Abstract
Epoxidation-esterification of fatty acid methyl ester obtained from Irvingia gabonensis kernel oil (IGKO), as well as its characterization, kinetics and thermodynamics were the main focus of this study. The methyl ester obtained via base catalyzed transesterification was used for epoxidation-esterification modification process. Epoxidation kinetics and thermodynamics parameters were also investigated. The properties of the IGKO and epoxidized-esterified Irvingia gabonensis kernel oil (IGKO) methyl ester (MIGKOe) were determined using standard methods. Rate constant K and activation energy Ea for the epoxidation process was found to be of the order Lmol−1s−1 and 46.02 kJ/mol, respectively. ΔG, ΔH, and ΔS values for the epoxidation process were (94.74–101.42 kJ mol−1), 43.30 kJ mol−1, and 167.20 J mol−1 K−1, respectively, indicating the non-spontaneous, endothermic, and endergonic nature of the process. The physicochemical characteristics of MIGKOe were: 9 °C, 298 °C, 840 kg/dm3, 13.84 mm2/s, 1.351 mg KOH/g oil, 1.01 mg/kg and 39.78 kV, for pour point, flash point, density, viscosity, acid value, moisture content and dielectric strength, respectively. The MIGKOe properties indicated its potential for use as a bio-transformer fluid, upon further treatment with pour point depressant.
Keywords: Irvingia gabonensis, Transesterification, Epoxidation-esterification, Kinetics, Theromdynamics
Irvingia gabonensis; Transesterification; Epoxidation-esterification; Kinetics; Theromdynamics.
1. Introduction
Increase in global energy demand, instability in world petroleum price, as well as the none-biodegradability of fossil fuels; have increased the demand for bio-oils used in bio-fuels production, as alternative to conventional fuels (Adepoju, 2014). Bio-oils used in the production of products like biodiesels, bio-transformer fluids and bio-lubricants, etc., which are substitute to convectional petroleum base fuels are gaining ground due to their high biodegradability nature, environmental friendliness, availability, energy conservation and management potentials (Adepoju and Olamide, 2014). Most bio-fuels and bio-products like the aforementioned ones are produced by transesterification or alcoholysis process (Knothe et al., 2007). Other processes include esterification, epoxidation, hydrotreatment and oleaginous microorganisms’ treatment (Shote et al., 2009; Agu et al., 2019). These bio-fuels and bio-products are often produced using vegetable oils extracted from seeds/nuts.
However, the primary challenges associated with the production of these bio-fuels and bio-products are limited raw materials and cost of production. Hence, these shortcomings, limit final products (biodiesels, bio-transformer fluids and bio-lubricants etc.) availability (Berhanu and Amare, 2012). In other words, to ensure production competitiveness and availability for commercial purposes, the readily availability and affordability of these vegetable oils raw materials becomes very essential. As a result, several countries like Brazil that depends so much on bio-oils for their energy needs have focused more on the use of vegetable oils for this purpose (Chhetri et al., 2008). Thus, the sustainable supply of less expensive vegetable oils, like Irvingia gabonensis kernel oil (IGKO) becomes very essential (Dorado et al., 2006).
Over the years, many researchers have produced the biodiesel, bio-transformer fluid and bio-lubricants from vegetable oil sources. For instance Ong et al. (2013), Sadia et al. (2013) and Rashid et al. (2014) produced biodiesel from Jatropha curcas oil, wild safflower (Carthamus oxyacantha Bieb) seed oil and milo (Thespesia populnea) seed oil, respectively. Similarly, in transformer fluid production, successes have been reported on bio-transformer oil production from Terminalia catappa L. kernel oil (Agu et al., 2019), Jatropha curcas oil (Beltran et al., 2017) and high oleic sunflower oil (Viertel et al., 2014). Also, in bio-lubricant production, Menkiti et al., 2017a, Menkiti et al., 2017b and Aji et al. (2015) produced bio-lubricant from Jatropha curcas oil and Neem seed oil, respectively. As such, there is need to evaluate the feasibility of modifying Irvingia gabonensis kernel oil (IGKO) and its methyl ester by the epoxidation-esterification process foruse as transformer fluid.
Irrespective of the modification method used, the need for modification of vegetable oil structure is critical, since it helps to ensure its applicability for use as different products. Therefore, some of the methods of vegetable oil modifications include, but not limited to estolides formation (Sharma et al., 2006), esterification (Mohammed et al., 2007), epoxidation (Campanella et al., 2010; Ciubota-Rosie et al., 2014; Goud et al., 2006) transesterification (Agu et al., 2019; Abdelmalik, 2014; Sanchez et al., 2014), and epoxidation-esterification (Abdelmalik et al., 2011; Agu et al., 2019). Irrespective of method(s) adopted, the unsaturated groups of the fatty acid chains in triglycerides in vegetable oils are intentionally changed to obtain more complex structures. This is to ensure improvements in the low-temperature properties of the oil samples, as well as their oxidative stability (Campanella et al., 2010; Agu et al., 2019).
Of all these methods of vegetable oil modification, classical epoxidation of vegetable oil procedure is of great interest to researchers because of its advantages. These benefits include; high product yield of the process, low cost of the peracid (often acetic acid) synthesis, recapture of carboxylic acid, and its comparative stability under epoxidation process (Wai et al., 2019). Furthermore, another advantage of epoxidation is the positive effect associated with the branching at the chain end on the low temperature properties and performance of the final product(s). This results in the formation of microcrystalline structures instead of macrocrystalline structures, which are the main causes of poor low temperature properties (Salimon et al., 2010; Sharma et al., 2006). Therefore, the epoxidation of vegetable oil becomes essential irrespective of the intended product.
For instance, with respect to bio-transformer oil production using epoxidation-esterification method, Agu et al. (2019) have assessed the possibility of using modified Terminalia catappa L. oil as transformer oil. This method was also used by Abdelmalik et al. (2011) for bio-transformer oil production using Palm kernel oil. For bio-lubricant production, Campanella et al. (2010) explored the possibility of producing lubricants from soybean and sunflower oils, by the chemical modification of the oils using epoxidation method. Also, Hwang et al. (2003) worked on the biolubricant production by acid-catalyzed oxirane ring-opening reactions using epoxidized soybean oil, using alcohols and subsequent esterification of the obtained hydroxyl group with acid anhydride. Similarly, Ciubota-Rosie et al. (2014) successfully evaluated the role of epoxidation reaction in the modification of Camelina sativa oil for possible use as biodiesel. Also, the epoxidation of waste used oil was successfully used in the synthesis of biodiesel by Kongyai et al. (2013).
Even though vegetable oils epoxidation has been comprehensively studied and reported in literature, it has been discovered that the epoxidation of alkyl ester of base oil, instead of the vegetable oil itself, gives better properties of the intended final product. This is attributed to better product stability (thermo-oxidative) and pour point (Agu et al., 2019; Abdelmalik et al., 2011). Also, the alkenyl group with different numbers of double bonds in the fatty acid methyl ester (FAME) significantly affects the epoxidation efficiency, as well as the product selectivity (Huang et al., 2015). As such, several works have been carried out on the epoxidation of alkyl ester of various vegetable oils. For example, Campanella et al. (2008) successfully carried out epoxidation of soybean fatty acid methyl ester (FAME) using performic acid. Similarly, Borugadda and Goud (2014) carried out castor oil methyl esters epoxidation using heterogeneous ion-exchange resin (IR-120) as a catalyst. Epoxidation of methyl esters was also successfully carried out by Shuangfei and Lisheng (2011) using SO3H-functional Brønsted acidic ionic liquid catalyst. Furthermore, the epoxidation of methyl esters obtained from olive and linseed oils was successfully carried out by Huang et al. (2015). During epoxidation reaction, it is essential to understand the kinetics and thermodynamics of the process.
A number of researchers have also successfully carried out research on the kinetics and thermodynamics of vegetable oil and methyl ester epoxidations. For instance, Ikhuoria et al. (2007) successfully carried out kinetics and thermodynamics of epoxidation of the methyl esters of Parkia Biglobosa seed oil and obtained the rate constant of the order, activation energy, enthalpy ΔH, entropy ΔS and free energy ΔG values of 10−7 L mol−1 S−1, 51.963 kJ/mol, 13.8 kJ/mol, KJ/mol and 1.51 kJ/mol. Similarly, Cai et al. (2008) successfully epoxidized sunflower oil and obtained rate constant of the order 10−6 L mol−1S−1 and activation energy of 85.21 kJ/mol. They also obtained thermodynamic parameter values of 82.73 kJ/mol, KJ/mol and 110.01 kJ/mol, for the enthalpy ΔH, entropy ΔS and free energy ΔG, respectively. In a similar manner, Okieimen et al. (2002) successfully carried out the epoxidation of rubber seed oil, with rate constant of the order, activation energy, enthalpy ΔH, entropy ΔS and free energy ΔG values of 10−6 L mol−1S−1, 15.7 kJ/mol, 15.2 kJ/mol, KJ/mol and 25.44 kJ/mol. Also, Naidir et al. (2012) studied the kinetics and thermodynamics of the epoxidation of trimethylolpropane ester, with the rate constant of the order and activation energy values of 10−2 L mol−1 S−1 and 69.4 kJ/mol. In the work of Cai and Wang (2011), they successfully studied the epoxidation of unsaturated fatty acid methyl ester (FAMEs) in the presence of SO3H-functional, and obtained the rate constant of the order, activation energy, enthalpy ΔH, entropy ΔS and free energy ΔG values of 104 L mol−1 S−1, 45.5 kJ/mol, 42.7 kJ/mol, KJ/mol and 112.1 kJ/mol. Goud et al. (2006), successfully studied of the epoxidation of mahua oil (Madhumica indica), and obtained the rate constant of the order, activation energy, enthalpy ΔH, entropy ΔS and free energy ΔG values of 10−6 L mol−1 S−1, 14.5 kJ/mol, 13.8 kJ/mol, KJ/mol and 30.6 kJ/mol.
However, to the best of our knowledge, there is no report available on the extensive study of the epoxidation of Irvingia gabonensis kernel oil (IGKO) methyl ester using peracetic acid (PAA). Also, there is no literature information on the kinetics and thermodynamics of Irvingia gabonensis kernel oil methyl ester epoxidation, hence, the justification and novelty of the present study. Thus, it has becomes paramount to close these existing research gaps. Therefore, this work focuses on the extensive study of the epoxidation kinetics and thermodynamics of Irvingia gabonensis kernel oil methyl ester, using peracetic acid (PAA). Also, the physicochemical properties of the obtained product were analyses as well.
2. Materials and methods
2.1. Materials
The primary raw material, Irvingia gabonensis kernels (IGK) were bought in Nkwo-Agu Market, Umuaga, Nigeria. Conraws laboratory chemical vendor located in Enugu, Nigeria supplied all analytical grade chemicals/reagents used in research and they were used without further purification. These reagents include n-hexane, KOH, acetic acid, H2SO4, Hydrogen peroxide, NaHCO3, NaCl, Magnesium sulphate, ethyl acetate, acid anhydride, boron trifluoro diethyletherate, and methanol. The entire reagents were of analytical grade with 99 % purity levels.
2.2. Extraction of Irvingia gabonensis kernel oil (IGKO)
Extraction of oil from IGK followed the procedure described in the work of Menkiti et al. (2015). The entire extraction process was repeated times, after which the average value determined and noted. N-hexane was used for the solvent extraction process using soxhlet apparatus to extract IGKO. Percentage oil yield determination was carried out in line with Association of Official Analytical Chemists AOAC method no. 963.15 (1979).
Eq. (1) was used in estimating the IGKO yield.
| (1) |
2.3. Transesterification experiment
2.3.1. Acid catalyzed esterification
The acid catalyzed esterification of the IGKO followed the procedure as described in our earlier work (Agu et al., 2019). In the earlier described procedure, a reaction glass vessel was used in heating IGKO sample at 60 °C. Thereafter, H2SO4 (1.0 % w/w) catalyst and 99 % pure methanol (30 % v/v) solution was heated at 30 °C for a period of 4 h. This solution was then poured into the glass vessel that contains IGKO to ensure esterification reaction. With the aid of a stirrer operating at 600 revolutions per minute (RPM), the mixture was stirred for an hour. Using a separating funnel, the content was decanted into it and allowed for 2 h, to ensure proper/complete separation. The top layer which consists of methanol-water fractions was discarded, while the oil phase (methyl ester) was used as starting material for base-catalyzed transesterification reaction.
2.3.2. Base catalyzed transesterification
The transesterification of the methyl ester followed the procedure described in the work Agu et al. (2019). 50 ml of methyl ester sample was decanted into a 150 ml conical flask. It was heated to 60 °C using a water bath. Afterwards, potassium methoxide solution was obtained by dissolving KOH pellets in methanol, contained in a 250 ml beaker. The potassium methoxide solution and methyl ester were mixed at a ratio of 6:1. The mixture was eventually stirred vigorously at 500 revolutions per minute (RPM) for 2 h using magnetic stirrer. Thereafter, it was kept in separating funnel for 24 h without further stirring, to ensure proper sedimentation. The obtained methyl ester was washed with distilled water. Demoisturization of the obtained methyl ester was carried out at 100 °C.
Eq. (2) was used to estimate % methyl ester yield.
| (2) |
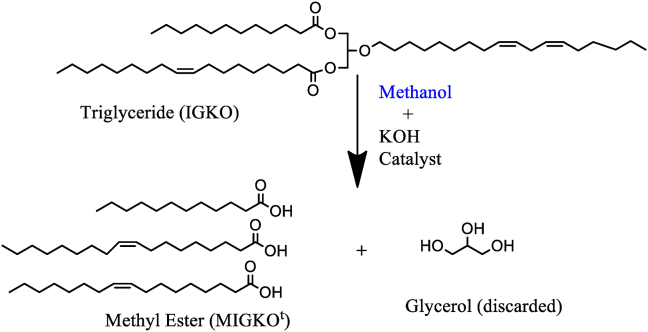
The obtained methyl ester sample is modified Irvingia gabonensis kernel oil (MIGKOt). The scheme is presented in Figure 1. A similar reaction scheme had earlier been presented in our previous publication Agu et al. (2019).
Figure 1.
Transesterification reaction for methyl ester (MIGKOt) production from Irvingia gabonensis kernel oil (IGKO).
2.4. Epoxidation experiments
2.4.1. Epoxidation reaction
The epoxidation reaction was carried out according to the method described by Agu et al. (2019). 100 g of the MIGKOt sample was measured into a flask and heated to 70 °C, prior to the addition of peracetic acid (PAA). The PAA was obtained by mixing 14 wt % of acetic acid and 2.5 % of H2SO4. Thereafter, hydrogen peroxide (16 wt %) was added. After the introduction, the reaction proceeded with constant stirring (1200 RPM) for 7 h. Afterwards, 5 % NaHCO3 was used to purify the sample, followed by saturated NaCl addition, so as to get the epoxide. Finally, anhydrous magnesium sulphate was used to dry the product, while a rotary evaporator was used for solvent removal.
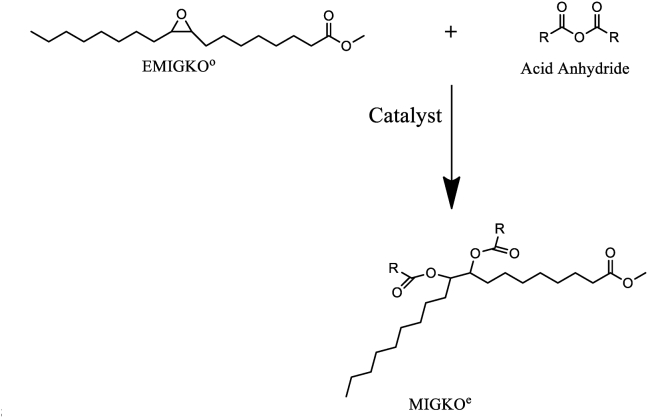
Figure 2 shows the MIGKOt epoxidation reaction for epoxide [epoxy methyl ester of Irvingia gabonensis kernel oil (EMIGKOo)] production. A similar reaction scheme had earlier been presented in our previous publication, Agu et al. (2019).
Figure 2.
Epoxidation reaction scheme for epoxide (EMIGKOo) production, from Irvingia gabonensis kernel oil methyl ester (MIGKOt).
2.4.2. Esterification ring opening reaction
The ring-opening reaction was carried out according to the method described in Agu et al. (2019). EMIGKOo (20 g) was measured and then added to 10 ml of ethyl acetate with constant stirring. 4 g acid anhydride was then introduced, followed by boron trifluoro diethyletherate (1 ml). The reaction proceeded with constant stirring (1200 RPM) for 7 h. Thereafter, 5 % NaHCO3 was used to purify the obtained branched methyl ester sample, followed by the addition of 10 % NaCl solution. Subsequently, the branched methyl ester sample was obtained, after NaCl addition. Finally, anhydrous magnesium sulphate was used to dry the product, while rotary evaporator was applied for solvent removal. Distillation method was used to remove the unreacted anhydrides at reduced pressure and 80 °C.
Figure 3 show the esterification ring-opening of the EMIGKOo (epoxide) reaction scheme for the production of modified/epoxidized Irvingia gabonensis kernel oil methyl ester (MIGKOe) sample. Similar reaction scheme had earlier been presented in our previous publication by Agu et al. (2019).
Figure 3.
Esterification ring-opening reaction scheme for epoxidized Irvingia gabonensis kernel oil methyl ester (MIGKOe) production from the epoxide (EMIGKOo).
2.5. Physicochemical characterization of IGKO and MIGKOe samples
Using AOAC approved techniques (1990), acidity/acid value (AOAC 969.17), iodine value (AOAC 993.20), density (AOAC 985.19) and moisture content (AOAC 926.12) of the samples were estimated. Similarly, ASTM D445 (2011) was used for viscosity determination, while the breakdown voltage (dielectric strength) was analyzed following the IEC 60156 (2003) standard. Furthermore, flash and pour points were determined using ASTM D93 (2012) and ASTM D97 (2012) standard procedures respectively. These properties were measured in triplicates while obtained average values were recorded.
2.6. Epoxidation kinetics
The in situ epoxidation reaction generally takes place in two steps: (i) formation of peroxy acid and (ii) reaction of peroxy acid with the unsaturated fatty acids of the methyl ester (MIGKOt). Eqs. (3) and (4) are the equations for the formation of peroxy acid and reaction of peroxy acid with the unsaturated fatty acids of the methyl esters respectively.
| (3) |
 |
(4) |
Assuming that the rate determining step for the epoxidation reaction is peroxy acid formation, while peracid concentration is assumed to be unchanged during the entire reaction process, the epoxidation rate is then expressed as shown in Eq. (5) (Arumugam et al., 2014; Gan et al., 1995).
| (5) |
Where subscript 0 denotes initial concentrations, k denotes the rate constant of the epoxidation reaction and EP denotes epoxides.
Hence,
| (6) |
According to Eq. (6), plot of vs. time should yield straight lines for those reactions with insignificant oxirane degradation. However, reactions with substantial ring opening were seen to deviate from linearity. As such, their respective rate constants were obtained using the initial slopes.
2.7. Activation energy calculation
The energy level of the molecules to initiate a chemical reaction is generally represented as the activation energy.
For a second order model, rate constants increase with temperature and may be described by the Arrhenius law in Eq. (7).
| (7) |
Where: K= the extraction rate constant (L g−1 min−1)
A= the temperature independent factor (L g−1min−1)
= the activation energy (J mol−1)
R= the gas constant (8.314J mol−1 K−1)
T= the absolute suspension temperature (K)
Eq. (8) is the Linearized form of Eq. (7). From the plot of In (K) against I/T plot derived from Eq. (8), the values of the Activation energy () and the temperature independent factor (A) would be obtained from the slope and the intercept, respectively.
| (8) |
2.8. Thermodynamics studies of epoxidation reaction
In the epoxidation of Irvingia gabonensis kernel oil methyl esters (MIGKOt), Eq. (9) was used to estimate the average enthalpy of activation ΔH of the reaction (Okieimen et al., 2002).
| (9) |
Where is the activation energy calculated from the Arrhenius plot, T is temperature, and R is the universal gas constant.
Similarly, the average entropy of activation, ΔS, as well as the Gibbs free energy of activation, ΔG, were obtained using equations, 10 and 11, respectively (Okieimen et al., 2002).
| (10) |
| (11) |
Similarly, can also be expressed as shown in Eq. (12)
| (12) |
Where k, N and h are the rate constant, Avogadro () constant and Planck (6.63 × 10−34 J) constant, respectively.
2.9. Thermogravimetric Analyses (TGA) of Irvingia gabonensis kernel
The thermal stability/behavior of IGK was investigated using a Mettler-Toledo TGA/SDTA 851e thermogravimetric analyzer under nitrogen atmosphere. A total of 8.96 mg of the Irvingia gabonensis IGK sample was placed in an aluminium vessel and covered with a pierceable cap. Prior to the measurement of the IGK sample, the cap was perforated and the sample was heated from 24.52 °C to 800 °C at a heating rate of 3–4 °C/min. Nitrogen N2 was used as an inert atmospheric gas at 100 ml min−1 flow rate. The different weight loss phases and loss-rate temperatures for moisture and other combustible materials loss steps, alongside the overall percentage weight loss were determined using STARe Thermal Analysis Software.
3. Results and discussions
3.1. Oil yield of Irvingia gabonensis kernel
As already reported in our earlier publications, the oil yield of Irvingia gabonensis kernel (IGK) was 68.80 % (by dry mass basis) (Agu et al., 2020). This oil yield obtained, differ from other IGK oil yields reported elsewhere, 67.33 % (Ekpe et al., 2018), 73.83 % (Matos et al., 2009) and 69.76 % (Zoué et al., 2013). Variations in oil yields were associated with the geographical location, solvents of extraction, varieties of Irvingia gabonensis kernel, as well as seeds/kernels harvest period. However, when compared to other oil seeds, the oil yield obtained for IGK was significantly higher than those of Terminalia catappa L. kernel (60.45 %), Soybean (20.4 %) Colocynthis vulgaris Shrad Seeds and Cottonseed (24.82 %), as reported by Agu et al. (2019), Nikolić et al. (2009), Agu et al. (2018) and Sharif et al. (2019). Hence, the economic viability of IGK oil has been established, as well as its high oil yield; unlike seeds like Chrysophyllum albidum with lower oil yield.
3.2. Physicochemical properties of Irvingia gabonensis kernel oil (IGKO) and the epoxidized methyl ester (MIGKOe)
The properties of Irvingia gabonensis kernel oil (IGKO) and the epoxidized methyl ester of Irvingia gabonensis kernel l oil (MIGKOe) samples are presented in Table 1.
Table 1.
Physicochemical properties of Modified Irvingia gabonensis kernel oil samples.
| Property | Unit | TOa | IGKOb | MIGKOe | Standard method |
|---|---|---|---|---|---|
| Dielectric strength | KV | 40–60 | 25.83 ± 0.001 | 39.78 ± 0.001 | IEC 60156 |
| Moisture content | mg/kg | <20 | 3.75 ± 0.001 | 1.01 ± 0.001 | AOAC 926.12 |
| Pour point | °C | -48 | 17 ± 0.5 | 9 ± 0.5 | ASTM D97 |
| Flash point | °C | 152 | 285 ± 0.5 | 298 ± 0.5 | ASTM D93 |
| Density, 20 °C | g/cm3 | 870 | 900 ± 0.5 | 840 ± 0.5 | AOAC 985.19 |
| Viscosity, 40 °C | mm2/s | 10 | 19.37 ± 0.001 | 13.84 ± 0.001 | ASTM D445 |
| Acidity/Acid value | mg KOH/g oil | <0.01 | 5.18 ± 0.001 | 1.351 ± 0.001 | AOAC 969.17 |
conventional mineral transformer oil.
Irvingia gabonensis kernels oil.
Modified Irvingia gabonensis kernels oil obtained by epoxidation-esterification of the methyl ester.
3.2.1. Dielectric strength
Dielectric strength is the maximum voltage needed to cause dielectric breakdown of the material. It is also expressed as the maximum electrical strength that a material, like oil can constantly withstand without failure of its properties. Its importance cannot be overemphasized because it is vital in maintaining reliable operation of power transformers, in order to avert transformer failure (Agu et al., 2019). The dielectric strength (DS) (breakdown voltage) values for IGKO and MIGKOe were 25.83 and 39.78 , respectively (see Table 1). With reference to the values of the DS of IGKO and MIGKOe, it was noticed that the breakdown voltage changed (increased) after the modification of the IGKO; with MIGKOe having higher DS value. Hence, the reason for the 39.78 value, obtained for MIGKOe sample. This increase in the DS, resulting from the modification of the IGKO, was associated to esterification and transesterification processes used for methyl ester production; as well as the choice of the methyl ester over the oil for the expoxidation-esterification modification process (Menkiti et al., 2017b). The breakdown voltage of IGKO, obtained in this work is comparable, though slightly higher than those of crude palm oil (17–23 kV) (Azis et al., 2014) and palm kernel oil (25 kV) (Usman et al., 2012). Although the breakdown voltage of IGKO was slightly less, compared to 30.61 kV for Terminalia catappa L. kernel (Agu, 2019); it was significantly lower, compared to 60 kV reported for coconut oil by Abeysundara et al. (2001). These variations in DS of IGKO and other vegetable oils in literature were linked to differences in triglycerides of various vegetable oils; as a result of their diverse polar natures (Agu et al., 2019; Shah and Tahir, 2011). Furthermore, for the epoxidized methyl ester of Irvingia gabonensis kernel oil (MIGKOe), its dielectric strength (DS) was found to be lower, compared to epoxy methyl esters of palm kernel oil (42.58 kV) (Abdelmalik et al., 2011) and Terminalia catappa L. kernel oil (50.05 kV) (Agu et al., 2019). However, it was higher, compared to that of purified calabash seed oil transformer fluid (24 kV) (Oyelaran et al., 2020). Just like in the base vegetable oils, the difference in the DS of MIGKOe, and other epoxy methyl esters of various vegetable oil in literature, was attributed to their respective diverse triglycerides polar natures (Agu et al., 2019). Also, this difference in the DS of MIGKOe, and other works in literature, could be associated to diverse modification process routes, and reagents used (Agu et al., 2019).
3.2.2. Moisture content
The primary cause of poor vegetable oil quality, when compared to mineral oils is high moisture. This is attributed to its potential to increase tendency for oil to conduct electricity, as against its primary role of insulation especially for oils like transformer fluids (Menkiti et al., 2017b). It is therefore important to state that high moisture content in oils, results in reduction in DS of oil, as well as its insulation/cooling properties. As noted in Table 1, moisture contents for IGKO and MIGKOe were 3.75 and 1.01 , respectively. Lower moisture value was noticed for MIGKOe, when compared to IGKO. This decrease in the moisture content of MIGKOe sample was associated with catalytic reactions of esterification, transesterification, and the epoxidation-esterification modification processes. This is because oil modification, as seen in this work, lowered the moisture content of the final product (MIGKOe) (Menkiti et al., 2017b). Furthermore, the moisture content of IGKO was found to be higher, compared to those of Terminalia catappa L. kernel oil (2.1 mg/kg) (Agu et al., 2019) and soya bean oil (2.0 mg/kg) (Usman et al., 2012). However, moisture content variations were attributed to the method used to extract oil from the seeds (Ikya et al., 2013). This is because it has been established that the moisture content of solvent (method) extracted oils, is less than the traditional cold maturation extracted oils (Agu et al., 2018). Nevertheless, for the modified oil sample obtained by epoxidation-esterification of Irvingia gabonensis kernel oil methyl ester (MIGKOe), its moisture content was found to be lower than the 1.9 , reported for bio-transformer fluids obtained from palm oil; but the same as the 1.0 obtained from coconut (Aimi and Hussin, 2014). However, the moisture content of MIGKOe in this work was higher, compared to 0.18 for epoxidized waste cooking oil methyl ester (Paul et al., 2021) and 0.65 for epoxidized Terminalia catappa L. kernel oil methyl ester (Agu et al., 2019). As earlier reported for vegetable oils, the differences in moisture contents of MIGKOe sample, and those of bio-transformer fluids produced from coconut, Terminalia catappa L. kernel and palm oils, were linked to extraction and modification methods adopted (Agu et al., 2019).
3.2.3. Flash and pour points
As it relates to transformer fluid, flash point is the temperature during which constituents (especially hydrocarbons) present in the oil begins to evaporate, leading to flash when in contact with source of light. The implication is that the flash point must be kept above temperature of 140 °C. Conversely, the least temperature at which oil begins to flow at specific standard condition is referred to as the pour point. In temperate regions with icy/snow climate particularly during winter, pour point of transformer oil is a vital property of valuable consideration. This is due to the fact that if oil temperature drops below the pour point, transformer oil functionality of convection flow stops, resulting in obstruction of the transformer cooling (Agu, 2019). As presented in Table 1, with the epoxidation-esterification modification process, flash point of Irvingia gabonensis kernel oil (IGKO) sample increased, while pour point decreased. Hence, there was increase in flash point, and corresponding decrease in pour point of modified oil (MIGKOe), after the modification process. It is vital to state that the decline in pour point of IGKO resulting from the oil modification was linked to decline in the unsaturation nature of the oil sample, emanating from the modification processes (Adekunle et al., 2016). From the Table 1, the flash points of IGKO and MIGKOe samples were 285 and 298 , respectively. Furthermore, the flash point of IGKO was found to be higher than those of coconut oil (225 ), Terminalia catappa L. kernel oil (260 ) and Jetropha oil (146 ), as reported by Aimi and Hussin (2014), Agu et al. (2019) and Aliyu and Tijjani et al. (2017), respectively. For Irvingia gabonensis kernel oil (IGKO) samples reported by other researchers, Bello et al. (2011) reported that a flash point value of 300 . Similarly, the flash point (298 of MIGKOe, was found to be higher, compared to those of palm kernel alkyl ester with 148 (Abdelmalik, 2014) and epoxidized Terminalia catappa L. kernel oil methyl ester 280 (Agu et al., 2019). Also, the flash point of MIGKOe was higher than mineral transformer fluid with 152 (Menkiti et al., 2017b). On the other hand, Table 1 shows that pour point values of IGKO and MIGKOe samples were 17 and 9 , respectively. However, these values were higher, compared to the -48 °C value, for conventional transformer fluid. Higher pour points of IGKO and MIGKOe were due to unsaturated fatty acid ester presence in oil, as well as its leftover in the MIGKOe sample; despite the ester enrichment processes (Agu et al., 2019). IGKO pour value was found to be less than 28 °C, reported for Irvingia gabonensis kernel oil by Bello et al. (2011). This value was also found to be greater than the 2 3 and -1.15, reported for Moringa seed, Terminalia catappa L. kernel and castor seed oils, by Aliyu and Tijjani (2017), Agu et al. (2019) and Aliyu and Tijjani (2017), respectively. Pour point of MIGKOe was less than -8 , 2.40 , 4 °C and 8 °C values, reported by Agu et al. (2019), Paul et al. (2021), Oyelaran et al. (2020) and Borugadda and Goud (2014), for epoxidized Terminalia catappa L. kernel oil methyl ester, epoxidized waste cooking oil methyl ester, purified calabash seed oil transformer fluid and epoxidized castor oil methyl ester, respectively. The difference in pour point of MIGKOe and other epoxidized methyl ester samples and the purified calabash seed oil sample, was linked to the adopted extraction and modification methods (Demirbas, 2009). However, using pour point depressants, there is possibility of improvement in Pour point of MIGKOe (Agu, 2019).
3.2.4. Density
Density is defined as the substance mass per unit volume. The density of oil is very important as it helps/guides in pump designs, since most pump systems are designed to pump a fluid of a specific density. With respect to transformer fluid, it helps to ensure that proper cooling takes place in the system during operation (Agu, 2019). From Table 1, the densities of IGKO and MIGKOe samples were 900 and 840, respectively. The density of MIGKOe was seen to be lower, compared to that of IGKO (Table 1). This reduction in IGKO density value after modification, which is evident in the obtained value for MIGKOe, was seen to be due to the improved purity of the obtained final product (in this case MIGKOe); since vegetable oil modification, lowers impurities in the desired final product (Menkiti et al., 2017b). With reference to other results in the literature, it was found that density of IGKO was lower, compared to 930 for Irvingia gabonensis kernel oil (Bello et al., 2011). The difference in density of IGKO in this study, compared to other IGKO reported in the literature, could be associated with diverse morphologies of the seeds/kernels, and methods of extracting the oils (Ikya et al., 2013). However, the density of IGKO (900 ) in this study was observed to be higher, compared to those of Jatropha (720 ), Moringa (520 ), Castor (740 ) and cotton (850 ) oils (Aliyu and Tijjani, 2017). Also, the difference in density of IGKO, compared to other vegetable oil seeds/nuts could be associated with diverse morphologies of the seeds, as well as the method of extracting the oils (Agu et al., 2019). This is associated with the fact that better purity levels, hence lower densities, are obtained when solvent extraction using soxhlet extractor is used, unlike the adoption of cold maturation method (Agu et al., 2018) Furthermore, the density (840 ) of the modified oil sample obtained by epoxidation-esterification of Irvingia gabonensis kernel oil methyl ester (MIGKOe) was found to be lower than the 850 and 956 values, which were reported for purified calabash seed oil used as bio-transformer fluids and epoxidized castor oil fatty acid methyl ester, by Oyelaran et al. (2020) and Borugadda and Goud (2014), respectively. On the other hand, the density (840 ) of MIGKOe sample was higher than 773.8 , reported for epoxidized waste cooking oil methyl ester, by Paul et al. (2021). Also, as earlier stated for vegetable oils, this difference in the density of the MIGKOe sample and those of other bio-transformer fluids, was linked to extraction methods adopted; and the modification routes used for the processes (Agu et al., 2019). From Table 1, it is worthy of mention that the density of the final product obtained by epoxidation-esterification of Irvingia gabonensis kernel oil methyl ester (MIGKOe), was close to the standard stipulated for the density (870 ) of conventional mineral transformer oil.
3.2.5. Viscosity
It is evident from Table 1 that the viscosity of IGKO, significantly decreased after the modification process, giving rise to the final product (MIGKOe sample), with much lower viscosity value. Viscosity of IGKO and MIGKOe samples were 19.379 and 13.84 , respectively (See Table 1). This was due to the modification reactions processes routes adopted, which improve product (MIGKOe sample) purity, viscosity, as well as its thermo-oxidative stability state (Agu et al., 2019; Abdelmalik et al., 2011). As observed in Table 1, the viscosity of the obtained IGKO (19.37 ) was found to be lower than 45 reported for Irvingia gabonensis kernel oil in literature, by Bello et al. (2011). As earlier inferred, the differences in viscosities of IGKO in this study, and other IGKO reported in literature was likened to the extraction method used, in addition to the differences in their respective species (Agu et al., 2019). IGKO viscosity (19.37 ) in this work was found to be higher than the viscosity of other seeds/nuts oils like, Jatropha (16.50 ), Moringa (11.80 ), and Castor (18.10 ) (Aliyu and Tijjani, 2017). However, the viscosity of IGKO in this work was found to be lower, compared to those of Sunflower oil (34.5 ) (Rouabeh et al., 2019), Coconut oil (29 ) (Aimi and Hussin, 2014), Cotton oil (29.40 ) (Aliyu and Tijjani, 2017), Palm oil (29.2 ) (Aimi and Hussin, 2014), Terminalia catappa L. kernel oil (20.29 ) (Agu et al., 2019) and Olive oil (110.3 ) (Rouabeh et al., 2019). The differences in viscosity of these other seeds/nuts reported in the literature and that of IGKO in this study, was attributed to the difference in the morphologies of the seeds/nuts, in addition to the extraction method chosen for extraction purposes (Agu et al., 2019). Furthermore, the viscosity (13.84 ) of the modified oil sample (MIGKOe) was found to be higher than 8.8 (Oyelaran et al., 2020), and 12.15 (Paul et al., 2021), reported for purified calabash seed oil used as bio-transformer fluids and epoxidized waste cooking oil methyl ester respectively. However, its viscosity was lower, compared to the 170.85 , 35.81 and 55.13 , reported for epoxidized soybean oil, epoxidized castor oil fatty acid methyl esterand epoxidized trimethylolpropane (TMP) ester, by Adhvarye and Erhan (2002), Borugadda and Goud (2014) and Naidir et al. (2012), respectively. However, the 13.84 value obtained for MIGKOe sample, was higher than 3.2 , reported for IGKO biodiesel, by Bello et al. (2011). These observable differences, was likened to the variations in the structural formations of the molecules of the triglyceride in the respective base oils, together with the differences in the modification methods/process routes (Agu et al., 2019).
3.2.6. Acid value
As observed in Table 1, the acid values of IGKO and MIGKOe samples were 5.18 and 1.351 , respectively. From Table 1, it was observed that MIGKOe sample had lower acid value, when compared to IGKO. This decrease in acid value as evident in the MIGKOe sample could be attributed to the fact that the prevalent free fatty acids in IGKO were significantly removed after the modification process (Agu et al., 2019). Acid value of IGKO was higher, compared to other IGKO reported in literature by 4.67 (Zoué et al., 2013) and 1.2 (Bello et al., 2011). However, IGKO acid value was less, compared to 9.40 , as reported for Irvingia gabonensis kernel oil, by Etong et al. (2014). As earlier inferred in other earlier discussed properties, the differences in acid values of IGKO in this study, and other IGKO reported in literature was due to the extraction method used, in addition to the differences in their respective species (Agu et al., 2019). Nevertheless, acid value of IGKO in this work was higher, compared to those of Jatropha (3.45 ), Moringa (4.47 ), Castor (2.12 ), and Cotton (3.72 ) oils, reported by Aliyu and Tijjani (2017). These differences in the acid values of these other seeds/nuts reported in literature and that of IGKO in this study, was attributed to the difference in the morphologies of the seeds/nuts, in addition to extraction method used, as oils obtained by solvent extraction method, have higher acid value, compared to those obtained by cold maturation. Also, in solvent extraction method, solvent type significantly influences acid value of obtained oil (Agu et al., 2019). Furthermore, for epoxidized-esterified Irvingia gabonensis kernel oil methyl ester (MIGKOe), its acid value (1.351 ) was found to be higher than 0.142 , 1.08 and 0.59 , reported for purified calabash seed oil used as bio-transformer fluids (Oyelaran et al., 2020), epoxidized castor oil fatty acid methyl ester (Borugadda and Goud, 2014) and epoxidized methyl ester of Parkia biglobosa seed oil (Ikhuoria et al., 2007), respectively. Also, the acid value of MIGKOe (1.351 ) was still higher, compared to 0.20 for epoxidized waste cooking oil methyl ester (Paul et al., 2021) and 0.09 for epoxidized soybean oil (Adhvarye and Erhan, 2002). Furthermore, acid value of MIGKOe was also greater, compared to <0.01 recommended for mineral transformer oil by IEC standard. As earlier reported for vegetable oils, the difference in acid value of MIGKOe and that of purified calabash seed oil used as bio-transformer fluids was associated with adopted modification procedure (Agu et al., 2019). However, it is important to state that high acid value in transformer fluid, results in its absorption by the paper insulating materials in the transformer; hence resulting in increased degradation of the entire transformer insulation system (Kouassi et al., 2018).
3.3. Kinetics of the epoxidation of Irvingia gabonensis kernel oil methyl ester (MIGKOt)
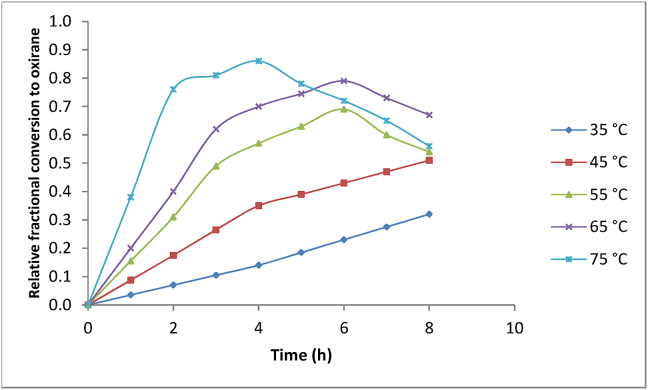
Figure 4 shows the plots for the in situ epoxidation of Irvingia gabonensis kernel oil methyl ester (MIGKOt) at different temperatures. According to Equ (6), the plot of against time results in straight lines, specifically for reactions that had insignificant degradation of oxirane [see Figure 4]. In this study, deviations from linearity were observed for the reactions (at 55, 65 and 75 °C) with substantial ring opening [Figure 4]. This was attributed to the epoxide degradation (Ikhuoria et al., 2007). In cases like this, epoxidation reaction rate constants were gotten using initial slopes.
Figure 4.
Plot of In [(H2O2)0 – (EP)] vs. time for the epoxidation of MIGKOt by peracetic acid.
The values of rate constants, gotten from linear portion of the plots for the studied sample (MIGKOt) at different temperatures are presented in Table 2. Rate constants gotten for MIGKOt sample are of the order of Lmol−1s−1 (Table 2). The rate constants obtained for the sample, were less than the Lmol−1s−1, as reported by Cai and Wang (2011) for the epoxidation of unsaturated fatty acid methyl ester. Although, this obtained rate constant for the epoxidation of the MIGKOt sample was of the same order of Lmol−1s−1, reported for oleic acid derived from palm kernel oil (PKO), by Jalil et al. (2018). However, the obtained rate constants of the studied sample was greater, compared to Lmol−1s−1 for epoxidations of rubber seed oil (Okieimen et al., 2002), palm olein methyl ester (Gan et al., 1992) and mahua oil (Goud et al., 2006). Similarly, the obtained rate constants in this work were also greater than the Lmol−1s−1, as reported by Ikhuoria et al. (2007) for the kinetics of epoxidation of methyl esters of Parkia Biglobosa seed oil. This difference in rate constants for epoxidation of MIGKOt sample, compared to other methyl esters and vegetable oils, was due to differences in hydrogen bonding present in molecular chains of these samples, as well as interaction between these molecules (Saalah et al., 2017; Blayo et al., 2001).
Table 2.
Rate constant for the epoxidation of MIGKOt sample at different temperatures.
| Temperature |
Rate constants for the epoxidation of MIGKOt sample |
|---|---|
| (°C) | K x 10−5 (Lmol−1S−1) |
| 35 | 3.02 |
| 45 | 4.83 |
| 55 | 12.4 |
| 65 | 15.8 |
| 75 | 21.8 |
Furthermore, it is important to state that the values of rate constants for the studied sample were temperature dependent. Hence, elevation of reaction temperature increases the rate constant. The reason behind this is that elevated temperature favors formation of peracetic acid, resulting in both accelerated reaction and increased oxirane conversion, in addition to enhanced rate of oxirane ring opening (Cai and Wang, 2011). For instance, there was over sevenfold increase in the rate constant for the epoxidation of MIGKOt, when temperature was increased from 35 to 75 °C. The results obtained in this study were slightly different from that obtained at temperature of 45–75 °C by Cai et al. (2008) with a fourfold increase in the rate constant value for the epoxidation of soybean oil. Also, Ikhuoria et al. (2007) reported a threefold increase in the rate constant value for the epoxidation of Parkia Biglobosa seed oil when the reaction temperature was increased from 50 to 70 °C.
3.4. Thermodynamics of the epoxidation of Irvingia gabonensis kernel oil methyl ester
The values of activation energy, calculated from the slope of the linear dependence of InK verses 1/T (K−1) (Eq. 8) [see Figure 5] for the epoxidation of the Irvingia gabonensis kernel oil methyl esters (MIGKOt) are presented in Table 3. From the table, the activation energy for MIGKOt sample is 46.02 kJ/mol. The value of the activation energy obtained for the studied sample was significantly lower, compared to those reported by Gan et al. (1992) (63.20 kJ/mol), Goud et al. (2006) (60.60 kJ/mol), Okieimen et al. (2002) (65.6 kJ/mol), Cai et al. (2008) (74.22 kJ/mol) and Ikhuoria et al. (2007) (51.96 kJ/mol), for epoxidations of palm olein methyl ester, mahua oil, rubber seed oil, corn oil and Parkia Biglobosa seed oil, respectively. However, the activation energy of MIGKOt sample was slightly higher, compared to those reported by Cai and Wang (2011) (45.5 kJ/mol) and Cai et al. (2008) (43.11 kJ/mol), for the epoxidations of unsaturated fatty acid methyl esters and soybean oil, respectively. Furthermore, the activation energy (46.02 kJ/mol), obtained for the epoxidation of MIGKOt, was significantly higher than 10.72 kJ/mol, 19.81 kJ/mol, 6.18 kJ/mol, 7.60 kJ/mol, and 7.58 kJ/mol, reported by Jalil et al. (2018), for soybean oil, sunflower oil, rapeseed oil, fatty acid methyl ester and oleic acid derived from palm kernel oil (PKO), respectively. The difference in activation energy obtained for epoxidation of MIGKOt, compared to other methyl esters and oils was associated with limited flexibility of the molecular chains and the interaction between the molecules in MIGKOt (Agu, 2019; Saalah et al., 2017; Blayo et al., 2001).
Figure 5.
Plot of Ink verses 1/T (K−1) for the epoxidation of MIGKOt sample, obtained by using Arrhenius equation.
Table 3.
Thermodynamics parameters for the epoxidation of MIGKOt sample.
| Temperature (K) | Ea |
ΔH |
ΔS |
ΔG |
|---|---|---|---|---|
| KJmol−1 | KJmol−1 | Jmol−1K−1 | KJmol−1K−1 | |
| 308 | 94.74 | |||
| 318 | 96.41 | |||
| 328 | 46.02 | 43.30 | -167.20 | 98.08 |
| 338 | 99.75 | |||
| 348 | 101.42 |
Thermodynamic parameters ΔH, ΔS and ΔG, for MIGKOt epoxidation, calculated from Eqs. (9), (10), and (11), respectively, are also presented in Table 3. From Table 3, the value of ΔH for the epoxidation of MIGKOt sample is 43.30 kJ mol−1. The obtained ΔH value for the epoxidation of the studied sample was similar in range to the values obtained for the epoxidations of unsaturated fatty acid methyl esters (42.70 kJ mol−1) (Cai and Wang, 2011) and soybean oil (40.63 kJ mol−1) (Cai et al., 2008). However, ΔH value obtained for the studied sample was much lower, compared to 60.5 kJ mol−1 for epoxidations of palm olein methyl ester (Gan et al., 1992), 63.5 kJ mol−1 for mahua oil (Goud et al., 2006) and 57.7 kJ mol−1 for rubber seed oil (Okieimen et al., 2002). The higher values of ΔH for the works reported in the literature by Gan et al. (1992), Goud et al. (2006) and Okieimen et al. (2002), compared to that for the epoxidation MIGKOt sample (43.30 kJ mol−1), could be attributed to pronounced hydrogen bonding in molecular chains of those samples reported in the literature, when compared to that of MIGKOt (Blayo et al., 2001). This is an indication that less energy (heat) was needed for the epoxidation of MIGKOt, unlike methyl esters and oils cited earlier by other authors. Conversely, the ΔH value obtained for MIGKOt (43.30 kJ mol−1) was much higher, compared to the 10.06, 19.16 and 6.18 kJ mol−1, reported by Jalil et al. (2018), for the epoxidations of soybean, sunflower, and rapeseed oils, respectively. The positive values of ΔH indicate that the energy input (heat) from external source is required to raise the energy level and transform the reactants to their transition states. This indicates that the process is endothermic in nature (Cai and Wang, 2011).
The value of ΔS for the epoxidation of MIGKOt is 167.20 J mol−1 K−1 (Table 3). From Table 3, it was observed that the value of ΔS for MIGKOt (.20 J mol−1 K−1) was significantly lower than those reported by Jalil et al. (2018) (.82 kJ mol−1 K−1), Gan et al. (1992) ( 146.0 kJ mol−1 K−1) and Goud et al. (2006) ( 146.0 kJ mol−1 K−1), for the epoxidations of soybean oil, palm olein methyl ester and mahua oil, respectively. However, in the works of Ikhuoria et al. (2007) and Okieimen et al. (2002), they reported that the ΔS value for epoxidation of Parkia Biglobosa seed oil and rubber seed oil were 3550 J mol−1 K−1 and 213.6 J mol−1 K−1, respectively. These values were lower than the ΔS value for MIGKOt epoxidation.
The value of ΔG for the epoxidation of MIGKOt sample was found to be in the range of 94.74–101.42 kJ mol−1 (Table 3). These positive values of ΔG indicate the non-spontaneous nature of the reaction (Cai and Wang, 2011). From Table 3, it was observed that the ΔG values in the MIGKOt sample studied, increased with temperature. Thus, indicating that the Gibb's free energy of a substance is temperature dependent (Lin et al., 2008; Cai and Wang, 2011). These values were similar in range with those reported by Cai et al. (2008) (110.01 kJ mol−1), Lin et al. (2008) (111.39 kJ mol−1), Cai and Wang (2011) (112.1 kJ mol−1), Goud et al. (2006) (106.3 kJ mol−1) and Okieimen et al. (2002) (127.9 kJ mol−1), for the epoxidation of sunflower oil, soybean oil, unsaturated fatty acid methyl ester, mahua and rubber seed oil, respectively. However, the ΔG range of values (94.74–101.42 kJ mol−1) for the epoxidation of MIGKOt sample was higher, compared to those for the epoxidation of soybean oil (25.98 kJ mol−1), sunflower oil (24.98 kJ mol−1) and rapeseed oil (22.78 kJ mol−1) (Jalil et al., 2018).
3.5. Thermogravimetric Analyses (TGA) of Irvingia gabonensis kernel (IGK)
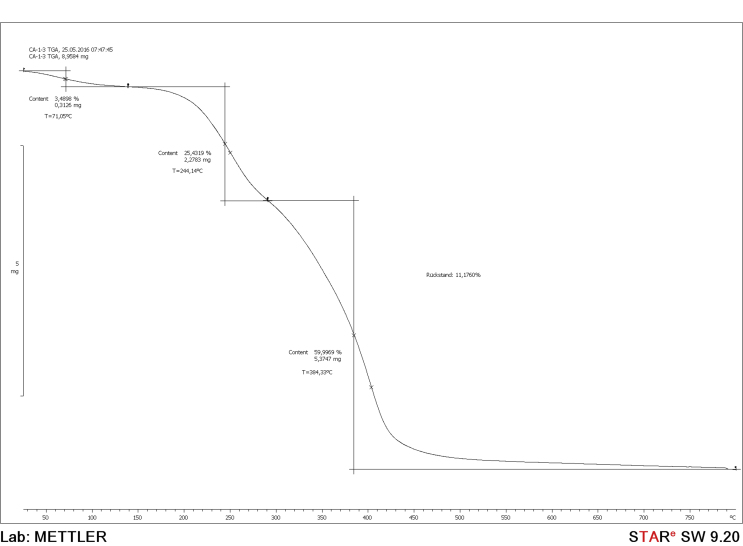
TGA was used to investigate the thermal decomposition and thermal stability behaviors of Irvingia gabonensis kernel (IGK) at heating rate of 3–4 °C/min. The decomposition of the IGK sample is shown in Figure 6. The thermograms show three weight loss phases for the IGK sample. Similar results have been reported by a number of researchers. Sugumaran et al. (2012) reported that during the thermal decomposition of banana empty fruit bunch and Delonix regia fruit pods, four and three weight losses were respectively observed. Similarly, Vichaphund et al. (2014) and Lotus et al. (2011) both reported three weight loss phases for the thermal decomposition of physic nut and as-spun fibers, respectively.
Figure 6.
Thermogravimetric Analysis (TGA) of Irvingia gabonensis kernel (IGK).
From Figure 6, the first phase for the IGK sample with range of 24.52–71.05 °C corresponded to the loss of moisture. This moisture loss represents 3.49 % initial weight losses in IGK. Similar results of moisture losses can be found in the works on the thermal decompositions of Chrosophyllum albidum (Sokoto et al., 2016), Jatropha curcus L. (Vichaphund et al., 2014), and banana empty fruit bunch (Sugumaran et al., 2012).
The second phase weight loss for the IGK (71.05–244.14 °C), could be attributed to (correspond to) the loss of residual moisture, oils and other volatile materials. This decomposed material at this phase represents 25.43 %, losses in the initial mass of the IGK sample. Furthermore, the third phase weight loss for the IGK (244.14–284.33 °C), corresponds to 60 % losses in the initial mass of the sample. At this phase, there was major decomposition of organic compounds like starches, fibers, cellulose biomass for IGK, as evident in its huge weight losses. Synonymous results have been reported in the literature for the thermal decompositions of Terminalia catappa L., Chrosophyllum albidum and natural fiber by Dos Santos et al. (2016), Sokoto et al. (2016) and Yao et al. (2008), respectively.
The thermal decomposition of IGK sample occurred within the temperature range of 244.14–384.33 °C, with maximum decomposition of 60 %. As could be seen in the curve in Figure 6, the complete pyrolysis of IGK sample occurred at 384.33 °C. This third phase thermal decomposition temperatures of the studied IGK sample is comparable to the degradation temperature range of 200–500 °C for Chrosophyllum albidun cake (Sokoto et al., 2016), safflower seed (Onay, 2007) and Cherry seed (Duman et al., 2011). However, this third phase decomposition temperature of the IGK sample was lower than 400–700 °C for rapeseeds cake (Onay and Koçkar, 2004).
Finally, beyond the decomposition temperature 384.33 °C, for the IGK sample, there was no significant change in the mass of the sample with further temperature increase. Hence, indicating the high thermal stability of the samples. Therefore, the residue left after the volatilization of combustible substances was 11.18 % of the initial mass of the IGK sample, used for the analyses. Practically, the high decomposition temperature of the sample is an indication of its high thermal stability.
4. Conclusion
Based on experimental conditions, after the modification of IGKO, MIGKOe had acceptable properties that were comparable to mineral transformer oil properties. Hence, the properties MIGKOe, significantly conformed to the stipulated IEC standard for transformer fluid. At extraction conditions of 55 °C, 150 min and 0.5 mm, 68.80 % was the maximum oil yield, extracted from Irvingia gabonensis kernel (IGK). Rate constant K and activation energy Ea for the epoxidation process was found to be of the order Lmol−1s−1 and 46.02 kJ/mol, respectively. The ΔG, ΔH, and ΔS values obtained for the epoxidation reaction indicates non-spontaneous, endothermic, and endergonic nature of the process. Finally, high decomposition temperature of the Irvingia gabonensis kernel (IGK) samples, as obtained from the Thermogravimetric Analyses (TGA) results, is an indication of the high thermal stability of the sample. In other words, IGKO and MIGKOe samples could be used in transformers as dielectric fluids.
Declarations
Author contribution statement
C.M. Agu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A.C. Agulanna, C.H. Kadurumba: Performed the experiments.
P.C. Nnaji, E.L Udokporo: Contributed reagents, materials, analysis tools or data.
M.C. Menkiti: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abdelmalik A.A. Chemically modified palm kernel oil ester: a possible sustainable alternative insulating fluid. Sustain. Mater. Technol. 2014;1–2:42–51. [Google Scholar]
- Abdelmalik A.A., Abbott A.P., Fothergill J.C., Dodd S., Harris R.C. Synthesis of a base stock for electrical insulating fluid based on palm kernel oil. Ind. Crop. Prod. 2011;33:532–536. [Google Scholar]
- Abeysundara D.C., Weerakon C., Lucas J.R., Gunatunga K.A.I., Obadage K.C. Coconut oil as an alternative to transformer oil. ERU Symposium. Sri Lanka For. 2001;1 – 11 [Google Scholar]
- Adekunle A.S., Oyekunle J.A.O., Obisesan O.R., Ojo O.S., Ojo O.S. Effects of degumming on biodiesel properties of some non-conventional seed oils. Energy Rep. 2016;2:188–193. [Google Scholar]
- Adepoju T.F. Response surface methodology (RSM) a good optimizer for transesterification reaction of chrysophyllum albidium seed oil to chrysophyllum albidium oil biodiesel. Int. J. Chem. Process Eng. Res. 2014;1(4):32–42. [Google Scholar]
- Adepoju T.F., Olamide O. Acid-catalyzed esterification of waste cooking oil (WCO) with high FFA for biodiesel production. Chem. Process Eng. Res. 2014;21:80–85. [Google Scholar]
- Adhvaryu A., Erhan S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crop. Prod. 2002;15:247–254. [Google Scholar]
- Agu C.M. Department of Chemical Engineering, Faculty of Engineering Nnamdi Azikiwe University; Awka, Anambra State, Nigeria: 2019. Synthesis and Modification of Selected Vegetable Oils for Transformer Oil Production Ph.D Thesis. March, 2019. [Google Scholar]
- Agu C.M., Kadurumba C.H., Agulanna A.C., Aneke O.O., Agu I.J., Eneh J.N. Nonlinear Kinetics, Thermodynamics, and parametric studies of Colocynthis vugaris Shrad seeds oil extraction. Ind. Crop. Prod. 2018;123:386–400. [Google Scholar]
- Agu C.M., Menkiti M.C., Ohale P.E., Ugonabo V.I. Extraction modeling, kinetics, and thermodynamics of solvent extraction of Irvingia gabonensis kernel oil, for possible industrial application. Eng. Rep. 2020;2020 [Google Scholar]
- Agu C.M., Menkiti M.C., Nwabanne J.T., Onukwuli O.D. Comparative assessment of chemically modified Terminalia catappa L. kernel oil samples – a promising ecofriendly transformer fluid. Ind. Crop. Prod. 2019;140:111727. [Google Scholar]
- Aimi A.H.Z., Hussin N. Comparative studies of vegetable oils as transformer insulating oil. Appl. Mech. Mater. 2014;679:200–206. [Google Scholar]
- Aji M.M., Kyari S.A., Zoaka G. Comparative studies between bio lubricants from jatropha oil, neem oil and mineral lubricant (Engen Super 20W/50) Appl. Res. J. 2015;1(4):252–257. [Google Scholar]
- Aliyu A.O., Tijjani A. Transesterification and epoxidation of oil extracts from selected plants for use as bio-transformer oil. Int. Res. J. Pure Appl. Chem. 2017;14(3):1–7. [Google Scholar]
- AOAC . fifteenth ed. Association of Official Analytical Chemists; Washington, D.C: 1990. Official Methods of Analysis. 1990. [Google Scholar]
- ASTM D445 . 2011. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (And Calculation of Dynamic Viscosity) 2011. [Google Scholar]
- ASTM D93 . 2012. Standard Test Methods for Flash Point by Pensky Martens Closed Cup Tester. 2012. [Google Scholar]
- ASTM D97 . ASTM International; West Conshohocken, PA: 2012. Standard Test Method for Pour Point of Petroleum Products. 2012. [Google Scholar]
- Arumugam S., Sriram G., Rajmohan T. Multi-Response Optimization of Epoxidation process parameters of rapeseed oil using response surface methodology (RSM)-Based Desirability Analysis. Arabian J. Sci. Eng. 2014;39:227–2287. [Google Scholar]
- Azis N., Jasni J., Abidin Ab Kadir M.Z., Mohtar M.N. Suitability of palm based oil as dielectric insulating fluid in transformers. J. Elect. Eng. Technol. 2014;9(2):662–669. [Google Scholar]
- Bello E.I., Fade-Aluko A.O., Anjorin S.A., Mogaji T.S. Characterization and evaluation of African bush mango Nut (Dika nut) (Irvingia gabonensis) oil biodiesel as alternative fuel for diesel engines. J. Petr. Technol. Altern. Fuels. 2011;2(9):176–180. [Google Scholar]
- Beltran N., Palacios E., Blass G. Potential of Jatropha curcas oil as a dielectric fluid for power transformers. IEEE Electr. Insul. Mag. 2017;3:8–15. [Google Scholar]
- Berhanu A., Amare G. Production and characterization of biodiesel from Brebra (M. Ferruginea) seed non-edible oil. Biotechnology. 2012;11:217–224. [Google Scholar]
- Blayo A., Gandini A., Nest Le J.-F. Chemical and rheological characterizations of some vegetable oils derivatives commonly used in printing inks. Ind. Crop. Prod. 2001;14:155–167. [Google Scholar]
- Borugadda V.B., Goud V.V. Epoxidation of castor oil fatty acid methyl esters (COFAME) as a lubricant base stock using heterogeneous ion-exchange resin (IR-120) as a catalyst. Energy Proc. 2014;54:75–84. [Google Scholar]
- Cai C., Dai H., Chen R., Su C., Xu X., Zhang S., Yang L. Studies on the kinetics of in situ epoxidation of vegetable oils. Eur. J. Lipid Sci. Technol. 2008;110:341–346. [Google Scholar]
- Cai S., Wang L. Epoxidation of unsaturated fatty acid methyl esters in the presence of SO3H-functional Bronsted acidic ionic liquid catalyst. Chin. J. Chem. Eng. 2011;19(1):57–63. [Google Scholar]
- Campanella A., Fontanini C., Baltanás M.A. High yield epoxidation of fatty acid methyl ester with performic acid generated in situ. Chem. Eng. J. 2008;144:466–475. [Google Scholar]
- Campanella A., Rustoy E., Baldessari A., Baltanás M.A. Lubricants from chemical modified vegetable oils. Bioresour. Technol. 2010;101:245–254. doi: 10.1016/j.biortech.2009.08.035. [DOI] [PubMed] [Google Scholar]
- Chhetri A.B., Tango M.S., Budge S.M., Watts K.C., Islam M.R. Non-edible plant oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008;9:169–180. doi: 10.3390/ijms9020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciubota-Rosie C., Díaz-Medino A., Ramos M.J., Pérez A., Carmona M., Rodríguez J.F. The role of epoxidation on Camelina sativa Biodiesel properties. Global Nest J. 2014;16(6):1076–1084. [Google Scholar]
- Demirbas A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009;50:14–34. [Google Scholar]
- Dorado M.P., Cruz F., Palomar J.M., Lopez F.J. An approach to the economics of two vegetable oil-based biofuels in Spain. Renew. Energy. 2006;31:1231–1237. [Google Scholar]
- Dos Santos O.V., Lorenzo N.D., Lannes S.C.D. Chemical, morphological, and thermogravimetric of Terminalia catappa Linn. Food Sci. Technol. 2016;36(1):151–158. [Google Scholar]
- Duman G., Okutucu C., Ucar S., Stahl R., Yanik J. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011;102:1869–1878. doi: 10.1016/j.biortech.2010.07.051. [DOI] [PubMed] [Google Scholar]
- Ekpe O.O., Bassey S.O., Udefa A.L., Essien N.M. Physicochemical properties and fatty acid profile of Irvingia gabonensis (Kuwing) seed oil. Int. J. Food Sci. Nutr. 2018;3(4):153–156. [Google Scholar]
- Etong D.I., Mustapha A.O., Taleat A.A. Physicochemical properties and fatty acid composition of Dikanut (IrvingiaGabonensis) seed oil. Res. J. Chem. Sci. 2014;4(12):70–74. [Google Scholar]
- Gan L.H., Goh S.H., Ooi K.S. Kinetics studies of epoxidation and oxirane cleavage of palm olein methyl esters. J. Am. Oil Chem. Soc. 1992;69(4):347–351. [Google Scholar]
- Gan L.H., Ooi K.S., Gan L.M., Goh S.H. Effect of epoxidation on the thermal oxidative stabilities of esters of fatty acids derived from palm olein. J. Am. Oil Chem. Soc. 1995;72(4):439–442. [Google Scholar]
- Goud V.V., Patwardhan A.V., Pradhan N.C. Studies on the epoxidation of mahua oil (Madhumica indica) by hydrogen peroxide. Bioresour. Technol. 2006;97:1365–1371. doi: 10.1016/j.biortech.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Huang Y.-B., Yao M.-Y., Xin P.-P., Zhou M.-C., Yang T., Pan H. Influence of alkenyl structures on the epoxidation of unsaturated fatty acid methyl esters and vegetable oils. RSC Adv. 2015;5:74783. [Google Scholar]
- Hwang H.S., Adhvaryu A., Erhan S.Z. Preparation and properties of lubricant basestocks from epoxidized soybean oil and 2-ethylhexanol. J. Am. Oil Chem. Soc. 2003;81:811–815. [Google Scholar]
- Ikhuoria E.U., Obuleke R.O., Okieimen F.E. Studies on the epoxidation of the methyl esters of Parkia biglobosa seed oil. J. Macromol. Sci., Pure Appl. Chem. 2007;44:235–238. [Google Scholar]
- Ikya J.K., Umenger L.N., Lorbee A. Effects of extraction methods on the yield and quality characteristics of oils from shea nut. J. Food Resour. Sci. 2013;2(1):1–12. [Google Scholar]
- Jalil M.J., Yamin A.F.M., Azmi I.S., Jamaludin S.K., Daud A.R.M. Mechanism and kinetics study in homogenous epoxidation of vegetable oil. Int. J. Eng. Technol. 2018;7(4.42):124–126. [Google Scholar]
- Knothe G., Krahl J., Gerpen J.V. AOCS Press; Champaign, IL: 2007. The Biodiesel Handbook. [Google Scholar]
- Kongyai C., Chalermsinsuwan B., Hunsom M. Epoxidation of waste used oil biodiesel: effect of reaction factors and its impact on the oxidative stability. Kor. J. Chem. Eng. 2013;30(2):327–336. [Google Scholar]
- Kouassi K.D., Fofana I., Cissé L., Hadjadj Y., Yapi K.M.L., Diby K.A. Impact of low molecular weight Acids on oil impregnated paper insulation degradation. Energies. 2018;11:1465. [Google Scholar]
- Lin B., Yang L., Dai H., Yi A. Kinetics studies on oxirane cleavage of epoxidized soybean oil by methanol and characterization of polyols. J. Am. Oil Chem. Soc. 2008;85:113–117. [Google Scholar]
- Lotus A.F., Tacastacas S.N., Pinti M.J., Britton L.A., Stojilovic N., Ramsier R.D., Chase G.G. Fabrication and characterization of TiO2 –ZnO composite nanofibers. Physica E. 2011;43:857–861. [Google Scholar]
- Matos L., Nzikou J.M., Matouba E., Pandzou-Yembe V.N., Mapepoulou T.G., Linder M., Desobry S. Studies of Irvingia gabonensis seeds kernels: oil technological applications. Pakistan J. Nutr. 2009;8(2):151–157. [Google Scholar]
- Menkiti M.C., Agu C.M., Udeigwe T.K. Extraction of oil from Terminalia catappa L.: process parameter impacts, kinetics, and thermodynamics. Ind. Crop. Prod. 2015;77:713–723. [Google Scholar]
- Menkiti M.C., Ocheje O., Agu C.M. Production of environmentally adapted lubricant basestock from Jatropha curcas specie seed oil. Int. J. Integrated Care. 2017 [Google Scholar]
- Menkiti M.C., Agu C.M., Ejikeme P.M., Onyelucheya O.E. Chemically improved Terminalia catappa L. oil: a possible renewable substitute for conventional mineraltransformer oil. J. Environ. Chem. Eng. 2017;5:1107–1118. [Google Scholar]
- Mohammed A., Samer C., Gary L., Syed M.I. Patent No.; 2007. Low Viscosity Oil-Based Dielectric Fluids. WO 2007041785 A1 (2007) [Google Scholar]
- Naidir F., Yunus R., Rashid U., Masood H., Ghazi T.I.M., Ramli I. The kinetics of epoxidation of trimethylolpropane ester. Eur. J. Lipid Sci. Technol. 2012 [Google Scholar]
- Nikolić N.Č., Cakić S.M., Novaković S.M., Cvetković M.D., Stanković M.Z. Effect of extraction techniques on yield and composition of soybean oil. Macedonian J. Chem. Chem. Eng. 2009;28(2):173–179. [Google Scholar]
- Okieimen F.E., Bakare O.I., Okieimen C.O. Studies on the epoxidation of rubber seed oil. Ind. Crop. Prod. 2002;15:139–144. [Google Scholar]
- Onay O. Influence of pyrolysis temperature and heating rate on the production of bio-oil and char from safflower seed by pyrolysis, using a well-swept fixed-bed reactor. Fuel Process. Technol. 2007;88:523–531. [Google Scholar]
- Onay O., Koçkar O.M. Fixed-bed pyrolysis of rapeseed (Brassica napus L.) Biomass Bioenergy. 2004;26:289–299. [Google Scholar]
- Ong H.C., Silitonga A.S., Masjuki H.H., Mahlia T.M.I., Chong W.T., Boosroh M.H. Production and comparative fuel properties of biodiesel from non-edible oils: Jatropha curcas, Sterculia foetida and Ceiba pentandra. Energy Convers. Manag. 2013;73:245–255. [Google Scholar]
- Oyelaran O.A., Bolaji B.O., Samuel O.D. Assessment of calabash seed oil as biobased insulating fluid for power transformers. J. Chem. Technol. Metallurgy. 2020;55(2):307–313. [Google Scholar]
- Paul A.K., Borugadda V.B., Goud V.V. In-situ epoxidation of waste cooking oil and its methyl esters for lubricant applications: characterization and rheology. Lubricants. 2021;9:27. [Google Scholar]
- Rashid U., Anwar F., Yunus R., Al-Muhtaseb A.H. Transesterification for biodiesel production using Thespesia populnea seed oil: an optimization study. Int. J. Green Energy. 2014;12(5):479–484. [Google Scholar]
- Rouabeh J., M’barki L., Hammami A., Jallouli I., Driss A. Studies of different types of insulating oils and their mixtures as an alternative to mineral oil for cooling power transformers. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e01159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalah S., Abdullah L.C., Aung M.M., Salleh M.Z., Biak D.R.A., Basri M., Jusoh E.R., Mamat S. Physicochemical properties of jatropha oil-based polyol produced by a two steps method. Molecules. 2017;22:551. doi: 10.3390/molecules22040551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadia H., Ahmad M., Zafar M., Sultana S., Azam A., Khan M.A. Variables effecting the optimization of non-edible wild safflower oil biodiesel using alkali catalyzed transesterification. Int. J. Green Energy. 2013;10(1):53–62. [Google Scholar]
- Salimon J., Salih N., Yousif E. Biolubricants: raw materials, chemical modifications and environmental benefits. Eur. J. Lipid Sci. Technol. 2010;112:519–530. [Google Scholar]
- Sanchez A.J.P., Hernandez C.M.U., Mendez S.F.C., Rios J.R.V., De Leon J.E.C., Zubiaga D.A.G. United States Patent; 2014. Vegetable oil of high dielectric purity, method for obtaining same and use in an electrical device. [Google Scholar]
- Shah Z.H., Tahir Q.A. Dielectric properties of vegetable oils. J. Sci. Res. 2011;3(3):481–492. [Google Scholar]
- Sharif I., Farooq J., Chohan S.M., Saleem S., Kainth R.A., Mahmood A., Sarwar G. Strategies to enhance cottonseed oil contents and reshape fatty acid profile employing different breeding and genetic engineering approaches. J. Integr. Agric. 2019;18(10):2205–2218. [Google Scholar]
- Sharma B.K., Adhvaryu A., Liu Z., Erhan S.Z. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006;83:129–136. [Google Scholar]
- Shote A., Betiku E., Asere A.A. Biodiesel production by transmethylation of Nigeria palm kernel oil. Ife J. Technol. 2009;18:1–4. [Google Scholar]
- Shuangfei C., Lisheng W. Epoxidation of unsaturated fatty acid methyl esters in the presence of SO3H-functional bronsted acidic ionic liquid as catalyst. Chin. J. Chem. Eng. 2011;19(1):57–63. [Google Scholar]
- Sokoto M.A., Singh R., Krishna B.B., Kumar J., Bhaskar T. Heliyon; 2016. Non-isothermal Kinetics Study of De-oiled Seeds Cakes of African star Apple (Chrosophyllum Albidum) Using Thermogravimerty. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran P., Priya Susan V., Ravichandran P., Seshadri S. Production and characterization of activated carbon from banana empty fruit bunch and Delonix regia fruit pod. J. Sustain. Energy Environ. 2012;3:125–132. [Google Scholar]
- Usman M.A., Olanipekun O.O., Henshaw U.T. A comparative study of soya bean oil and palm kernel oil as alternatives to transformer oil. J. Emerg. Trends Eng. Appl. Sci. 2012;3(1):33–37. [Google Scholar]
- Vichaphund S., Aht-ong D., Sricharoenchaikul V., Atong D. Chemical Engineering Research and Design; 2014. Effectofcrystallizationtemperatureontheinsitu Valorization ofphysicnut(JatrophacurcusL.) wastesusingsyntheticHZSM-5catalyst. [Google Scholar]
- Viertel J., Ohlsson K., Singha S. 2014 IEEE 18th Int. Conf. Dielectr. Liq. 2014. Studies of the improvement of the viscosity of natural ester liquids; pp. 1–4. [Google Scholar]
- Wai P.T., Jiang P., Shen Y., Zhang P., Gu Q., Leng Y. Catalytic developments in the epoxidation of vegetable oils and the analysis methods of epoxidized products. RSC Adv. 2019;9:38119. doi: 10.1039/c9ra05943a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao F., Wu Q., Lei Y., Guo W., Xu Y. Thermal decomposition kinetics of natural fibers: activation energy with dynamic thermogravimetric analysis. Polym. Degrad. Stabil. 2008;93:90–98. [Google Scholar]
- Zoue´ L.T., Bedikou M.E., Faulet B.M., Gonnety J.T., Niamke´ S.L. Characterisation of highly saturated Irvingia gabonensis seed kernel oil with unusual linolenic acid content. Food Sci. Technol. Int. 2013;19(1):79–87. doi: 10.1177/1082013212442190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.