Abstract
Cashew nut production generates large amounts of cashew apple as residue. In Colombia, cashew cultivation is increasing together with the concerns on residue management. The objective of this study was to provide the first chemical, physical and thermal decomposition characterization of cashew apple from Colombian varieties harvested in Vichada, Colombia. This characterization was focused to identify the important bioactive and natural compounds that can be further valorized in the formulation of food, nutraceuticals, and pharmacological products. The results obtained in this study are helpful to portray the cashew apple as a potential by-product due to its renewable nature and valuable composition, instead of seeing it just as an agricultural residue. For that, cashew apples of Regional 8315 and Mapiria varieties were studied. The natural juice (cashew apple juice) that was extracted from the cashew apples and the remanent solids (cashew apple bagasse) were separately analyzed. The HPLC analytical technique was used to determine the concentration of bioactive compounds, structural carbohydrates, and soluble sugars that constitute this biomass. Spectrophotometric techniques were used to determine the concentration of tannins, carotenoids, and total polyphenols. Mineral content and antioxidant activity (DPPH and ABTS assays) were determined in the biomass. Also, the thermal decomposition under an inert atmosphere or pyrolysis was performed on cashew apple bagasse. The varieties of cashew apple studied in this work showed similar content of bioactive compounds, total phenolic content, and structural carbohydrates. However, the Mapiria variety showed values slightly higher than the Regional 8315. Regarding cashew apple juice, it is rich in tannins and ascorbic acid with values of 191 mg/100 mL and 70 mg/100 mL, respectively, for Mapiria variety. Additionally, the principal reservoir of bioactive compounds and constitutive carbohydrates was the cashew apple bagasse. About 50 wt.% of it was composed of cellulose and hemicellulose. Also, in the bagasse, the ascorbic acid content was in a range of 180–200 mg/100 g, which is higher than other fruits and vegetables. Moreover, alkaloids were identified in cashew apples. The maximum value of antioxidant activity (DPPH assay: 405 TEs/g) was observed in the bagasse of Mapiria variety. The bagasse thermal decomposition started around 150 °C when the structural carbohydrates and other constitutive substances started to degrade. After thermogravimetric analysis, a remanent of 20% of the initial weight suggested the formation of a rich-carbon solid, which could correspond to biochar. Therefore, the cashew apple harvested in Vichada is a valuable reservoir of a wide range of biomolecules that potentially could be valorized into energy, foods, and pharmacologic applications. Nevertheless, future work is necessary to describe the complex compounds of this residual biomass that are still unknown.
Keywords: Cashew apple, Characterization, Antioxidant activity, Bioactive compounds, Carbohydrates
Cashew apple, characterization, antioxidant activity, bioactive compounds, carbohydrates.
1. Introduction
Cashew nut is one of the most important agro-industrial crops worldwide due to the economic value of this product; the market size of this nut was around 7.46 billion dollars in 2019 [1,2]. The cashew nut is obtained from the Anacardium occidentale tree, native to Brazil but introduced to other countries in the world [3]. Colombia is an agricultural country that seeks to increase its participation in the cashew nut market. The Altillanura of Colombia is a region with a significant potential for agricultural exploitation where the cashew crop is a promissory economic activity [4]. Vichada (Coordinates: 6°11′N 67° 28′W) department is the most extensive territory that belongs to the Altillanura region, and it has focused the attention of farmers, investors, and local and national government. This attention resulted in the interest in increasing the cashew nut production in Vichada [4]. Nowadays, the Mapiria and Regional 8315 varieties of cashew tree have been widely cultivated in Vichada. These tree varieties were developed in Colombia to improve the production yield of nuts. Even though cashew nut production is a promissory activity, its agricultural residue management is a concern in Vichada.
Nowadays, the relevance of this problem has focused the attention of the scientific community not only concerns Colombia. The residues of cashew nut production are (1) cashew apple (CA) which is edible, and (2) cashew nut shell (CNS). Regarding CNS, this is the part of the fruit that protects the nut. This is a nonedible residue that contains toxic and complex phenolic compounds [5]. Because of that, the CNS is not part of the present study since the focus of this article was the characterization of edible residues of cashew nut production.
Conversely, the cashew nut grows attached to the CA, also known as the peduncle, and represents about 90 wt.% of the whole fruit [6]. According to FAO data [7], in 2018, cashew nut production in the world amounted to 6 million tonnes. Although the CA is an edible part of the fruit, the low acceptance for its flavor generally makes it biomass without economic value [8]. Approximately 50 million tonnes of CA were generated in the world in 2018. This considerable generation of agricultural residues increases the contamination of farms, water sources, and air. Nevertheless, the chemical composition of CA has shown that it is rich in valuable compounds such as vitamins, sugars, amino acids, dietary fibers, minerals, carotenes, and polyphenols, among other phytonutrients [5, 6]. For instance, the vitamin C (ascorbic acid) content that has been found in CA [9] is higher than observed in oranges [10]. Sugars such as fructose, sucrose, and glucose have been found in CA [6]. Also, compounds such as cellulose, hemicellulose, and pectin are part of the CA composition [11]. Additionally, polyphenols with antioxidant activity [12] and tannins have been observed in CA from different varieties worldwide [6]. However, high tannin content is attributed to a strong astringent flavor, which reduces the value of this biomass [13]. The chemical composition of CA and the occurrence of these components are related to the tree variety, place of cultivation, and storage conditions. Consequently, the main challenge in the valorization of CA is that requires transformation processes to obtain value-added products such as cashew apple juice (CAJ), syrups, jams and jellies, nutraceutical powders, or fermented beverages [6, 14]. Recently, some research has used CA in formulating vegan burger meat after a laboratory-scale cooking process with flavor acceptance [15]. Usually, chemical [8], physical [9], and biological [16] processing are performed to modify the CA composition to develop edible products. It is an important research field that seeks to reduce the negative impact of cashew nut production. To exploit the potential of CA, its chemical composition must be analyzed as a primary step to identify valorization pathways. Though, to the best of our knowledge, a rigorous chemical and physical characterization of CA from Colombian varieties has not been reported yet. Therefore, the potential of this agricultural residue in Colombia is still un-exploited to be used in value-added products in food, nutraceutical, and pharmaceutical applications.
Then, this study aimed to determine the chemical, physical characterization, and thermal decomposition of the two varieties of CA, the most harvested in Vichada. Therefore, this work was the first detailed study that had the objective to establish the chemical composition of CA (Mapiria and Regional 8315 varieties) cultivated in Vichada, Colombia. Then, the CA was analyzed in two parts (i) the natural cashew apple juice (CAJ) that can be extracted from the fruit, and (ii) the remaining solids, defined as cashew apple bagasse (CAB). The chemical characterization of bioactive compounds and constitutive carbohydrates was performed using advanced analytical techniques such as high-performance liquid chromatography. Additionally, nutraceutical attributes and antioxidant activities were determined in CAJ and CAB to evaluate their potential in the elaboration of edible products, for instance, syrups, juices, fermented beverages, or jellies.
2. Materials and methods
2.1. Chemicals
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)), galacturonic acid, gallic acid, tannic acid, DNS (3.5-dinitrosalicylic acid), β-carotene, sodium carbonate, sodium chloride, and EDTA were purchased from Sigma Aldrich (Saint Louis, USA). Hexane, potassium chloride, xylose, Folin-Ciocalteu's reagent, and disodium phosphate were purchased from PanReac AppliChem (Chicago, USA). DPPH (2,2-diphenyl-1-picrylhydrazyl) was purchased from Santa Cruz Biotechnology (Texas, USA). Ethanol, acetone, sodium hydroxide, and sodium tartrate were purchased from Merck (Darmstadt, Germany). Fructose and glucose were purchased from Scharlab (Barcelona, Spain).
Organic acids: (i) lactic, tartaric, acetic, and fumaric were purchased from PanReac AppliChem (Chicago, USA), and (ii) glyoxylic monohydrated, itaconic, citric, and malic were purchased from Sigma Aldrich (Saint Louis, USA). Ascorbic acid was purchased from Chemi (Patrica, Italy). Oxalic acid was purchased from Carlo Erba Reagents (Milano, Italy).
2.2. Cashew apple juice and cashew apple bagasse
Florez Rojas company (Puerto Carreño, Vichada, Colombia) kindly donated the CA of the varieties (i) Regional 8315 (orange) and (ii) Mapiria (yellow) (Figure 1) that were characterized in this work. The fruits were stored at -20 °C to avoid degradation of components. For the juice extraction, a Vitamix ® blender was used. First, 3 kg of fresh CA was blended for 5 s, and 900 mL of distilled water was added to improve the filtration and dilution of bio-active compounds. Consequently, 3500 mL of juice were obtained from 3 kg of fresh CA, which was stored at -20 °C to avoid degradation and microorganism proliferation. After filtering, the resulting CAB was recovered and stored at -20 °C for further analysis.
Figure 1.
Cashew apple varieties: a) Regional 8315 and b) Mapiria.
2.3. Extraction of bioactive compounds from cashew apple bagasse
Ultrasonic treatment was performed to recover the bioactive compounds from CAB. This process improves the solubilization of bioactive compounds using the cavitation effect on the sample [17]. The extraction was performed using CAB (room temperature around 17 °C) and distilled water (in a ratio of 1:4 g/mL) for 10 min at the maximum power delivered by the ultrasonic equipment (Bransonic® CPXH 3800). The samples were stored at 4 °C for 24 h to avoid degradation. Subsequently, the supernatant was recovered after a centrifugation and filtration procedure.
2.4. Preliminary identification of phytochemicals
Qualitative tests were performed on the juice and bagasse to identify common phytochemicals. Tests were run in duplicate. Table 1 lists each test and the color change expected if the evaluated phytochemical is present in the sample.
Table 1.
Methodology for assessment of phytochemicals.
| Test | Phytochemical | Color change (Presence) | Reference |
|---|---|---|---|
| Shinoda | Flavonoids | Magenta | [18] |
| Rosenheim | Leucoantocianydins | Crimson/Pale pink | [19] |
| Ferric Chloride | Tannins | Between blue and black precipitate | [20] |
| Foam | Saponins | Emulsion formation | [20] |
| Wagner's reagent | Alkaloids | Brown precipitate | [18] |
| Liebermann Burchard | Sterols | From violet/blue to green | [21] |
| Bontrager | Naphthoquinones and Anthraquinones | From pink to red in the ammonium layer | [22] |
| Chloroform and sulfuric acid | Carotenoids | Green (vanishes after 2 min) | [23, 24] |
2.5. Cashew apple juice characterization
2.5.1. Titratable acidity, pH, and total soluble solids content
Titratable acidity was measured following the AOAC 942.15 method. About 5 mL of sample was dissolved in 100 mL of distilled water. The titration was performed with a 0.1N sodium hydroxide solution, and the pH changes were measured by a multimeter (SevenMulti™, Mettler-Toledo, Switzerland). Titration was stopped when a pH of around 8.2 was reached. These tests were run in triplicate for each juice variety. The pH measurements were performed with a multimeter (SevenMulti™, Mettler-Toledo, Switzerland). The content of total soluble solids was determined using an analog Brix portable refractometer (Model RF20, Extech Instruments, USA). The assays were carried out in triplicate.
2.5.2. Reducing sugars and protein content
The concentration of reducing sugars in the natural juice from CA was determined by following Miller's method. Briefly, this method is based on the reaction of a reducing sugar with the DNS reagent catalyzed by temperature. This reaction changes de color of the solution from yellow to brown, which is measured by spectrophotometry [25]. In this work, the reaction mixture consisted of 3 mL of DNS reagent and 0.5 mL of juice sample. The reaction mixture was boiled in a water bath (92 °C) for 5 min, and the reaction was stopped by transferring the test tubes to an icy water bath for 10 min. Then, the absorbance was measured at 540 nm using a UV–vis spectrophotometer (Genesys TM 10S UV–vis, Thermo Scientific, USA). Glucose was used as standard over a range of 0.1–1 g/L in a calibration curve [25]. The results were expressed as wet basis percent (wb. %). This assay was done in triplicate for each type of sample. The BCA Protein Assay Kit (ThermoScientific TM) was used to determine the total protein content. Accurately 0.05 mL was transferred to a test tube from the aqueous sample, and then 1 mL of BCA reagent was added. After that, the sample was incubated (Barnstead Lab-line 305 Imperial III incubator, Barnstead International, Dubuque, IL, USA) for 30 min at 37 °C. Absorbance was measured at 562 nm using a UV–vis spectrophotometer (Genesys TM 10S UV–vis, Thermo Scientific, USA). Calibration was done with albumin in a range of 0–2000 μg/mL. The protein determination was run in four replicates for each CA variety.
2.6. Organic acids and sugars content
The CAJ and CAB extracts were analyzed to determine the organic acids and sugars content, based on the method detailed in the NREL/TP-510-42623 technical report and the methodology used by J.K.S. Andrade, et al. [26, 27]. For that, 1 mL of each sample was accurately transferred to 2 mL glass vials. The High-Performance Liquid Chromatography (HPLC) technique was carried out with a Biorad Aminex HPX-87H column, 1300 × 7.8 mm, 9 μm particle size, and 8% cross-linkage (Bio-Rad, USA). The column was operated in an Agilent Series 1200 HPLC system (Agilent Technologies, Santa Clara, USA). Both DAD (for organic acids detection) and RID (for sugars detection) detectors were used. The method specifications used in this work were: (i) column temperature: 55 °C, (ii) sample injection volume: 0.2 μL, (iii) flow rate of mobile phase: 0.6 mL/min, and iv) sulfuric acid 0.005 M as the mobile phase. Each organic acid that was analyzed in this work and its calibration range are specified next: (i) ascorbic acid (100–1000 mg/mL), (ii) acetic acid (250–2500 mg/mL), (iii) citric acid (200–1200 mg/mL), (iv) tartaric acid (100–1000 μg/mL), (v) oxalic acid (40–400 mg/mL), (vi) itaconic acid (250–3000 mg/mL), (vii) fumaric acid (40–300 μg/mL), (viii) lactic acid (500–2500 mg/mL), and (ix) glyoxylic acid (250–2000 mg/mL). In addition, the sugars (i) glucose (calibration range of 10–100 mg/mL) and (ii) fructose (calibration range of 10–100 mg/mL) were analyzed. Five replicates of each CAJ and CAB extract were performed.
2.7. Total phenolic and total tannin content
Phenolic content was determined using a modified version of the Folin-Ciocalteu method [28]. From the aqueous samples (of CAJ and CAB extract), 0.25 mL were accurately transferred to a test tube and mixed with (1) 4.8 mL of distilled water, (2) 0.5 mL of Folin-Ciocalteu´s phenol reagent, and (3) 1.5 mL of 20% (w/v) sodium carbonate solution after 2 min incubation at room temperature. The reaction mixture was allowed to react for 2 h at room temperature in darkness, and the absorbance was measured at 765 nm. A calibration curve with gallic acid over a concentration range of 25 mg/L to 500 mg/L was used as standard. The results were reported in milligrams of gallic acid equivalents (mg GAE) per 100 mL of juice or 100 g of dry matter for CAJ and CAB, respectively. The assay was done in triplicate for each variety of CA.
Total tannin content was determined using the method mentioned above with some modifications. The wavelength used for tannins was 700 nm, and tannic acid as standard was used in a calibration curve over a concentration range of 5 mg/L to 200 mg/L [29]. This assay was done in triplicate for each variety of CA.
2.8. Total carotenoid content
The carotenoid extraction was performed on the CAJ and CAB extracts. Accurately, 0.5 mL of sample was transferred to test tubes. Then, 25 mL of a solution of acetone: hexane: ethanol, at a ratio of 1:2:2, were added and mixed by vortex for 5 seg. The mixture was allowed to interact for 30 min at 37 °C. Next, the mixture was separated at 5000 rpm for 5 min at 4 °C in a centrifuge (ThermoFisher Scientific Legend XTR). The supernatant corresponding to the hexane with carotenoids (organic phase) was recovered. The concentration of total carotenoids was determined by spectrophotometry using a UV–vis spectrophotometer (Genesys TM 10S UV–vis, Thermo Scientific, USA). The absorbance was measured at 540 nm with hexane as blank [30]. A standard calibration curve with β-carotene in a range of 0.5–14 μg/mL was used. This assay was run in triplicate for each variety of CA.
2.9. Mineral content
The quantification of minerals was performed following the U.S. EPA Method 3051A (Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils) and EPA Method 6010D (SW-846) procedures. For the digestion of liquid samples, about 45 mL of juice were transferred to a flask. Accurately, 4 mL of HNO3 and 1 mL of HCl were added. The mixture was heated up to 170 °C in a microwave oven (CEM model MARS6) for 10 min. Next, the aqueous solution was filtered and washed with NHO3 5 v.% solution. The filtrate was transferred to the ICP equipment (Inductively Coupled Plasma Atomic Emission Spectrometry, ICP-OES Thermo Electron ICAP 6500 Duo, Thermo Scientific, USA) for mineral determination. Zinc (Zn), copper (Cu), and iron (Fe) contents were measured at 213 nm, 324 nm, and 259 nm, respectively. The calibration was in the range of 0.05 mg/L to 10 mg/L for each mineral. The procedure was run in duplicate.
For solid samples, accurately 0.5 g of bagasse was mixed with 9 mL of HNO3 and 3 mL of HCl. The digestion was performed in a microwave oven (CEM model MARS6) at 175 °C for 10 min. After cooling to room temperature, the sample is filtered and washed with HNO3 5 v.% solution. Then, the sample was taken to the IPC equipment (Inductively Coupled Plasma Atomic Emission Spectrometry, ICP-OES Thermo Electron ICAP 6500 Duo, Thermo Scientific, USA) for mineral measurement. Zinc (Zn), copper (Cu), and iron (Fe) contents were measured at 213 nm, 324 nm, and 259 nm, respectively. The calibration was in the range of 0.05 mg/L to 10 mg/L. The procedure was run in duplicate.
2.10. Cashew apple bagasse characterization
2.10.1. Sample preparation
The moist CAB was obtained after the juice filtration and was dried following the NREL/TP-510-42620 protocol. The CAB sample was dried in a convection oven at 45 °C for 36 h. After that, the CAB was ground until the entire sample passed the 1mm screen [31].
2.10.2. Moisture content
The moisture content of the CAB was determined by gravimetry. Accurately, 2 g of CAB were weighed in a thermobalance Precisa XM 60, d 0.001 g (Precisa instruments AG, 8953 Dietikon, Switzerland). The sample was heated for 30–45 min at 105 °C until reaching a constant weight [32]. For further calculations, the oven-dry weight (ODW) was calculated under the NREL/TP-510-42621 procedure by follows by Eq. (1).
| (1) |
Where W0 is the initial sample weight (g), and %M is the moisture percent (%) obtained from the thermobalance. This measurement was done in triplicate for each analyzed variety of CAB.
2.10.3. Extractives content
The waxes, gums, and free sugars extracted with solvents are known as extractives. The extractive content of CAB was determined following the NREL/TP-510-42619 protocol [33]. About 10 g of dried CAB were transferred to a cellulose thimble. Then, Soxhlet extraction with water was performed for 24 h, followed by extraction with ethanol (96 % purity) for another 24 h. The solids were recovered and washed with distilled water for further analysis. The flask with the solvent plus extractives of each extraction was transferred to a rotary evaporator (BÜCHI Rotavapor R-114). After solvent remotion, the content of extractives was weighted. To calculate the extractives, Eq. (2) was used.
| (2) |
Where Wf+e is the weight of the flask plus extractives after solvent remotion (g), Wf is the weight of the empty flask before extraction (g), and ODWsample corresponds to the oven-dry weight of the sample (g). The assay was done in triplicate for each variety of CAB analyzed.
2.10.4. Protein content
Kjeldahl's method was used to determine the nitrogen content of the samples [34]. The protein content was measured following the ISO 11261:1995 (E) method. Accurately, 0.5 g of sample was digested in micro Kjeldahl equipment (Velp Scientifica model DKL12, Usmate, Italy). A solution of NaOH was added and mixed well with the digested sample. Next, the mixture was separated in a steam distillation unit (Buchi model K-355, Switzerland). The recovered sample was titrated with H2SO4 in an automatic titrator (Methrom model 702 SC Titrino). The protocol was run in duplicate. A conversion factor of 6.25 was used to find the protein content in the solid sample [35].
2.10.5. Lignin and constitutive carbohydrates content
Lignin and constitutive carbohydrates content was determined following the NREL procedure (TP-510-42618) [36]. Accurately, 300 mg ± 10 mg of free extractives samples of CAB were transferred to a test tube, and 3 mL of H2SO4 solution (72 wt.%) were added. The mixture was allowed to react for 1 h at 30 °C with periodic agitation. Next, the acid solution was diluted to a concentration of 4 wt.%, and the mixture was allowed to react for 1 h at 121 °C. After that, the solids were separated from liquids by filtering with a dry crucible. The solid fraction was used to determine the lignin content by gravimetric analysis of the sample. Cellulose and hemicellulose content were determined in the aqueous phase by HPLC. The column used in this work was a Rezex RCM-Monosaccharide Ca+2 (8 %) (300 × 7.8 mm) (Phenomenex, Torrance, CA, USA) operated by an Agilent Series 1200 HPLC system (Agilent Technologies, Santa Clara, USA). The method details were (1) column temperature: 85 °C, (2) sample injection volume: 10 μL, (3) flow rate of mobile phase: 0.6 mL/min, and (4) ultrapure water as the mobile phase. Glucose and xylose concentration was related to cellulose and hemicellulose concentration in the CAB, as is described by Sluiter [36]. Calibration curves of glucose and xylose were used in a range of 0.5 g/L to 4 g/L. Five replicates were run for each variety of CAB.
2.10.6. Ash content
Ash content was determined following the protocol NREL/TP-510-42622 [37]. Accurately, 2 g of moisture-free samples were placed in previously cured crucibles and transferred to a muffle for 5 h following the heating ramp: (1) from room temperature to 105 °C (10 °C/min) for 12 min, (2) from 105 °C to 250 °C (10 °C/min) for 30 min, and finally, (3) from 250 °C to 575 °C (20 °C/min) for 180 min. After that, the sample was allowed to cold to 105 °C and transferred to a desiccator recipe. The ash content calculations were done using Eq. (3), as detailed in the protocol.
| (3) |
Where Wc+a is the weight of crucible plus ashes (g), Wc corresponds to the weight of dry, empty crucible (g), and ODWsample corresponds to the oven-dry weight of the sample (g). Five replicates were analyzed in this work for each variety of CAB.
2.10.7. Pectin content
A colorimetric method based on the reaction of carbazole with galacturonic acid was implemented. For that, 1 g of CAB was washed with ethanol (1:2 w/v) and filtered to remove remnant sugars and other ethanol-soluble compounds. Then, samples were treated for 30 min with a solution of EDTA (5%). After that, NaOH (1M) was added until a pH of 11.5 was achieved. The mixture was allowed to interact for 30 min. Then, acetic acid solution (0.25 M) was added until a pH between 5 to 5.5 was reached. Pectinase from Aspergillus niger (0.5 g) was added to perform complete hydrolysis of galacturonic acid. The supernatant of the hydrolysate was retrieved and used for the colorimetric quantification by treating samples with H2SO4 (98 wt%) at 5 °C, cooled in an ice bath until the sample reached 3 °C, and then heated up to 90 °C in a water bath. The colorimetric reaction occurred for 25 min in the presence of carbazole solution (0.15%). Absorbance was measured using a UV–vis spectrophotometer (Genesys TM 10S UV–vis, Thermo Scientific, USA) at 530 nm. Finally, 0.1 g of pectin was treated to find the ratio between the amount of galacturonic acid per unit of pectin [38, 39]. A calibration curve with galacturonic acid in a range of 10–100 μg/mL was used as standard. Four replicates were analyzed in this work for each variety of CAB.
2.10.8. Thermal decomposition
The thermal decomposition of CAB was studied by thermogravimetry analysis (TGA). Tests were performed using a TA Instruments Q600 thermogravimetric analyzer with alumina sample pans. The temperature ranged from 17 °C to 1000 °C with a constant heating rate of 10 °C/min in a nitrogen atmosphere. Two sample runs were performed under the same experimental conditions.
2.11. Antioxidant activity
Antioxidant activity was determined by ABTS and DPPH radical cation decolorization assays. For the first-mentioned method, non-oxidized ABTS was dissolved in water to a 7 nM concentration. Then, it was allowed to react in the dark at room temperature for 16 h with a potassium persulfate solution (0.45 nM) to form the ABTS+ solution. Following, this solution with a dark-green color was diluted in a phosphate-buffered saline (PBS) buffer (pH 7.4) until reaching an absorbance of 0.700 at 734 nm. This final solution is the radical working ABTS+ solution.
To assess the antioxidant activity, 1 mL of the working solution was transferred to a spectrophotometer plastic cell. Accurately, 10 μL of the aqueous sample was added, and it was allowed to react in darkness for 5 min at room temperature. The absorbance values were measured at 734 nm using a UV–vis spectrophotometer (Genesys TM 10S UV–vis, Thermo Scientific, USA) with the work solution as control [40]. A calibration curve with Trolox in a range of 10–600 μM was used to determine the antioxidant activity. Four replicates for each CAJ and CAB extract sample were run.
The DPPH assay was performed accordingly to the method reported by Brand-Williams [41] with some modifications. A stock solution of DPPH was prepared using 0.24 mg of DPPH diluted in 10mL of ethanol (99 v.%). Next, more ethanol was added to the DPPH solution until achieving an absorbance of 1.1 ± 0.02 at 517 nm (about 60 mL of ethanol was needed) to prepare the working DPPH solution. To assess the antioxidant activity, 150 μL of the aqueous sample was mixed with 2850 μL of the working DPPH solution. The resulting solution was allowed to react for 30 min in darkness at room temperature. Then, the absorbance was measured at 517 nm in a UV-vis spectrophotometer (Genesys TM 10S UV–vis, Thermo Scientific, USA) with pure ethanol as blank. A standard curve was prepared using Trolox solutions in 80–800 μM. Results of antioxidant activity were expressed in Trolox Equivalents (TEs) which refers to mmol of Trolox. Four replicates for each sample of CAJ and CAB extracts were run.
2.12. Statistical analysis
Obtained data were processed using the analysis of variance (ANOVA). The significance levels of the differences between means of each variety were determined using the t-test of Tukey (p < 0.05).
3. Results and discussion
3.1. Preliminary identification of phytochemicals
Phytochemicals are important metabolites that are present in vegetable food and biomass. They are non-nutritive chemical compounds that have been proved to have health protection benefits [42]. Therefore, the identification of phytochemicals provides a first screening of the content of these valuable substances in CA.
Table 2 presents the results of the preliminary identification of phytochemicals in CAJ and CAB. Regarding the identified phytochemicals, leucoanthocyanins that belong to the flavonoid family were found in CAB extracts. These components are related to anthocyanins and contribute to a particular color in plants. Also, they have a positive impact on human health. These compounds possess strong scavenging reactive oxygen species [43]. This effect protects the cell DNA from oxidative damage. Some plants that contain anthocyanins are Solanaceous fruits, including tomato, eggplant, and pepper [44].
Table 2.
Results of identification of phytochemicals.
| Phytochemical | Cashew apple juice |
Cashew apple bagasse |
||
|---|---|---|---|---|
| Mapiria | Regional 8315 | Mapiria | Regional 8315 | |
| Flavonoids | (–) | (–) | (–) | (–) |
| Leucoanthocyanins | (–) | (–) | (+) | (+) |
| Tannins | (+) | (+) | (+) | (+) |
| Saponins | (–) | (–) | (–) | (–) |
| Alkaloids | (+) (+) | (+) (+) | (–) | (–) |
| Sterols | (–) | (–) | (+) | (+) |
| Naphthoquinones and anthraquinones | (–) | (–) | (–) | (–) |
| Carotenoids | (+) | (+) | (+) | (+) |
∗ Interpretation: (+) color observed (presence), (+) (+) dark-color observed (relative high presence), (–) no color observed (not detected).
Moreover, tannins were identified in the samples of CAJ and CAB. Tannins are a diverse group of polyphenolic compounds widely distributed among medicinal plants. An essential property of tannins is to form stable complexes with proteins, starches, and other macromolecules. Tannins in plant extracts have been used as astringents against diarrhea, anti-inflammatory drugs, and antiseptic pharmaceuticals [45]. The cashew tree (Anacardium occidentale) belongs to the family Anacardiaceae, also the mango tree (Mangifera indica). The Mangifera indica tree has been studied for tannin identification. Available data showed that the leaves of Mangifera indica have tannins such as penta-O-galloylglucose, epigallocatechin-3-O-gallate-(4β→8)-epigallocatechin(4β→8)-catechin, and maclurin-3-C′-(2″,3″, 6″-o-galloyl)-β-d-glucoside [46, 47]. This tree is genetically related to cashew; then, similar tannins could be found in CA.
The qualitative test marked positive for alkaloids in the juice of both varieties of CA analyzed in this work. Alkaloids are defined as bioactive substances with nitrogen-containing organic compounds. In plants, alkaloids are considered part of their defense mechanism since they act as poisons and repellents to predators [48]. Alkaloids are a diverse family of bioactive compounds and are mainly classified into pyrrolidines (hygrine), tropanes (cocaine), pyrrolizidines (retronecine), pyridines (nicotine), isoquinolines (papaverine), and purine derivatives (caffeine), among others [49].
The isoquinoline alkaloids have one of the most abundant occurrences in the plant kingdom, and they are found in a vast number of family plants. Some of them are Berberidaceae, Fumariaceae, Rubiaceae, Magnoliaeace, Ranunculacea, Cactacear, Lauraceae, and Leguminosae. The importance of this family of alkaloids is that they have shown pharmacological and biological activities. Some effects such as anticancer, antioxidant, anti-inflammatory, antifungal, antihyperglycemic, and antiviral have been observed related to isoquinolines [50].
Alkaloid-related compounds that have been found in mango tree steam bark are Cis-zeatin riboside, trans-zeatin, and trans-zeatin ribose [46]. The genetic relation between mango and cashew trees could indicate the type of alkaloids that could be found in both plants. As far as we know, no further information has been published about this topic at the moment of writing this work. Therefore, alkaloid characterization in cashew trees is an open field of investigation. More studies are needed to explore all the potential of alkaloids in Colombian cashew varieties.
Additionally, sterols were also found in CAB extracts. They are bioactive compounds present in plants and are similar to cholesterol [51]. Functional properties of sterols are attributed to anti-inflammatory [52], anti-apoptotic [53], and anticancer [54] effects. Other plants commonly used in the human diet where sterols can be found are Pimpinella anisum, Coriandrum sativum, Carum carvi, or Sinapis alba [55].
Regarding carotenoids that were found in the samples, they are non-toxic compounds with potent antioxidant bioactivity, and they are considered radical-free oxygen scavengers. This effect can reduce the oxidative stress in cells, which is the desired effect that helps to reduce the incidence of cancer [56]. Consequently, carotenoids are desirable compounds in edible products, and they can be found in plants and fruits from species such as Anacardium spp, Byrsonima crassifolia, Caryocar coriaceum, Genipa americana, Spinacia oleracea, Vitis labrusca, and Solanum lycopersicum, among others [57, 58].
Finally, with the information obtained from the phytochemical identification, it was observed that Colombian CAs have a wide range of valuable natural compounds. Therefore, this agricultural residue is a reservoir of essential substances that can be used to formulate pharmaceuticals and edible products due to their bioactive perspective.
3.2. Characterization of cashew apple juice
3.2.1. Titratable acidity, pH, and total soluble solids content
Table 3 summarizes the titratable acidity, pH, and total soluble solids that were measured in CAJ. Titratable acidity and pH values are related to the concentration and variety of organic acids found in fruit beverages [59]. The titratable acidity and pH were similar between the Regional 8315 and Mapiria varieties studied in this work. Nevertheless, the titratable acidity of natural juices from Colombian varieties was below compared to some reports of CAJ from Brazil, India, or Nigeria. This last indicates that preserving agents and acidity regulators could be added to the juices to improve their characteristics, as is commonly made in the fruit juice production industry [60].
Table 3.
Physical properties of cashew apple juice. Adapted from [6].
| Parameter | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
| Titratable acidity | g/100 mL | 0.150 ± 0.005a | Colombia (Regional 8315) | Present study |
| 0.170 ± 0.008a | Colombia (Mapiria) | Present study | ||
| 0.28–0.43 | Brazil (Commercial juices and pulps) | [9, 61, 62] | ||
| 0.32 | India (Andhra Pradesh, and others) | [14, 63] | ||
| 0.48–0.63 | Nigeria (Kosoniola farm, Oro) | [13, 64, 65] | ||
| pH | - | 4.56 | Colombia (Regional 8315) | Present study |
| 4.61 | Colombia (Mapiria) | Present study | ||
| 3.77–4.40 | Brazil (Commercial juices and pulps) | [9, 62, 66, 67] | ||
| 3.40–4.14 | India (Andhra Pradesh, and others) | [14, 63, 68] | ||
| 3.70–4.15 | Nigeria (Kosoniola farm, Oro) | [64, 65, 69, 70] | ||
| Total soluble solids | Brix | 23 | Colombia (Regional 8315) | Present study |
| 22 | Colombia (Mapiria) | Present study | ||
| 7.4–12.2 | Brazil (Commercial juices and pulps) | [9, 62, 66, 67] | ||
| 10–13.8 | India (Andhra Pradesh, and others) | [14, 63, 68] | ||
| 11–13.1 | Nigeria (Kosoniola farm, Oro) | [64, 65, 69, 70] |
Values are expressed in means ± standard deviation (n = 3).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
Commercial juices and pulps could be processed to improve the characteristics of the final product.
Regarding total soluble solids content, this parameter was found similar between the natural juices obtained from Regional 8315 (23°Brix) and Mapiria (22°Brix) varieties. Compared to commercial references of juices found in Brazil or India, the total soluble solids values in Colombian CAJs were found higher. This high content of soluble solids causes turbidity in the juice and can be related to a high presence of non-structural sugars that can be highly solubilized in water during the juice elaboration [32].
These observations indicate that this parameter should be modified to improve the aspect of commercial juices that would be obtained from CA, as commercial references indicate.
3.2.2. Reducing sugars and protein content
The measurements of reducing sugars and protein content in CAJ are summarized in Table 4. In the analyzed Colombian varieties, the reducing sugar content was in a range of 9.80–11.8 wb. %, which fits in values reported in Brazilian [9, 61, 62] and Indian [14, 63] CAJs. By contrast, Nigerian juice has a lower reducing sugar content (3.2 wb. %) than the other ones referenced. This suggests that modifications are necessary for the natural CAJ to improve its acceptance. During the elaboration of commercial juices, some joint operations such as filtering, dilution, or pasteurization can change the sugar concentration in the final product [8]. Conversely, the sensorial perception can be modified due to sugar reduction, as Tsitlakidou, et al. described for orange juice soft drinks [71].
Table 4.
Reducing sugars and protein content in cashew apple juice.
| Component | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
| Reducing sugars | wb. % | 9.99 ± 0.17a | Colombia (Regional 8315) | Present study |
| 10.6 ± 0.42a | Colombia (Mapiria) | Present study | ||
| 9.8–10.45 | Brazil (Commercial juices and pulps) | [9, 61, 62] | ||
| 1.76–11.8 | India (Andhra Pradesh, and others) | [14, 63] | ||
| 3.20 | Nigeria (Kosoniola farm, Oro) | [13, 64, 65] | ||
| Protein | g/100 mL | 2.54 ± 0.20a | Colombia (Regional 8315) | Present study |
| 3.37 ± 0.16b | Colombia (Mapiria) | Present study | ||
| 7.85–30∗ | India (Chatrapur, Odisha) | [72] |
Values are expressed in means ± standard deviation (n = 3).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
Commercial juices and pulps could be processed to improve the characteristics of the final product.
∗This work used enzymes to extract the components.
Regarding protein content, values of 2.54 g/100 mL and 3.30 g/100 mL were found in the Regional 8315 and Mapiria CAJs, respectively. These values are lower than Indian CAJ obtained from CA varieties harvested in Odisha. The differences are attributed to the extraction process performed by S. Abdullah et al., which was assisted by enzymes in that case. Then, proteins were extracted more efficiently; however, enzymes can increase the complexity and cost of processing. Moreover, proteins can strongly interact with bioactive compounds such as tannins. These interactions are influenced by protein characteristics (including size, amino acid composition, and posttranslational modifications) and conditions in media (pH, temperature, and solvent composition) [68]. The protein content in orange, lemon, and ginger juices (0.01–0.56 g/100 mL) [10] is under the values obtained for CAJ, studied in the present work. This last indicates that CA could be a reservoir of vegetable proteins. Though, the amino acid profile in CAJ and the protein interactions between other compounds should be studied to address the development of nutraceutical or nutraceutical products from this agricultural residue [73].
Fermented beverages and bioethanol production from CAJ are open fields for further investigations. Regarding fermentation processes to produce ethanol, reducing sugars and protein contents are important variables to consider [16, 74, 75]. It is known that sugars are transformed into ethanol by yeasts. Furthermore, the nitrogen source in the culture media must be enough to ensure yeast growth [76]. The initial sugar concentration for producing ethanol with yeast varies from 33 to 500 g/L (3.3–50 wb. %) in the culture media, and the ethanol productivity varies from 0.17 to 3.46 g/L/h [77]. These observations highlight the importance of fermentation variables such as initial sugar and protein content, yeast strain, or fermentation time in ethanol productivity. Accordingly, CAJ from Regional 8315 and Mapiria varieties could be a suitable source of sugars for alcoholic fermentation processes. Nevertheless, adjusting sugar concentration, protein content, or the addition of any other component may be necessary during fermentation to obtain value-added products such as vinegar, fermented beverages, or bio-ethanol [75].
3.2.3. Organic acids and sugars content
Organic acids are bioactive compounds required in the human diet because of their beneficial effect on health. These substances are naturally present in small quantities in plant products and lipid-rich foods [78]. Table 5 presents the organic acids profile in CAJ studied in this work. The juices of Regional 8315 and Mapiria varieties showed significant differences between the content of ascorbic, oxalic, and fumaric acids. This could be related to genetic differences between the varieties of CAs analyzed in this work. Itaconic, acetic, and lactic acids were not detected in any of the samples analyzed.
Table 5.
Organic acids profile and non-structural sugars quantification in juice.
| Compound | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
|
Organic acids |
mg/100 mL |
|||
| Ascorbic acid | 51.8 ± 1.82a | Colombia (Regional 8315) | Present study | |
| 70.5 ± 3.03b | Colombia (Mapiria) | Present study | ||
| 49.3 ± 2.05 | Brazil (commercial) | [12] | ||
| 63.9 ± 2.20 | Brazil (commercial, Kipolpa ®) | [87] | ||
| 79–256 | India (Chatrapur, Odisha) | [72] | ||
| Oxalic acid | 9.88 ± 0.23a | Colombia (Regional 8315) | Present study | |
| 16.1 ± 0.45b | Colombia (Mapiria) | Present study | ||
| Tartaric acid | 67.9 ± 7.98a | Colombia (Regional 8315) | Present study | |
| 75.4 ± 14.9a | Colombia (Mapiria) | Present study | ||
| Citric acid | 53.3 ± 2.47a | Colombia (Regional 8315) | Present study | |
| 56.6 ± 3.46a | Colombia (Mapiria) | Present study | ||
| Fumaric acid | 18.2 ± 0.18a | Colombia (Regional 8315) | Present study | |
| 12.1 ± 0.30b |
Colombia (Mapiria) |
Present study |
||
|
Sugars |
g/L |
|||
| Glucose | 45.1 ± 0.29a | Colombia (Regional 8315) | Present study | |
| 45.6 ± 0.32a | Colombia (Mapiria) | Present study | ||
| 36.5 | Brazil (clarified) | [88] | ||
| Fructose | 13.9 ± 1.14a | Colombia (Regional 8315) | Present study | |
| 15.4 ± 0.56a | Colombia (Mapiria) | Present study |
Values are expressed in means ± standard deviation (n = 5).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
Itaconic, acetic, glyoxylic, and lactic acids were not detected.
Commercial juices and pulps could be processed to improve the characteristics of the final product.
One of the most important organic acids is ascorbic acid, also called vitamin C. This compound is considered an effective natural antioxidant, which is necessary for the normal function of the human body. Also, it is involved in physiological functions such as catabolism and reduction of oxidative stress in cells [79, 80, 81]. Some studies have suggested that a daily intake of 90 mg of ascorbic acid can reduce the incidence of respiratory diseases and other illnesses in men and women [82]. Ascorbic acid content observed in the juice from Mapiria variety (70.5 ± 3.03 mg/100 mL of juice) exhibited a higher value than Regional 8315 variety (51.8 ± 1.82 mg/100 mL of juice). Compared to commercial references of CAJs from Brazil, they showed ascorbic acid values between 44 to 50 mg/100 mL of juice.
In contrast, the ascorbic acid content of CAJ and pulps from India can be found in a range of 70–250 mg/100 mL of sample. This last can be related to the varieties cultivated in this country, the maturation state of CA, the storage conditions, and the processing of the apples [83]. Hence, CA is an agricultural residue rich in ascorbic acid that should be more studied for addressing nutraceutical and pharmacological applications.
Oxalic acid was identified in Regional 8315 (9.88 ± 0.23 mg/100 mL of juice) and Mapiria (16.1 ± 0.45 mg/100 mL of juice) juices. This compound is an important metabolic product of plants because it possesses important biological functions. Accordingly, oxalic acid plays an important role in living organisms, such as regulating calcium concentration in plant tissues, heavy metal tolerance [83], and reducing chilling injury in vegetables [84]. Tartaric acid was identified in Regional 8315 (67.9 ± 7.98 mg/100 mL of juice) and Mapiria (75.4 ± 14.9 mg/100 mL of juice) juices, with values of tartaric acid statistically similar between both varieties of CA. This compound was identified as the representative organic acid with the higher concentration in the analyzed samples of CAJ. In grape berries, tartaric acid is also the main organic acid [85], similar to the CA evaluated in the present work. Also, citric acid was identified in Regional 8315 (53.3 ± 2.47 mg/100 mL of juice) and Mapiria (56.6 ± 3.46 mg/100 mL of juice) juices. Citric acid is an acidulant agent in fruit beverages [85] and is commonly found in citric fruits. The fumaric acid in CAJs was found in 12–18 mg/100 mL of sample. This organic acid is used as a food preserving agent because it has strong bactericidal activity due to its low pKa values and it is considered safe by the U.S. FDA [86].
Bioactive compounds have pharmacologic properties such as antioxidant effects and inhibition or induction of specific gene expressions [89]. Consequently, these compounds have shown positive effects on human health. It has been proved that they can diminish the incidence of diseases, such as cancer, heart disease, diabetes, and age-related functional decadence [90, 91, 92].
Regarding non-structural sugars, glucose and fructose were found in the juices of Regional 8315 (45.1 ± 0.29 g/L and 13.9 ± 1.14 g/L, respectively) and Mapiria (45.6 ± 0.32 g/L and 15.4 ± 0.56 g/L respectively) CA varieties. These values are in concordance with the concentration of reducing sugars reported in the previous section. Comparing these values with a clarified Brazilian commercial CAJ [88], it can be seen that natural juices present higher glucose values. Clarification of natural juices can reduce the content of solids, including sugars, which can modify the flavor characteristics of the juice [93].
3.2.4. Total phenolic, total tannin, and total carotenoid content
Table 6 summarizes the results of phenolic compounds identified in the CAJ varieties. In the analyzed CAJs, the tannin content was 178 and 191 mg/100 mL of juice. Statistical differences between both varieties of CA were identified. The differences found can be attributed to each type of CA [68] since the higher tannin content was observed in the juice of the Mapiria variety (yellow apple). The total phenolic content for Regional 8315 and Mapiria juices were 190 and 196 mg/100 mL, respectively. Contrary, carotenoid concentration was not detected under the conditions of the assay. Even though carotenoids were detected in the qualitative analysis of CAJ, the carotenoid content was below the quantification limit of the analytical technique (<0.5 μg/mL).
Table 6.
Phenolic compounds in cashew apple juice.
| Compound | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
| Phenolics | ||||
| Tannins | mg TA/100 mL | 178 ± 7.08a | Colombia (Regional 8315) | Present study |
| 191 ± 1.66b | Colombia (Mapiria) | Present study | ||
| 12.2–12.4 | Thailand | [93] | ||
| 270 | Brazil (natural juice) | [94] | ||
| Carotenoids | μg/100 mL | N.D. | Colombia (Regional 8315) | Present study |
| N.D. | Colombia (Mapiria) | Present study | ||
| Total phenols | mg GAE/100 mL | 190 ± 9.02a | Colombia (Regional 8315) | Present study |
| 196 ± 5.72a | Colombia (Mapiria) | Present study | ||
| 111–245 | India (Chatrapur, Odisha) | [72] | ||
| 12.2–12.4 | Thailand | [93] | ||
Values are expressed in means ± standard deviation (n = 3).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
TA: Tannic acid, GAE: Gallic Acid Equivalents. N.D.: Not Detected (under assay conditions).
Comparing the natural juices with commercial juices from Thailand or India, it is evident that the content of tannins is lower in commercial products. The tannins and total phenolic content were similar between the CAJs of Regional 8315 and Mapiria varieties, suggesting that tannins are the primary type of phenolics in the sample. Concerning tannins, their presence in juices is associated with astringency [95]. Also, they could inhibit the growth of microorganisms during fermentation [96]. Therefore, processes such as clarification can remove these components that interfere with fermentation [97].
3.2.5. Mineral content
Minerals such as Cu, Fe, and Zn are essential elements for metabolic processes due to their interaction with enzymes, defined as natural catalysts for cells. The deficiency of some minerals in the human body causes anemia, deficiencies in fetal and infant development, dermatitis, and growth retardation [98]. Table 7 presents the mineral content measured in CAJ. Zn, Cu, and Fe contents in the CAJ were 0.413, 0.218, 0.263 mg/L, and 0.462, 0.358, 0.378 for Regional 8315 and Mapiria varieties, respectively. Compared to a commercial juice from Brazil, these values are lower, being the Brazilian ones in a 1.8–11 mg/L range. These differences can be attributed to the variety of the CA, cultivation conditions, juice extraction processes, and modification during the commercial juice elaboration.
Table 7.
Mineral content in cashew apple juice.
| Compound | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
| Minerals | ||||
| Zinc (Zn) | mg/L Zn | 0.413 | Colombia (Regional 8315) | Present study |
| 0.462 | Colombia (Mapiria) | Present study | ||
| 4.70 ± 0.31 | Brazil (commercial) | [12] | ||
| 11.20 | Brazil | [94] | ||
| Copper (Cu) | mg/L Cu | 0.218 | Colombia (Regional 8315) | Present study |
| 0.358 | Colombia (Mapiria) | Present study | ||
| 2.10 ± 0.14 | Brazil (commercial) | [12] | ||
| L.D. | Brazil | [94] | ||
| Iron (Fe) | mg/L Fe | 0.263 | Colombia (Regional 8315) | Present study |
| 0.378 | Colombia (Mapiria) | Present study | ||
| 1.82 ± 0.12 | Brazil (commercial) | [12] | ||
L.D.: below the detection limit of the analytical method.
In some fermentation processes with white-rot fungi, the content of Cu in the culture media is essential for synthesizing enzymes such as laccases and other oxidases [99]. These enzymes can promote the degradation of phenolic compounds such as lignin, tannins, and other complex molecules [96]. Therefore, juices obtained from CA can be a suitable substrate for white-rot fungi cultivation, which could be a novel research field of investigation in biotechnological applications such as fungal biomass [99], and enzyme production [100], and bioremediation [101].
3.3. Cashew apple bagasse characterization
The CAB corresponds to about 10 wt.% of the whole weight of CA. This is the by-product obtained after the juice extraction. The chemical characterization of CA is important to find interesting and sustainable ways to use this biomass in further valorization routes.
3.3.1. Organic acids content
Table 8 summarizes the results for organic acid characterization in CAB. Ascorbic, citric, and lactic acids were the principal organic acids measured in the CAB of Regional 8315 and Mapiria varieties. The ascorbic content of Mapiria (204 ± 4.26 mg/100 g) was statistically different from Regional 8315 (188 ± 3.81 mg/100 g). These results were in concordance with the observations in CAJ, mentioned and discussed previously. Compared to CAB from Brazilian investigations, the content of ascorbic acid is variable between the commercial or industrialized products (range of 20–900 mg/100 g) [102,103]. This can be attributed to transformation processes made on the pulps that contain the dietary fibers of CAB.
Table 8.
Organic acids in cashew apple bagasse and pulps.
| Component | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
|
Organic acids |
mg/100 g |
|||
| Ascorbic acid | 188 ± 3.81a | Colombia (Regional 8315) | Present study | |
| 204 ± 4.26b | Colombia (Mapiria) | Present study | ||
| N.D. | Brazil (commercial) | [12] | ||
| 901 ± 74.8 | Brazil (artisanal) | [15] | ||
| 20.3 ± 0.02 | Brazil (industrialized) | [15] | ||
| 109–115 | Brazil | [102] | ||
| 189–200 | Brazil (Ceará, commercial) | [104] | ||
| Oxalic acid | 64.2 ± 1.74a | Colombia (Regional 8315) | Present study | |
| 74.9 ± 2.69b | Colombia (Mapiria) | Present study | ||
| Citric acid | 698 ± 60.6a | Colombia (Regional 8315) | Present study | |
| 1385 ± 269.8b | Colombia (Mapiria) | Present study | ||
| Fumaric acid | 58.2 ± 0.38a | Colombia (Regional 8315) | Present study | |
| 28.5 ± 1.03b | Colombia (Mapiria) | Present study | ||
| Lactic acid | 5177 ± 536.1a | Colombia (Regional 8315) | Present study | |
| 4803 ± 370.8a | Colombia (Mapiria) | Present study |
Values are expressed in means ± standard deviation (n = 5).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
Itaconic, acetic, glyoxylic, and tartaric acids were not detected.
The concentration of organic acids and phenolics is reported per 100 g of dry bagasse.
Commercial cashew apple bagasse and pulps could be processed to improve the characteristics of the final product.
Furthermore, the concentrations of oxalic acid from Colombian CAB varieties were statistically different, being Mapiria (74.9 ± 2.69 mg/100 g) the higher one. The CA processing could be associated with these variations since the artisanal pulps present a higher content of ascorbic acid than industrialized ones. It is believed that oxalic acid improves the vegetable membrane integrity due to its antioxidant activity, which increases the storage life in post harvested fruits. Therefore, oxalic acid has been studied as an effective additive that reduces chilling injury in harvested mangoes [105] and peaches [106]. These observations suggest that CA could be resistant to chilling injury, a helpful attribute in the cold preservation chain of this fruit. Then, the oxalic acid of CA could have a protective effect against chilling injury during freezing storage, especially in CA of Mapiria variety.
Similarly, the citric acid content in CAB was found higher in the Mapiria variety (1385 ± 269.8 mg/100 g). Other studies measuring the citric acid content in CAB were not found at the moment of writing this article. The content of citric acid that was found in this work is comparable to fruits with high content, such as the baobab (Adansonia digitata) fruit (3345 mg/100 g) [105] and guava (Psidium guajava) (2120 mg/100 g) [106].
In addition, fumaric acid content was statistically different between the analyzed samples of CAB, with the Regional 8315 (58.2 ± 0.38 mg/100g) being the one with higher content. Tartaric acid was the organic acid with the lowest concentration in the analyzed bagasse samples. Contrary, in Colombian CAB samples, lactic acid was the representative organic acid, valued between 4500 and 5000 mg/100 g. This result could be related to a fermentation process mediated by bacteria that occurs in the last maturation state of the CA. During fermentation, malic acid is transformed into lactic acid [107]. The natural microbiota in CA before the freezing storage can promote the transformation of bioactive compounds. This unavoidable effect is also observed in wines and grapes [108].
3.3.2. Total phenolic, total tannin, and carotenoid content in cashew apple bagasse
Table 9 presents the results for total phenolic content measurements in bagasse varieties studied in this work. The total phenolic content in CAB was 293 ± 2.84 mg GAE/100 g and 265 ± 3.88 mg GAE/100 g for Regional 8315 and Mapiria varieties, respectively. Similarly, as observed in CAJ, the content of total phenols and total tannins were similar, indicating that tannins are the representative phenolics in the samples. The Brazilian references of CA pulps have a lower content of total phenolic compounds, especially in the industrialized one. Compared to other exotic plants cultivated in Colombia, the values of total phenolics content found in Colombian varieties are higher than pulps or edible part of borojo (Borojoa patinoi) (41.8 ± 1.54 mg/100 g), peach tomato (70.9 ± 2.57 mg/100 g), guava (192 ± 11.5 mg/100 g), and American oil palm (Elaeis oleifera) (80.5 ± 3.44 mg/100 g) [109].
Table 9.
Phenolic compounds in cashew apple bagasse.
| Component | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
|
Phenolics | ||||
| Tannins | mg TA/100 g | 299 ± 3.09a | Colombia (Regional 8315) | Present study |
| 288 ± 4.50b | Colombia (Mapiria) | Present study | ||
| Carotenoids | μg/100 g | 1.71 ± 0.28a | Colombia (Regional 8315) | Present study |
| 4.72 ± 0.60b | Colombia (Mapiria) | Present study | ||
| 5.24 ± 0.37 | Brazil (artisanal) | [15] | ||
| 5.27 ± 0.04 | Brazil (industrialized) | [15] | ||
| Total phenols | mg GAE/100 g | 293 ± 2.84a | Colombia (Regional 8315) | Present study |
| 265 ± 3.88b | Colombia (Mapiria) | Present study | ||
| 97.4 ± 6.65 | Brazil (artisanal) | [15] | ||
| 49.8 ± 0.22 | Brazil (industrialized) | [15] | ||
| 118 ± 3.70 | Brazil (Pacajus) | [110] | ||
Values are expressed in means ± standard deviation (n = 3).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
TA: Tannic acid, GAE: Gallic Acid Equivalents. N.D.: Not Detected (under assay conditions).
In addition, carotenoids were identified in a concentration of 1.71 ± 0.28 and 4.72 ± 0.60 μg/100 g for Regional 8315 and Mapiria varieties, respectively. Compared to Brazilian pulps, the carotenoid content of Colombian varieties was lower. This result can be attributed to genotype diversity in cashew trees cultivated in each country [95].
3.3.3. Mineral content
The results of the mineral analysis in CAB are summarized in Table 10. Zn content in both varieties of CAB was about 9.5 mg/kg. The content of Zn in CAB was higher in comparison to other exotic fruits such as passion fruit pulp (3 mg/kg) [111] and lower in comparison to exotic fruits from Africa (range of 17–191 mg/kg) [112]. The Cu content in the Regional 8315 CAB variety (33 mg/kg) was higher than in the Mapiria variety (26 mg/kg). Compared to the Cu content of orange (0.6 mg/kg), pomelo (0.5 mg/kg), and lemon (0.4 mg/kg) pulps [112], with the observed in the varieties analyzed in the present work is much higher. Regarding Fe content, it was below the detection limit of the analytical technique.
Table 10.
Mineral content in cashew apple bagasse from Colombian varieties.
| Component | Unit | Value | Country (details) | Reference |
|---|---|---|---|---|
|
Minerals | ||||
| Zinc (Zn) | mg/kg Zn dry basis | 9.41 | Colombia (Regional 8315) | Present study |
| 9.87 | Colombia (Mapiria) | Present study | ||
| Copper (Cu) | mg/kg Cu dry basis | 33.9 | Colombia (Regional 8315) | Present study |
| 26.1 | Colombia (Mapiria) | Present study | ||
| Iron (Fe) | mg/kg Fe dry basis | <59.5 | Colombia (Regional 8315) | Present study |
| <59.5 | Colombia (Mapiria) | Present study | ||
The mineral content that can be found in vegetable biomass depends on the cultivation conditions, minerals in the soil and chemical products applied during the growing process of the plant [111].
3.3.4. Chemical characterization of CAB
The chemical composition of CAB is presented in Table 11. It is important to note that CAB contains around 7–7.5 wt. % of extractive content that remains after juice extraction. Compared to reported CAB characterizations, the extractive content measured in Colombian CAB varieties fits in the range of 6.5–12 wt. %. The content of extractives found for the Regional 8315 (7.14 ± 0.51%) and Mapiria (7.52 ± 0.28%) varieties are comparable to the data reported by Correia et al. [113] and Silva et al. (7.3 ± 0.6%) [114]. Also, the values obtained in the present work are slightly higher than those reported by de Araujo Padilha et al. (6.5 ± 0.2%) [115], which can be attributed to differences between the varieties. As Restrepo-Serna et al. reported, the extractive fraction can contain bioactive compounds with antioxidant effects [116]. Nevertheless, the extractive content varies depending on the plant species, environmental conditions, and specific parts. For instance, mango peel contains around 5–10 wt. % of extractives [117], and mandarin peel about 38 wt. % [118]. Therefore, extractive fraction should be analyzed in future investigations to find compounds that can still be further valorized or removed if the valorization of the remaining substances present in CAB is desired.
Table 11.
Chemical composition of cashew apple bagasse.
| Component (wt.%) ∗ | Present work | References |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [113, 114] | [115] | [119] | [120] | [120] | [11] | [11] | [11] | |||
| Variety | Regional 8315 | Mapiria | NR | NR | NR | NR | NR | Red | Yellow | Mixture |
| Total solids | 27.7 ± 0.54 | 31.2 ± 3.90 | NR | NR | NR | NR | NR | 90.7 ± 0.03 | 90.9 ± 0.06 | 91.7 ± 0.01 |
| Extractives | 7.14 ± 0.51 | 7.52 ± 0.28 | 7.39 ± 0.60 | 6.5 ± 0.2 | NR | NR | NR | 10.4 ± 0.05∗∗ | 12.0 ± 0.23∗∗ | 7.59 ± 0.37∗∗ |
| Ash | 1.510 ± 0.008 | 1.480 ± 0.016 | 1.62 ± 0.20 | NR | NR | NR | NR | 2.22 ± 0.06 | 2.20 ± 0.09 | 3.71 ± 0.11 |
| Cellulose | 25.3 ± 0.26 | 26.6 ± 0.50 | 20.5 ± 2.20 | 34.7 ± 0.20 | 18.3 ± 0.07 | 20.5 ± 0.70 | 19.2 ± 0.35 | 18.0 ± 0.61 | 19.9 ± 0.36 | 19.1 ± 0.30 |
| Hemicellulose | 28.4 ± 0.27 | 26.8 ± 0.54 | 16.3 ± 0.90 | 17.6 ± 0.20 | 27.1 ± 0.01 | NR | 12.0 ± 0.37 | 51.6 ± 0.11 | 51.1 ± 0.18 | 41.1 ± 0.24 |
| Lignin | 15.2 ± 1.22 | 18.2 ± 1.40 | 35.2 ± 0.90 | 41.4 ± 3.20 | 23.3 ± 0.02 | 33.8 ± 1.30 | 38.1 ± 0.08 | 4.27 ± 0.41 | 3.65 ± 0.24 | 4.86 ± 0.23 |
| Protein | 8.69 ± 0.05 | 9.13 ± 0.05 | NR | NR | NR | NR | NR | 16.8 ± 0.05 | 18.2 ± 0.02 | 16.3 ± 0.08 |
| Pectin | 11.2 ± 2.58 | 10.3 ± 1.80 | NR | NR | NR | NR | NR | 10.1 ± 0.48 | 8.56 ± 0.39 | 9.28 ± 0.77 |
| Total | 97.6 ± 4.89 | 100 ± 4.57 | 73.3 ± 3.08 | 100 ± 3.08 | 68 ± 0.1 | 54.3 ± 2.00 | 69.3 ± 0.80 | 113 ± 1.77 | 115.±1.51 | 102 ± 2.10 |
Values are expressed in means ± standard deviation.
∗In dry weight basis, ∗∗Lipids, NR: Not Reported.
Ash content was approximately 1.50 wt. % and 1.48 wt. % in Regional 8315 and Mapiria varieties of CAB. Ashes are the least abundant component in the chemical composition of the analyzed bagasse. These values are in the range reported for other varieties, which is lower than 3 wt. %. Regarding the chemical composition of ashes, this fraction can contain minerals such as potassium, aluminum, silicon, and magnesium [121].
Concerning structural carbohydrates, the cellulose and hemicellulose content in the bagasse is around 52 to 54 wt. %. Compared to other varieties of CAB summarized in Table 11, the reports of structural carbohydrate fraction vary in a range of 20–60 wt. %. These variations could be attributed to genetic differences, cultivation conditions, and even owed to the analytical techniques used. The relevance of this fraction is due it can be hydrolyzed into fermentable sugars to produce ethanol [120], xylitol [122], and organic acids [123], among other value-added chemicals. Furthermore, this carbohydrate-rich biomass could be used for biogas production through anaerobic digestion [124].
Regarding the lignin content of CAB (around 15 to 18 wt. %), it is a substance that confers recalcitrance [125]. The lignin content found in the present study is slightly lower than the one reported by dos Santos Lima et al. (∼23 wt. %) [119]. Lignin can also be valorized and further used for obtaining substances such as vanillin, vanillin acid, mucolactone, veratric acid, and others [126].
The protein content in Colombian CAB (8.69 ± 0.05 and 9.13 ± 0.05 wt.%) was lower than reported by E.K.A. Kouassi et al. [11]. These variations may be attributed to differences in the varieties of each CAB. Nevertheless, since protein content was not measured in the works of Correia et al. [113], Silva et al. [114], de Araujo Padilha et al. [115], Rodrigues et al. [120], and dos Santos Lima et al. [119], there is limited information on the protein content of CAB. The determination of the protein profile in CAB is an open field of investigation. The content of pectin obtained in our study for the Regional 8315 (11.22 ± 2.58%) and Mapiria (10.31 ± 1.80%) varieties is similar to the one reported by Kouassi et al. (∼8–10%) [11]. Pectin is widely used in the formulation of products in the pharmaceutical, cosmetic, and food sectors [127]. Then, this compound could be recovered to be further valorized. Interestingly, the mass closure of all identified components in this work was around 100 wt. %. Therefore, the analyses performed were able to explain the complete composition of the bagasse. This methodology of quantification that included HPLC could be used as a reference for vegetable biomass characterization.
3.3.5. Thermal decomposition of CAB
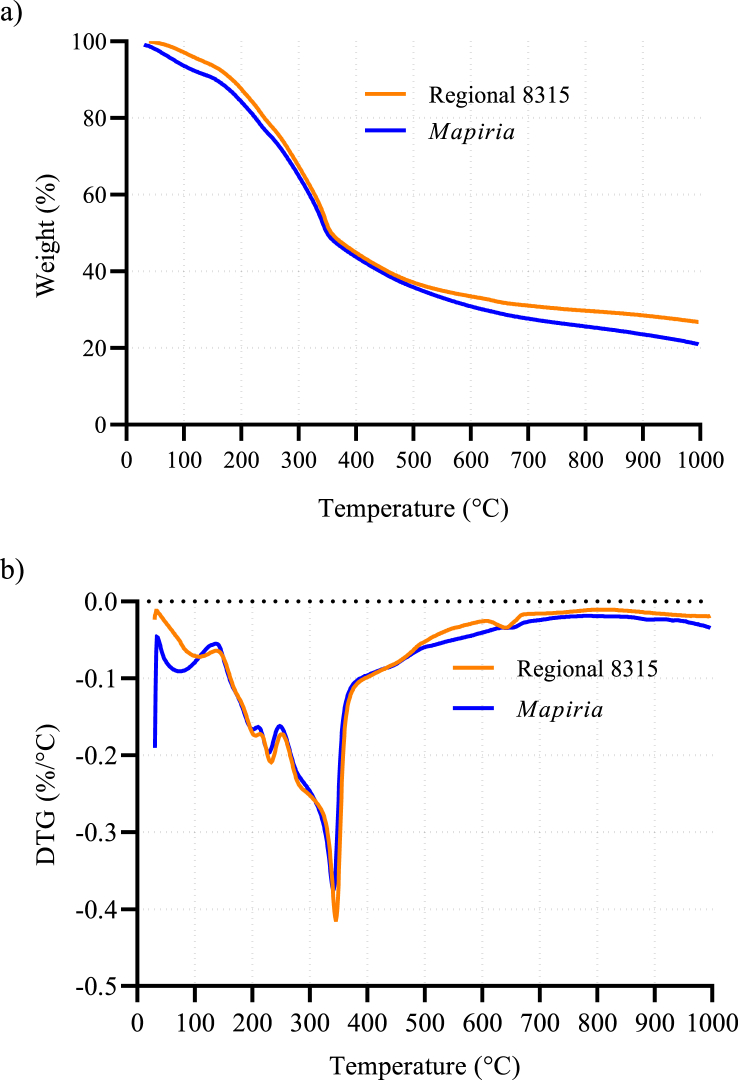
The TG curve of the CAB is presented in Figure 2 a. In both varies of CAB, the TG curves were similar in number and occurrence of weight losses. The first loss step observed in the TG curve occurred around 100 °C, with about 5% and 9% of the total weight for Regional 8315 and Mapiria varieties, respectively. This loss is attributable to the moisture evaporation that remains in the sample. The second important weight loss was observed in a range of 150 °C–250 °C with a loss of around 20% of total initial weight for both varieties. The loss of approximately 50% of the total weight occurred near 350 °C in both varieties of CAB. Moreover, a remanent of around 20% of the total weight was observed after 500 °C. The DTG curves are shown in Figure 2 b. Regarding the thermal decomposition of CAB, it occurred in steps that can be confirmed in the TG curve. In the first step of mass loss, the moisture and highly volatile compounds were lost up to a temperature of around 150 °C [128]. This moisture loss peak reported in the present study is in concordance with the one reported by Ticiane C. de Souza et al. [129], also performed in CAB. The study of Ticiane C. de Souza et al. was carried out until 200 °C. Therefore, the comparison beyond this temperature is not available. Neither any additional reported data of TG/DTG of this biomass was available at the moment of writing this article.
Figure 2.
Thermal analysis of Regional 8315 (orange line) and Mapiria (blue line) varieties of cashew apple bagasse, a) TG curves, b) DTG curves.
In CAB, the first decomposition step was observed at temperatures in a range of 180 °C–250 °C. In this temperature range, pectin and hemicellulose are typically lost [130]. The DTG curves also showed a thick peak in this range of temperature. This last indicates a decomposition of various substances which is in concordance with the chemical composition of CAB. The second step of decomposition occurred at temperatures between 250 °C to 400 °C. This peak is related to cellulose and lignin degradation [128].
Additionally, the decomposition behavior under inert atmosphere conditions corresponds to a pyrolysis process [131]. The complexity of this biomass can be reduced with this process. After thermal decomposition, about 20 wt.% of CAB corresponded to a solid pyrolytic product, which could be a rich-carbon solid obtained from CAB. This pyrolytic product could have high adsorption properties, which can be suitable in a wide range of sustainable applications, for instance, increasing nutrient availability in soils [132].
Finally, the thermal degradation of constitutive components in CAB from Vichada's varieties was observed at 200 °C. The thermal decomposition in agricultural residues is an important parameter in further valorization processes. For instance, some treatments where biomass transformation requires high temperatures are i) cooking operations [15], ii) manufacturing of materials [128], iii) thermochemical pretreatments [133], and iv) pyrolysis [134].
3.4. Antioxidant activity
Table 12 presents the results of antioxidant activity. Regarding natural juices studied in this work, the Regional 8315 and Mapiria varieties presented similar values of antioxidant activity in ABTS (around 7 TEs/mL) and DPPH (around 2.5 TEs/mL) assays. The ABTS antioxidant activity reported by A.C.S. De Lima et. al was 0.018 TEs/mL in a commercial juice [12]. These differences could be attributed to clarification processes performed in commercial juices that can reduce the availability of bioactive compounds [9]. The antioxidant effect in edible products and fruits is related to the presence of polyphenols and vitamins [135]. Also, the interaction of these compounds with additives usually added to edible products, can alter the polyphenol availability and, therefore, the antioxidant effect [136]. Regarding the antioxidant activity of CAB, the ABTS assay showed results (around 7–8 TEs/g) of the same magnitude as the ones observed in juices. Contrary, the antioxidant activity measured with the DPHH method was observed in a range of 335–450 TEs/g.
Table 12.
Antioxidant activity of cashew apple juice and cashew apple bagasse.
| Variety origin | Antioxidant activity |
Reference | ||
|---|---|---|---|---|
| ABTS | DPPH | |||
| Cashew apple juice1 | Colombia (Regional 8315) | 7.83 ± 0.80a | 2.96 ± 0.34a | Present study |
| Colombia (Mapiria) | 7.26 ± 0.77a | 2.83 ± 0.85a | Present study | |
| Brazil (commercial) | 0.018 | N.R. | [12] | |
| Brazil (Fortaleza) | 1.73 | 4.07 | [137] | |
| Cashew apple bagasse2 | Colombia (Regional 8315) | 8.06 ± 0.64a | 335 ± 3.75a | Present study |
| Colombia (Mapiria) | 7.17 ± 0.10b | 405 ± 19.4b | Present study | |
| Brazil (commercial) | N.D. | N.R. | [12] | |
| Brazil (artisanal) | 0.049 | 0.109 | [15] | |
| Brazil (industrialized) | 0.032 | 0.031 | [15] | |
Values are expressed in means ± standard deviation (n = 4).
Data with the same letter are similar statistically in the Tukey test (p < 0.05).
TEs = Trolox Equivalents (mmol Trolox).
N.R.: Not Reported. N.D.: Not Detected.
Cashew apple juice antioxidant activity expressed as TEs/mL of juice.
Cashew apple bagasse antioxidant activity expressed as TEs/g of bagasse on a dry basis.
Concerning the differences observed in antioxidant activities in Colombian CAB, it could be explained based on the oxidation mechanisms for each radical for ABTS and DPPH methods. The radical DPPH accepts electrons or hydrogen radicals from donors [138]. In contrast, the ABTS radical defines the scavenging of antioxidant ability by the reaction of a strong antioxidant agent. Then, the ABTS method is based on inhibiting the radical by one-electron oxidants [139]. Therefore, the antioxidant activity is related to the type and properties of the polyphenolics, which can interact differently with free radicals, as reported by Villaño et al. [140].
Additionally, compared to antioxidant activities of commercial pulps from Brazil (range of 0.03–0.1 TEs/g) [15], the differences were found in three magnitude order below of values measured in CAB from Vichada. This was observed for both DPPH and ABTS methods. Similar differences were observed in CAJ, which can be related to transformation processes that were performed in natural pulps.
The antioxidant activity in exotic fruits from Colombia was studied by José Contreras-Calderón et al. [141]. They reported that banana passion fruit (144 ± 1.88 μmol TEs/g), arrayana (Citrus reticulata) (48.8 ± 1.40 μmol TEs/g), and cashew fruit (collected in Cúcuta, Colombia) (115 ± 15.2 μmol TEs/g) presented the higher antioxidant activities among other fruits. Compared to the measurements obtained in this work, the antioxidant activities for Colombian varieties of CA were quite higher (antioxidant activities in this work were expressed in mmol of Trolox). This last indicates that the varieties of CA harvested in Vichada have high antioxidant activities, which could be related to the presence of bioactive compounds that can be easily recovered from this biomass, such as ascorbic acid, tannins, and carotenes.
4. Perspectives and future work
The results of the investigation with Regional 8315 and Mapiria varieties of cashew apples that were harvested in Vichada allowed identifying the potential for the valorization of this agricultural residue. The perspectives can be summarized as follows:
-
i)
The CAJ is a rich natural juice that can be obtained at a low price and can be used in beverage formulations. The high concentration of bioactive compounds related to the antioxidant activities of edible juice could have beneficial effects on human health, and today, this potential is not fully exploited in Colombia. Nevertheless, the identification of the substances responsible for the high tannin and alkaloid content in CAJ presents a challenge in the formulation of value-added products. Therefore, more studies should be performed to characterize and quantify these components.
-
ii)
The CAB is rich in bioactive compounds, carbohydrates, and pectin. It is a renewable source of valuable compounds that can be used in multiple applications. Though, the protein profile and fatty acid composition of CAB should be studied in detail in the upcoming work.
-
iii)
Under inert thermal decomposition or pyrolysis, the TGA showed that near 20 wt.% of CAB is not decomposed (at 1000 °C). Pyrolysis is used to produce biochar as a solid product [142]. Therefore, the production of adsorbent biochar from CAB is an open field of investigation.
-
iv)
Efficient and selective extractions of bioactive compounds from residual biomass are an innovative trend. Novel and sustainable extraction technologies such as supercritical fluid extraction [141], pressurized fluid extraction [142], and ultrasound-assisted extraction [143], can be used for selective extraction of a diverse range of bioactive compounds for food, nutraceuticals, and pharmacologic applications. Then, the extraction and quantification of organic acids, fatty acids, and alkaloids, which are still unknown in cashew residues using advanced and novel techniques, should be further investigated.
5. Conclusions
This work established the first exploratory and rigorous characterization of Colombian cashew apples harvested in Vichada. This agricultural residue was demonstrated to be a renewable and valuable reservoir of bioactive compounds. Additionally, pharmacological applications of this residue can be addressed due to its high antioxidant activity. Alkaloid identification in cashew apple revealed a challenge that requires further analysis to characterize the alkaloids in this biomass. The solid fraction of cashew apple is composed mainly of carbohydrates and pectin, which can be used in multiple industrial applications. Therefore, the cashew apple is a promissory and renewable feedstock that can be valorized to produce a wide range of value-added products. Nevertheless, regarding edible products from the cashew apple, the integration of technologies that ensure the safety of food and nutraceutical products must be considered in the valorization routes. It is important to avoid toxic or allergic reactions in people due to the consumption of artisanal or industrial products derived from the cashew apple owed to the presence of tannins, alkaloids, and unknown substances.
Declarations
Author contribution statement
Luis J. Cruz Reina, Chiara Carazzone, Rocío Sierra: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Daniel David Durán-Aranguren: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Laura Fernanda Forero-Rojas, Luisa Fernanda Tarapuez-Viveros: Performed the experiments.
Dinary Durán-Sequeda: Conceived and designed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by Ministerio de Ciencia, Tecnología e Inovación (MinCiencias) and Gobernación del Vichada (CO) (Bicentennial Doctoral Excellence Scholarship Program), and by Centro de Estudios Sobre Desarrollo Económico (CEDE) Facultad de Economía Universidad de los Andes and Centro de Investigación y Fomento Estudiantil (CIFE) Universidad de los Andes (CO) (Contest for research projects for doctoral students CEDE-CIFE fund 2021).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Nair K.P.P. Agron. Econ. Important Tree Crop. Dev. World. Elsevier; 2010. Cashew nut (anacardium occidentale L.) pp. 21–66. [Google Scholar]
- 2.The O. 2019. Observatory of Economic Complexity, Cashew Nuts, Fresh or Dried (HS: 080130) Product Trade, Exporters and Importers.https://oec.world/en/profile/hs92/cashew-nuts-fresh-or-dried [Google Scholar]
- 3.Assunção R.B., Mercadante A.Z. Carotenoids and ascorbic acid from cashew apple (Anacardium occidentale L.): variety and geographic effects. Food Chem. 2003;81:495–502. [Google Scholar]
- 4.Fontanilla-Díaz C.A., Preckel P.V., Lowenberg-DeBoer J., Sanders J., Peña-Lévano L.M. Identifying profitable activities on the frontier: the Altillanura of Colombia. Agric. Syst. 2021;192:103199. [Google Scholar]
- 5.Sharma P., Gaur V.K., Sirohi R., Larroche C., Kim S.H., Pandey A. Valorization of cashew nut processing residues for industrial applications. Ind. Crop. Prod. 2020;152:112550. [Google Scholar]
- 6.Das I., Arora A. Post-harvest processing technology for cashew apple – a review. J. Food Eng. 2017;194:87–98. [Google Scholar]
- 7.F. and A.O. of the U. Nations . 2018. Resources | Family Farming Knowledge Platform.https://www.fao.org/family-farming/resources/en/?freetext=cashew+&year=2018&country_iso3=&ncl=&main_topic=&sub_topic=&related=&tx_dynafef_search=1&rec_uid=&act_Submit=Submit&form_build_id=form-d20139f97f332a3d1530f9616ceee091dd45514bdf474b0fa82c90b95bae60 [Google Scholar]
- 8.Emmanuelle D., Joseph D., Victor A., Mohamed M.S. A review of cashew (Anacardiumoccidentale L.) apple: effects of processing techniques, properties and quality of juice. Afr. J. Biotechnol. 2016;15:2637–2648. [Google Scholar]
- 9.Campos D.C.P., Santos A.S., Wolkoff D.B., Matta V.M., Cabral L.M.C., Couri S. Cashew apple juice stabilization by microfiltration. Desalination. 2002;148:61–65. [Google Scholar]
- 10.Tiencheu B., Nji D.N., Achidi A.U., Egbe A.C., Tenyang N., Tiepma Ngongang E.F., Djikeng F.T., Fossi B.T. Nutritional, sensory, physico-chemical, phytochemical, microbiological and shelf-life studies of natural fruit juice formulated from orange (Citrus sinensis), lemon (Citrus limon), Honey and Ginger (Zingiber officinale) Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e07177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kouassi E.K.A., Soro Y., Vaca-Garcia C., Yao K.B. Chemical composition and specific lipids profile of the cashew apple bagasse. Rasayan J. Chem. 2018;11:386–391. [Google Scholar]
- 12.De Lima A.C.S., Soares D.J., Da Silva L.M.R., De Figueiredo R.W., De Sousa P.H.M., De Abreu Menezes E. In vitro bioaccessibility of copper, iron, zinc and antioxidant compounds of whole cashew apple juice and cashew apple fibre (Anacardium occidentale L.) following simulated gastro-intestinal digestion. Food Chem. 2014;161:142–147. doi: 10.1016/j.foodchem.2014.03.123. [DOI] [PubMed] [Google Scholar]
- 13.Deenanath E.D., Rumbold K., Daramola M., Falcon R., Iyuke S. Evaluation of physicochemical properties of South African cashew apple juice as a biofuel feedstock. Scientifica. 2015;2015(Cairo):1–9. doi: 10.1155/2015/764196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohanty S., Ray P., Swain M.R., Ray R.C. Fermentation of cashew (anacardium occidentale L.) “apple” into wine. J. Food Process. Preserv. 2006;30:314–322. [Google Scholar]
- 15.Sucupira N.R., Sabino L.B. de S., Gondim Neto L., Gouveia S.T., de Figueiredo R.W., Maia G.A., de Sousa P.H.M. Evaluation of cooking methods on the bioactive compounds of cashew apple fibre and its application in plant-based foods. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro Á.D.T., Rocha M.V.P., MacEdo G.R., Gonçalves L.R.B. Evaluation of cashew apple juice for the production of fuel ethanol. Appl. Biochem. Biotechnol. 2008;148:227–234. doi: 10.1007/s12010-007-8118-7. [DOI] [PubMed] [Google Scholar]
- 17.Fonteles T.V., Leite A.K.F., da Silva A.R.A., Fernandes F.A.N., Rodrigues S. Sonication effect on bioactive compounds of cashew apple bagasse. Food Bioprocess Technol. 2017;10:1854–1864. [Google Scholar]
- 18.Cs V., Vb H., Sr S., V V., Nm K., Pg L. Comparative preliminary phytochemical analysis of ethanolic extracts of leaves of Olea dioica Roxb., infected with the rust fungus Zaghouania oleae (E.J. Butler) Cummins and non-infected plants. J. Pharmacogn. Phytochem. 2014;3:69–72. [Google Scholar]
- 19.Ardoino S.M., Toso R.E., Boeris M.A. Caracterización fitoquímica de Prosopis flexuosa var. flexuosa (algarrobo) y Prosopis flexuosa var. depressa (alpataco), plantas con acción farmacológica. Cienc. Vet. 2013;15:115–125. [Google Scholar]
- 20.Mir M.A., Parihar K., Tabasum U., Kumari E. Estimation of alkaloid, saponin and flavonoid, content in various extracts of Crocus sativa. J. Med. Plants Stud. 2016;4:171–174. [Google Scholar]
- 21.Nath M.C., Chakravorty M.K., Chowdhury S.R. Liebermann-burchard reaction for steroids. Nature. 1946;157:103–104. doi: 10.1038/157103b0. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal E., Salim K.A., Lim L.B.L. Phytochemical screening, total phenolics and antioxidant activities of bark and leaf extracts of Goniothalamus velutinus (Airy Shaw) from Brunei Darussalam. J. King Saud Univ. Sci. 2015;27:224–232. [Google Scholar]
- 23.Levine V.E., Bien G.E. Liebermann-burchard reaction with carotene. Exp. Biol. Med. 1934;31:804–808. [Google Scholar]
- 24.Sharma A.K., Gangwar M., Kumar D., Nath G., Kumar Sinha A.S., Tripathi Y.B. Phytochemical characterization, antimicrobial activity and reducing potential of seed oil, latex, machine oil and presscake of Jatropha curcas. Avicenna J. Phytomed. 2016;6:366–375. [PMC free article] [PubMed] [Google Scholar]
- 25.Gusakov A.V., Kondratyeva E.G., Sinitsyn A.P. Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int. J. Anal. Chem. 2011;2011:1–4. doi: 10.1155/2011/283658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrade J.K.S., Barros R.G.C., Rezende Y.R.R.S., Nogueira J.P., de Oliveira C.S., Gualberto N.C., Narain N. Evaluation of bioactive compounds, phytochemicals profile and antioxidant potential of the aqueous and ethanolic extracts of some traditional fruit tree leaves used in Brazilian folk medicine. Food Res. Int. 2021;143:110282. doi: 10.1016/j.foodres.2021.110282. [DOI] [PubMed] [Google Scholar]
- 27.Lee H.S. HPLC method for separation and determination of nonvolatile organic acids in orange juice. J. Agric. Food Chem. 2002;41:1991–1993. [Google Scholar]
- 28.Obanda M., Owuor P.O., Taylor S.J. Flavanol composition and caffeine content of green leaf as quality potential indicators of Kenyan black teas. J. Sci. Food Agric. 1997;74:209–215. [Google Scholar]
- 29.Scalbert A. Plant Polyphenols. Springer US; 1992. Quantitative methods for the estimation of tannins in plant tissues; pp. 259–280. [Google Scholar]
- 30.Castro-López C., Sánchez-Alejo E.J., Saucedo-Pompa S., Rojas R., Aranda-Ruiz J., Martínez-Avila G.C.G. Fluctuations in phenolic content, ascorbic acid and total carotenoids and antioxidant activity of fruit beverages during storage. Heliyon. 2016;2 doi: 10.1016/j.heliyon.2016.e00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hames B., Ruiz R., Scarlata C., Sluiter A., Sluiter J., Templeton D. 2008. Preparation of Samples for Compositional Analysis, Golden, Colorado. [Google Scholar]
- 32.Sluiter A., Hames B., Hyman D., Payne C., Ruiz R., Scarlata C., Sluiter J., Templeton D., Wolfe J. 2008. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples, Golden, CO. [Google Scholar]
- 33.Sluiter A., Ruiz R., Scarlata C., Sluiter J., Templeton D. 2008. Determination of Extractives in Biomass, Golden, CO. [Google Scholar]
- 34.Hames B., Scarlata C., Sluiter A. 2008. Determination of Protein Content in Biomass, Golden, CO. [Google Scholar]
- 35.Sáez-Plaza P., Navas M.J., Wybraniec S., Michałowski T., Asuero A.G. An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013;43:224–272. [Google Scholar]
- 36.Sluiter A., Hames B., Ruiz R., Scarlata C., Sluiter J., Templeton D., Crocker D., Hyman D., Payne C., Ruiz R., Scarlata C., Sluiter J., Templeton D., Wolfe J. 2008. Determination of Structural Carbohydrates and Lignin in Biomass, Golden, CO.https://www.nrel.gov/docs/gen/fy13/42618.pdf [Google Scholar]
- 37.Sluiter A., Hames B., Ruiz R., Scarlata C., Sluiter J., Templeton D. 2008. Determination of Ash in Biomass, Golden, CO. [Google Scholar]
- 38.Barazarte H., García T., Garrido E., Pérez H., Terán Y. Evaluación de dos métodos colorimétricos para cuantificar sustancias pécticas en parchita (passiflora edulis) Bioagro. 2010;22(2):163–166. [Google Scholar]
- 39.McCready R.M., McComb E.A. Extraction and determination of total pectic materials in fruits. Anal. Chem. 1952;24:1986–1988. [Google Scholar]
- 40.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 41.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1995;28:25–30. [Google Scholar]
- 42.Craig W.J. Phytochemicals: guardians of our health. J. Am. Diet Assoc. 1997;97 doi: 10.1016/s0002-8223(97)00765-7. [DOI] [PubMed] [Google Scholar]
- 43.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61 doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S., He Y., Li L., Li D., Chen H. New insights on the regulation of anthocyanin biosynthesis in purple Solanaceous fruit vegetables. Sci. Hortic. (Amsterdam) 2022;297:110917. [Google Scholar]
- 45.Jiamboonsri P., Pithayanukul P., Bavovada R., Chomnawang M.T. The inhibitory potential of Thai mango seed kernel extract against methicillin-resistant Staphylococcus aureus. Molecules. 2011;16:6255. doi: 10.3390/molecules16086255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Derese S., Guantai E.M., Yaouba S., Kuete V. Mangifera indica L. (Anacardiaceae), med. Spices Veg. From Africa ther. Potential against Metab. Inflammatory. Infect. Syst. Dis. 2017:451–483. [Google Scholar]
- 47.Ediriweera M.K., Tennekoon K.H., Samarakoon S.R. A review on ethnopharmacological applications, pharmacological activities, and bioactive compounds of Mangifera indica (mango) Evid. Based. Complement. Alternat. Med. 2017;2017 doi: 10.1155/2017/6949835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robinson T. Metabolism and function of alkaloids in plants. Science (80-. ) 1974;184:430–435. doi: 10.1126/science.184.4135.430. [DOI] [PubMed] [Google Scholar]
- 49.Chen C., Lin L. Handb. Diet. Phytochem. Springer Singapore; 2020. Alkaloids in diet; pp. 1–35. [Google Scholar]
- 50.Singh S., Pathak N., Fatima E., Negi A.S. Plant isoquinoline alkaloids: advances in the chemistry and biology of berberine. Eur. J. Med. Chem. 2021;226:113839. doi: 10.1016/j.ejmech.2021.113839. [DOI] [PubMed] [Google Scholar]
- 51.Makran M., Barberá R., Cilla A. Gene-diet interaction in plasma lipid response to plant sterols and stanols: a review of clinical trials. J. Funct.Foods. 2021;87:104751. [Google Scholar]
- 52.López-García G., Cilla A., Barberá R., Alegría A. Anti-inflammatory and cytoprotective effect of plant sterol and galactooligosaccharides-enriched beverages in caco-2 cells. J. Agric. Food Chem. 2019;68:1862–1870. doi: 10.1021/acs.jafc.9b03025. [DOI] [PubMed] [Google Scholar]
- 53.Alvarez-Sala A., López-García G., Attanzio A., Tesoriere L., Cilla A., Barberá R., Alegría A. Effects of plant sterols or β-cryptoxanthin at physiological serum concentrations on suicidal erythrocyte death. J. Agric. Food Chem. 2018;66:1157–1166. doi: 10.1021/acs.jafc.7b05575. [DOI] [PubMed] [Google Scholar]
- 54.López-García G., Cilla A., Barberá R., Alegría A. Antiproliferative effect of plant sterols at colonic concentrations on Caco-2 cells. J. Funct.Foods. 2017;39:84–90. [Google Scholar]
- 55.Kozłowska M., Gruczyńska E., Ścibisz I., Rudzińska M. Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem. 2016;213:450–456. doi: 10.1016/j.foodchem.2016.06.102. [DOI] [PubMed] [Google Scholar]
- 56.Fiedor J., Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.de Assis R.C., de Lima Gomes Soares R., Siqueira A.C.P., de Rosso V.V., de Sousa P.H.M., Mendes A.E.P., de Alencar Costa E., de Góes Carneiro A.P., Maia C.S.C. Determination of water-soluble vitamins and carotenoids in Brazilian tropical fruits by High Performance Liquid Chromatography. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hammond B.R., Renzi L.M. Carotenoids. Adv. Nutr. 2013;4:474–476. doi: 10.3945/an.113.004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Julian H., Khoiruddin K., Julies N., Edwina V., Wenten I.G. Pineapple juice acidity removal using electrodeionization (EDI) J. Food Eng. 2021;304:110595. [Google Scholar]
- 60.Ephrem E., Najjar A., Charcosset C., Greige-Gerges H. Encapsulation of natural active compounds, enzymes, and probiotics for fruit juice fortification, preservation, and processing: an overview. J. Funct.Foods. 2018;48:65–84. [Google Scholar]
- 61.Sousa A.D., de Brito E.S. Effect of treatment with adsorbent resin on the volatile profile and physicochemical characteristics of clarified cashew apple juice. Food Sci. Technol. 2013;33:619–623. [Google Scholar]
- 62.Damasceno L.F., Fernandes F.A.N., Magalhães M.M.A., Brito E.S. Evaluation and optimization of non enzymatic browning of “cajuina” during thermal treatment. Braz. J. Chem. Eng. 2008;25:313–320. [Google Scholar]
- 63.Attri B.L. Effect of initial sugar concentration on the physico-chemical characteristics and sensory qualities of cashew apple wine. Nat. Product. Radiance. 2009:374–397. [Google Scholar]
- 64.Akinwale T.O. Cashew apple juice: its use in fortifying the nutritional quality of some tropical fruits. Eur. Food Res. Technol. 2000;211:205–207. [Google Scholar]
- 65.Akinwale T.O., Olubamiwa O., Ajav E.A. Cottage processing of cashew apple juice in Nigeria: physico-chemical and sensory evaluation of product. J. Food Technol. Afr. 2001;6 [Google Scholar]
- 66.Azoubel P.M., Cipriani D.C., El-Aouar Â.A., Antonio G.C., Murr F.E.X. Effect of concentration on the physical properties of cashew juice. J. Food Eng. 2005;66:413–417. [Google Scholar]
- 67.Azoubel P.M., Murr F.E.X. Optimisation of osmotic dehydration of cashew apple (anacardium occidentale L.) in sugar solutions. Food Sci. Technol. Int. 2003;9:427–433. [Google Scholar]
- 68.Talasila U., Vechalapu R.R., Shaik K.B. Clarification, preservation, and shelf life evaluation of cashew apple juice. Food Sci. Biotechnol. 2012;21:709–714. [Google Scholar]
- 69.Daramola D. Assessment of some aspects of phytonutrients of cashew apple juice of domestic origin in Nigeria. Afr. J. Food Sci. 2013;7:107–112. [Google Scholar]
- 70.Kabuo N.O., Ojukwu M., Omeire G.C., Ibeabuchi J.C. Extraction and preservation of cashew juice using sorbic and benzoic acids. Am. J. Food Sci. Technol. 2015;3:48–54. [Google Scholar]
- 71.Tsitlakidou P., Van Loey A., Methven L., Elmore J.S. Effect of sugar reduction on flavour release and sensory perception in an orange juice soft drink model. Food Chem. 2019;284:125–132. doi: 10.1016/j.foodchem.2019.01.070. [DOI] [PubMed] [Google Scholar]
- 72.Abdullah S., Pradhan R.C., Pradhan D., Mishra S. Modeling and optimization of pectinase-assisted low-temperature extraction of cashew apple juice using artificial neural network coupled with genetic algorithm. Food Chem. 2021;339:127862. doi: 10.1016/j.foodchem.2020.127862. [DOI] [PubMed] [Google Scholar]
- 73.Crépin L., Truong N.M., Bloem A., Sanchez I., Dequin S., Camarasa C. Management of multiple nitrogen sources during wine fermentation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2017;83 doi: 10.1128/AEM.02617-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cardona C.A., Quintero J.A., Paz I.C. Production of bioethanol from sugarcane bagasse: status and perspectives. Bioresour. Technol. 2010;101:4754–4766. doi: 10.1016/j.biortech.2009.10.097. [DOI] [PubMed] [Google Scholar]
- 75.Moawad E.Y. Optimizing bioethanol production by regulating yeast growth energy. Syst. Synth. Biol. 2012;6:61–68. doi: 10.1007/s11693-012-9099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Albers E., Larsson C., Lidén G., Niklasson C., Gustafsson L. Influence of the nitrogen source on Saccharomyces cerevisiae anaerobic growth and product formation. Appl. Environ. Microbiol. 1996;62:3187–3195. doi: 10.1128/aem.62.9.3187-3195.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohd Azhar S.H., Abdulla R., Jambo S.A., Marbawi H., Gansau J.A., Mohd Faik A.A., Rodrigues K.F. Yeasts in sustainable bioethanol production: a review. Biochem. Biophys. Reports. 2017;10:52–61. doi: 10.1016/j.bbrep.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Griel A.E., Etherton T.D. Am. J. Med. Elsevier Inc.; 2002. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer; pp. 71–88. [DOI] [PubMed] [Google Scholar]
- 79.Barba F.J., Esteve M.J., Frígola A. Stud. Nat. Prod. Chem. Elsevier B.V.; 2014. Bioactive components from leaf vegetable products; pp. 321–346. [Google Scholar]
- 80.Davey M.W., Van Montagu M., Inze D., Sanmartin M., Kanellis A., Smirnoff N., Benzie I.J., Strain J.J., Favell D., Fletcher J. PlantL-ascorbic acid: chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000;80:825–860. [Google Scholar]
- 81.Hamrick I., Counts S.H. Vitamin and mineral supplements. Prim. Care Clin. Off. Pract. 2008;35:729–747. doi: 10.1016/j.pop.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 82.Carr A.C., Frei B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999;69:1086–1107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 83.de Oliveira Alves Sena E., da Silva P.S.O., de Araujo H.G.S., de Aragão Batista M.C., Matos P.N., Sargent S.A., de Oliveira Junior L.F.G., Carnelossi M.A.G. Postharvest quality of cashew apple after hydrocooling and coold room. Postharvest Biol. Technol. 2019;155:65–71. [Google Scholar]
- 84.Li P., Yin F., Song L., Zheng X. Alleviation of chilling injury in tomato fruit by exogenous application of oxalic acid. Food Chem. 2016;202:125–132. doi: 10.1016/j.foodchem.2016.01.142. [DOI] [PubMed] [Google Scholar]
- 85.Scherer R., Rybka A.C.P., Ballus C.A., Meinhart A.D., Filho J.T., Godoy H.T. Validation of a HPLC method for simultaneous determination of main organic acids in fruits and juices. Food Chem. 2012;135:150–154. [Google Scholar]
- 86.Jeon M.J., Ha J.W. Inactivating foodborne pathogens in apple juice by combined treatment with fumaric acid and ultraviolet-A light, and mechanisms of their synergistic bactericidal action. Food Microbiol. 2020;87:103387. doi: 10.1016/j.fm.2019.103387. [DOI] [PubMed] [Google Scholar]
- 87.Leite A.K.F., Fonteles T.V., Miguel T.B.A.R., Silvestre da Silva G., Sousa de Brito E., Alves Filho E.G., Fernandes F.A.N., Rodrigues S. Atmospheric cold plasma frequency imparts changes on cashew apple juice composition and improves vitamin C bioaccessibility. Food Res. Int. 2021;147:110479. doi: 10.1016/j.foodres.2021.110479. [DOI] [PubMed] [Google Scholar]
- 88.Ramos J.E.T., Duarte T.C., Rodrigues A.K.O., Silva I.J., Cavalcante C.L., Azevedo D.C.S. On the production of glucose and fructose syrups from cashew apple juice derivatives. J. Food Eng. 2011;102:355–360. [Google Scholar]
- 89.Santos D.I., Saraiva J.M.A., Vicente A.A., Moldão-Martins M. Innov. Therm. Non-Thermal Process. Bioaccessibility Bioavailab. Nutr. Bioact. Compd. Elsevier; 2019. Methods for determining bioavailability and bioaccessibility of bioactive compounds and nutrients; pp. 23–54. [Google Scholar]
- 90.Siriwardhana N., Kalupahana N.S., Cekanova M., LeMieux M., Greer B., Moustaid-Moussa N. Modulation of adipose tissue inflammation by bioactive food compounds. J. Nutr. Biochem. 2013;24:613–623. doi: 10.1016/j.jnutbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 91.Hassimotto N.M.A., Genovese M.I., Lajolo F.M. Antioxidant capacity of Brazilian fruit, vegetables and commercially-frozen fruit pulps. J. Food Compos. Anal. 2009;22:394–396. [Google Scholar]
- 92.Ahmed F.A., Ali R.F.M. Bioactive compounds and antioxidant activity of fresh and processed white cauliflower. BioMed Res. Int. 2013;2013 doi: 10.1155/2013/367819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaprasob R., Kerdchoechuen O., Laohakunjit N., Sarkar D., Shetty K. Fermentation-based biotransformation of bioactive phenolics and volatile compounds from cashew apple juice by select lactic acid bacteria. Process Biochem. 2017;59:141–149. [Google Scholar]
- 94.de A.C. Vergara C.M., Honorato T.L., Maia G.A., Rodrigues S. Prebiotic effect of fermented cashew apple (Anacardium occidentale L) juice. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2010;43:141–145. [Google Scholar]
- 95.Braga D.C., Alves Filho E.G., Ribeiro P.R.V., Araújo Í.M. da S., de Brito E.S., Garruti D. dos S. Multivariate correlation of the astringency sensory perception with the phenolic profiling of cashew apple genotypes. Food Biosci. 2021;41:100931. [Google Scholar]
- 96.Prommajak T., Leksawasdi N., Rattanapanone N. Tannins in fruit juices and their removal. Chiang Mai Univ. J. Nat. Sci. 2020;19:76–90. [Google Scholar]
- 97.Lu C., Bao Y., Huang J.Y. Fouling in membrane filtration for juice processing. Curr. Opin. Food Sci. 2021;42:76–85. [Google Scholar]
- 98.Konoha K., Sadakane Y., Kawahara M. Zinc neurotoxicity and its role in neurodegenerative diseases. J. Health Sci. 2006;52:1–8. [Google Scholar]
- 99.Baldrian P., Valášková V., Merhautová V., Gabriel J. Degradation of lignocellulose by Pleurotus ostreatus in the presence of copper, manganese, lead and zinc. Res. Microbiol. 2005;156:670–676. doi: 10.1016/j.resmic.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 100.Durán-Aranguren D.D., Meléndez-Melo J.P., Covo-Ospina M.C., Díaz-Rendón J., Reyes-Gutiérrez D.N., Reina L.C., Durán-Sequeda D., Sierra R. Biological pretreatment of fruit residues using the genus Pleurotus: a review. Bioresour. Technol. Reports. 2021;16:100849. [Google Scholar]
- 101.Rodríguez E., Nuero O., Guillén F., Martínez A.T., Martínez M.J. Degradation of phenolic and non-phenolic aromatic pollutants by four Pleurotus species: the role of laccase and versatile peroxidase. Soil Biol. Biochem. 2004;36:909–916. [Google Scholar]
- 102.Scherer R., Rybka A.C.P., Godoy H.T. Simultaneous determination of tartaric, malic, ascorbic and citric acids in acerola, açai and cashew pulps, and stability evaluation in cashew juices. Quim. Nova. 2008;31:1137–1140. [Google Scholar]
- 103.Fonteles T.V., Leite A.K.F., Silva A.R.A., Carneiro A.P.G., Miguel E.D.C., Cavada B.S., Fernandes F.A.N., Rodrigues S. Ultrasound processing to enhance drying of cashew apple bagasse puree: influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016;31:237–249. doi: 10.1016/j.ultsonch.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Fonteles T.V., Leite A.K.F., Silva A.R.A., Carneiro A.P.G., Miguel E.D.C., Cavada B.S., Fernandes F.A.N., Rodrigues S. Ultrasound processing to enhance drying of cashew apple bagasse puree: influence on antioxidant properties and in vitro bioaccessibility of bioactive compounds. Ultrason. Sonochem. 2016;31:237–249. doi: 10.1016/j.ultsonch.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 105.Adetola O.Y., Kruger J., White Z., Taylor J.R.N. Comparison between food-to-food fortification of pearl millet porridge with moringa leaves and baobab fruit and with adding ascorbic and citric acid on iron, zinc and other mineral bioaccessibility. LWT. 2019;106:92–97. [Google Scholar]
- 106.Guevara M., Tejera E., Granda-Albuja M.G., Iturralde G., Chisaguano-Tonato M., Granda-Albuja S., Jaramillo-Vivanco T., Giampieri F., Battino M., Alvarez-Suarez J.M. Chemical composition and antioxidant activity of the main fruits consumed in the western coastal region of Ecuador as a source of health-promoting compounds. Antioxidants. 2019;8:387. doi: 10.3390/antiox8090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scherer R., Rybka A.C.P., Godoy H.T. Simultaneous determination of tartaric, malic, ascorbic and citric acids in acerola, açai and cashew pulps, and stability evaluation in cashew juices. Quim. Nova. 2008;31:1137–1140. [Google Scholar]
- 108.Son H.S., Hwang G.S., Park W.M., Hong Y.S., Lee C.H. Metabolomic characterization of malolactic fermentation and fermentative behaviors of wine yeasts in grape wine. J. Agric. Food Chem. 2009;57:4801–4809. doi: 10.1021/jf9005017. [DOI] [PubMed] [Google Scholar]
- 109.Contreras-Calderón J., Calderón-Jaimes L., Guerra-Hernández E., García-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011;44:2047–2053. [Google Scholar]
- 110.do S.M. Rufino M., Alves R.E., de Brito E.S., Pérez-Jiménez J., Saura-Calixto F., Mancini-Filho J. Bioactive compounds and antioxidant capacities of 18 non-traditional tropical fruits from Brazil. Food Chem. 2010;121:996–1002. [Google Scholar]
- 111.Pacheco A.L.V., Pagliarini M.F., de Freitas G.B., Santos R.H.S., Serrão J.E., Zanuncio J.C. Mineral composition of pulp and production of the yellow passion fruit with organic and conventional fertilizers. Food Chem. 2017;217:425–430. doi: 10.1016/j.foodchem.2016.08.068. [DOI] [PubMed] [Google Scholar]
- 112.Sibiya N.P., Kayitesi E., Moteetee A. Mineral composition of selected indigenous wild southern African fruits. South Afr. J. Bot. 2020;132:87–94. [Google Scholar]
- 113.Correia J.A. da C., Júnior J.E.M., Gonçalves L.R.B., Rocha M.V.P. Alkaline hydrogen peroxide pretreatment of cashew apple bagasse for ethanol production: study of parameters. Bioresour. Technol. 2013;139:249–256. doi: 10.1016/j.biortech.2013.03.153. [DOI] [PubMed] [Google Scholar]
- 114.Silva J.S., Mendes J.S., Correia J.A.C., Rocha M.V.P., Micoli L. Cashew apple bagasse as new feedstock for the hydrogen production using dark fermentation process. J. Biotechnol. 2018;286:71–78. doi: 10.1016/j.jbiotec.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 115.de Araújo Padilha C.E., da Costa Nogueira C., Oliveira Filho M.A., de Santana Souza D.F., de Oliveira J.A., dos Santos E.S. Valorization of cashew apple bagasse using acetic acid pretreatment: production of cellulosic ethanol and lignin for their use as sunscreen ingredients. Process Biochem. 2020;91:23–33. [Google Scholar]
- 116.Restrepo-Serna D.L., Cardona Alzate C.A. Economic pre-feasibility of supercritical fluid extraction of antioxidants from fruit residues. Sustain. Chem. Pharm. 2022;25:100600. [Google Scholar]
- 117.Banerjee J., Vijayaraghavan R., Arora A., MacFarlane D.R., Patti A.F. Lemon juice based extraction of pectin from mango peels: waste to wealth by sustainable approaches. ACS Sustain. Chem. Eng. 2016;4:5915–5920. [Google Scholar]
- 118.Choi I.S., Kim J.H., Wi S.G., Kim K.H., Bae H.J. Bioethanol production from Mandarin (Citrus unshiu) peel waste using popping pretreatment. Appl. Energy. 2013;102:204–210. [Google Scholar]
- 119.Cristina dos Santos Lima F., Luiz Honorato da Silva F., Palmeira Gomes J., Mariano da Silva Neto J. Chemical composition of the cashew apple bagasse and potential use for ethanol production. Adv. Chem. Eng. Sci. 2012;2:519–523. [Google Scholar]
- 120.Rodrigues T.H.S., Rocha M.V.P., De MacEdo G.R., Gonçalves L.R.B. Ethanol production from cashew apple bagasse: improvement of enzymatic hydrolysis by microwave-assisted alkali pretreatment. Appl. Biochem. Biotechnol. 2011;164:929–943. doi: 10.1007/s12010-011-9185-3. [DOI] [PubMed] [Google Scholar]
- 121.Royo J., Canalís P., Quintana D. Chemical study of bottom ash sintering in combustion of pelletized residual agricultural biomass. Fuel. 2022;310:122145. [Google Scholar]
- 122.Narisetty V., Castro E., Durgapal S., Coulon F., Jacob S., Kumar D., Kumar Awasthi M., Kishore Pant K., Parameswaran B., Kumar V. High level xylitol production by Pichia fermentans using non-detoxified xylose-rich sugarcane bagasse and olive pits hydrolysates. Bioresour. Technol. 2021;342:126005. doi: 10.1016/j.biortech.2021.126005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bressani A.P.P., Martinez S.J., Sarmento A.B.I., Borém F.M., Schwan R.F. Organic acids produced during fermentation and sensory perception in specialty coffee using yeast starter culture. Food Res. Int. 2020;128:108773. doi: 10.1016/j.foodres.2019.108773. [DOI] [PubMed] [Google Scholar]
- 124.Valenti F., Zhong Y., Sun M., Porto S.M.C., Toscano A., Dale B.E., Sibilla F., Liao W. Anaerobic co-digestion of multiple agricultural residues to enhance biogas production in southern Italy. Waste Manag. 2018;78:151–157. doi: 10.1016/j.wasman.2018.05.037. [DOI] [PubMed] [Google Scholar]
- 125.Sethupathy S., Murillo Morales G., Gao L., Wang H., Yang B., Jiang J., Sun J., Zhu D. Lignin valorization: status, challenges and opportunities. Bioresour. Technol. 2022;347:126696. doi: 10.1016/j.biortech.2022.126696. [DOI] [PubMed] [Google Scholar]
- 126.Poveda-Giraldo J.A., Solarte-Toro J.C., Cardona Alzate C.A. The potential use of lignin as a platform product in biorefineries: a review. Renew. Sustain. Energy Rev. 2021;138:110688. [Google Scholar]
- 127.Santos E.E., Amaro R.C., Bustamante C.C.C., Guerra M.H.A., Soares L.C., Froes R.E.S. Extraction of pectin from agroindustrial residue with an ecofriendly solvent: use of FTIR and chemometrics to differentiate pectins according to degree of methyl esterification. Food Hydrocolloids. 2020;107:105921. [Google Scholar]
- 128.Thomason J.L., Rudeiros-Fernández J.L. Thermal degradation behaviour of natural fibres at thermoplastic composite processing temperatures. Polym. Degrad. Stabil. 2021;188:109594. [Google Scholar]
- 129.De Souza T.C., De Fonseca T.S., Da Costa J.A., Rocha M.V.P., De Mattos M.C., Fernandez-Lafuente R., Gonçalves L.R.B., Dos Santos J.C.S. Cashew apple bagasse as a support for the immobilization of lipase B from Candida Antarctica: application to the chemoenzymatic production of (R)-Indanol. J. Mol. Catal. B Enzym. 2016;130:58–69. [Google Scholar]
- 130.Raveendran K., Ganesh A., Khilar K.C. Pyrolysis characteristics of biomass and biomass components. Fuel. 1996;75:987–998. [Google Scholar]
- 131.Sfakiotakis S., Vamvuka D. Study of co-pyrolysis of olive kernel with waste biomass using TGA/DTG/MS. Thermochim. Acta. 2018;670:44–54. [Google Scholar]
- 132.Anca-Couce A., Tsekos C., Retschitzegger S., Zimbardi F., Funke A., Banks S., Kraia T., Marques P., Scharler R., de Jong W., Kienzl N. Biomass pyrolysis TGA assessment with an international round robin. Fuel. 2020;276:118002. [Google Scholar]
- 133.Leng L., Yang L., Chen J., Hu Y., Li H., Li H., Jiang S., Peng H., Yuan X., Huang H. Valorization of the aqueous phase produced from wet and dry thermochemical processing biomass: a review. J. Clean. Prod. 2021;294:126238. [Google Scholar]
- 134.Chun D.D., Ni D., Simson A. The effect of inherent inorganics and CO2 co-pyrolysis on biochar production from biowastes and their gasification reactivity. Biomass Bioenergy. 2022;158:106361. [Google Scholar]
- 135.Tereucan G., Ercoli S., Cornejo P., Winterhalter P., Contreras B., Ruiz A. Stability of antioxidant compounds and activities of a natural dye from coloured-flesh potatoes in dairy foods. LWT. 2021;144:111252. [Google Scholar]
- 136.Li Q., Li J., Duan M., Liu L., Fu Y., McClements D.J., Zhao T., Lin H., Shi J., Chen X. Impact of food additive titanium dioxide on the polyphenol content and antioxidant activity of the apple juice. LWT. 2022;154:112574. [Google Scholar]
- 137.Rodríguez Ó., Gomes W.F., Rodrigues S., Fernandes F.A.N. Effect of indirect cold plasma treatment on cashew apple juice (Anacardium occidentale L.) LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2017;84:457–463. [Google Scholar]
- 138.Chen Z., Bertin R., Froldi G. EC50 estimation of antioxidant activity in DPPH∗ assay using several statistical programs. Food Chem. 2013;138:414–420. doi: 10.1016/j.foodchem.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Tirzitis G., Bartosz G. Determination of antiradical and antioxidant activity: basic principles and new insights. Acta Biochim. Pol. 2010;57:139–142. [PubMed] [Google Scholar]
- 140.Villaño D., Fernández-Pachón M.S., Moyá M.L., Troncoso A.M., García-Parrilla M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta. 2007;71:230–235. doi: 10.1016/j.talanta.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 141.Contreras-Calderón J., Calderón-Jaimes L., Guerra-Hernández E., García-Villanova B. Antioxidant capacity, phenolic content and vitamin C in pulp, peel and seed from 24 exotic fruits from Colombia. Food Res. Int. 2011;44:2047–2053. [Google Scholar]
- 142.Rasool T., Kumar S. Kinetic and thermodynamic evaluation of pyrolysis of plant biomass using TGA. Mater. Today Proc. 2020;21:2087–2095. [Google Scholar]
- 143.Deng Y., Wang W., Zhao S., Yang X., Xu W., Guo M., Xu E., Ding T., Ye X., Liu D. Ultrasound-assisted extraction of lipids as food components: Mechanism, solvent, feedstock, quality evaluation and coupled technologies – A review. Trends Food Sci. Technol. 2022 doi: 10.1016/J.TIFS.2022.01.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.