Abstract
Background
In 2021, the US Preventive Services Task Force (USPSTF) revised its lung cancer screening guidelines to expand screening eligibility. We evaluated screening sensitivities and racial and ethnic disparities under the 2021 USPSTF criteria vs alternative risk-based criteria in a racially and ethnically diverse population.
Methods
In the Multiethnic Cohort, we evaluated the proportion of ever-smoking lung cancer cases eligible for screening (ie, screening sensitivity) under the 2021 USPSTF criteria and under risk-based criteria through the PLCOm2012 model (6-year risk ≥1.51%). We also calculated the screening disparity (ie, absolute sensitivity difference) for each of 4 racial or ethnic groups (African American, Japanese American, Latino, Native Hawaiian) vs White cases.
Results
Among 5900 lung cancer cases, 43.3% were screen eligible under the 2021 USPSTF criteria. Screening sensitivities varied by race and ethnicity, with Native Hawaiian (56.7%) and White (49.6%) cases attaining the highest sensitivities and Latino (37.3%), African American (38.4%), and Japanese American (40.0%) cases attaining the lowest. Latino cases had the greatest screening disparity vs White cases at 12.4%, followed by African American (11.2%) and Japanese American (9.6%) cases. Under risk-based screening, the overall screening sensitivity increased to 75.7%, and all racial and ethnic groups had increased sensitivities (54.5%-91.9%). Whereas the screening disparity decreased to 5.1% for African American cases, it increased to 28.6% for Latino cases and 12.8% for Japanese American cases.
Conclusions
In the Multiethnic Cohort, racial and ethnic disparities decreased but persisted under the 2021 USPSTF lung cancer screening guidelines. Risk-based screening through PLCOm2012 may increase screening sensitivities and help to reduce disparities in some, but not all, racial and ethnic groups. Further optimization of risk-based screening strategies across diverse populations is needed.
Lung cancer remains the leading cause of cancer-related mortality in the United States. Low-dose computed tomography (LDCT) screening has demonstrated mortality reductions across randomized screening trials in adults aged 50-74 years with various smoking histories (1-3). In 2020, the US Preventive Services Task Force (USPSTF) drafted a recommendation to expand screening eligibility to individuals aged 50-80 years with a minimum 20 pack-year smoking history and active smoking within 15 years (4,5). This recommendation was finalized as the national standard in March 2021 (6). Lowering the pack-year threshold from 30 to 20 pack-years addresses some limitations of the 2013 USPSTF criteria, which may miss high-risk individuals with lower smoking exposures, such as among African American individuals (7). Thus, the 2021 USPSTF criteria are expected to reduce racial and ethnic screening disparities.
An alternative strategy for screening uses a risk-based approach in which eligibility is determined through risk prediction models that incorporate risk factors beyond age and smoking history. The PLCOm2012 model has been well-validated in selecting high-risk individuals for lung cancer screening (8,9) and includes the following variables: age, race or ethnicity, education, body mass index, chronic obstructive pulmonary disease (COPD), personal history of cancer, and family history of lung cancer as well as smoking status, intensity, duration, and quit time. Risk-based screening through PLCOm2012 reduced racial and ethnic disparities in screening eligibility within observational cohort and simulation studies compared with the 2013 (10,11) and 2021 USPSTF criteria (12). However, these assessments primarily evaluated disparities among African American individuals (12); further assessments among other racial and ethnic groups are lacking.
In drafting the 2021 USPSTF guidelines for lung cancer screening, a comparative modeling-based decision analysis identified 5 alternate efficient strategies using the 20 pack-year criterion (13). By varying the minimum age (50, 55 years) and extending the time from smoking cessation (15, 20, 25 years), these strategies increased screening eligibility in the general population compared with the 2013 USPSTF criteria (13,14). However, an assessment of screening eligibility among lung cancer cases across racial and ethnic groups is lacking, as are comparisons with risk-based screening.
In this study, we evaluated the screening eligibility (ie, screening sensitivity) and racial and ethnic disparities under the 2021 USPSTF criteria and its variations compared with risk-based screening through PLCOm2012 in a racially and ethnically diverse population of lung cancer cases.
Methods
Study Population
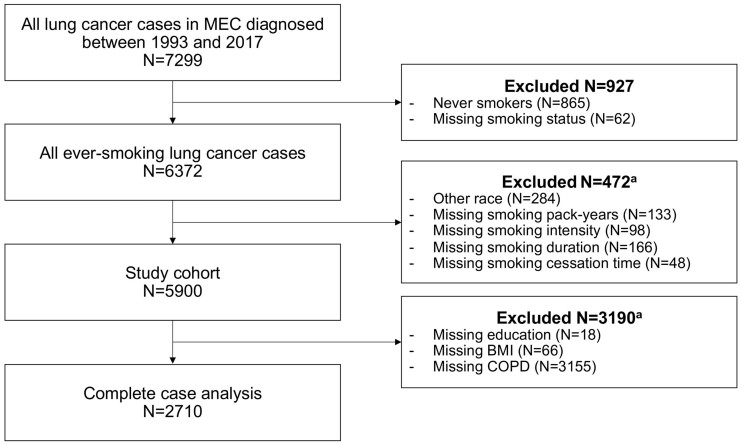
The Multiethnic Cohort (MEC) is a prospective population-based cohort that follows >215 000 residents of California and Hawaii aged 45-75 years at enrollment (1993-1996) for the development of chronic diseases, including cancer (15). This study included all MEC participants with an ever-smoking history who were diagnosed with incident lung cancers from 1993 to 2017, as identified via linkage to California and Hawaii Surveillance, Epidemiology, and End Results (SEER) registries (N = 5900; Figure 1).
Figure 1.
Study diagram. BMI = body mass index; COPD = chronic obstructive pulmonary disease; MEC = Multiethnic Cohort. aListed variables were not mutually exclusive among study participants.
Baseline data including smoking history were collected through a self-reported questionnaire at enrollment. A subset of cases additionally reported their smoking use on a 10-year follow-up questionnaire (2003-2008) before the occurrence of their lung cancer (n = 1526). For these participants, we used the updated smoking information in our analyses (Supplementary Methods, Supplementary Table 1, available online). Given the temporal gap between smoking assessment (at enrollment or 10-year follow-up) and lung cancer diagnosis, we projected the time from smoking assessment to diagnosis to derive the smoking parameters for determining screening eligibility (Supplementary Methods, available online). All other variables were analyzed as self-reported except for COPD status, which was ascertained via linkage of MEC to Medicare claims data from 1999-2016 (16) (Supplementary Methods, available online).
USPSTF Screening Eligibility and Consensus-Efficient Strategies
For each lung cancer case, we evaluated screening eligibility at diagnosis through the 2021 USPSTF criteria with the following age and smoking thresholds: 50–80/20/15 (age range [years]/minimum pack-years/maximum quit years) (5). We also examined eligibility through the 2013 USPSTF criteria (55-80/30/15) and 5 alternate strategies that informed the USPSTF recommendations (13): 55-80/20/15, 55-80/20/20, 55-80/20/25, 50-80/20/20, and 50-80/20/25. Here, the proportion of cases eligible for screening reflects the “screening sensitivity” of the approach (ie, number of screen-eligible lung cancer cases divided by the total number of lung cancer cases).
Risk-Based Screening Through PLCOm2012
PLCOm2012 is a logistic regression model developed to predict the 6-year lung cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial control group of ever-smoking participants, which has been validated in multiple populations (17-25) (Supplementary Table 2, available online). For each lung cancer case, we calculated the PLCOm2012 risk score to determine risk-based screening eligibility at diagnosis. For the primary analysis, we used a 1.51% risk threshold such that individuals with a calculated risk of at least 1.51% were deemed eligible for screening, because this is where the survival benefit with LDCT over chest radiograph was historically demonstrated (8). In sensitivity analyses, we used a 1.7% risk threshold, which corresponds to a similar number of LDCT screens required (or number of screen-eligible individuals) as the 2013 USPSTF criteria (26), and a stringent 2% risk threshold, which is expected to select fewer individuals for screening compared with the 2021 USPSTF criteria in the general population (26).
Participants of all ages at diagnosis (N = 5900) were included for the primary study analysis. In sensitivity analyses, the cohort was restricted to individuals aged 50-80 years at diagnosis (n = 4428) to match the age range used in the 2021 USPSTF criteria. Given the potential for increasing age to artificially inflate the PLCOm2012 risk score—as the regression coefficient for every 1-year increase in age is positive (beta = 0.078, odds ratio [OR] = 1.08)—and to reduce USPSTF screening eligibility by exceeding the upper age limit, we conducted a sensitivity analysis to evaluate screening sensitivities 6 years before each participant’s lung cancer diagnosis.
Study Outcomes
The primary outcome was the screening sensitivity of the 2021 USPSTF criteria vs risk-based screening via PLCOm2012 at the 1.51% risk threshold. The secondary outcome was the screening disparity (ie, absolute difference in screening sensitivity) between each racial or ethnic group—African American, Japanese American, Latino, and Native Hawaiian—and White cases.
Missing Data
We handled missing data (Supplementary Table 3, available online) as follows. First, among all ever-smoking lung cancer cases in MEC, we selected participants who self-reported belonging to 1 of 5 racial or ethnic groups and who had complete smoking data (Figure 1). We excluded participants with missing smoking data given the importance of accurate smoking information in determining USPSTF screening eligibility and their low missing rates (<3%). We performed multiple imputation by chained equations to impute missing data 20 times using all PLCOm2012 predictors because the data were missing at random or harbored a relationship between the propensity of missingness and the observed covariate data (Supplementary Table 4, available online) (27,28). Each participant’s PLCOm2012 risk score was calculated and combined across all iterations to yield the final risk score using Rubin’s rules (29).
In a series of sensitivity analyses, we first examined individuals who had complete data for all variables in PLCOm2012 (n = 2710). Next, we conducted a conservative analysis in which we classified individuals with missing COPD data as having “no COPD,” which yields a conservative PLCOm2012 risk estimate because the regression coefficient for COPD is positive (beta = 0.355, OR = 1.43).
Descriptive Statistics
Differences in categorical variables were assessed using the χ2 test, and continuous variables were compared using 1-way analysis of variance. Statistical significance was defined at a 2-sided P less than .05. Statistical analyses were performed using R version 4.0.2.
Results
Participant Characteristics
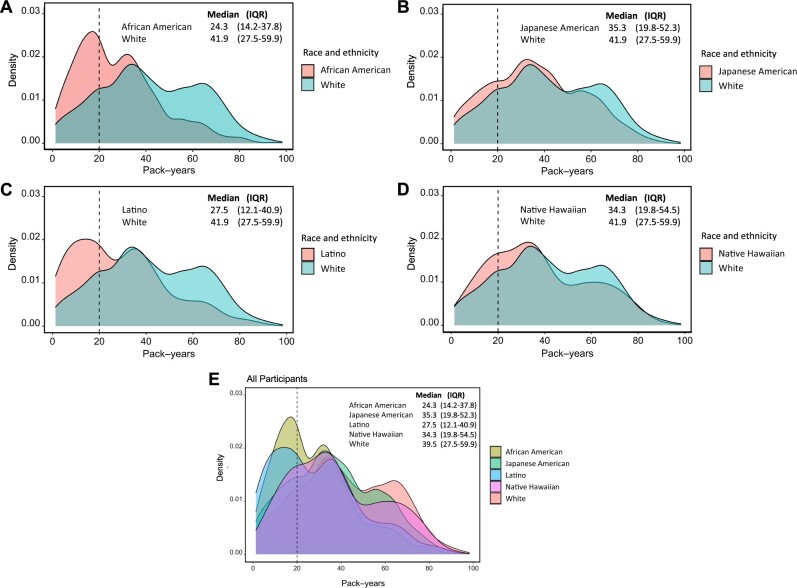
The mean age at lung cancer diagnosis was 74.2 years, and the cohort was comprised of 28.1% African American, 22.5% Japanese American, 13.6% Latino, 9.0% Native Hawaiian, and 26.8% White cases (Table 1). African American and Latino cases had the lowest smoking exposures with a mean 27.9 pack-years and 29.1 pack-years, respectively. White cases had the highest smoking exposure with a mean 41.9 pack-years. Pack-year density curves by racial and ethnic group are presented in Figure 2.
Table 1.
Characteristics of participants with lung cancer in the Multiethnic Cohorta
| Race and ethnicity |
||||||
|---|---|---|---|---|---|---|
| Characteristic | Overall | African American | Japanese American | Latino | Native Hawaiian | White |
| (N = 5900) | (n = 1660) | (n = 1328) | (n = 800) | (n = 533) | (n = 1579) | |
| Age at diagnosis, mean (SD), y | 74.2 (8.2) | 74.1 (8.4) | 75.6 (7.9) | 75.1 (7.4) | 70.5 (8.5) | 73.8 (8.3) |
| Age at diagnosis (categorical), No. (%) | ||||||
| ≤50 | 31 (0.5) | 14 (0.8) | 3 (0.2) | 1 (0.1) | 7 (1.3) | 6 (0.4) |
| 51–60 | 360 (6.1) | 103 (6.2) | 57 (4.3) | 21 (2.6) | 70 (13.1) | 109 (6.9) |
| 61–70 | 1552 (26.3) | 419 (25.2) | 287 (21.6) | 220 (27.5) | 196 (36.8) | 430 (27.2) |
| 71–80 | 2732 (46.3) | 807 (48.6) | 627 (47.2) | 369 (46.1) | 208 (39.0) | 721 (45.7) |
| 81–90 | 1162 (19.7) | 283 (17.0) | 349 (26.3) | 179 (22.4) | 52 (9.8) | 299 (18.9) |
| ≥91 | 63 (1.1) | 34 (2.0) | 5 (0.4) | 10 (1.2) | 0 (0.0) | 14 (0.9) |
| BMIa, mean (SD), kg/m2 | 26.0 (4.7) | 26.9 (5.0) | 24.5 (3.8) | 26.5 (4.0) | 28.0 (6.0) | 25.6 (4.5) |
| Sex, No. (%) | ||||||
| Male | 3523 (59.7) | 892 (53.7) | 986 (74.2) | 579 (72.4) | 274 (51.4) | 792 (50.2) |
| Female | 2377 (40.3) | 768 (46.3) | 342 (25.8) | 221 (27.6) | 259 (48.6) | 787 (49.8) |
| Education, No. (%) | ||||||
| High school or less | 3014 (51.1) | 796 (48.0) | 691 (52.0) | 570 (71.2) | 377 (70.7) | 580 (36.7) |
| Some college or graduate | 2382 (40.4) | 718 (43.3) | 537 (40.4) | 199 (24.9) | 137 (25.7) | 791 (50.1) |
| Postgraduate | 486 (8.2) | 136 (8.2) | 98 (7.4) | 27 (3.4) | 18 (3.4) | 207 (13.1) |
| Unknown | 18 (0.3) | 10 (0.6) | 2 (0.2) | 4 (0.5) | 1 (0.2) | 1 (0.1) |
| Personal history of cancer, No. (%) | ||||||
| No | 4373 (74.1) | 1205 (72.6) | 1023 (77.0) | 595 (74.4) | 433 (81.2) | 1117 (70.7) |
| Yes | 1527 (25.9) | 455 (27.4) | 305 (23.0) | 205 (25.6) | 100 (18.8) | 462 (29.3) |
| Family history of lung cancer, No. (%) | ||||||
| No | 5369 (91.0) | 1503 (90.5) | 1213 (91.3) | 737 (92.1) | 494 (92.7) | 1422 (90.1) |
| Yes | 531 (9.0) | 157 (9.5) | 115 (8.7) | 63 (7.9) | 39 (7.3) | 157 (9.9) |
| COPD, No. (%) | ||||||
| No | 1217 (20.6) | 272 (16.4) | 354 (26.7) | 137 (17.1) | 124 (23.3) | 330 (20.9) |
| Yes | 1528 (25.9) | 377 (22.7) | 351 (26.4) | 212 (26.5) | 139 (26.1) | 449 (28.4) |
| Unknown | 3155 (53.5) | 1011 (60.9) | 623 (46.9) | 451 (56.4) | 270 (50.7) | 800 (50.7) |
| Smoking status, No. (%) | ||||||
| Former | 3071 (52.1) | 770 (46.4) | 828 (62.3) | 415 (51.9) | 194 (36.4) | 864 (54.7) |
| Current | 2829 (47.9) | 890 (53.6) | 500 (37.7) | 385 (48.1) | 339 (63.6) | 715 (45.3) |
| Pack-years, mean (SD)b,c | 34.7 (20.7) | 27.9 (17.7) | 36.6 (20.0) | 29.1 (20.0) | 37.7 (21.1) | 41.9 (21.4) |
| Pack-years (categorical), No. (%) | ||||||
| 1–10 | 756 (12.8) | 278 (16.7) | 139 (10.5) | 174 (21.8) | 48 (9.0) | 117 (7.4) |
| 11–20 | 1186 (20.1) | 471 (28.4) | 225 (16.9) | 174 (21.8) | 97 (18.2) | 219 (13.9) |
| 21–30 | 711 (12.1) | 237 (14.3) | 161 (12.1) | 93 (11.6) | 71 (13.3) | 149 (9.4) |
| 30+ | 3247 (55.0) | 674 (40.6) | 803 (60.5) | 359 (44.9) | 317 (59.5) | 1094 (69.3) |
| Cigarettes per day, mean (SD)c | 17.8 (8.7) | 14.6 (7.5) | 19.2 (8.5) | 14.7 (8.3) | 18.1 (8.3) | 21.5 (8.8) |
| Cigarettes per day (categorical), No. (%) | ||||||
| 1–10 | 1556 (26.4) | 641 (38.6) | 242 (18.2) | 333 (41.6) | 128 (24.0) | 212 (13.4) |
| 11–20 | 2308 (39.1) | 714 (43.0) | 552 (41.6) | 291 (36.4) | 217 (40.7) | 534 (33.8) |
| 21–30 | 1178 (20.0) | 195 (11.7) | 324 (24.4) | 109 (13.6) | 109 (20.5) | 441 (27.9) |
| 30+ | 858 (14.5) | 110 (6.6) | 210 (15.8) | 67 (8.4) | 79 (14.8) | 392 (24.8) |
| Quit years, median (IQR)b,c | 5.2 (0–20) | 0.0 (0–16) | 11.0 (0–24) | 5.9 (0–21) | 0.0 (0–13) | 6.6 (0–21) |
| Quit years (categorical), No. (%) | ||||||
| 0 | 2829 (47.9) | 890 (53.6) | 500 (37.7) | 385 (48.1) | 339 (63.6) | 715 (45.3) |
| 1–15 | 1165 (19.7) | 344 (20.7) | 287 (21.6) | 134 (16.8) | 81 (15.2) | 319 (20.2) |
| 16–25 | 1018 (17.3) | 245 (14.8) | 263 (19.8) | 146 (18.2) | 68 (12.8) | 296 (18.7) |
| 26+ | 888 (15.1) | 181 (10.9) | 278 (20.9) | 135 (16.9) | 45 (8.4) | 249 (15.8) |
| Stage at diagnosis, No. (%) | ||||||
| I-III | 2354 (39.9) | 634 (38.2) | 552 (41.6) | 279 (34.9) | 204 (38.3) | 685 (43.4) |
| IV | 3183 (53.9) | 918 (55.3) | 704 (53.0) | 449 (56.1) | 305 (57.2) | 807 (51.1) |
| Unknown | 363 (6.2) | 108 (6.5) | 72 (5.4) | 72 (9.0) | 24 (4.5) | 87 (5.5) |
| Histology, No. (%) | ||||||
| Adenocarcinoma | 2186 (37.1) | 601 (36.2) | 524 (39.5) | 274 (34.2) | 181 (34.0) | 606 (38.4) |
| Squamous cell carcinoma | 1295 (21.9) | 385 (23.2) | 298 (22.4) | 171 (21.4) | 122 (22.9) | 319 (20.2) |
| Large cell carcinoma | 180 (3.1) | 66 (4.0) | 40 (3.0) | 30 (3.8) | 10 (1.9) | 34 (2.2) |
| Small cell lung carcinoma | 670 (11.4) | 161 (9.7) | 134 (10.1) | 87 (10.9) | 100 (18.8) | 188 (11.9) |
| Non-small cell lung carcinoma NOS | 463 (7.8) | 125 (7.5) | 102 (7.7) | 72 (9.0) | 38 (7.1) | 126 (8.0) |
| Other | 1106 (18.7) | 322 (19.4) | 230 (17.3) | 166 (20.8) | 82 (15.4) | 306 (19.4) |
| Age at MEC enrollment (categorical), No. (%) | ||||||
| ≤50 | 491 (8.3) | 148 (8.9) | 85 (6.4) | 38 (4.8) | 91 (17.1) | 129 (8.2) |
| 51–55 | 627 (10.6) | 181 (10.9) | 99 (7.5) | 83 (10.4) | 93 (17.4) | 171 (10.8) |
| 56–60 | 950 (16.1) | 249 (15.0) | 177 (13.3) | 164 (20.5) | 103 (19.3) | 257 (16.3) |
| 61–65 | 1268 (21.5) | 268 (16.1) | 322 (24.2) | 228 (28.5) | 125 (23.5) | 325 (20.6) |
| 66–70 | 1526 (25.9) | 477 (28.7) | 373 (28.1) | 188 (23.5) | 67 (12.6) | 421 (26.7) |
| ≥71 | 1038 (17.6) | 337 (20.3) | 272 (20.5) | 99 (12.4) | 54 (10.1) | 276 (17.5) |
| Year of diagnosis, No. (%) | ||||||
| 1993–1997 | 920 (15.6) | 310 (18.7) | 180 (13.6) | 95 (11.9) | 74 (13.9) | 261 (16.5) |
| 1998–2002 | 1403 (23.8) | 438 (26.4) | 279 (21.0) | 168 (21.0) | 127 (23.8) | 391 (24.8) |
| 2003–2007 | 1589 (26.9) | 445 (26.8) | 365 (27.5) | 203 (25.4) | 132 (24.8) | 444 (28.1) |
| 2008–2012 | 1423 (24.1) | 283 (17.0) | 405 (30.5) | 195 (24.4) | 166 (31.1) | 374 (23.7) |
| 2013–2017 | 565 (9.6) | 184 (11.1) | 99 (7.5) | 139 (17.4) | 34 (6.4) | 109 (6.9) |
BMI was unknown in 66 participants: 45 (2.7%) African American, 5 (0.4%) Japanese American, 4 (0.5%) Latino, 6 (1.1%) Native Hawaiian, and 6 (0.4%) White (P < .001, χ2 test). BMI = body mass index; COPD = chronic obstructive pulmonary disease; IQR = interquartile range; MEC = Multiethnic Cohort; NOS = not otherwise specified.
Smoking-related variables were projected to time at initial diagnosis of lung cancer.
Smoking-related variables were measured at baseline and updated with 10-year follow-up data before lung cancer diagnosis, if available (N = 1526).
Figure 2.
Smoking pack-year density curves. Smoking pack-year density curves are presented (A) between African American and White cases, (B) between Japanese American and White cases, (C) between Latino and White cases, (D) between Native Hawaiian and White cases, and (E) across all racial and ethnic groups. The vertical line at 20 pack-years distinguishes the participants who meet the 20 pack-year smoking threshold in the 2021 US Preventive Services Task Force guidelines for lung cancer screening. IQR = interquartile range.
Screening Sensitivity and Disparities Through the 2021 USPSTF Criteria
Overall, 43.3% of lung cancer cases were eligible for screening under the 2021 USPSTF criteria (Table 2). The sensitivity was lowest among Latino (37.3%) and African American (38.4%) cases, intermediate among Japanese American (40.0%) cases, and highest among White (49.6%) and Native Hawaiian (56.7%) cases. Under the 2013 USPSTF criteria, 35.1% of lung cancer cases were eligible for screening (Supplementary Table 5, available online). For the 2021 USPSTF criteria, the primary source of ineligibility was the quit-years criterion, with 71.2% and 70.3% of ineligible White and Japanese American cases above the 15 quit-year threshold, respectively (Table 2). The 20 pack-year criterion was the second-most common source of ineligibility, with 70.0% of African American and 65.7% of Latino ineligible cases falling below this threshold.
Table 2.
2021 USPSTF criteria screening eligibility (ie, screening sensitivity) and reasons for ineligibility among all and ineligible lung cancer casesa
| Source | Overall | Race and ethnicity |
||||
|---|---|---|---|---|---|---|
| African American | Japanese American | Latino | Native Hawaiian | White | ||
| All lung cancer cases, No. | 5900 | 1660 | 1328 | 800 | 533 | 1579 |
| Age <50 y | 18 (0.3) | 5 (0.3) | 2 (0.2) | 1 (0.1) | 7 (1.3) | 3 (0.2) |
| Age >80 y | 1454 (24.6) | 393 (23.7) | 413 (31.1) | 216 (27.0) | 71 (13.3) | 361 (22.9) |
| Pack-years <20 | 1865 (31.6) | 715 (43.1) | 356 (26.8) | 330 (41.2) | 138 (25.9) | 326 (20.6) |
| Quit-years >15 | 1998 (33.9) | 457 (27.5) | 560 (42.2) | 294 (36.8) | 120 (22.5) | 567 (35.9) |
| Eligible lung cancer cases, No. (%a) | 2552 (43.3) | 638 (38.4) | 531 (40.0) | 298 (37.3) | 302 (56.7) | 783 (49.6) |
| Ineligible lung cancer cases, No. (%a) | 3348 (56.7) | 1022 (61.6) | 797 (60.0) | 502 (62.7) | 231 (43.3) | 796 (50.4) |
| Age <50 y | 18 (0.5) | 5 (0.5) | 2 (0.3) | 1 (0.2) | 7 (3.0) | 3 (0.4) |
| Age >80 y | 1454 (43.4) | 393 (38.5) | 413 (51.8) | 216 (43.0) | 71 (30.7) | 361 (45.4) |
| Pack-years <20 | 1865 (55.7) | 715 (70.0) | 356 (44.7) | 330 (65.7) | 138 (59.7) | 326 (41.0) |
| Quit-years >15 | 1998 (59.7) | 457 (44.7) | 560 (70.3) | 294 (58.6) | 120 (51.9) | 567 (71.2) |
Percentages correspond to the number of eligible or ineligible lung cancer cases divided by all lung cancer cases overall and within each race and ethnicity stratum. USPSTF = United States Preventive Services Task Force.
The screening disparity vs White cases was largest among Latino (12.4%) and African American (11.2%) cases under the 2021 USPSTF criteria (Table 3). Compared with the 2013 USPSTF criteria, the 2021 criteria reduced screening disparities across racial and ethnic groups. In the age-restricted cohort (50-80 years), similar results were observed (Table 4).
Table 3.
Screening eligibilities (ie, screening sensitivities) and racial and ethnic disparities through risk-based screening and the USPSTF criteria in the primary study cohorta
| Screening assessment | Overall | Race and ethnicity |
|||||
|---|---|---|---|---|---|---|---|
| African American | Japanese American | Latino | Native Hawaiian | White | P b | ||
| (N = 5900) | (n = 1660) | (n = 1328) | (n = 800) | (n = 533) | (n = 1579) | ||
| Screening eligibility or sensitivity, no. (%) | |||||||
| Risk-based screening | |||||||
| Risk ≥1.51% | 4466 (75.7) | 1294 (78.0) | 934 (70.3) | 436 (54.5) | 490 (91.9) | 1312 (83.1) | <.001 |
| Risk ≥1.7% | 4291 (72.7) | 1255 (75.6) | 880 (66.3) | 408 (51.0) | 475 (89.1) | 1273 (80.6) | <.001 |
| Risk ≥2.0% | 4036 (68.4) | 1191 (71.7) | 815 (61.4) | 353 (44.1) | 468 (87.8) | 1209 (76.6) | <.001 |
| USPSTF criteria | |||||||
| 2013 USPSTF | 2068 (35.1) | 460 (27.7) | 443 (33.4) | 231 (28.9) | 247 (46.3) | 687 (43.5) | <.001 |
| 2021 USPSTF | 2552 (43.3) | 638 (38.4) | 531 (40.0) | 298 (37.3) | 302 (56.7) | 783 (49.6) | <.001 |
| Alternate USPSTF strategies | |||||||
| 55–80/20/15 | 2491 (42.2) | 624 (37.6) | 521 (39.2) | 296 (37.0) | 294 (55.2) | 756 (47.9) | <.001 |
| 55–80/20/20 | 2731 (46.3) | 671 (40.4) | 577 (43.4) | 330 (41.2) | 318 (59.7) | 835 (52.9) | <.001 |
| 55–80/20/25 | 2903 (49.2) | 697 (42.0) | 638 (48.0) | 349 (43.6) | 329 (61.7) | 890 (56.4) | <.001 |
| 50–80/20/20 | 2792 (47.3) | 685 (41.3) | 587 (44.2) | 332 (41.5) | 326 (61.2) | 862 (54.6) | <.001 |
| 50–80/20/25 | 2964 (50.2) | 711 (42.8) | 648 (48.8) | 351 (43.9) | 337 (63.2) | 917 (58.1) | <.001 |
| Racial and ethnic disparity, % | |||||||
| Risk-based screening | |||||||
| Risk ≥1.51% | — | 5.1 | 12.8 | 28.6 | −8.8 | Ref | |
| Risk ≥1.7% | — | 5.0 | 14.3 | 29.6 | −8.5 | Ref | |
| Risk ≥2.0% | — | 4.9 | 15.2 | 32.5 | −11.2 | Ref | |
| USPSTF criteria | |||||||
| 2013 USPSTF | — | 15.8 | 10.1 | 14.6 | −2.8 | Ref | |
| 2021 USPSTF | — | 11.2 | 9.6 | 12.4 | −7.1 | Ref | |
| Alternate USPSTF strategies | |||||||
| 55–80/20/15 | — | 10.3 | 8.7 | 10.9 | −7.3 | Ref | |
| 55–80/20/20 | — | 12.5 | 9.5 | 11.7 | −6.8 | Ref | |
| 55–80/20/25 | — | 14.4 | 8.4 | 12.8 | −5.3 | Ref | |
| 50–80/20/20 | — | 13.3 | 10.4 | 13.1 | −6.6 | Ref | |
| 50–80/20/25 | — | 15.3 | 9.3 | 14.2 | −5.1 | Ref | |
Risk-based screening was evaluated through the PLCOm2012 model. Racial and ethnic disparity is defined as the absolute difference in screening sensitivities between each racial and ethnic group and White cases. USPSTF = United States Preventive Services Task Force.
P value was calculated across racial and ethnic strata using the χ2 test.
Table 4.
Screening eligibilities (ie, screening sensitivities) and racial and ethnic disparities through risk-based screening and the USPSTF criteria in the age-restricted (50-80 years) cohorta
| Screening assessment | Overall | Race and ethnicity |
|||||
|---|---|---|---|---|---|---|---|
| African American | Japanese American | Latino | Native Hawaiian | White | P b | ||
| (N = 4428) | (n = 1262) | (n = 913) | (n = 583) | (n = 455) | (n = 1215) | ||
| Screening eligibility or sensitivity, no. (%) | |||||||
| Risk-based screening | |||||||
| Risk ≥1.51% | 3288 (74.3) | 946 (75.0) | 621 (68.0) | 310 (53.2) | 421 (92.5) | 990 (81.5) | <.001 |
| Risk ≥1.7% | 3148 (71.1) | 916 (72.6) | 578 (63.3) | 289 (49.6) | 409 (89.9) | 956 (78.7) | <.001 |
| Risk ≥2.0% | 2949 (66.6) | 865 (68.5) | 527 (57.7) | 249 (42.7) | 402 (88.4) | 906 (74.6) | <.001 |
| USPSTF criteria | |||||||
| 2013 USPSTF | 2068 (46.7) | 460 (36.5) | 443 (48.5) | 231 (39.6) | 247 (54.3) | 687 (56.5) | <.001 |
| 2021 USPSTF | 2552 (57.6) | 638 (50.6) | 531 (58.2) | 298 (51.1) | 302 (66.4) | 783 (64.4) | <.001 |
| Alternate USPSTF strategies | |||||||
| 55–80/20/15 | 2491 (56.3) | 624 (49.4) | 521 (57.1) | 296 (50.8) | 294 (64.6) | 756 (62.2) | <.001 |
| 55–80/20/20 | 2731 (61.7) | 671 (53.2) | 577 (63.2) | 330 (56.6) | 318 (69.9) | 835 (68.7) | <.001 |
| 55–80/20/25 | 2903 (65.6) | 697 (55.2) | 638 (69.9) | 349 (59.9) | 329 (72.3) | 890 (73.3) | <.001 |
| 50–80/20/20 | 2792 (63.1) | 685 (54.3) | 587 (64.3) | 332 (56.9) | 326 (71.6) | 862 (70.9) | <.001 |
| 50–80/20/25 | 2964 (66.9) | 711 (56.3) | 648 (71.0) | 351 (60.2) | 337 (74.1) | 917 (75.5) | <.001 |
| Racial and ethnic disparity, % | |||||||
| Risk-based screening | |||||||
| Risk ≥ 1.51% | − | 6.5 | 13.5 | 28.3 | −11.0 | Ref | |
| Risk ≥ 1.7% | − | 6.1 | 15.4 | 29.1 | −11.2 | Ref | |
| Risk ≥ 2.0% | − | 6.1 | 16.9 | 31.9 | −13.8 | Ref | |
| USPSTF criteria | |||||||
| 2013 USPSTF | − | 20.0 | 8.0 | 16.9 | 2.2 | Ref | |
| 2021 USPSTF | − | 13.8 | 6.2 | 13.3 | −2.0 | Ref | |
| Alternate USPSTF strategies | |||||||
| 55–80/20/15 | − | 12.8 | 5.1 | 11.4 | −2.4 | Ref | |
| 55–80/20/20 | − | 15.5 | 5.5 | 12.1 | −1.2 | Ref | |
| 55–80/20/25 | − | 18.1 | 3.4 | 13.4 | 1.0 | Ref | |
| 50–80/20/20 | − | 16.6 | 6.6 | 14.0 | −0.7 | Ref | |
| 50–80/20/25 | − | 19.2 | 4.5 | 15.3 | 1.4 | Ref | |
Risk-based screening was evaluated through the PLCOm2012 model. Racial and ethnic disparity is defined as the absolute difference in screening sensitivities between each racial and ethnic group and White cases. USPSTF = United States Preventive Services Task Force.
P value was calculated across race and ethnicity strata using the χ2 test.
Risk-Based Screening vs the 2021 USPSTF Criteria
Risk-based screening (risk ≥1.51%) through PLCOm2012 increased the overall sensitivity to 75.7% compared with the 2021 USPSTF criteria (43.3%; Table 3). Increases in screening sensitivity occurred across all racial and ethnic groups, ranging from 17.2% to 39.6%. Compared with the 2021 USPSTF criteria, the screening disparity decreased from 11.2% to 5.1% for African American cases. However, the screening disparity increased from 9.6% to 12.8% for Japanese American cases and from 12.4% to 28.6% for Latino cases. Risk-based screening using more stringent risk thresholds of 1.7% or 2.0% decreased the overall screening sensitivities to 72.7% and 68.4%, respectively (Table 3). Increasing the risk thresholds decreased the screening disparity for African American cases but widened the disparities for all other racial and ethnic groups vs White cases.
In the age-restricted (50-80 years) cohort, the overall results were consistent (Table 4). However, the absolute gains in screening sensitivity attained by risk-based screening (risk ≥1.51%) vs the 2021 USPSTF criteria decreased in the age-restricted cohort for all racial and ethnic groups. Similar patterns were observed in the sensitivity analyses evaluating complete cases (Supplementary Table 6, available online) and with the conservative COPD analysis (Supplementary Table 7, available online), with risk-based screening achieving a greater overall sensitivity compared with the 2021 USPSTF criteria. However, the screening sensitivities across racial and ethnic groups varied.
In evaluating eligibility 6 years before lung cancer diagnosis, the screening sensitivities decreased through risk-based screening (risk ≥1.51%) but increased through the 2021 USPSTF criteria overall and across racial and ethnic groups, as expected (Table 5). Risk-based screening maintained a higher sensitivity over the 2021 USPSTF criteria across the study cohort (60.4% vs 51.8%) and among African American (64.3% vs 43.9%), Native Hawaiian (82.9% vs 60.2%), and White (69.8% vs 60.2%) cases. However, the sensitivity gains with risk-based screening over the 2021 USPSTF criteria were lost among Japanese American (52.0% vs 52.5%) and Latino (32.8% vs 44.6%) cases, with risk-based screening underperforming in the latter population. Trends in screening disparities were comparable with those observed in the primary study cohort.
Table 5.
Screening eligibilities (ie, screening sensitivities) and racial and ethnic disparities through risk-based screening and the USPSTF criteria in the primary study cohort 6 years before lung cancer diagnosisa
| Screening assessment | Overall | Race and ethnicity |
|||||
|---|---|---|---|---|---|---|---|
| African American | Japanese American | Latino | Native Hawaiian | White | P b | ||
| N = 5900 | n = 1660 | n = 1328 | n = 800 | n = 533 | n = 1579 | ||
| Screening eligibility or sensitivity, No. (%) | |||||||
| Risk-based screening | |||||||
| Risk ≥1.51% | 3563 (60.4) | 1067 (64.3) | 690 (52.0) | 262 (32.8) | 442 (82.9) | 1102 (69.8) | <.001 |
| Risk ≥1.7% | 3378 (57.3) | 1019 (61.4) | 633 (47.7) | 233 (29.1) | 430 (80.7) | 1063 (67.3) | <.001 |
| Risk ≥2.0% | 3083 (52.3) | 942 (56.7) | 556 (41.9) | 201 (25.1) | 420 (78.8) | 964 (61.1) | <.001 |
| USPSTF criteria | |||||||
| 2013 USPSTF | 2081 (35.3) | 421 (25.4) | 500 (37.7) | 250 (31.3) | 201 (37.7) | 709 (44.9) | <.001 |
| 2021 USPSTF | 3055 (51.8) | 729 (43.9) | 697 (52.5) | 357 (44.6) | 321 (60.2) | 951 (60.2) | <.001 |
| Alternate USPSTF strategies | |||||||
| 55–80/20/15 | 2874 (48.7) | 701 (42.2) | 661 (49.8) | 348 (43.5) | 281 (52.7) | 883 (55.9) | <.001 |
| 55–80/20/20 | 3118 (52.8) | 739 (44.5) | 739 (55.6) | 374 (46.8) | 298 (55.9) | 968 (61.3) | <.001 |
| 55–80/20/25 | 3247 (55.0) | 754 (45.4) | 786 (59.2) | 388 (48.5) | 305 (57.2) | 1014 (64.2) | <.001 |
| 50–80/20/20 | 3299 (55.9) | 767 (46.2) | 775 (58.4) | 383 (47.9) | 338 (63.4) | 1036 (65.6) | <.001 |
| 50–80/20/25 | 3428 (58.1) | 782 (47.1) | 822 (61.9) | 397 (49.6) | 345 (64.7) | 1082 (68.5) | <.001 |
| Racial and ethnic disparity, % | |||||||
| Risk-based screening | |||||||
| Risk ≥1.51% | — | 5.5 | 17.8 | 37.0 | −13.1 | Ref | |
| Risk ≥1.7% | — | 5.9 | 19.6 | 38.2 | −13.4 | Ref | |
| Risk ≥2.0% | — | 4.4 | 19.2 | 36.0 | −17.7 | Ref | |
| USPSTF criteria | |||||||
| 2013 USPSTF | — | 19.5 | 7.2 | 13.6 | 7.2 | Ref | |
| 2021 USPSTF | — | 16.3 | 7.7 | 15.6 | 0.0 | Ref | |
| Alternate USPSTF strategies | |||||||
| 55–80/20/15 | — | 13.7 | 6.1 | 12.4 | 3.2 | Ref | |
| 55–80/20/20 | — | 16.8 | 5.7 | 14.5 | 5.4 | Ref | |
| 55–80/20/25 | — | 18.8 | 5.0 | 15.7 | 7.0 | Ref | |
| 50–80/20/20 | — | 19.4 | 7.2 | 17.7 | 2.2 | Ref | |
| 50–80/20/25 | — | 21.4 | 6.6 | 18.9 | 3.8 | Ref | |
Risk-based screening was evaluated through the PLCOm2012 model. Racial and ethnic disparity is defined as the absolute difference in screening sensitivities between each racial and ethnic group and White cases. USPSTF = United States Preventive Services Task Force.
P value was calculated across racial and ethnic strata using the χ2 test.
Five Alternate USPSTF Strategies
Under the alternate efficient strategies identified by the USPSTF decision analysis (13), the overall screening sensitivities were generally greater (42.2%-50.2%) than that under the 2021 USPSTF criteria (43.3%; Table 3). Yet, the screening disparities under these strategies were generally larger, except for the 55-80/20/15 strategy. For example, the most inclusive strategy, 50-80/20/25, had the largest disparities for African American (15.3%) and Latino (14.2%) cases.
In comparing the sensitivities of the alternate strategies vs risk-based screening (risk ≥1.51%), the sensitivity was highest with risk-based screening overall and by racial and ethnic group (Table 3). Screening disparities for African American cases were reduced under risk-based screening compared with the alternate strategies, but as with the 2021 USPSTF criteria, screening disparities increased for Japanese American and Latino cases. Similar patterns were observed in the age-restricted cohort (Table 4).
Discussion
In this study, 43.3% of ever-smoking lung cancer cases in MEC were eligible for screening at diagnosis through the 2021 USPSTF criteria, which is increased relative to the 2013 criteria. However, screening sensitivities of the 2021 USPSTF criteria differed by race or ethnicity and remained lowest among African American and Latino cases, who also bore the greatest screening disparities vs White cases. Under risk-based screening (risk ≥1.51%), the overall screening sensitivity increased to 72.9%, and the screening disparity for African American cases decreased relative to the 2021 USPSTF criteria, although it increased for Japanese American and Latino cases. When examining eligibility 6 years before lung cancer diagnosis, risk-based screening achieved a greater screening sensitivity vs the 2021 USPSTF criteria among African American cases but was equivocal among Japanese American and underperformed among Latino cases. In contrast, risk-based screening consistently performed better than the 2021 USPSTF criteria among Native Hawaiian cases. Thus, risk-based screening may improve screening sensitivities compared with the USPSTF criteria, but the benefits appear variable across racial and ethnic groups.
Studies have uncovered racial and ethnic disparities in lung cancer screening when using criteria based solely on age and smoking history (7,10,11,30–32). A considerable proportion of African American individuals are ineligible for screening under the 2013 (7,11) and 2021 USPSTF criteria (12). However, these analyses focused on the disparity between African American and White populations, whereas potential disparities for other racial and ethnic groups were not fully assessed. One study showed that the 2021 USPSTF criteria can reduce racial and ethnic disparities in modeled screening outcomes for African American and Latino individuals if implemented with prediction models (14); however, screening sensitivities among lung cancer cases were not examined. Another study evaluated the screening sensitivity of PLCOm2012 and the 2021 USPSTF criteria among 497 African American and 258 White lung cancer cases (12). Like our study, risk-based screening across various risk thresholds (1.51%-2.0%) achieved a statistically significantly greater sensitivity among African American cases compared with the 2021 USPSTF criteria. However, this study did not examine screening sensitivities among other racial and ethnic groups.
In evaluating screening sensitivities across 5 racial and ethnic groups in MEC, we found that African American and Latino cases still bear the largest disparities under the 2021 USPSTF criteria. Risk-based screening increased the screening sensitivity for all racial and ethnic groups at diagnosis vs the 2021 USPSTF 2021. This may be because the PLCOm2012 model measures smoking exposures more comprehensively with 4 smoking variables, incorporates risk factors beyond age and smoking history, and avoids the loss of information through dichotomization of continuous age and smoking variables, as with the USPSTF criteria.
However, sensitivity gains with risk-based screening vs the 2021 USPSTF criteria varied by racial or ethnic group, with Latino cases in particular experiencing fewer gains, resulting in a widened screening disparity vs White cases. In the analysis occurring 6 years before lung cancer diagnosis, risk-based screening underperformed among Latino cases, and Japanese American cases also did not derive a sensitivity gain. These limitations may arise because in the PLCOm2012 model, the 6-year lung cancer risk is reduced in the Asian American (beta = −0.467, OR = 0.63) and Hispanic (beta = −0.743, OR = 0.48) groups, whereas the risk is increased or equivocal in the other non-White racial and ethnic groups (Supplementary Table 2, available online). Thus, PLCOm2012 may inadvertently widen screening disparities for these populations. One potential solution is to eliminate the race and ethnicity variable from PLCOm2012, as has been evaluated in a previous analysis (11). Another option is to retain the PLCOm2012 race and ethnicity variable with the positive beta coefficients for African American and Native Hawaiian individuals but have all other racial and ethnic groups equal non-Hispanic White individuals (the reference group) in risk. With this modification, the screening sensitivities increased and screening disparities decreased for Japanese American and Latino cases both at diagnosis and 6 years before in an exploratory analysis of our study cohort (Supplementary Tables 8 and 9, available online). Altogether, these data support the sensitivity gains attained by risk-based screening over the 2021 USPSTF criteria but caution against reductions in calculated lung cancer risk based on racial or ethnic group, as has recently been contended in the estimation of renal glomerular filtration rates in equations that incorporate race (33).
This study compared risk-based screening and the 2021 USPSTF criteria in a large, prospective, racially and ethnically diverse, population-based cohort of lung cancer cases. Nonetheless, this study has several limitations. Selection bias is possible; for example, lung cancer cases in MEC have a low current smoking prevalence (41%) compared with cases in previous studies (42%-83%) (34). However, because this analysis focused exclusively on ever-smoking cases, differential biases based on smoking status should be minimized. Missing values were low except for COPD status, which was obtained through Medicare claims, thus with data only available for participants aged 65 years and older. Linkage of MEC to Medicare claims data has been previously validated (35,36). To address the missing data, we conducted multiple imputation and performed several sensitivity analyses, which demonstrated consistent results. Almost 25% of cases were older than 80 years of age at diagnosis and may be considered at risk of overdiagnosis (17). Nonetheless, the results in the age-restricted cohort were largely consistent. PLCOm2012 has been validated among participants aged 50-75 years (19). This analysis applied the model to participants up to age 80 years; thus, this extrapolation requires further validation. In this MEC analysis, follow-up was completed over a maximal period of 24 years; thus, relative changes over time in environment, dietary exposures, and smoking patterns could affect the diagnosis of lung cancer. The feasibility of collecting patient data for the PLCOm2012 model in the real-world setting needs to be examined; whereas most variables are routinely collected in a clinical encounter, others such as detailed smoking measures may be challenging to procure given the sensitive nature of the information. However, data on these variables were successfully collected in a prospective study evaluating lung cancer screening through PLCOm2012 (37).
Additionally, although we evaluated screening sensitivities in detail, we were unable to evaluate screening specificity—or the number of screen-ineligible noncases divided by the total number of noncases—because our study population consisted solely of lung cancer cases. Our approach of evaluating screening sensitivities alone is like that in previous studies (11,12). To address this limitation, we compared the 2021 USPSTF criteria with risk-based strategies that have higher specificities by increasing the PLCOm2012 risk threshold. For example, a stringent PLCOm2012 risk threshold at 2% is expected to select fewer individuals vs the 2021 USPSTF criteria, based on a previous study that showed that the 1.7% risk threshold would select a similar number of individuals for screening compared with the 2013 criteria (26). Because the 2021 criteria expanded eligibility from the 2013 guidelines, we expect that the specificity of risk-based screening at the 2% threshold will be higher than the 2021 USPSTF criteria, although this requires further validation.
In summary, the updated 2021 USPSTF guidelines for lung cancer screening still give rise to racial and ethnic disparities in MEC, especially among African American and Latino cases. Risk-based screening may achieve a greater screening sensitivity and help to reduce screening disparities in some, but not all, racial and ethnic groups. Further optimization of risk-based strategies is warranted to improve lung cancer screening efficiency in diverse populations and reduce racial and ethnic disparities.
Funding
This work was supported by a National Institutes of Health grant (grant number 1R37CA226081). Dr Aredo was supported by a Stanford Medical Scholars Research Grant. MEC is supported by NIH grant U01CA164973.
Notes
Role of the funder: The funders had no role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Disclosures: Dr Tammemägi developed the PLCOm2012 lung cancer risk prediction model, which is open access and available free of charge to noncommercial users; to date, he has not received any money for the use of the PLCOm2012 model nor does he anticipate receiving any money in the future. Dr Tammemägi is also a consultant for Johnson & Johnson/Janssen, Medial EarlySign, Nucleix, bioAffinity Technologies, and AstraZeneca. Dr ten Haaf reports grants from University of Zurich, Switzerland, nonfinancial support from International Association for the Study of Lung Cancer, nonfinancial support from International Association for the Study of Lung Cancer, grants from Cancer Research UK, nonfinancial support from Russian Society of Clinical Oncology, nonfinancial support and other from BIOMEDICAL RESEARCH IN ENDSTAGE AND OBSTRUCTIVE LUNG DISEASE HANNOVER (BREATH), grants from NIH/National Cancer Institute, grants from European Union (Horizon 2020), outside the submitted work. Dr Le Marchand reports grants from NIH/National Cancer Institute. Dr Wakelee reports personal fees from AstraZeneca, personal fees from Janssen, personal fees from Daiichi Sankyo, personal fees from Helsinn, personal fees from Mirati, personal fees from Blueprint, grants from ACEA Biosciences, grants from Arrys Therapeutics, grants from AstraZeneca/MedImmune, grants from BMS, grants from Celgene, grants from Clovis Oncology, grants from Exelixis, grants from Lilly, grants from Pfizer, grants from Pharmacyclics, outside the submitted work. Dr Meza reports grants from NIH. No other disclosures were reported.
Author contributions: Conceptualization, JVA, MCT, SSH; Data Curation, JVA, EC, SSH; Formal Analysis, JVA, EC, VYD, SJL, SSH; Resources, LRW, LL, SLP, IC, SSH; Writing–original draft, JVA, EC; Writing–review & editing, all authors; Validation, all authors; Supervision, SSH.
Data Availability
The data underlying this analysis were provided by the Multiethnic Cohort (MEC) Study under a data use agreement. Researchers interested in the MEC data may submit an inquiry online: https://www.uhcancercenter.org/for-researchers/mec-data-sharing.
Supplementary Material
References
- 1. Aberle DR, Adams AM, Berg CD, et al. ; National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening-results from the randomized German LUSI trial. Int J Cancer. 2020;146(6):1503-1513. [DOI] [PubMed] [Google Scholar]
- 3. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med. 2020;382(6):503-513. [DOI] [PubMed] [Google Scholar]
- 4. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. [DOI] [PubMed] [Google Scholar]
- 5. US Preventive Services Task Force. Lung cancer: screening. 2020. https://www.uspreventiveservicestaskforce.org/uspstf/draft-update-summary/lung-cancer-screening. Accessed December 2, 2020.
- 6. US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962-970. [DOI] [PubMed] [Google Scholar]
- 7. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD.. Evaluation of USPSTF lung cancer screening guidelines among African American adult smokers. JAMA Oncol. 2019;5(9):1318-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tammemagi MC, Church TR, Hocking WG, et al. Evaluation of the lung cancer risks at which to screen ever- and never-smokers: screening rules applied to the PLCO and NLST cohorts. PLoS Med. 2014;11(12):e1001764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han SS, Chow E, Ten Haaf K, et al. Disparities of National Lung Cancer screening guidelines in the US population. J Natl Cancer Inst. 2020;112(11):1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk prediction model versus United States Preventive Services Task Force lung cancer screening eligibility criteria: reducing race disparities. J Thorac Oncol. 2020;15(11):1738-1747. [DOI] [PubMed] [Google Scholar]
- 12. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Brief report: risk prediction model versus United States Preventive Services Task Force 2020 draft lung cancer screening eligibility criteria - reducing race disparities. JTO Clin Res Rep. 2021;2(3):100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meza R, Jeon J, Toumazis I, et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: modeling study for the US Preventive Services Task Force. JAMA. 2021;325(10):988-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Landy R, Young CD, Skarzynski M, et al. Using prediction-models to reduce persistent racial/ethnic disparities in draft 2020 USPSTF lung-cancer screening guidelines. J Natl Cancer Inst. 2021;113(11):1590-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol. 2000;151(4):346-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Setiawan VW, Virnig BA, Porcel J, et al. Linking data from the Multiethnic Cohort Study to Medicare data: linkage results and application to chronic disease research. Am J Epidemiol. 2015;181(11):917-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ten Haaf K, Jeon J, Tammemagi MC, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017;14(4):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weber M, Yap S, Goldsbury D, et al. Identifying high risk individuals for targeted lung cancer screening: independent validation of the PLCO(m2012) risk prediction tool. Int J Cancer. 2017;141(2):242-253. [DOI] [PubMed] [Google Scholar]
- 19. Tammemagi MC, Schmidt H, Martel S, et al. Participant selection for lung cancer screening by risk modelling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. Lancet Oncol. 2017;18(11):1523-1531. [DOI] [PubMed] [Google Scholar]
- 20. Li K, Husing A, Sookthai D, et al. Selecting high-risk individuals for lung cancer screening: a prospective evaluation of existing risk models and eligibility criteria in the German EPIC cohort. Cancer Prev Res (Phila). 2015;8(9):777-785. [DOI] [PubMed] [Google Scholar]
- 21. Hüsing A, Kaaks R.. Risk prediction models versus simplified selection criteria to determine eligibility for lung cancer screening: an analysis of German federal-wide survey and incidence data. Eur J Epidemiol. 2020;35(10):899-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crosbie PA, Balata H, Evison M, et al. Second round results from the Manchester ‘Lung Health Check’ community-based targeted lung cancer screening pilot. Thorax. 2019;74(7):700-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crosbie PA, Balata H, Evison M, et al. Implementing lung cancer screening: baseline results from a community-based ‘Lung Health Check’ pilot in deprived areas of Manchester. Thorax. 2019;74(4):405-409. [DOI] [PubMed] [Google Scholar]
- 24. Kavanagh J, Liu G, Menezes R, et al. Importance of long-term low-dose CT follow-up after negative findings at previous lung cancer screening. Radiology. 2018;289(1):218-224. [DOI] [PubMed] [Google Scholar]
- 25. Aggarwal R, Lam ACL, McGregor M, et al. Outcomes of long-term interval rescreening with low-dose computed tomography for lung cancer in different risk cohorts. J Thorac Oncol. 2019;14(6):1003-1011. [DOI] [PubMed] [Google Scholar]
- 26. Ten Haaf K, Bastani M, Cao P, et al. A comparative modeling analysis of risk-based lung cancer screening strategies. J Natl Cancer Inst. 2020;112(5):466-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8(1):3-15. [DOI] [PubMed] [Google Scholar]
- 29. Miles A. Obtaining predictions from models fit to multiply imputed data. Sociol Methods Res. 2016;45(1):175-185. [Google Scholar]
- 30. Annangi S, Nutalapati S, Foreman MG, Pillai R, Flenaugh EL.. Potential racial disparities using current lung cancer screening guidelines. J Racial Ethn Health Disparities. 2019;6(1):22-26. [DOI] [PubMed] [Google Scholar]
- 31. Li CC, Matthews AK, Rywant MM, Hallgren E, Shah RC.. Racial disparities in eligibility for low-dose computed tomography lung cancer screening among older adults with a history of smoking. Cancer Causes Control. 2019;30(3):235-240. [DOI] [PubMed] [Google Scholar]
- 32. Pinsky PF, Kramer BS.. Lung cancer risk and demographic characteristics of current 20-29 pack-year smokers: implications for screening. J Natl Cancer Inst. 2015;107(11):djv226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Parsons A, Daley A, Begh R, Aveyard P.. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: systematic review of observational studies with meta-analysis. BMJ. 2010;340:b5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farias AJ, Wu AH, Porcel J, et al. Diabetes-related complications and pancreatic cancer incidence in the multiethnic cohort. JNCI Cancer Spectr. 2020;4(5):pkaa035. pkaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L, Noureddin M.. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016;64(6):1969-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tammemagi MC, Ruparel M, Tremblay A, et al. USPSTF2013 versus PLCOm2012 lung cancer screening eligibility criteria (International Lung Screening Trial): interim analysis of a prospective cohort study. Lancet Oncol. 2022;23(1):138-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this analysis were provided by the Multiethnic Cohort (MEC) Study under a data use agreement. Researchers interested in the MEC data may submit an inquiry online: https://www.uhcancercenter.org/for-researchers/mec-data-sharing.