Summary
Background
Multisystemic inflammatory syndrome in children (MIS-C) has increasingly been documented globally with the progression of the COVID-19 pandemic and a significant proportion of cases have been noted in children of Black descent. There has been a noticeable discrepancy in the presentation and outcomes of COVID-19 infection in sub-Saharan Africa compared to the rest of the world. We documented the demography, clinical features, laboratory and imaging findings, therapeutic management, and short-term outcomes of paediatric patients with MIS-C diagnosed during the COVID-19 pandemic in Lagos, Nigeria.
Methods
We carried out a retrospective review of MIS-C cases seen in nine public and private hospitals in Lagos from July 10, 2020 to July 30, 2021. Data on clinical presentation, laboratory investigations, therapy as well as outcomes at 2 weeks, 6 weeks, 3 months and 6 months were analyzed.
Findings
28 children and adolescents with median age of 7·5 (IQR 2·3 - 9·4) years were diagnosed with MIS-C. MIS-C was suspected in 24 patients (85·7%) at initial clinical evaluation and mucocutaneous, gastrointestinal and cardiovascular manifestations were identified in 75·0%, 71·4% and 89·3% of patients respectively. Acute kidney injury and aseptic meningitis were noted in 32·1% and 17·9% of patients respectively. Cardiac manifestations at presentation included coronary dilatation and pericardial effusion in 46·4% each, ventricular dysfunction (32·1%), atrioventricular valve regurgitation (25·0%), prolonged QTc interval (40·0%) and first-degree atrioventricular block (16·0%). Therapy included aspirin in 89·3%, steroids in 75·0% and intravenous immunoglobulin (IVIG) infusion in 60·7%. All patients survived and were discharged after a mean of 11·14 (SD 5·65) days. Frequency of coronary dilatation had reduced from 46·4% to 7·1% by 3 months follow up and prolonged QTc interval persisted until the 6 week follow up in 4.5% of patients. Echocardiogram and electrocardiogram findings were normal in all patients assessed at 6 months follow up.
Interpretation
MIS-C is an important diagnosis in children presenting with prolonged fever during the COVID-19 pandemic. Cardiovascular manifestations occurred in several children with MIS-C and improved by 6 months follow up. Early diagnosis and prompt institution of a combination of antiplatelet therapy, steroids and IVIG appear to be beneficial.
Funding
None.
Keywords: MIS-C, PMIS, COVID-19, Kawasaki disease, Nigeria
Research in context.
Evidence before this study
There is a myriad of clinical, laboratory and imaging findings of multisystemic inflammatory syndrome in children (MIS-C) and treatment strategies are varied. Cardiovascular findings are key manifestations of this disease and follow up is usually to exclude cardiac complications. Coronary dilatation may be a presenting feature; may persist beyond the acute phase of the illness and duration of required follow up is unclear. We searched PubMed for articles published between Feb 1, 2020 and Dec 31, 2021 using search terms “multisystemic inflammatory syndrome”, “Kawasaki disease”, “coronary abnormalities”, “COVID-19 sequelae”, “MIS-C manifestations in organ systems” and “MIS-C treatment”.
Added value of this study
This study shows the varied manifestations of MIS-C in a native Black African population including cardiac manifestations, the utility of readily accessible investigations, the available and affordable therapy in this environment and also documents short term outcome data of patients up to six months post-acute illness.
Implications of all the available evidence
The findings of this study support the need to consider this diagnosis in children presenting with a plethora of clinical manifestations. Current global guidelines for diagnosis, treatment and follow up may be challenging in resource constrained settings; and information from this study may guide establishment of local protocols in environments like ours where comprehensive health care is still largely poorly accessible. Prospective follow up studies to document long term outcomes in patients with MIS-C are essential.
Alt-text: Unlabelled box
Introduction
Multisystemic inflammatory syndrome in children (MIS-C) is a debilitating and potentially fatal illness manifesting commonly with a cytokine storm that causes widespread multi organ involvement.1 The features of MIS-C include fever, gastrointestinal symptoms, rash, conjunctivitis, cardiac and renal dysfunction, shock and coagulopathy; severe enough to require treatment in the intensive care unit.2 There has been a notable upsurge in MIS-C cases globally since the onset of the COVID-19 pandemic and a temporal association between both entities has been hypothesised.2 Since the report of the first case of COVID-19 infection in Nigeria in February 2020, there have been 250,000 confirmed cases of the disease and 3,000 deaths documented as of Jan 12, 2022. Lagos, one of the 36 states in Nigeria; is in the southwestern part of the country. The city is home to about one tenth of the country's populace but accounts for 40% of the total cases of COVID-19 and up to 25% of attributable deaths documented nationwide.3
The asymptomatic or minimally symptomatic nature of COVID-19 in children has been previously reported.4 An early report of a Kawasaki disease-like toxic shock syndrome (TSS) documented among children presenting at a hospital in the United Kingdom1 drew attention to the deleterious effect of this viral disease even in previously well children. One of the earliest reports of severe Kawasaki-like disease from Bergamo Italy, which was one of the epicentres of the COVID-19 pandemic globally, documented up to a 30-fold increase in the incidence of Kawasaki-like disease. This increase occurred within a month of the COVID-19 epidemic and progressed exponentially compared to the 5 years preceding the pandemic.5 MIS-C as a clinical entity was first described as paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS-TS) by the Royal College of Paediatrics and Child Health (RCPCH)6 and it has now been termed MIS-C by the Centres for Disease Control and Prevention (CDC) and the World Health Organization (WHO) with similar criteria put forward to define cases of the syndrome.7,8
MIS-C has been associated with the COVID-19 infection and has been noted to occur in children about two to four weeks after the onset of the COVID-19 infection.1,22 From previous reports, up to a third to three quarter of cases of MIS-C have been noted in children of Black descent 4,5,9, 10, 11, 12 and there may likely be a genetic predilection for the occurrence of this disease entity in children of Black ethnicity as reported in a French series.13 Although the pathogenesis of hyperinflammation which typifies MIS-C is largely unknown, a postinfectious immune activation has been hypothesised as well as a direct effect of SARS–CoV-2 spike protein structure on immune activation.1,14 Evidence of immune response to the virus has been noted in older children with a higher rate of cardiac involvement.5
The clinical presentation of MIS-C mirrors that of Kawasaki disease (KD) and presents with features of TSS in some children. Both KD and TSS are important differential diagnoses in any child presenting with features of MIS-C and these three entities have been compared previously.11 The earliest definition of MIS-C issued by WHO includes clinical and laboratory features, with evidence of COVID-19, or likely contact with a person who has or has had COVID-19 infection.15
The first report of MIS-C associated with COVID-19 infection was in the United Kingdom where eight cases were documented among previously fit and well children1, and subsequently, there have been several reports from other regions including the United States, Europe and Africa.10,12,16,17 There have however been fewer reports of MIS-C from Asia which is known to be the hub of typical KD globally and several reasons have been postulated.18,19 A case of MIS-C was reported in a 12-year-old Nigerian child and called for more vigilance on the part of health care providers in Africa.20
Given that the pandemic peaked later in sub-Saharan Africa compared to Europe and America, it is expected that the occurrence of MIS-C was also later in this African population. There is a need to document the occurrence and spectrum of presentation of this syndrome in native black regions especially Nigeria, which is the most populous black African nation; this will provide key data on MIS-C in children of African ancestry. The Nigerian population, like most other parts of Africa has received a less severe deleterious effect of the pandemic than Europe and America. It may thus be logical to extrapolate that post infectious sequelae such as MIS-C may likewise be a less significant cause of morbidity and mortality among the Nigerian paediatric population; however, this may not be the case.
Follow up data is also essential to aid the establishment of guidelines for evidence based, meticulous, yet cost effective post hospitalization care of paediatric patients who have suffered this disease in resource limited settings. To the best of our knowledge, this report is the first cluster series and analysis of cases documenting MIS-C among Nigerian children collated from health care facilities managing paediatric patients in the metropolitan city of Lagos; the epicentre of the COVID -19 pandemic in Nigeria.
Methods
Study design and participants
This was a retrospective case series which included all cases of MIS-C reported by a total of nine consenting participating institutions in Lagos, Nigeria between July 10, 2020 and July 30, 2021. All cases were defined using WHO criteria as previously described.15 The study was conducted in Lagos, the commercial capital of Nigeria which remains the epicentre of COVID-19 infection in the country. Ethical approval was obtained from the Health Research and Ethics Committee of the Lagos University Teaching Hospital, Idi Araba, one of the major teaching hospitals in Nigeria which served as the co-ordinating centre for data collection, collation and analysis of cases seen in participating institutions. Consent to publish forms were completed by parents/guardians of participants whose photographs were used in the publication according to the Declaration of Helsinki for clinical research.
Procedures
Data was formally requested by invited survey from private and public hospitals that manage paediatric patients in the Lagos Metropolis, and all participating institutions were requested to sign a consent form to participate. Two public and seven private hospitals responded to the request and sent in data of patients treated for MIS-C during the specified study period. After obtaining permission from the medical director and/or other relevant authorities within the participating institution, information was obtained from the managing paediatrician directly caring for these patients. All institutions who gave consent to participate were required to submit anonymous data of patients using appropriately filled WHO and/or CDC case report forms21,22 or medical records of patients diagnosed with MIS-C for review; and formal documentation was done by an appointed data manager at the co-ordinating institution. The case report forms included relevant bio data, clinical history and symptoms, clinical signs, duration from symptom onset to hospital admission in the participating centre, investigation findings (laboratory and imaging), treatment regimens and patient outcomes. In all cases, MIS-C was suspected by the attending paediatrician, necessitating a cardiology and/or infectious disease or respiratory medicine consult after which the diagnosis was established.
Outcomes
Study outcomes included clinical and laboratory data, cardiac findings, therapeutic measures, and patient outcomes. Follow up was scheduled for all participants at 2 weeks and 4-6 weeks after diagnosis. The decision to follow up at 3 months and 6 months was determined by the outcomes of the two previous echocardiograms and electrocardiograms. Follow up was discontinued if clinical and imaging findings were normal by the 6 week follow up visit. Available follow up clinical, electrocardiographic and echocardiography data as well as clinical and imaging photographs were also obtained. Abnormal echocardiography findings were defined as previously documented.23 Left ventricular (LV) dysfunction was defined as LV ejection fraction <55% and/or fractional shortening <28%; right ventricular dysfunction was defined as Tricuspid annular plane systolic excursion (TAPSE) < 2SD of expected; coronary artery dilatation was defined as coronary artery z score >+2.5; moderate atrioventricular valve regurgitation was characterised by vena contracta >0.3cm; pulmonary hypertension was defined by tricuspid regurgitant gradient >25.0mmHg, diastolic dysfunction was defined and graded using E/A ratios across the atrioventricular valves. Abnormal ECG findings were defined as previously documented.24 PR interval > 200msecs was defined as first degree atrioventricular block and prolonged QTc was defined as QTc interval >440msecs. All case report forms had no means of patient identification. All relevant clinical photographs were accompanied by a signed informed consent from the parent/guardian of the participant.
Statistical analysis
Data was analysed using the Statistical Package for the Social Sciences (SPSS) SPSS v25 (SPSS, Chicago, IL, USA). Patients’ characteristics were described using mean, median, ranges and percentages as appropriate. Frequency data were computed using percentages for categorical data and other data represented using figures.
Role of the funding source
There was no funding source for this study. OS, YA, GO, OK, UO, EO, EI, OA, EO, OA, ML, EE and CO had full access to the data and were responsible for the decision to submit for publication.
Results
Sociodemographic findings
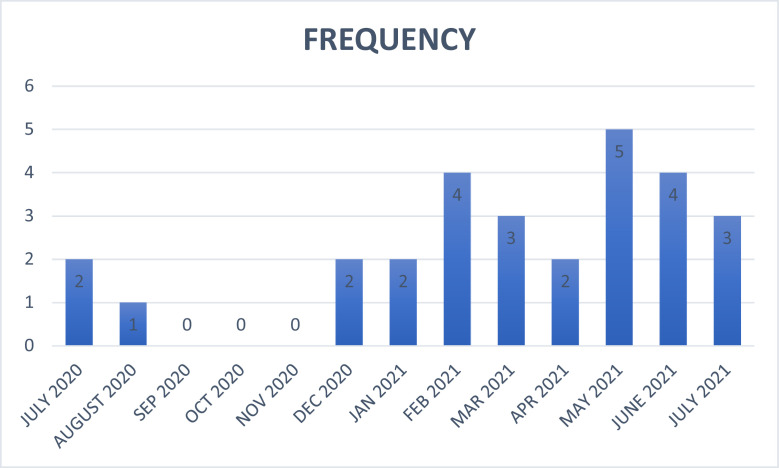
A total of 28 patients with features of MIS-C from nine participating institutions who responded to the invited survey were enrolled into the study. The frequency of cases categorised by month is shown in Figure 1.
Figure 1.
Frequency distribution of cases of MIS-C by month from July 2020 to July 2021.
Three of the private hospitals had the capacity to offer tertiary level care and the two public owned hospitals were teaching hospitals. The mean age (SD) of patients was 6·77± 4·2 years (range of six weeks to 13 years) with a male female ratio of 1·55:1. The age group of patients were as follows: < 1 year (10·7%), 1 to 5 years (28·6%), 6 to 10 years (39·3%), 10 to 13 years (21·4%).
Diagnosis
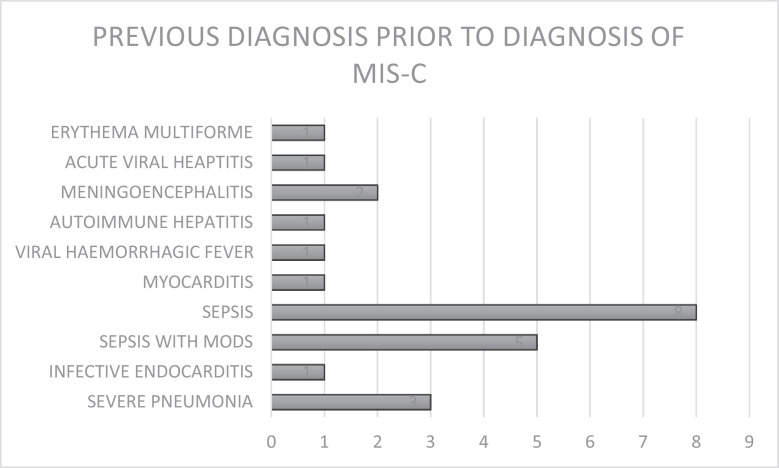
MIS-C was suspected in 85·7% of cases at initial clinical evaluation in the participating health institutions, although all patients had been previously treated in at least one health facility prior to referral. Previous treatments received included administration of antibiotics, antimalarials and/or antivirals, transfusion of blood and blood products, correction of electrolyte derangements, inotropic support, and conservative management according to diagnoses indicated in Figure 2.
Figure 2.
Figure showing the previous diagnoses made in referring hospitals prior to the diagnosis of MIS-C in the participating health institutions. MODS: Multiple Organ Dysfunction Syndrome.
Preceding COVID-19 infection
About half of enrolled participants in this study (42·8%) had a previous history of COVID-19 infection in the preceding 6 weeks; and there was evidence of antibodies to the SARS- CoV 2 virus in about one third of patients (28·5%) while about two third of patients (60·7%) had negative SARS-CoV 2 polymerase chain reaction (PCR) tests at presentation. Previous COVID-19 exposure could not be verified in 46·4% of study participants.
Clinical presentation
Systemic manifestations and the frequency of affected systems is summarised in Table 1.
Table 1.
Frequency of systemic manifestations of MIS-C in study participants.
| Systemic manifestation | Frequency (%) |
|---|---|
| Hematologic | 89·3 |
| Mucocutaneous/Dermatologic | 89·3 |
| Gastrointestinal | 75·0 |
| Cardiovascular | 71·4 |
| Musculoskeletal | 67·9 |
| Respiratory | 60·7 |
| Neurologic | 32·1 |
| Renal | 32·1 |
Fever
The most common presentation was fever which was universal in all patients with an average maximum temperature (T max) of 39·4 ± 0·7 °C. The mean (SD) duration of fever was 11·6 ± 5·7 days (range 7 to 33 days).
Hematologic
Hematologic manifestations included anaemia (60·7%), thrombocytopenia (21·4%) and thrombocytosis (78·6%).
Dermatologic/Mucocutaneous
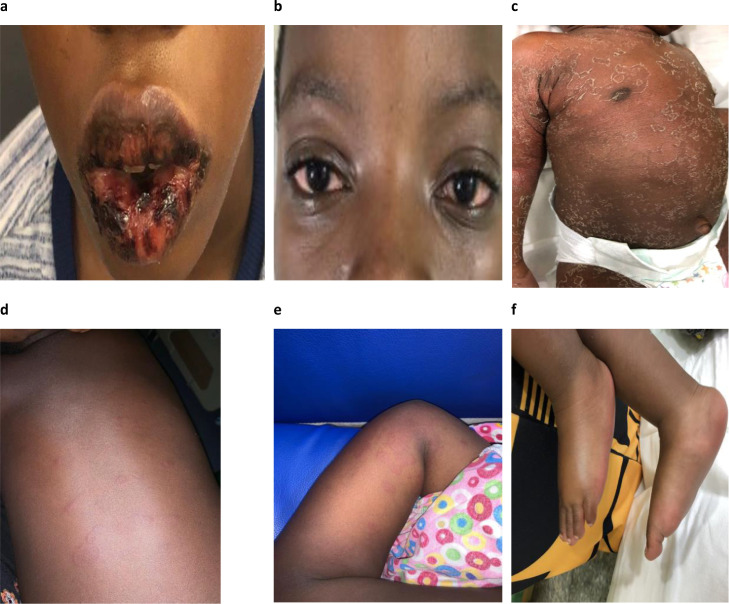
Mucocutaneous lesions were one of the most common presenting features and were seen in up to 89·3% of enrolled participants. These mucocutaneous lesions include oral lesions such as ulcers, cheilosis, oral erythema and strawberry tongue shown in Figure 3a; non-suppurative conjunctivitis seen in 89·3% as shown in Figure 3b and pharyngitis seen in 39·3%. Generalised polymorphous rash seen in 21 patients (75·0%) included desquamating rash in 23 patients (82·1%) shown in Figure 3c; and ten patients (35·7%) reported a transient erythematous rash also shown in Figure 3d and e. Palmar and plantar erythema which occurred in 75·0% of children is shown in Figure 3f; and 82·1% of the children experienced desquamation of the palms and soles about one to two weeks into the illness.
Figure 3.
(a) – (f): Mucocutaneous manifestations of MIS-C. (a) oral ulcers and sores (b) non suppurative conjunctivitis; (c) Generalized maculopapular desqaumating rash; (d) and (e) Transient erythematous rash; (f) plantar erythema and edema.
Gastrointestinal
Vomiting and diarrhoea were reported in 21 patients (75·0%). Nineteen of 28 patients had abdominal pain and hepatomegaly was documented in 67·9% of patients. Acute liver failure manifesting as jaundice with transaminitis occurred in five patients (17·9%).
Cardiovascular
Cardiovascular manifestations were seen in 71·4% of patients. These included peripheral oedema (60·7%), heart failure (57·1%), pericarditis (46·4%) and hypertension (14·3%).
Shock
Four children (14·2%) presented with features of septic shock; one of whom was admitted in the intensive care unit of the managing hospital, intubated, and mechanically ventilated for about 72 hours while three patients were monitored in the high dependency unit requiring continuous monitoring and high flow oxygen supplementation with or without continuous positive airway pressure (CPAP). In three patients, due to non-accessibility of ICU services primarily because of lack of funds; these children were nursed in the general ward with close monitoring as much as possible after the parents and caregivers had been counselled about the risk of deterioration in the absence of continuous monitoring and the possible need for resuscitation with limited facilities in an open ward.
Musculoskeletal
Eight children (28·6%) reported neck pain either in isolation or in combination with neurological features and 19 patients (67·9%) reported myalgia and arthralgia.
Respiratory
Cough and shortness of breath were documented in 53·6 % and 50·0% of study participants respectively and 28·6% of participants complained of chest pain. Half of the patients (50·0%) had clinical and/or radiologic features of pneumonia and two patients had radiological features of acute respiratory distress syndrome: one requiring continuous positive airway pressure (CPAP) ventilation and another requiring mechanical ventilation. Pleural effusion was seen in 12 patients (42·9%), pulmonary oedema occurred in 2 patients (7·1%) and atelectasis in one patient.
Neurological
Headache was a presenting symptom in 50·0% of patients. Five patients (17·9%) presented as aseptic meningitis. Three patients (10·7%) presented with features of meningoencephalitis including photophobia with neck stiffness and an altered mental state. One of the children developed cranial nerve VI palsy manifesting as diplopia and a squint which persisted for about ten days despite normal brain imaging but resolved remarkably following commencement of anti-inflammatory medications.
Renal
Ten patients (35·7%) presented with history of passage of dark urine. Acute kidney injury presenting as oliguria with proteinuria and /or haematuria occurred in nine patients (32·1%). Oliguria and haematuria were seen in 32·1% and 28·6% respectively, dysuria in 17·9%, periorbital oedema in 60·7%, and ascites in 39·2%.
Cervical lymphadenopathy
Significant unilateral cervical lymphadenopathy was documented in 67·9% of participants.
Laboratory investigations
All patients had full blood counts, renal function tests and at least one acute phase reactant assay done. Nineteen patients (67·9%) had liver function tests done, and three patients (10·7%) had coagulation profile assay done. Urinalysis and urine microscopy, culture and sensitivity (mcs) results were reported in 20 (71·4%) and 17 (60·7%) of patients respectively. Other laboratory tests carried out to rule out alternative diagnoses included blood culture in 20 (71·4%) participants; viral markers such as hepatitis B surface antigen (HbsAg) and anti-hepatitis C antibody (anti HCV) in eight (28·6%) patients respectively; and Human immunodeficiency virus (HIV I and II) in 13 (46·4%) study participants. Thrombocytosis was noted in 100% of children whose illness duration had exceeded one week prior to presentation. Thrombocytosis was noted at an average (SD) of 14·3 ± 4.7 days from the first day of the illness (range 7 to 22 days). Electrolytes, urea and creatinine were normal in 42·9%. D dimer was elevated in all of five patients assayed (range: 5·0-10·0mg/dl); Ferritin was elevated in one of two patients assayed and lactate dehydrogenase (LDH) was elevated in the only patient assayed. Viral markers were negative in all patients tested and blood culture showed no growth in 18 out of 20 patients who were sampled. Moderate growth of Acinetobacter baumani was cultured in one patient who had been hospitalised for about one month prior to presentation and sparse growth of Staphylococcus haemolyticus which was reported as a possible contaminant was cultured in another patient. Urine mcs was positive for Candida spp and Klebsiella pneumoniae in one patient each. Laboratory parameters of study participants are shown in Table 2.
Table 2.
Laboratory parameters of study participants.
| Parameters | Values |
|---|---|
| Acute phase reactants | |
| Mean CRP (mg/L) (n = 25) | 142·1 ± 87·8 (range: 3·2 to 350·3) |
| Elevated CRP n/N (%) | 24/25 (96·0) |
| Mean ESR (mm in the 1st hour) (n = 26) | 62·3±34·8 (range: 24·0 to 143·0) |
| Elevated ESR n/N (%) | 26/26 (100.0) |
| Mean Procalcitonin (ng/ml) (n = 6) | 4·2±4·9 (range: 0·1 to 11·6) |
| Elevated procalcitonin n (%) | 3/6 (50·0) |
| Mean D-dimer (mg/dl) | 4·1±3·6 (range: 5·0-10·0) |
| Elevated D-dimer (>0.5 mg/L) n/N (%) | 5/5 (100·0) |
| Mean Ferritin (ug/L) (n = 2) | 307·3 (range:177·2-437·4) |
| Elevated Ferritin (>300ug/L) n/N (%) | 1/2 (50·0) |
| Electrolytes Urea and Creatinine | |
| Hyponatremia (<135mmol/L) n/ N (%) | 9/28 (32·1) |
| Hypokalaemia (<3.5mmol/L) n/N (%) | 7/28 (25·0) |
| Uraemia n/ N (%) | 9/28 (32·1) |
| Elevated creatinine n/ N (%) | 9/28 (32·1) |
| Haemogram | |
| Mean total leucocyte count (/uL) | 25,234 (range 5,900-55,000) |
| Leucocytosis (>11,000/uL) n/ N (%) | 26/28 (92.9) |
| Mean Neutrophil differential (%) | 77·5 (range of 61·0 to 95·0). |
| Neutrophilia (>60%) | 28/28 (100) |
| Mean platelet count (x 109/uL) | 581 (range of 218 to 1,200) |
| Thrombocytosis at presentation (>400 × 109/uL) n/N (%) | 20/28 (78·6) |
| Thrombocytopaenia at presentation (>150 × 109/uL) n/N (%) | 6/28 (21·4) |
| Anaemia (Hb <10.0g/dl) n (%) | 17/28 (60·7) |
| Liver function tests | |
| Deranged liver enzymes n/N (%) | 16/19 (84·2) |
| Mean SGOT (IU/L) | 120·8 (range of 12 to 757) |
| Elevated SGOT n/N (%) | 8/19 (42·9) |
| Mean SGPT (IU/L) | 77·6 (range of 10 to 391) |
| Elevated SGPT n/N (%) | 8/19 (42·9) |
| Mean GGT (IU/L) | 103·5 (range of 18 to 528) |
| Elevated GGT n/N (%) | 8/19 (42·9) |
| Mean ALP (IU/L) | 148·2 (range of 68 to 569) |
| Elevated ALP n/N (%) | 1/19 (5·3) |
| Mean serum total protein (g/L) | 58·0 ± 10·2 (range: 36·7 to 80·0) |
| Hypoproteinaemia (< 60.0g/L) | 11/19 (57·9) |
| Mean serum albumin (g/L) | 30·2 ± 6·5 (range: 21 to 49·6) |
| Hypoalbuminemia (<30.0g/L) | 11/19 (57.9) |
| Mean total bilirubin (µmol/L) | 23·1 (range: 2·0 to 166·7) |
| Mean indirect bilirubin (µmol/L) | 10·2 (range: 2·0 to 166·7) |
| Hyperbilirubinemia n/N (%) | 3/19 (15·8) |
| Urinalysis | |
| Proteinuria n/N (%) | 10/20 (50·0) |
| Pyuria n/N (%) | 8/20 (40·0) |
| Haematuria n/N (%) | 5/20 (25·0) |
| Bilirubinuria n/N (%) | 2/20 (10·0) |
| Ketonuria n/N (%) | 2/20 (10·0) |
Imaging
Chest radiographs were done in 67·9% of patients and the most common findings included pleural effusion, cardiomegaly and lung consolidation. Pneumonic changes were seen in 36·8% of these patients, cardiomegaly in 26·3%, interstitial pneumonitis in 10·5% and atelectasis and pulmonary oedema in one patient each. Pleural effusion was seen in 12 patients (42·9%) which was bilateral in seven and left sided in five out of 12 patients respectively. Chest computerised tomography (CT) scan was carried out in four patients; three of whom showed evidence of consolidation. Abdomino -pelvic ultrasound scan carried out in 15 patients (53·6%) showed evidence of ascites and hepatomegaly in six patients, splenomegaly in two patients, renomegaly with at least grade two renal parenchymal disease in two patients and mesenteric adenitis in one patient respectively.
Cardiac investigations at presentation
All patients had an echocardiogram; and 89·3% had an electrocardiogram (ECG) at the time of diagnosis. Echocardiographic findings at presentation included coronary dilatation and pericardial effusion in 46·4% of patients respectively; ventricular dysfunction in 32·1%; at least moderate atrioventricular valve regurgitation in 25·0%; pulmonary hypertension in 21·4% and left ventricular hypertrophy in 10·7% of participants, some of which are shown in Figure 5. All patients with ventricular dysfunction had coronary abnormalities as well. Left coronary artery dilatation was 1·22 times more frequently seen than right coronary artery dilatation. The average coronary artery z score of participants at presentation was + 3·9. Left ventricular systolic and diastolic dysfunction occurred in 32·1% and 39·3% of study participants respectively, while right ventricular systolic and diastolic dysfunction occurred in 21·4% and 25·0 % of study participants respectively. The mean (SD) left ventricular ejection fraction (LVEF) was 59·2 ± 11·8% and mean (SD) left ventricular fractional shortening was 31·1 ± 7·5%. ECG findings at presentation included prolonged QTc interval in 40·0% of patients; first degree atrioventricular block in 16·0%; evidence of myocardial ischaemia in 8·0% and complete right bundle branch block in 8·0% of patients.
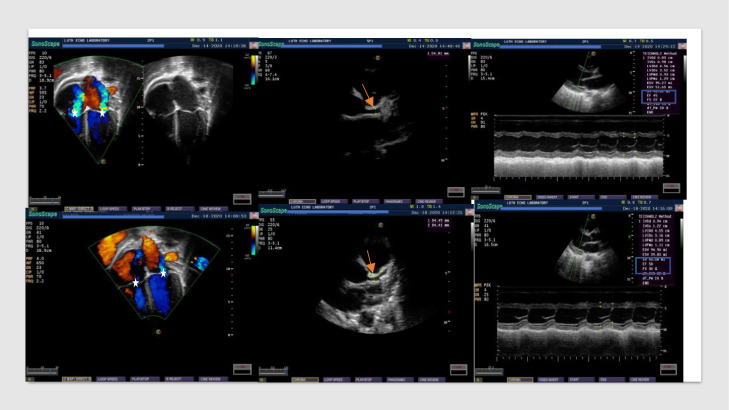
Figure 4.
Echocardiographic findings in MIS-C at diagnosis (upper row) and four days after administration of intravenous immunoglobulin (lower row) in the same patient. Upper row (left to right): moderate atrioventricular valve regurgitation (white star); dilated left main coronary artery (red arrow); and left ventricular systolic function [Ejection fraction {EF} of 45% and fractional shortening {FS} of 22%] (highlighted in blue rectangle) . Lower row (left to right): trace atrioventricular valve regurgitation (white star); dilated left main coronary artery (red arrow); and improved left ventricular systolic function [Ejection fraction {EF} of 58% and fractional shortening {FS} 30%] (highlighted in blue rectangle).
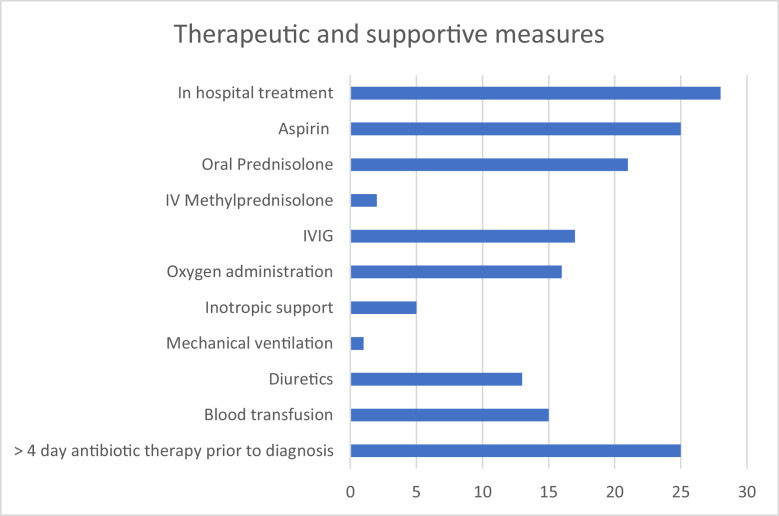
Treatment
All patients were diagnosed and treated on in-patient basis. Therapeutic agents used are shown in Figure 5. High dose aspirin was given at 30-60mg/kg/day in four divided doses for five to seven days then reduced to low dose Aspirin (3-5mg/kg daily as a single dose) for at least 6 weeks. Prednisolone was given at 1-2mg/kg daily as a single dose (up to a maximum of 60mg daily) for five to seven days and tapered down over the next one week; intravenous Methylprednisolone was given at a dose of 10mg/kg for two days followed by oral Prednisolone at a dose as stated above and intravenous immunoglobulin (IVIG) was administered as an infusion at a dose of 1-2g/kg over 12 to 24 hours and after a test dose. All patients received intravenous Omeprazole for gastric protection during therapy with high dose steroids and Aspirin. Recommended treatment regimen for all patients was a combination of anti-platelet, steroids, and immunoglobulin. Aspirin and steroids were readily available to all patients; but aspirin was not administered in three patients due to presence of thrombocytopenia or previous history of aspirin allergy. In one patient, high dose aspirin was commenced but withheld after 48 hours on account of significant upper gastrointestinal bleeding manifesting as hematemesis and melena. Administration of IVIG was mainly determined by the ability of caregivers to procure the medication which was very expensive and not readily available. Supportive therapy included oxygen, inotropic support with intravenous Dobutamine infusion for at least 48 hours, mechanical ventilation, diuretic therapy with intravenous frusemide and oral spironolactone; and blood transfusion. Most patient had received at least a five day course of intravenous antibiotics without clinical improvement prior to diagnosis of MIS-C and these antibiotics included single medication or combined therapy using Ceftriaxone (25·0%), Levofloxacin (21·4%), Amikacin (14·3%), Vancomycin (14·3%), Meropenem (14·3%), Cefotaxime (10·7%), Cefuroxime (10·7%), Azithromycin (7·1%), Gentamycin (3·6%) and Metronidazole (3·6%).
Figure 5.
Therapeutic and supportive measures administered to participantsIVIG: intravenous immunoglobulin.
Immediate treatment response
The immediate outcome of our study population was 100% recovery with all patients discharged one day to three weeks after treatment initiation. Defervescence occurred within 24 hours following the commencement of IVIG administration in 14 out of 17 patients (82·4%) treated with this medication and between 24 to 48 hours in three patients (17·6%). Kidney function recovered spontaneously after IVIG and/or steroid administration in all patients with acute kidney injury and no patient required dialysis. Lateral squint in one patient resolved within one week of steroid therapy. Other symptoms which resolved within 48 hours of commencing anti-inflammatory therapy included improvement in activity (50·0%), marked drop in acute phase reactants (17·6%) and rapid resolution of florid skin lesions (11·8%). The treatment response of cardiac function seen in one of the study participants is shown in Figure 4.
Details of hospital stay, immediate outcomes and follow up
All patients were managed in-hospital with an average duration of hospitalization of 11·14 ± 5·65 days. The mean (SD) duration from symptom to hospital admission was 6·9 ± 5·5 days. Three out of 28 patients were managed in the intensive care unit for an average (SD) of 5·7 ± 3·5 days (range 2 to 9 days) and required respiratory support by continuous positive airway pressure ventilation or mechanical ventilation. All patients were discharged alive and were asymptomatic and clinically well at follow up visits. Twenty-two children were followed up at two weeks post illness, 22 children at six weeks post illness, 14 patients at three months, and eight patients at six months post illness respectively.
Cardiac investigations on follow up
Patients were followed up primarily for cardiac evaluation. Seven patients required follow up echocardiograms and ECGs one week after the first due to previously identified severe cardiac dysfunction. In these subgroups of patients, coronary dilatation and ventricular dysfunction were noted in 42·9%; at least moderate atrioventricular valve regurgitation and pericardial effusion in 28·5% respectively; prolonged QTc interval in 75·0% and first-degree heart block in 40·0% of these patients respectively. Cardiology assessments were recommended for all patients at two weeks and six weeks after the first evaluation; however, several patients were lost to follow up. Three month and six month follow up assessments were determined by the outcomes of the two previous follow up assessments at two weeks and six weeks. 22 patients (78·5%) were followed up at two weeks, and six weeks after the first echocardiogram; 14 participants (50·0%) and eight participants (28·5%) were reviewed three months and six months after the first cardiac evaluation respectively. Coronary artery dilatation did not develop in patients with previously normal coronary artery sizes. Table 3 shows the serial echocardiogram and ECG data of participants at various follow up visits.
Table 3.
Follow up Echocardiogram and ECG findings of participants.
| Echocardiogram findings | ||||
|---|---|---|---|---|
| Frequency of findings | Coronary dilatation (%) | Ventricular dysfunction (%) | Pathologic AVVR (%) | Pericardial effusion (%) |
| Presentation (n = 28) | 46·4 | 39·3 | 25·0 | 46·4 |
| 2 week follow up (n = 22) | 63·6 | 22·7 | 0·0 | 0·0 |
| 6 week follow up (n = 22) | 13·6 | 4·5 | 0·0 | 0·0 |
| 3 month follow up (n = 14) | 7·1 | 0·0 | 0·0 | 0·0 |
| 6 month follow up (n = 8) | 0·0 | 0·0 | 0·0 | 0·0 |
| Electrocardiographic findings | ||||
|---|---|---|---|---|
| Frequency of findings | Prolonged QTc interval | 10 AVB | ST depression+/- abnormal T waves | Complete RBBB |
| Presentation (n = 25) | 40·0 | 16·0 | 8·0 | 8·0 |
| 2 week follow up (n = 22) | 22·7 | 4·5 | 0·0 | 0·0 |
| 6 week follow up (n = 22) | 4·5 | 0·0 | 0·0 | 0·0 |
| 3 month follow up (n = 14) | 0·0 | 0·0 | 0·0 | 0·0 |
| 6 month follow up (n = 8) | 0·0 | 0·0 | 0·0 | 0·0 |
Discussion
This study shows the spectrum of cases of MIS-C seen in Nigeria's COVID-19 epicentre and the similarities and/or differences in its clinical, laboratory and imaging characteristics compared to findings in other geographical regions. MIS-C has been associated with an antecedent COVID-19 infection.10,12,16,17 Although the mechanisms underlying MIS-C could not be elucidated from this study data, about half of enrolled participants in this study had a previous history of COVID-19 infection; and there was evidence of antibodies to SARS-CoV-2 in about one third of patients while approximately two thirds of patients had negative PCR tests at presentation; suggesting an immunologic pathogenesis of the disease entity in which the antigen is not present and antibodies persist. Sub-analysis could not be performed to establish association between MIS-C and previous COVID-19 exposure in this cohort due to the small sample size and because almost half of the study population could not verify previous exposure to COVID-19 infection.
MIS-C bears a phenotypic similarity to KD, and although differences in both disease entities have been documented previously, it is likely that both conditions share similar pathogenetic mechanisms.11 Verdoni et al5 documented a 30-fold increase in cases of KD in Italy since the onset of the COVID-19 pandemic. This increase was similar to the recent exponential rise in the prevalence of this Kawasaki like disease seen in Lagos, Nigeria since the onset of the pandemic compared to the prevalence documented in a previous study25 in the same geographical location over a five-year period between 2011 and 2016. The upsurge of cases of this Kawasaki-like disease during the pandemic likely supports its temporal association with COVID-19 infection and is further supported by the monthly variation in frequency of cases akin to the waves of the pandemic.
The school age group was predominantly affected in this cohort as seen in earlier studies which documented older children than those usually affected in typical KD.5,17,26 There are infrequent reports of the occurrence of the syndrome in adults27 and male preponderance has also been shown in several study groups26,28,29 as was seen in our cohort.
The multisystemic nature of the pathology is reflected in the plethora of affected systems in study participants. In this study, fever was a universal finding; with gastrointestinal, cardiac and mucocutaneous symptoms being the most frequently described manifestations in these patients, similar to other researchers.1,29, 30, 31, 32 Cardiovascular manifestations have been described as frequent and prominent in children with MIS-C manifesting as coronary dilatation and/or aneurysms, ventricular dysfunction and biochemical evidence of myocardial injury.31 In our cohort, neurological symptoms including signs of meningism were documented among a third of our cohort similar to other studies.26,33 One of these patients had a palsy of the sixth cranial nerve despite normal brain imaging which resolved remarkably after anti-inflammatory therapy was commenced. Gastrointestinal symptoms were seen in about three quarter of the children in this study and transaminitis in about half of the study population in similar proportions documented by Miller et al.34 Renal involvement has been reported in 10% to 60% of MIS-C patients across studies and in keeping with this, one third of patients in this cohort had abnormalities of renal function.35
Erythrocyte sedimentation rate and C-reactive protein were the main acute phase reactants assayed in this population as these markers are more readily accessible and affordable compared to other inflammatory markers such as ferritin, LDH and D-dimers. In this environment where health care is primarily out of pocket, funds largely determine the extent of investigation as well as the treatment modalities instituted and this may account for the wide variation in the investigative results available for analysis among enrolled participants. Although all patients had full blood counts and renal function tests conducted and most of them had at least one acute phase reactant assayed, other ancillary tests were only performed based on strict clinical evidence of the need for the same; and not based on the premise that such tests may likely be abnormal in a disease of this nature. This strategy, though cost effective and desirable for patient care; but may have led to unrecognised subtle organ involvement.
Electrocardiogram findings in children with MIS-C documented by other researchers include non-specific ST/T changes, reduced QRS voltage and dysrhythmias.31,32 We found prolonged QTc to be an almost consistent finding in patients with echocardiographic evidence of myocardial dysfunction. Whereas negative ECG findings cannot completely rule out significant cardiac disease, prolonged QTc may considerably prompt early further cardiac evaluation and institution of relevant care even when such services are few and far between.
Several echocardiographic findings which have been frequently documented in children with MIS-C including coronary artery dilatation and/or aneurysms, pathological atrioventricular regurgitations, reduced ejection fraction and pericardial effusion36; were also seen frequently in our study group and are representative of the inflammatory effects of the disease on the heart.
The optimal treatment of MIS-C is still undefined but current treatment guidelines recommend intravenous immunoglobulin and high dose corticosteroids as first line therapy. McArdle et al37 documented that escalation to immunomodulators was less common among the patients who received IVIG plus glucocorticoids than among those who received IVIG alone. Intravenous immunoglobulin was utilised in a significant proportion of patients in this study along with steroids, similar to treatment regimens documented in previous populations of children and adolescents with MIS-C1,17,32; with demonstrable improvement in the clinical state of these patients from as early as 24 hours after instituting treatment. As suggested by McArdle et al,37 the use of initial combination therapy in our cohort may have accounted for the successful management of all cases without the use of immunomodulatory biologic agents. Cardiac, as well as other systemic effects resolved early and remarkably after immunosuppressive therapy with steroids and/or immunoglobulin as previously described by other authors who documented this disease entity in their populations.26,36 This significant improvement with immunosuppressant therapy strengthens the hypothesis that the disease is primarily immune mediated.
Aspirin has been used previously in the initial studies documenting MIS-C 1,36; and at similar doses to that used in this cohort of Nigerian patients. The presence of coronary artery dilatation at presentation may likely have influenced the addition of antiplatelet therapy to immunomodulatory therapy early in the course of the disease. More so, the high mean platelet counts in these patients noted up to the third week after the onset of fever may be beneficial as a precaution against significant morbidity from thromboembolic events; none of which was seen in our study group.
A small proportion of participants in this study were nursed in the high dependency and intensive care units similar to the experience from other resource constrained settings like ours;26 and in contrast to reports from other climes where ICU admissions as high as 83·0% were documented in some studies.17 This may not reflect the severity of the syndrome in Nigerian children, but rather likely represents the care available to critically ill children in resource limited settings like ours where the ICU is more readily available to critically ill adults than paediatric patients.
Two-third of the patients in this study received IVIG, but more patients were treated with steroids and aspirin which are more readily accessible medications. A significant proportion of these families funded their health care out-of- pocket and even when health insurance was available, most schemes did not accommodate the high cost of immunoglobulin which is approximately $500 per 10-gram vial.
MIS-C may mimic severe sepsis in its clinical presentation and thus, most children in this cohort had been treated with more than four-day course of antibiotics without significant clinical improvement prior to MIS-C diagnosis. From our experience, basic available and accessible laboratory parameters including leucocytosis, neutrophilia, thrombocytosis, elevated ESR and CRP, deranged liver enzymes especially transaminases, hypoproteinaemia, hypoalbuminaemia and proteinuria were present in a considerable number of study participants. Due to the limited size of this cohort, findings in this study may not be absolutely generalised to a wider study population; however, in resource limited climes like ours, clinical suspicion of this disease should be considered in children presenting with a combination of these commonly deranged; yet cost effective laboratory findings of MIS-C especially if fever and other systemic features persist despite appropriate use of antibiotics.
The sequelae of MIS-C especially on the heart, is still a subject of intense research. Like a previous Indian study,26 most of our study participants had coronary artery dilatation at presentation and although several patients were lost to follow up, the frequency of this abnormality reduced steadily at six-week, three-month and six-month follow up assessments. This may suggest that coronary dilatation in this syndrome may be primarily a consequence of inflammation rather than an alteration of the integrity of the arterial wall; thus, dimensions expectedly returned to normal quickly. In this study, new coronary artery abnormalities were not detected on follow up in patients who had normal coronaries at presentation, and this may raise concerns about the necessity of serial follow up echocardiograms in such patients. The cost of cardiology consultations and investigations are prohibitive for many Nigerian families and may have accounted for the significant drop out rate on follow up in our cohort. From our findings, patients with coronary artery abnormalities at presentation may likely not require serial cardiac evaluations beyond 3 months post illness. However, longer, and more comprehensive follow up studies are required to firmly establish this.
This study was hospital based and likely excluded the subset of patients with mild manifestations of MIS-C who did not require hospitalization. Due to the retrospective nature of the study, data available for review were inconsistent across the participating hospitals because of variation in investigations, management strategies and timing of follow-up assessments. Study findings could not lead to generalizations about this population as the small number of participants hampered rigorous sub analysis of data. Government regulations prohibited serologic testing in the country at a point during the pandemic and thus, relevant testing to confirm previous COVID-19 infection in some study participants could not be done. There was an appreciable number of patients lost to follow up even after appointments were advised and scheduled, due to several reasons including unwillingness to return to a hospital environment during the period of a pandemic, lack of funds for follow up investigations and poor health seeking behaviour, more so for a well child. The array of investigations available and therapeutic options in our cohort was largely limited by the cost of these services, thus medications such as immune modulators e.g. anakinra, tocilizumab which are not readily available could not be used in any of the study participants.
MIS-C is an important diagnosis in children presenting with prolonged fever during the COVID-19 pandemic. Cardiovascular manifestations occurred in several children with MIS-C, which had improved by 6-month follow up. Early diagnosis and prompt institution of a combination of antiplatelet therapy, steroids and IVIG appear to be beneficial.
Contributors
All authors contributed to the preparation of this manuscript and accept responsibility for publication and submission. The following authors had access to and verified data and manuscript submission: OS, YA, GO, OK, UO, EO, EI, OA, EO, OA, ML, EE and CO. SO and AY were responsible for conceptualization, data curation and analysis, investigations, methodology, validation, writing the original draft, reviewing and editing; AP, OO, UC and OB were responsible for investigations, methodology, supervision, draft review and editing; OG and AO were responsible for data curation, formal analysis, project administration, draft review and editing; KO, OU, OE, IE, OE, FAM, AO, LM were responsible for data collection, data curation and validation, draft review and editing; AA, EE, OC were responsible for conceptualization, investigations, methodology, project administration and supervision, draft review and editing.
Declaration of interests
We declare no competing interests.
Acknowledgments
Funding
None.
Data sharing
Deidentified patient data obtained during this study will be made available upon reasonable request to the corresponding author immediately after article publication with no end date.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang L, Tang K, Levin M, et al. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect Dis. 2020;20(11):e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://covid19.ncdc.gov.ng/report/ Accessed 30 July 2020
- 4.Dong Y, Mo X, Hu Y, Qi X, Jiang F, Tong S. Epidemiological characteristics of 2143 pediatric patients with 2019 coronavirus disease in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [Google Scholar]
- 5.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Royal College of Paediatrics and Child Health. Guidance: paediatric multisystem inflammatory syndrome temporally associated withCOVID-19. www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19 Accessed 5 August 2020.
- 7.Centers for Disease Control and Prevention. Emergency preparedness and response: health alert network. Published May 14, 2020. emergency.cdc.gov/han/2020/han00432.asp Accessed 5 August 2020.
- 8.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Published May 15, 2020. www.who.int/publications-detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 Accessed 5 August 2020.
- 9.Godfred-Cato S, Bryant B, Leung J, et al. California MIS-C Response Team. COVID-19-associated multisystem inflammatory syndrome in children - United States, March-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1074–1080. doi: 10.15585/mmwr.mm6932e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dufort E, Koumans E, Chow E, et al. New York state and centers for disease control and prevention multisystem inflammatory syndrome in children investigation team. Multisystem inflammatory syndrome in children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020 doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webb K, Abraham D, Faleye A, Cape Town MISC-Team Multisystem inflammatory syndrome in children in South Africa. Lancet Child Adolesc Health. 2020;4(10):e38. doi: 10.1016/S2352-4642(20)30272-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toubiana J, Poirault C, Corsia A, et al. Outbreak of Kawasaki disease in children during COVID-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowley A, Shulman S, Arditi M. Immune pathogenesis of COVID-19–related multisystem inflammatory syndrome in children. J Clin Invest. 2020;130(11):5619–5621. doi: 10.1172/JCI143840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.15 May 2020. WHO Multisystem Inflammatory Syndrome in Children and Adolescents Temporally Related to COVID-19 Scientific Brief.https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19 [Google Scholar]
- 16.DeBiasi RL, Song X, Delaney M, et al. Severe COVID-19 in children and young adults in the Washington, DC Metropolitan Region. J Pediatr. 2020 doi: 10.1016/j.jpeds.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS–CoV-2 pandemic. Circulation. 2020;142:429–436. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Tang Y, Shi Y, Chen Y, Liu E. Why multisystem inflammatory syndrome in children has been less commonly described in Asia? Transl. Pediatr. 2020;9(6):873–875. doi: 10.21037/tp-20-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choe Y, Choi E, Choi J, et al. Surveillance of COVID-19–associated multisystem inflammatory syndrome in children, South Korea. Emerg. Infect. Dis. 2021;27(4):1196–1200. doi: 10.3201/eid2704.210026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onyeaghala C, Alasia D, Eyaru O, et al. Multisystem inflammatory syndrome (MIS-C) in an adolescent Nigerian girl with COVID-19: A call for vigilance in Africa. Int. J. Infect. Dis. 2021;105:124–129. doi: 10.1016/j.ijid.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization . World Health Organization; 2020. Global COVID-19 clinical platform: case report form for suspected cases of multisystem inflammatory syndrome (MIS) in children and adolescents temporally related to COVID-19.https://apps.who.int/iris/handle/10665/332121 License: CC BY-NC-SA 3.0 IGO Accessed 18 May 2020. [Google Scholar]
- 22.2020. Center for Disease Control and Prevention Multisystem Inflammatory Syndrome.https://www.cdc.gov/mis-c/hcp Accessed 20 July 2020. [Google Scholar]
- 23.Lopez L, Colan S, Frommelt P, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the pediatric measurements writing group of the American Society of echocardiography pediatric and congenital heart disease Council. J Am Soc Echocardiogr. 2010;23:465–495. doi: 10.1016/j.echo.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Park M, Guntheroth W. Mosby; Missouri: 2006. Ventricular Conduction Disturbances. How to Read Paediatric ECGs; pp. 85–86. [Google Scholar]
- 25.Animasahun A, Adekunle M, Kusimo O, Fadipe C. The diagnosis of Kawasaki disease among Nigerian children: a nightmare for the caregivers and the doctors. J Public Health Emerg. 2017;1:69. doi: 10.21037/jphe.2017.06.06. [DOI] [Google Scholar]
- 26.Sethy G, Mishra B, Jain MK, et al. Clinical profile and immediate outcome of multisystem inflammatory syndrome in children associated with COVID-19: a multicentric study. J Glob Infect Dis. 2021;13(4):159–163. doi: 10.4103/jgid.jgid_85_21. 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elouardi Y, Rebahi H, Zarrouki Y, Ziadi A, Younous S, Samkaoui MA. COVID-19 associated Kawasaki-like multisystem inflammatory syndrome in an adult. Rev Esp Anestesiol Reanim. 2022 doi: 10.1016/j.redare.2020.11.009. S2341-1929(22)00003-8 Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehra B, Pandey M, Gupta D, et al. COVID-19-associated multisystem inflammatory syndrome in children: a multicentric retrospective cohort study. Indian J. Crit. Care Med. 2021;25(10):1176–1182. doi: 10.5005/jp-journals-10071-23996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feldstein L, Rose E, Horwitz S, et al. CDC COVID-19 response team multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383(4):334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma C, Ganigara M, Galeotti C, et al. Multisystem inflammatory syndrome in children and Kawasaki disease: a critical comparison. Nat. Rev. Rheumatol. 2021;17(12):731–748. doi: 10.1038/s41584-021-00709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperotto F, Friedman K, Son M, VanderPluym C, Newburger J, Dionne A. Cardiac manifestations in SARS-CoV-2-associated multisystem inflammatory syndrome in children: a comprehensive review and proposed clinical approach. Eur J Pediatr. 2021;180(2):307–322. doi: 10.1007/s00431-020-03766-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung E, Zachariah P, Gorelik M, et al. Multisystem inflammatory syndrome related to COVID-19 in previously healthy children and adolescents in New York City. JAMA. 2020;324(3):294–296. doi: 10.1001/jama.2020.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T. Neurological involvement associated with COVID-19 infection in children. J. Neurol. Sci. 2020;418 doi: 10.1016/j.jns.2020.117096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller J, Cantor A, Zachariah P, Ahn D, Martinez M, Margolis KG. Gastrointestinal symptoms as a major presentation component of a novel multisystem inflammatory syndrome in children that is related to coronavirus disease 2019: a single center experience of 44 cases. Gastroenterology. 2020;159(4) doi: 10.1053/j.gastro.2020.05.079. 1571-1574.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sethi S, Rana A, Adnani H, et al. Kidney involvement in multisystem inflammatory syndrome in children: a pediatric nephrologist's perspective. Clin Kidney J. 2021;14(9):2000–2011. doi: 10.1093/ckj/sfab073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramcharan T, Nolan O, Lai C, et al. Paediatric inflammatory multisystem syndrome: temporally associated with SARS-CoV-2 (PIMS-TS): cardiac features, management and short-term outcomes at a UK tertiary paediatric hospital. Pediatr. Cardiol. 2020;41(7):1391–1401. doi: 10.1007/s00246-020-02391-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McArdle AJ, Vito O, Patel H, et al. Treatment of multisystem inflammatory syndrome in children. N Engl J Med. 2021;385(1):11–22. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]