Abstract
Purpose
To inquire into clinical practices perceived to mitigate patients’ intraoperative distress during awake craniotomies.
Methods
This mixed-methods study involved administration of Amsterdam Preoperative Anxiety and Information Scale and PTSD Checklist prior to the awake craniotomy to evaluate anxiety and information-seeking related to the procedure and symptoms of PTSD. Generalized Anxiety Disorder Scale and Depression Module of the Patient Health Questionnaire were administered before and after the procedure to evaluate generalized anxiety and depression. Patient interviews were conducted 2-weeks postprocedure and included a novel set of patient experience scales to assess patients’ recollection of intraoperative pain, overall distress, anxiety, distress due to noise, perception of empowerment, perception of being well-prepared, overall satisfaction with anaesthesia management, and overall satisfaction with the procedure. Qualitative data were analysed using conventional content analysis.
Results
Participants (n = 14) had undergone an awake craniotomy for tissue resection due to primary brain tumours or medically-refractory focal epilepsy. Validated self-report questionnaires demonstrated reduced levels of generalized anxiety (pre mean = 8.66; SD = 6.41; post mean=4.36; SD = 4.24) following the awake craniotomy. Postprocedure interviews revealed very high satisfaction with the awake craniotomy and anaesthesia management and minimal levels of intraoperative pain, anxiety, and distress. The most stressful aspects of the procedure included global recognition of medical diagnosis, anxiety provoked by unfamiliar sights, sounds, and sensations, a perception of a lack of information or misinformation, and long periods of immobility. Important factors in alleviating intraoperative distress included the medical team’s ability to promote patient perceptions of control, establish compassionate relationships, address unfamiliar intraoperative sensations, and deliver effective anaesthesia management.
Conclusion
Compassion, communication, and patient perception of control were critical in mitigating intraoperative distress. Clinical practice recommendations with implications for all clinicians involved in patient care during awake craniotomies are provided. Use of these interventions and strategies to reduce distress are important to holistic patient care and patient experiences of care and may improve the likelihood of optimal brain mapping procedures to improve clinical outcomes during awake craniotomies.
Keywords: Awake craniotomy, intracranial mapping, functional localization, patient-centred care, qualitative research
Introduction
Awake craniotomies (ACs) are a well-established and safe technique to maximize lesion resection while optimizing postoperative function.1–4 During ACs, intraoperative functional mapping using direct electrical stimulation (DES) affords a real-time investigation of cortical and white matter function. The objective of DES is to mimic a transient lesion by disrupting a neural subcircuit for a few seconds.5 During this stimulation, cognitive testing can be conducted to assess for associated disruption in cognitive function with the purpose of localizing function to predict potential deficits that might result from resection of the stimulated area. Cognitive testing can also be conducted without DES throughout the resection, providing surgeons with continuous real-time feedback regarding patients’ cognitive processing and informing intraoperative decision-making. Patients undergoing an AC for lesion resection, when compared to those who receive general anaesthesia, have fewer postoperative neurological and cognitive deficits, shorter hospital stays, and lower total inpatient costs.2,3,6,7
The success of intraoperative functional mapping during ACs requires a high level of patient participation during a lengthy surgical procedure, and this prolonged patient involvement comes with challenges.8,9 The patient is exposed to a range of preoperative and intraoperative stressors, including the recent diagnosis of a potentially malignant brain tumour, hospital admittance, and the intraoperative discomfort and distress that may occur due to clamp head fixation, immobility, cranial drilling, evoked seizures, and loss of functioning during DES mapping.10 Though previous studies indicate that ACs are generally well-tolerated by patients, it is also reported that up to 20–50% of patients report moderate to severe intraoperative distress,11,12 10–15% report severe intraoperative anxiety,13,14 and up to 12% report sequel symptoms of post-traumatic stress disorder (PTSD). Intraoperative distress, specifically anxiety and fear, can reduce a patient’s ability to collaborate and impede optimal brain mapping procedures, and may result in a failed AC in which the patient must be converted to an asleep procedure.15,16
To increase optimal brain mapping during ACs, and therefore improve clinical outcomes, intraoperative distress should be proactively minimized. Understanding the patient’s perspective is key to achieving this clinical objective.17 To date, however, there are no studies that have reported patients’ perspectives on clinical practices for alleviating intraoperative distress. To fill this knowledge gap, we conducted a mixed-methods study using patient-reported outcome measures to explore the subjective experiences of patients undergoing ACs, and specifically inquired into clinical practices and processes that were perceived to mitigate intraoperative distress.
Methods
Design
This mixed-methods study involved the administration of patient-reported outcome measures both before and after AC, as well as semi-structured interviews conducted with patients two weeks after AC. Participant recruitment and data collection occurred at Oregon Health & Science University from January 2018 to January 2019. Data collection ended after 14 postoperative interviews.
Participants
Study participation was offered to all patients between the ages of 18 and 89 who were scheduled for an AC with author AR or SJH for tissue resection due to primary brain tumours or medically-refractory focal epilepsy. Patients were informed about the study during a routine preoperative neuropsychological evaluation conducted one to four days prior to the AC. Individuals who identified as a member of a vulnerable population (e.g. pregnant women, decisionally-impaired adults, prisoners) or patients unavailable for a follow-up interview were excluded from participation. All participants provided written informed consent via a process approved by the Oregon Health & Science University Institutional Review Board. No compensation was provided.
Procedures
Preoperative evaluation
A clinical psychologist (CP; author DDC) or a speech-language pathologist (SLP; author BP) conducted a cognitive evaluation one to four days prior to the surgical procedure. Preoperative evaluations established baseline performance in expressive and receptive language, attention and concentration, auditory and visual working memory, visual-spatial and visuo-constructional processing, executive functioning, processing speed, calculation, visual field loss, and colour blindness. The clinical interview included a battery of mental health screeners for anxiety, depression, PTSD, and procedure-related anxiety and information-seeking, as well as an assessment of risk factors for failed AC (Table 1). During the evaluation, the clinician described AC and intraoperative mapping procedures and administered the testing paradigms to be used during intraoperative functional mapping. The CP or SLP described the goals of the medical team, including ensuring the patient’s comfort, clear communication, minimal movement, and optimal mapping and monitoring, and explained the patient’s role in accessing control and empowerment during the procedure through communication. The clinician and patient discussed stress management techniques (e.g. slow breathing, distraction, visualization, prayer) to be used during the procedure, and patients’ priorities for post-procedure outcomes (e.g. to play the guitar, preserve or improve speech or language, maintain independent ambulation to allow walks in the woods with a spouse, or live long enough to see a child graduate from high school). The preoperative evaluation provided an opportunity for the CP or SLP to establish a therapeutic rapport with the patient, with the goals of increasing both the patient’s comfort with the procedure and the probability of optimal mapping and monitoring.30,31
Table 1.
Preoperative evaluation battery.
| Tool | Function or characteristic assessed |
|---|---|
| Edinburgh Handedness Inventory – Short Form18 | Handedness |
| Boston Diagnostic Aphasia Examination – Short Form19 | Conversational speech, auditory comprehension, oral expression, reading, confrontational naming |
| Pyramids and Palm Trees Test20 | Semantic appreciation and reasoning |
| Montreal Cognitive Assessment21 | General cognitive function |
| Forward, backward, and sequence digit span tasks from the Wechsler Adult Intelligence Scale – IV22 | Auditory working memory |
| Trail Making Test, Parts A and B23 | Visual attention, processing speed, executive function |
| Navon Figures24 | Visual processing |
| Line Bisection Test25 | Visual processing |
| Depression module of the Patient Health Questionnaire (PHQ-9)26 | Depression |
| Generalized Anxiety Disorder (GAD-7)27 | Generalized anxiety |
| Amsterdam Preoperative Anxiety and Information Scale (APAIS)28 | Preoperative anxiety and need-for-information regarding the anaesthetic and surgical procedure |
| Abbreviated PTSD Checklist – Civilian (PCL-C), 6-item version29 | Symptoms related to post-traumatic stress disorder |
| Clinical Interview | Patients’ self-reported strengths, interests, and social supports Frequently used stress management strategies Description of chronic pain history, if present Patients’ post-operative priorities |
A summary of the evaluation results, along with additional information obtained from a review of the patient’s medical records, was provided to the surgical team prior to the procedure, including neurosurgeons, the neuro-monitoring team, stereotactic surgery coordinators, operating room nurses, and attending anaesthesiologists. The summary informed the medical team’s decision regarding the propriety of the patient as a candidate for an AC vs. a conventional craniotomy under general anaesthesia, provided valuable data for the anaesthesiologists to tailor the anaesthetic plan to a given patient, and apprised the team of important individual differences that may affect the procedure (e.g. trauma history, cognitive or sensory impairment, post-surgical priorities, previous experiences during an AC, preferred stress management techniques, and personal strengths and interests).32,33
Surgical procedure
Our group’s protocol does not include administration of general anaesthesia with endotracheal intubation to protect the airway and control of ventilation to regulate arterial PCO2. Instead, patients were variably sedated by a combination of intravenous infusions of sedative hypnotic agent dexmetetomidine with or without propofol, and analgesic opioids sufentanil and/or remifentanil, with or without small boluses of propofol or fentanyl; titrated to effect according to the patient’s requirement. In general, we used a ‘sleep-awake-sleep’ protocol, in which sedation was titrated from deep sedation for more stimulating aspects of the procedure that did not require patient cooperation. Sedation was initially deeper for cannulations and percutaneous needle placement including scalp nerve blocks, then lightened for patient positioning to ensure that the patient was comfortable in the fixed position in which they would be held during the subsequent critical ‘awake’ component of the procedure, then deepened again for skin incision and initial extradural dissection including raising of the craniotomy bone flap. Once the bone flap was removed, tumour boundaries were identified using neuronavigation, the dura was opened, and sedation was minimized to allow recovery to an awake state in a cooperative patient.
Once the patient had recovered to an awake state, a series of multimodality mapping techniques were performed, beginning with SSEP N20/P20 phase reversal to identify the central sulcus, followed by passive gamma mapping for motor sites or language sites using the cortiQ mapping system (g.tec medical engineering GmbH, Austria).34 Finally, an Ojemann bipolar stimulator (Integra Life Sciences, Plainsboro, NJ, USA) was used during active stimulation mapping and in conjunction with electrocorticography (ECoG) to allow detection of after-discharges or early epileptiform activities which may be induced by DES. A bipolar electrode delivering a 2–3 s pulse at 60 Hz was applied to the brain. The current intensity used for individual patients started at a baseline of 2 mA for cortical stimulation or 4 mA for subcortical simulation, and was progressively increased by 1 mA increments until a functional response was elicited or a maximum of 4 mA (cortical) or 8 mA (subcortical) was reached. ECoG recorded from the cortical surface helped to rule out triggered after-discharges and seizures that result in false mapping results, as well as to identify the limits of current delivery to prevent those complications. After cortical stimulation, tissue was removed with continuous cognitive monitoring and/or alternating resection and cognitive testing without DES. Once the critical awake phase of the procedure was completed, sedation was deepened for the third stage duration of the surgical procedure although the patient’s airway was never instrumented. After skin closure, sedation was discontinued and the monitored awake patient was transferred directly to the neuro-intensive care unit.
Data collection and analysis
Preoperative evaluations were conducted one to four days prior to the surgical procedure. Post-operative interviews were conducted at each patient’s two-week follow-up appointment, which is standard clinical practice in the Department of Neurosurgery following an AC.
Quantitative
Quantitative data were collected using four widely-used patient-reported outcome measure questionnaires and a novel set of experience scales designed specifically for AC patients. The four existing questionnaires were chosen for their ease of use, quick administration, and usefulness in assessing the mental state of patients before and after AC.
The Generalized Anxiety Disorder Scale (GAD-7)27 is a validated seven-item scale designed to assess levels and severity of generalized anxiety. Scores of 5, 10 and 15 represent cut-off points for mild, moderate and severe anxiety, respectively.27 The GAD-7 was administered by the interviewer pre and postoperatively and demonstrates excellent reliability (a = 0.91).
The Depression Module of the Patient Health Questionnaire (PHQ-9)26 is validated nine-item scale designed to assess levels and severity of depression. Scores of 5, 10, 15 and 20 represent cut-off points for mild, moderate, moderately-severe, and severe depression, respectively.26 The PHQ-9 was administered by the interviewer pre and postoperatively and demonstrates excellent reliability (a = 0.89).
The Amsterdam Preoperative Anxiety and Information Scale (APAI)28 is a validated 6-item scale designed to assess procedure related anxiety and information seeking. A score greater than 11 represents the cut-off for clinically-elevated anxiety. The APAI was administered by the interviewer preoperatively and demonstrates good reliability (a = 0.87).28
The Abbreviated Post-Traumatic Stress Disorder Checklist29 is a validated six-item scale designed to assess symptoms of PTSD. A score of 14 has been proposed for screening positive for PTSD. The scale was administered by the interviewer preoperatively and demonstrates good reliability (a = 0.80).35
Due to the lack of existing tools specific to the unique experience of undergoing an AC, a novel set of patient experience scales were developed and administered by the interviewer to assess patients’ recollection of intraoperative pain, overall distress, anxiety, distress due to noise, perception of empowerment/agency during the procedure, perception of being well-prepared prior to the procedure, overall satisfaction with anaesthesia management, and overall satisfaction with the procedure (Table 2). Patients rated their experiences on a scale of 0 to 10, with higher scores indicating greater levels of the described phenomena.
Table 2.
Interview questions and patient experience rating scales.
| Interview questions |
|---|
| Please tell me about your overall experience. |
| Please describe your emotions and thoughts as best as you can remember. |
| Do you remember any loss of functioning during the procedure? |
| Do you remember the motor mapping? What was that experience like for you? |
| What was the most stressful or uncomfortable aspect of the procedure? |
| Was anything helpful/beneficial in alleviating that stress or discomfort? |
| Did you experience any pain during the procedure? |
| Was it addressed? |
| Did you experience any anxiety during the procedure? |
| Was anything helpful/beneficial in alleviating or easing the anxiety? |
| Can you speak of your sense of agency/empowerment during procedure? |
| Was there anything that fostered this sense of agency? |
| Was there anything that may have diminished this sense of agency? |
| Did you use any of the stress management strategies we discussed before the procedure? |
| Did you find them helpful/unhelpful? |
| If you had to do this again, would you rather an awake craniotomy or an asleep craniotomy? |
| Is there anything else you would like to share about this procedure? |
| Patient experience rating scales (all ratings on a scale of 0 to 10) |
| Please rate the pre-operative preparation provided. |
| Please rate your overall pain during the procedure. |
| Please rate your overall anxiety during the procedure. |
| Please rate your overall distress/discomfort during the procedure. |
| Please rate how disturbing the noise was to your experience. |
| Please rate your overall sense of agency/empowerment during procedure. |
| Please rate how satisfied you were with the anaesthetic management. |
| Please rate your overall satisfaction with the procedure. |
Descriptive statistics, including mean, standard deviation, median, interquartile range (IQR), and percentages were used to summarize the data. Statistical comparisons (e.g. t-tests) were not conducted due to limited sample size.
Qualitative
A trained researcher and CP (author DDC) conducted one-on-one individual interviews using a semi-structured interview guide (Table 2). Patients were interviewed alone or accompanied by a family member, and were invited to ask questions and provide additional details or feedback throughout the interview. Interviews were de-identified, digitally audiotaped, and transcribed verbatim by a secure transcription service. Transcribed interviews were analysed by four researchers using a conventional content analysis. This method systematically examined material and obtained a condensed description of content, as described in Hsieh and Shannon; Triangulation was employed to increase the validity of the results.36
Results
Consecutive sampling was used and 17 participants were approached. One individual was not enrolled due to status as a member of a vulnerable population (i.e. prisoner). Two patients were unavailable for a follow-up interview due to postsurgical expressive aphasia (n = 1) or fatigue following medical appointments (n = 1). Participants that completed postoperative interviews (n = 14) had undergone an AC for tissue resection due to primary brain tumours (n = 12) or medically-refractory focal epilepsy (n = 2). Patient demographics and baseline characteristics are presented in Table 3. All patients tolerated the intraoperative functional mapping procedures. There were no failed ACs, no surgery-related mortalities, and no significant surgical complications. Among the 14 patients, all but one reported clearly remembering the procedure and could describe their experiences in detail. Twelve participants completed all pre- and postoperative questionnaires, while one did not complete the postoperative PHQ-9 and GAD-7 due to time limitations. The remaining participant did not like answering with a numeric rating scale, and instead, described his experience at length. The number of participants who completed each questionnaire is indicated in Table 4. Missing data were handled using pairwise deletion.
Table 3.
Participant demographics (n = 14).
| Measure | n | % |
|---|---|---|
| Gender | ||
| Cisgender male | 11 | 79 |
| Cisgender female | 2 | 14 |
| Transgender male | 1 | 7 |
| Race/ethnicity | ||
| Non-Hispanic/Latinx | 13 | 94 |
| Hispanic/Latinx | 1 | 6 |
| Age | ||
| Mean | 44 | |
| Range | 27–83 | |
| Diagnosis | ||
| Refractory focal epilepsy | 2 | 14 |
| Brain tumour | 12 | 86 |
| Benign neoplasm | 2 | |
| Low grade | 3 | |
| High grade | 7 | |
| Tumour/lesion location | ||
| L frontal | 5 | 36 |
| L temporal | 6 | 43 |
| R frontal | 1 | 7 |
| R temporo-occipital | 1 | 7 |
| R parietal | 1 | 7 |
Table 4.
Summary of results on study specific validated measures and novel patient experience scales.
| Range | |||||||
|---|---|---|---|---|---|---|---|
| Validated measures | n | M | SD | Median | Low | High | IQR |
| PHQ-9 (pre-op) | 13 | 6.16 | 4.78 | 5.00 | 0.00 | 14.0 | 9.00 |
| PHQ-9 (post-op) | 12 | 6.00 | 4.02 | 5.00 | 1.00 | 13.0 | 6.00 |
| GAD-7 (pre-op) | 13 | 8.66 | 6.41 | 7.50 | 0.00 | 21.0 | 11.00 |
| GAD-7 (post-op) | 12 | 4.36 | 4.24 | 2.00 | 0.00 | 12.0 | 8.00 |
| APIAS (pre-op) | 13 | 15.46 | 7.27 | 15.00 | 6.00 | 30.0 | 10.00 |
| Patient experience scales | |||||||
| Perception of preparedness | 13 | 8.42 | 1.88 | 9.00 | 5.00 | 10.00 | 2.75 |
| Intraoperative self-agency | 13 | 6.16 | 4.04 | 8.00 | 0.00 | 10.00 | 8.00 |
| Satisfaction, AM | 13 | 9.41 | .99 | 10.0 | 7.00 | 10.00 | 1.00 |
| Satisfaction, overall | 13 | 9.36 | .80 | 10.0 | 8.00 | 10.00 | 1.00 |
| Intraoperative anxiety | 13 | 1.33 | 2.31 | 0.00 | 0.00 | 7.00 | 1.75 |
| Intraoperative pain | 13 | .87 | 1.34 | 0.00 | 0.00 | 3.50 | 2.00 |
| Intraoperative distress, drill noise | 13 | 2.75 | 3.41 | 1.00 | 0.00 | 9.00 | 5.75 |
| Intraoperative distress, overall | 13 | 1.35 | 2.35 | 0.00 | 0.00 | 7.00 | 2.75 |
Notes. SD: standard deviation; Med: median; PHQ-9: Depression module of the Patient Health Questionnaire (scoring range: 0–27); GAD-7: Generalized Anxiety Disorder-7 (scoring range: 0–21); APAIS: Amsterdam Preoperative Anxiety and Information Scale (scoring range: 6–30); AM: anaesthesia management.
Quantitative
Results from pre and postoperative standardized rating scales of generalized anxiety27 and depression26 and postoperative patient experience rating scales are displayed in Table 4 and in supplemental materials. Prior to the procedure, 46% of patients reported a trauma history, 75% reported clinically-elevated levels of anxiety regarding the procedure (APAIS score ≥ 11; mean = 15.46, SD = 7.27), 33% reported moderate to severe generalized anxiety (GAD-7 score ≥ 10; mean = 8.55, SD = 6.41), and 33% reported moderate to severe symptoms of depression (PHQ-9 score ≥ 10; mean = 6.16, SD = 4.78). Following the procedure, 20% reported moderate to severe generalized anxiety (GAD-7 score ≥ 10; mean = 4.36, SD = 4.24), and 20% reported moderate to severe symptoms of depression (PHQ-9 score ≥ 10; mean = 6.00, SD = 4.02). Patients reported very high satisfaction with the AC (median = 10.0; IQR = 1) and anaesthesia management (median = 10.0; IQR = 1). Patients reported feeling well-prepared (median = 9.0; IQR = 2.75), though approximately 20% (n = 3) noted that they would have liked more information regarding what to expect during the post-procedure recovery. Of the 14 patients interviewed, 12 (86%) reported experiencing stress and/or discomfort, while only 2 (14%) denied any stress or discomfort during the procedure. Patients reported minimal levels of intraoperative pain (median = 0; IQR =2), intraoperative anxiety (median = 0; IQR = 1.75), and overall intraoperative distress (median = 0; IQR = 2.75) and moderate levels of agency (median = 8; IQR = 8). Half of patients reported that the noise of the cranial drill was bothersome, but ratings of distress due to noise remained low (median = 1.0; IQR = 5.75).
Qualitative
Interview duration ranged from 36 to 68 min. Two main themes emerged from data analysis.
Theme one: the most stressful aspects of the procedure
Patients reported that the most stressful aspects of the procedures included: (1a) global recognition of their medical diagnosis and prognosis, (1b) anxiety provoked by unfamiliar sights, sounds, and sensations experienced in the operating room (e.g. sound of the cranial drill, seeing blood on the plastic drapes that surrounded the patient), (1c) a perception of a lack of information or misinformation before or during the procedure, and (1d) long periods of immobility.
Theme two: clinical practices and procedures that alleviated intraoperative distress
Three factors that alleviated intraoperative distress included the medical team’s ability to: (2a) promote patient empowerment and perceptions of control, (2b) establish compassionate and authentic relationships during pre-surgical appointments and in the surgical suite, and (2c) deliver effective anaesthesia management. Examples of patients’ statements supporting our interpretation are displayed in supplemental Appendix 4.
Promote patient empowerment and self-agency
Patient empowerment is the process and result of patients being educated, informed, and empowered to actively participate in their own care.37, 38 Self-agency is the perception of having some sort of control over one’s own actions or the confidence that one can successfully execute a course of action to produce a desired outcome.39,40 In this sample, thorough preoperative counselling and a sense of purpose and teamwork promoted patient empowerment and self-agency. One patient stated, ‘I felt like it [self-agency] started in the preoperative appointment… between what Dr. X and yourself had told me beforehand about the exercises we would be going through and what I could expect from it. I felt very involved and prepared for both what we were going to be doing, why were we going to be doing it and…that felt very empowering and like I was part of it…and that continued into the surgery where I knew why we were doing each part of it and I felt involved and respected in all of that. So that was very positive.’
Providers’ responsiveness in the surgical suite also aided in promoting a sense of empowerment and perceptions of control. Most patients acknowledged the responsiveness in anaesthesia management by describing providers responding quickly and administering additional medication as requested. Other examples of provider responsiveness included medical staff providing water, consistent feedback during testing, and giving reassurance in response to distress as needed. One patient responded to the question about what fostered a sense of intraoperative agency as, ‘I think just the quick responses from people when I asked for water. I felt immediately that I was getting some water. And the anaesthesiologist too. It felt like he was responding right away when I said something.’
Finally, patients described the benefit of having tasks and timeframes presented to them. Tasks included neuropsychological tests, breathing practices (if identified in the pre-surgical evaluation and usually prompted by the CP, SLP, or anaesthesiologist), requesting and receiving the moist oral swab as needed for a dry mouth, and focusing on what was communicated to them by the providers. These tasks were reported as a means of diverting focus from their experience of stress and/or pain. One patient noted, ‘I think being tasked is really important, even if they’re simplistic things that are meaningless to the team and to the surgeon, it sure meant a lot to me psychologically, being in that process of, ‘Okay, what’s next? What do you need me to do? You need me to count, say words, count backwards, name the days, whatever…’ so that I’m focused on something other than the pain, and the discomfort, and the anxiety. The little sponge water actually was a really successful thing for me because I felt like it was something I could depend on, you know, it was a system that we had going of like, ‘okay, I need a little bit water’ and I could focus on that and think about that and forget about some of the other things.’
Creating and communicating time frames was also helpful in fostering a sense of control. As stated by one patient, ‘If I know that that’s the timeline, I can set my mind for that, I can make it through that process. But if there’s some indefinite, you know, period of really open-ended here, that’s hard for me. That’s very challenging. But I think I can make a process better when I know it’s going to be like there’s a timeline associated to it. And then I feel like I can kind of just make it through the bank because it’ll end. Right, that predictability, that control.’
Establish compassionate and authentic relationships
Patients reported that the medical team’s ability to establish compassionate and authentic relationships was significant in reducing intraoperative distress. Multiple patients reported that the reliable and attentive presence of a provider (e.g. a CP, SLP, or anaesthesiologist stationed near the patient and within his/her field of view) elicited a sense of calm and safety that was critical in promoting patient comfort and compliance. One patient stated, ‘Thank you for allowing me to hold your hand. It was just very comforting to have a human there that was very present with me. Yeah, made me feel safe in, like, an environment where there’s a lot going on and there’s a lot of machines connected to me and a lot of people not necessarily paying attention to me, but doing a lot of different things. To have that connection with one other person was a very positive part of it, and that was not insignificant. I remember that this was very significant in feeling safe through the surgery process.’ Another noted, ‘Your calmness was good. I realized when I was getting scared that I think you were holding my hand and that helped me…kind of bring me back… to be like ‘Okay, if she’s this calm, it can’t be an emergency situation.”
Thorough and frequent communication was described as an important aspect of the compassionate and authentic relationship and a significant factor in alleviating distress. One patient stated, The communication between myself and the team I thought was really positive. I felt pretty calm for the most part and I think that’s attributable to the team again, of just keeping me in a spot of knowing what’s happening, what’s about to happen, and then what’s going on as it happened.’
Finally, patients perceived the frequent inquiries about their comfort level as evidence that team members truly understood the patient’s remarkable situation, which created a more humanized experience. One patient noted, ‘It was really helpful for me to almost have a more humanized experience with people in the room who are here to help you [when they told me] ‘We understand this is tough. We understand that you’re not comfortable. What can we do to help you?”
Deliver effective, patient-centred anaesthesia management
Patients reported high levels of satisfaction with anaesthesia management. One patient noted, ‘The anaesthesiologists were remarkable. If there was any slight issue and I spoke up, they were on it.’ Most patients acknowledged the responsiveness in anaesthesia management by describing providers responding quickly and administering additional medication as requested. Notably, provider responsiveness was described as helpful not just in alleviating the physical pain, but importantly, fostered a perception of being heard. One patient noted, ‘It was just a reassuring that they’re listening to me’, underscoring the importance, and even possible analgesic effects, of acknowledging the patient’s pain, even if no additional anaesthetic medicine can be administered in that moment.
Discussion
The success of intraoperative functional mapping during ACs requires that the patient is comfortable, calm, and able to fully attend to and participate in cognitive testing. Yet previous studies indicate that many patients report moderate to severe intraoperative distress, increasing the risk of sub-optimal brain mapping and even failed procedures.11 Previous studies that have investigated patient-reported experiences during ACs, discussed the importance of adequate preoperative counselling and described the critical nature of multidisciplinary communication; however, less is known about patients’ perspectives on how to mitigate intraoperative distress. Therefore, the aim of this mixed-methods study was to explore the subjective experiences of patients undergoing ACs and gain insight into clinical practices and procedures that were perceived to mitigate intraoperative distress. With implications for all clinicians involved in patient care during ACs (e.g. neurosurgeons, anaesthesiologists, clinical neuropsychologists, speech-language pathologists, neurologists, members of the neuro-monitoring team, stereotactic surgery coordinators, and pre-operating and operating room nurses), results from this mixed-methods study suggest that promoting patients’ perception of control, establishing compassionate and authentic relationships, addressing unfamiliar intraoperative sensations, and delivering patient-centred anaesthesia are critical to diminish intraoperative distress.
Overall, patients in the current study reported high levels of satisfaction with the procedure and anaesthesia management, described feeling well-prepared, and, compared to previous studies, reported low intraoperative anxiety, pain, and overall distress.11 Patients reported diminished levels of anxiety at postprocedure, thereby, complementing data from semi-structured interviews. Similar to previous studies, we found that the long period of immobility was one of the most stressful aspects of the procedure.11,12,41 In addition to immobility, cognitive-affective factors were the most frequently reported causes of intraoperative distress.
Interestingly, no patient reported pain as the most distressing experience and no patient described more than mild intraoperative pain. Current results are in contrast to previous studies that reported up to 30% of patients undergoing ACs recalled at least moderate pain.11 Current theories of pain emphasizes that pain perception is not necessarily related to noxious input, but is strongly influenced by psychological variables, including anxiety, fear, catastrophizing, and self-agency.42–47 Relatedly, in this sample, only 17% of patients endorsed moderate anxiety and no patients reported severe anxiety (See supplemental materials). Findings are in contrast to Danks et al.48 and Whittle et al.11 who reported 10%–14% of interviewed patients endorsed severe levels of intraoperative anxiety. We argue that proactively promoting patient empowerment and perceptions of control, establishing compassionate and authentic relationships prior and during the procedure, and addressing unfamiliar intraoperative sensations will likely contribute to reduced intraoperative distress and pain, limit the need for additional sedation to mitigate pain, and result in improved participation during brain mapping procedures.
Promote patient empowerment and perceptions of control
Patient empowerment and perceptions of control have been previously associated with increased patient satisfaction, better clinical outcomes,37,38 reduced anxiety,42 fear,49 and pain severity,50 and predict how much effort and persistence individuals exhibit within the context of aversive experiences.51 A sense of control is especially important for individuals with a trauma history, as it is not only associated with lower distress and anxiety, but also reduces the probability of sequel symptoms of post-traumatic stress.52
In this sample, patient empowerment and perceptions of control were promoted by thoroughly preparing the patient, cultivating a sense of purpose and teamwork prior to and during the procedure, and responding quickly to the patient’s intraoperative needs. Thorough preoperative counselling is the key to successful awake surgery, is essential to gaining the patient’s confidence and trust, and has been shown to facilitate a faster recovery.53–58 Notably, provider responsiveness was described as helpful not just in alleviating patients’ intraoperative pain, but importantly, fostered a perception of being heard. In this way, provider responsiveness may increase a sense of self-agency, thereby, potentially reducing intraoperative anxiety,42 fear,49 and pain.50
Establish compassionate and authentic relationships
There is strong evidence supporting the importance of provider compassion in medical care.59 Patients easily sense compassionate behaviour by providers and exhibit measurable physiologic responses to compassionate interactions.60 Provider compassion has been shown to be significantly associated with patient empowerment, the ability to cope with illness,61 positive clinical outcomes,62,63 and even reduced perceptions of experimental pain.43,44 Expressing compassion does not require providers to join and share in patients’ suffering or distress, which may lead to provider distress or burnout and threaten personal boundaries crucial for clinical objectivity. Instead, compassion is demonstrated through an awareness of another’s experience64 and paired with meaningful action to relieve the observed suffering.65 Success is achieved when the patient no longer feels isolated by their situation and takes comfort in providers’ motivation to improve their experience. In this study, providers’ compassion and authenticity were experienced and described by patients as a sense of support, an attentive and reliable human presence, and thorough and frequency intraoperative communication.
It is also important to note that patients present with various coping styles. For some patients, receiving too much detailed information during the procedure can unintentionally provoke anxiety. To inquire into our patients’ diverse coping styles, during the preoperative evaluation, the CP or SLP assesses the patient’s need for information using the APAIS.28 If a patient identifies as a non-information seeker on this two-item subscale, the CP or SLP will subsequently inquire into the patient’s desired amount of information provided during the procedure. Previous studies suggest that individuals who identified as a non-information seeker may benefit from receiving no more information than legally required during the procedure, unless they specifically ask for the information.28,66 For non-information seekers, necessary, but conservative, amounts of information provided intraoperatively may reduce the likelihood of an overload of information that provokes anxiety. This, of course, does not preclude trauma-informed care in which providers directly answer patients’ questions and concerns and always inform them of new sensations they are about to experience (e.g. muscle contractions due to phase reversal, movement of the table, new noises introduced to the room).
Address unfamiliar intraoperative sensations
In this sample, half of patients reported that the noise of the cranial drill was mildly to severely bothersome, in-line with previously studies that reported most intraoperative memories recalled by patients are auditory.51,67,68 If the patient is awake during the bone flap removal, Hansen and colleagues suggest that, prior to the procedure, providers offer suggestions for ‘reframing’ noises based on patients interests or experience.69 For example, the noise of the cranial drill could be reframed as driving a motorcycle down a country road or riding in a motor boat on a beautiful lake. In this context, positive suggestions are given to introduce and propose the consideration of new or different views, ideas, or options. Alternatively, based on a patient’s previous history (e.g. significant trauma, chronic pain, anxiety disorders) or specific request, the anaesthesiologist may deem it appropriate to provide slightly more sedation during bone flap removal. Finally, during positioning and draping, ensure that the patient will not see blood, surgical instruments, or any other potentially distressing items in their line of vison.
Deliver patient-centered anaesthesia management
While describing the anaesthesia management approach in detail is beyond the scope of this manuscript, our protocol does not include induction of general anaesthesia with endotracheal intubation. Instead, all patients in this study were sedated to a depth that was titrated according the needs of the patient and the surgical team for successful completion of the procedure. It is noteworthy that sedation was initiated in the preoperative suite even before transfer to the operating room to minimize anxiety. The light sedation periods were for patient positioning after the head frame was in place, and the critical awake period for functional localization and cognitive monitoring during resection, both of which required full patient cooperation for success of the procedure. Following resection, deep sedation was resumed for the duration of the surgical procedure. Modern fast-acting and short-acting anaesthetics, such as propofol, remifentanil, and dexmedetomedine, allow rapid recovery from sedation and control of stressful phases during surgery.69 Our protocol spares the patient unnecessary exertion during the craniotomy and may improve the patient’s ability to focus attention on cognitive tests.8 On the other hand, we have contraindicated sedative-hypnotic anxiolytic benzodiazepines, which, although potentially useful as amnestics to prevent intraoperative recall, compromise ability of the patient to fully cooperate during the critical awake portion of the procedure. An important aspect of our anaesthesia management is the multi-disciplinary communication that takes place prior to the procedure between the CP or SLP and anaesthesiologists, which informs our patient-centred anaesthesia management.
Consideration of postoperative distress
Of note, while the aim of the interviews was to elicit patients’ views on intraoperative stressors and the mitigation of intraoperative distress, up to 20% of patients reported experiences in the post-procedure ICU as most stressful aspects of AC. One patient explained, ‘Then I was just put to one side for a few days. That was the hardest part for me, the follow-up. Because in there, nobody did anything wrong, I [just] didn’t feel any strong connectivity with anyone. People would come in and then go away and I wasn’t really sure. The attention kind of dissipated, [the attention] that was there before and during [the procedure] but then nothing.’ Findings again signify the importance of the patient-provider relationship and the critical role that compassionate, attentive, and responsive providers play in patients’ perceptions of well-being. Future research should investigate clinical practices to foster health, wellbeing, and recovery post-procedure.
Clinical practice recommendations
Clinical practice recommendations informed by patient interviews are listed below and summarized in Table 5.
Table 5.
Summary of clinical practice recommendations for awake craniotomies.
| Timing | |||
|---|---|---|---|
| Clinical Practice | Recommendations | Pre | Intra |
| Promote perception of control | Thoroughly prepare the patient | ||
| Describe the progression of the procedure in detail | X | ||
| Describe the operating room, expected discomforts, and unfamiliar sights and sounds the patient may hear | X | X | |
| Describe postoperative practices and procedures | X | ||
| Cultivate a sense of purpose and teamwork | |||
| Explain the value of the patient’s participation during the AC | X | X | |
| Describe the goals of the medical team | X | X | |
| Administer testing paradigms to be used during intraoperative mapping | X | X | |
| Explain that loss of function during mapping is temporary and informative | X | X | |
| Elicit the patient’s preferred stress management techniques | X | X | |
| Assess the patient’s coping style and need for information | X | ||
| Teach and coach patients to assert control during the procedure through communication | X | X | |
| Be responsive to the patient’s intraoperative needs | |||
| Administer additional medication as needed for pain management | X | ||
| Provide water and adjust temperature or positioning, when possible, at the patient’s request | X | ||
| Acknowledge the patient’s experience, even if there is no action that can be taken to address their concern | X | ||
| Provide focused tasks and time frames | |||
| Use focused tasks (e.g. neuropsychological tests, breathing exercises, requesting a moist oral swab) to divert attention from painful or stressful experiences | X | ||
| Communicate expected time frames during the procedure | X | X | |
| Establish compassionate and authentic relationships | Cultivate a sense of support | ||
| Frequently inquire about the patient’s comfort level | X | ||
| Repeat soothing statements of support and encouragement and avoid negative statements | X | ||
| For providers working behind the patient, introduce yourself face-to-face after draping but before onset of the procedure (or after the patient is awake) | X | ||
| Use nonverbal gestures, such holding the patient’s hand or placing a hand on their shoulder, to demonstrate compassion | X | ||
| Provide an attentive and reliable human presence | |||
| Station a provider (e.g. CP, SLP, or anaesthesiologist) near the patient and within his/her field of view | X | ||
| If possible, have a CP or SLP conduct most direct patient interaction, allowing the anaesthesiologist to focus on anaesthesia management | X | ||
| Provide consistency by having the same clinician conduct the preoperative evaluation and work with the patient in the OR | X | X | |
| Provide thorough and frequent communication | |||
| Throughout the procedure, orient the patient what is happening, directly answer their questions and concerns, and inform them of new sensations they are about to experience | X | ||
| Tailor the amount of detail provided to the patient’s coping style and need for information | X | ||
| Address intraoperative sensations | Offer suggestions to “reframe” distressing noises based on patient’s interests | X | X |
| Ensure patient will not see potentially distressing items in his/her line of vison | X | ||
| Provide patient-centred anaesthesia management | Engage in multidisciplinary communication when planning patient-centred anaesthesia management | X | X |
Preoperative recommendations
Describe the progression of the procedure in detail, from procedures in the preoperative waiting room to intraoperative positioning, infiltration of local anaesthesia, head pinning, bone flap removal (and associated noise), phase reversal (and associated sensation), the phases of brain mapping (in our program that includes passive gamma mapping using the cortiQ, cortical stimulation, and cognitive monitoring during resection) and closure. Patients should be provided with a realistic description of the operating room, expected discomforts (e.g. unchangeable position, aphasia during cortical mapping, dry mouth), potential risks, and safety precautions. Discuss the sounds the patient may hear in the operating room (e.g. monitor alarms, cranial drilling, elektroknife, ultrasonic surgical aspirator). Repeated reassurance and careful explanation of the procedure by multiple members of the clinical team may foster patients’ confidence and trust in their medical teams.
Explain to the patient the reason for the awake vs. asleep procedure. This conversation focuses on the value of the patient’s input to optimize the safety and extent of resection, creates a sense of purpose for the patient, and identifies the patient as a respected member of his/her medical team.
Administer testing paradigms to be used during intraoperative mapping to increase familiarity with instructions and expectations. A critical component of administering intraoperative testing paradigms is to explain the benefits of positive findings, or the temporary loss of cognitive function during active stimulus mapping (i.e. DES). Patients need to be aware that during this phase of surgery, loss of functioning (a) is temporary, (b) indicates a positive finding, and (c) informs the ‘brain map’ that guides the neurosurgeons. By sharing this information, the medical team promotes a sense of partnership and purpose within the patient and eliminates the unnecessary fear provoked by concern about a permanent deficit or worries about disappointing the medical team by not being able to perform tasks as a result of DES.
Elicit patients’ preferred stress management techniques to be used during the procedure.33 Examples of stress management techniques may include breathing practices, visualization, prayer, or even distraction via conversations about the patients’ interests or hobbies. It is important to note that for individuals with high levels of anxiety, an explicit focus on sensations of breath may actually trigger more, not less, anxiety.
Explicitly teach patients that they can assert a sense of control during the procedure through communication. Coach them to share their intraoperative experiences with the medical team so that members can respond accordingly.
Assess patients’ diverse coping styles, during the preoperative evaluation28 to adjust the amount and delivery of information to be provided to the patient throughout the procedure accordingly.
Intraoperative recommendations
We suggest that the neurosurgeon(s), or other providers who will be speaking to the patient from the other side of the drape, introduce themselves to the patient face-to-face in the surgical suite, after the draping is complete and prior to the onset of the craniotomy. This one small act of pairing the voice with a face during this very stressful experience can be significant in establishing a human connection and cultivating a sense of trust within the surgical suite.
Repeat soothing statements (e.g. ‘The procedure is going very well’) and expressions of encouragement (i.e. ‘You’re doing really well’ or ‘Excellent job keeping your body still and relaxed’). Negative statements or sentiments of agitation or frustration should be avoided.8
Provide small nonverbal gestures, such as holding the patient’s hand or placing a hand on his/her shoulder, to assist in creating a sense of human connection. Employ non-verbal techniques to demonstrate compassion and understanding, such as keeping silent while sitting next to the patient, using a kind tone of voice and eye contact, nodding while listening, smiling, and talking while attending to the patient.70,71
Given the importance of establishing relationships, we recommend that the same CP or SLP who conducts the preoperative evaluation, also conducts the intraoperative cognitive testing. Such preoperative support, intraoperative coaching, and continuity of care from the CP or SLP has been shown to increase the overall success of surgery by increasing compliance and reducing fear.31 In our program, the presence of the CP or SLP allows the anaesthesiologist to focus on his/her role of ensuring adequate sedation and anxiolysis during the surgical steps, preventing nausea, vomiting, and seizures, and assuring adequate ventilation, hemodynamic stability, and normal intracranial process. Further, if and when adverse events do take place (i.e. seizure activity), the anaesthesiologists can conduct necessary actions to cease seizure activity, while the CP or SLP can be attentive and present during and following the seizure activity to promote the patient’s sense of safety, emotional regulation, and a successful transition back to brain mapping procedures, as appropriate.
Limitations
First, though the results can be transferable, they cannot be generalized because they were taken from the singular experiences of a limited number of patients. Further, approximately 60% of participants with tumours were diagnosed with high grade tumours, which are associated with distinct clinical presentations when compared to low grade or benign tumours.72 Nevertheless, it is important for medical teams to consider patients’ experiences, perceptions, and insights, in order to better understand how to reduce intraoperative distress. Second, the timing of patient questionnaire administration (before and two weeks after surgery) may have led to response bias and poor recall. Third, the authors did not find an applicable validated questionnaire for the central questions of this study, and therefore a new approach had to be chosen. Authors developed a novel set of patient experience scales based on validated questionnaires, but these were not validated before this study. Fourth, for some patients, the CP who conducted the post-operative interview was also the primary person interacting with the patient during AC, which may have influenced their responses to questions about their AC experiences. Finally, the simple demonstration of interest in how the patient experienced the perioperative period may have produced a positive effect on patient satisfaction.
Summary
The current study describes patients’ perspectives of clinical practices that mitigated intraoperative distress during ACs. Previous studies have investigated patient-reported experiences during ACs, discussed the importance of adequate preoperative counselling, and described the critical nature of multidisciplinary communication; however, less is known about patients’ perspectives on how to mitigate intraoperative distress. Results from this mixed-methods study suggest that compassion, communication, and patient perception of control were critical. Use of the interventions and strategies presented above may improve the likelihood of optimal brain mapping procedures to improve clinical outcomes during ACs. Future research should evaluate patients’ perspectives intraoperatively, in addition to assessing post-procedure perspectives, and consider including physiological markers of intraoperative distress. Future studies should also evaluate post-procedural factors that promote a sense of wellness and recovery.
Supplementary Material
Acknowledgements
We want to acknowledge the many team members of the Oregon Health & Science University Awake Craniotomy Program, including Dan Klee, Ilker Yaylali, Aaron Kawamoto, Chris Timpa, Aubry Raney, Ann Mitchell, Faye Mulcahy, Amber Welze, and resident neurosurgeons.
Oregon Health & Science UniversityNational Institutes of Health [10.13039/100000002T32 AT002688This study was supported by Oregon Health & Science University and by a grant from the National Institutes of Health [T32 AT002688].
Appendix 1. Scores on validated measures of depression, generalized anxiety, and pre-operative anxiety.
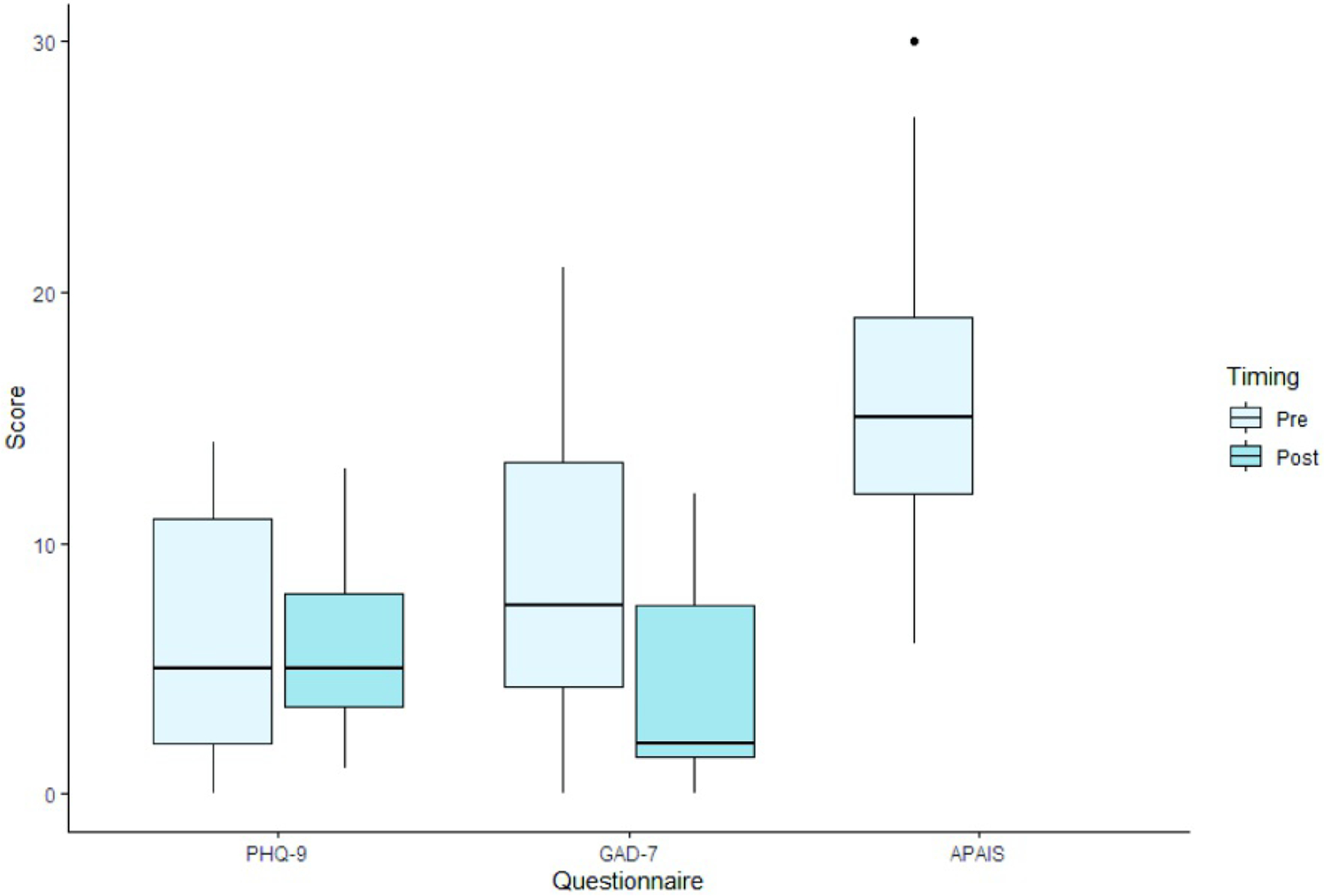
Notes. PHQ-9 = Depression module of the Patient Health Questionnaire (scoring range: 0–27); GAD-7 = Generalized Anxiety Disorder-7 (scoring range: 0–21); APAIS: Amsterdam Preoperative Anxiety and Information Scale (scoring range: 6–30). Dots represent outliers.
Appendix 2.
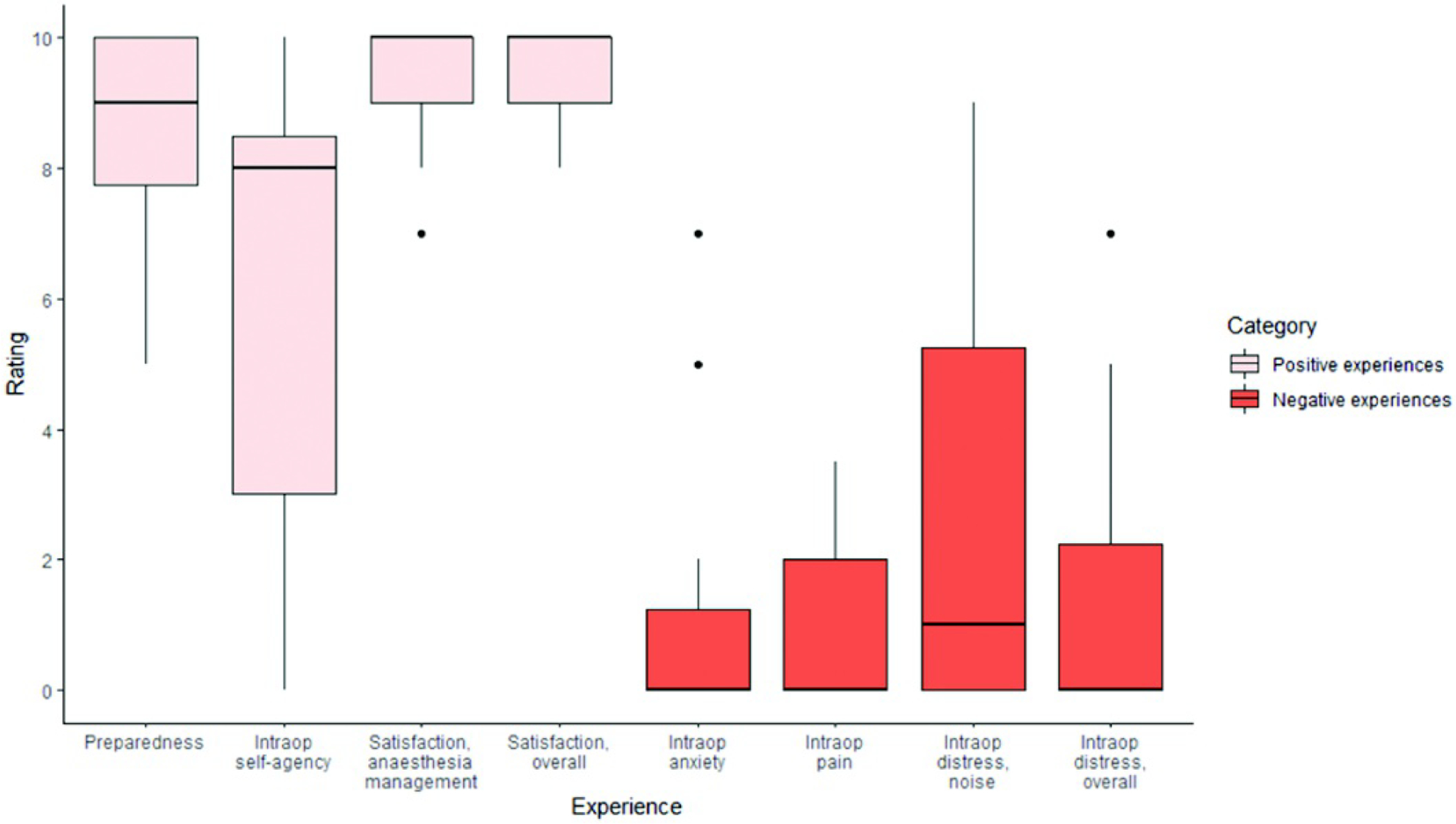
Ratings on patient experience scales
Appendix 3.
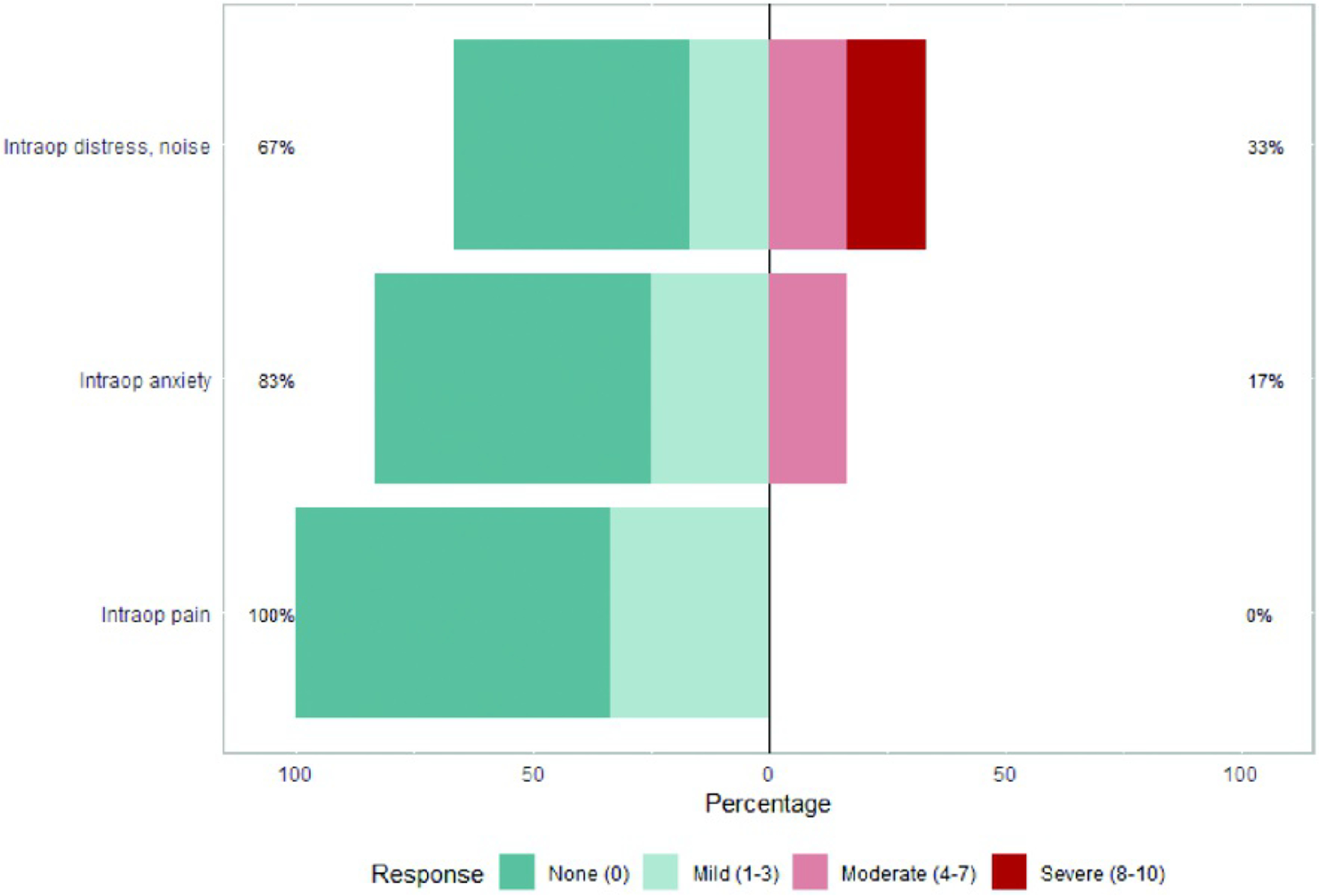
Reported severity of intraoperative pain, anxiety, and distress due to noise as measured by the study-specific questionnaire
Footnotes
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Bello L, Gallucci M, Fava M, et al. Intraoperative subcortical language tract mapping guides surgical removal of gliomas involving spee ch areas. Neurosurgery 2007;60:67–82. [DOI] [PubMed] [Google Scholar]
- 2.Duffau H, Lopes M, Arthuis F, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. Journal of Neurology, Neurosurgery & Psychiatry 2005;76:845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hamer PDW, Robles SG, Zwinderman AH, Duffau H, Berger M. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 2012;30:2559–65. [DOI] [PubMed] [Google Scholar]
- 4.Ruis C neuropsychology eMonitoring cognition during awake brain surgery in adults: a systematic review. J Clin Exp Neuropsychol 2018;40: 1081–104. [DOI] [PubMed] [Google Scholar]
- 5.Duffau H Stimulation mapping of white matter tracts to study brain functional connectivity. Nat Rev Neurol 2015;11:255–65. [DOI] [PubMed] [Google Scholar]
- 6.De Benedictis A, Moritz-Gasser S, Duffau H. Awake mapping optimizes the extent of resection for low-grade gliomas in eloquent areas. Neurosurgery 2010;66:1074–84. [DOI] [PubMed] [Google Scholar]
- 7.Capelle L, Fontaine D, Mandonnet E, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg 2013;118:1157–68. [DOI] [PubMed] [Google Scholar]
- 8.Deras P, Moulinié G, Maldonado IL, Moritz-Gasser S, Duffau H, Bertram L. Intermittent general anesthesia with controlled ventilation for asleep-awake-asleep brain surgery: a prospective series of 140 gliomas in eloquent areas. Neurosurgery 2012;71:764–72. [DOI] [PubMed] [Google Scholar]
- 9.Stevanovic A, Rossaint R, Veldeman M, Bilotta F, Coburn M. Anaesthesia management for awake craniotomy: systematic review and meta-analysis. PLoS One 2016;11:e0156448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazanwy M, Chakrabarti R, Tewari A, Sinha A. Awake craniotomy: a qualitative review and future challenges. Saudi J Anaesth 2014;8:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milian M, Tatagiba M, Feigl GC. Patient response to awake craniotomy - a summary overview. Acta Neurochir (Wien) 2014;156:1063–70. [DOI] [PubMed] [Google Scholar]
- 12.Wahab S, Grundy P, Weidmann C. Patient experience and satisfaction with awake craniotomy for brain tumours. Br J Neurosurg 2011;25:606–13. [DOI] [PubMed] [Google Scholar]
- 13.Beez T, Boge K, Wager M, et al. Tolerance of awake surgery for glioma: a prospective European Low Grade Glioma Network multicenter study. Acta Neurochir (Wien) 2013;155:1301–8. [DOI] [PubMed] [Google Scholar]
- 14.Milian M, Luerding R, Ploppa A, et al. “Imagine your neighbor mows the lawn”: a pilot study of psychological sequelae due to awake craniotomy: clinical article. J Neurosurg 2013;118:1288–95. [DOI] [PubMed] [Google Scholar]
- 15.Klimek M, van der Horst PH, Hoeks SE, Stolker RJ. Quality and quantity of memories in patients who undergo awake brain tumor resection. World Neurosurg 2018;109:e258–e264. [DOI] [PubMed] [Google Scholar]
- 16.van Ark TJ, Klimek M, de Smalen P, Vincent AJ, Stolker RJ. Anxiety, memories and coping in patients undergoing intracranial tumor surgery. Clin Neurol Neurosurg 2018;170:132–9. [DOI] [PubMed] [Google Scholar]
- 17.Siriwardena AN, Gillam S. Patient perspectives on quality. Qual Prim Care 2014;22:11–5. [PubMed] [Google Scholar]
- 18.Veale JF. Edinburgh handedness Inventory - Short Form: a revised version based on confirmatory factor analysis. Laterality: Asymmetries of Body, Brain and Cognition 2014;19:164–77. [DOI] [PubMed] [Google Scholar]
- 19.Goodglass H, Kaplan E, Weintraub S, BDAE: The Boston diagnostic aphasia examination. Philadelphia (PA): Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 20.Howard D, Patterson K. The Pyramids and Palm Trees Test: A test of semantic access from words and pictures. Pearson assessment; 1992. [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–9. [DOI] [PubMed] [Google Scholar]
- 22.Drozdick LW, Raiford SE, Wahlstrom D, Weiss LG, The Wechsler Adult Intelligence Scale—Fourth Edition and the Wechsler Memory Sc ale—Fourth Edition. 2018.
- 23.Bowie CR, Harvey PD. Administration and interpretation of the Trail Making Test. Nat Protoc 2006;1:2277–81. [DOI] [PubMed] [Google Scholar]
- 24.Navon D Forest before trees: The precedence of global features in visual perception. Perception and Psychophysics 1969;5:197–200. [Google Scholar]
- 25.Schenkenberg T, Bradford D, Ajax EJN. Line bisection and unilateral visual neglect in patients with neurologic impairment. Neurology 1980;30:509. [DOI] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. [DOI] [PubMed] [Google Scholar]
- 28.Moerman N, van Dam FS, Muller MJ, Oosting H. The Amsterdam preoperative anxiety and information scale (APAIS). Anesth Analg 1996;82:445–51. [DOI] [PubMed] [Google Scholar]
- 29.Lang AJ, Stein MB. An abbreviated PTSD checklist for use as a screening instrument in primary care. Behav Res Ther 2005;43:585–94. [DOI] [PubMed] [Google Scholar]
- 30.Nossek E, Matot I, Shahar T, et al. Failed awake craniotomy: a retrospective analysis in 424 patients undergoing craniotomy for brain tumor. JNS 2013;118:243–9. [DOI] [PubMed] [Google Scholar]
- 31.Kelm A, Sollmann N, Ille S, Meyer B, Ringel F, Krieg SM. Resection of gliomas with and without neuropsychological support during awake Craniotomy-Effects on Surgery and Clinical Outcome. Front Oncol 2017;7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santini B, Talacchi A, Casagrande F, et al. Eligibility criteria and psychological profiles in patient candidates for awake craniotomy: a pilot study. J Neurosurg Anesthesiol 2012;24:209–16. [DOI] [PubMed] [Google Scholar]
- 33.Johans SJ, Garst JR, Burkett DJ, et al. Identification of preoperative and intraoperative risk factors for complications in the elderly undergoing elective craniotomy. World Neurosurgery 2017;107:216–25. [DOI] [PubMed] [Google Scholar]
- 34.Kapeller C, Korostenskaja M, Prueckl R, et al. CortiQ-based real-time functional mapping for epilepsy surgery. J Clin Neurophysiol 2015;32:e12–e22. [DOI] [PubMed] [Google Scholar]
- 35.Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety 2011;28:596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Health 2020: a European policy framework supporting action across government and society for health and well-being. Copenhagen: World Health Organization Regional Officie for Europe. 2012. [Google Scholar]
- 38.Bravo P, Edwards A, Barr PJ, Scholl I, Elwyn G, McAllister M. Conceptualising patient empowerment: a mixed methods study. BMC Health Serv Res 2015;15:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bandura A Guide for constructing self-efficacy scales. Self-Efficacy Beliefs of Adolescents 2006;5:307–37. [Google Scholar]
- 40.Bandura A Self-efficacy mechanism in human agency. American Psychologist 1982;37:122–47. [Google Scholar]
- 41.Whittle I, Midgley S, Georges H, Pringle A-M, Taylor R. Patient perceptions of “awake” brain tumour surgery. Acta Neurochir (Wien) 2005;147:275–7. [DOI] [PubMed] [Google Scholar]
- 42.Meredith P, Strong J, Feeney JA. Adult attachment, anxiety, and pain self-efficacy as predictors of pain intensity and disability. Pain 2006;123:146–54. [DOI] [PubMed] [Google Scholar]
- 43.Krahé C, Springer A, Weinman JA, Fotopoulou AK. The social modulation of pain: others as predictive signals of salience - a systematic review. Front Hum Neurosci 2013;7:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fauchon C, Faillenot I, Perrin A, et al. Does an observer’s empathy influence my pain? Effect of perceived empathetic or unempathetic support on a pain test. Eur J Neurosci 2017;46:2629–37. [DOI] [PubMed] [Google Scholar]
- 45.Turk DC, Wilson H, Swanson KS, Ebert M, Kerns R. The biopsychosocial model of pain and pain management. Behavioral and Psychopharmacologic Pain Management 2011;16–43. [Google Scholar]
- 46.Villemure C, Bushnell CM. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain 2002;95:195–9. [DOI] [PubMed] [Google Scholar]
- 47.Hejrati N, Spieler D, Samuel R, Regli L, Weyerbrock A, Surbeck W. Conscious experience and psychological consequences of awake craniotomy. World Neurosurgery 2019;129:e381–6. [DOI] [PubMed] [Google Scholar]
- 48.Danks RA, Rogers M, Aglio LS, Gugino LD, Black PM. Patient tolerance of craniotomy performed with the patient under local anesthesia and monitored conscious sedation. Neurosurgery 1998;42:28–36. [DOI] [PubMed] [Google Scholar]
- 49.Costa L. d C M, Maher CG, McAuley JH, Hancock MJ, Smeets RJEM. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain 2011;15:213–9. [DOI] [PubMed] [Google Scholar]
- 50.Buescher KL, Johnston JA, Parker JC, et al. Relationship of self-efficacy to pain behavior. J Rheumatol 1991;18:968–72. [PubMed] [Google Scholar]
- 51.Palese A, Skrap M, Fachin M, Visioli S, Zannini L. The experience of patients undergoing awake craniotomy: in the patients’ own words. A qualitative study. Cancer Nurs 2008;31:166–72. [DOI] [PubMed] [Google Scholar]
- 52.Katon W, Sullivan M, Walker E. Medical symptoms without identified pathology: relationship to psychiatric disorders, childhood and adult trauma, and personality traits. Ann Intern Med 2001;134:917–25. [DOI] [PubMed] [Google Scholar]
- 53.Jääskeläinen J, Randell T, Awake craniotomy in glioma surgery. Local therapies for glioma present status and future developments. Springer; 2003:31–5. [DOI] [PubMed] [Google Scholar]
- 54.Sarang A, Dinsmore J. Anaesthesia for awake craniotomy-evolution of a technique that facilitates awake neurological testing. Br J Anaesth 2003;90:161–5. [DOI] [PubMed] [Google Scholar]
- 55.Conte V, Baratta P, Tomaselli P, Songa V, Magni L, Stocchetti N. Awake neurosurgery: an update. Minerva Anestesiol 2008;74:289–92. [PubMed] [Google Scholar]
- 56.Suls J, Wan CK. Effects of sensory and procedural information on coping with stressful medical procedures and pain: a meta-analysis. J Consult Clin Psychol 1989;57:372–9. [DOI] [PubMed] [Google Scholar]
- 57.Webber GC. Patient education: a review of the issues. Med Care 1990;28:1089–103. [PubMed] [Google Scholar]
- 58.Devine EC. Effects of psychoeducational care for adult surgical patients: a meta-analysis of 191 studies. Patient Educ Couns 1992;19:129–42. [DOI] [PubMed] [Google Scholar]
- 59.Asch SM, Atkins DV, Walling A. If kindness were a drug, the FDA would approve it. Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marci CD, Ham J, Moran E, Orr SP. Physiologic correlates of perceived therapist empathy and social-emotional process during psychotherapy. J Nerv Ment Dis 2007;195:103–11. [DOI] [PubMed] [Google Scholar]
- 61.Merluzzi TV, Nairn RC, Hegde K, Martinez Sanchez MA, Dunn L. Self-efficacy for coping with cancer: revision of the Cancer Behavior Inventory (version 2.0). Psychooncology 2001;10:206–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hojat M, Louis DZ, Markham FW, Wender R, Rabinowitz C, Gonnella JS. Physicians’ empathy and clinical outcomes for diabetic patients. Acad Med 2011;86:359–64. [DOI] [PubMed] [Google Scholar]
- 63.Weng H-C, Steed JF, Yu S-W, et al. The effect of surgeon empathy and emotional intelligence on patient satisfaction. Adv in Health Sci Educ 2011;16:591–600. [DOI] [PubMed] [Google Scholar]
- 64.Dewar B, Nolan M. Caring about caring: Developing a model to implement compassionate relationship centred care in an older people care setting. Int J Nurs Stud 2013;50:1247–58. [DOI] [PubMed] [Google Scholar]
- 65.Halifax J The precious necessity of compassion. J Pain Symptom Manage 2011;41:146–53. [DOI] [PubMed] [Google Scholar]
- 66.Spanner S, Sayer L. Is the Amsterdam Preoperative Anxiety and Information Scale (APAIS) a valid tool in guiding the management of preoperative anxiety in adult patients. A Literature Review. Journal of Nursing and Practice 2019;3. [Google Scholar]
- 67.Khu KJ, Doglietto F, Radovanovic I, et al. Patients’ perceptions of awake and outpatient craniotomy for brain tumor: a qualitative study. J Neurosurg 2010;112:1056–60. [DOI] [PubMed] [Google Scholar]
- 68.Manchella S, Khurana VG, Duke D, Brussel T, French J, Zuccherelli L. The experience of patients undergoing awake craniotomy for intracranial masses: expectations, recall, satisfaction and functional outcome. Br J Neurosurg 2011;25:391–400. [DOI] [PubMed] [Google Scholar]
- 69.Hansen E, Seemann M, Zech N, Doenitz C, Luerding R, Brawanski A. Awake craniotomies without any sedation: the awake-awake-awake technique. Acta Neurochir (Wien) 2013;155:1417–24. [DOI] [PubMed] [Google Scholar]
- 70.Cameron CD, Fredrickson BL. Mindfulness facets predict helping behavior and distinct helping-related emotions. Mindfulness 2015;6:1211–8. [Google Scholar]
- 71.Cameron RA, Mazer BL, DeLuca JM, Mohile SG, Epstein RM. In search of compassion: a new taxonomy of compassionate physician behaviours. Health Expect 2015;18:1672–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen R, Smith-Cohn M, Cohen AL, Colman H. Glioma subclassifications and their clinical significance. Neurotherapeutics 2017;14:284–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


