Highlights
-
•
Immune checkpoint inhibitors can be safely administered to patients with dermatomyositis.
-
•
Immunosuppressants did not impact the efficacy of treatment with an immune check point inhibitor.
-
•
Patients with autoimmune disorders who require immune check point inhibitors should be monitored by a rheumatologist.
Keywords: Dermatomyositis, Ovarian cancer, Pembrolizumab, MMR deficiency, Microsatellite instability
1. Introduction
Dermatomyositis (DM) is a rare systemic autoimmune disease characterized by progressive muscle weakness and/or skin rashes. Although, the etiology is not well understood, it has been suspected that interplay of genetic, immune, environmental factors could trigger its development (Krathen et al., 2008). Most importantly, it has been ascertained that DM can present as a paraneoplastic syndrome preceding, developing simultaneously or after initial diagnosis of internal malignancies, in particular ovarian cancer (OC) which is thought to be related to autoimmune cross-reactivity between similar autoantigens within the cancer tissue and normal tissue in muscle and skin (Whitmore et al., May 1994, Bohan et al., 1977). Immune checkpoint inhibitors have become a staple in the management of many tumor types including ovarian cancer where pembrolizumab is approved in patients with microsatellite instability-high, mismatch repair-deficient, or patients with tumor mutational burden-high ≥ 10 mutations/megabase and no satisfactory alternative treatment options. Immunotherapy is known to cause immune related adverse effects; therefore, their use in patients with autoimmune disorders may result in exacerbation of their underlying condition. In addition, patients who are on active immunosuppressive therapy are generally excluded from clinical trials involving immune checkpoint inhibitors making their safety and effectiveness unclear. In this case report, we describe a patient with cancer associated dermatomyositis associated with a mismatch repair (MMR)-deficient mixed endometrioid and clear cell carcinoma of the ovaries, who achieved a complete response to pembrolizumab while on immunosuppressive therapy and with no exacerbation of the life-threatening dermatomyositis.
2. Case report presentation
A 48-year-old female patient presented with abdominal pain and fullness, early satiety, myalgias and pyrexia. Initial computed tomography (CT) scan of the abdomen and pelvis revealed an 8.0 × 8.8 × 5.8 cm left pelvic mass. Serum cancer antigen-125 (CA-125) levels were 500.7 U/mL. A total abdominal hysterectomy and bilateral salpingo-oophorectomy (TAH-BSO) with omentectomy, lymphadenectomy and partial colectomy was performed with no gross residual disease and pathology revealing mixed grade 1 endometrioid (90%) and clear cell carcinoma (10%) of the left ovary measuring 9.8 cm with implants on the sigmoid colon (Stage IIB) and focal endometriosis in the left fallopian. Immunohistochemistry revealed tumor cells positive for PAX-8, napsin and estrogen receptor; p53 showed partial staining in scattered cells. DNA mismatch repair (MMR) protein immunohistochemistry showed loss of expression of MSH2 and MSH6 in the tumor cells while MLH1 and PMS2 were retained. Foundation One analysis demonstrated MSH2 (E132 and E786) pathogenic mutations, high microsatellite instability (MSI) and a high tumor mutational burden (TMB) of 21 Muts/Mb. Other pathogenic mutations were identified in other genes including ARID1A, ASXL1, CASP8 MAP3K1, NOTCH1, NOTCH3, PAX5, PIK3R1, PTEN, and TP53; Loss of heterozygosity score could not be determined. Germline genetic analysis showed no pathogenic mutations for ATM, BARD1, BRIP1, CHEK2, MRE11A, NBN, PALB2, RAD51C, RAD51D, BRCA1, BRCA2, APC, BMPR1A, CDH1, CDKN2A, DICER1, MLH1, MSH2, MSH6, MUTYH, PMS2, PTEN, SMAD4, STK11, TP53, CDK4, NF1, POLD1, POLE, SMARCA4, HOXB13, EPCAM, or GREM1. A variant of unknown significance was seen in RAD50.
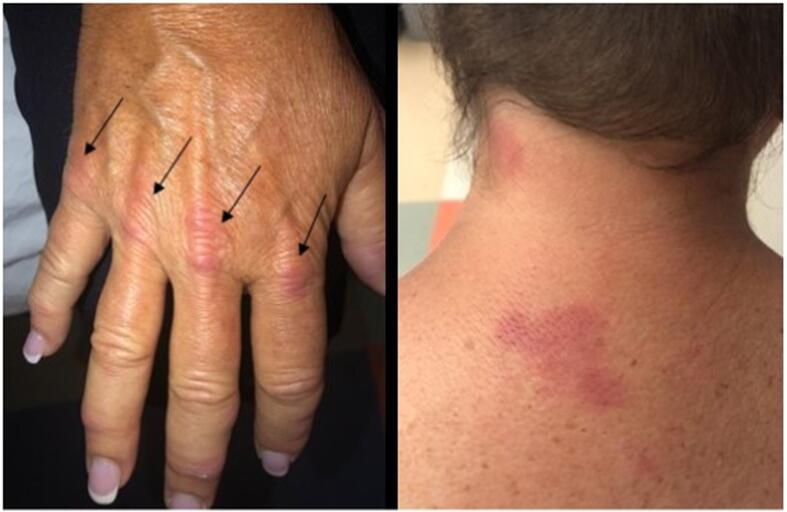
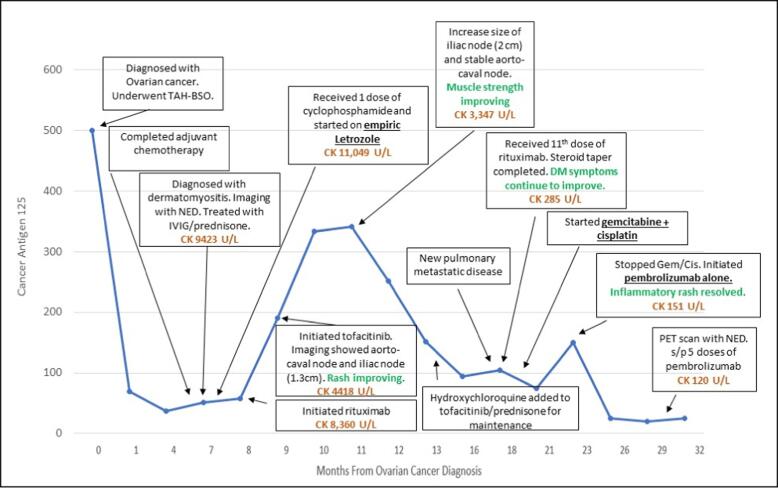
Adjuvant chemotherapy with carboplatin and paclitaxel was given every 3 weeks. Paclitaxel was replaced by docetaxel because of an anaphylactic reaction to the first dose of paclitaxel. After 4 cycles of chemotherapy, the patient developed progressive bilateral symmetrical proximal muscle weakness and rashes, approximately 5 months after her original cancer diagnosis. After completing 5 cycles of chemotherapy and 6 months from her original ovarian cancer diagnosis, her weakness had progressed to the point where she was not able to ambulate without assistance and she developed dysphagia with nasal regurgitation and presence of mucous production causing shortness of breath and dysphonia. Patient was admitted to the hospital and rheumatology was consulted. Physical examination revealed mild inflammation of her bilateral wrists and proximal interphalangeal joints as well as a Gottron papules, heliotrope rash, shawl sign, diffuse erythema throughout her body, Raynaud’s phenomenon, peripheral edema and severe proximal muscle weakness with the inability to hold her head up (Fig. 1 A-B). Evaluation showed increased serum levels of creatinine kinase of 9,423 U/L and aldolase of 122.9 U/L and negative antinuclear antibody (ANA) test, myositis specific and associated antibodies, anti-double stranded DNA and rheumatoid factor. Repeat CT scans of the abdomen and pelvis at the end of chemotherapy did not show any evidence of malignancy; serum CA-125 was 76 U/mL (normal range < 40 U/mL) (Fig. 2). An electromyogram (EMG) test demonstrated evidence of diffuse myopathy with fibrillation potentials confirming the diagnosis of dermatomyositis. A clinical diagnosis of dermatomyositis was made based on the above findings. Patient was admitted to the hospital and her DM was treated with corticosteroids with no improvement followed by 5 daily intravenous immunoglobulin (IVIG) infusions and then one dose of intravenous cyclophosphamide with no improvement. Rituximab was given next with significant improvement noted after the second dose. Tofacitinib, hydroxychloroquine and doxycycline were added to rituximab while the prednisone dose was tapered with gradual improvement in her muscle enzymes and improvement in her profound muscle weakness, peripheral edema, and skin rash. Five months following completion of the initial chemotherapy, a PET scan showed an enlarging aorto-caval node and left iliac node. At this point her DM associated muscle weakness was improving while on tofacitinib, prednisone daily and after receiving two infusions of rituximab. Given these lymph nodes seen on imaging, she was initiated on letrozole, based on her original tumor being estrogen receptor positive. In the setting of continued improvement of her DM muscle weakness and rash while on tofacitinib, prednisone, hydroxychloroquine, and after receiving a total of 11 doses of rituximab (six months after her initial rituximab infusions), she developed biopsy proven recurrence to the lymph nodes and lungs. Gemcitabine with cisplatin was initiated. She initially had stable disease and then progression after 4 months of treatment. Pembrolizumab was initiated with continuation of rituximab (every 2 months), tofacitinib, and hydroxychloroquine. Patient completed 7 doses of pembrolizumab (200 mg IV every 3 weeks) with a complete resolution of her adenopathy noted after 5 doses of pembrolizumab and no exacerbation of her dermatomyositis or immunotherapy-related adverse events. Repeat imaging studies 7 weeks after completion of pembrolizumab revealed no evidence of recurrence. Patient reported gradual improvement in her DM and was able to walk without assistance.
Fig. 1.
A-B: Images of patient demonstrating physical exam findings. A) Gottron papules of the left hand. B) Erythematous rash of back/neck consistent with dermatomyositis.
Fig. 2.
Timeline of tumor marker (CA-125), creatine kinase (CK), DM symptom trend with dermatomyositis and ovarian cancer directed treatment. TAH-BSO, total abdominal hysterectomy and bilateral salpingo-oophorectomy; NED, no evidence of disease; IVIG, intravenous immunoglobulin.
3. Discussion
DM is characterized by symmetrical proximal muscle weakness, elevated muscle enzymes, violaceous periorbital (heliotropic) erythema, commonly accompanied with diffuse erythematous rash, typically with a V-shaped eruption around the neck, and hyperpigmentation of metacarpophalangeal joints (Gottrön’s papules). In addition, severe cases of DM present distinctively with Raynaud’s phenomenon, dysfunction of bulbar muscles causing dysphagia and increasing the risk of aspiration pneumonia, and calcinosis (Bohan et al., 1977). Pathognomonic clinical signs and findings were all present in our case, and an abnormal electromyogram (EMG) further confirmed the diagnosis of DM. Classification criteria for DM by Peter and Bohan, require the presence of 4 out 5 manifestations for definite DM: 1) proximal muscle weakness, 2) elevated serum enzymes, 3) abnormal EMG, 4) abnormal muscle biopsy, and 5) characteristic skin manifestations (Bohan et al., 1977). The patient described here met the criteria for definite DM, which allowed for management directed at DM.
The treatment for primary DM consists of glucocorticoids and/or glucocorticoid-sparing regimens guided by a rheumatologist. Glucocorticoids are initiated at high doses until improvement of symptoms, which can take weeks, followed by a slow taper over 1 year (Kohsaka et al., 2019). When DM fails to respond to glucocorticoids or individuals have contraindications to steroids, other options include methotrexate, cyclophosphamide, cyclosporin A, rituximab, and IVIG. Responses to IVIG infusions are usually short lived (Bohan et al., 1977, Levine, 2005). Rituximab, a monoclonal antibody causing depletion of CD20 + B-cells, has proven to be successful in regaining muscle strength and significant improvement in skin erythema. In a clinical trial of 7 patients with DM, 6 patients achieved evident improvement by 4 weeks of treatment with rituximab achieving movement without assistance, by 12 to 36 weeks. Two of the patients had relapsed muscle weakness, coinciding with elevated CD20 + B cells, leading to resuming rituximab with responses.
The relationship of myositis with malignancy was first reported in 1916 by Stertz in a patient with gastric adenocarcinoma (Stertz, 1916). In several case studies conducted with patients with myositis, DM tends to present a higher risk of malignancy association than polymyositis (PM). Specifically, in large literature reviews, DM, has been known to develop with higher prevalence as a paraneoplastic syndrome prior to OC diagnosis than following the onset of OC (Whitmore et al., May 1994, Bohan et al., 1977). When considering paraneoplastic related dermatomyositis laboratory studies such as Anti-TIF-1gamma Ab (Anti-p155/140) has exhibited cancer DM association, but presenting only in 13–21% of adults with paraneoplastic DM (Gunawardena et al., Jun. 2009). This marker was negative in the patient presented here, however; timing was suggestive of a paraneoplastic process. In addition to a paraneoplastic syndrome, providers should be aware of other causes of myositis in patients with malignancy. Case reports and studies have described taxane medications inducing transient myositis in patients (Perel-Winkler et al., 2015). Discontinuing the taxane chemotherapy and treatment with corticosteroids, is associated with improvement (Bohan et al., 1977, Perel-Winkler et al., 2015).
The management of patients with DM as part of a paraneoplastic syndrome remains unclear in terms of order of treatment. Case reports and series have described treating these two conditions simultaneously, or sequentially (Kohsaka et al., 2019). It is suggested that treatment directed towards DM first should take place in patients with severe DM symptoms, as in our patient. There have been several reports of myositis going into remission after treating the malignancy alone(Yoshinaga et al., 2005, Hirai et al., Oct. 2007, Takahashi et al., 2008). Therefore, timing of treatment should be based on each individual patient with particular attention to managing the underlying malignancy expeditiously. The introduction of checkpoint inhibitor therapy into the management of solid tumors has led to challenges with how to manage patients with autoimmune conditions, since immunotherapy has the potential to exacerbate the underlying autoimmune diseases or cause immunotherapy-related adverse events, such as myositis. Pembrolizumab, a humanized monoclonal antibody immune checkpoint inhibitor (ICI), was first FDA approved in May of 2017, for solid tumors showing loss of MMR proteins, however, patients with autoimmune disorders or patients on immunosuppressive therapies are historically excluded from clinical trials using immune checkpoint inhibitors. In ovarian cancer, MMR deficient tumors have been seen in 7% of patients with ovarian endometrioid carcinoma and 2–6% of patients with ovarian clear cell carcinoma(Bennett et al., Feb. 2019, Ge et al., 2021). In the KEYNOTE-158 trial 15 patients with ovarian cancer and MMR deficiency were treated with pembrolizumab with 5 patients (33.3%) achieving a response with 3 patients (20%) patients achieving a complete response. Fortunately, the patient presented here achieved a complete response with pembrolizumab without exacerbation of her dermatomyositis.
4. Conclusion
This case illustrates the safe administration of pembrolizumab in a patient on immunosuppressive therapy with severe paraneoplastic DM and MMR-deficient mixed endometrioid and clear cell carcinoma of the ovaries. On the basis of this case presentation, the presence of paraneoplastic DM should not preclude the potentially curative treatment with pembrolizumab in MMR-deficient ovarian cancers, despite its association with immune related adverse events including myositis.
Consent
Informed consent was obtained from the patient for the publication of any potential identifiable images or data included in this case report
CRediT authorship contribution statement
Maia L. Valls: Writing – original draft. Adam M. Kase: Writing – original draft, Conceptualization. Rina Patel: . Benjamin Wang: . Rohit Aggarwal: Conceptualization. Gerardo Colon-Otero: Conceptualization, Writing – original draft.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Krathen M., Fiorentino D., Werth V. Dermatomyositis. Curr Dir Autoimmun. 2008;10:313–332. doi: 10.1159/000131751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmore S.E., Rosenshein N.B., Provost T.T. Ovarian cancer in patients with dermatomyositis. Medicine (Baltimore) May 1994;73(3):153–160. doi: 10.1097/00005792-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Bohan ANTHONY, Peter J.B., Bowman R.L., Pearson C.M. A computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine. 1977;56(4):255–286. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- Kohsaka H., Mimori T., Kanda T., Shimizu J., Sunada Y., Fujimoto M., Kawaguchi Y., Jinnin M., Muro Y., Ishihara S., Tomimitsu H., Ohta A., Sumida T. Treatment consensus for management of polymyositis and dermatomyositis among rheumatologists, neurologists and dermatologists. Neurology and Clinical Neuroscience. 2019;7(1):3–21. doi: 10.1111/ncn3.12223. [DOI] [PubMed] [Google Scholar]
- Levine T.D. Rituximab in the treatment of dermatomyositis: An open-label pilot study. Arthritis & Rheumatism. 2005;52(2):601–607. doi: 10.1002/art.20849. [DOI] [PubMed] [Google Scholar]
- Stertz G. Polymyositis. Berl klin Wschr. 1916;53:489. [Google Scholar]
- Gunawardena H., Betteridge Z.E., McHugh N.J. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology. Jun. 2009;48(6):607–612. doi: 10.1093/rheumatology/kep078. [DOI] [PubMed] [Google Scholar]
- Perel-Winkler A., Belokovskaya R., Amigues I., Larusso M., Hussain N. A Case of Docetaxel Induced Myositis and Review of the Literature. Case Reports in Rheumatology. 2015;2015:1–8. doi: 10.1155/2015/795242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinaga ATSUSHI, Hayashi TETSUO, Ishii NOBUYUKI, Ohno RENA, Watanabe TORU, Yamada TAKUMI. Successful cure of dermatomyositis after treatment of nonseminomatous testicular cancer. International Journal of Urology. 2005;12(6):593–595. doi: 10.1111/j.1442-2042.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- Hirai T., Tsujihata M., Ueda T., Nonomura N., Okuyama A. A case of polymyositis associated with adrenal carcinoma. Int J Urol. Oct. 2007;14(10):952–953. doi: 10.1111/j.1442-2042.2007.01868.x. [DOI] [PubMed] [Google Scholar]
- Takahashi F., Tsuta K., Nagaoka T., Miyamoto H., Saito Y., Amano H., Uchida K., Morio Y., Shimizu K., Sasaki S., Tominaga S., Uekusa T., Izumi H., Anami Y., Matsuno Y., Takahashi K., Fukuchi Y. Successful resection of dermatomyositis associated with thymic carcinoma: report of a case. Surg Today. 2008;38(3):245–248. doi: 10.1007/s00595-007-3601-x. [DOI] [PubMed] [Google Scholar]
- Bennett J.A., Pesci A., Morales-Oyarvide V., Da Silva A., Nardi V., Oliva E. Incidence of Mismatch Repair Protein Deficiency and Associated Clinicopathologic Features in a Cohort of 104 Ovarian Endometrioid Carcinomas. Am J Surg Pathol. Feb. 2019;43(2):235–243. doi: 10.1097/PAS.0000000000001165. [DOI] [PubMed] [Google Scholar]
- Ge H., Xiao Y., Qin G., Gu Y., Cai X.u., Jiang W., Tu X., Yang W., Bi R. Mismatch repair deficiency is associated with specific morphologic features and frequent loss of ARID1A expression in ovarian clear cell carcinoma. Diagnostic Pathology. 2021;16(1) doi: 10.1186/s13000-021-01071-w. [DOI] [PMC free article] [PubMed] [Google Scholar]