From the management of microtubules to the production of pathological species: liquid–liquid phase separation may tune the behavior of the protein tau in health and neurodegenerative disease. In this issue of The EMBO Journal, Hochmair et al (2022) demystify important aspects of tau condensate compilation.
Subject Categories: Neuroscience, RNA Biology
New findings show how macromolecular crowding and RNA synergize in generating Tau droplets with distinct physiological and pathological properties.
On a daily basis, cells must orchestrate a plethora of routine processes as well as react to fluctuations in intracellular and extracellular signals. The biomolecules needed to execute these tasks are tightly packed in the confined intracellular space (Fulton, 1982). Cells create order in their crowded interior by compartmentalization in membrane‐delineated and membraneless organelles (preprint: Keber et al, 2021). The latter are highly dynamic organizational units that emerge upon the de‐mixing of biomolecules from the intracellular fluid into condensed—even more crowded—liquid “droplets,” a process termed liquid–liquid phase separation (LLPS). Membraneless organelles play crucial roles in cellular physiology that we are only starting to understand, yet their formation comes at a risk, as proteins involved in neurodegenerative disease pathogenesis undergo LLPS and their high concentration within droplets may foster irreversible pathological aggregation (Zbinden et al, 2020).
One of these proteins is tau, an intrinsically disordered microtubule‐associated protein that deposits in pathognomonic aggregates in patients with Alzheimer’s disease (AD), frontotemporal lobar degeneration, and other neurodegenerative tauopathies. The progressive nature of these incurable disorders has been attributed to the intercellular propagation of aggregation‐inducing tau “seeds” that wander through neural circuitries (Gibbons et al, 2019). Yet, the mechanisms causing tau to abandon its important job as axonal microtubule‐bundler and instead jeopardize neural integrity by mislocalizing, aggregating, and spreading are incompletely understood (Fig 1A and B). In 2017, two laboratories independently showed the ability of full‐length tau to undergo LLPS in vitro (Hernández‐Vega et al, 2017; Zhang et al, 2017), sparking the idea that condensation plays a central role in tau physiology and its transition to pathology. Indeed, tau droplets form in cultured neurons (Wegmann et al, 2018) and facilitate microtubule polymerization (Hernández‐Vega et al, 2017), but more gloomy outlooks include the “aging” of tau droplets to a nondynamic state, coinciding with the emergence of seed‐competent aggregates (Zhang et al, 2017; Wegmann et al, 2018).
Figure 1. Condensate properties affect physiological and pathological tau activity.
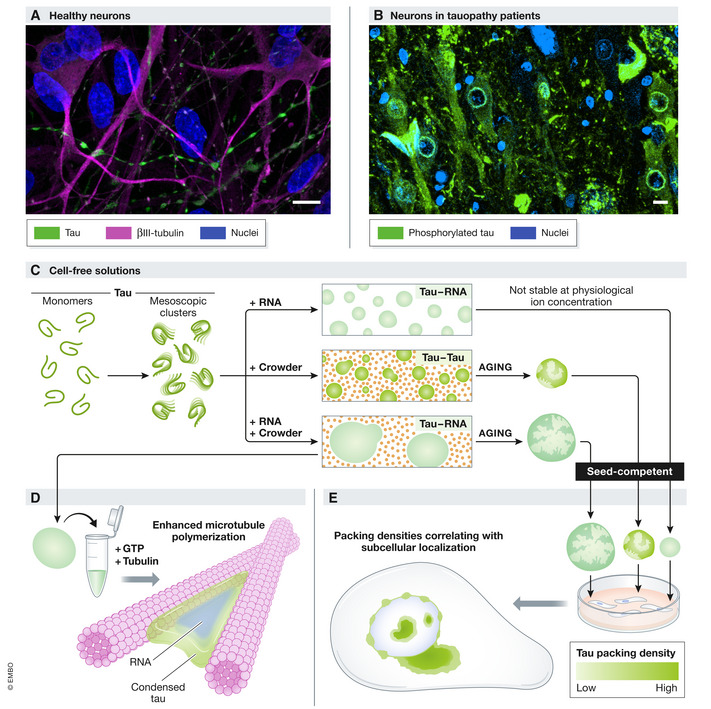
Tau associates with axonal microtubules in healthy neurons (A) but aggregates in the soma and at the nuclear membrane in tauopathy patients (B). Evidence is mounting that tau LLPS plays an important role in both processes. In cell‐free solutions (C), tau monomers spontaneously assemble into mesoscopic clusters. Macromolecular crowding and/or RNA induce microscopic tau droplets with distinct physiological (microtubule bundling (D)) and pathological (aging) properties. All tau droplets can form seed‐competent species and trigger intracellular tau aggregation that harbors variable packing densities correlating with subcellular localization (E) and recapitulates the neuropathological spectrum of tau inclusions (B). (A) Tau (green, Tau‐5, Invitrogen, AHB0042) and βIII‐tubulin (magenta, ProteinTech, 10068‐1‐AP) immunolabeling in human neural networks (preprint: Hruska‐plochan et al, 2021). (B) phosphorylated tau (green, AT8, ThermoScientific, MN1020) immunolabeling in the hippocampus of an AD patient. DAPI‐stained nuclei are shown in blue in (A–B). Bars: 10 µm.
What drives the formation of these functional but risky tau droplets? In vitro data indicate that homotypic tau–tau droplets form via tau self‐coacervation, a process inducible by inert molecular crowding agents such as polyethylene glycol (PEG) (Hernández‐Vega et al, 2017; Wegmann et al, 2018). In addition, tau‐attracting polyanions can initiate complex coacervation, giving rise to heterotypic (e.g., tau‐RNA or tau‐tubulin) droplets (Hernández‐Vega et al, 2017; Zhang et al, 2017). Employing state‐of‐the‐art optical techniques, Hochmair et al (2022) now untangle the relevance of these tau LLPS modes under conditions simulating the intracellular fluid.
As the intracellular milieu is both crowded and comprises a multitude of negatively charged biomolecules, the authors mixed tau with the crowding agent PEG, RNA, or both and followed the size distribution of droplets by dynamic light scattering (DLS) (Fig 1C). At ion levels, mimicking those in the intracellular fluid, tau‐RNA droplets were unable to assemble. However, when PEG was added, heterotypic tau–RNA droplets did form and were larger, more numerous, and more resistant to an increase in ionic buffer strength compared to homotypic tau–tau droplets formed in the presence of PEG alone. Importantly, in a cell‐free functional assay (Hernández‐Vega et al, 2017), tau–RNA droplets nucleated microtubule bundle polymerization more efficiently than tau–tau droplets under crowded conditions (Fig 1D). Therefore, molecular crowding and RNA team up to generate robust and operational tau condensates.
Dynamic light scattering allows the detection of particles with a size well below the resolution limit of confocal microscopes commonly used to visualize droplets. This sensitive method revealed the presence of not only tau monomers (~ 10 nm) but also mesoscopic tau clusters (~ 50–150 nm) that spontaneously formed prior to the addition of PEG and/or RNA to the solution but vanished upon the formation of microscopic droplets (~ 1,000 nm). Although the lack of detection of the mesoscopic tau clusters in buffers with higher ionic strength warrants further examination of their physiological relevance, this observation is of high interest in the light of recent data pointing to a major contribution of mesoscopic liquid‐like assemblies (~ 100 nm) to cytoplasmic organization (preprint: Keber et al, 2021). It is therefore important to determine whether mesoscopic tau assemblies also exist in the crowded interior of neurons. Perhaps, such clustering at the mesoscale brings tau in a ready‐to‐react state, allowing the rapid formation of visible tau droplets to execute on‐demand cellular functions, such as microtubule nucleation.
Hochmair et al (2022) next questioned whether crowding and RNA also join forces during tau condensation in a pathological context. As expected (Wegmann et al, 2018), tau–tau droplets lost their dynamic character over time, as evidenced by minimal fluorescence recovery after photobleaching (FRAP) of labeled tau after a day in a PEG‐packed environment. Tau–RNA coacervates similarly hardened in the presence of the crowding agent, yet they retained over 60% of their original mobility in a PEG‐free solution. Would the diminished aging of these tau‐RNA droplets translate into a lower pathological potential? Perhaps unexpectedly, this was not the case: 24 h‐old droplets of any type induced aggregation of naïve tau in vitro and in commonly used HEK293 tau biosensor cells. These data indicate that droplet maturation is not essential for the sprouting of seed‐competent tau species. Therefore, tau condensates formed in the presence of crowding, RNA, or both pose an equal risk of generating tau pathology.
The role of LLPS in pathological tau transitions was further probed in the droplet‐exposed tau biosensor cells. In addition to bright intranuclear and cytoplasmic aggregates, the intracellular tau pathology elicited by tau–tau and tau–RNA droplets included a fainter inclusion type that presented as a beaded ring around the nucleus and consistently overlapped with nuclear pore proteins. Employing elegant fluorescence lifetime imaging microscopy (FLIM) strategies, the molecular packing density in these peculiar nuclear envelope inclusions was found to be looser than in the core, but similar to the shell of intranuclear and cytoplasmic tau aggregates (Fig 1E). These data align with a recent study (Kang et al, 2021) and jointly suggest that nuclear envelope inclusions represent an early phase in the tau aggregation cascade. Importantly, an identical, relatively faint, perinuclear tau accumulation pattern is observed in neurons in the AD brain and classified as “pre‐tangle” tau pathology, a stage inferred to precede the formation of characteristic endpoint neurofibrillary tangles (Bancher et al, 1993). Furthermore, a sensitive proximity ligation assay yielded labeling around and inside neuronal nuclei in brain areas free of classical tau pathology and in asymptomatic AD stages (Bengoa‐Vergniory et al, 2021), indicating that tau multimerization at the nuclear envelope is among the earliest events in human pathology. Notably, like droplet exposure, also seeding with AD patient‐derived brain homogenate elicited nuclear membrane inclusions in the tau biosensor cells. Whether these loosely packed perinuclear tau inclusions are condensates formed via LLPS—as speculated (Kang et al, 2021; Hochmair et al, 2022)—and whether their formation is a prerequisite to the development of larger cytoplasmic inclusions requires further investigation.
Taken together, Hochmair et al (2022) combine a flurry of optical techniques in cell‐free and cellular systems to unravel novel aspects of physiological and pathological tau LLPS, including the functional alliance between molecular crowding and RNA, the spontaneous assembly of mesoscopic tau clusters and the evolution of seed‐competent species in the absence of droplet aging. Advances in our understanding of the LLPS behavior of hazardous proteins include discoveries on proteins like TDP‐43, FUS, and α‐synuclein that constitute the hallmark inclusions in other neurodegenerative disorders (Zbinden et al, 2020), and, like tau, often present with a juxtanuclear localization. The latter may result from aberrant LLPS and faulty interactions with nuclear import receptors and/or nuclear pore proteins, leading to errors in nucleocytoplasmic trafficking, an emerging theme in neurodegeneration (Moore et al, 2020). Future work will disentangle how aberrant LLPS of neurotoxic proteins contributes to tipping the balance between coordination and chaos in the crowded cellular milieu.
Acknowledgments
The authors thankfully acknowledge the support of the Swiss National Science Foundation grants: Sinergia (CRSII5_205922) and National Centre of Competence in Research RNA & Disease (205601). V.I.W. is supported by the FEBS Long‐Term Fellowship. The authors thank Dr. W. Scheper and Dr. J.J.M. Hoozemans (Amsterdam UMC location Vrije Universiteit Amsterdam, the Netherlands) for their contribution to acquiring the image of AT8 immunolabeling in the AD hippocampus (Fig 1B). Human brain tissue was supplied by the Netherlands Brain Bank (NBB; Amsterdam, the Netherlands, http://brainbank.nl). The authors thank Anna Maria Reuss and Dr. Adriano Aguzzi for their kind donation of the Tau‐5 antibody.
The EMBO Journal (2022) 41: e111425.
See also: J Hochmair et al (June 2022)
References
- Bancher C, Braak H, Fischer P, Jellinger KA (1993) Neuropathological staging of Alzheimer lesions and intellectual status in Alzheimer’s and Parkinson’s disease patients. Neurosci Lett 162: 179–182 [DOI] [PubMed] [Google Scholar]
- Bengoa‐Vergniory N, Velentza‐Almpani E, Silva AM, Scott C, Vargas‐Caballero M, Sastre M, Wade‐Martins R, Alegre‐Abarrategui J (2021) Tau‐proximity ligation assay reveals extensive previously undetected pathology prior to neurofibrillary tangles in preclinical Alzheimer’s disease. Acta Neuropathol Commun 9: 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton AB (1982) How crowded is the cytoplasm? Cell 30: 345–347 [DOI] [PubMed] [Google Scholar]
- Gibbons GS, Lee VMY, Trojanowski JQ (2019) Mechanisms of cell‐to‐cell transmission of pathological tau: a review. JAMA Neurol 76: 101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández‐Vega A, Braun M, Scharrel L, Jahnel M, Wegmann S, Hyman BT, Alberti S, Diez S, Hyman AA (2017) Local nucleation of microtubule bundles through tubulin concentration into a condensed tau phase. Cell Rep 20: 2304–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochmair J, Exner C, Franck M, Dominguez‐Baquero A, Diez L, Brognaro H, Kraushar ML, Mielke T, Radbruch H, Kaniyappan S et al (2022) Molecular crowding and RNA synergize to promote phase separation, microtubule interaction, and seeding of Tau condensates. EMBO J 41: e108882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska‐plochan M, Betz KM, Ronchi S, Wiersma VI, Maniecka Z, Hock E, Laferriere F, Sahadevan S, Hoop V, Delvendahl I et al (2021) Human neural networks with sparse TDP‐43 pathology reveal NPTX2 misregulation in ALS/FTLD. bioRxiv 10.1101/2021.12.08.471089 [PREPRINT] [DOI] [Google Scholar]
- Kang S‐G, Han ZZ, Daude N, McNamara E, Wohlgemuth S, Molina‐Porcel L, Safar JG, Mok S‐A, Westaway D (2021) Pathologic tau conformer ensembles induce dynamic, liquid‐liquid phase separation events at the nuclear envelope. BMC Biol 19: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keber FC, Nguyen T, Brangwynne CP, Wühr M (2021) Evidence for widespread cytoplasmic structuring into mesoscopic condensates. bioRxiv 10.1101/2021.12.17.473234 [PREPRINT] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S, Rabichow BE, Sattler R (2020) The hitchhiker’s guide to nucleocytoplasmic trafficking in neurodegeneration. Neurochem Res 45: 1306–1327 [DOI] [PubMed] [Google Scholar]
- Wegmann S, Eftekharzadeh B, Tepper K, Zoltowska KM, Bennett RE, Dujardin S, Laskowski PR, MacKenzie D, Kamath T, Commins C et al (2018) Tau protein liquid–liquid phase separation can initiate tau aggregation. EMBO J 37: e98049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zbinden A, Pérez‐Berlanga M, De Rossi P, Polymenidou M (2020) Phase separation and neurodegenerative diseases: a disturbance in the force. Dev Cell 55: 45–68 [DOI] [PubMed] [Google Scholar]
- Zhang X, Lin Y, Eschmann NA, Zhou H, Rauch JN, Hernandez I, Guzman E, Kosik KS, Han S (2017) RNA stores tau reversibly in complex coacervates. PLoS Biol 15(7): e2002183 [DOI] [PMC free article] [PubMed] [Google Scholar]


