Summary
Research on the gut microbiome and related diseases is rapidly growing with the development of sequencing technologies. An increasing number of studies offer new perspectives on disease development or treatment. Among these, the mechanisms of gut microbial metabolite-mediated effects merit better understanding. In this review, we first summarize the shifts in gut microbial metabolites within complex diseases, in which metabolites have correlational and occasionally causal effects on diseases and discuss the reported mechanisms. We further investigate the interactions between gut microbes and drugs, providing insights for precision medication as well as limitations of current research. Finally, we provide new research directions and research strategies for the development of drugs from gut microbial metabolites.
Funding statement
None
Keywords: Gut microbial metabolites, Drug-microbiome interaction, Health and diseases, Host-microbiome cross-talk
Introduction
There is accumulating evidence that the gut microbiome closely engages with host health and disease. Many studies have uncovered differences in the gut microbiome between patients and healthy individuals, with (to some extent) reproducible signatures for particular diseases.1 Gut microbiota-derived metabolite differences have also been observed in diseased and healthy individuals.2
The relationship between these differential metabolites and disease can be two-fold: 1) Diseases lead to alterations in gut microbiome metabolites. These altered metabolites can therefore be used as biomarkers for the disease. 2) Gut microbes incite disease through their metabolites, thus being risk factors for certain diseases. In this review, we first summarize a list of metabolites associated with disease and then focus on those gut microbial metabolites that can protect the host from diseases, with a particular interest in metabolites that have pharmaceutical potentials. We further discuss the cases in drug interaction with and metabolism by gut microbes.
Small molecules implicated in representative human diseases
Gut microbiota-derived metabolites, especially short-chain fatty acids (SCFAs), have been shown to be involved and play an important role in host-microbiome cross-talk. To date, a mounting number of gut microbial metabolites have been shown to be associated with various diseases. We focused on metabolic disorders, cardiovascular disease, and central neural diseases for the burden they place on global health.3 Here, we summarize the altered gut microbial metabolites in the patients with these diseases, particularly the ones serving as risk factors. Table 1 presents metabolites elevated or reduced in the disease, and Table 2 lists the known effects of metabolites in disease.
Table 1.
Changes in gut microbial metabolites in representative diseases.
| Disease | Metabolites | Levels in patients |
|---|---|---|
| Inflammatory Bowel Disease | Primary bile acids | Increased6 |
| Fatty Liver | N, N, N-trimethyl-5-aminovaleric acid | Increased7 |
| Hepatocellular carcinoma | Linoleic acid and phenol | Decreased24 |
| Blood pressure | Acetate, butyrate, and propionate | Increased24 |
| Chronic kidney disease | Indole, indole-3-carboxaldehyde and indole-3-propionic acid | Decreased8 |
| Obesity |
SCFA | Decreased10 |
| Succinic acid | Increased12 | |
| Type 2 diabetes/prodromal diabetes | Imidazole Propionate | Increased16 |
Table 2.
Examples of roles of gut microbial metabolites in disease.
| Disease | Metabolites | Features |
|---|---|---|
| Hepatitis | Tryptophan metabolites | Neutralizing8 |
| Impaired Liver Function | DL-3-phenyllactic acid, L-tryptophan, glycocholic acid and 1-methylnicotinamide | Significant correlation7 |
| Atherosclerosis | TMAO | Promoting18, 19, 20 |
| Chronic Kidney Disease |
Advanced glycation end products (AGEs), phenylacetic acid, indole-3-acetic acid, p-Cresol sulfate | Inducing inflammation8 |
| Indoxyl sulfate | Risk-related9 | |
| Indoxyl sulfate and p-Cresol sulfate | Pathological process related9 | |
| Obesity |
BCAAs | Positive correlation10 |
| Succinic acid | Positive correlation12 | |
| δ-valerobetaine (N, N, N-trimethyl-5-aminovaleric acid, δVB) | Exacerbating obesity manifestations13 | |
| Insulin Resistance |
SCFA | Improvement14 |
| Succinic acid | Improvement14 | |
| p-Cresol | Inducing effects15 | |
| Type 2 Diabetes |
Dimethylglycine | Increased risk associated with16 |
| 1-linoleoylglycerophosphocholine (18:2) | Reduced risk16 | |
| Indole-3-propionic acid | Negative correlation17 | |
| Tryptophan, four kynurenine-pathway metabolites (kynurenine, kynurenate, xanthurenate and quinolinate) and indolelactate | Positive correlation17 | |
| Type 2 Diabetes/prodromal diabetes | Imidazole propionate | Significant correlation16 |
Metabolic disorders
Because of the anatomical link with the intestine, the liver is the first major metabolic organ to receive gut microbial metabolites, and the hepatic portal vein is the channel through which the liver receives and transports microbial metabolites from the gut. Gut microbial metabolites play an important role in liver diseases, such as alcoholic/non-alcoholic fatty liver disease, as well as hepatocellular carcinoma. Gut microbiota-derived SCFAs, bile acids, and aromatic amino acid metabolites are all associated with liver pathology. Specifically, SCFAs are considered to be able to alleviate the development of nonalcoholic fatty liver diseases (NAFLD), which may derive from their potential contribution to regulating fatty acid oxidation, inflammation, and insulin resistance.4
Additionally, patients with severe alcoholic cirrhosis have a significantly altered bile acid profile and gut microbiome dysbiosis, accelerating the liver disease process.5 Recent studies have also reported various gut microbial metabolites other than SCFAs and bile acids associated with liver diseases, such as microbial aromatic acid metabolites, N, N, N-trimethyl-5-aminopentanoic acid (TMAVA, also known as δ-valerobetaine),6 linoleic acid, and phenol.7 Specifically, levels of aromatic amino acid metabolites, such as phenylacetic acid (PLA), are altered in patients with liver disease, while tryptophan metabolites can directly reduce inflammation in the liver or indirectly affect liver function by modulating the intestinal barrier.8 Zhao et al. found elevated levels of TMAVA in patients with fatty liver and that TMAVA treatment exacerbated fatty liver induced by a high-fat diet by affecting carnitine synthesis and subsequent fatty acid oxidation in a mouse model.6 Liu et al. reported metabolites enriched in hepatocellular carcinoma patients, such as DL-3-phenyl lactate, L-tryptophan, and 1-methylnicotinamide, which led to impaired liver function and poorer survival rates. Conversely, hepatocellular carcinoma patients had significantly decreased linoleic acid and phenol levels in serum and feces, two metabolites that significantly inhibit the growth of hepatocellular carcinoma cells.7 Overall, patients with liver diseases have altered gut microbial metabolites and these changes are associated with the disease process.
A large proportion of gut metabolites enter the circulation to perform their functions and are eventually cleared by the kidneys. Studies have shown that in states of impaired renal function, uremic toxins produced by gut microbes such as advanced glycation end products (AGEs), the aromatic amino acid derivatives phenylacetic acid (PAA), indole-3-acetic acid (IAA), and p-cresol sulfate accumulate, thereby inducing inflammation and leading to chronic kidney disease (CKD).8 Moreover, elevated levels of indoxyl sulfate (microbial metabolite of tryptophan) were observed in patients with CKD and in a partially nephrectomized mice model, indoxyl sulfate was shown to activate the intrarenal renin–angiotensin–aldosterone system and further interstitial fibrosis and glomerulosclerosis.9 Therefore, microbial uremic toxins can act as risk factors for kidney diseases, while altering these metabolites can be useful for exploratory treatment of kidney diseases.
Similarly, the gut microbial metabolites in the systemic circulation are capable of affecting host obesity and diabetes. In a study in which fecal microbiota collected from four twin pairs with obesity were transplanted into germ-free mice, the mice then showed obesity phenotypes. SCFAs levels in their feces declined and circulating levels of branched-chain and aromatic amino acids (BCAAs) were elevated,10 which hints at the significant roles of gut microbiota-derived metabolites in obesity. Moreover, SCFAs have been reported to have potential to enhance intestinal gluconeogenesis, with hepatic glucose production declining and energy-spending increasing.2 Furthermore, SCFAs are capable of facilitating peptide YY (PYY) and glucagon-like peptide-1 (GLP-1) secretion, which can promote satiety and increase peripheral glucose catabolism.11 Therefore, gut microbiota-derived SCFAs play an important role in regulating energy balance and weight. Other associations were also found between important metabolites and obesity, although there were few established causal relationships. For example, study have reported the increased levels of succinate (an important metabolite of glucose metabolism) in plasma and feces of obese patients and a positive correlation between succinate levels and body weight.12 Recently, an additional microbiome-derived metabolite δ-valerobetaine (TMAVA), a trimethylamine N-oxide (TMAO) structural analogue, was indicated to be a diet-dependent obesogen that could exacerbate obesity in mice induced by the western diet.13 Moreover, studies have shown that gut microbial metabolites can influence insulin resistance. Specifically, SCFAs-induced improvements in adipose tissue metabolism can prevent insulin resistance, while succinate improves the host insulin sensitivity while preventing obesity.14 In addition, p-cresol was shown to have the potential to induce diabetes, with mice given p-cresol sulfate for four consecutive weeks showing an insulin-resistant phenotype.15 Large-scale cohorts have also revealed a range of gut microbial metabolites associated with type 2 diabetes (T2D), such as dimethylglycine, imidazole propionate,16 tryptophan, kynurenine and indolelactate,17 which are associated with increased risk of T2D, as well as 1-linoleoylglycerophosphocholine (18:2)16 and indole-3-propionic acid (indolepropionic),17 with potential for significantly reducing T2D risk.
Cardiovascular diseases
Gut microbiota-derived bioactive metabolites in the circulatory system influence host cardiovascular health, particularly TMAO, the hepatic oxidation product of the microbial trimethylamine (TMA), has been established in several large-scale cohort studies to be a potential promoter of atherosclerosis.18, 19, 20 Initially, TMAO was identified as a cardiovascular disease risk factor in a study involving >1,800 patients with stable cardiac profiles undergoing elective coronary angiography, and dietary supplementation with TMAO was shown to promote atherogenesis and development of atherosclerosis in mice.18 Later, in a study involving 4,007 patients with stable cardiac profiles undergoing elective coronary angiography, elevated circulating TMAO levels were again shown to be associated with an increased risk of adverse cardiovascular events.19 Recently, carotid atherosclerosis was demonstrated to be associated with gut microbial metabolites (especially TMAO and p-cresol sulfate) in >3,000 patients, which could serve as an independent predictor of the disease.20 Additionally, TMAO levels are also associated with heart failure21 and coronary artery disease.22 Animal models suggest a possible mechanism for TMAO promoting cardiovascular disease, that TMAO enhances the responsiveness of platelets to multiple agonists, promoting platelet hyperactivity and thus thrombosis and subsequent cardiovascular disease.23
In addition to TMAO, gut microbial metabolites such as SCFAs and tryptophan derivatives also influence cardiovascular health. SCFAs are physiologically potent and functionally diverse, acting as signaling molecules that activates the G-protein-coupled receptor pathway to regulate host blood pressure.21 There is accumulating evidence to support the correlation between SCFAs and blood pressure. For example, Huart et al. investigated the metabolome of individuals with different blood pressure levels and found significantly different SCFAs levels, with significantly higher levels of acetate, propionate, and butyrate in hypertensive patients than in subjects with normal blood pressure.24 However, tryptophan metabolites may play an opposite role, for example, Cason et al. compared plasma metabolomes of patients with advanced atherosclerosis and healthy controls and showed that levels of microbial metabolites of tryptophan, indole, indole-3-carboxaldehyde, and indole-3-propionic acid were significantly lower in patients.25 Altogether, gut microbes can produce bioactive metabolites that affect host cardiovascular health through various pathways.
Central neural diseases
It is established as well that microbial metabolites also have important effects on the host nervous system, known as “gut-brain axis” that has been extensively studied. It has been shown that metabolites, such as SCFAs, serotonin, kynurenine, indole and its derivatives, and tryptamine, can bridge the gut and nervous system.26 In detail, kynurenine and indole derivatives might be associated with neurogenic depression, while SCFAs had the potential to alleviate chronic stress-induced insomnia and enhanced stress response, as well as stress-induced increases in intestinal permeability in mouse models.26 Additionally, SCFAs can promote microglia maturation and restore microglia defects.27 Furthermore, reduced levels of SCFAs were observed in patients with Alzheimer's disease, and they also promoted depression-like behaviors and impairments of short-term memory in mice.28 However, in children with autism, SCFA levels were differentially altered, with an increase in propionate and acetate and a decrease in butyrate levels.29 Based on the current evidence, we found that SCFA supplementation had inconsistent effects on central neural diseases; for example, rats fed propionate exhibited phenotypic features similar to those of autism.30 In contrast, SCFAs can restore the function of the blood-brain barrier in patients with multiple sclerosis.31 Moreover, indole-3-propionic acid has been shown to have neuroprotective and antioxidant effects, protecting primary neurons and neuroblastoma cells from oxidative damage and death caused by amyloid β protein (Aβ).8 Elevated levels of glutamine and γ-aminobutyric acid (GABA) and reduced glutamate were observed in the hippocampus of germ-free mice that received fecal transplants from schizophrenic mice and showed schizophrenia-like behaviors.32
Gut microbial metabolites with treatment potential
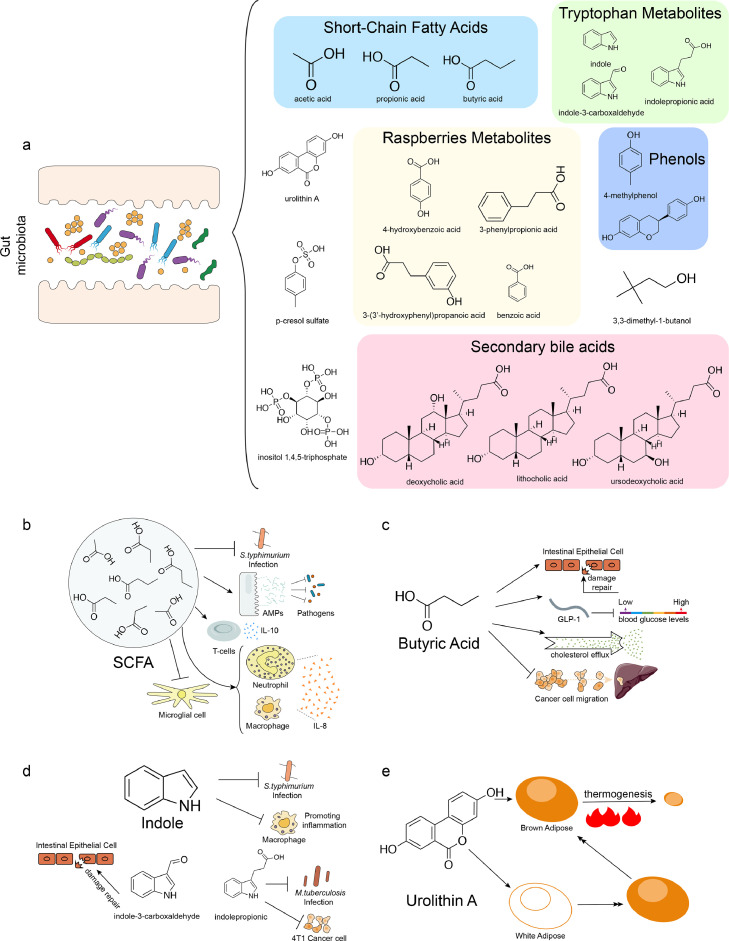
Through the study of gut microbiome and host health, gut microbial metabolites are increasingly being explored for their potential pharmaceutical value (Figure 1a). For example, SCFAs and tryptophan have been shown to improve the health status of patients in a variety of different diseases, and various small molecule metabolites of food origin and metabolized by gut microbes have also been shown to have effects on host health.
Figure 1.
Examples of gut microbial metabolites with pharmaceutical potential. a. The types and structures of major functional gut microbial metabolites. b. The physiological activities of short-chain fatty acids (SCFAs), against Salmonella typhimurium (S. typhimurium) infection, were by promoting the production of antimicrobial peptides of intestinal epitheliums, regulating IL-10 and IL-8 production, and inhibiting activation of microglial cell. c. The major physiological functions of butyric acid, including promoting intestinal barrier repairmen, regulating blood glucose levels, promoting cholesterol efflux, and inhibiting liver metastases from colorectal cancer. d. The potential protective effects of indole and their derivatives on host health. Indole has anti-infection potential and anti-inflammatory properties, and their derivatives have anti-infection activities against Mycobacterium tuberculosis (M. tuberculosis) and anticancer activities, as well as the intestinal epithelial cell damage repair capacity. e. The beneficial effects of urolithin A on host health, in promoting thermogenesis of brown adipose tissue (BAT), and browning of white adipose tissue (WAT).
Short-chain fatty acids
Gut microbiota-derived metabolites with the earliest and most recognized pharmaceutical potential are SCFAs, mainly produced by microbes metabolizing dietary fiber (Figure 1b). SCFAs have been confirmed to display antibacterial activity by disturbing the intracellular acid-alkaline balance or acylating certain virulence factors of Salmonella typhimurium.33 Furthermore, SCFAs could also promote the production of antimicrobial peptides in the intestinal epithelium, thus helping the host to resist broad-spectrum pathogenic infections.34 For gastrointestinal diseases such as inflammatory bowel disease (IBD), SCFAs have been shown to alleviate inflammatory phenotypes by regulating IL-10 production by T cells in both humans and the mouse model.35 In patients with respiratory inflammation, SCFAs downregulate IL-8 expression by targeting the activation of free fatty acid receptors 2 and 3 (FFAR2 and FFAR3) on macrophages and neutrophils, thereby reducing inflammation.36 In addition, effects of SCFAs can extend to the nervous system; specifically, SCFAs could promote recovery after stroke by acting on microglia to inhibit their activation.37
Butyrate or butyric acid, a member of SCFAs, in particular can serve as a palliative or therapeutic strategy for certain diseases alone (Figure 1c). Studies have shown that gut microbial butyrate has a direct impact on the intestine, promoting intestinal barrier function, accelerating repair of intestinal epithelial cell damage, and maintaining intestinal homeostasis.38 Moreover, in a mouse model with hyperglycemia, butyrate has also been shown to increase diverse circulating glucose-regulating hormones by promoting intestinal expression of GLP-1, ultimately lowering blood glucose levels.39 In addition to direct action on the intestine, butyrate can also be transported to other tissues where it plays an important role; for example, use of butyrate has been shown to be a potential therapeutic strategy for atherosclerosis, specifically by its promoting cholesterol efflux through upregulation of ABCA1 expression in macrophages, thereby ameliorating atherosclerosis.40 Furthermore, butyrate can modulate anti-regulate levels of pro-inflammatory factors in patients with respiratory inflammation, exert anti-inflammatory effects,36 and reduce autoreactive T cell-mediated apoptosis in allogeneic transplant recipients41; altogether, butyrate plays an important role in regulating host immune response. Additionally, it has been shown that butyrate bridges the gut microbiome and nervous system, and studies have shown that butyrate supplementation can suppress appetite and reduce food intake in a high-fat diet (HFD) mouse model, thereby preventing obesity and dyslipidemia induced by HFD.42
Indole and derivatives
Gut microbial metabolites of tryptophan, mainly indole and its derivatives, are crucial for host health as well (Figure 1d). First, indole has antipathogenic potential toward Salmonella by reducing the expression of virulence genes.43 Indole also exerts anti-inflammatory properties, that is, counteracting the lipopolysaccharide (LPS)-induced inflammatory response and inhibiting pro-inflammatory activation of macrophages,44 thus protecting the liver. Indole-3-propionic acid, an indole derivative, exhibits biological activities similar to those of indoles, such as affecting host susceptibility to Mycobacterium tuberculosis,45 anticancer activity.46 Indole-3-carboxyaldehyde, an indole derivative with resistance to intestinal epithelial damage and anti-inflammatory activity, has been shown to alleviate adverse symptoms of graft-versus-host disease (GvHD) and reduce mortality in GvHD.47 Clostridium perfringens also metabolizes tryptophan to tryptamine, a metabolite that decreases macrophage inflammatory indicators and reduces pro-inflammatory cytokines stimulated by fatty acids and LPS.48 Together, tryptophan metabolites form a large class of metabolites and play an important role in modulating inflammatory responses and development of diseases.
Food derivatives metabolized by gut microbes
Many of the gut microbial metabolites originate from food and are then metabolized by gut microbiome into various functional metabolites; in return, those metabolites have specific effects on the host that have been linked to food itself. For instance, urolithin A (UA), a gut microbial metabolite of ellagic acid from certain fruits, walnuts, and wine, has been demonstrated to benefit host health, with the capability of reducing intestinal inflammation, repairing the intestinal barrier, and preventing colitis49 (Figure 1e). Moreover, UA has also been shown to prevent hereditary or HFD-induced obesity by increasing energy expenditure mainly through promoting thermogenesis of brown adipose tissue (BAT) and browning of white adipose tissue (WAT).50 More importantly, studies indicate that UA is closely related to mitochondrial function, that is, it is currently the only compound that can rebuild cellular recirculation of defective mitochondria, improving mitochondrial conditions in myocytes and muscle health.51
Xenobiotics from drug-microbiome interactions
It has been widely accepted in pharmaceutical studies that some drugs are effective only for a proportion of patients, leading to suboptimal final clinical outcomes. Recent studies on gut microbes have provided one of the possible reasons for this phenomenon, where gut microbes interact with drugs, leading to perturbation of gut microbes and gut microbes’ metabolizing drugs, causing changes in drug activity and thus altering the ultimate therapeutic effect. Disturbance of the gut microbial community structure has shown confounding effects in the treatment of different diseases. The direct alteration of drug activity, for better or worse, is a major concern, as this affects the therapeutic potential and side effects of the drugs.
Drugs with influences on microbiome
The antibiotics used in the treatment of disease often have a non-negligible disturbance of the gut microbiota. The disrupted gut microbial structure often has different, unintended, and negative effects. For example, antibiotics cause a rapid loss of gut microbial oxalate metabolism, and while disturbed bacteria can partially recover over time, the ability to metabolize oxalate does not.52 This results in the accumulation of oxalates in the body and increases the risk of kidney stones. In a population study by Ronald et al., there was a significant positive correlation between antibiotic consumption and the risk of colon cancer in people under 50 years old.53 Thus, the potential negative effects of antibiotics need to be fully considered when establishing treatment regimens.
Apart from antibiotics, targeting gut microbes to modulate the effects of drugs or alleviate their side effects is also an area that deserves further research. The main drug for Parkinson's disease is levodopa (L-dopa),54 and it has been shown that only about 1% to 5% of L-dopa crosses the blood-brain barrier to reach the brain. Tyrosine decarboxylase (TyrDC), present in gut microbes, converts L-dopa into dopamine, which does not cross the blood-brain barrier and accumulates in the gut, causing many side effects such as cardiac arrhythmias and nausea.55 In addition, levodopa can be metabolized by Clostridium sporogenes to 3-(3,4-dihydroxyphenyl) propionic acid (DHPPA), a metabolite that inhibits muscle contraction in the ileum, reduces intestinal movement, and impairs absorption in the small intestine.56 Also, in a mice model, oral intake of the tyrosine analogue α-fluoromethyltyrosine (AFMT) can inhibit TyrDC activity, thus decreasing the side effects of levodopa in the treatment of Parkinson's disease.57
In addition, manipulating microbes with differential drug metabolism properties may also achieve a therapeutic effect along with removing certain gut microbial metabolites that are not beneficial to treatment. For example, TMAO as mentioned above is a risk metabolite for cardiovascular disease,19 thus providing implications for treatment. Study has shown that Bilophila, a genus of gut microbes, can metabolize TMA, resulting in lower TMAO,58 suggesting that differences in TMA-metabolizing gut bacteria in individuals lead to different ultimate effects, and replacement of relevant bacteria may reduce the risk from TMAO. Another possible option besides targeting gut microbes is to target metabolites that affect the efficacy of the drug, e.g., metformin is the main drug currently used in the treatment of T2D; however, in some patients the reduction in blood glucose is not significant and studies have shown that these patients have high levels of the gut microbiota metabolite imidazole propionate. It has been demonstrated in mouse models that imidazole propionate derived from gut microbes can impair the glucose-lowering effect of metformin59; therefore, developing inhibitors that effectively inhibit imidazole propionate could be effective in restoring the glucose-lowering effect of metformin.
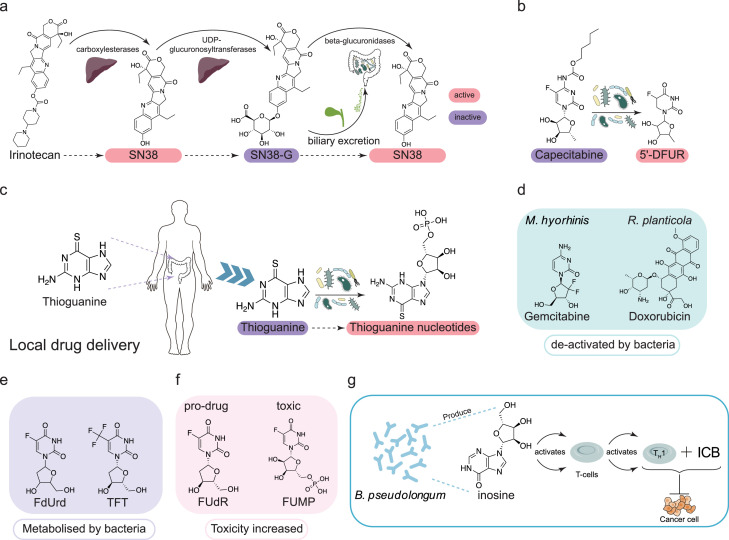
Microbiome-metabolized products of drugs
Gut microbes can metabolize oral drugs and alter their activity. Irinotecan, a drug for advanced colorectal cancer, is metabolized by the host carboxylesterases to its active form, SN38, which is then converted by UDP-glucuronosyltransferases to the inactive form of SN38G and excreted into the intestine via the bile. After entering the intestine, β-glucuronidases of gut microbes can reduce it again to SN38, thus restoring the drug activity60 (Figure 2a). Moreover, capecitabine, an adjuvant chemotherapeutic agent for colon cancer, was shown to be metabolized by mycoplasma-encoded thymidine phosphorylase (TP) in gut microbes to 5-fluoro-5′-deoxyuridine (5′DFUR), and this metabolite possessed enhanced cancer cytotoxicity driven by activation of this prodrug by TP61 (Figure 2b).
Figure 2.
Examples of facilitation or interruption of gut microbial metabolism for drug therapy or other therapeutic approaches. a. The metabolism of irinotecan in the body and the transformation of its anticancer activity. Specially, it can be metabolized by the host carboxylesterases to its active form, SN38, which is then de-activated into SN38G by host UDP-glucuronosyltransferases. The SN38G can enter the intestine and be converted into SN38 by β-glucuronidases of gut microbe. b. The activation of the capecitabine by gut microbes. The capecitabine can be metabolized into its active form, the 5-fluoro-5’-deoxyuridine (5’DFUR), by thymidine phosphorylase (TP) of gut microbes. c. The activation of the thioguanine by gut microbes. The thiopurine was administrated local to the gut and activated into functionally thioguanine nucleotides. d,e. De-active metabolism of drugs by gut microbes. Four anticancer drugs, gemcitabine, doxorubicin, 5-fluoro-2’-deoxyuridine (FdUrd), and 5-trifluorothymidine (TFT), can be degraded by gut microbes to their inactive forms. f. The bacterial toxicity-enhancing effects of some toxic substances. Gut microbes can convert the FUdR to the toxic 5-fluorouridine-5’-monophosphate (FUMP). g. Adjunctive effect of gut microbial inosine on immune checkpoint blockade (ICB). Inosine produced by Bifidobacterium pseudolongum (B. pseudolongum) can promote the derivation and activation of Th1 cells and enhance the therapeutic effect of ICB.
There is also the potential for antibiotic treatment to reduce the efficacy of certain drugs. The potential Parkinson's disease drug FLZ, formulated as N-2-[(4-hydroxyphenyl)-ethyl]-2-(2,5-dimethoxy-phenyl)-3-(3-methoxy-4-hydroxyphenyl)-acrylamide, was shown to be metabolized by gut microbes, and its metabolites can be rapidly absorbed into the blood where they are methylated to FLZ, and it has been shown that significant reduction in the improvement of Parkinson's disease was found with the use of antibiotics and interference of gut microbiota.62 Oxaliplatin, an anti-tumor drug, has been shown to be influenced by gut microbes; treatment with oxaliplatin in SPF mice on antibiotics or in germ-free mice often does not have a significant therapeutic effect. This is because butyrate, a metabolite of gut microbes, promotes the efficacy of the anti-tumor drug oxaliplatin by modulating CD8+ T cells, a mechanism that is attenuated or even eliminated in mice lacking some/all gut microbes.63 Cyclophosphamide is a drug commonly used to treat P815 mast cell tumors. Studies have shown that cyclophosphamide mediates the accumulation of TH17 and TH1 cells in conjunction with gram-positive bacteria (Lactobacillus, segmented filamentous bacteria) in the gut, and that long-term antibiotic treatment disrupts the composition of the gut microbiota, thus reducing the effectiveness of cyclophosphamide in tumor treatment.64
Some metabolites have no therapeutic effect in the intestine and do not exert their true therapeutic effect until they enter the blood. Immune checkpoint blockade (ICB)-treated colon cancer mice have an impaired intestinal barrier, which allows inosine, a metabolite of B. pseudolongum, to cross the intestinal barrier into the blood. When inosine enters the blood, it acts on T cells and promotes the derivation and activation of Th1 cells, thus enhancing the therapeutic effect of ICB (Figure 2g). Similarly, the use of inosine together with ICB in mouse models of intestinal cancer, bladder cancer, and melanoma has been shown to enhance the therapeutic effect.65 T cell-specific responses to B. thetaiotaomicron/B. fragilis correlate with the efficacy of CTLA-4 blockade in mouse models and patients. Tumor-model mice treated with antibiotics or germ-free mice did not respond to CTLA blockade.66
Different bacterial strains may have completely different responses to the same drug. A study by Lee et al. demonstrated that two different Bifidobacterium bifidum strains (synergistic B. bifidum KCTC3357 and non-synergistic B. bifidum Bb-06) in conjunction with oxaliplatin had different effects in patients with non-small cell lung cancer.67 The strain B. bifidum KCTC3357 with oxaliplatin significantly increased the number of anti-tumor lymphocytes (including CD8+ T and effector CD8+ T cells) and enhanced the ratio of CD8+ T/Treg cells to effector CD8+ T/Treg cells in spleen and tumors. Strain B. bifidum Bb-06 with oxaliplatin treatment had weaker effects, and increased L-tryptophan, uric acid, and N-acetyl zonisamide in the metabolites of mice. It was also demonstrated in an in vitro assay that L-tryptophan-treated CD8+T increased IFN-γ production.
In addition, drugs with side effects, such as thiopurine (an effective treatment for IBD), can cause DNA damage in host cells when delivered systemically. Studies have shown that the gut microbes have the ability to convert thioguanine into its active form, and therefore, local administration of thioguanine in the rectum can be an effective treatment for colitis while reducing the risk of serious side effects of being delivered systemically68 (Figure 2c). These results suggest that the composition of the gut microbes plays a crucial role in drug efficacy.
In addition to activation, gut microbes can also inactivate drugs through metabolism, such as the pancreatic adenocarcinoma treatment gemcitabine (2′,2′-difluoro-2′-deoxycytidine), as M. hyorhinis (ATCC 17981) in the gut can phosphorylate gemcitabine into inactive derivatives69 (Figure 2d). Riquelme et al. found that Gammaproteobacteria could also metabolize gemcitabine to an inactive form, leading to a reduction in efficacy, which was reversed by co-administration with antibiotics. Subsequently, the presence of Gammaproteobacteria in the mouse intestine and its metabolism of gemcitabine were shown to induce resistance to gemcitabine in pancreatic tumors.70 The intestinal bacterium Raoultella planticola can metabolize the anti-tumor drug doxorubicin under anaerobic conditions, rendering it inactive71 (Figure 2d). In addition, studies have shown that some nucleoside analogues, which are widely used as chemotherapeutic agents for the treatment of cancer, such as 5-fluoro-2′-deoxyuridine (FdUrd), 5-trifluorothymidine (TFT), and 5-halogenated 2′-deoxyuridines, can be degraded to inactive bases by TP in the intestine61 (Figure 2e). In addition to activating and nullifying the drug, gut microbes can also potentiate the action of toxic substances. For example, thymidine supplementation in the diet promotes the conversion of the prodrug floxuridine (FUdR) to the toxic 5-fluorouridine-5′-monophosphate (FUMP) by gut microbes, leading to host death as a result of mitochondrial RNA and DNA depletion and lethal activation of autophagy72 (Figure 2f).
Gut microbiome structure can also influence drug metabolism and its efficacy. Recently, Javdan et al. established a subject-specific gut microbial community in vitro and co-cultured 575 drugs with this system, showing that a total of 438 (76%) drugs could be metabolized and degraded by gut microbes.73 The study also found that inter-individual differences in gut microbial composition and the extensive metabolism of drugs by gut microbes (76% of drugs are metabolized and degraded) resulted in different efficacy of the same drug in different patients. Similarly, Zimmermann et al.74 co-cultured 271 drugs with 76 gut bacteria and found that about two-thirds of the drugs were metabolized and degraded by at least one gut bacterial strain, while 11-95 drugs were metabolized by each bacterium, and drugs with a common core structure were more likely to be metabolized by the same bacterial enzyme. These studies suggest an important role of gut microbial composition on drug metabolism and drug efficacy.
Conclusion
In this review, we first summarized the altered gut microbial metabolites in patients within three categories of representative and important diseases. However, these significant alterations do not directly indicate a causal link between these metabolites and health. The metabolites observed in these studies require further experimental to confirm their functions. For example, central neural diseases patients with reduced SCFAs, whereas SCFAs have been shown to have neuroprotective effects, such as promoting microglia maturation and restoring its defects.27 These prior insights indicate that SCFAs appear to be beneficial metabolites for alleviating some central neural diseases, and supplementation of SCFAs in patients with multiple sclerosis indeed helped alleviate the disease.31 Furthermore, the variations in the gut microbiome among individuals mentioned above indicate that sample size is critical for the screening of metabolites with therapeutic potential, and a sufficiently large sample size or a meta-analysis of multiple independent studies are required to achieve reliable conclusions. We need to avoid over-interpreting metabolite changes in patients, but they do offer a list of candidates to be investigated.
We then summarized the evidence for gut microbial metabolites interacting with the host. Studies indicate there are a few metabolites with pleiotropic effects, they may exhibit beneficial effects in various diseases, and have multiple biological activities. Butyrate, for example, has been proven biologically effective in maintaining the functional integrity of the intestinal barrier,38 lowering blood glucose levels,39 alleviating atherosclerotic symptoms,40 and having synergistic antitumor effects.63 Such of metabolites is ever increasing, and we have summarized them in the second part. However, it remains to be answered regarding their biological activities in healthy populations and the relative importance of multiple targets in disease alleviation. We believe partial answer can be obtained through further investigations, for example dietary manipulations of metabolite levels in healthy germ-free mice and observation of the changes in gene expression, can provide a more comprehensive understanding of metabolite functions.
We finally described cases where gut microbes and drugs interacted and drug efficacy was altered. In drug-microbe interactions, drugs are metabolized by gut microbes, altered therapeutic effects. Moreover, it is necessary to identify the enzymes and genes via functional genomics and narrow down the exact species or strains, as in Lee et al., different strains of Bifidobacterium bifidum had different therapeutic effects when synergized with oxaliplatin.67 Furthermore, the measurement of metabolic abilities of drugs in the patient's gut may provide a better prediction of drug efficacy than the traditional evaluation of alterations in the gut microbial composition. For instance, there are studies evaluated the metabolic abilities in individuals by in vitro culturing gut species and co-culturing with candidate drugs.73,74
To summarize, we have compiled examples wherein the gut microbial metabolites play an important role in the host health and disease. Studying the systematic effects of metabolites in patients also requires monitoring of the metabolites across different tissues, which will be important perspectives for metabolomic studies. Meanwhile, the impact of gut microbes on cancer treatment response through drug metabolism and immunomodulation varies widely. The interaction between gut microbes and hosts in cancer therapy has been well characterized recently by Jun Yu's group,75 suggesting that gut microbes play an important role in cancer treatment. Combining metagenomics, metabolome, and other omics will thus lead to improved resolution in studying the etiology, treatment, and prevention of many diseases.
Outstanding questions
To further improve the understanding of the interactions between gut microbiota metabolites and hosts and to apply these insights to disease prevention and treatment, there are still multiple questions needed to be addressed, including:
More refined and precise mechanisms of how gut microbial metabolites affect host health. Our understanding of gut microbes is still at its most basic level, and researches into their metabolites is progressively shedding light on some of the underlying mechanisms. However, most of these researches are still at the stage of correlational analysis, but lack further mechanistic studies. Such information is greatly helpful to be applied in development of novel treatment options.
Better metabolite identification systems need to be developed. At present, absolute quantification of metabolites on a large scale is difficult to achieve due to the high cost of quantitative metabolomes. Therefore, more convenient quantification techniques are needed to reduce the cost of quantitative metabolomes if we want to obtain more accurate and reliable quantitative indicators for disease diagnosis and prevention, drug development, efficacy screening, and independent pharmacology of drugs. Moreover, many gut microbial metabolites cannot be accurately identified currently as the limitations of the current metabolome identification database, which hinders the discovery and mechanistic study of novel functional metabolites. Therefore, it is also important to improve the database to identify as many metabolites as possible.
Search strategy and selection criteria
Data for this review were collected using PubMed searches between 2008-2022, using the terms “Gut microbial metabolites”, “Short-chain fatty acids”, “Indole”, “Metabolic disorders”, “Cardiovascular diseases”, “Central neural diseases”, “Cancer”, “TMAO”, “type 2 diabetes”.
Contributions
Jun Wang contributed to the conception and design of the review. The first draft of the manuscript was written by Yue Ma and Xiaolin Liu. Yue Ma and Xiaolin Liu created all the Figures and tables. All authors contributed to the article and approved the submitted version.
Declaration of interests
The authors declare no competing interest.
Acknowledgement
This work was supported by the National Key Research and Development Program of China (2021YFA1301000), the National Science Foundation of China (91857101) and the Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDB29020000),.
References
- 1.Liu Y, Méric G, Havulinna AS, et al. Early prediction of incident liver disease using conventional risk factors and gut-microbiome-augmented gradient boosting. Cell Metab. 2022;34:719–730. doi: 10.1016/j.cmet.2022.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou M, Johnston LJ, Wu C, Ma X. Gut microbiota and its metabolites: bridge of dietary nutrients and obesity-related diseases. Crit Rev Food Sci Nutr. 2021:1–18. doi: 10.1080/10408398.2021.1986466. [DOI] [PubMed] [Google Scholar]
- 3.WHO. The top 10 causes of death: World Health Organization; 2020 [Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 4.Zhang S, Zhao J, Xie F, et al. Dietary fiber-derived short-chain fatty acids: a potential therapeutic target to alleviate obesity-related nonalcoholic fatty liver disease. Obes Rev. 2021;22(11):e13316. doi: 10.1111/obr.13316. [DOI] [PubMed] [Google Scholar]
- 5.Ciocan D, Voican CS, Wrzosek L, et al. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol Ther. 2018;48(9):961–974. doi: 10.1111/apt.14949. [DOI] [PubMed] [Google Scholar]
- 6.Zhao M, Zhao L, Xiong X, et al. TMAVA, a metabolite of intestinal microbes, is increased in plasma from patients with liver steatosis, inhibits γ-butyrobetaine hydroxylase, and exacerbates fatty liver in mice. Gastroenterology. 2020;158(8):2266–2281. doi: 10.1053/j.gastro.2020.02.033. e27. [DOI] [PubMed] [Google Scholar]
- 7.Liu J, Geng W, Sun H, et al. Integrative metabolomic characterisation identifies altered portal vein serum metabolome contributing to human hepatocellular carcinoma. Gut. 2021 doi: 10.1136/gutjnl-2021-325189. gutjnl-2021-325189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu Y, Hou Y, Wang G, Zheng X, Hao H. Gut microbial metabolites of aromatic amino acids as signals in host-microbe interplay. Trends Endocrinol Metab. 2020;31(11):818–834. doi: 10.1016/j.tem.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Van Treuren W, Dodd D. Microbial contribution to the human metabolome: implications for health and disease. Ann Rev Pathol. 2020;15(1):345–369. doi: 10.1146/annurev-pathol-020117-043559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150) doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers ES, Morrison DJ, Frost G. Control of appetite and energy intake by SCFA: what are the potential underlying mechanisms? Proc Nutr Soc. 2015;74(3):328–336. doi: 10.1017/S0029665114001657. [DOI] [PubMed] [Google Scholar]
- 12.Serena C, Ceperuelo-Mallafré V, Keiran N, et al. Elevated circulating levels of succinate in human obesity are linked to specific gut microbiota. ISME J. 2018;12(7):1642–1657. doi: 10.1038/s41396-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mossad O, Nent E, Woltemate S, et al. Microbiota-dependent increase in δ-valerobetaine alters neuronal function and is responsible for age-related cognitive decline. Nature Aging. 2021;1(12):1127–1136. doi: 10.1038/s43587-021-00141-4. [DOI] [PubMed] [Google Scholar]
- 14.Canfora EE, Meex RCR, Venema K, Blaak EE. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat Rev Endocrinol. 2019;15(5):261–273. doi: 10.1038/s41574-019-0156-z. [DOI] [PubMed] [Google Scholar]
- 15.Koppe L, Pillon NJ, Vella RE, et al. p-Cresyl sulfate promotes insulin resistance associated with CKD. J Am Soc Nephrol. 2013;24(1):88–99. doi: 10.1681/ASN.2012050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vangipurapu J, Fernandes Silva L, Kuulasmaa T, Smith U, Laakso M. Microbiota-Related Metabolites and the Risk of Type 2 Diabetes. Diabetes Care. 2020;43(6):1319–1325. doi: 10.2337/dc19-2533. [DOI] [PubMed] [Google Scholar]
- 17.Qi Q, Li J, Yu B, et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut. 2021 doi: 10.1136/gutjnl-2021-324053. gutjnl-2021- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogiatzi C, Gloor G, Allen-Vercoe E, et al. Metabolic products of the intestinal microbiome and extremes of atherosclerosis. Atherosclerosis. 2018;273:91–97. doi: 10.1016/j.atherosclerosis.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120(7):1183–1196. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Li J, Li Y, et al. Gut microbiota–derived metabolites and risk of coronary artery disease: a prospective study among US men and women. Am J Clin Nutr. 2021;114(1):238–247. doi: 10.1093/ajcn/nqab053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu W, Gregory JC, Org E, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165(1):111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huart J, Leenders J, Taminiau B, et al. Gut Microbiota and Fecal Levels of Short-Chain Fatty Acids Differ Upon 24-Hour Blood Pressure Levels in Men. Hypertension. 2019;74(4):1005–1013. doi: 10.1161/HYPERTENSIONAHA.118.12588. [DOI] [PubMed] [Google Scholar]
- 25.Cason CA, Dolan KT, Sharma G, et al. Plasma microbiome-modulated indole- and phenyl-derived metabolites associate with advanced atherosclerosis and postoperative outcomes. J Vasc Surg. 2018;68(5):1552–1562. doi: 10.1016/j.jvs.2017.09.029. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erny D, Dokalis N, Mezö C, et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021;33(11):2260–2276. doi: 10.1016/j.cmet.2021.10.010. e7. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Wang Y, Xiayu X, et al. Altered gut microbiota in a mouse model of Alzheimer's disease. J Alzheimers Dis. 2017;60(4):1241–1257. doi: 10.3233/JAD-170020. [DOI] [PubMed] [Google Scholar]
- 29.De Angelis M, Francavilla R, Piccolo M, De Giacomo A, Gobbetti M. Autism spectrum disorders and intestinal microbiota. Gut Microbes. 2015;6(3):207–213. doi: 10.1080/19490976.2015.1035855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shultz SR, MacFabe DF, Ossenkopp KP, et al. Intracerebroventricular injection of propionic acid, an enteric bacterial metabolic end-product, impairs social behavior in the rat: implications for an animal model of autism. Neuropharmacology. 2008;54(6):901–911. doi: 10.1016/j.neuropharm.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 31.Braniste V, Al-Asmakh M, Kowal C, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6(263):263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng P, Zeng B, Liu M, et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci Adv. 2019;5(2):eaau8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang ZJ, Pedicord VA, Peng T, Hang HC. Site-specific acylation of a bacterial virulence regulator attenuates infection. Nat Chem Biol. 2020;16(1):95–103. doi: 10.1038/s41589-019-0392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Chen F, Wu W, et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018;11(3):752–762. doi: 10.1038/mi.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun M, Wu W, Chen L, et al. Microbiota-derived short-chain fatty acids promote Th1 cell IL-10 production to maintain intestinal homeostasis. Nat Commun. 2018;9(1):3555. doi: 10.1038/s41467-018-05901-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinolo MA, Rodrigues HG, Hatanaka E, Sato FT, Sampaio SC, Curi R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J Nutr Biochem. 2011;22(9):849–855. doi: 10.1016/j.jnutbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 37.Sadler R, Cramer JV, Heindl S, et al. Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J Neurosci. 2020;40(5):1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang RX, Lee JS, Campbell EL, Colgan SP. Microbiota-derived butyrate dynamically regulates intestinal homeostasis through regulation of actin-associated protein synaptopodin. Proc Natl Acad Sci U S A. 2020;117(21):11648–11657. doi: 10.1073/pnas.1917597117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiao W, Zhang Z, Xu Y, et al. Butyric acid normalizes hyperglycemia caused by the tacrolimus-induced gut microbiota. Am J Transplant. 2020;20(9):2413–2424. doi: 10.1111/ajt.15880. [DOI] [PubMed] [Google Scholar]
- 40.Du Y, Li X, Su C, et al. Butyrate protects against high-fat diet-induced atherosclerosis via up-regulating ABCA1 expression in apolipoprotein E-deficiency mice. Br J Pharmacol. 2020;177(8):1754–1772. doi: 10.1111/bph.14933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathewson ND, Jenq R, Mathew AV, et al. Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat Immunol. 2016;17(5):505–513. doi: 10.1038/ni.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Z, Yi CX, Katiraei S, et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut. 2018;67(7):1269–1279. doi: 10.1136/gutjnl-2017-314050. [DOI] [PubMed] [Google Scholar]
- 43.Kohli N, Crisp Z, Riordan R, Li M, Alaniz RC, Jayaraman A. The microbiota metabolite indole inhibits salmonella virulence: involvement of the PhoPQ two-component system. PLoS One. 2018;13(1) doi: 10.1371/journal.pone.0190613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beaumont M, Neyrinck AM, Olivares M, et al. The gut microbiota metabolite indole alleviates liver inflammation in mice. Faseb J. 2018;32(12) doi: 10.1096/fj.201800544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negatu DA, Yamada Y, Xi Y, et al. Gut microbiota metabolite indole propionic acid targets tryptophan biosynthesis in mycobacterium tuberculosis. mBio. 2019;10(2):e02781. doi: 10.1128/mBio.02781-18. -18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sári Z, Mikó E, Kovács T, et al. Indolepropionic Acid, a Metabolite of the Microbiome, Has Cytostatic Properties in Breast Cancer by Activating AHR and PXR Receptors and Inducing Oxidative Stress. Cancers (Basel) 2020;12(9):2411. doi: 10.3390/cancers12092411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swimm A, Giver CR, DeFilipp Z, et al. Indoles derived from intestinal microbiota act via type I interferon signaling to limit graft-versus-host disease. Blood. 2018;132(23):2506–2519. doi: 10.1182/blood-2018-03-838193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krishnan S, Ding Y, Saedi N, et al. Gut microbiota-derived tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23(4):1099–1111. doi: 10.1016/j.celrep.2018.03.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh R, Chandrashekharappa S, Bodduluri SR, et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat Commun. 2019;10(1):89. doi: 10.1038/s41467-018-07859-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xia B, Shi XC, Xie BC, et al. Urolithin A exerts antiobesity effects through enhancing adipose tissue thermogenesis in mice. PLoS Biol. 2020;18(3) doi: 10.1371/journal.pbio.3000688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ryu D, Mouchiroud L, Andreux PA, et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22(8):879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 52.Miller AW, Orr T, Dearing D, Monga M. Loss of function dysbiosis associated with antibiotics and high fat, high sugar diet. Isme j. 2019;13(6):1379–1390. doi: 10.1038/s41396-019-0357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDowell R, Perrott S, Murchie P, Cardwell C, Hughes C, Samuel L. Oral antibiotic use and early-onset colorectal cancer: findings from a case-control study using a national clinical database. Br J Cancer. 2021;126:957–967. doi: 10.1038/s41416-021-01665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hornykiewicz O.L-DOPA. J Parkinsons Dis. 2017;7(s1):S3–s10. doi: 10.3233/JPD-179004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldenberg MM. Medical management of Parkinson's disease. P t. 2008;33(10):590–606. [PMC free article] [PubMed] [Google Scholar]
- 56.van Kessel SP, de Jong HR, Winkel SL, et al. Gut bacterial deamination of residual levodopa medication for Parkinson's disease. BMC Biol. 2020;18(1):137. doi: 10.1186/s12915-020-00876-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maini Rekdal V, Bess EN, Bisanz JE, Turnbaugh PJ, Balskus EP. Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism. Science. 2019;364(6445):eaau6323. doi: 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kivenson V, Giovannoni SJ. An expanded genetic code enables trimethylamine metabolism in human gut bacteria. mSystems. 2020;5(5):e00413–e00420. doi: 10.1128/mSystems.00413-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koh A, Mannerås-Holm L, Yunn NO, et al. Microbial imidazole propionate affects responses to metformin through p38γ-dependent inhibitory AMPK phosphorylation. Cell Metab. 2020;32(4):643–653. doi: 10.1016/j.cmet.2020.07.012. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guthrie L, Gupta S, Daily J, Kelly L. Human microbiome signatures of differential colorectal cancer drug metabolism. NPJ Biofilms Microbiomes. 2017;3:27. doi: 10.1038/s41522-017-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bronckaers A, Balzarini J, Liekens S. The cytostatic activity of pyrimidine nucleosides is strongly modulated by Mycoplasma hyorhinis infection: implications for cancer therapy. Biochem Pharmacol. 2008;76(2):188–197. doi: 10.1016/j.bcp.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 62.Shang J, Ma S, Zang C, Bao X, Wang Y, Zhang D. Gut microbiota mediates the absorption of FLZ, a new drug for Parkinson's disease treatment. Acta Pharm Sin B. 2021;11(5):1213–1226. doi: 10.1016/j.apsb.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He Y, Fu L, Li Y, et al. Gut microbial metabolites facilitate anticancer therapy efficacy by modulating cytotoxic CD8(+) T cell immunity. Cell Metab. 2021;33(5):988–1000. doi: 10.1016/j.cmet.2021.03.002. e7. [DOI] [PubMed] [Google Scholar]
- 64.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mager LF, Burkhard R, Pett N, et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science. 2020;369(6510):1481–1489. doi: 10.1126/science.abc3421. [DOI] [PubMed] [Google Scholar]
- 66.Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee SH, Cho SY, Yoon Y, et al. Bifidobacterium bifidum strains synergize with immune checkpoint inhibitors to reduce tumour burden in mice. Nat Microbiol. 2021;6(3):277–288. doi: 10.1038/s41564-020-00831-6. [DOI] [PubMed] [Google Scholar]
- 68.Oancea I, Movva R, Das I, et al. Colonic microbiota can promote rapid local improvement of murine colitis by thioguanine independently of T lymphocytes and host metabolism. Gut. 2017;66(1):59–69. doi: 10.1136/gutjnl-2015-310874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vande Voorde J, Sabuncuoğlu S, Noppen S, et al. Nucleoside-catabolizing enzymes in mycoplasma-infected tumor cell cultures compromise the cytostatic activity of the anticancer drug gemcitabine. J Biol Chem. 2014;289(19):13054–13065. doi: 10.1074/jbc.M114.558924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riquelme E, Maitra A, McAllister F. Immunotherapy for pancreatic cancer: more than just a gut feeling. Cancer Discov. 2018;8(4):386–388. doi: 10.1158/2159-8290.CD-18-0123. [DOI] [PubMed] [Google Scholar]
- 71.Yan A, Culp E, Perry J, et al. Transformation of the anticancer drug doxorubicin in the human gut microbiome. ACS Infect Dis. 2018;4(1):68–76. doi: 10.1021/acsinfecdis.7b00166. [DOI] [PubMed] [Google Scholar]
- 72.Ke W, Saba JA, Yao CH, et al. Dietary serine-microbiota interaction enhances chemotherapeutic toxicity without altering drug conversion. Nat Commun. 2020;11(1):2587. doi: 10.1038/s41467-020-16220-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Javdan B, Lopez JG, Chankhamjon P, et al. Personalized mapping of drug metabolism by the human gut microbiome. Cell. 2020;181(7):1661–1679. doi: 10.1016/j.cell.2020.05.001. e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zimmermann M, Zimmermann-Kogadeeva M, Wegmann R, Goodman AL. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature. 2019;570(7762):462–467. doi: 10.1038/s41586-019-1291-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ting NL, Lau HC, Yu J. Cancer pharmacomicrobiomics: targeting microbiota to optimise cancer therapy outcomes. Gut. 2022 doi: 10.1136/gutjnl-2021-326264. gutjnl-2021-326264. [DOI] [PMC free article] [PubMed] [Google Scholar]