Abstract
Globally, malaria and human immunodeficiency virus (HIV) are both independently associated with a massive burden of disease and death. While their co-infection has been well studied for Plasmodium falciparum, scarce data exist regarding the association of P. vivax and HIV. In this cohort study, we assessed the effect of HIV on the risk of vivax malaria infection and recurrence during a 4-year follow-up period in an endemic area of the Brazilian Amazon. For the purpose of this study, we obtained clinical information from January 2012 to December 2016 from two databases. HIV screening data were acquired from the clinical information system at the tropical hospital Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD). The National Malaria Surveillance database (SIVEP malaria) was utilized to identify malaria infections during a 4-year follow-up period after diagnosis of HIV. Both datasets were combined via data linkage. Between 2012 and 2016, a total of 42,121 people were screened for HIV, with 1569 testing positive (3.7%). Out of all the patients diagnosed with HIV, 198 had at least one episode of P. vivax malaria in the follow-up. In the HIV-negative group, 711 participants had at least one P. vivax malaria episode. When comparing both groups, HIV patients had a 6.48 [(5.37–7.83); P < 0.0001] (adjusted relative risk) greater chance of acquiring P. vivax malaria. Moreover, being of the male gender [ARR = 1.41 (1.17–1.71); P < 0.0001], Amerindian ethnicity [ARR = 2.77 (1.46–5.28); P < 0.0001], and a resident in a municipality of the Metropolitan region of Manaus [ARR = 1.48 (1.02–2.15); P = 0.038] were independent risk factors associated with an increased risk of clinical malaria. Education ≥ 8 years [ARR = 0.41 (0.26–0.64); P < 0.0001] and living in the urban area [ARR = 0.44 (0.24–0.80); P = 0.007] were associated to a lower risk of P. vivax malaria. A total of 28 (14.1%) and 180 (25.3%) recurrences (at least a second clinical malaria episode) were reported in the HIV-positive and HIV-negative groups, respectively. After adjusting for sex and education, HIV-positive status was associated with a tendency towards protection from P. vivax malaria recurrences [ARR = 0.55 (0.27–1.10); P = 0.090]. HIV status was not associated with hospitalizations due to P. vivax malaria. CD4 + counts and viral load were not associated with recurrences of P. vivax malaria. No significant differences were found in the distribution of parasitemia between HIV-negative and HIV-positive P. vivax malaria patients. Our results suggest that HIV-positive status is a risk factor for vivax malaria infection, which represents an additional challenge that should be addressed during elimination efforts.
Subject terms: HIV infections, Malaria
Introduction
Malaria and HIV/AIDS are major public health problems and globally have a great geographical overlap, with most affected people living in sub-Saharan Africa, the Indian subcontinent and Southeast Asia, Latin America, and the Caribbean1. This overlap favors Plasmodium-HIV co-infections, which are responsible for deaths of millions of individuals each year2–4. Although the consequences of co-infection with HIV-Plasmodium are not fully understood, the available evidence suggests that infections act synergistically and together may result in worse clinical outcomes3–5. Recent studies show that people living with HIV have more episodes of severe and lethal P. falciparum malaria, and more malaria treatment failures3–9. HIV can increase parasitic loads in malaria patients and consequently increase malaria transmission rates7,10–12. Moreover, P. falciparum infection may increase HIV plasma viral load and reduce TCD4 + cells13,14; this, in turn, suggests that malaria can lead to a faster progression from HIV infection to AIDS and increased risk of therapeutic failure after antimalarial chemotherapy15. However, the evidence on HIV-Plasmodium co-infection is mostly restricted to P. falciparum endemic areas in sub-Saharan Africa where the two diseases have the highest burden16.
The impact of HIV on susceptibility to non-P. falciparum malaria is unknown, and further research should be conducted in other settings to assess the interaction of HIV infection with other Plasmodium species, such as P. vivax17. Globally, ~ 229 million malaria cases were reported in 2019, with at least 6.9 million cases due to P. vivax (3%)18. Despite its comparatively lower numbers, P. vivax is geographically the most widely distributed cause of malaria, with its highest endemicity found in Southeast Asia, Central Asia, and Latin America, and a much less frequent presence in Africa19. P. vivax is a challenging parasite to eliminate due to its peculiar biology, which causes the different origins of a P. vivax malaria episode that include acquired infection by mosquito bites, relapse from liver-stage hypnozoites, and recrudescence from blood-stage treatment failure20. Clinically, P. vivax infection ranges from asymptomatic individuals carrying low parasitemia to severe disease and death21. Reports of HIV-P. vivax co-infection are scarce in the literature, with only a few studies describing their clinical outcomes22–27. In one study in the Brazilian Amazon, of 21 patients with HIV-P. vivax co-infection hospitalized in a referral hospital, severe malaria was diagnosed in 5 (23.8%), and one patient died (4.8%)27.
Although over the past three decades, there has been clear evidence of an association between the increased risk of falciparum malaria among HIV-infected patients, research on co-infection with the most widely distributed vivax malaria has so far been neglected. In this study, we evaluated the impact of HIV infection on P. vivax malaria incidence and the frequency of recurrences in a malaria endemic area of the Brazilian Amazon. The hypothesis of this study was that HIV infection may increase P. vivax malaria susceptibility and recurrence rates.
Results
Participant characteristics
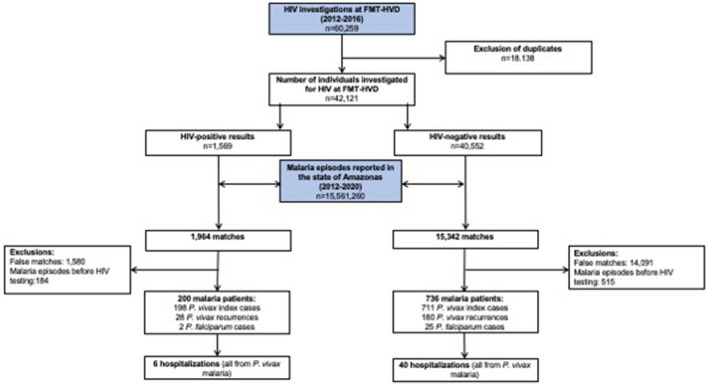
A total of 60,259 HIV tests were performed at the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (FMT-HVD) between January 2012 and December 2016. After removing duplicates for individuals tested more than once, 42,121 were included in the study, with 1569 being classified as HIV- positive (3.7%) and 40,552 as HIV-negative (96.3%) (Fig. 1). Compared with the HIV-negative group, the HIV-positive group had a higher proportion of males (P < 0.001) and a younger mean age (P < 0.001). Regarding municipality of residence, HIV-positive status was less prevalent in Manaus compared to the municipalities of the metropolitan region (P = 0.046) (Table 1).
Figure 1.
Flowchart of participants’ inclusion and linkage between HIV status and malaria episode databases, Amazonas state, Brazil.
Table 1.
Demographic characteristics of the included participants according to HIV status, tested at the FMT-HVD, Brazil, between 2012 and 2016.
| Variable | HIV status | P-value | ||
|---|---|---|---|---|
| Total (n = 42,121) | Negative (40,552) | Positive (1569) | ||
| Male | 23,110/42,121 (54.9%) | 22,046/40,552 (54.4%) | 1064/1569 (67.8%) | < 0.001 |
| Mean age (years; ± SD) | 37.7 ± 15.5 | 37.7 ± 15.5 | 35.7 ± 14.2 | < 0.001 |
| Education (years) | 0.036 | |||
| < 3# | 385/20,410 (2%) | 361/19,341 (2%) | 24/1069 (2%) | |
| 4–7 | 7702/20,410 (37.7%) | 7263/19,341 (37.5%) | 439/1069 (41%) | |
| ≥ 8 | 12,323/20,410 (60.3%) | 11,717/19,341(60.5%) | 606/1069 (57%) | |
| Ethnicity (self-declared) | 0.43 | |||
| Admixed# | 21,906/24,395(89.8%) | 20,818/23,194(89.8%) | 1088/1201 (90.6%) | |
| White | 1918/24,395(7.9%) | 1832/23,194 (7.9%) | 86/1201 (7.2%) | |
| Black | 421/24,395(1.7%) | 398/23,194 (1.7%) | 23/1201 (1.9%) | |
| Amerindian | 150/24,395(0.6%) | 146/23,194(0.6%) | 4/1201 (0.3%) | |
| Place of residence | 0.20 | |||
| Urban área | 40,847/40,983 (99.7%) | 39,298/39,426 (99.7%) | 1549/1557 (99.5%) | |
| Rural área | 136/40,983 (0.3%) | 128/39,426 (0.3%) | 8/1557 (0.5%) | |
| Residence municipality | 0.064 | |||
| Manaus# | 38,358/41,021 (93.5%) | 36,924/39,464 (93.6%) | 1434/1557 (92.1%) | |
| Metropolitan region | 1381/41,021 (3.4%) | 1315/39,464 (3.3%) | 66/1557 (4.2%) | |
| Others | 1282/41,021 (3.1%) | 1225/39,464 (3.1%) | 57/1557 (3.7%) | |
#Reference groups.
HIV-status and malaria episode database matches
A total of 15,561,260 malaria tests were performed in the state of Amazonas between January 2012 and December 2020, according to the SIVEP-malaria database. The linkage process between the databases for malaria and HIV resulted in 1,954 and 15,342 matches between HIV-positive and HIV-negative groups, respectively. After a detailed analysis and further exclusion of false matches and malaria episodes reported before HIV testing, a total of 936 participants were identified as having had at least one episode of P. vivax malaria during follow-up, 198 in the HIV-positive group and 711 in the HIV-negative group (Fig. 1).
Impact of HIV status and other variables on malaria incidence
Table 2 summarizes the results of the univariate and multivariate logistic regression models and evaluates the factors associated with P. vivax malaria incidence. HIV-positive status [ARR = 6.48 (5.37–7.83); P < 0.0001], male gender [ARR = 1.41 (1.17–1.71); P < 0.0001], Amerindian ethnicity [ARR = 2.77 (1.46–5.28); P = 0.002], and residence in a municipality in the Metropolitan region of Manaus [ARR = 1.48 (1.02–2.15); P = 0.038] were independently associated with an increased risk of P. vivax malaria. Education ≥ 8 years [ARR = 0.41 (0.26–0.64); P < 0.0001] and living in the urban area [ARR = 0.44 (0.24–0.80); P = 0.007] were independently associated to a decreased risk of P. vivax malaria.
Table 2.
Impact of HIV-status and other variables on P. vivax malaria incidence in the state of Amazonas, Brazil, 2012–2020.
| Variable | RR (CI95%) | P | ARR (CI95%) | P |
|---|---|---|---|---|
| HIV-positive | 7.20 (6.20–8.36) | < 0.0001 | 6.48 (5.37–7.83) | < 0.0001 |
| Male | 1.59 (1.39–1.82) | < 0.0001 | 1.41 (1.17–1.71) | < 0.0001 |
| Age (years; ± SD) | 1.00 (0.99–1.01) | 0.412 | ||
| Education (years) | ||||
| < 3# | ||||
| 4–7 | 0.68 (0.43–1.06) | 0.092 | 0.75 (0.48–1.16) | 0.192 |
| ≥ 8 | 0.34 (0.22–0.54) | < 0.0001 | 0.41 (0.26–0.64) | < 0.0001 |
| Ethnicity | ||||
| Admixed# | ||||
| White | 0.80 (0.36–1.77) | 0.577 | ||
| Black | 1.17(0.85–1.61) | 0.336 | ||
| Amerindian | 4.47 (2.49–8.04) | < 0.0001 | 2.77 (1.46–5.28) | 0.002 |
| Place of residence | ||||
| Urban area | ||||
| Rural area | 0.23 (0.13–0.38) | < 0.0001 | 0.44 (0.24–0.80) | 0.007 |
| Residence municipality | ||||
| Manaus# | ||||
| Metropolitan region | 1.65 (1.24–2.20) | 0.001 | 1.48 (1.02–2.15) | 0.038 |
| Others | 1.59 (1.18–2.16) | 0.002 | 1.36 (0.91–2.03) | 0.135 |
#Reference groups.
Impact of HIV status on recurrences of P. vivax malaria
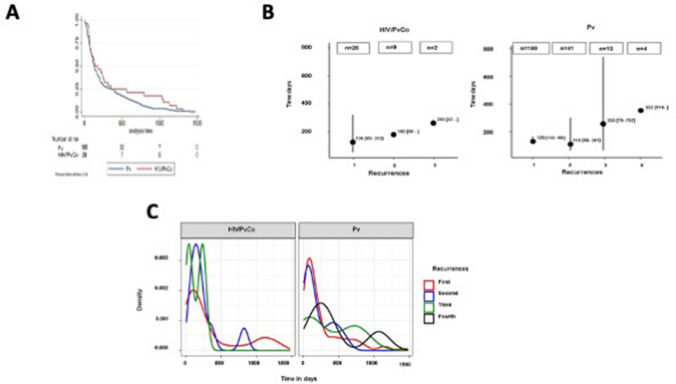
A total of 28 (14.1%) and 180 (25.3%) recurrences were reported in the HIV-positive and HIV-negative groups, respectively. In the univariate analysis, HIV-positive status was associated with protection from recurrences of P. vivax malaria [RR = 0.57 (0.40–0.82); P = 0.003]. After adjusting for sex and education, the association between HIV-positive status with protection from recurrences was not detected [ARR = 0.55 (0.27–1.10); P = 0.090] (Table 3 and Fig. 2).
Table 3.
Impact of HIV-status and other variables on P. vivax recurrences in the state of Amazonas, Brazil, 2012–2020.
| Variable | RR (CI95%) | P | ARR (CI95%) | P |
|---|---|---|---|---|
| HIV-positive | 0.57 (0.40–0.82) | 0.003 | 0.55 (0.27–1.10) | 0.090 |
| Male | 0.80 (0.63–1.03) | 0.078 | 0.75 (0.47–1.19) | 0.215 |
| Age (years; ± SD) | 1.00 (0.99–1.01) | 0.935 | ||
| Education (years) | ||||
| < 3# | ||||
| 4–7 | 0.63 (0.31–1.27) | 0.197 | 1.06 (0.29–3.88) | 0.928 |
| ≥ 8 | 0.59 (0.30–1.17) | 0.131 | 1.06 (0.30–3.77) | 0.922 |
| Ethnicity | ||||
| Admixed# | ||||
| White | 0.52 (0.08–3.41) | 0.495 | ||
| Black | 0.72 (0.42–1.22) | 0.221 | ||
| Amerindian | 1.04 (0.39–2.74) | 0.938 | ||
| Place of residence | ||||
| Urban area | ||||
| Rural area | 0.78 (0.34–1.80) | 0.561 | ||
| Residence municipality | ||||
| Manaus# | ||||
| Metropolitan region | 1.11 (0.67–1.84) | 0.694 | ||
| Others | 0.70 (0.35–1.41) | 0.321 |
#Reference groups.
Figure 2.
(A) Kaplan–Meier survival estimates comparing the time to first Plasmodium vivax malaria recurrence (in days). (B) Median of the number of Plasmodium vivax malaria recurrences by HIV status groups throughout the follow-up. (C) Graphical representation of Plasmodium vivax malaria recurrences densities throughout the follow-up. Time zero was the date of the index case for all recurrences.
Impact of HIV status on malaria hospitalizations
Six hospitalizations due to P. vivax malaria were documented in the HIV-positive group (2.7%) and 40 in the HIV-negative group (4.5%) [RR = 0.58 (0.22–1.32); P = 0.216]. No deaths from malaria were reported.
Impact of CD4+ counts and viral load on malaria incidence and recurrence of P. vivax in HIV-positive patients
Among individuals with HIV-positive status, no association was found between CD4+ counts at baseline and the rates of total malaria and P. vivax malaria. Individuals carrying a viral load that was < 50 copies/mL at baseline presented a higher risk of malaria [ARR = 3.43 (2.01–5.85); P < 0.001] and P. vivax malaria [ARR = 3.40 (1.99–5.80); P < 0.001] compared to those with a viral load > 100,000 copies/mL, after adjusting by gender, ethnicity, municipality of residence, years of education and zone of residence (rural or urban) (Table 4). CD4 + counts and viral load were not associated with recurrences in P. vivax malaria (Table 4).
Table 4.
Impact of HIV-status and other variables on P. vivax recurrences in HIV-positive patients in the state of Amazonas, Brazil, 2012–2020.
| Variable | RR (CI95%) | P | ARR (CI 95%)¶ | P |
|---|---|---|---|---|
| CD4 cell counts (/mm3) | ||||
| < 200# | 1 | – | 1 | – |
| 200–350 | 1.10 (0.32–3.76) | 0.875 | 1.07 (0.30–3.97) | 0.692 |
| 350–500 | 1.78 (0.54–5.85) | 0.344 | 2.01 (0.40–6.37) | 0.317 |
| > 500 | 0.89 (0.26–3.06) | 0.852 | 0.76 (0.22–3.14) | 0.890 |
| Viral load (copies/mL) | ||||
| > 100,000# | 1 | – | 1 | – |
| 10,000–100,000 | 0.59 (0.12–2.93) | 0.519 | 0.57 (0.12–2.67) | 0.479 |
| 1000–10,000 | 1.77 (0.47–6.64) | 0.395 | 2.01 (0.54–7.51) | 0.302 |
| 50–1000 | 0.59 (0.07–4.71) | 0.619 | 0.545 (0.08–3.94) | 0.548 |
| < 50 | 0.83 (0.26–2.68) | 0.755 | 0.749 (0.24–2.36) | 0.623 |
#Reference groups.
¶Adjusted by gender and years of education.
Impact of HIV status on malaria parasitemia
No significant differences were found in the distribution of parasitemia between HIV-negative and HIV-positive P. vivax malaria patients.
Discussion
The high prevalence of HIV and P. vivax malaria in tropical and subtropical regions may increase the simultaneous co-infection by these two pathogens. In this study, HIV-positive status was associated to increased risk of P. vivax malaria, as previously observed in Africa with P. falciparum malaria3,13,37. To the best of our knowledge, there is no information on the effect of HIV infection on P. vivax susceptibility, but as occurs in P. falciparum malaria38–40, abnormalities in the immune response may increase susceptibility to P. vivax malaria by compromising naturally acquired immunity41–43. A mixed pattern of proinflammatory and regulatory biomarkers involving a robust IL-10 and IL-6 axis is produced in P. vivax malaria43 and, in recurrent P. vivax episodes, an unbalanced CD4+/CD8+ T-cell ratio was found and is associated with a significant increase in IL-10 levels44. Genetic polymorphisms in TLRs play a role in P. vivax susceptibility and parasitemia42, possibly by activation of IL-6, IFN-γ, IL-10 pathways45. From an integrated perspective, mechanisms behind recurrences and subclinical P. vivax infections are related to inhibitory receptors, T regulatory cells, and IL‐10, with a poor induction of immunological memory cells and inefficient T effector cells46. Since HIV infects CD4 + T cells, a disruption of the components of both the cellular and humoral aspects of the immune response is observed, thus predisposing individuals to many infections43.
Evidence from modelling studies47 and historical observations suggests that fevers from other systemic parasitic and bacterial infections can activate P. vivax hypnozoites48. If this is true, opportunistic infections in HIV/AIDS patients could also trigger new episodes of P. vivax malaria through relapse. Additionally, reports also suggest that antimalarial treatment failure in P. falciparum malaria is more common in HIV-infected patients with low CD4-cell counts compared to those not infected with HIV49. In this study, HIV-positive status was associated with a tendency towards protection from recurrences of P. vivax malaria. Although some preliminary evidence demonstrated antiplasmodial50 and hypnozoiticidal51 activities of HIV protease inhibitors, our results point to another explanation for a lower frequency of recurrences in HIV patients, since, overall, P. vivax malaria was significantly less common in this group. Moreover, prophylaxis of opportunistic infections using trimethoprim/sulfamethoxazole would possibly minimize the clinical manifestations of recurrences, as seen in P. falciparum malaria52. As the HIV patients in this study were followed in a tertiary institution for infectious diseases, including malaria, they should have received more information about the need to complete antimalarial treatment, in a more rigorous clinical follow-up to reach a radical cure. It should also be considered that participants with HIV/AIDS may have a lower body weight than those in the HIV-negative group, which could lead to proportionally higher plasma levels of primaquine and its active metabolites, resulting in higher radical cure rates.
In this study, no significant differences were found between HIV-negative and HIV-positive P. vivax malaria patients in the distribution of parasitemia and severity. This differs from studies linking HIV to P. falciparum parasitemia, which demonstrate that HIV is associated with an increase in parasitemia and severity53–56. However, the number of severe cases was very small, which may have limited the statistical power of this comparison.
To the best of our knowledge, this is the only cohort that has been studied to assess the influence of HIV infection on P. vivax infections. Regardless of the large number of coinfections, some limitations were imposed by the study design, and these should be stated. HIV patients may be more likely than their HIV negative counterparts to seek treatment for a febrile illness than HIV-negative individuals. HIV patients are more likely to need ongoing outpatient monitoring and therefore will interact with the medical system more frequently.
In accordance with the case reporting procedures, only positive results for malaria are registered in SIVEP-malaria, which prevents to determine the universe of tested individuals, both in HIV positive and controls, potentially generating a substantial detection bias. In the Brazilian Amazon, however, fever is closely associated with malaria by the inhabitants and, in the case of the presence of this sign, testing for malaria is routine in all health units. As a retrospective cohort study, such confounders related to different access to the health system could not be collected. Additionally, as per the Brazilian Ministry of Health and local protocols, the treatment for both diseases is provided free-of-charge and under prescription, which means the diseases are necessarily conditioned to case reporting, and this is expected to minimize underreporting and related bias. Another weakness is regarding the absence of a unique identifier that allows for a deterministic linkage between distinct databases. In order to ensure high accuracy in this process, we used a high throughput algorithm that was previously described35 and that has both high sensitivity and specificity when used for probabilistic linkage of public health datasets.
This study hypothesizes that HIV-infected patients are more likely to present P. vivax malaria. HIV counselling and testing programs, as well as approaching patients with febrile illnesses for the diagnosis of malaria should be strengthened to improve HIV/AIDS control strategies and the determination of malaria and HIV co-infection to help and articulate prevention and HIV/malaria control. With continuing interest in the elimination of malaria, it is necessary to have a better understanding of HIV-P. vivax co-infection, with the goal of reducing the burden of P. vivax malaria. Prospective studies are needed to confirm our hypothesis.
Methods
Ethics statement
This study was conducted in accordance with the principles of the Declaration of Helsinki and the guidelines of Good Clinical Practice of the International Harmonization Conference. The study was approved by the Ethics Review Board (ERB) of the Fundação de Medicina Tropical Dr. Heitor Vieira Dourado (CAAE: 09,714,619.2.0000.0005). The ERB gave a waiver for informed consent. After database linkages, the final dataset was anonymized before statistical analysis.
Study site
Out of all malaria cases in Brazil, more than 99% occur in the Brazilian Amazon region, of which about 89% are attributable to P. vivax infections28. The national HIV prevalence is estimated at 0.4%, with a noticeably higher HIV incidence rate of 34.8/100,000 population in the Brazilian Amazon, compared to the overall national rate of 17.8/100,00029.
FMT-HVD is a tertiary, referral healthcare center for infectious diseases in Manaus, state of Amazonas, western Brazilian Amazon and, in its outpatient clinic and emergency department, it receives all patients seeking care for infectious and parasitic diseases or those referred from other health units in the city of Manaus and the surrounding areas. HIV testing is provided after careful health screening for possible epidemiological risk and exposure. Diagnosis and treatment are free of charge. For both diseases, any positive HIV or malaria diagnoses are compulsorily recorded in structured forms available on-line as part of the Brazilian Ministry of Health's notification system. FMT-FVD is responsible for the follow-up and management of ~ 80% of the HIV/AIDS cases in the state of Amazonas.
Study design and exposure
This is a concurrent, cohort study, based on surveillance data from patients tested for HIV at FMT-HVD and malaria diagnosis data and case recurrences recorded in the National Malaria Epidemiological Surveillance Information System (SIVEP Malaria) between January 2012 and December 2016. In this study, exposure was defined as a confirmed HIV-positive status, according to the national HIV infection diagnosis guideline30, as reported on the FMT-HVD Information System (iDoctor).
In short, a rapid diagnostic test (RDT) (Bio-Manguinhos, Fiocruz) with 100% sensitivity and 99.8% specificity31 was used in mobile testing campaigns and at the FMT-HVD outpatient clinics. For each positive test, blood samples were collected at the FMT-HVD laboratory, and the western blot technique was used to confirm the diagnosis, and the patient was then referred to the HIV ward for consultations, psychological support and ART therapy. All the remaining individuals with HIV-negative test results were included as non-exposed controls. HIV-positive patients were stratified by CD4 cell counts (/mm3) and viral load (copies/mL), via data gathered from the FMT-HVD SISCEL (Laboratory Test Control System) database. The predictor variables sex, age, education (in years of schooling), self-reported ethnicity, place of residence (rural/urban), and municipality of residence were also used. All HIV-positive patients were treated according to the Brazilian Ministry of Health Guidelines32.
Outcomes
The present study was designed to determine the risk of (i) malaria infection, (ii) P. vivax malaria, (iii) P. falciparum infection, (iv) P. vivax malaria recurrences up to 90, 180 days and 4-years, (v) malaria-associated hospitalizations, (vi) malaria-associated deaths, in a 4-year follow-up, and (vii) parasitemia. Recurrence was defined as the occurrence of at least one additional malaria infection during the follow-up period. Furthermore, the study aimed to determine any association between age, sex or parasitemia as a potential additional risk factor for malaria reinfection. In Brazil, thick blood smears (TBS) are routinely performed for the diagnosis of malaria, and are prepared according to the Walker technique33 and then evaluated by a local microscopist. The results of peripheral parasitemia are given using the following semi-quantitative system: < 1/2 + (< 200 parasites/mm3); 1/2 + (200–300 parasites/mm3); 1 + (301–500 parasites/mm3); 2 + (501–10,000 parasites/mm3); 3 + (10,001–100,000 parasites/mm3); and 4 + (> 100,000 parasites/mm3). All positive slides and 10% of negative slides are routinely reviewed at a referral unit with experienced microscopists. In case of divergence, the reviewed result is updated on the on-line system. Only participants with a positive TBS for Plasmodium spp. are treated according to the Brazilian Anti-Malarial Treatment Guidelines34. The malaria patients' data are entered into the SIVEP Malaria (National Malaria Surveillance) database. Data on malaria-associated hospitalizations and deaths were extracted from HIS (Hospitalization Information System) and MIS (Mortality Information System) databases, respectively.
Data processing and record linkage strategy
Duplicated HIV tests were excluded from the iDoctor database (HIV-status database; 2012–2016 period) and, in the case of individuals that had had more than one HIV test in the period, the first positive result was considered. Patients exposed to disease (HIV-positive) and the controls (HIV-negative) were included in the study according to the day of confirmation of HIV-status. Patients entered and left the study at different points in time. The HIV-status database and the SIVEP-malaria database were both deduplicated and further the record linkage was performed (Malaria database; 2012–2020) of Tucuxi-BLAST software, which uses the DNA-encoded approach35 and was installed on a Linux Workstation, Intel Core i7-8700, with 32 GB of RAM and 12 physical processing cores. In the absence of a unique identifier, Tucuxi-BLAST used the patient's name, date of birth and mother's name. Malaria episodes reported before the participants' entries were then excluded from the generated database. All remaining matches were double-checked and false matches were also excluded. This inspection included visual confirmation of homonyms, possible siblings (twins), and duplicity. Finally, the list of malaria cases obtained was linked to the HIS (Hospitalization Information System) and MIS (Mortality Information System) databases to investigate these outcomes. We obtained a final selection of pairs identified as likely to be from the same patients by automatic verification, applying a probability threshold (probability > 0.7) for all linkages. A final identification (ID) was created for all included participants.
Statistical analysis
Descriptive statistics were used for demographic variables. Student's t test was used to compare means while Fisher's exact or the Chi-squared (X2) test were used to compare proportions, as appropriate. Crude relative risk (RR) with its respective 95% confidence interval (95% CI) was determined in a univariate analysis. Logistic regression was used for the multivariate analyses and the adjusted RR (ARR) with 95% CI were also estimated. A log binomial multivariate generalized linear regression was performed using an automated backward and forward stepwise estimation. All variables that were associated with dependent variables at a significance level of P < 0.200 in the univariate analysis were included in the multivariate analysis. Statistical significance was considered when P < 0.05 in the Hosmer–Lemeshow goodness-of-fit test. Kaplan–Meier survival estimates were used to compare recurrences over follow-up time. Additionally, a survival analysis for recurrent events was performed36. A two-tailed P < 0.05 was considered significant. The statistical analyses were carried out using R software (version 4.1.0), R Studio (version 1.4.17), and Stata v.13.0 (Stata Corp. LP, College Station, TX).
Supplementary Information
Acknowledgements
AGCM was the beneficiary of postdoctoral fellowships from CAPES (PNPD, Grant No. 88887.353550/2019-00). JCSS received a doctoral fellowship from FAPESP (Grant No. 2019/27139-5). AGCM was the beneficiary of postdoctoral fellowships from CAPES (PNPD, Grant No. 88887.353550/2019-00). JCSS received a doctoral fellowship from FAPESP (Grant No. 2019/27139-5).This study was funded by Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM- Resolução N. 002/2008, 007,/2018 e 005/2019-PRÓ-ESTADO AND POSGRAD 2021). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
Concept and design: C.V.C.G., V.S.S., W.M., F.V. Acquisition, analysis, or interpretation of data: C.V.C.G., V.S.S., W.M., F.V., P.M., B.M.S., M.A.S.M., G.C.M., H.I.N., F.E.M.E., J.D.A.A., J.C.S.S., D.C.B.S., A.G.C., Q.B., M.L. Critical revision of the manuscript for important intellectual content: C.V.C.G., V.S.S., W.M., F.V., P.M., B.M.S., M.A.S.M., G.C.M., H.I.N., F.E.M.E., J.D.A.A., J.C.S.S., D.C.B.S., A.G.C., Q.B., M.L. Statistical analysis: C.V.C.G., V.S.S., W.M., A.A.S.B., A.V.S.N. Obtained funding: M.L., V.S.S., W.M., Supervision: C.V.C.G., V.S.S., W.M., M.L.
Data availability
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-13256-4.
References
- 1.Frischknecht F, Fackler OT. Experimental systems for studying plasmodium/HIV coinfection. FEBS Lett. 2016;590:2000–2013. doi: 10.1002/1873-3468.12151. [DOI] [PubMed] [Google Scholar]
- 2.Secretaria de Vigilância em Saúde|Ministério da Saúde. Boletim Epidemiológico HIV/Aids|2019, 72 (2019)
- 3.Drotman DP, Mcdade JE, Potter P, Beard CB, Collins F, Bell D, et al. Malaria attributable to the HIV-1 epidemic; sub-Saharan Africa. Emerg. Infect. Dis. 2005;11:1410–1419. doi: 10.3201/eid1109.050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skinner-Adams TS, McCarthy JS, Gardiner DL, Andrews KT. HIV and malaria co-infection: interactions and consequences of chemotherapy. Trends Parasitol. 2008;24:264–271. doi: 10.1016/j.pt.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Perrault SD, Hajek J, Zhong K, Owino SO, Sichangi M, et al. Human immunodeficiency virus co-infection increases placental parasite density and transplacental malaria transmission in Western Kenya. Am. J. Trop. Med. Hyg. 2009;80:119–125. doi: 10.4269/ajtmh.2009.80.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, et al. Childhood malaria in a region of unstable transmission and high human immunodeficiency virus prevalence. Pediatr. Infect. Dis. J. 2003;22:1057–1063. doi: 10.1097/01.inf.0000101188.95433.60. [DOI] [PubMed] [Google Scholar]
- 7.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, et al. HIV infection as a cofactor for severe falciparum malaria in adults living in a region of unstable malaria transmission in South Africa. AIDS. 2004;18:547–554. doi: 10.1097/00002030-200402200-00023. [DOI] [PubMed] [Google Scholar]
- 8.Cohen C, Karstaedt A, Frean J, Thomas J, Govender N, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin. Infect. 2005;41:1631–1637. doi: 10.1086/498023. [DOI] [PubMed] [Google Scholar]
- 9.Otieno RO, Ouma C, Ong'echa JM, Keller CC, Were T, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–280. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 10.Gasasira, A.F. Interactions between HIV infection and malaria in children living in sub-Saharan Africa in the Era of widening access to improved interventions, 80 (2010).
- 11.Hoffman IF, Jere CS, Taylor TE, Munthali P, Dyer JR, et al. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 12.ter Kuile FO, Parise ME, Verhoeff FH, Udhayakumar V, Newman RD, et al. The burden of co-infection with human immunodeficiency virus type 1 and malaria in pregnant women in sub-saharan Africa. Am. J. Trop. Med. Hyg. 2004;71:41–54. doi: 10.4269/ajtmh.2004.71.41. [DOI] [PubMed] [Google Scholar]
- 13.Patnaik P, Jere CS, Miller WC, Hoffman IF, Wirima J, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J. Infect. Dis. 2005;192:984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
- 14.Van Geertruyden JP, Mulenga M, Kasongo W, Polman K, Colebunders R, et al. CD4 T-cell count and HIV-1 infection in adults with uncomplicated malaria. J. Acquir. Immune. Defic. Syndr. 2006;43:363–367. doi: 10.1097/01.qai.0000243125.98024.da. [DOI] [PubMed] [Google Scholar]
- 15.Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, et al. Effect of HIV-1 infection on antimalarial treatment outcomes in uganda: a population-based study. J. Infect. Dis. 2006;193:9–15. doi: 10.1086/498577. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO. World Malaria Report 2017. World Health Organization. (2017)
- 17.González R, Ataíde R, Naniche D, Menéndez C, Mayor A. HIV and malaria interactions: where do we stand? Exp. Rev. Anti. Infect. Ther. 2012;10:153–165. doi: 10.1586/eri.11.167. [DOI] [PubMed] [Google Scholar]
- 18.Malaria Report WM. World malaria report 2020. 20, avenue Appia CH-1211 Geneva 27 (2020).
- 19.Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller I, Galinski MR, Baird JK, Carlton JM, Kochar DK, et al. Key gaps in the knowledge of Plasmodium vivax, a neglected human malaria parasite. Lancet Infect. Dis. 2009;9:555–566. doi: 10.1016/S1473-3099(09)70177-X. [DOI] [PubMed] [Google Scholar]
- 21.Lacerda MVG, Fragoso SCP, Alecrim MGC, Alexandre MAA, Magalhães BML, et al. Postmortem characterization of patients with clinical diagnosis of plasmodium vivax malaria: to what extent does this parasite kill? Clin. Infect. Dis. 2012;55:67–74. doi: 10.1093/cid/cis615. [DOI] [PubMed] [Google Scholar]
- 22.Katongole-Mbidde E, Banura C, Kizito A. Blackwater fever caused by Plasmodium vivax infection in the acquired immune deficiency syndrome. Br. Med. J. 1988;296:827. doi: 10.1136/bmj.296.6625.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McIver LJ, Kippin AN, Parish ST, Whitehead OG. HIV, Malaria and pneumonia in a Torres Strait Islander male: a case report. Commun. Dis. Intell. 2010;34:448–449. [PubMed] [Google Scholar]
- 24.Tano ZN, Filho CEK, Breganó RM, Pavanelli WR, Ruzon UG. Hyperreactive malarious splenomegaly and aids: a case report. Braz. J. Infect. Dis. 2014;18:565–567. doi: 10.1016/j.bjid.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ranaweera, D., Kanchana Rajapaksha, R.M.J., Silva, P., Hettiarachchi, R., Gunasekera, W.M.K.T.A.W., et al. Severe Plasmodium vivax malaria, HIV, tuberculosis co-infection in a Sri Lankan traveller: case management and challenges during the prevention of malaria reintroduction phase. Malaria J.17, 1–8 (2018). [DOI] [PMC free article] [PubMed]
- 26.Montenegro-Idrogo JJ, Vargas-Gonzales R, Sihuincha M. Malaria in HIV-Infected patients: a series of cases in a peruvian hospital. Rev. Peru Med. Exp. Salud Publica. 2019;36:520–524. doi: 10.17843/rpmesp.2019.363.4370. [DOI] [PubMed] [Google Scholar]
- 27.Del-Tejo PL, Cubas-Veja N, Caraballo-Guerra C, da Silva BM, da Silva Valente J, et al. Should we care about Plasmodium vivax and HIV co-infection? A systematic review and a cases series from the Brazilian Amazon. Malar J. 2021;20:13. doi: 10.1186/s12936-020-03518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ministério da Saúde. Boletim Epidemiológico Malária. Secretaria de Vigilância em Saúde. Brasília. (2020).
- 29.Boletim Epidemiologico HIV/Aids 2020. Boletim Epidemiológico HIV/Aids2020. Secretaria de Vigilância em Saúde. 1, 68 (2020)
- 30.Manual Técnico para Diagnóstico da Infecção pelo HIV em Adultos e Crianças|Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. (2018)
- 31.Barroso. Validação De Reagentes Nacionais Para a Produção Do Tampão De Corrida Para O Teste Rápido Hiv-1/2. (2012)
- 32.Protocolos clínicos e manuais|Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis. (2017)
- 33.Basic malaria microscopy [Recurso electrónico]. Volume 2, Tutor’s guide/World Health Organization. (2010)
- 34.Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Imunização e Doenças Transmissíveis. Guia de tratamento da malária no Brasil [recurso eletrônico]/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Imunização e Doenças Transmissíveis. 2 ed. Brasília: Ministério da Saúde (2020)
- 35.Araújo, J.D.A. Integração de bases de dados administrativos com ferramentas genômicas [doi:10.11606/T.95.2021.tde-23072021-132101]. São Paulo: Bioinformática, Universidade de São Paulo (2021)
- 36.Thenmozhi M, Jeyaseelan V, Jeyaseelan L, Isaac R, Vedantam R. Survival analysis in longitudinal studies for recurrent events: applications and challenges. Clin. Epidemiol. Global Health. 2019;7:253–260. doi: 10.1016/j.cegh.2019.01.013. [DOI] [Google Scholar]
- 37.Ukibe N, Onyenekwe C, Ahaneku J, Meludu S, Ukibe S, et al. CD 4 + T-cells count in HIV-malaria co-infection in adult population in Nnewi, South Eastern Nigeria. Int. J. Biol. Chem. Sci. 2010;4:1593–1601. [Google Scholar]
- 38.Mount AM, Mwapasa V, Elliott SR, Beeson JG, Tadesse E, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet. 2004;363:1860–1867. doi: 10.1016/S0140-6736(04)16354-X. [DOI] [PubMed] [Google Scholar]
- 39.Hasang W, Dembo EG, Wijesinghe R, Molyneux ME, Kublin JG, et al. HIV-1 infection and antibodies to Plasmodium falciparum in adults. J. Infect. Dis. 2014;210:1407–1414. doi: 10.1093/infdis/jiu262. [DOI] [PubMed] [Google Scholar]
- 40.Musimbi ZD, Rono MK, Otieno JR, Kibinge N, Ochola-Oyier LI, et al. Peripheral blood mononuclear cell transcriptomes reveal an over-representation of down-regulated genes associated with immunity in HIV-exposed uninfected infants. Sci. Rep. 2019;9:18124. doi: 10.1038/s41598-019-54083-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costa AG, Ramasawmy R, Ibiapina HNS, Sampaio VS, Xábregas LA, et al. Association of TLR variants with susceptibility to Plasmodium vivax malaria and parasitemia in the Amazon region of Brazil. PLoS ONE. 2017;12:1–14. doi: 10.1371/journal.pone.0183840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa AG, Ramasawmy R, Val FFA, Ibiapina HNS, Oliveira AC, et al. Polymorphisms in TLRs influence circulating cytokines production in Plasmodium vivax malaria: TLR polymorphisms influence cytokine productions in malaria-vivax. Cytokine. 2018;110:374–380. doi: 10.1016/j.cyto.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 43.Antonelli LR, Junqueira C, Vinetz JM, Golenbock DT, Ferreira MU, et al. The immunology of Plasmodium vivax malaria. Immunol. Rev. 2020;293:163–189. doi: 10.1111/imr.12816. [DOI] [PubMed] [Google Scholar]
- 44.Chaves YO, Da Costa AG, Pereira MLM, De Lacerda MVG, Coelho-Dos-Reis JG, et al. Immune response pattern in recurrent Plasmodium vivax malaria. Malaria J. BioMed. Central. 2016;15:1–13. doi: 10.1186/s12936-016-1501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soares RR, Nakaie CR, Rodrigues-da-Silva RN, da Silva RL, Lima-Junior JDC, et al. Main B-cell epitopes of PvAMA-1 and PvMSP-9 are targeted by naturally acquired antibodies and epitope-specific memory cells in acute and convalescent phases of vivax malaria. Parasite Immunol. 2020;42:e12705. doi: 10.1111/pim.12705. [DOI] [PubMed] [Google Scholar]
- 46.da Costa AG, Antonelli LR, Costa PA, Pimentel JP, Garcia NP, et al. The robust and modulated biomarker network elicited by the Plasmodium vivax infection is mainly mediated by the IL-6/IL-10 axis and is associated with the parasite load. J. Immunol. Res. 2014;2014:318250. doi: 10.1155/2014/962047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White MT, Karl S, Battle KE, Hay SI, Mueller I, et al. Modelling the contribution of the hypnozoite reservoir to Plasmodium vivax transmission. Elife. 2014;3:1–19. doi: 10.7554/eLife.04692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shanks GD, White NJ. The activation of vivax malaria hypnozoites by infectious diseases. Lancet Infect. Dis. 2013;13:900–906. doi: 10.1016/S1473-3099(13)70095-1. [DOI] [PubMed] [Google Scholar]
- 49.Van Geertruyden JP, Mulenga M, Mwananyanda L, Chalwe V, Moerman F, et al. HIV-1 immune suppression and antimalarial treatment outcome in Zambian adults with uncomplicated malaria. J. Infect. Dis. 2006;194:917–925. doi: 10.1086/507310. [DOI] [PubMed] [Google Scholar]
- 50.Andrews KT, Fairlie DP, Madala PK, Ray J, Wyatt DM, et al. Potencies of human immunodeficiency virus protease inhibitors in vitro against Plasmodium falciparum and in vivo against murine malaria. Antimicrob. Agents Chemother. 2006;50:639–648. doi: 10.1128/AAC.50.2.639-648.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hobbs CV, Dixit S, Penzak SR, Sahu T, Orr-Gonzalez S, Lambert L, et al. Neither the HIV protease inhibitor lopinavir-ritonavir nor the antimicrobial trimethoprim-sulfamethoxazole prevent malaria relapse in plasmodium cynomolgi-infected non-human primates. PLoS ONE. 2014;9:e115506. doi: 10.1371/journal.pone.0115506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khalil IF, Ronn AM, Alifrangis M, Gabar HA, Jelinek T, et al. Response of Plasmodium falciparum to cotrimoxazole therapy: relationship with plasma drug concentrations and dihydrofolate reductase and dihydropteroate synthase genotypes. Am. J. Trop. Med. Hyg. 2005;73:174–177. doi: 10.4269/ajtmh.2005.73.174. [DOI] [PubMed] [Google Scholar]
- 53.Briand V, Badaut C, Cot M. Placental malaria, maternal HIV infection and infant morbidity. Ann. Trop. Paediat. 2009;29:71–83. doi: 10.1179/146532809X440699. [DOI] [PubMed] [Google Scholar]
- 54.Kwenti TE. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies. Res. Rep. Trop. Med. 2018;279:123–136. doi: 10.2147/RRTM.S154501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Berg A, Patel S, Aukrust P, David C, Gonca M, et al. Increased severity and mortality in adults co-infected with malaria and HIV in Maputo, Mozambique: a prospective cross-sectional study. PLoS ONE. 2014;9:e88257. doi: 10.1371/journal.pone.0088257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mouala C, Guiguet M, Houzé S, Damond F, Pialoux G, et al. Impact of HIV infection on severity of imported malaria is restricted to patients with CD4 cell counts < 350 cells/microl. AIDS. 2009;23:1997–2004. doi: 10.1097/QAD.0b013e32832f4215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].