Abstract
Hyperbaric oxygen therapy (HBOT), a technique through which 100% oxygen is provided at a pressure higher than 1 atm absolute (ATA), has become a well-established treatment modality for multiple conditions. The noninvasive nature, favorable safety profile, and common clinical application of HBOT make it a competitive candidate for several new indications, one of them being aging and age-related diseases. In fact, despite the conventional wisdom that excessive oxygen accelerates aging, appropriate HBOT protocols without exceeding the toxicity threshold have shown great promise in therapies against aging. For one thing, an extensive body of basic research has expanded our mechanistic understanding of HBOT. Interestingly, the therapeutic targets of HBOT overlap considerably with those of aging and age-related diseases. For another, pre-clinical and small-scale clinical investigations have provided validated information on the efficacy of HBOT against aging from various aspects. However, a generally applicable protocol for HBOT to be utilized in therapies against aging needs to be defined as a subsequent step. It is high time to look back and summarize the recent advances concerning biological mechanisms and therapeutic implications of HBOT in promoting healthy aging and shed light on prospective directions. Here we provide the first comprehensive overview of HBOT in the field of aging and geriatric research, which allows the scientific community to be aware of the emerging tendency and move beyond conventional wisdom to scientific findings of translational value.
Keywords: Hyperbaric oxygen therapy, Aging intervention, Age-related disease, Oxidative stress, Cellular senescence
1. Introduction
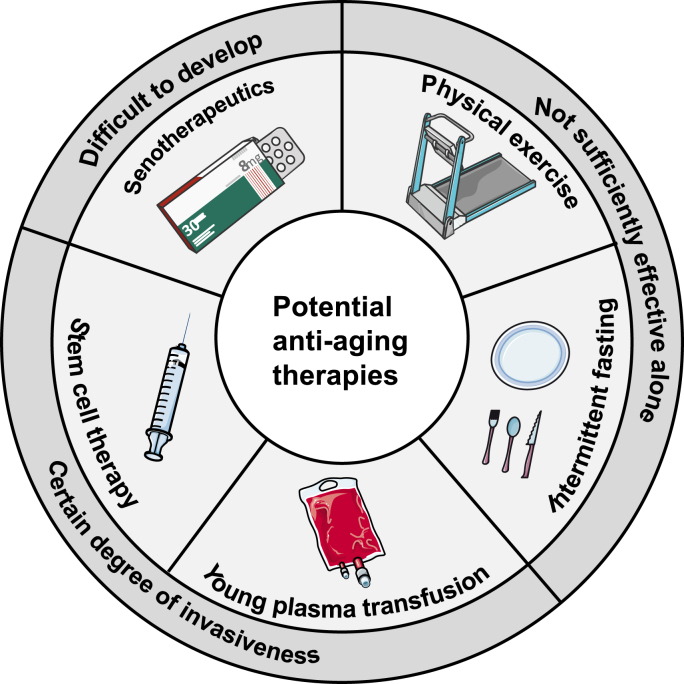
Aging is characterized by a progressive loss of physiological functions over time. Not only does aging substantially affect the quality of life, but it also represents a major risk factor for a number of age-related diseases. Effective approaches are required to sustain better health in old age by slowing down the natural aging process and preventing age-related conditions. At present, there are limited options to interfere with the aging process, such as stem cell therapy, young plasma transfusion, physical exercise, intermittent fasting and senotherapeutics [1]. These mainstream strategies have inspired great interest in the scientific community and shown considerable promise in combating aging. There are, however, a few flies in the ointment (Fig. 1). First, some of the known therapies, such as young plasma transfusion and stem cell grafting, involve a certain degree of invasiveness. Second, despite adequate safety, lifestyle changes alone, such as physical exercise and intermittent fasting, may not be sufficient to ensure definitive efficacy. Third, senotherapeutics, as another modality of noninvasive strategy, are not yet fully understood in humans and still in their infancy before routine clinical practice, mainly due to the complex, time-consuming and expensive procedure of pharmacological development. Therefore, while effectively advancing existing strategies, researchers are also in search of novel strategies to achieve healthy aging that are noninvasive, sufficiently effective, and easy to use, among which hyperbaric oxygen therapy (HBOT) is a competitive candidate.
Fig. 1.
Potential strategies against aging and their deficiencies. Strategies under development to intervene aging include stem cell therapy, young plasma transfusion, physical exercise, intermittent fasting and senotherapeutics. Despite the great promise of these mainstream strategies, there are three deficiencies among them. (1) Stem cell transplantation and young plasma transfusion involve a certain degree of invasiveness. (2) Physical exercise and intermittent fasting alone may not be sufficient enough to ensure definitive efficacy. (3) The efficacy of senotherapeutics is not yet fully understood in humans and the development pipelines are complex, time-consuming and expensive.
HBOT is a noninvasive technique to allow 100% oxygen supplied at a pressure greater than 1 atm absolute (ATA). The treatment was originally used for conditions related to hypoxia [2]. Up to now, it has become a well-established treatment modality for diverse conditions, including non-healing wounds, infections and medical emergencies [3]. Through providing a sealed environment with high pressure and rich oxygen, HBOT can effectively increase the oxygen content dissolved in plasma and arterial oxygen partial pressure [4]. Oxygen is a pivotal player in numerous physiological processes, reaching all tissues and cells through blood circulation. Hence, HBOT can induce a wide range of cellular, biochemical, and physiological changes throughout the body. Proven biological mechanisms through which HBOT exerts its beneficial effects in traditional indications include angiogenesis promotion, inflammation alleviation, antioxidant defense enhancement, stem cell stimulation and so forth. Nowadays, proposals for new indications for HBOT continue to arise, among them are aging and age-related diseases, which draw our attention. As with most established indications, the employment of HBOT in aging intervention is based on its multiple effects on the organism. The advantages of HBOT as a novel therapy for healthy aging include its noninvasiveness, established safety profile and common clinical application in diverse populations [5]. This article aims to provide an overview of the mechanisms by which HBOT targets the aging process, as well as its potential therapeutic implications supported by pre-clinical and small-scale clinical studies.
2. The oxygen paradox in aging
There is a paradoxical relationship between oxygen and aging. Despite the indispensable role that oxygen plays in tissue homeostasis and organismal survival, oxygen is considered a key driver of the aging process as well. Before we discuss the therapeutic mechanisms of HBOT in aging intervention, it is necessary to delve into the delicate balance of protection versus damage by oxygen in living organisms.
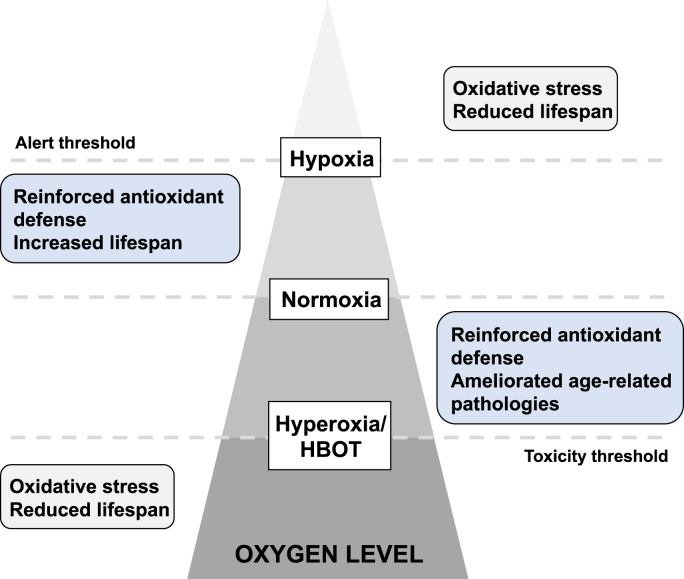
Oxygen serves as a source of reactive oxygen species (ROS). Though ROS can be beneficial in some circumstances, overproduction of ROS is able to induce cumulative macromolecular oxidative damage including lipid peroxidation, protein dysfunction and DNA damage [6,7], all of which contribute to aging. Hence, it is not surprising that hypoxic conditions can ameliorate multiple hallmarks of aging in cell culture, including senescence-associated secretory phenotype (SASP) production, mitochondrial dysfunction and replicative senescence [[8], [9], [10]]. However, while it is often inappropriately assumed that the rate of aging and oxygen levels are directly proportional, the biological consequences of aging with respect to oxygen levels are actually complex and remain poorly understood. As demonstrated in Drosophila, there is a non-linear response of oxidative damage and lifespan to atmospheric oxygen levels [11]. Both extreme high and low atmospheric oxygen levels lead to increased oxidative stress and reduced longevity. On the other hand, a reduction in oxidative stress has been attributed to both increases and decreases in oxygen levels [12,13]. In another word, the truth is not a duality when it comes to the trade-off between hypoxia and hyperoxia (Fig. 2), especially when issues such as free radicals, oxidative stress and scavengers are involved [14,15]. There is actually a biphasic response induced by HBOT: although the accumulation of ROS does exist, the subsequent cytoprotective antioxidant responses tend to be more pronounced after repeated exposures, which is discussed in detail in Section 3.3. In fact, it has been already reported that systemic levels of oxidative stress are largely unaltered in healthy young volunteers after multiple HBOT sessions, with signs of depletion of ROS generation capacity [16]. Likewise, a recent study of HBOT in middle-aged males reported attenuation of oxidative stress, as reflected by circulating biomarkers [17]. These encouraging findings help alleviate concerns that HBOT results in oxidative damage. More importantly, fluctuations in oxygen concentration levels are perceived by tissues as a hypoxia trigger, allowing HBOT over several cycles to stimulate cellular protection characterized by hypoxia-inducible factor-1 (HIF-1) activation without additional detrimental effects of hypoxia [18,19]. We will discuss this problem further in Section 3.1 and Section 3.3.
Fig. 2.
The biological consequences of aging with respect to oxygen levels. In terms of aging, the truth is not a duality when it comes to the trade-off between hypoxia and hyperoxia, especially when issues such as oxidative stress and scavengers are involved. First, large deviations from normoxia (either increases or decreases in oxygen levels) generally lead to increased oxidative stress and reduced longevity. To the contrary, modest modulation of oxygen levels (either increases or decreases in oxygen levels) can enhance the antioxidant defenses and slow the aging process. These facts suggest both an alert threshold in hypoxia and a toxicity threshold in hyperoxia in the biological consequences of aging with respect to oxygen levels.
The contradictory roles of oxygen in aging can be attributed to the phenomenon of “Hormesis”. The term describes the fact that treatment with sub-toxic and non-damaging doses of a certain toxicant can actually induce adaptations that prevent subsequent damage by the same agent [14]. The oxygen in HBOT may represent such a sub-toxic substance. Doubtless, there exists a “toxicity threshold” in terms of quantity and duration [20], beyond which oxygen administration will speed up the aging process instead. The thresholds vary by species, age and tissue, depending on the different cellular sensitivity to oxygen. This would be helpful in explaining the seemingly paradoxical results obtained under hyperoxic conditions in different settings. Together, we hold that oxygen plays an active role against aging under appropriate protocols of HBOT without exceeding the toxicity threshold. It forms the basis for our subsequent discussion about the role of HBOT in the field of aging and geriatric research.
3. The mechanisms by which HBOT intervenes aging
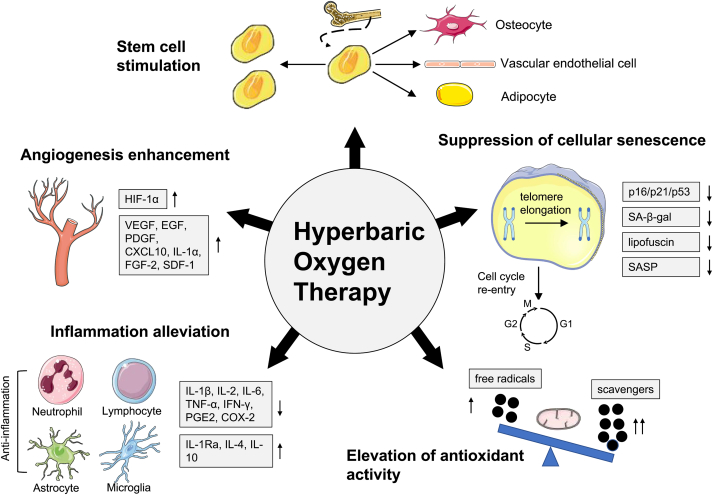
Substantial progress has been made in comprehending the molecular mechanisms of the aging process over the past few decades. Obviously, this provides clinicians with a wide array of therapeutic targets for aging and age-related diseases. At the same time, there is growing evidence for the benefits of HBOT in tissue homeostasis and regeneration. The fact that the therapeutic targets of HBOT overlap considerably with those of aging and age-related diseases is beginning to gain attention. In a recent prospective trial, HBOT was found to induce transcriptome changes in whole-blood samples from healthy aging subjects, with 1342 genes upregulated and 570 genes downregulated [21]. Changes in these age genetic signatures in vivo suggest remarkable effects of HBOT on the elderly, at least at the molecular level. In this section, we aim to place HBOT in the context of various aging theories and summarize possible mechanisms by which HBOT promotes healthy aging (Fig. 3).
Fig. 3.
The mechanisms by which HBOT promotes healthy aging. HBOT can cause a wide range of cellular, biochemical and physiological changes. The proven biological mechanisms by which HBOT may promote healthy aging can be summarized into five categories. (1) HBOT enhances angiogenesis mainly by increasing the expression of HIF-1α and a series of angiogenic markers. (2) HBOT reduces inflammation by regulating the number and activity of extensive inflammatory cell types such as neutrophils, lymphocytes, astrocytes and microglia. At the molecular level, HBOT can inhibit pro-inflammatory factors while promoting anti-inflammatory factors. (3) HBOT enhances antioxidant defenses by modulating the balance between free radicals and scavengers. The process is closely correlated with the regulation of mitochondrial function. (4) HBOT interferes with the detrimental effects of cellular senescence, manifested by cell cycle re-entry and attenuation of senescence markers such as p16/p21/p53, SA-β-gal, lipofuscin and the SASP. HBOT also plays a role in inhibiting telomere shortening, one of the major stimuli of cellular senescence. (5) HBOT increases the number of circulating stem cells by stimulating stem cell mobilization, and changes stem cell properties by promoting proliferation and differentiation.
3.1. Angiogenesis enhancement
Impaired vascular homeostasis and angiogenesis, one of the hallmarks of aging, leads to reduced capillary density throughout the body, which in turn contributes to fading physical functions in the elderly [22,23]. The corollary to this “angiogenesis hypothesis of aging” recommends pro-angiogenesis therapy for symptoms and signs of aging [23]. Meanwhile, oxygen is essential for angiogenesis. Exposure to oxygen increases angiogenesis in a dose-dependent manner [24]. And the stimulus for angiogenesis seems not to be the increased oxygen availability by itself, but to be most related to the pressure at which it is delivered [25]. Thus, both the hyperoxia and pressure components of HBOT play an indispensable role in promoting angiogenesis. To date, numerous studies have reported the pro-angiogenesis effects of HBOT in different tissues with compromised blood perfusion such as skin [[26], [27], [28], [29]], brain [[30], [31], [32]], penile [33], bone [34], and even tumor [35,36]. These results firmly establish the pro-angiogenesis effects of HBOT, implying its possible advantages in preventing age-related microcirculation impairments.
The mechanisms of HBOT impacting angiogenesis have been explored in various animal models. There is an age-related signaling decline in HIF-1, contributing to the defective neovascularization with natural aging [37]. Interestingly, it has been widely reported in the literature that HBOT induces an increase in HIF-1 [38,39]. HIF-1, consisting of HIF-1α and HIF-1β subunits, is an essential mediator of oxygen homeostasis, whose biological activity is determined by the expression of HIF-1α subunit [40]. Hypoxia is the main regulator of its function and activity. Aside from that, there are many other regulatory factors, among which are free radicals including ROS and reactive nitrogen species (RNS) [41]. Correspondingly, there are generally two approaches to modulating HIF-1α by HBOT. For one thing, a unique protocol of repeated intermittent hyperoxia exposures, with a 5-min air break every 20 min, can induce some cellular mechanisms usually induced during hypoxia, including the release of HIFs and increase in their stability and activity [27,42]. This is because the return to normoxia following a hyperoxia exposure results in fluctuations in the dissolved oxygen, which are interpreted by tissues as a lack of oxygen though hypoxia does not actually occur, namely the so-called “hyperoxic-hypoxic paradox” [19]. The same applies to the general HBOT protocols since intermittent fluctuations in oxygen occur between daily treatments [43]. For another, it has been widely acknowledged that HBOT leads to elevated partial pressure of oxygen in blood and tissues, which in turn increases the production of ROS [39,43,44] and RNS [44,45]. The transiently increased ROS and RNS serve as signaling molecules to stabilize HIF-1 in its active form [44]. Following the stabilization and activation of HIF-1, HIF-dependent vascular endothelial growth factor (VEGF) stimulation contributes to blood vessel formation directly [40,46,47]. In addition to the commonly observed increase in VEGF after HBOT in the literature [26,38,48,49], several reports have revealed other angiogenic markers induced by HBOT, such as EGF, PDGF, CXCL10, IL- 1α, FGF-2 and SDF-1 [26,48]. These angiogenic factors, especially the most prominent proangiogenic factor VEGF, work together to activate vascular cells to promote angiogenesis and arteriogenesis [50]. Among them, HIF-1-regulated VEGF and SDF-1 can also reach the circulation and stimulate bone marrow-derived endothelial progenitor cells (EPCs) mobilization and recruitment, thus promoting angiogenesis and vasculogenesis [50,51], which has been demonstrated in human subjects receiving HBOT [50,52,53]. Another nuclear factor E2-related factor 2 (Nrf2) signaling pathway induced by HBOT has also been shown to stimulate angiogenesis, possibly through its interaction with VEGF and other angiogenic factors [48].
3.2. Immunomodulatory properties
Immune dysregulation and activation of inflammatory pathways have been postulated to be essential contributors to tissue dysfunction in the course of aging [54]. The chronic, sterile, low-grade inflammatory state during aging, also known as inflammaging, plays a vital role in the pathogenesis of age-related diseases. In recent years, interventions targeting inflammatory pathways have shown great potential in the rejuvenation of different tissues, thereby preventing age-related tissue dysfunction [54]. Coincidentally, HBOT has shown its immunomodulatory properties from the beginning of its use, suggesting its benefits in ameliorating age-related immune dysregulation.
At the cellular level, HBOT can exert immunomodulatory effects on a variety of inflammatory cell types. Neutrophil apoptosis plays a crucial part in the resolution of inflammation, while enhanced apoptosis of neutrophil-like cells is observed after a single 90 min exposure to hyperbaric oxygen, as evidenced by promoted caspase 3/7 activity and morphological changes associated with apoptosis [55]. In another study, neutrophils from severely injured patients or healthy volunteers respectively showed no significant reduction in apoptosis but a decline in ROS production, MAPKs activation and NETs release after exposure to hyperbaric oxygen [56]. Despite the contradiction, both in vitro studies suggest a role for HBOT in limiting neutrophil-mediated systemic inflammation. This has been confirmed by in vivo experiments using different animal models, in which HBOT reduces neutrophil recruitment and activation [57,58]. Apart from its effects on neutrophils, hyperbaric oxygen can induce the apoptosis of lymphocytes as well via a mitochondrion-associated mechanism, demonstrated by caspase-9 activation and loss of mitochondrial membrane potential [59,60]. Besides, HBOT can reduce inflammation by regulation of iNOS activity/expression and nitrite/nitrate production in lymphocytes, as observed in T1DM patients [61]. These results illustrate that HBOT suppresses lymphocyte-mediated inflammatory responses both quantitatively and qualitatively. For T lymphocytes, a single exposure to hyperbaric oxygen can result in a transient reduction in the CD4:CD8 ratio in blood from healthy volunteers [62], reinforcing the role of HBOT in reversing immunosenescence as an augment in the CD4:CD8 ratio with aging was previously reported in human peripheral blood [63,64]. The brain is known as an immune-privileged organ, in which astrocytes act in concert with microglia in neuroinflammation during normal aging, leading to cognitive impairment [65]. HBOT can attenuate neuroinflammatory processes by reducing astrocytes and microglia activation in different animal models including aging [66], Alzheimer's Disease (AD) [67] and brain injury [68]. These results confirm that HBOT's effects on inflammatory cells are not limited to those in the blood circulation.
At the molecular level, HBOT can target the inflammatory process through its extensive effects on the expression of cytokines and other mediators. Various pro-inflammatory cytokines and inflammatory mediators are reduced in the peripheral blood following HBOT, including IL-1β, IL-2, IL-6, TNF-α, IFN-γ, PGE2, COX-2 [60,[69], [70], [71], [72]]. The same goes for the levels of inflammatory markers in different tissues. Meanwhile, HBOT can lead to increases in some anti-inflammatory cytokines, including IL-1Ra, IL-4 and IL-10 [48,67,[72], [73], [74]]. Among them the most frequently reported is IL-10, the major mediator of protective effects of HBOT against sepsis [75] and traumatic brain injury [76]. There are conflicting results pertaining to acute phase proteins [77], with most reports showing a decrease in CRP while a few showing stimulation of G-CSF and inhibition of IGF-1. As an overall effect, it has been reported that HBOT can exert protective effects against multi-organ damage following generalized inflammation [78]. Mechanisms include that hyperbaric oxygen can interfere with the TLR/NF-κB pathway, accounting for a downregulation of pro-inflammatory cytokine release [78]. Given that the aging process is accompanied by the activation of multiple inflammatory pathways throughout the body, the results suggest that HBOT may protect various tissues from chronic inflammatory damage during aging in a similar manner.
Collectively, HBOT appears to exert anti-inflammatory effects in a variety of both physiological and pathological conditions. So far, researchers have preliminarily observed benefits of HBOT in slowing down tissue aging by attenuating inflammation, while more comprehensive research is expected to clarify the systemic effects of HBOT on age-related inflammatory state.
3.3. Elevation of antioxidant activity
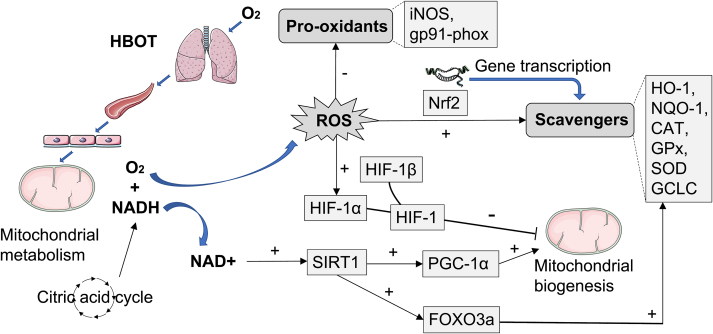
Oxidative stress is induced when ROS production exceeds antioxidant capacity. The oxidative stress theory of aging takes it as a major mechanism responsible for age-related functional losses and longevity limitation. At the same time, the decrease in mitochondrial efficiency plays a significant role in oxidative stress induced with aging [79], since mitochondrion is a primary site for ROS production in the cell. We have summarily described the biological consequences of excessive oxygen on oxidative stress and antioxidant defense in Section 2. In this section, we discuss in detail the role of HBOT on oxidative stress balance, as well as its effect on mitochondrial function (Fig. 4). We will pay particular attention to the antioxidant potential of HBOT when applied appropriately as an alternative theoretical mechanism.
Fig. 4.
The effects of HBOT on oxidative stress balance and mitochondrial properties. In HBOT, the inhaled oxygen passes through the lungs, effectively elevating the content of oxygen dissolved in the plasma, which in turn causes a plethora of oxygen within tissues. In mitochondria of tissue cells, the citric acid cycle is boosted under hyperoxia. NADH, a product of the citric acid cycle, can react directly with oxygen to produce ROS in the mitochondria. The overproduced ROS activates HIF-1α, which conjugates with HIF-1β to stabilize HIF-1 in its active form (Another way HBOT stabilizes HIF-1 arises from the hypoxic-like state during intermittent periods). HIF-1 inhibits mitochondrial biogenesis. On the other hand, consumption of more NADH by mitochondria results in higher NAD + levels. In the presence of elevated NAD+, SIRT1 is activated, which improves mitochondrial biogenesis via acetylation of PGC-1α and induces antioxidant responses via deacetylation of FOXO3a. Notably, as an adaptive mechanism, high ROS levels can produce more endogenous scavengers as well. The elimination half-life of scavengers is much longer than that of ROS, underlying the antioxidant effects of HBOT. The molecular mechanisms by which HBOT stimulates antioxidant defenses include activation of Nrf2 and its downstream targets such as HO-1, NQO-1, CAT, GPx, SOD and GCLC, as well as decreased expression of pro-oxidant enzymes such as iNOS and gp91-phox.
Under different protocols and pathological conditions, studies over the past few decades on the effects of HBOT on mitochondrial properties and oxidative stress balance have yielded mixed results [43]. On the one hand, HBOT is thought to cause excessive ROS production and induce oxidative stress, implying adverse effects while being therapeutic under certain circumstances [77,80]. This is accompanied by a reduction in mitochondrial function [81]. On the other hand, HBOT has been found to improve mitochondria activity as well as increase free radical scavengers, thereby providing effective antioxidant defense [13,17,43,48,49]. Despite the multifactorial process, the contradiction has mainly been attributed to the number of sessions [43]. Generally, a single exposure to hyperbaric oxygen or short-term HBOT may create oxidative stress. Via mitochondrion-dominated mechanisms, HBOT leads to elevated ROS production. Although there is an increase in scavenger production as an adaptive response to ROS accumulation, the compensation is inadequate and gradual after limited exposures [19]. In parallel, mitochondrial respiration is reduced in order to counteract additional ROS production [81]. Sirtuin1 (SIRT1) is considered a major metabolic stimulator of mitochondrial biogenesis and part of a cellular defense mechanism against oxidative stress [82,83]. Its reduction with aging contributes to age-related disorders. After repeated intermittent hyperoxia exposures or long-term HBOT, SIRT1 is significantly activated through increased NAD + levels from the hyperoxic state during HBOT [84,85], improving mitochondrial biogenesis via acetylation of PGC-1α and inducing antioxidant responses via deacetylation of FOXO3a [19]. Notably, HIF-1, which is also activated after HBOT as described in Section 3.1, antagonizes the promoting effect of SIRT1 on mitochondrial biogenesis [86]. The crosstalk between the HIF-1α and SIRT1 pathways in HBOT remains to be further elucidated. Synergistically with SIRT1-mediated beneficial effects, there is a distinct increase in scavenging activity induced by ROS production following repeated exposures, while the elimination half-life of scavengers is much longer than that of ROS [19]. The biphasic response has been confirmed by a study revealing that HBOT-induced DNA damage can only be detected immediately after the first treatment but not after subsequent treatments under the same conditions, suggesting that repeated exposures can result in an increased antioxidant protection but not an accumulation of oxidative damage [87]. Taken together, contrary to short-term protocols, long-term HBOT or repeated intermittent hyperbaric oxygen exposures can enhance antioxidant defenses via adaptive mechanisms. Apart from that, exposure pressure and frequency have been proposed as important factors to consider when investigating antioxidant responses induced by HBOT [88]. The contributions of variables other than the number of sessions warrant further investigation.
Mechanistically, HBOT activates a series of transcription factors and gene expression to increase endogenous antioxidant enzymes [13]. Nrf2, a redox sensor, represents the master regulator of cellular defenses against oxidative stress [89]. The age-dependent decline in antioxidant enzyme responses is supposed to result from decreased expression of Nrf2 and its target genes [90]. Accumulating data suggest that HBOT induces antioxidant responses by upregulating Nrf2 and its downstream targets, such as HO-1, NQO-1, CAT, GPx, SOD and GCLC [48,[91], [92], [93]]. Reversal of age-related decline in Nrf2 signaling by HBOT makes it a viable therapeutic option for aging of the antioxidase system. Besides increased expression of antioxidant enzymes, decreased levels of enzymatic pro-oxidants, such as iNOS and gp91-phox, have also been observed in two separate studies [74,94]. In conclusion, HBOT induces an increase in antioxidant enzymes and a decrease in pro-oxidant enzymes through a negative feedback, thereby enhancing the antioxidant defenses.
3.4. Suppression of cellular senescence
The accumulation of senescent cells in various tissues is regarded as a significant contributor to aging as well as age-related diseases. Meanwhile, cellular senescence can be characterized by multiple features, which have emerged as potentially effective targets for therapeutic exploitation [95]. Here we review the effects of HBOT on cellular senescence from multiple perspectives.
So far, no single marker with absolute specificity for senescent cells has been established [96]. Two classic tools to identify senescent cells in vivo include senescence-associated beta galactosidase (SA-β-gal) and cyclin-dependent kinase inhibitors including p16 and p21 [97]. Intriguingly, in two independent reports, HBOT respectively showed its ability to attenuate aging markers in the hippocampus of d-galactose (D-gal)-induced aging mice, as demonstrated by decreased number of SA-β-gal positive cells [66] and reduced expression of key components of the senescence program, such as p16, p21 and p53 [71]. It has also been shown that HBOT can reduce the number of SA-β-gal positive cells in cardiomyocytes in aging pre-diabetic rats [98]. In another study, researchers evaluated cellular senescence by lipofuscin, another established biomarker, and found senescent cells cleared from skin after HBOT [27]. However, new indicators for senescence such as LINE1 have been recently developed [99], which are absent from existing HBOT studies. Besides, a multi-parametric strategy for identification of senescent cells is currently being advocated [96]. For example, the use of SA-β-gal assay in combination with nuclear HMGB1 staining allows a more accurate evaluation of senescence than SA-β-gal staining alone [100]. Therefore, the pleiotropic phenotypes of senescent cells have not been considered in the existing literature, which is clearly a limitation.
In tissues, senescent cells are heterogeneous but share a number of common features, among which the most widely recognized are the permanent cell cycle arrest and a bioactive secretome, namely the SASP [97]. Critical components of pathways involved in senescence-mediated cell cycle arrest include p16, p21 and p53 [101], which, as noted above, tend to decrease after HBOT [71], reflecting the potential of HBOT to resume cell cycle progression in senescent cells. In prostate cancer cells, a single exposure to hyperbaric oxygen causes senescent cells to enter cell cycle [102]. Likewise, exposure to hyperbaric oxygen can prevent cell cycle arrest in malignant glioma cells [36]. Nevertheless, the current results are insufficient to illustrate the general effects of HBOT on senescent cells, as cancer cells are more likely to overcome the senescence-associated cell cycle arrest. Senescent cells secrete the SASP, which usually consists of pro-inflammatory cytokines and chemokines, angiogenic factors, growth factors, and matrix metalloproteinases (MMPs) [96,97]. The downregulation of a variety of factors involved in the SASP after HBOT has been described in Section 3.1 and Section 3.2. Moreover, the declined expression of MMPs after HBOT has also been well documented [94,103,104]. Therefore, there is sufficient evidence to support the role of HBOT in blocking SASP. Since development of the SASP can partially explain the deleterious, pro-aging effects of senescent cells [96], the downregulation of SASP expression by HBOT may alleviate the pro-aging effects of senescent cells to a certain extent, or more optimally, reflect the result of senescent cell clearance. In general, specific therapies to target senescent cells are referred to as senotherapeutics, consisting of senomorphics and senolytics [105]. The former indirectly impedes the appearance of senescence phenomenon through blocking the SASP, while the latter selectively clears senescent cells [100,106]. Some of them have been shown to markedly intervene with the aging process in animal models. In practice, however, only a few senolytics have entered early-stage clinical trials, while senomorphics have yet to enter clinical trials with greater potential risks [105]. It seems that the role of HBOT coincides with theirs in intervening in cellular senescence. To some extent, this raises the possibility that HBOT can be utilized as an available alternative to senotherapeutics, or as an adjunct therapy.
One of the major pro-senescence stimuli is telomere shortening [97], which can be either a direct inherited trait or the result of multiple environmental factors [42]. Telomere length in blood cells is a proxy for telomere length in various tissues and thus a useful biomarker of human aging [107]. For divers exposed to intense hyperbaric oxygen, a previous study showed telomere elongation in leukocytes over a 12-month interval [108]. Similarly, a recent clinical trial in healthy aging populations has revealed that HBOT can target cellular senescence in isolated leukocytes in terms of telomere shortening and accumulation of senescent cells [42]. Unfortunately, neither telomerase activity nor the expression of CD57, the most reliable surface marker for T cell senescence, was evaluated in this study, complicating the interpretation of the results. In addition to the effect of HBOT on circulating immune cells, the restoration of telomere length was also observed in the hippocampus after HBOT in aging and obese rats with shortened telomeres [66]. By and large, HBOT makes a difference in inhibiting telomere shortening, but it remains to be clarified how hyperbaric oxygen targets telomeres and how its response relates to different conditions.
It appears to be an attractive rationale to blunt the pro-aging effects of senescent cells with HBOT. At the present stage, the study on understanding the role of HBOT in senescent cells is still in its infancy, but with rapid progress. Future work is essential and expected to examine suitable biomarkers of cellular senescence. Given the diversity of senescent cells of different origins, it is necessary to evaluate the effects of HBOT on cellular senescence in various systems in vivo.
3.5. Stem cell regulation
Stem cell can promote tissue regeneration not only by replacing dead cells with new ones, but also by secreting cytokines and growth factors, making them prime targets in aging and regenerative medicine. Based on that, an attractive theory of aging holds that the loss of stem cell number and activity over time drives organismal aging [109]. Interestingly, studies in various tissues and diseases have established stem cells as key players in the regenerative effects of HBOT. It can be inferred that regulating stem cell biology to slow aging by HBOT may be feasible in at least three aspects.
First, HBOT can stimulate stem/progenitor cell (SPC) mobilization and recruitment from bone marrow. As described in Section 3.1, HBOT mobilizes SPCs by stimulating NOS, increasing its circulating population and intracellular regulatory protein content [52,110]. The mobilized SPCs have been found to be engaged in wound healing [110,111] and cognition enhancement [112,113]. Second, HBOT can cause changes intrinsic to SPCs, including the promotion of stem cell proliferation and differentiation, as well as the regulation of protein secretion. In vivo, HBOT has been reported to stimulate proliferation of neural stem cell [114,115] and intestinal stem cell [116], growth and differentiation of vasculogenic stem cell [117], and activation of colonic stem cells [118]. In vitro, HBOT can promote proliferation and differentiation of adipose-derived stem cells [119], as well as osteogenic and vasculogenic differentiation of mesenchymal stem cells (MSCs) [120,121]. The secretion profile of proteins is also modulated by HBOT in MSCs, with some upregulated proteins being neuroprotective and others involved in cellular mechanisms against oxidative stress [122]. While the above two mechanisms potentially ensure the efficacy of HBOT alone by stimulating inherent stem cells, the third mechanism we summarize here is that combining HBOT with stem cell transplantation can enhance the therapeutic value of stem cells. Compared with monotherapy, a combined treatment of stem cell transplantation and HBOT has shown better therapeutic effects on cardiac regeneration [123], neurological function recovery [124], and metabolic control [125]. Considering the great promise of stem cell transplantation in aging or geriatric medicine, it is speculated that HBOT can be used as an adjunct therapy to improve survival and function of the transplanted stem cells. Despite the encouraging findings, research gaps in the effects of HBOT on stem cell properties in the elderly are worth filling through continued studies in the future.
4. Therapeutic implications of HBOT in aging intervention
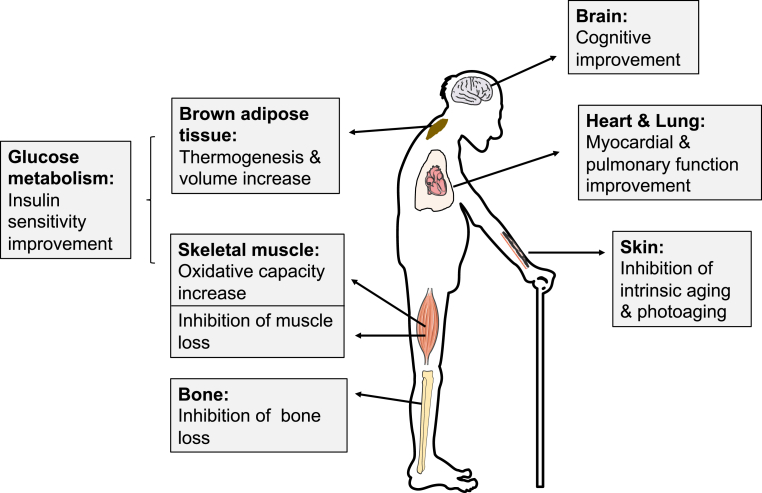
Supported by the multiple mechanisms described above, the past decade has seen an explosion of interest in the rejuvenation potential of HBOT that goes far beyond its traditional use in medicine. We hereby set out to review the potential therapeutic implications of HBOT in aging intervention from the existing literature (Fig. 5).
Fig. 5.
The effects of HBOT on aging in different organs or tissues. In pre-clinical and clinical investigations, HBOT has shown great potential in improving cognition, inhibiting intrinsic skin aging and photoaging, improving glucose metabolism (by increasing thermogenesis and volume of brown adipose tissue and promoting oxidative ability of skeletal muscle), preventing bone and muscle loss, and enhancing myocardial and pulmonary function. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4.1. Cognitive improvement
As the largest consumer of oxygen, the brain comprises only 2% of the body's weight and yet utilizes 20% of the total oxygen and consumes 25% of the total glucose [126]. Considering the importance of oxygen to the brain, researchers have displayed great interest in applying HBOT to neurological disorders. Cognitive decline begins as early as the third decade even in the absence of pathologic neurodegeneration, making it a symbol of both healthy and neurodegenerative brain aging [126]. Here we review the role of HBOT in cognitive improvement, especially in the context of normal aging as well as neurodegenerative brain aging.
Early studies showed that transient oxygen administration to healthy young adults, even at normobaric conditions, significantly enhances cognitive function through increased brain activation, as tested by different cognitive measures including word recall [127], n-back task [128], visuospatial task [129], verbal cognitive test [130], etc. Later, HBOT emerged as a novel approach to temporarily enhance cognitive function in healthy adults. It was reported that HBOT could improve memory performance in young healthy adults [131]. In another study including healthy adults aged 22 to 68, HBOT significantly improved cognitive, motor as well as multitasking performance [132], implying that HBOT benefits the brain beyond what was previously known. The study was further continued by the same team to examine the effects of HBOT on major cognitive domains, with the most pronounced enhancement of episodic memory [133]. On the other hand, some other measured cognitive domains yielded neutral results, consistent with previous studies on single oxygen exposure that not all cognitive domains can be improved by oxygen administration [127,134].
Back in 1970s, researchers first investigated the effects of HBOT on cognitive impairment in the elderly, with conflicting results though [135,136]. Only in recent years have researchers begun to refocus on the cognitive protective effects of HBOT in the context of normal aging. It is widely acknowledged that D-gal-induced model can mimic natural aging associated with neurodegeneration [137]. In D-gal-induced preclinical aging model [66,71,138] or combined model of D-gal-induced aging and obesity [66], HBOT has shown the ability to prevent cognitive impairments and attenuate hippocampal pathologies. The demonstrated mechanisms include improvement of cholinergic and anti-apoptotic functions [138], inhibition of oxidative damage and inflammatory responses [66,71], as well as modulation of age-related gene expression [71]. However, despite the convenience of D-gal-induced aging model compared with naturally aging model, a meta-analysis of D-gal-induced brain aging models reported significant heterogeneity between studies on neurobehavioral and neurochemical outcomes [139], suggesting that such results should be interpreted with great caution. Despite the flaw, attempts have been made to apply HBOT to improve cognition in the aging populations. In a randomized controlled trial involving 63 healthy aging subjects, HBOT, utilized in a repeated 60 daily sessions protocol, was shown to induce cognitive enhancements in clinical aspects including attention, information processing speed and executive functions, likely by an increase in regional cerebral blood flow (CBF) [140]. It's worth noting that the study excluded transient effects of oxygen, as all changes were evaluated at 1–2 weeks after the last session. The increase in CBF was later confirmed by another study of HBOT in elderly individuals suffering from significant memory loss [141].
Over the years, studies investigating the effects of HBOT on various neurological disorders including traumatic brain injury [32], anoxic brain damage [142], and stroke [143], have provided satisfactory neurotherapeutic effects, one of which is enhanced cognitive function. Of particular relevance to the current review, apart from being a direct cause of the chronological cognitive decline, aging is also the greatest risk factor for dementia [144], which is characterized by pathological and progressive cognitive decline. Among the various types of dementia, AD is the most prevalent one, followed by vascular dementia (VD) [144,145]. To date, mounting evidence has supported the notion that HBOT should be considered an effective treatment for both AD [67,141,146,147] and VD [145,148], given the desirable cognitive-improving results in animal models and clinical trials. Interestingly, the pathology and biochemistry of late-life dementia, especially AD, share some common features with those of normal aging [149,150]. We also note in the literature that the effects of HBOT in dementia somehow resemble those in normal aging, such as attenuating neuroinflammation [67] and increasing CBF [141], which further confirms the benefits of HBOT for the aging brain, in both physiological and pathological settings.
4.2. Skin rejuvenation
Unlike other organs of the human body, skin aging is not only inevitably affected by the intrinsic aging process, as happens in all tissues, but also subject to the unique process of extrinsic aging or photoaging, resulting from exposure to environmental agents, particularly ultraviolet (UV) radiation [27,151]. Photoaged skin has several clinical and histologic manifestations distinct from those of intrinsic aged skin [151]. Here, we review the role of hyperbaric oxygen in skin rejuvenation in terms of intrinsic aging and photoaging respectively.
Like any other tissue, the intrinsic physiological aging of the skin is accompanied by the accumulation of senescent cells [151]. Moreover, the dermis of intrinsically aged skin shows degradation of collagen and elastic fibers [152], as well as diminished blood supply [153]. A recent clinical trial demonstrated that repeated intermittent hyperbaric exposures had dramatic aging-modulating effects on the skin, illustrated as decreased senescent cells, increased elastic fiber length and stability and collagen density, and induced angiogenesis [27]. Notably, this study focused on intrinsic aging by taking skin biopsies from a light protected area. The increase in angiogenesis and collagen density was in line with previous reports of HBOT in skin conditions including wound healing [26,28] and compromised grafts and flaps [29]. In the epidermis, the intrinsic physiological aging can be characterized by a generalized epidermal atrophy [151] mostly due to a decrease in proliferative activity and turnover rate of epidermal basal cells [152,154]. It is reported that exposure to mild hyperbaric oxygen with 36% oxygen reversed the age-related decline in proliferative activity of epidermal basal cells in the skin of aged mice [154]. These results supported the point that the intrinsically aged skin can be partially revitalized by hyperbaric oxygen, whose potentiality has not been sufficiently realized.
In contrast to intrinsic aging, photoaging is featured by a thicker skin, with deep marked wrinkles and irregular pigmentation [155]. Photoaging of the skin is mostly attributable to UV irradiation, particularly UVA and UVB. UVA (320–400 nm) have a strong penetrating ability, interacting with both epidermal keratinocytes and dermal fibroblasts [156]. Pretreatment with HBOT protected skin from UVA-induced oxidative damage in a mouse model, manifested by reductions in apoptosis and proliferation of the skin, as well as prevention from deleterious structural changes such as creasing and decreased elasticity [157]. As a highly energetic component of UV light, UVB(280–320 nm) are the major environmental threat to the skin, increasing the risk of long-term damage [156]. A previous study found that exposure to hyperoxia (90% oxygen) immediately after UVB irradiation attenuated acute UVB-induced skin angiogenesis and wrinkle formation in adult mice [158]. Moreover, in healthy individuals, it was reported that hyperbaric exposure at 1.25 ATA with 32% oxygen led to both the fading in melanin pigmentation induced by UVB irradiation and the decrease in senile spot size [159]. Taken together, high oxygen concentration has demonstrated surprising potential in treatment of skin photoaging.
It is worth noting that strictly speaking, some of the studies mentioned above involving hyperbaric exposure with moderate atmospheric pressure and oxygen concentration [154,159] or simply hyperoxic exposure [158], are actually beyond the definition of HBOT. Yet these studies have confirmed the importance of oxygen to revitalize the aging skin. Up to now, the role of HBOT in skin aging has not been fully understood. More supporting evidence is needed to determine how high oxygen concentration affect the aging skin from every aspect of intrinsic and extrinsic aging both clinically and histologically. Given the varying test conditions in the existing literature, it is also necessary to figure out the optimal atmospheric pressure, oxygen concentration and time of treatment for maximal skin benefit. However, with the few positive outcomes, the use of HBOT or hyperoxia conditions for skin rejuvenation has turned out to be promising and effective, at least to a certain extent.
4.3. Metabolism regulation
It has been commonly accepted that aging is associated with a loss of metabolic homeostasis and plasticity. Accordingly, greater metabolic diseases incidence rate estimates have been also observed in the elderly. The main mechanism of HBOT includes the regulation of oxidative stress balance, which is closely related to the pathogenesis of age-related metabolic conditions. Therefore, the effects of HBOT on metabolic parameters, especially glucose metabolism, have been extensively addressed in recent years.
Glucose homeostasis depends on appropriate insulin secretion and the sensitivity of receptors to insulin, both of which are impaired with advancing age [160] In two studies involving animal models of D-gal-induced aging and combined model of D-gal-induced aging and obesity, HBOT attenuated disturbances of glucose metabolism, mediated by restored insulin sensitivity instead of enhanced insulin secretion [66,98]. In another study, following a reduction in AGEs, the levels of TNF-α and IL-6, independent biomarkers predicting the development of insulin resistance and type 2 diabetes mellitus [161], were decreased significantly after HBOT in D-gal-induced aging mice [71]. To the best of our knowledge, however, no clinical trials to date have tapped into the effects of HBOT on glucose metabolism in healthy aging populations. In existing clinical trials involving healthy individuals of a wide range of ages, HBOT has shown a tendency to lower serum insulin [162], increase peripheral insulin sensitivity [163] and reduce HbA1C [163], indicating that HBOT modulates glucose metabolism by increasing insulin sensitivity in healthy adults [162]. Meanwhile, no significant reduction in blood glucose levels was observed in those studies, implying a low risk of hypoglycemia when HBOT was applied to individuals without diabetes [163,164]. Given the age-related impairments of insulin sensitivity in humans [160], these findings, along with the results from animal models of aging, indicate that HBOT may benefit the aging populations in terms of glucose metabolism by attenuating age-related insulin resistance.
In addition to the possible positive effects on glucose metabolism during normal aging, HBOT has shown clinical efficacy in lowering blood glucose and improving insulin sensitivity in type 2 diabetes mellitus [[164], [165], [166], [167]], the most common age-related metabolic disorder. Notably, the majority of participants in these studies received HBOT for treatment of diverse conditions including non-healing ulcers and diabetic foot ulcers, leading to an older age range [165]. It suggests that these conclusions may be more applicable to the elderly diabetic population. Relevant mechanisms by which HBOT improves glucose metabolism, as shown in animal models, include enhancements in oxidative capacity and GLUT4 expression in skeletal muscle [168,169], as well as increases in brown adipose tissue volume and thermogenesis by upregulating protein levels of UCP1 and PGC−1α [168,170]. Moreover, HBOT has been proven to be beneficial for treating diabetic kidney disease (DKD) in animal studies [171,172] and preliminary clinical trials [173,174]. Considering the well-established role of HBOT in diabetic foot ulcers, along with the potential benefits of HBOT on blood glucose and DKD, there is a strong case for further research on role of HBOT in treatment and prevention of the development of diabetes mellitus.
Insulin resistance is often accompanied by central obesity, hyperglycaemia, dyslipidaemia and hypertension, collectively known as a pathological condition called metabolic syndrome, which is also common in the elderly. In rats with metabolic syndrome, apart from enhanced insulin sensitivity, researchers also observed improvements in biochemical parameters of dyslipidemia after HBOT [175,176] or exposure to mild hyperbaric oxygen [169], consistent with the alternations of lipid profiles in diabetic subjects receiving HBOT [166,177]. Interestingly, HBOT also ameliorated obesity by reducing adipocyte hypertrophy in rats with metabolic syndrome [175] and diabetic rats with obesity [178], and in the former even significantly decreased body weight together with abdominal and epididymal fat [175]. However, no effect on lipid profile or body weight was observed in reports of aging or aging-obese rats [66,98]. It is unclear whether this contradiction is due to the assumption that diabetic rats are more sensitive to the effects of HBOT on lipid metabolism and obesity, or simply due to the differences in experimental methods.
Collectively, HBOT has shown potential in alleviating impaired insulin sensitivity in normal aging and age-related metabolic conditions including type 2 diabetes mellitus and metabolic syndrome. These findings are going to be the basis for further work on detailed effects of HBOT on age-related metabolic alternations.
4.4. Musculoskeletal restoration
Musculoskeletal aging, resulting from various age-related changes in body composition, inflammation and hormonal imbalance, is closely correlated with high morbidity and healthcare rates in the elderly. In recent years, researchers have demonstrated that mild hyperbaric oxygen under 1.25 ATA with 36% oxygen, can be effective against degenerative changes in the musculoskeletal system. The rodent model of hindlimb unloading, which was developed to simulate microgravity conditions, has become a commonly used method for modelling a natural decrease in muscle mass defined as sarcopenia [179,180], as well as disuse osteoporosis frequently found in the bedridden elderly [181]. Exposure to mild hyperbaric oxygen can reverse age-related decline in the oxidative capacity of skeletal muscles [182]. Since impaired oxidative metabolism is associated with muscle atrophy with aging, researchers explored the effects of mild hyperbaric oxygen on muscle loss in hindlimb unloading rats. As a result, a combination of preconditioning and postconditioning with mild hyperbaric oxygen reversed atrophy and decreased oxidative capacity of the soleus muscle [183]. Later, the same research team found that exposure to mild hyperbaric oxygen during unloading partially inhibited unloading-induced decrease in soleus muscle weight and type shift from type I to type IIA fibers [184]. Given the interactions between bone and muscle health [185], researchers next explored the effects of mild hyperbaric oxygen on bone loss. As a result, mild hyperbaric oxygen partially protected from osteoporosis in hindlimb unloading rats by inhibiting the increase of osteoclasts and enhancing bone formation [186]. In conclusion, mild hyperbaric oxygen can prevent from bone and muscle loss induced by unloading in rats. Thus, one can speculate that hyperbaric oxygen may be beneficial in preventing degenerative changes in the musculoskeletal system during aging. Yet much uncertainty remains, given the single source of data and current lack of direct clinical evidence. More research is needed to further confirm the significance of mild hyperbaric oxygen for the musculoskeletal system in different animal models of aging, and ultimately, in the elderly.
In addition to disuse osteoporosis simulated by unloading, a more common type of osteoporosis in the elderly arises from aging-related dyshomeostasis of bone metabolism. Interestingly, previous studies on complete spinal transection have demonstrated beneficial effects of HBOT on bone metabolism, which in turn lead to increased bone hardness and flexibility [187,188]. These results have helped establish the role for HBOT in maintaining bone homeostasis. Better yet, it was revealed in a recent study that HBOT not only restored bone structure/strength in aged non-obese rats, but it ameliorated bone dyshomeostasis in aged rats with obesity [189]. This study provides direct evidence that HBOT can be considered as a potential intervention in age-related bone pathology to reduce the risk of osteoporosis and fractures.
4.5. Cardiopulmonary function improvement
Aging of the heart and lung is accompanied by declining functions and increasing vulnerability to diseases. Considering that cardiovascular and pulmonary dysfunction are closely connected during aging [190], we here set out to review the effects of HBOT on aging of these two systems in parallel.
Both animal and human studies have demonstrated the positive effects of HBOT on the aging heart. The myocardium of elderly diabetic patients is particularly vulnerable to the adverse effects of local and systemic factors caused by diabetes. In pre-diabetic rats after D-gal-induced aging, HBOT ameliorated the aggravation of cardiac dysfunctions [98], consistent with a previous clinical trial involving elderly diabetic patients, in which HBOT resulted in improved myocardial diastolic function [191]. Researchers also examined the effects of HBOT on myocardial function during normal aging. It was found that HBOT restored cardiac senescence marker expression and cardiac function parameters in D-gal-induced aging rats, even to the same level of the vehicle group [98]. HBOT used under different protocols yields inconsistent results in elderly subjects. In a clinical study of asymptomatic elderly, HBOT, utilized in a repeated 60 daily sessions protocol at 2 ATA, improved left ventricular and right ventricular systolic function, and resulted in better cardiac performance, while no significant changes were observed in diastolic parameters [192]. In contrast, a slight improvement in diastolic function in the elderly was previously observed after a single exposure to hyperbaric oxygen while the EF result reflecting cardiac systolic function showed a negative trend [193]. Despite the controversy, these results indicate that HBOT, when used in an appropriate manner, can reverse age-related deterioration of myocardial function. In terms of pulmonary function, there were concerns about the potential pulmonary oxygen toxicity of HBOT given the particularity of the therapy. However, a prospective cohort study revealed that HBOT, utilized in a repeated 60 daily sessions protocol at 2 ATA, resulted in a modest though statistically significant improvement in PEF and FVC in subjects with a mean age of 60 years without chronic lung diseases [194]. Yet, using two different administration protocols, we were unable to show a positive role of HBOT on pulmonary function [195,196]. The reason for such a discrepancy mainly lies in the HBOT protocols, in which a prolonged period with lower oxygen pressure is critical. Taken together, appropriate use of HBOT can resist the degenerative changes of cardiopulmonary function in the elderly.
5. Limitations and future directions
The benefits of HBOT for healthy aging in both mechanistic and therapeutic aspects are comprehensively summarized in this review. However, a key issue remains for existing HBOT research. First of all, HBOT protocols vary widely, making it difficult to compare and integrate different results. Moreover, updates on published clinical protocols are warranted so as to accommodate the general aging populations rather than currently approved indications. Therefore, a generally applicable HBOT protocol needs to be defined in the subsequent step.
In recent years, HBOT has been used for various new medical conditions with protocols based on lower oxygen pressure (2 ATA or less) and more daily sessions (40–60 sessions) [194]. One specific emerging protocol, which utilizes repeated intermittent hyperbaric exposures, can lead to a series of changes in the aging populations including transcriptome changes [21], telomere elongation [42], cognitive enhancement [140], skin rejuvenation [27], and pulmonary function parameters improvement [194]. The protocol includes 60 daily HBOT sessions of 100% oxygen at 2 ATA for 90 min with intermittent air breaks. Through a mechanism previously referred to as the hyperoxic-hypoxic paradox, it induces physiological effects that classically occur in hypoxia state, excluding an increase in mitochondrial metabolism by activation of SIRT1 [19,85]. These findings will lay a solid foundation for further research. Another protocol, a modified version of HBOT called mild hyperbaric oxygen, provides appropriately high atmospheric pressure and 35–40% oxygen [197]. Although not within the usual definition of HBOT, this therapy has been shown to reverse degenerative changes in skin [154,159], bone [186], muscle [183,184] and metabolism [169] in experimental animals, primarily by promoting oxidative metabolism [197]. Despite its great potential demonstrated in preclinical studies, there is still a lack of supporting data from clinical trials regarding this modified version of therapy. Investigators need to further determine not only the efficacy and sustainability of existing protocols, but also the dose-response curves related to oxygen pressure, exposure time, frequency of intervals and number of sessions in order to optimize treatment conditions. Also, the optimal age range for HBOT to yield significant protective effects against aging needs to be described.
Concerns about the adverse effects of HBOT remain, possibly due to the exposure to a special atmospheric environment. Indeed, HBOT cannot be considered an entirely benign intervention because of the risk of some mild complications during HBOT, including claustrophobia, barotrauma and visual effects [3,198]. Fortunately, the vast majority of subjects can recover spontaneously from common complications and serious complications are fairly exceptional, suggesting that the current procedure is relatively safe [198]. Despite the guaranteed safety, the acceptable rate of side effects may be even lower when targeting healthy aging populations rather than patients with specific pathologies. In determining an applicable protocol of HBOT for healthy aging, the potential benefits must be carefully weighed against the corresponding risks, and the cost if necessary. To put it another way, HBOT may be absolutely or relatively contraindicated in some cases. In addition to the commonly considered contraindications such as pneumothorax, special attention must be paid to some chronic conditions that frequently occur in the aging populations. For example, it is demonstrated in a retrospective study that standard HBOT protocols lead to an absolute rise in arterial blood pressure (ABP), especially in a hypertensive subgroup [199]. Since a higher baseline ABP is commonly observed in the elderly, appropriate pre-session ABP levels should be described as thresholds for strict ABP monitoring during HBOT. In a nutshell, for clinical application in healthy older adults, technically safer HBOT protocols with more stringent guidelines are required.
To conclude, previous findings on HBOT have provided valid and sufficient information on its protective effects against aging. With many remaining questions beginning to be answered and protocols to be optimized, there are important discoveries yet to come. Obviously, HBOT has significant and beneficial implications for aging and age-related conditions, with great potential for clinical applications to be explored in the future.
Author contributions
QL provided direction and guidance throughout the preparation of the manuscript. QF performed the literature search and wrote the original manuscript. YS provided constructive suggestions and made significant revisions to the manuscript. RD helped revise the manuscript. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work is financially supported by grants from the Innovative Research Team of High-level Local Universities in Shanghai (SHSMU-ZDCX20210400) and Shanghai Municipal Key Clinical Specialty (shslczdzk00901) to QL, and National Natural Science Foundation of China (NSFC) (31871380, 82130045) to YS.
Contributor Information
Qiaoyu Fu, Email: dr_fuqiaoyu@163.com.
Ran Duan, Email: tracydr@126.com.
Yu Sun, Email: sunyu@sibs.ac.cn.
Qingfeng Li, Email: dr.liqingfeng@shsmu.edu.cn.
Abbreviations
- HBOT
hyperbaric oxygen therapy
- ATA
atmosphere absolute
- ROS
reactive oxygen species
- SASP
senescence-associated secretory phenotype
- HIF-1
hypoxia-inducible factor-1
- RNS
reactive nitrogen species
- VEGF
vascular endothelial growth factor
- EPCs
endothelial progenitor cells
- Nrf2
nuclear factor E2-related factor 2
- SIRT1
sirtuin1
- SA-β-gal
senescence-associated β-galactosidase
- D-gal
d-galactose
- MMPs
matrix metalloproteinases
- SPC
stem/progenitor cell
- MSCs
mesenchymal stem cells
- CBF
cerebral blood flow
- AD
Alzheimer's disease
- VD
vascular dementia
- UV
ultraviolet
- DKD
diabetic kidney disease
- ABP
arterial blood pressure
References
- 1.Shetty A.K., Kodali M., Upadhya R., Madhu L.N. Emerging anti-aging strategies – scientific basis and efficacy. Aging Dis. 2018;9:1165–1184. doi: 10.14336/AD.2018.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tibbles P.M., Edelsberg J.S. Hyperbaric-oxygen therapy. N. Engl. J. Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- 3.Ortega M.A., Fraile-Martinez O., García-Montero C., et al. A general overview on the hyperbaric oxygen therapy: applications, mechanisms and translational opportunities. Med. Plus. 2021;57:1–25. doi: 10.3390/medicina57090864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer I., Barak B. Molecular and therapeutic aspects of hyperbaric oxygen therapy in neurological conditions. Biomolecules. 2020;10:1–17. doi: 10.3390/biom10091247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhingra S., Buckey J.C.C.R. Hyperbaric oxygen reduces Aspergillus fumigatus proliferation in vitro and influences in vivo disease outcomes. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/AAC.01953-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buonocore G., Perrone S., Tataranno M.L. Oxygen toxicity: chemistry and biology of reactive oxygen species. Semin. Fetal Neonatal Med. 2010;15:186–190. doi: 10.1016/j.siny.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Dubreuil M.M., Morgens D.W., Okumoto K., et al. Systematic identification of regulators of oxidative stress reveals non-canonical roles for peroxisomal import and the pentose phosphate pathway. Cell Rep. 2020;30:1417–1433. doi: 10.1016/j.celrep.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Vliet T., Varela-Eirin M., Wang B., et al. Physiological hypoxia restrains the senescence-associated secretory phenotype via AMPK-mediated mTOR suppression. Mol. Cell. 2021;81:2041–2052. doi: 10.1016/j.molcel.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 9.Lee S.H., Lee J.H., Yoo S.Y., Hur J., Kim H.S., Kwon S.M. Hypoxia inhibits cellular senescence to restore the therapeutic potential of old human endothelial progenitor cells via the hypoxia-inducible factor-1α-TWIST-p21 Axis. Arterioscler. Thromb. Vasc. Biol. 2013;33:2407–2414. doi: 10.1161/ATVBAHA.113.301931. [DOI] [PubMed] [Google Scholar]
- 10.Parrinello S., Samper E., Krtolica A., Goldstein J., Melov S.C.J. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nat. Cell Biol. 2003;5:741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rascón B., Harrison J.F. Lifespan and oxidative stress show a non-linear response to atmospheric oxygen in Drosophila. J. Exp. Biol. 2010;213:3441–3448. doi: 10.1242/jeb.044867. [DOI] [PubMed] [Google Scholar]
- 12.Honda Y.H.S. Oxidative stress and life span determination in the nematode Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2002;959 doi: 10.1111/j.1749-6632.2002.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 13.Godman C.A., Chheda K.P., Hightower L.E., Perdrizet G., Shin D.G., Giardina C. Hyperbaric oxygen induces a cytoprotective and angiogenic response in human microvascular endothelial cells. Cell Stress Chaperones. 2010;15:431–442. doi: 10.1007/s12192-009-0159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies J.M.S., Cillard J., Friguet B., et al. The oxygen paradox, the French paradox, and age-related diseases. Gero. Sci. 2017;39:499–550. doi: 10.1007/s11357-017-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baik A.H., Jain I.H. Turning the oxygen dial: balancing the highs and lows. Trends Cell Biol. 2020;30:516–536. doi: 10.1016/j.tcb.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Wolde S.D., Hulskes R.H., de Jonge S.W., et al. The effect of hyperbaric oxygen therapy on markers of oxidative stress and the immune response in healthy volunteers. Front. Physiol. 2022;13:1–8. doi: 10.3389/fphys.2022.826163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang J.S., Chang E., Lee Y., et al. Hyperbaric oxygen exposure attenuates circulating stress biomarkers: a pilot interventional study. Int. J. Environ. Res. Publ. Health. 2020;17:1–9. doi: 10.3390/ijerph17217853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fratantonio D., Virgili F., Zucchi A., et al. Increasing oxygen partial pressures induce a distinct transcriptional response in human pbmc: a pilot study on the “normobaric oxygen paradox”. Int. J. Mol. Sci. 2021;22:1–13. doi: 10.3390/ijms22010458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hadanny A., Efrati S. The hyperoxic-hypoxic paradox. Biomolecules. 2020;10:958. doi: 10.3390/biom10060958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Vliet T., Casciaro F., Demaria M. To breathe or not to breathe: understanding how oxygen sensing contributes to age-related phenotypes. Ageing Res. Rev. 2021;67 doi: 10.1016/j.arr.2021.101267. [DOI] [PubMed] [Google Scholar]
- 21.Hadanny A., Forer R., Volodarsky D., et al. Hyperbaric oxygen therapy induces transcriptome changes in elderly: a prospective trial. Aging (Albany NY) 2021;13:24511–24523. doi: 10.18632/aging.203709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lähteenvuo J., Rosenzweig A. Effects of aging on angiogenesis. Circ. Res. 2012;110:1252–1263. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ambrose C.T. Pro-angiogenesis therapy and aging: a mini-review. Gerontology. 2017;63:393–400. doi: 10.1159/000477402. [DOI] [PubMed] [Google Scholar]
- 24.Hopf H.W., Gibson J.J., Angeles A.P., et al. Hyperoxia and angiogenesis. Wound Repair Regen. 2005;13:558–564. doi: 10.1111/j.1524-475X.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 25.Marx R.E., Ehler W.J., Tayapongsak P., Pierce L.W. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am. J. Surg. 1990;160:519–524. doi: 10.1016/s0002-9610(05)81019-0. [DOI] [PubMed] [Google Scholar]
- 26.Huang X., Liang P., Jiang B., et al. Hyperbaric oxygen potentiates diabetic wound healing by promoting fibroblast cell proliferation and endothelial cell angiogenesis. Life Sci. 2020;259 doi: 10.1016/j.lfs.2020.118246. [DOI] [PubMed] [Google Scholar]
- 27.Hachmo Y., Hadanny A., Mendelovic S., et al. The effect of hyperbaric oxygen therapy on the pathophysiology of skin aging: a prospective clinical trial. Aging (Albany NY) 2021;13:24500–24510. doi: 10.18632/aging.203701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oropallo A.R., Serena T.E., Armstrong D.G., Niederauer M.Q. Molecular biomarkers of oxygen therapy in patients with diabetic foot ulcers. Biomolecules. 2021;11:1–10. doi: 10.3390/biom11070925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kleban S., Baynosa R.C. The effect of hyperbaric oxygen on compromised grafts and flaps. Undersea Hyperb. Med. 2020;47:635–648. [PubMed] [Google Scholar]
- 30.Duan S., Shao G., Yu L.R.C. Angiogenesis contributes to the neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Int. J. Neurosci. 2015;125:625–634. doi: 10.3109/00207454.2014.956101. [DOI] [PubMed] [Google Scholar]
- 31.Lin K.C., Niu K.C., Tsai K.J., Kuo J.R., Wang L.C., et al. Attenuating inflammation but stimulating both angiogenesis and neurogenesis using hyperbaric oxygen in rats with traumatic brain injury. J. Trauma. Acute Care Surg. 2012;72:650–659. doi: 10.1097/TA.0b013e31823c575f. [DOI] [PubMed] [Google Scholar]
- 32.Tal S., Hadanny A., Sasson E., Suzin G., Efrati S. Hyperbaric oxygen therapy can induce angiogenesis and regeneration of nerve fibers in traumatic brain injury patients. Front. Hum. Neurosci. 2017;11:1–12. doi: 10.3389/fnhum.2017.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hadanny A., Lang E., Copel L., et al. Hyperbaric oxygen can induce angiogenesis and recover erectile function. Int. J. Impot. Res. 2018;30:292–299. doi: 10.1038/s41443-018-0023-9. [DOI] [PubMed] [Google Scholar]
- 34.Grassmann J.P., Schneppendahl J., Sager M., Hakimi A.R., et al. The effect of bone marrow concentrate and hyperbaric oxygen therapy on bone repair. J. Mater. Sci. Mater. Med. 2015;26:5331. doi: 10.1007/s10856-014-5331-0. [DOI] [PubMed] [Google Scholar]
- 35.Chen S.Y., Tsuneyama K., Yen M.H., Lee J.T., Chen J.L., Huang S.M. Hyperbaric oxygen suppressed tumor progression through the improvement of tumor hypoxia and induction of tumor apoptosis in A549-cell-transferred lung cancer. Sci. Rep. 2021;11:1–15. doi: 10.1038/s41598-021-91454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y.G., Zhan Y.P., Pan S.Y., et al. Hyperbaric oxygen promotes malignant glioma cell growth and inhibits cell apoptosis. Oncol. Lett. 2015;10:189–195. doi: 10.3892/ol.2015.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoenig M., Bianchi C., Rosenzweig A., Sellke F. Decreased vascular repair and neovascularization with ageing: mechanisms and clinical relevance with an emphasis on hypoxia- inducible factor-1. Curr. Mol. Med. 2008;8:754–767. doi: 10.2174/156652408786733685. [DOI] [PubMed] [Google Scholar]
- 38.Peng Z.R., Yang A.L., Yang Q.D. The effect of hyperbaric oxygen on intracephalic angiogenesis in rats with intracerebral hemorrhage. J. Neurol. Sci. 2014;342:114–123. doi: 10.1016/j.jns.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 39.Hu Q., Liang X., Chen D., et al. Delayed hyperbaric oxygen therapy promotes neurogenesis through reactive oxygen species/hypoxia-inducible factor-1α/β-catenin pathway in middle cerebral artery occlusion rats. Stroke. 2014;45:1807–1814. doi: 10.1161/STROKEAHA.114.005116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Semenza G L. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J. Appl. Physiol. 2000;88:1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 41.Dewhirst Mark W., Yiting Cao B.M. Cycling hypoxia and free radicals regulate angiogenesis and radiotherapy response. Nat. Rev. Cancer. 2008;8:425–437. doi: 10.1038/nrc2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hachmo Y., Hadanny A., Hamed R.A., et al. Hyperbaric oxygen therapy increases telomere length and decreases immunosenescence in isolated blood cells : a prospective trial. Aging (Albany NY) 2020;12:22445–22456. doi: 10.18632/aging.202188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schottlender N., Gottfried I., Ashery U. Hyperbaric oxygen treatment: effects on mitochondrial function and oxidative stress. Biomolecules. 2021;11:1827. doi: 10.3390/biom11121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poff A.M., Kernagis D., D'Agostino D.P. Hyperbaric environment: oxygen and cellular damage versus protection. Compr. Physiol. 2017;7:213–234. doi: 10.1002/cphy.c150032. [DOI] [PubMed] [Google Scholar]
- 45.Joseph V., Boykin C.B., Jr. Hyperbaric oxygen therapy mediates increased nitric oxide production associated with wound healing: a preliminary study. Adv. Skin Wound Care. 2007;20:382–388. doi: 10.1097/01.ASW.0000280198.81130.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Francis A., Baynosa R. Ischaemia-reperfusion injury and hyperbaric oxygen pathways: a review of cellular mechanisms. Diving Hyperb. Med. 2017;47:110–117. doi: 10.28920/dhm47.2.110-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cramer T., Schipani E., Johnson R.S., Swoboda B., Pfander D. Expression of VEGF isoforms by epiphyseal chondrocytes during low-oxygen tension is HIF-1α dependent. Osteoarthr. Cartil. 2004;12:433–439. doi: 10.1016/j.joca.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 48.Dhamodharan U., Karan A., Sireesh D., et al. Tissue-specific role of Nrf2 in the treatment of diabetic foot ulcers during hyperbaric oxygen therapy. Free Radic. Biol. Med. 2019;138:53–62. doi: 10.1016/j.freeradbiomed.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 49.Sureda A., Batle J.M., Martorell M., et al. Antioxidant response of chronic wounds to hyperbaric oxygen therapy. PLoS One. 2016;11:1–14. doi: 10.1371/journal.pone.0163371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenza G.L. Oxygen sensing, homeostasis, and disease. N. Engl. J. Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 51.Milovanova T.N., Bhopale V.M., Sorokina E.M., et al. Lactate stimulates vasculogenic stem cells via the thioredoxin system and engages an autocrine activation loop involving hypoxia-inducible factor 1. Mol. Cell Biol. 2008;28:6248–6261. doi: 10.1128/MCB.00795-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thom S.R., Bhopale V.M., Velazquez O.C., Goldstein L.J., Thom L.H., Buerk D.G. Stem cell mobilization by hyperbaric oxygen. Am. J. Physiol. Heart Circ. Physiol. 2006;290:1378–1386. doi: 10.1152/ajpheart.00888.2005. [DOI] [PubMed] [Google Scholar]
- 53.Velazquez O.C. Angiogenesis and vasculogenesis: inducing the growth of new blood vessels and wound healing by stimulation of bone marrow-derived progenitor cell mobilization and homing. J. Vasc. Surg. 2007;45:A39–A47. doi: 10.1016/j.jvs.2007.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neves J., Sousa-Victor P. Regulation of inflammation as an anti-aging intervention. FEBS J. 2020;287:43–52. doi: 10.1111/febs.15061. [DOI] [PubMed] [Google Scholar]
- 55.Almzaiel A.J., Billington R., Smerdon G., Moody A.J. Effects of hyperbaric oxygen treatment on antimicrobial function and apoptosis of differentiated HL-60 (neutrophil-like) cells. Life Sci. 2013;93:125–131. doi: 10.1016/j.lfs.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Grimberg-Peters D., Büren C., Windolf J., Wahlers T., Paunel-Görgülü A. Hyperbaric oxygen reduces production of reactive oxygen species in neutrophils from polytraumatized patients yielding in the inhibition of p38 MAP kinase and downstream pathways. PLoS One. 2016;11:1–14. doi: 10.1371/journal.pone.0161343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Q., Chang Q., Cox R.A., Gong X., Gould L.J. Hyperbaric oxygen attenuates apoptosis and decreases inflammation in an ischemic wound model. J. Invest. Dermatol. 2008;128:2102–2112. doi: 10.1038/jid.2008.53. [DOI] [PubMed] [Google Scholar]
- 58.Tjarnstrom J., Wikstrom T., Bagge U., Risberg B.B.M. Effects of hyperbaric oxygen treatment on neutrophil activation and pulmonary sequestration in intestinal ischemia-reperfusion in rats. Eur. Surg. Res. 1999;31:147–154. doi: 10.1159/000008633. [DOI] [PubMed] [Google Scholar]
- 59.Weber S.U., Koch A., Kankeleit J., et al. Hyperbaric oxygen induces apoptosis via a mitochondrial mechanism. Apoptosis. 2009;14:97–107. doi: 10.1007/s10495-008-0280-z. [DOI] [PubMed] [Google Scholar]
- 60.Bai X., Song Z., Zhou Y., et al. The apoptosis of peripheral blood lymphocytes promoted by hyperbaric oxygen treatment contributes to attenuate the severity of early stage acute pancreatitis in rats. Apoptosis. 2014;19:58–75. doi: 10.1007/s10495-013-0911-x. [DOI] [PubMed] [Google Scholar]
- 61.Resanovic I., Gluvic Z., Zaric B., et al. Early effects of hyperbaric oxygen on inducible nitric oxide synthase activity/expression in lymphocytes of type 1 diabetes patients: a prospective pilot study. Internet J. Endocrinol. 2019 doi: 10.1155/2019/2328505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bitterman N., Bitterman H., Kinarty A., Melamed Y.L.N. Effect of a single exposure to hyperbaric oxygen on blood mononuclear cells in human subjects. Undersea Hyperb. Med. 1993;20:197–204. [PubMed] [Google Scholar]
- 63.Li M., Yao D., Zeng X., et al. Age related human T cell subset evolution and senescence. Immun. Ageing. 2019;16:1–7. doi: 10.1186/s12979-019-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu K., Miao L., Chen W., et al. Establishment of the reference intervals of lymphocyte subsets for healthy Chinese Han adults and its influencing factors. Ann. Transl. Med. 2021;9 doi: 10.21037/atm-21-4031. 1495–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Navakkode S., Liu C., Soong T.W. Altered function of neuronal L-type calcium channels in ageing and neuroinflammation: implications in age-related synaptic dysfunction and cognitive decline. Ageing Res. Rev. 2018;42:86–99. doi: 10.1016/j.arr.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Shwe T., Bo-Htay C., Ongnok B., et al. Hyperbaric oxygen therapy restores cognitive function and hippocampal pathologies in both aging and aging-obese rats. Mech. Ageing Dev. 2021;195 doi: 10.1016/j.mad.2021.111465. [DOI] [PubMed] [Google Scholar]
- 67.Shapira R., Solomon B., Efrati S., Frenkel D., Ashery U. Hyperbaric oxygen therapy ameliorates pathophysiology of 3xTg-AD mouse model by attenuating neuroinflammation. Neurobiol. Aging. 2018;62:105–119. doi: 10.1016/j.neurobiolaging.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Lavrnja I., Parabucki A., Brkic P., Jovanovic T., et al. Repetitive hyperbaric oxygenation attenuates reactive astrogliosis and suppresses expression of inflammatory mediators in the rat. Mediat. Inflamm. 2015:1–17. doi: 10.1155/2015/498405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weisz G., Lavy A., Adir Y., Melamed Y., Rubin D., Eidelman S.P.S. Modification of in vivo and in vitro TNF-alpha, IL-1, and IL-6 secretion by circulating monocytes during hyperbaric oxygen treatment in patients with perianal Crohn's disease. J. Clin. Immunol. 1997;17:154–159. doi: 10.1023/a:1027378532003. [DOI] [PubMed] [Google Scholar]
- 70.Al-Waili N.S., Butler G.J. Effects of hyperbaric oxygen on inflammatory response to wound and trauma: possible mechanism of action. Sci. World J. 2006;6:425–441. doi: 10.1100/tsw.2006.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen X., Li Y., Chen W., Nong Z., Huang J., Chen C. Protective effect of hyperbaric oxygen on cognitive impairment induced by d-galactose in mice. Neurochem. Res. 2016;41:3032–3041. doi: 10.1007/s11064-016-2022-x. [DOI] [PubMed] [Google Scholar]
- 72.Arıcıgil M., Dündar M.A., Yücel A., et al. Anti-inflammatory effects of hyperbaric oxygen on irradiated laryngeal tissues. Braz J Otorhinolaryngol. 2018;84:206–211. doi: 10.1016/j.bjorl.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hao Y., Dong X., Zhang M., Liu H., Zhu L., Wang Y. Effects of hyperbaric oxygen therapy on the expression levels of the inflammatory factors interleukin-12p40, macrophage inflammatory protein-1b, platelet-derived growth factor-BB,and interleukin-1 receptor antagonist in keloids. Medicine (Baltim.) 2020;99 doi: 10.1097/MD.0000000000019857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen L.F., Tian Y.F., Lin C.H., Huang L.Y., Niu K.C.L.M. Repetitive hyperbaric oxygen therapy provides better effects on brain inflammation and oxidative damage in rats with focal cerebral ischemia. J. Formos. Med. Assoc. 2014;113:620–628. doi: 10.1016/j.jfma.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Buras J.A., Holt D., Orlow D., Belikoff B., Pavlides S., Reenstra W.R. Hyperbaric oxygen protects from sepsis mortality via an interleukin-10-dependent mechanism. Crit. Care Med. 2006;34:2624–2629. doi: 10.1097/01.CCM.0000239438.22758.E0. [DOI] [PubMed] [Google Scholar]
- 76.Chen X., Duan X.S., Xu L.J., et al. Interleukin-10 mediates the neuroprotection of hyperbaric oxygen therapy against traumatic brain injury in mice. Neuroscience. 2014;266:235–243. doi: 10.1016/j.neuroscience.2013.11.036. [DOI] [PubMed] [Google Scholar]
- 77.De Wolde S.D., Hulskes R.H., Weenink R.P., Hollmann M.W., Van Hulst R.A. The effects of hyperbaric oxygenation on oxidative stress, inflammation and angiogenesis. Biomolecules. 2021;11:1–47. doi: 10.3390/biom11081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rinaldi B., Cuzzocrea S., Donniacuo M., et al. Hyperbaric oxygen therapy reduces the toll-like receptor signaling pathway in multiple organ failures. Intensive Care Med. 2011;37:1110–1119. doi: 10.1007/s00134-011-2241-1. [DOI] [PubMed] [Google Scholar]
- 79.Vatner S.F., Zhang J., Oydanich M., Berkman T., Naftalovich R., Vatner D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Memar M.Y., Yekani M., Alizadeh N., Baghi H.B. Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019;109:440–447. doi: 10.1016/j.biopha.2018.10.142. [DOI] [PubMed] [Google Scholar]
- 81.Tezgin D., Giardina C., Perdrizet G.A., Hightower L.E. The effect of hyperbaric oxygen on mitochondrial and glycolytic energy metabolism: the caloristasis concept. Cell Stress Chaperones. 2020;25:667–677. doi: 10.1007/s12192-020-01100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan Y., Cruzat V.F., Newshome P., Cheng J., Chen Y., Lu Y. Regulation of SIRT1 in aging: roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016;155:10–21. doi: 10.1016/j.mad.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Singh V., Ubaid S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflammation. 2020;43:1589–1598. doi: 10.1007/s10753-020-01242-9. [DOI] [PubMed] [Google Scholar]
- 84.Hu Q., Manaenko A., Bian H., et al. Hyperbaric oxygen reduces infarction volume and hemorrhagic transformation through ATP/NAD+/Sirt1 pathway in hyperglycemic middle cerebral artery occlusion rats. Stroke. 2017;48:1655–1664. doi: 10.1161/STROKEAHA.116.015753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamat S.M., Mendelsohn A.R., Larrick J.W. Rejuvenation through oxygen, more or less. Rejuvenation Res. 2021;24:158–163. doi: 10.1089/rej.2021.0014. [DOI] [PubMed] [Google Scholar]
- 86.Yeo E.J. Hypoxia and aging. Exp. Mol. Med. 2019;51:1–15. doi: 10.1038/s12276-019-0233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dennog C., Hartmann A., Frey G., Speit G. Detection of DNA damage after hyperbaric oxygen (HBO) therapy. Mutagenesis. 1996;11:605–609. doi: 10.1093/mutage/11.6.605. [DOI] [PubMed] [Google Scholar]
- 88.Körpınar S., Uzun H. The effects of hyperbaric oxygen at different pressures on oxidative stress and antioxidant status in rats. Med. Plus. 2019;55:1–8. doi: 10.3390/medicina55050205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tonelli C., Chio I.I.C., Tuveson D.A. Transcriptional regulation by Nrf2. Antioxidants Redox Signal. 2018;29:1727–1745. doi: 10.1089/ars.2017.7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Hongqiao, Kelvin J., Davies A., Hjf Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015;176:314–336. doi: 10.1016/j.freeradbiomed.2015.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu X., Liang F., Song W., Diao X., Zhu W., Yang J. Effect of Nrf2 signaling pathway on the improvement of intestinal epithelial barrier dysfunction by hyperbaric oxygen treatment after spinal cord injury. Cell Stress Chaperones. 2021;26:433–441. doi: 10.1007/s12192-020-01190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xue F., Huang J wen, Ding P yan, et al. Nrf2/antioxidant defense pathway is involved in the neuroprotective effects of Sirt1 against focal cerebral ischemia in rats after hyperbaric oxygen preconditioning. Behav. Brain Res. 2016;309:1–8. doi: 10.1016/j.bbr.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 93.Meng X., Zhang Y., Li N., et al. Effects of hyperbaric oxygen on the Nrf2 signaling pathway in secondary injury following traumatic brain injury. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15016933. [DOI] [PubMed] [Google Scholar]
- 94.Zhang Q., Gould L.J. Hyperbaric oxygen reduces matrix metalloproteinases in ischemic wounds through a redox-dependent mechanism. J. Invest. Dermatol. 2014;134:237–246. doi: 10.1038/jid.2013.301. [DOI] [PubMed] [Google Scholar]
- 95.Martini H., Passos J.F. Cellular senescence: all roads lead to mitochondria. FEBS J. 2022 doi: 10.1111/febs.16361. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorgoulis V., Adams P.D., Alimonti A., et al. Cellular senescence: defining a path forward. Cell. 2019;179:813–827. doi: 10.1016/j.cell.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 97.Childs B.G., Gluscevic M., Baker D.J., et al. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017;16:718–735. doi: 10.1038/nrd.2017.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bo-Htay C., Shwe T., Jaiwongkam T., et al. Hyperbaric oxygen therapy effectively alleviates D-galactose-induced-age-related cardiac dysfunction via attenuating mitochondrial dysfunction in pre-diabetic rats. Aging (Albany NY) 2021;13:10955–10972. doi: 10.18632/aging.202970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.De Cecco M., Ito T., Petrashen A.P., et al. L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature. 2019;566:73–78. doi: 10.1038/s41586-018-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kirkland J.L., Tchkonia T. Senolytic drugs: from discovery to translation. J. Intern. Med. 2020;288:518–536. doi: 10.1111/joim.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumari R., Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front. Cell Dev. Biol. 2021;9:1–24. doi: 10.3389/fcell.2021.645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalns J.E., Piepmeier E.H. Exposure to hyperbaric oxygen induces cell cycle perturbation in prostate cancer cells. In Vitro Cell. Dev. Biol. Anim. 1999;35:98–101. doi: 10.1007/s11626-999-0008-6. [DOI] [PubMed] [Google Scholar]
- 103.Silva F.S., Canêdo V.S.R., Abreu B.J., Oliveira M.F. Responses of matrix metalloproteinases to hyperbaric oxygen treatment: changing for good or ill? Connect. Tissue Res. 2021;62:249–262. doi: 10.1080/03008207.2020.1821675. [DOI] [PubMed] [Google Scholar]
- 104.Vlodavsky E., Palzur E., Soustiel J.F. Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model of traumatic brain injury. Neuropathol. Appl. Neurobiol. 2006;32:40–50. doi: 10.1111/j.1365-2990.2005.00698.x. [DOI] [PubMed] [Google Scholar]
- 105.Lagoumtzi S.M., Chondrogianni N. Senolytics and senomorphics: natural and synthetic therapeutics in the treatment of aging and chronic diseases. Free Radic. Biol. Med. 2021;171:169–190. doi: 10.1016/j.freeradbiomed.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Kim E.C., Kim J.R. Senotherapeutics: emerging strategy for healthy aging and age-related disease. BMB Rep. 2019;52:47–55. doi: 10.5483/BMBRep.2019.52.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Demanelis K., Jasmine F., Chen L.S., et al. Determinants of telomere length across human tissues. Science. 2020;369 doi: 10.1126/science.aaz6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shlush Liran I., Skorecki Karl L., Itzkovitz Shalev, et al. Telomere elongation followed by telomere length reduction, in leukocytes from divers exposed to intense oxidative stress – implications for tissue and organismal aging. Mech. Ageing Dev. 2011;132:123–130. doi: 10.1016/j.mad.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 109.Schultz M.B., Sinclair D.A. When stem cells grow old: phenotypes and mechanisms of stem cell aging. Devenir. 2016;143:3–14. doi: 10.1242/dev.130633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhopale M., Sorokina E.M., Uzun G., Malay D.S., Logue C.J., Margolis D.J. Vasculogenic stem cell mobilization and wound recruitment in diabetic patients: increased cell number and intracellular regulatory protein content associated with hyperbaric oxygen therapy. Wound Repair Regen. 2011;19:149–161. doi: 10.1111/j.1524-475X.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forner L., Berkowicz A., Dickmeiss E., Hyldegaard O., Jansen E.C., Fischer-Nielsen A. Only minor stem cell mobilization in head and neck irradiated patients treated with hyperbaric oxygen. Diving Hyperb. Med. 2019;49:175–185. doi: 10.28920/dhm49.3.175-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang L., Sun Q., Xin Q., et al. Hyperbaric oxygen therapy mobilized circulating stem cells and improved delayed encephalopathy after acute carbon monoxide poisoning with up-regulation of brain-derived neurotrophic factor. Am. J. Emerg. Med. 2021;42:95–100. doi: 10.1016/j.ajem.2021.01.021. [DOI] [PubMed] [Google Scholar]
- 113.Shandley S., Wolf E.G., Schubert-Kappan C.M., Baugh L.M., Richards M.F., Prye J., Arizpe H.M.K.J. Increased circulating stem cells and better cognitive performance in traumatic brain injury subjects following hyperbaric oxygen therapy. Undersea Hyperb. Med. 2017;44:257–269. doi: 10.22462/5.6.2017.6. [DOI] [PubMed] [Google Scholar]
- 114.Yang Y., Wei H., Zhou X., Zhang F., Wang C. Hyperbaric oxygen promotes neural stem cell proliferation by activating vascular endothelial growth factor/extracellular signal-regulated kinase signaling after traumatic brain injury. Neuroreport. 2017;28:1232–1238. doi: 10.1097/WNR.0000000000000901. [DOI] [PubMed] [Google Scholar]
- 115.Feng Z., Liu J., Ju R. Hyperbaric oxygen treatment promotes neural stem cell proliferation in the subventricular zone of neonatal rats with hypoxic-ischemic brain damage. Neural Regen. Res. 2013;8:1220–1227. doi: 10.3969/j.issn.1673-5374.2013.13.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Peña-Villalobos I., Casanova-Maldonado I., Lois P., et al. Hyperbaric oxygen increases stem cell proliferation, angiogenesis and wound-healing ability of WJ-MSCs in diabetic mice. Front. Physiol. 2018;9:1–15. doi: 10.3389/fphys.2018.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milovanova T.N., Bhopale V.M., Sorokina E.M., et al. Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. J. Appl. Physiol. 2009;106:711–728. doi: 10.1152/japplphysiol.91054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bekheit M., Baddour N., Katri K., Taher Y., Tobgy K El, Mousa E. Hyperbaric oxygen therapy stimulates colonic stem cells and induces mucosal healing in patients with refractory ulcerative colitis: a prospective case series. BMJ Open Gastroenterol. 2016;3:1–8. doi: 10.1136/bmjgast-2016-000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yoshinoya Y., Böcker A.H., Ruhl T., et al. The effect of hyperbaric oxygen therapy on human adipose-derived stem cells. Plast. Reconstr. Surg. 2020;146:309–320. doi: 10.1097/PRS.0000000000007029. [DOI] [PubMed] [Google Scholar]
- 120.Lin S.S., Ueng S.W., Niu C.C., et al. Effects of hyperbaric oxygen on the osteogenic differentiation of mesenchymal stem cells. BMC Muscoskel. Disord. 2014;15:1–10. doi: 10.1186/1471-2474-15-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gardin C., Bosco G., Ferroni L., et al. Hyperbaric oxygen therapy improves the osteogenic and vasculogenic properties of mesenchymal stem cells in the presence of inflammation in vitro. Int. J. Mol. Sci. 2020;21:1–22. doi: 10.3390/ijms21041452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schulze J., Kaiser O., Paasche G., et al. Effect of hyperbaric oxygen on BDNF-release and neuroprotection: investigations with human mesenchymal stem cells and genetically modified NIH3T3 fibroblasts as putative cell therapeutics. PLoS One. 2017;12:1–23. doi: 10.1371/journal.pone.0178182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khan M., Meduru S., Gogna R., et al. Oxygen cycling in conjunction with stem cell transplantation induces NOS3 expression leading to attenuation of fibrosis and improved cardiac function. Cardiovasc. Res. 2012;93:89–99. doi: 10.1093/cvr/cvr277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou H.X., Liu Z.G., Liu X.J., Chen Q.X. Umbilical cord-derived mesenchymal stem cell transplantation combined with hyperbaric oxygen treatment for repair of traumatic brain injury. Neural Regen. Res. 2016;11:107–113. doi: 10.4103/1673-5374.175054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Estrada E.J., Decima J.L., Bortman G., et al. Combination treatment of autologous bone marrow stem cell transplantation and hyperbaric oxygen therapy for type 2 diabetes mellitus: a randomized controlled trial. Cell Transplant. 2019;28:1632–1640. doi: 10.1177/0963689719883813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zauner A., Daugherty W.P., Bullock M.R., Warner D.S. Brain oxygenation and energy metebolism : Part I — biological function and pathophysiology. Neurosurgery. 2003;52:1508–1509. doi: 10.1227/01.neu.0000068349.22859.c6. Ph D. [DOI] [PubMed] [Google Scholar]
- 127.Scholey A.B., Moss M.C., Wesnes K. Oxygen and cognitive performance: the temporal relationship between hyperoxia and enhanced memory. Psychopharmacology (Berl) 1998;140:123–126. doi: 10.1007/s002130050748. [DOI] [PubMed] [Google Scholar]
- 128.Chung S.C., Kwon J.H., Lee H.W., et al. Effects of high concentration oxygen administration on n-back task performance and physiological signals. Physiol. Meas. 2007;28:389–396. doi: 10.1088/0967-3334/28/4/005. [DOI] [PubMed] [Google Scholar]
- 129.Chung Soon-Cheol, Tack Gye-Rae, Lee Bongsoo, et al. The effect of 30% oxygen on visuospatial performance and brain activation: an fMRI study. Brain Cognit. 2004;56:279–285. doi: 10.1016/j.bandc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 130.Chung Soon-Cheol, Sohn Jin-Hun, Lee Bongsoo, et al. The effect of transient increase in oxygen level on brain activation and verbal performance. Int. J. Psychophysiol. 2006;62:103–108. doi: 10.1016/j.ijpsycho.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Yu R., Wang B., Li S., et al. Cognitive enhancement of healthy young adults with hyperbaric oxygen: a preliminary resting-state fMRI study. Clin. Neurophysiol. 2015;126:2058–2067. doi: 10.1016/j.clinph.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 132.Vadas D., Kalichman L., Hadanny A., Efrati S. Hyperbaric oxygen environment can enhance brain activity and multitasking performance. Front. Integr. Neurosci. 2017;11:1–6. doi: 10.3389/fnint.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suzin G., Frolinger T.H., Yogev D., et al. Oxygen: the rate-limiting factor for episodic memory performance, even in healthy young individuals. Biomolecules. 2020;10:1–10. doi: 10.3390/biom10091328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Moss M.C., Scholey A.B., Wesnes K. Oxygen administration selectively enhances cognitive performance in healthy young adults: a placebo-controlled double-blind crossover study. Psychopharmacology (Berl) 1998;138:27–33. doi: 10.1007/s002130050641. [DOI] [PubMed] [Google Scholar]
- 135.Jacobs E.A., Winter P.M., Alvis H.J., et al. Hyperoxygenation effect on cognitive functioning in the aged. N. Engl. J. Med. 1969:753–757. doi: 10.1056/NEJM196910022811402. [DOI] [PubMed] [Google Scholar]
- 136.Raskin A., Gershon S., Crook T.H., Sathananthan G., Ferris S. The effects of hyperbaric and normobaric oxygen on cognitive impairment in the elderly. Arch. Gen. Psychiatr. 1978;35:50–56. doi: 10.1001/archpsyc.1978.01770250052005. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y., Ding C., Cai Y., et al. Astilbin ameliorates oxidative stress and apoptosis in D-galactose-induced senescence by regulating the PI3K/Akt/m-TOR signaling pathway in the brains of mice. Int. Immunopharm. 2021;99 doi: 10.1016/j.intimp.2021.108035. [DOI] [PubMed] [Google Scholar]
- 138.Chen C., Huang L., Nong Z., et al. Hyperbaric oxygen prevents cognitive impairments in mice induced by d-galactose by improving cholinergic and anti-apoptotic functions. Neurochem. Res. 2017;42:1240–1253. doi: 10.1007/s11064-016-2166-8. [DOI] [PubMed] [Google Scholar]
- 139.Sadigh-Eteghad S., Majdi A., McCann S.K., Mahmoudi J., Vafaee M.S., Macleod M.R. D-galactose-induced brain ageing model: a systematic review and meta-analysis on cognitive outcomes and oxidative stress indices. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Amir H., Malka D.K., Gil S., et al. Cognitive enhancement of healthy older adults using hyperbaric oxygen: a randomized controlled trial. Aging (Albany NY) 2020;12:13740–13761. doi: 10.18632/aging.103571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shapira R., Gdalyahu A., Gottfried I., et al. Hyperbaric oxygen therapy alleviates vascular dysfunction and amyloid burden in an Alzheimer's disease mouse model and in elderly patients. Aging (Albany NY) 2021;13:20935–20961. doi: 10.18632/aging.203485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hadanny A., Golan H., Fishlev G., et al. Hyperbaric oxygen can induce neuroplasticity and improve cognitive functions of patients suffering from anoxic brain damage. Restor. Neurol. Neurosci. 2015;33:471–486. doi: 10.3233/RNN-150517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hadanny A., Rittblat M., Bitterman M., et al. Hyperbaric oxygen therapy improves neurocognitive functions of post-stroke patients-a retrospective analysis. Restor. Neurol. Neurosci. 2020;38:93–107. doi: 10.3233/RNN-190959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Livingston G., Sommerlad A., Orgeta V., et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 145.You Q., Li L., Xiong S.Q., et al. Meta-analysis on the efficacy and safety of hyperbaric oxygen as adjunctive therapy for vascular dementia. Front. Aging Neurosci. 2019;11 doi: 10.3389/fnagi.2019.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shapira R., Efrati S., Ashery U. Hyperbaric oxygen therapy as a new treatment approach for Alzheimer's disease. Neural Regen. Res. 2018;13:817–818. doi: 10.4103/1673-5374.232475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Somaa F. A review of the application of hyperbaric oxygen therapy in alzheimer's disease. J. Alzheim. Dis. 2021;81:1361–1367. doi: 10.3233/JAD-210157. [DOI] [PubMed] [Google Scholar]
- 148.Chen C., Chen W., Nong Z., et al. Hyperbaric oxygen alleviated cognitive impairments in mice induced by repeated cerebral ischemia-reperfusion injury via inhibition of autophagy. Life Sci. 2020;241:117170. doi: 10.1016/j.lfs.2019.117170. [DOI] [PubMed] [Google Scholar]
- 149.Maarouf C.L., Daugs I.D., Kokjohn T.A., et al. Alzheimer's disease and non-demented high pathology control nonagenarians: comparing and contrasting the biochemistry of cognitively successful aging. PLoS One. 2011;6:1–18. doi: 10.1371/journal.pone.0027291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Duong M.T., Nasrallah I.M., Wolk D.A., Chang C.C.Y., Chang T.Y. Cholesterol, atherosclerosis, and APOE in vascular contributions to cognitive impairment and dementia (VCID): potential mechanisms and therapy. Front. Aging Neurosci. 2021;13:1–12. doi: 10.3389/fnagi.2021.647990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gu Y., Han J., Jiang C., Zhang Y. Biomarkers, oxidative stress and autophagy in skin aging. Ageing Res. Rev. 2020;59:101036. doi: 10.1016/j.arr.2020.101036. [DOI] [PubMed] [Google Scholar]
- 152.Zhang S., Duan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27:729–738. doi: 10.1177/0963689717725755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gunin A.G., Petrov V.V., Vasil’eva O.V.G.N. Blood vessels in human dermis during aging. Adv. Gerontol. 2014;27:54–61. [PubMed] [Google Scholar]
- 154.Nishizaka T., Nomura T., Higuchi K., Takemura A., Ishihara A. Mild hyperbaric oxygen activates the proliferation of epidermal basal cells in aged mice. J. Dermatol. 2018;45:1141–1144. doi: 10.1111/1346-8138.14484. [DOI] [PubMed] [Google Scholar]
- 155.Debacq-Chainiaux F., Leduc C., Verbeke A., Toussaint O. UV, stress and aging. Dermatoendocrinol. 2012;4:236–240. doi: 10.4161/derm.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cavinato M., Jansen-Dürr P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017;94:78–82. doi: 10.1016/j.exger.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 157.Fuller A.M., Giardina C., Hightower L.E., Perdrizet G.A., Tierney C.A. Hyperbaric oxygen preconditioning protects skin from UV-A damage. Cell Stress Chaperones. 2013;18:97–107. doi: 10.1007/s12192-012-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kawada S., Ohtani M., Ishii N. Increased oxygen tension attenuates acute ultraviolet-B-induced skin angiogenesis and wrinkle formation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;299:694–701. doi: 10.1152/ajpregu.00199.2010. [DOI] [PubMed] [Google Scholar]
- 159.Nishizaka T., Nomura T., Sano T., Higuchi K., Nagatomo F., Ishihara A. Hyperbaric oxygen improves ultraviolet B irradiation-induced melanin pigmentation and diminishes senile spot size. Skin Res. Technol. 2011;17:332–338. doi: 10.1111/j.1600-0846.2011.00502.x. [DOI] [PubMed] [Google Scholar]
- 160.Kalra S., Sharma S.K. Diabetes in the elderly. Diabetes Ther. 2018;9:493–500. doi: 10.1007/s13300-018-0380-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Akash M.S.H., Rehman K., Liaqat A., Numan M., Mahmood Q., Kamal S. Biochemical investigation of gender-specific association between insulin resistance and inflammatory biomarkers in types 2 diabetic patients. Biomed. Pharmacother. 2018;106:285–291. doi: 10.1016/j.biopha.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 162.Chen S.J., Yu C.T., Cheng Y.L., Yu S.Y., Lo H.C. Effects of hyperbaric oxygen therapy on circulating interleukin-8, nitric oxide, and insulin-like growth factors in patients with type 2 diabetes mellitus. Clin. Biochem. 2007;40:30–36. doi: 10.1016/j.clinbiochem.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 163.Wilkinson D., Chapman I.M., Heilbronn L.K. Hyperbaric oxygen therapy improves peripheral insulin sensitivity in humans. Diabet. Med. 2012;29:986–989. doi: 10.1111/j.1464-5491.2012.03587.x. [DOI] [PubMed] [Google Scholar]
- 164.Guarino M.P., Conde S.V. Hyperbaric oxygen therapy improves glucose homeostasis in type 2 diabetes patients:A likely involvement of the carotid bodies. Adv. Exp. Med. Biol. 2015;860:221–225. doi: 10.1007/978-3-319-18440-1_24. [DOI] [PubMed] [Google Scholar]
- 165.Baitule S., Patel A.H., Murthy N., et al. A systematic review to assess the impact of hyperbaric oxygen therapy on glycaemia in people with diabetes mellitus. Med. Plus. 2021;57:1–12. doi: 10.3390/medicina57101134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karadurmus N., Sahin M., Tasci C., et al. Potential benefits of hyperbaric oxygen therapy on atherosclerosis and glycaemic control in patients with diabetic foot. Endokrynol. Pol. 2010;61:275–279. [PubMed] [Google Scholar]
- 167.Xu Q., Wei Y.T., Fan S.B., Wang L., Zhou X.P. Repetitive hyperbaric oxygen treatment increases insulin sensitivity in diabetes patients with acute intracerebral hemorrhage. Neuropsychiatric Dis. Treat. 2017;13:421–426. doi: 10.2147/NDT.S126288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu Y., Zhang D., Yuan J., et al. Hyperbaric oxygen ameliorates insulin sensitivity by increasing GLUT4 expression in skeletal muscle and stimulating UCP1 in Brown adipose tissue in T2DM mice. Front. Endocrinol. 2020;11:1–11. doi: 10.3389/fendo.2020.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Takemura A., Ishihara A. Mild hyperbaric oxygen inhibits growth-related decrease in muscle oxidative capacity of rats with metabolic syndrome. J. Atherosclerosis Thromb. 2017;24:26–38. doi: 10.5551/jat.34686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee C.H., Choi Y.A., Heo S.J., Song P. The effect of hyperbaric therapy on brown adipose tissue in rats. Int. J. Environ. Res. Publ. Health. 2021;18:1–8. doi: 10.3390/ijerph18179165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nørlinger T.S., Nielsen P.M., Qi H., et al. Hyperbaric oxygen therapy reduces renal lactate production. Phys. Rep. 2017;5:1–8. doi: 10.14814/phy2.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Verma R., Chopra A., Giardina C., et al. Hyperbaric oxygen therapy (HBOT) suppresses biomarkers of cell stress and kidney injury in diabetic mice. Cell Stress Chaperones. 2015;20:495–505. doi: 10.1007/s12192-015-0574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Harrison L.E., Giardina C., Hightower L.E., Anderson C., Perdrizet G.A. Might hyperbaric oxygen therapy (HBOT) reduce renal injury in diabetic people with diabetes mellitus? From preclinical models to human metabolomics. Cell Stress Chaperones. 2018;23:1143–1152. doi: 10.1007/s12192-018-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cardenas Ureña K.G., Ramírez Nava J.C., Márquez Celedonio F.G., et al. Clinical efficacy of adjuvant therapy with hyperbaric oxygen in diabetic nephropathy. Undersea Hyperb. Med. 2020;47:415–422. [PubMed] [Google Scholar]
- 175.Cruz-Villanueva S.R., Ramirez-Nava J.C., Moreno-Luna J.A., et al. Effect of hyperbaric oxygen therapy (HBOT) on insulin resistance associated with abdominal obesity in wistar rats with dietary sucrose-induced metabolic syndrome. J. Nutr. Sci. Vitaminol. 2021;67:292–300. doi: 10.3177/jnsv.67.292. [DOI] [PubMed] [Google Scholar]
- 176.Kahraman C., Yaman H. Hyperbaric oxygen therapy affects insulin sensitivity/resistance by increasinadiponectin, resistin, and plasminogen activator inhibitor-i in rats. Turk. J. Med. Sci. 2021;51:1572–1578. doi: 10.3906/sag-2011-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Resanović I., Gluvić Z., Zarić B., et al. Effect of hyperbaric oxygen therapy on fatty acid composition and insulin-like growth factor binding protein 1 in adult type 1 diabetes mellitus patients: a pilot study. Can. J. Diabetes. 2020;44:22–29. doi: 10.1016/j.jcjd.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 178.Fujita N., Nagatomo F., Murakami S., Kondo H., Ishihara A., Fujino H. Effects of hyperbaric oxygen on metabolic capacity of the skeletal muscle in type 2 diabetic rats with obesity. Sci. World J. 2012 doi: 10.1100/2012/637978. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.qing Xie W., He M., Yu D jie, et al. Mouse models of sarcopenia: classification and evaluation. J. Cachexia Sarcopenia Muscle. 2021;12:538–554. doi: 10.1002/jcsm.12709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Brioche T., Pagano A.F., Py G., Chopard A. Muscle wasting and aging: experimental models, fatty infiltrations, and prevention. Mol. Aspect. Med. 2016;50:56–87. doi: 10.1016/j.mam.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 181.Komori T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015;759:287–294. doi: 10.1016/j.ejphar.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 182.Nishizaka T., Nagatomo F., Fujino H., et al. Hyperbaric oxygen exposure reduces age-related decrease in oxidative capacity of the tibialis anterior muscle in mice. Enzym. Res. 2010 doi: 10.4061/2010/824763. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Takemura A., Roy R.R., Yoshihara I., Ishihara A. Unloading-induced atrophy and decreased oxidative capacity of the soleus muscle in rats are reversed by pre- and postconditioning with mild hyperbaric oxygen. Phys. Rep. 2017;5:1–11. doi: 10.14814/phy2.13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ishihara A. Effects of exposure to mild hyperbaric oxygen during unloading on muscle properties in rats. J. Muscle Res. Cell Motil. 2019;40:365–372. doi: 10.1007/s10974-019-09530-0. [DOI] [PubMed] [Google Scholar]
- 185.Edwards M.H., Dennison E.M., Sayer A.A., Fielding R., Cooper C. Osteoporosis and sarcopenia in older age. Bone. 2015;80:126–130. doi: 10.1016/j.bone.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Takemura A., Divieti P., Tatsuro P., Rika E., Tatsuya T., Akihiko H. Effects of mild hyperbaric oxygen on osteoporosis induced by hindlimb unloading in rats. J. Bone Miner. Metabol. 2020;38 doi: 10.1007/s00774-020-01100-6. 631-618. [DOI] [PubMed] [Google Scholar]
- 187.Liu M., Chen H., Tong M., Zhou J., Wu X. Effects of ultra-early hyperbaric oxygen therapy on femoral calcitonin gene-related peptide and bone metabolism of rats with complete spinal transection. Spine. 2018;43:E919–E926. doi: 10.1097/BRS.0000000000002581. [DOI] [PubMed] [Google Scholar]
- 188.Liu M., Wu X., Tong M., Wu X., Zhou J. Impacts of ultra-early hyperbaric oxygen therapy on bone mass of rats with complete spinal cord transection. Spine. 2016;41:E837–E843. doi: 10.1097/BRS.0000000000001458. [DOI] [PubMed] [Google Scholar]
- 189.Imerb N., Thonusin C., Pratchayasakul W., et al. Hyperbaric oxygen therapy improves age induced bone dyshomeostasis in non-obese and obese conditions. Life Sci. 2022;295 doi: 10.1016/j.lfs.2022.120406. [DOI] [PubMed] [Google Scholar]
- 190.Ma S., Sun S., Li J., et al. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. 2021;31:415–432. doi: 10.1038/s41422-020-00412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Aparci M., Kardesoglu E., Suleymanoglu S., et al. Hyperbaric oxygen therapy improves myocardial diastolic function in diabetic patients. Tohoku J. Exp. Med. 2008;214:281–289. doi: 10.1620/tjem.214.281. [DOI] [PubMed] [Google Scholar]
- 192.Leitman M., Efrati S., Fuchs S., Hadanny A., Vered Z. The effect of hyperbaric oxygenation therapy on myocardial function. Int. J. Cardiovasc. Imag. 2020;36:833–840. doi: 10.1007/s10554-020-01773-0. [DOI] [PubMed] [Google Scholar]
- 193.Wunderlich T., Frey N., Kahler W., et al. Influence of hyperoxia on diastolic myocardial and arterial endothelial function. Undersea Hyperb. Med. 2017;44:521–533. doi: 10.22462/11.12.2017.3. [DOI] [PubMed] [Google Scholar]
- 194.Hadanny A., Zubari T., Tamir-Adler L., et al. Hyperbaric oxygen therapy effects on pulmonary functions: a prospective cohort study. BMC Pulm. Med. 2019;19:1–6. doi: 10.1186/s12890-019-0893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pott F., Westergaard P., Mortensen J.J.E. Hyperbaric oxygen treatment and pulmonary function. Undersea Hyperb. Med. 1999;26:225–228. [PubMed] [Google Scholar]
- 196.Thorsen E., Aanderud L.A.T. Effects of a standard hyperbaric oxygen treatment protocol on pulmonary function. Eur. Respir. J. 1998;12:1442–1445. doi: 10.1183/09031936.98.12061442. [DOI] [PubMed] [Google Scholar]
- 197.Ishihara A. Mild hyperbaric oxygen: mechanisms and effects. J. Physiol. Sci. 2019;69:573–580. doi: 10.1007/s12576-019-00678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kranke P., Bennett M.H., Martyn-St James M., Schnabel A., Debus S.E., Weibel S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst. Rev. 2015 doi: 10.1002/14651858.CD004123.pub4. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Schiavo S., Djaiani C., Debacker J., et al. Magnitude and clinical predictors of blood pressure changes in patients undergoing hyperbaric oxygen therapy: a retrospective study. Int. J. Environ. Res. Publ. Health. 2020;17:1–14. doi: 10.3390/ijerph17207586. [DOI] [PMC free article] [PubMed] [Google Scholar]