. Arbuscular mycorrhizal fungi are abundant and widely distributed in an ample range of environments and may significantly enhance the stress tolerance of host plants15. It is also important to note that the presence of different AMF species is related to the type of host plant and edaphoclimatic conditions43. Colonization by mycorrhizae is the principal route of symbiosis with the host plant44, which favors both the organisms involved in the relationship. The application of AMF is a potential strategy for the enhancement of the capacity of a plant to tolerate drought in arid ecosystems45,46.
The potential for colonization depends on the plant and the type of fungus, in addition to the cultivation conditions and exposure time47–50. The Anta82 cultivar is more sensitive to drought stress (DS) and presented an increase in mycorrhizal colonization, especially under drought stress on days 3 and 7. However, the Desafio cultivar presented a pronounced increase in mycorrhizal colonization after 7 days of drought stress. Moreira et al.51 found that coffee plants inoculated with R. clarus had a higher mycorrhizal colonization (39%) and root dry matter than noninoculated plants, in soils at 71% of field capacity. Inoculation with AMF of different species has also been shown to potentially mitigate the effects of drought stress and increase resistance in bean52, rice53, tomato54, wheat55, and in orange15 and apple56 trees.
The mechanisms associated with the maintenance of the water status of a plant are triggered rapidly when hydrological conditions become limited, which means that drought stress is one of the principal factors affecting plant growth and production6,57. These mechanisms include stomatal regulation, which responds rapidly to water stimuli10,58. As the root-bound hyphae of mycorrhizal fungi improve water uptake, their association with plants is a potential ally for the maintenance of their water status47. Under conditions of drought stress, there is an increase in the space and accumulation of air between the soil particles and the roots, which can be compensated for the presence of AMF, guaranteeing the transport of water15. Our results show that AMF mitigated the adverse effects of drought stress in both cultivars. Similarly, the water potential of Poncirus trifoliata plants was also increased by 20% under conditions of water deficit when they were inoculated with mycorrhizal fungi59. Under drought conditions, AMF helps the host plants to absorb more water, which means that inoculation with these fungi may provide an important strategy for the improvement of the productivity of plants grown in semi-arid regions60.
A mechanism that maintains plant turgor, the closure of the stomata may limit photosynthetic processes and compromise crop yields61–63. Low photosynthetic yields related to stomatal limitations have been observed in plants exposed to drought stress, although inoculation with AMF increases stomatal conductance under drought stress in comparison with noninoculated plants. Photosynthesis and transpiration were also higher in the presence of mycorrhizae under drought stress conditions in both cultivars in the present study, which indicates that plants associated with AMF can improve their water status under drought stress through their ability to use water more efficiently. Quiroga et al.64 found that maize plants in symbiosis with AMF R. irregularis under drought conditions had increased stomatal conductance and photosynthesis parameters. Inoculation with AMF may have beneficial effect, even in the absence of drought stress. Coffee plants of three different cultivars, which were well-watered and inoculated with the spores of R. clarus and/or Acaulospora colombiana presented an increase in photosynthetic rates, stomatal conductance, transpiration, water use efficiency, and the percentage of mycorrhizal colonization65. These findings indicate that the AMF had an active role in this process, permitting greater stomatal opening and higher photosynthetic rates, associated with higher turgor, as recently observed in eggplant27, tomato54, and Olea europaea66. This was possible because the AMF enhanced water uptake, even under limiting conditions67,68, thereby inducing changes in the critical substrate water potential and increasing the water transport in the colonized substrates26, which would permit greater water-use efficiency. The maintenance of water status also favors the fixation of atmospheric CO2 and increases the movement of photoassimilates (the "sink effect") from the aerial parts of the plant to its roots48.
Photosynthetic limitation is directly related to the photochemical responses of the plant, as demonstrated by the chlorophyll a fluorescence variables69. Drought stress may compromise the functionality of the chloroplast electron chain9,70,71, limit the production of energy, and reduce the energy required for the completion of the photosynthetic process. In the present study, the inoculation of soybean plants with AMF under drought stress permitted the maintenance of the maximum quantum yield of PSII (Fv/Fm) in comparison with well-watered plants, as observed previously in rice72, watermelon73, wheat74 and maize14. By day 3 of the present study, the plants under drought stress used a thermal dissipation mechanism (NPQ) to avoid excess energy expenditure. Thermal dissipation by the xanthophyll cycle, which is activated in response to the pH gradient formed by the cyclic electron cycle75,76, is known to be an initial response to abiotic stress and permits the regulation of the amount of excitation energy directed to the reaction centers of the photosystems77,78. Higher NPQ rates are important early protection mechanisms for the photosystem, as they avoid the effects of photo-oxidative stress on the photosynthetic photochemical protein complex. The increase in NPQ was efficient in the presence of AMF, especially in plants of the Desafio cultivar, even after 7 days of exposure to drought stress.
The exposure of plants to drought stress can cause an excess of energy expenditure that is not devoted to the photosynthetic process. This results in the increased production of reactive oxygen species (ROS), which promotes the peroxidation of lipids, proteins, and chloroplast pigments79,80. There was also a reduction in the concentration of photosynthetic pigments, in particular chlorophyll a, which also acts on the photosystem reaction centers. In the present study, drought stress contributed to a reduction in the photosynthetic pigments of the soybean plants, although this damage was mitigated in the plants inoculated with AMF. These results are consistent with the findings of Baslam and Goicoechea81, who observed that the association of the AMF Rhizophagus intraradices (Glomus intraradices) and Funneliformis mosseae associated with Lactuca sativa plants resulted in an increase in the chlorophyll and carotenoid content, even after exposure to drought stress. In addition, R. clarus and a mixture of AMF spores (including those of R. clarus) induced an increase in the activity of antioxidant enzymes and the concentration of malondialdehyde in strawberry82 and soybean plants83 under drought conditions, which may also contribute to the avoidance of oxidative stress and the degradation of pigments.
The impairment of the photochemical stage, associated with the oxidative stress caused by drought stress, may limit plant growth. Under unfavorable water conditions, the plant tends to initially expands its root system to increase the area of contact with the soil12,84,85. This compromises aerial growth, including the development of new leaves and shoots86. In the present study, even after a short period of stress, it was possible to observe a reduction in the vegetative growth of the soybean plants exposed to drought stress, which was mediated in part in the plants inoculated with R. clarus. Inoculation with Funneliformis geosporus and Funneliformis mosseae contributed to the growth of Fragaria ananassa plants under drought stress, as observed by Boyer et al.87. Oliveira et al.88 found an increase in the yield of Cicer arietinum following inoculation with the AMF Rhizophagus irregularis and the bacterium Mesorhizobium mediterraneum in the absence of drought conditions. Under drought stress, however, these authors observed an increase in plant biomass and the crude protein content of the grains in comparison with the control plants. Bernardo et al.89 found that wheat plants (Triticum spp.) inoculated with Funneliformis mosseae (Glomus mosseae) accumulated more dry matter in the aerial parts under drought stress than the noninoculated plants. Plant growth occurs through the accumulation of nutrients, which is enhanced by the interaction between the soybean plants and the AMF. Under drought stress, nutrients may be accumulated in or adsorbed by the roots, due to the reduction in the flow of mass that is normally promoted by transpiration. When associated with AMF, plants increase the efficiency of their water use, which permits the transfer of nutrients to the shoots. The symbiosis between AMF and legumes may be relevant to the rhizobia nodulation of N2-fixing bacteria. This interaction may be responsible for nutrient recycling and favor nutrient uptake67, as well as improving the tolerance of abiotic stress90. Rhizophagus clarus is also known to increase the effectiveness of chemical fertilizers in soybean plants under field conditions, and to increase the P and N contents in inoculated plants91. This is an economically important finding, given that it would favor a reduction in the application of fertilizers to farm crops.
Overall, inoculation with R. clarus increased the capacity of soybean plants to tolerate drought stress, by modifying their metabolism to permit the maintenance of or even an increase in their development under moderate drought conditions. The potential mitigation of the effects of drought stress by R. clarus provides important insights for the development of further research, including field experiments to verify the response of the plants under natural conditions, considering the potential occurrence of multiple abiotic stressors, to support the development of more efficient agricultural practices in regions subject to moderate water stress.
Conclusions
The arbuscular mycorrhizal fungus Rhizophagus clarus supported the maintenance of the water status of Anta82, a drought-sensitive soybean cultivar, mitigating the negative effects of drought stress on the photosynthetic apparatus of this plant. In the case of the Desafio cultivar, which is moderately drought-tolerant, greater colonization by Rhizophagus clarus increased the concentration of photosynthetic pigments and improved the physiological performance of the plant and its growth. These data indicate that inoculation with Rhizophagus clarus is a potentially important tool for the improvement of soybean yields, especially in regions with low precipitation that are subject to drought.
Materials and methods
Plant material and experimental conditions
The experiment was conducted in a climated-controlled greenhouse (~ 27 °C and relative humidity of ~ 75%) at the Laboratory of Ecophysiology and Plant Productivity at the Rio Verde campus of the Goiano Federal Institute of Science and Technology in Rio Verde, Goiás, Brazil. In the greenhouse, soybean plants (Glycine max (L.) Merrill) of two cultivars—Anta82 RR (Anta82; Geneze Seeds, São Paulo, Brazil), which is drought-sensitive, and the moderately drought-tolerant Desafio 8473 RSF (Desafio; Brasmax Sementes, Cambé, PR, Brazil)—were grown in 3-dm3 pots. Each pot contained a mixture (2:1) of Red Latosol (LVdf), which is the typical soil of the Brazilian Cerrado savanna, and sand, which had been corrected to 60% of base saturation with PNRT 100 dolomitic limestone. This substrate was fertilized based on the results of the chemical analysis (Supplementary Table S1) and the recommendation for Cerrado soils92.
The arbuscular mycorrhizal fungus (AMF) Rhizophagus clarus was donated by the germplasm bank of the Soil Microbiology Laboratory at the Ilha Solteira campus of São Paulo State University (UNESP) in Ilha Solteira, São Paulo, Brazil. To multiply the inoculum, the sand/soil (2:1) substrate was first placed in cotton bags, which were sterilized in an autoclave at 1.5 atm at a temperature of 121 °C for two hours. The substrate was then dried in an oven at 105 °C for 24 h, and rehydrated with distilled and sterilized water for 24 h93,94. Each plastic pot (1 dm3) containing sterilized substrate received approximately 10 g of the AMF inoculum together with Sorghum bicolor seeds, which acted as host plants. After 3 months, the soil was collected and stored in an ultrafreezer prior to use in the experiment. The density of spores was determined by placing a sample of the substrate on an acrylic plate with concentric rings, and examined under a SteREO Discovery.V8 stereomiscroscope (Zeiss, Göttingen, Germany) to count the spores, following Gerdemann and Nicolson95 and Jenkins96. The spores were stored in an ultrafreezer. For the experiment, each seeding hole in the 3-dm3 pots was inoculated with 10 g of the inoculum containing 3.5 g of R. clarus spores during the sowing of the soybean97.
The experimental was based on a random-block design, with four replicates. Each pot containing four plants was considered to be an experimental unit. The experimental water treatment (drought stress) was applied when the plants reached vegetative developmental stage V3, approximately 30 days after germination, when the water-holding capacity (WHC) was maintained at 60%, based on the gravimetric method. The plants in the control treatment (well-watered) were maintained at 100% WHC. The treatments were as follows: well-watered (WW); well-watered and inoculated with R. clarus (AMF); drought stress (DS); and DS plants inoculated with R. clarus (DS + AMF). The plants in all four groups were evaluated on days 3 and 7 after the initiation of the treatment.
Mycorrhizal colonization rate
Mycorrhizal colonization rates were determined following Koskey and Gemma98. Root samples (~ 0.4 g), were collected and stored in 50% ethanol. For analysis, the samples were initially immersed in 2% KOH, heated in a stove at 90 °C for 120 min, and then washed in distilled water. The samples were then immersed in 1% HCl for 30 min, washed in water, and stained with 0.05% trypan blue in a lactoglycerol solution (1:1:1 lactic acid, glycerol, water) at 90 °C for 10 min99. The root fragments were mounted on microscope slides and observed at a magnification of 200 × under an Olympus BX61 microscope (Tokyo, Japan) to determine the percentage of root volume colonized by the fungus100.
Water potential
The predawn leaf water potential (Ψw) was measured using a Scholander 3005–1414 pressure chamber (Soil moisture Equipment Corp., Goleta, CA, USA).
Physiological traits
Gas exchange was measured in fully expanded leaves to determine the net photosynthetic assimilation rate (A, µmol CO2 m−2 s−1), stomatal conductance (gs, mol H2O m−2 s−1), transpiration rate (E, mmol H2O m−2 s−1), and the ratio between internal and external CO2 concentrations (Ci/Ca). The measurements were obtained using an Infrared Gas Analyzer (IRGA; LI-6400xt, Licor, Lincoln, NE, USA). The instantaneous water-use efficiency (WUE, in µmol CO2 mmol−1 H2O) was calculated as the ratio between A and E. All measurements were obtained under a constant photosynthetic photon flux density (PFFD, 1000 µmol photons m–2 s −1) and at the ambient atmospheric CO2 concentration (Ca, ~ 400 µmol mol−1), temperature (~ 25 °C), and relative humidity (~ 50%).
Chlorophyll a fluorescence
The fluorescence of chlorophyll was evaluated using a 6400–40 LCF fluorometer coupled to the IRGA. The leaves were initially acclimated in the dark for 40 min to obtain the minimum (F0) and maximum chlorophyll fluorescence (Fm) values and to calculate the maximum quantum yield of photosystem II (PSII) [Fv/Fm = (Fm − F0)/Fm]. The leaf tissue was then exposed to actinic light and a saturating pulse to obtain the steady-state fluorescence (F) and the maximum fluorescence in a light-adapted state (Fm′), respectively. This permitted the determination of the effective quantum yield of PSII [YII = (Fm′ − F)/Fm′], and non-photochemical quenching [NPQ = (Fm − Fm′)/Fm′]. The YII values were used to calculate the electron transport rate (ETR = YII.PFFD.LeafABS.0.5), where PFFD is the photon flow (µmol m−2 s−1) in the leaves, LeafABS is the fraction of incident light that is absorbed by the leaves, and 0.5 is the excitation energy fraction directed to PSII.
The photosynthetic pigments were extracted from the leaf discs (~ 2-cm2) immersed in dimethyl sulfoxide solution with calcium carbonate (40 g L−1) in a water bath at 65 °C. After 24 h, the solution absorbance was read at 480.0, 649.1, and 665.1 nm using an Evolution 60S UV–VIS spectrophotometer (Thermo Fisher Scientific, Madison, WI, USA). Chlorophyll a (Chla = 12.4.A665.1 – 3.62.A649.1), chlorophyll b (Chlb = 25.06.A649.1 – 6.50.A665.1), and total carotenoids (Carot = [1000A480 – 1.29 Chla – 53.78Chlb]/220) were calculated following Wellburn101 and the pigment concentrations were expressed as µg cm−2. Total chlorophyll was obtained by summing Chla and Chlb.
Morphological traits and nutrient content
The plants were measured to determine the plant height (PH, cm) and stem diameter (SD, mm). The leaves were separated out to obtain the leaf area (LA, cm2). The leaves and roots were dried to a constant weight at 65 °C in a forced-air circulating oven to obtain the leaf dry matter (LDM, g) and root dry matter (RDM, g).
To quantify the nutrient content of the leaves and roots, the content of the dry material (~ 500 mg) was extracted by nitric-perchloric (3:1) digestion and analysed following Embrapa102. Nitrogen (N) was measured by the Kjeldahl titration method using a nitrogen distiller (TE-0364, Tecnal, Piracicaba, Brazil). Phosphorus (P) was determined by the molybdenum blue method, and sulfur (S) was determined by the barium chloride turbidity approach, using molecular absorption spectrophotometry (SP1105, Tecnal, Piracicaba, Brazil). Potassium (K) was analyzed using flame photometry (B462, Tecnal, Piracicaba, Brazil) and calcium (Ca) and magnesium (Mg) were determined using atomic absorption spectrophotometry (SavantAA, GBC Scientific Equipment, Braeside, Australia).
Statistical analysis
The variation in the data were evaluated using analysis of variance (ANOVA) and pairs of means were compared using Tukey’s post hoc test (p < 0.05). These analyses were run in SISVAR software (v. 5.6, Lavras, MG, Brazil).
Principal components analyses (PCA) were run on the whole dataset, and for each cultivar separately. These analyses were run in the FactoMineR103 and factoextra104 packages in R software105. The data were first scaled using the scale function and then analyzed using the PCA function. The eigenvalues were evaluated to determine the number of axes that should be evaluated. We used the screen plot to visualize the data, based on Cattell's rule, which states that the components that correspond to the eigenvalues to the left of the straight line (eigenvalues lying on the straight line correspond to random variation) should be retained106. To better understand the variables represented in each component, we used the fviz_contrib and dimdesc functions, which determine the contribution of the variable to each component and its correlation with the component, respectively.
Ethics approval and consent to participate
Manuscripts reporting on studies do not involve any human participants, human data, or the analysis of human tissue are exempt from these considerations.
Complies with international, national and/or institutional guidelines
The experimental research reported here complies with all the relevant institutional, national, and international guidelines and legislation.
Supplementary Information
Acknowledgements
This work was supported financially by the National Council for Scientific and Technological Development (CNPq, grant nos. 552689/2011-4 to A.C.C. and 406197/2016-4 to G.C.M.), the Goiás Federal Institute of Education, Science and Technology (IFGoiano-RV, grant no. DPPG 031/2019), and the Santa Catarina Federal Institute of Education, Science and Technology (IFSC-Lages). T.C.O. and C.M. are grateful to the Brazilian Coordination for Higher Education Personnel Training (CAPES), while L.R..S and G.G.T. thank IFGoiano, and L.D.S.S. is grateful to CNPq, for the scholarships conceded. The authors also thank Dr. Stephen Ferrari for language editing.
Author contributions
G.C.M., C.M., E.L.S., and J.S.R.C. designed and supervised the research. T.C.O., J.S.R.C., L.R.S., G.G.T., L.D.S.S., G.C.M., and C.M. prepared the material and collected the data. T.C.O., J.S.R.C., T.P.P., and A.C.C. analyzed the data, and F.G.S. collaborated on the physiological analyses of plants. The first draft of the manuscript was written by T.C.O. with contributions from G.C.M., C.M., J.S.R.C., T.P.P., and E.L.S. G.C.M. and C.M. revised and edited the manuscript substantially. All the authors read and approved the final version of the manuscript.
Data availability
All the data generated or analyzed during the present study are included in this published manuscript. The raw datasets are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13059-7.
References
- 1.Grassini P, et al. Soybean, Chapter 8. In: Sadras VO, Calderini DF, et al., editors. Crop Physiology Case Histories for Major Crops. Elsevier Inc.; 2021. pp. 282–319. [Google Scholar]
- 2.Bragagnolo FS, Funari CS, Ibáñez E, Cifuentes A. Metabolomics as a tool to study underused soy parts: In search of bioactive compounds. Foods. 2021;10:1308. doi: 10.3390/foods10061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittencourt G, et al. Soybean hulls as carbohydrate feedstock for medium to high-value biomolecule production in biorefineries: A review. Bioresour. Technol. 2021;339:125594. doi: 10.1016/j.biortech.2021.125594. [DOI] [PubMed] [Google Scholar]
- 4.Habibzadeh Y. Arbuscular mycorrhizal fungi in alleviation of drought stress on grain yield and yield components of mungbean (Vigna radiata L.) plants. Int. J. Sci. 2015;4:34–40. [Google Scholar]
- 5.Cera JC, et al. Soybean yield in future climate scenarios for the state of Rio Grande do Sul, Brazil. Pesqui. Agropecu. Bras. 2017;52:380–392. doi: 10.1590/s0100-204x2017000600002. [DOI] [Google Scholar]
- 6.Rising J, Devineni N. Crop switching reduces agricultural losses from climate change in the United States by half under RCP 8.5. Nat. Commun. 2020;11:1–7. doi: 10.1038/s41467-020-18725-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao C, et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA. 2017;114:9326–9331. doi: 10.1073/pnas.1701762114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IPCC-Intergovernmental Panel on Climate Change . Climate Change 2013: The PHYSICAL Science Basis. Contribution of Working Group I to the Fifth Assessment Report. Cambridge University Press; 2013. [Google Scholar]
- 9.Batista PF, et al. Biochemical and physiological impacts of zinc sulphate, potassium phosphite and hydrogen sulphide in mitigating stress conditions in soybean. Physiol. Plant. 2020;168:456–472. doi: 10.1111/ppl.13034. [DOI] [PubMed] [Google Scholar]
- 10.Bharath P, Gahir S, Raghavendra AS. Abscisic acid-induced stomatal closure: An important component of plant defense against abiotic and biotic stress. Front. Plant Sci. 2021;12:615114. doi: 10.3389/fpls.2021.615114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Müller C, Hodecker BER, Barros NF, Merchant A. A physiological approach for pre-selection of eucalyptus clones resistant to drought. IForest. 2020;13:16–23. doi: 10.3832/ifor3185-012. [DOI] [Google Scholar]
- 12.Fang Y, et al. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017;8:1–14. doi: 10.3389/fpls.2017.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oliveira TC, et al. Production of soybean in association with the arbuscular mycorrhizal fungi Rhizophagus clarus cultivated in field conditions. Rev. Ciencias Agroveterinarias. 2019;18:530–535. doi: 10.5965/223811711832019530. [DOI] [Google Scholar]
- 14.Hu Y, Xie W, Chen B. Arbuscular mycorrhiza improved drought tolerance of maize seedlings by altering photosystem II efficiency and the levels of key metabolites. Chem. Biol. Technol. Agric. 2020;7:1–14. doi: 10.1186/s40538-020-00186-4. [DOI] [Google Scholar]
- 15.Cheng HQ, Zou YN, Wu QS, Kuča K. Arbuscular mycorrhizal fungi alleviate drought stress in trifoliate orange by regulating H+-ATPase activity and gene expression. Front. Plant Sci. 2021;12:659694. doi: 10.3389/fpls.2021.659694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latef AAHA, et al. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016;59:407–426. doi: 10.1007/s12374-016-0237-7. [DOI] [Google Scholar]
- 17.Yang Y, He C, Huang L, Ban Y, Tang M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE. 2017;12:e0182264. doi: 10.1371/journal.pone.0182264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang WX, et al. Nutrient exchange and regulation in Arbuscular mycorrhizal symbiosis. Mol. Plant. 2017;10:1147–1158. doi: 10.1016/j.molp.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Matos PF, et al. Beneficial services of glomalin and arbuscular mycorrhizal fungi in degraded soils in Brazil. Sci. Agric. 2022;79:e20210064. doi: 10.1590/1678-992x-2021-0064. [DOI] [Google Scholar]
- 20.Liu CY, et al. Mycorrhiza stimulates root-hair growth and IAA synthesis and transport in trifoliate orange under drought stress. Sci Rep. 2018;8:1978. doi: 10.1038/s41598-018-20456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsoata E, Njock SR, Youmbi E, Nwaga D. Early effects of water stress on some biochemical and mineral parameters of mycorrhizal Vigna subterranea (L.) Verdc. (Fabaceae) cultivated in Cameroon. Int. J. Agron. Agric. Res. 2015;7:21–35. [Google Scholar]
- 22.Wang W. Glomalin contributed more to carbon, nutrients in deeper soils, and differently associated with climates and soil properties in vertical profiles. Sci. Rep. 2017;7:13003. doi: 10.1038/s41598-017-12731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ingraffia R, Amato G, Frenda AS, Giambalvo D. Impacts of arbuscular mycorrhizal fungi on nutrient uptake, N2 fixation, N transfer, and growth in a wheat/faba bean intercropping system. PLoS ONE. 2019;14:1–16. doi: 10.1371/journal.pone.0213672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kameoka H, Maeda T, Okuma N, Kawaguchi M. Structure-specific regulation of nutrient transport and metabolism in arbuscular mycorrhizal fungi. Plant Cell Physiol. 2019;60:2272–2281. doi: 10.1093/pcp/pcz122. [DOI] [PubMed] [Google Scholar]
- 25.Kohler J, Roldán A, Campoy M, Caravaca F. Unraveling the role of hyphal networks from arbuscular mycorrhizal fungi in aggregate stabilization of semiarid soils with different textures and carbonate contents. Plant Soil. 2017;410:273–281. doi: 10.1007/s11104-016-3001-3. [DOI] [Google Scholar]
- 26.Bitterlich M, Sandmann M, Graefe J. Arbuscular mycorrhiza alleviates restrictions to substrate water flow and delays transpiration limitation to stronger drought in tomato. Front. Plant Sci. 2018;9:1–15. doi: 10.3389/fpls.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Badr MA, El-Tohamy WA, Abou-Hussein SD, Gruda NS. Deficit irrigation and arbuscular mycorrhiza as a water-saving strategy for eggplant production. Horticulturae. 2020;6:1–17. doi: 10.3390/horticulturae6030045. [DOI] [Google Scholar]
- 28.Etemadi M, et al. Auxin perception is required for arbuscule development in arbuscular mycorrhizal symbiosis. Plant Physiol. 2014;166:281–292. doi: 10.1104/pp.114.246595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Querejeta JI, Egerton-Warburton LM, Prieto I, Vargas R, Allen MF. Changes in soil hyphal abundance and viability can alter the patterns of hydraulic redistribution by plant roots. Plant Soil. 2012;355:63–73. doi: 10.1007/s11104-011-1080-8. [DOI] [Google Scholar]
- 30.Worrich A, et al. Mycelium-mediated transfer of water and nutrients stimulates bacterial activity in dry and oligotrophic environments. Nat. Commun. 2017;8:15472. doi: 10.1038/ncomms15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu QS, Xia RX. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J. Plant Physiol. 2006;163:417–425. doi: 10.1016/j.jplph.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Igiehon ON, Babalola OO. Rhizobium and mycorrhizal fungal species improved soybean yield under drought stress conditions. Curr. Microbiol. 2021;78:1615–1627. doi: 10.1007/s00284-021-02432-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YM, et al. Mycorrhizal-induced calmodulin mediated changes in antioxidant enzymes and growth response of drought-stressed trifoliate orange. Front. Microbiol. 2014;5:682. doi: 10.3389/fmicb.2014.00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Begum N, et al. Arbuscular mycorrhizal fungi improve growth, essential oil, secondary metabolism, and yield of tobacco (Nicotiana tabacum L.) under drought stress conditions. Environ. Sci. Pollut. Res. 2021;28:45276–45295. doi: 10.1007/s11356-021-13755-3. [DOI] [PubMed] [Google Scholar]
- 35.Bahadur A, et al. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019;20:4199. doi: 10.3390/ijms20174199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson NC, Gibson KS. Understanding multilevel selection may facilitate management of arbuscular mycorrhizae in sustainable agroecosystems. Front. Plant Sci. 2021;11:627345. doi: 10.3389/fpls.2020.627345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaefer DA, Gui H, Mortimer PE, Xu J. Arbuscular mycorrhiza and sustainable agriculture. Circ. Agric. Syst. 2021;1:1–7. [Google Scholar]
- 38.Schübler A, Walker C. The Glomeromycota. A Species List with New Families and New Genera. The Royal Botanic Garden, Botanische Staatssammlung Munich, Oregon State University; 2010. [Google Scholar]
- 39.Omirou M, Ioannides IM, Ehaliotis C. Mycorrhizal inoculation affects arbuscular mycorrhizal diversity in watermelon roots, but leads to improve colonization and plant response under water stress only. Appl. Soil Ecol. 2013;63:112–119. doi: 10.1016/j.apsoil.2012.09.013. [DOI] [Google Scholar]
- 40.INVAM: International Culture Collection of (Vesicular) Arbuscular Mycorrhizal Fungi. http://fungi.invam.wvu.edu. (West Virginia University, 2022).
- 41.Sugiura Y, et al. Myristate can be used as a carbon and energy source for the asymbiotic growth of arbuscular mycorrhizal fungi. PNAS. 2020;117:25779–25788. doi: 10.1073/pnas.2006948117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka S, et al. Asymbiotic mass production of the arbuscular mycorrhizal fungus Rhizophagus clarus. Commun. Biol. 2022;5:43. doi: 10.1038/s42003-021-02967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lenoir I, Fontaine J, Sahraoui AL-H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry. 2016;123:4–15. doi: 10.1016/j.phytochem.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Delavaux CS, et al. Mycorrhizal types influence island biogeography of plants. Commun. Biol. 2021;4:1–8. doi: 10.1038/s42003-021-02649-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Lozano JM, Aroca R. Host response to osmotic stresses: stomatal behaviour and water use efficiency of arbuscular mycorrhizal plants. In: Koltai H, Kapulnik Y, editors. Arbuscular Mycorrhizas: Physiology and Function. Springer; 2010. pp. 239–256. [Google Scholar]
- 46.Vasar M, et al. Arbuscular mycorrhizal fungal communities in the soils of desert habitats. Microorganims. 2021;9:229. doi: 10.3390/microorganisms9020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdalla M, Ahmed MA. Arbuscular mycorrhiza symbiosis enhances water status and soil plant hydraulic conductance under drought. Front. Plant Sci. 2021;12:722954. doi: 10.3389/fpls.2021.722954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Begum N, et al. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019;10:1–15. doi: 10.3389/fpls.2019.01068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peña Venegas RA, et al. The phosphate inhibition paradigm: Host and fungal genotypes determine arbuscular mycorrhizal fungal colonization and responsiveness to inoculation in cassava with increasing phosphorus supply. Front. Plant Sci. 2021;12:693037. doi: 10.3389/fpls.2021.693037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silva Júnior JMT, Mendes Filho PF, Gomes VFF, Almeida AMM, Garcia KGV. Morphological pattern of colonization by mycorrhizal fungi and the microbial activity observed in Barbados cherry crops. Ciência Rural. 2017;47:e20160660. doi: 10.1590/0103-8478cr20160660. [DOI] [Google Scholar]
- 51.Moreira SD, França AC, Rocha WW, Tibães ESR, Neiva Júnior E. Inoculation with mycorrhizal fungi on the growth and tolerance to water deficit of coffee plants. Rev. Bras. Eng. Agríc. Ambient. 2018;22:747–752. doi: 10.1590/1807-1929/agriambi.v22n11p747-752. [DOI] [Google Scholar]
- 52.Al-Amri SM. Application of bio-fertilizers for enhancing growth and yield of common bean plants grown under water stress conditions. Saudi J. Biol. Sci. 2021;28:3901–3908. doi: 10.1016/j.sjbs.2021.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruiz Sánchez M, et al. Categorization of the water status of rice inoculated with arbuscular mycorrhizae and with water deficit. Agron. Mesoam. 2021;32:339–355. doi: 10.15517/am.v32i2.42066. [DOI] [Google Scholar]
- 54.Duc NH, Csintalan Z, Posta K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018;132:297–307. doi: 10.1016/j.plaphy.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 55.Tereucán G, et al. Shifts in biochemical and physiological responses by the inoculation of arbuscular mycorrhizal fungi in Triticum aestivum growing under drought conditions. J. Sci. Food Agric. 2022;102:1927–1938. doi: 10.1002/jsfa.11530. [DOI] [PubMed] [Google Scholar]
- 56.Huang D, et al. Silencing MdGH3-2/12 in apple reduces drought resistance by regulating AM colonization. Hortic. Res. 2021;8:84. doi: 10.1038/s41438-021-00524-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilbert ME, Medina V. Drought adaptation mechanisms should guide experimental design. Trends Plant Sci. 2016;21:639–647. doi: 10.1016/j.tplants.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Tombesi S, et al. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Sci. Rep. 2015;5:1–12. doi: 10.1038/srep12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zou YN, et al. Mycorrhizal trifoliate orange has greater root adaptation of morphology and phytohormones in response to drought stress. Sci. Rep. 2017;7:1–10. doi: 10.1038/s41598-016-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Z, Zhang J, Xu G, Zhou L, Li Y. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Zenia insignis seedlings under drought stress. New For. 2018;50:593–604. doi: 10.1007/s11056-018-9681-1. [DOI] [Google Scholar]
- 61.Flexas J, Medrano H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002;89:183–189. doi: 10.1093/aob/mcf027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lavergne A, et al. Historical changes in the stomatal limitation of photosynthesis: Empirical support for an optimality principle. New Phytol. 2020;225:2484–2497. doi: 10.1111/nph.16314. [DOI] [PubMed] [Google Scholar]
- 63.Sakoda K, Yamori W, Groszmann M, Evans JR. Stomatal, mesophyll conductance, and biochemical limitations to photosynthesis during induction. Plant Physiol. 2021;185:146–160. doi: 10.1093/plphys/kiaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Quiroga G, Erice G, Aroca R, Chaumont F, Ruiz-Lozano JM. Contribution of the arbuscular mycorrhizal symbiosis to the regulation of radial root water transport in maize plants under water deficit. Environ. Exp. Bot. 2019;167:103821. doi: 10.1016/j.envexpbot.2019.103821. [DOI] [Google Scholar]
- 65.Cruz RS, Araújo FHV, França AC, Sardinha LT, Machado CMM. Physiological responses of Coffea arabica cultivars in association with arbuscular mycorrhizal fungi. Coffee Sci. 2020;15:e151641. [Google Scholar]
- 66.Ouledali S, Ennajeh M, Zrig A, Gianinazzi S, Khemira H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea) Acta Physiol. Plant. 2018;40:81. doi: 10.1007/s11738-018-2656-1. [DOI] [Google Scholar]
- 67.Azcón-Aguilar C, Barea JM. Nutrient cycling in the mycorrhizosphere. J. Soil Sci. Plant Nutr. 2015;15:372–396. [Google Scholar]
- 68.Zhou Q, Ravnskov S, Jiang D, Wollenweber B. Changes in carbon and nitrogen allocation, growth and grain yield induced by arbuscular mycorrhizal fungi in wheat (Triticum aestivum L.) subjected to a period of water déficit. Plant Growth Regul. 2015;75:751–760. doi: 10.1007/s10725-014-9977-x. [DOI] [Google Scholar]
- 69.Alemu ST. Photosynthesis limiting stresses under climate change scenarios and role of chlorophyll fluorescence: A review article. Cogent Food Agric. 2020;6:1785136. doi: 10.1080/23311932.2020.1785136. [DOI] [Google Scholar]
- 70.Dahal K, Vanlerberghe GC. Improved chloroplast energy balance during water deficit enhances plant growth: More crop per drop. J. Exp. Bot. 2018;69:1183–1197. doi: 10.1093/jxb/erx474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sade N, et al. Delaying chloroplast turnover increases water-deficit stress tolerance through the enhancement of nitrogen assimilation in rice. J. Exp. Bot. 2018;69:867–878. doi: 10.1093/jxb/erx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Porcel R, et al. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem II and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 2015;185:75–83. doi: 10.1016/j.jplph.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Mo Y, et al. Regulation of plant growth, photosynthesis, antioxidation and osmosis by an arbuscular mycorrhizal fungus in watermelon seedlings under well-watered and drought conditions. Front. Plant Sci. 2016;7:1–15. doi: 10.3389/fpls.2016.00644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathur S, Tomar RS, Jajoo A. Arbuscular Mycorrhizal fungi (AMF) protects photosynthetic apparatus of wheat under drought stress. Photosynth. Res. 2019;139:227–238. doi: 10.1007/s11120-018-0538-4. [DOI] [PubMed] [Google Scholar]
- 75.García-Plazaola JI, Hernández A, Artetxe U, Becerril JM. Regulation of the xanthophyll cycle pool size in duckweed (Lemna minor) plants. Physiol. Plant. 2002;116:121–126. doi: 10.1034/j.1399-3054.2002.1160115.x. [DOI] [PubMed] [Google Scholar]
- 76.Strand DD, et al. Activation of cyclic electron flow by hydrogen peroxide in vivo. Proc. Natl. Acad. Sci. USA. 2015;112:5539–5544. doi: 10.1073/pnas.1418223112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carbonera D, Gerotto C, Posocco B, Giacometti GM, Morosinotto T. NPQ activation reduces chlorophyll triplet state formation in the moss Physcomitrella patens. Biochim. Biophys. Acta Bioenergy. 2012;1817:1608–1615. doi: 10.1016/j.bbabio.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 78.Demmig-Adams B, Stewart JJ, López-Pozo M, Polutchko SK, Adams WW. Zeaxanthin, a molecule for photoprotection in many different environments. Molecules. 2020;25:5825. doi: 10.3390/molecules25245825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huseynova IM, et al. Drought-induced changes in photosynthetic apparatus and antioxidant components of wheat (Triticum durum Desf.) varieties. Photosynth. Res. 2016;130:215–223. doi: 10.1007/s11120-016-0244-z. [DOI] [PubMed] [Google Scholar]
- 80.Sachdev S, Ansari SA, Ansari MI, Fujita M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants. 2021;10:277. doi: 10.3390/antiox10020277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baslam M, Goicoechea N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza. 2012;22:347–359. doi: 10.1007/s00572-011-0408-9. [DOI] [PubMed] [Google Scholar]
- 82.Moradtalab N, Hajiboland R, Aliasgharzad N, Hartmann TE, Neumann G. Silicon and the association with an arbuscular-mycorrhizal fungus (Rhizophagus clarus) mitigate the adverse effects of drought stress on strawberry. Agronomy. 2019;9:41. doi: 10.3390/agronomy9010041. [DOI] [Google Scholar]
- 83.Sheteiwy MS, et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021;21:195. doi: 10.1186/s12870-021-02949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Comas LH, Becker SR, Cruz VMV, Byrne PF, Dierig DA. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013;4:1–16. doi: 10.3389/fpls.2013.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lozano YM, Aguilar-Trigueros CA, Flaig IC, Rillig MC. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 2020;34:2224–2235. doi: 10.1111/1365-2435.13656. [DOI] [Google Scholar]
- 86.Jin K, et al. Wheat root growth responses to horizontal stratification of fertiliser in a water-limited environment. Plant Soil. 2015;386:77–88. doi: 10.1007/s11104-014-2249-8. [DOI] [Google Scholar]
- 87.Boyer LR, Brain P, Xu XM, Jeffries P. Inoculation of drought-stressed strawberry with a mixed inoculum of two arbuscular mycorrhizal fungi: Effects on population dynamics of fungal species in roots and consequential plant tolerance to water deficiency. Mycorrhiza. 2015;25:215–227. doi: 10.1007/s00572-014-0603-6. [DOI] [PubMed] [Google Scholar]
- 88.Oliveira RS, et al. Increased protein content of chickpea (Cicer arietinum L.) inoculated with arbuscular mycorrhizal fungi and nitrogen fixing bacteria under water deficit conditions. J. Sci. Food Agric. 2017;97:4379–4385. doi: 10.1002/jsfa.8201. [DOI] [PubMed] [Google Scholar]
- 89.Bernardo L, et al. Proteomic insight into the mitigation of wheat root drought stress by arbuscular mycorrhizae. J. Proteomics. 2017;169:21–32. doi: 10.1016/j.jprot.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 90.Sun Z, Song J, Xin X, Xie X, Zhao B. Arbuscular mycorrhizal fungal 14-3-3 proteins are involved in arbuscule formation and responses to abiotic stresses during AM symbiosis. Front. Microbiol. 2018;9:1–17. doi: 10.3389/fmicb.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cely MV, et al. Inoculant of arbuscular mycorrhizal fungi (Rhizophagus clarus) increase yield of soybean and cotton under field conditions. Front. Microbiol. 2016;7:720. doi: 10.3389/fmicb.2016.00720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sousa, D. M. G. & Lobato, E. Correção do solo e adubação da cultura da soja. Planaltina: EMBRAPA-CPAC. Vol. 33, (1996).
- 93.Souza, F. A. Banco Ativo de Glomales da Embrapa Agrobiologia: catalogação e introdução de novos isolados desde 1995. Seropédica. Documentos, Embrapa Agrobiologia, Vol. 123, (2000).
- 94.Saggin-Junior OJ, Borges WL, Novais CB, Silva EMR. Manual de curadores de germoplasma—micro-organismos: fungos micorrízicos arbusculares. Embrapa Recursos Genéticos e Biotecnologia; 2011. [Google Scholar]
- 95.Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans. Br. Mycol. Soc. 1963;46:235–244. doi: 10.1016/S0007-1536(63)80079-0. [DOI] [Google Scholar]
- 96.Jenkins WR. A rapid centrifugal—flotation technique for separating nematodes from soil. Plant Dis. Report. 1964;48:692. [Google Scholar]
- 97.Tiepo AN, et al. Enhanced drought tolerance in seedlings of Neotropical tree species inoculated with plant growth-promoting bacteria. Plant Physiol Biochem. 2018;130:277–288. doi: 10.1016/j.plaphy.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 98.Koskey RE, Gemma JN. A modified procedure for staining roots to detect VA mycorrhizas. Mycol. Res. 1989;92:486–488. doi: 10.1016/S0953-7562(89)80195-9. [DOI] [Google Scholar]
- 99.Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970;55:158-IN18. doi: 10.1016/S0007-1536(70)80110-3. [DOI] [Google Scholar]
- 100.McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA. A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytol. 1990;115:495–501. doi: 10.1111/j.1469-8137.1990.tb00476.x. [DOI] [PubMed] [Google Scholar]
- 101.Wellburn AR. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994;144:307–313. doi: 10.1016/S0176-1617(11)81192-2. [DOI] [Google Scholar]
- 102.Empresa Brasileira De Pesquisa Agropecuária – EMBRAPA . Manual de análises químicas de solos, plantas e fertilizantes. 2. Informação Tecnológica; 2009. [Google Scholar]
- 103.Le S, Josse J, Husson F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008;25:1–18. doi: 10.18637/jss.v025.i01. [DOI] [Google Scholar]
- 104.Kassambara, A. & Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7. https://CRAN.R-project.org/package=factoextra (2020).
- 105.R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org/. (R Foundation for Statistical Computing, 2021).
- 106.Cattell R. The scree test for the number of factors. Multivariate Behav. Res. 1966;1:245–276. doi: 10.1207/s15327906mbr0102_10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analyzed during the present study are included in this published manuscript. The raw datasets are available from the corresponding author on reasonable request.