Abstract
Purpose
To assess the effect of radiation therapy on osteocyte apoptosis, osteocyte death, and bone marrow adipocytes in the human mandible and its contribution to the pathophysiology of radiation damage to the mandibular bone.
Methods and Materials
Mandibular cancellous bone biopsies were taken from irradiated patients and nonirradiated controls. Immunohistochemical detection of cleaved caspase-3 was performed to visualize apoptotic osteocytes. The number of apoptotic osteocytes per bone area and per total amount of osteocytes, osteocytes per bone area, and empty lacunae per bone area were counted manually. The percentage fibrotic tissue and adipose tissue per bone marrow area, the percentage bone marrow of total area, and the mean adipocyte diameter (μm) was determined digitally from adjacent Goldner stained sections.
Results
Biopsies of 15 irradiated patients (12 men and 3 women) and 7 nonirradiated controls (5 men and 2 women) were assessed. In the study group a significant increase was seen in the number of empty lacunae, the percentage of adipose tissue of bone marrow area, and the adipocyte diameter. There was no significant difference in bone marrow fibrosis nor apoptotic osteocytes between the irradiated group and the controls.
Conclusions
Irradiation alone does not seem to induce excessive bone marrow fibrosis. The damage to bone mesenchymal stem cells leads to increased marrow adipogenesis and decreased osteoblastogenic potential. Early osteocyte death resulting in avital persisting bone matrix with severely impaired regenerative potential may contribute to the vulnerability of irradiated bone to infection and necrosis.
Introduction
Osteoradionecrosis (ORN) of the jaw is a serious complication of radiation therapy in patients with head and neck cancer. To date, the events leading up to radiation-induced bone damage and ORN have not been fully elucidated.1 The primary findings of radiation damage to bone is local tissue atrophy, loss of functional osteoblasts, marrow adiposity, and microvascular impairments.2 The resulting effect of these independent findings on bone homeostasis and regeneration capacity is not clear.
Many theories on the pathophysiology of ORN of the jaw have been proposed. In 1983, Marx3 proposed the well-known hypothesis that states that irradiation leads to a sequence of hypoxic-hypocellular-hypovascular tissue, tissue breakdown, and chronic nonhealing wound. Marx found progressive loss of capillaries and fibrosis of marrow spaces in irradiated mandibular bone. His theory formed the cornerstone for the hyperbaric oxygen treatment, although its clinical efficacy is controversial.4,5 Delanian and Lefaix6 postulated another well-established theory in 2011, stating that ORN occurs because of a radiation-induced fibroatrophic mechanism, including free radical formation, endothelial dysfunction, inflammation, microvascular thrombosis, fibrosis and remodeling, and finally bone and tissue necrosis. A possible role for osteoclast deficiency as crucial mechanism in the pathophysiology of ORN has been proposed by several authors, arising from the resemblance with medication-related osteonecrosis of the jaw that is typically caused by drugs that inhibit osteoclast function.7,8
In the past decades, the view on the role of osteocytes and bone marrow adipocytes have changed from silent bystander to having their own important role in bone metabolism. Osteocytes are formed during bone formation when osteoblasts are encapsulated in the bone matrix and play a critical role in regulating bone turnover.9 A canicular network in the bone matrix enables communication between osteocytes and the cells on the bone surface, which is essential for the regulation of osteoblasts and osteoclasts.10 The adaptation of bone in relation to mechanical forces has been largely investigated and osteocytes are thought to have a function as mechano-sensors.11 Osteocytes produce receptor activator of nuclear factor-κB ligand that stimulates differentiation of osteoclasts.10,12,13 Apoptotic death of osteocytes is thought to be a critical event in the recruitment of osteoclasts to sites where bone resorption is needed, such as areas of fatigue damage, estrogen deficiency, skeletal unloading, and possibly other states that necessitate bone to be removed.14
Marrow adipocytes originate from the mesenchymal stem cells, like osteoblasts. Studies have shown that increased bone marrow adiposity is associated with diseases such as osteoporosis, obesity, and diabetes and is often associated with a deterioration of bone mass.15 An unbalanced shift to adipogenesis in the bone marrow is thought to have detrimental effects on bone through the release of different factors that can promote apoptosis, osteoclastogenesis, alter osteoblastogenesis, and favor adipogenesis and release of saturated fatty acids that impair osteoblast function and survival.16 Irradiation is known to induce bone marrow adipogenesis in postcranial sites,17 but little is known about this effect in the mandible.
Radiation therapy affects all cells in the targeted area and, therefore, ORN is a multifactorial disease. As the exact pathophysiology of ORN remains unclear, studies targeting on the effect of irradiation on mandibular bone homeostasis and bone marrow composition could provide better insight in the process leading up to ORN. We hypothesize that irradiation alters bone marrow composition and disrupts bone homeostasis on different levels, making the bone vulnerable and potentially susceptible for ORN. The present study sought to evaluate 2 aspects in the field of bone metabolism that are underrepresented in current literature on mandibular bone radiation damage: osteocyte death and bone marrow adiposity.
Methods and Materials
Patients
Fifteen patients with a history of radiation therapy for head and neck malignancy were compared with 7 edentulous patients with no history of oral cancer or radiation therapy. All irradiated patients were edentulous with an indication for oral rehabilitation with mandibular dental implants and were treated between August 1, 2012, and April 1, 2016. Patients without radiation dose on the mandible and patients who had undergone mandibular reconstruction with bone grafts were excluded from this study. Patients in the control group were treated with dental implants in the mandible between August 1, 2012, and December 31, 2014. Exclusion criteria were a history of bisphosphonate medication, impaired bone metabolism (eg, hyperparathyroidism, osteomalacia), or systemic immunosuppressive medication up to 3 months before dental implant surgery. All participants had blood calcium, phosphate, parathyroid hormone, and HbA1c levels within the normal range.
All patients were fully informed and signed a written consent form for study participation. Before the study, approval for the research was provided by the Medical Ethical Committee of the Amsterdam University Medical Centers, location VUmc (registration No. 2011/220). All methods were performed in accordance with the relevant guidelines and regulations.
Hyperbaric oxygen therapy
In accordance with the department´s protocol, hyperbaric oxygen (HBO) therapy is administered to patients who undergo surgical procedures in the area of the maxilla or mandible that have been irradiated with 50 Gy or more. Edentulous patients typically are treated with 2 or 4 dental implants in the interforaminal region of the anterior mandible to accommodate retention of an overdenture. Therefore, for all irradiated patients the radiotherapist was preoperatively consulted to estimate the maximum radiation dose in the anterior mandible. Patients who had received an estimated dose of 50 Gy or more on the anterior mandible were treated with 20 sessions of HBO therapy preoperatively and 10 sessions postoperatively according to the “Marx-protocol.”3 Thirty HBO sessions were administered on consecutive days excluding the weekends, that is, for a total duration of 6 weeks. Sessions consisted of administration of a total of 80 minutes of 100 per cent oxygen at 243 to 253 kPa.
Dental implant surgery and bone biopsy retrieval
Dental rehabilitation of all patients from the irradiated group was performed in the Amsterdam University Medical Centers, location VUmc, by a single oral and maxillofacial surgeon. Patients in the control group were treated in Alrijne Hospital, Leiderdorp, by a single oral and maxillofacial surgeon. Dental implants were placed in the interforaminal region of the anterior mandible.
The dental implant surgical procedure was the same in both groups. Implant preparations were made under copious irrigation with a 3.5 mm trephine burr (2.5 mm inner diameter; Straumann Dental Implant System, Straumann Holding AG, Basel, Switzerland) to a depth of 10 or 12 mm. An ejector pin was used to carefully remove the bone cylinder from the trephine drill. One bone cylinder (biopsy specimen) per patient was selected and prepared for further analysis.
Determination of radiation dose
All 15 patients were treated with intensity modulated radiation therapy. To determine the maximum radiation dose (Dmax) at the site of the dental implant, the radiation therapy treatment planning computed tomography (CT) image was merged with a postoperative cone beam CT image. In this way the dose administered at the site of the implant (corresponding with the site of the biopsy) was estimated.
Two patients were treated with radiation therapy in clinics outside the Amsterdam University Medical Centers. The total radiation dose was known for these patients. However, despite efforts to contact these clinics to gather the intensity modulated radiation therapy treatment plans, this information could not be retrieved. Therefore, in these 2 patients, the Dmax at the biopsy site could not be determined. These 2 patients were only included in comparisons between irradiated and nonirradiated groups but not in the dosimetry statistics.
Processing and measurements of the bone biopsies
Bone cylinders were immediately fixed by immersion in 4% phosphate-buffered formaldehyde, dehydrated in ascending series of ethanols, and embedded in 83% methyl methacrylate (BDH Chemicals) supplemented with 17% dibutyl phthalate (Merck), 8 g/L lucidol CH-50L (Akzo Nobel), and 22 µL/10 mL N,N-dimethyl-p-toluidine (Merck). Undecalcified biopsies were cut into sections of 5 µm with a microtome (Polycut 2500 S, Reichert-Jung). Immunohistochemical detection of cleaved caspase-3 was performed to visualize apoptotic osteocytes on 2 sections per biopsy, spaced by 50 µm. Sections were transferred to poly-L-lysine–coated slides and stained according to the following method:
The sections were deplastificated, rehydrated, and decalcified in 1% acidic acid for 10 minutes. Sections were incubated with Saponin (0.05%) in phosphate-buffered saline incubation for 30 minutes and 10 minutes with DNAse (3.5 μg/mL DNAse II (Sigma) in 25 mM Tris + 10 mM MgSO4) for antigen retrieval. Endogenous peroxidase was blocked with 3% H2O2 in methanol for 15 minutes. Primary antibody incubation was performed for 3 hours with 1/300 rabbit anticleaved caspase-3 antibody (Cell Signaling Technology, Beverly, MA) in phosphate-buffered saline + 0.05% Tween. Sections were incubated with EnVision-rabbit (Agilent Dako products, Santa Clara, CA) for 1 hour. Staining was performed for 10 minutes with the Nova Red kit (Vector Labs, Burlingame, CA). The sections were counterstained with 10% Toluidine blue in ethanol 60%. Sections were then dehydrated and sealed in DEPEX mounting medium (BDH Chemicals). An adjacent (undecalcified) section was selected for each section, with a maximum distance of 3 sections, and Goldner trichrome staining was performed.18
Histomorphometrical analysis
Bone samples were analyzed blinded. Bone volume, the number of adipocytes, the total adipose area, the total fibrotic marrow area, the total bone marrow area, and the total area of the section was measured in the Goldner section. A Nikon eclipse E800 microscope with 40 × magnification and NIS-Elements AR 4.10.01 (Nikon GmbH) was used to photograph and analyze the sections. From these measurements, the percentage fibrotic tissue of bone marrow area, the percentage adipose tissue of bone marrow area, the percentage bone marrow of total area of the section, and the mean adipocyte diameter (μm) were calculated. A magnification of × 200 was used to count the total number of osteocytes per bone area, the total number of empty lacunae per bone area in the Goldner sections, and the number of cleaved caspase-3 positive osteocytes per bone area, and to observe the percentage of positive osteocytes per total amount of osteocytes in the cleaved caspase-3 sections (Fig. 1).
Figure 1.
Histologic sections (× 200 magnification) of irradiated mandibular bone from an irradiated patient (Dmax = 34 Gy). (A) Goldner trichrome stain. Osteocyte nuclei are stained dark purple. Arrowheads point toward empty lacunae, indicating osteocyte death. (B) Cleaved caspase-3 stain. Arrowheads point toward cleaved caspase-3 positive osteocytes, indicating osteocyte apoptosis.
Statistical analysis
All statistical analyses were performed using SPSS Statistics (version 25; IBM). P < .05 was considered statistically significant. Mann-Whitney nonparametric tests were used to compare the median of the parameters against the hypothetical value 1.0 (no difference in parameter between the 2 groups; irradiated and nonirradiated, <50 Gy and ≥50 Gy). Correlations between histomorphometrical parameters and clinical data were analyzed with correlation coefficients and nonparametric tests. The Spearman correlation coefficient was used to analyze relations between the bone turnover and microarchitectural parameters with radiation dose and time interval between last radiation dose and biopsy.
Results
The characteristics of the irradiated patients are summarized in Table 1. The irradiated group consisted of 12 men and 3 women with a mean age of 66 years (range, 56-76 years). The mean total radiation dose was 66 Gy (range, 54-70 Gy) and the median Dmax at the biopsy site was 39 Gy (range, 3-63 Gy). The median interval between radiation therapy and biopsy was 24 months (range, 10-197 months). The control group consisted of 5 men and 2 women, with a mean age of 64 years (range, 34-73 years). Smoking and drinking habits of the irradiated and control groups are summarized in Table 2.
Table 1.
Patient and treatment characteristics of the irradiated group (n = 15)
| Age/sex | Tumor site | Total RT dose (Gy) | RT Dmax (Gy) | Interval RT biopsy (mo) |
|---|---|---|---|---|
| 69/Male | Submandibular gland | 56 | 63 | 10 |
| 68/Male | Oropharynx | 60 | 3 | 13 |
| 58/Male | Supraglottic larynx | 70 | 13 | 28 |
| 56/Male | Hypopharynx | 70 | 18 | 17 |
| 62/Male | Oropharynx | 62.5 | 50 | 197 |
| 68/Female | Oropharynx | 70 | 31 | 24 |
| 63/Male | Lower lip | 54 | 53 | 11 |
| 63/Male | Supraglottic larynx | 70 | NA* | 171 |
| 76/Male | Tongue base | 70 | 39 | 31 |
| 71/Male | Oropharynx | 70 | 25 | 30 |
| 74/Male | Tonsil | 70 | 41 | 70 |
| 58/Male | Tonsil | 70 | 34 | 23 |
| 61/Female | Lateral tongue | 70 | NA* | 88 |
| 70/Male | Floor of mouth | 66 | 51 | 17 |
| 67/Female | Retromolar trigone | 66 | 57 | 10 |
Abbreviations: Dmax = maximum radiation dose at biopsy site; NA = not available; RT = radiation therapy.
Radiation therapy treatment plans could not be retrieved from 2 patients.
Table 2.
Smoking and drinking characteristics of control group (n = 7) and irradiated group (n = 15)
| Current smoker | Pack years | Alcohol units per week | |
|---|---|---|---|
| Control 1 | No | 0 | 4 |
| Control 2 | No | 12 | 21 |
| Control 3 | Yes | 10 | 10 |
| Control 4 | No | 36 | 14 |
| Control 5 | No | 35 | 18 |
| Control 6 | No | 0 | 0 |
| Control 7 | No | 0 | 3 |
| Irradiated 1 | Yes | 25 | 0 |
| Irradiated 2 | No | 40 | 0 |
| Irradiated 3 | No | 5 | 0 |
| Irradiated 4 | No | 26 | 35 |
| Irradiated 5 | Yes | 23 | 28 |
| Irradiated 6 | No | 30 | 30 |
| Irradiated 7 | No | 0 | 5 |
| Irradiated 8 | No | 30 | 0 |
| Irradiated 9 | Yes | 80 | 0 |
| Irradiated 10 | Yes | 70 | 20 |
| Irradiated 11 | No | 0 | 14 |
| Irradiated 12 | Yes | 41 | 12 |
| Irradiated 13 | Yes | 50 | 30 |
| Irradiated 14 | No | 28 | 40 |
| Irradiated 15 | No | 0 | 21 |
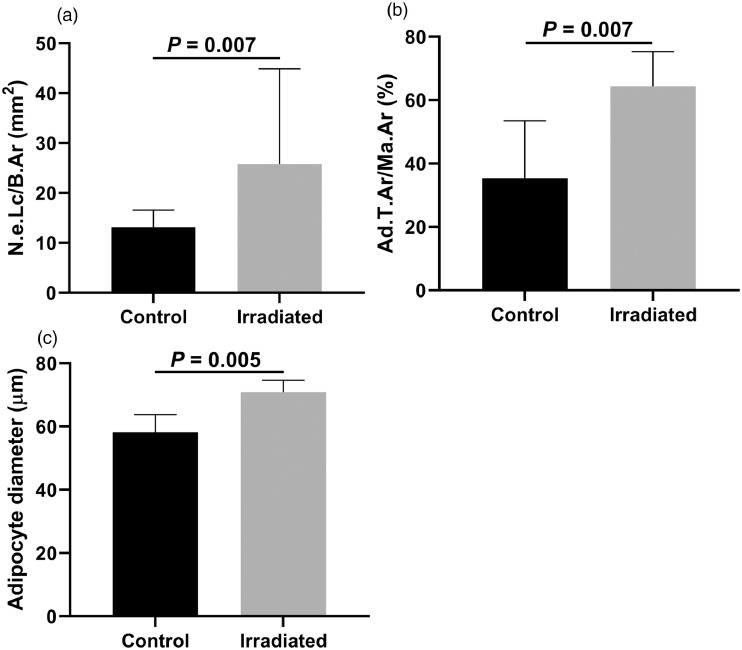
The histologic measurements of the irradiated bone versus nonirradiated (control) bone are summarized in Table 3. There was no significant difference in number or percentage of cleaved caspase-3 positive osteocytes between the irradiated group and the controls. The empty lacunae are expressed as number per mm2 bone area. In percentages, this corresponds with a median of 13.13% (range, 7.16) of lacunae in the control group and 25.81% (range, 48.3) in the irradiated group, which is a significant difference (P = .007). The percentage of adipose tissue in bone marrow area and the adipocyte diameter were significantly higher in the irradiated group (P = .007 and P = .005, respectively; Fig. 2). There was no significant difference in bone marrow fibrosis between irradiated and nonirradiated specimens, although the visual aspect of fibrosis as well as the adipose content in the irradiated samples have a distinct appearance (Fig. 3). No correlations between the histologic measurements and Dmax, total radiation dose or time interval between radiation therapy and biopsy were observed.
Table 3.
Measurements of nonirradiated versus irradiated mandibular cancellous bone biopsies
| Parameter | Control group (n = 7), median (IQR) | Irradiated group (n = 15), median (IQR) | P value |
|---|---|---|---|
| N.Ot/B.Ar (n/mm2) | 134.95 (60.94) | 127.47 (59.15) | .837 |
| N.e.Lc/B.Ar (n/mm2) | 13.13 (5.06) | 25.81 (24.93) | .007* |
| N.Pos.Ot/B.Ar (n/mm2) | 2.09 (5.64) | 4.84 (8.36) | .267 |
| N.Pos.Ot/N.Tt.Ot (%) | 0.981 (6.42) | 4.08 (8,21) | .237 |
| Fb.T.Ar/Ma.Ar (%) | 9.1 (17.25) | 13.7 (9.63) | .630 |
| Ad.T.Ar/Ma.Ar (%) | 35.34 (28.93) | 64.38 (27.55) | .007* |
| Ma.Ar/Tt.Ar (%) | 45.77 (16.20) | 50.98 (21.34) | .447 |
| Adipocyte diameter (µm) | 58.14 (13.69) | 70.88 (9.51) | .005* |
Abbreviations: Ad.T.Ar/Ma.Ar = percentage of adipose tissue per bone marrow area; Fb.T.Ar/Ma.Ar = percentage of fibrotic tissue per bone marrow area ; IQR = interquartile range; Ma.Ar/Tt.Ar = percetage of bone marrow per total area; N.e.Lc/B.Ar = number of empty lacunae per bone area; N.Ot/B.Ar = number of osteocytes per bone area; N.Pos.Ot/B.Ar = number of apoptotic osteocytes per bone area; N.Pos.Ot/N.Tt.Ot = number of apoptotic osteocytes per total number of osteocytes
Significant at P < .05 level (Mann-Whitney U test P value).
Figure 2.
(A) Number of empty lacunae per bone area (N.e.Lc/B.Ar) in the control and irradiated groups (Mann-Whitney U test; P = .007). (B) Percentage of adipose tissue of bone marrow area (Ad.T.Ar/Ma.Ar) in the control and irradiated groups (Mann-Whitney U test; P = .007). (C) Adipocyte diameter in the control and irradiated groups (Mann-Whitney U test; P = .005).
Figure 3.
Histologic sections with Goldner trichrome stain of mandibular bone from an unirradiated control patient (A: × 40 magnification, B: × 100 magnification) and from an irradiated patient, Dmax = 53 Gy (C: × 40 magnification, D: × 100 magnification). In both specimens, fibrotic areas as well as adipose tissue is present. The nonirradiated bone marrow has more abundant nuclei and the fibrosis is more localized. The irradiated bone marrow is hypocellular with smaller fibrotic patches scattered throughout the marrow space.
In follow-up, 6 of the irradiated patients died. Of the 9 patients who survived, 1 was lost to follow-up. The remaining 8 are still in follow-up (years in follow-up mean, 5.8; range, 4.2-6.7 years). None of the irradiated patients included in this study developed ORN.
Discussion
This study shows an increase in empty lacunae in irradiated mandibles, which is likely a result of osteocyte death. However, no significant increase in apoptotic osteocytes in the irradiated group was observed. Few studies have investigated irradiation-induced osteocyte apoptosis, and the available literature is based on in vitro experiments. Osteocytes irradiated in vitro are relatively sensitive to radiation-induced apoptosis, which is time- and dose-dependent, and detected as early as 48 hours after radiation.19,20 Although the results of these in vitro studies must be interpreted with caution, it is conceivable that osteocyte apoptosis is an early sequence of irradiation and is therefore not increased in our irradiated samples, as in the present study the time interval between the last radiation therapy and biopsy ranged from 10 to 197 months.
Osteocyte apoptosis is known to increase osteoclastogenesis.14,19 In animal and in vitro studies, it has been revealed that irradiation causes an initial increase of osteoclast recruitment and activity.20, 21, 22, 23, 24, 25 However, in murine models, this early, transient increase in osteoclasts is followed by long-term osteoclast depletion.2,25,26 In human irradiated mandibles, a significant reduction as well as an absence of osteoclasts is observed.27, 28, 29 The bone samples in human studies were derived from resections of osteoradionecrotic lesions28,29 and from trephine biopsies taken during implant surgery in irradiated bone,27 which represents bone harvested months to years after radiation therapy and captures the “late stage” radiation injury. As osteocyte apoptosis is thought to recruit osteoclasts to areas in need of bone resorption, perhaps the early radiation-induced apoptotic osteocyte death induces an initial increased osteoclast recruitment. Possibly this effect extinguishes in later stages of radiation damage when osteocyte apoptosis is no longer increased. Furthermore, as irradiation is known to deplete bone marrow osteoclast precursors, the combination of a lack of osteoclastogenic stimuli and local marrow suppression may cause the absence of osteoclasts in the irradiated human mandible.
Empty lacunae as an indication of earlier osteocyte death have been more commonly investigated in studies on the effects of irradiation on bone. In the present study, a significant increase of empty lacunae was seen in the irradiated group. No correlation between radiation dose and histologic findings was observed. Rodent models have been widely used to investigate dose- and time-dependent effects of irradiation on mandibular bone. Irradiation causes an increase in empty lacunae that seem to be dose and time dependent, with most osteocyte death occurring in the first weeks after irradiation and dependent on the administered dose.30, 31, 32
The literature on osteocyte viability in irradiated human mandibles is scarce. The available studies on human material have been performed on bone specimens from ORN lesions, with empty lacunae mentioned as a qualitative rather than a quantitative outcome.28,29 The persistent presence of empty lacunae in irradiated bone, even many years after irradiation, could in part be due to the absence of osteoclasts, which leads to the preservation of mineralized matrix that should have been resorbed.27
All patients with a Dmax of more than 50 Gy at the dental implant site are treated with HBO therapy in accordance with the department's protocol. All patients receive 20 sessions before and 10 sessions after dental implant placement. In the present study the patients did not start HBO until the implant surgery was indicated. No dental implant surgery is undertaken in the first 9 months after radiation therapy according to protocol. Because no control group of irradiated patients without HBO treatment was investigated in the present study, it is not possible to interpret the effect that HBO therapy could have in the examined specimens.
Spiegelberg et al22 found in a study in mice that HBO therapy reduced the number of empty lacunae in irradiated specimens 24 weeks after treatment. No effect was observed on bone marrow adiposity.22 The mice did, however, start with HBO treatment 1 day after radiation therapy. No conclusive evidence from double-blind randomized controlled trials on the efficacy of HBO on preventing ORN is available to date.4,5,30, 31, 32, 33, 34 Because osteocyte death seems to be an early sequence of radiation therapy rather than a late one, the HBO therapy administered to patients in the present study cannot influence the osteocytes that have already perished.
It should be mentioned that the irradiated group contained more patients with heavier smoking and drinking habits. The amount and duration of smoking and drinking habits as well as the number of years of cessation vary greatly, which makes it extremely difficult to determine and interpret the potential confounding effect. It is known that heavy smoking and drinking is associated with decreased bone volume.35,36 The specific effect on the mandible is not well known. In a previous study, mandibular bone samples from irradiated patients and healthy edentulous patients were compared, and no significant difference in bone volume and bone mineral density was observed between the groups.27
Unexpectedly, no significant increase in bone marrow fibrosis is seen in irradiated specimens compared with control biopsies. In edentulous mandibles, fibrosis in the bone marrow is a physiological finding as a result of alveolar healing.37 The control biopsies show fibrotic areas in the bone marrow as well, although the visual aspect is different. Bone marrow fibrosis is considered a key event in the theories of Marx3 as well of Delanian and Lefaix6 for the pathogenesis of ORN. Marx described progressive fibrosis over time after irradiation in a qualitative assessment of biopsies taken from patients irradiated for oral malignancies. However, the specific sites, technique, and circumstances under which the biopsies were taken is not further specified. In the study by Fenner et al38 describing histologic changes in an 4 × 15 Gy fractionated irradiated rat model, progressive fibrosis in the bone marrow cavity in the irradiated site was seen at both 6 and 12 weeks after radiation, and the overlying skin and mucosa were intact. The scarce histomorphometric studies that describe bone marrow fibrosis on human irradiated mandibles are performed on specimens from segmental resections that were performed because of osteoradionecrosis or tumor resection. Bras et al39 compared histologic findings in mandibles with manifest osteoradionecrosis, nonosteoradionecrotic irradiated mandibles, and nonirradiated mandibles (the latter 2 from tumor resections). The nonosteoradionecrotic irradiated mandibles exhibited fibrosis of the periosteum and adjacent submucosa but no fibrosis of the bone marrow. The bone marrow adjacent to ORN lesions was replaced by a dense fibrous, less-vascularized tissue with a gradual decrease of fibrosis toward the periphery. Multiple other histologic studies have reported bone marrow fibrosis in human mandibular osteoradionecrosis lesions.28,40 To the authors’ knowledge, no previous studies quantitatively assessing the extent of fibrosis in irradiated human mandibular bone without other pathology, such as recurrent tumor or ORN, are available. The present study shows that even in areas of the mandible that received a high dose of irradiation, abundant marrow fibrosis is not a characteristic finding.
In the present study, a marked increase in adipocyte area in the bone marrow as well as adipocyte diameter in the irradiated group was found. No relation with dose or interval between radiation and biopsy was demonstrated. Bone marrow mesenchymal stem cells can differentiate into either osteoblasts or bone marrow adipocytes. Irradiation inhibits proliferation and differentiation of surviving mesenchymal stem cells and osteoprecursor cells into osteoblast cell lineages.41 Osteogenic differentiation potential of human mesenchymal stem cells is less resistant to irradiation than adipogenic potential.42 Osteoblastic activity is significantly decreased in irradiated human mandibles.27
It is well established that irradiation causes myelosuppression, damage to bone marrow populations, and increased marrow fat cellularity.17,43 This phenomenon is studied extensively in postcranial sites rather than in mandibular bone. Multiple studies in mice have demonstrated that bone marrow adiposity, or fatty substitution, triggers bone loss and other skeletal alterations.44,45 In humans, bone marrow adiposity has been demonstrated in patients after radiation therapy in postcranial sites.46, 47, 48 However, the mandibular bone has distinct features that differ from other skeletal sites, including developmental origin, osteoclastic activity, osteogenic potential of mesenchymal stem cells, the rate of bone turnover, and collagen properties.49, 50, 51, 52, 53, 54, 55, 56, 57 Therefore, experimental data on bone from postcranial sites may not translate entirely to the mandibular bone.
Few animal studies have investigated the effect of radiation on bone marrow adipocytes in mandibular bone. Spiegelberg et al found a significant increase in adipocyte density in bone marrow of irradiated mice mandibles 10 and 24 weeks after irradiation with 15 Gy.22 Hyperbaric oxygen therapy that was also included in this study had no effect on adipocyte density. Marx et al58 did observe increased mandibular marrow adiposity and fibrosis in rabbits 6 months after irradiation and in rabbits treated with HBO, but to a qualitatively lesser extent.
No quantitative data on marrow adipocytes in irradiated human mandibles have been previously reported. Few studies on irradiated human mandibles report data on marrow adiposity. Marx et al28 described histologic findings in 45 ORN specimens in a qualitative report and found an absence of fat cells in the bone marrow. Instead, the bone marrow in these specimens was replaced with acellular collagen.28 Conversely, Curi et al40 investigated 40 bone specimens from mandibular ORN and found an increase in marrow fat besides fibrosis. A significant relation was found between the increased amount of marrow fat and increased time after radiation.40
Conclusion
The increased adipocytes (shown in this study) and the reduced presence of osteoblastic activity (demonstrated in a previous study27) that is observed in irradiated human mandibles together with absent osteocytes (shown in this study) and osteoclasts (demonstrated in a previous study27) lead to the hypothesis that irradiation disrupts bone homeostasis at different levels. Radiation causes early death of osteocytes, persistent suppression of osteoclastogenesis by lack of signaling from osteocytes and osteoblasts, as well as deprivation of osteoclast precursors, leading to persistence of fragile bone matrix void of osteocytes. The damage to bone mesenchymal stem cells leads to increased adipogenesis in the bone marrow and decreased osteoblastogenic potential. The nonvital persisting bone matrix with severely impaired regenerative and remodeling potential may contribute to the vulnerability of the bone to infection and necrosis, particularly when a “porte d'entrée” is introduced by disruption to the overlying soft tissue barrier, and could as such be a key event in the pathogenesis of ORN.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Frankart AJ, Frankart MJ, Cervenka B, Tang AL, Krishnan DG, Takiar V. Osteoradionecrosis: Exposing the evidence not the bone. Int J Radiat Oncol Biol Phys. 2021;109:1206–1218. doi: 10.1016/j.ijrobp.2020.12.043. [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Qiu X, Xi K, et al. Therapeutic ionizing radiation induced bone loss: A review of in vivo and in vitro findings. Connect Tissue Res. 2018;59:509–522. doi: 10.1080/03008207.2018.1439482. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE. Osteoradionecrosis: A new concept of its pathophysiology. J Oral Maxillofac Surg. 1983;41:283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 4.Sultan A, Hanna GJ, Margalit DN, et al. The use of hyperbaric oxygen for the prevention and management of osteoradionecrosis of the jaw: A Dana-Farber/Brigham and Women's Cancer Center multidisciplinary guideline. Oncologist. 2017;22:1413. doi: 10.1634/theoncologist.2016-0298erratum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shaw RJ, Butterworth CJ, Silcocks P, et al. HOPON (Hyperbaric Oxygen for the Prevention of Osteoradionecrosis): A randomized controlled trial of hyperbaric oxygen to prevent osteoradionecrosis of the irradiated mandible after dentoalveolar surgery. Int J Radiat Oncol Biol Phys. 2019;104:530–539. doi: 10.1016/j.ijrobp.2019.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: Therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Rivero JA, Shamji O, Kolokythas A. Osteoradionecrosis: A review of pathophysiology, prevention and pharmacologic management using pentoxifylline, alpha-tocopherol, and clodronate. Oral Surg Oral Med Oral Pathol Oral Radiol. 2017;124:464–471. doi: 10.1016/j.oooo.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson AS, Buchbinder D, Hu K, Urken ML. Paradigm shifts in the management of osteoradionecrosis of the mandible. Oral Oncol. 2010;46:795–801. doi: 10.1016/j.oraloncology.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Goldring SR. The osteocyte: Key player in regulating bone turnover. RMD Open. 2015;1(suppl 1) doi: 10.1136/rmdopen-2015-000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: Cytokines and the skeletal system. BMB Rep. 2008;41:495–510. doi: 10.5483/bmbrep.2008.41.7.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noble BS, Reeve J. Osteocyte function, osteocyte death and bone fracture resistance. Mol Cell Endocrinol. 2000;159:7–13. doi: 10.1016/s0303-7207(99)00174-4. [DOI] [PubMed] [Google Scholar]
- 12.Nakashima T, Hayashi M, Fukunaga T, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 2011;17:1231–1234. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 13.Bellido T. Osteocyte-driven bone remodeling. Calcif Tissue Int. 2014;94:25–34. doi: 10.1007/s00223-013-9774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jilka RL, Noble B, Weinstein RS. Osteocyte apoptosis. Bone. 2013;54:264–271. doi: 10.1016/j.bone.2012.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veldhuis-Vlug AG, Rosen CJ. Clinical implications of bone marrow adiposity. J Intern Med. 2018;283:121–139. doi: 10.1111/joim.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rharass T, Lucas S. Mechanisms in endocrinology: Bone marrow adiposity and bone, a bad romance? Eur J Endocrinol. 2018;179:R165–R182. doi: 10.1530/EJE-18-0182. [DOI] [PubMed] [Google Scholar]
- 17.Costa S, Reagan MR. Therapeutic irradiation: Consequences for bone and bone marrow adipose tissue. Front Endocrinol (Lausanne) 2019;10:587. doi: 10.3389/fendo.2019.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldner J. A modification of the masson trichrome technique for routine laboratory purposes. Am J Pathol. 1938;14:237–243. [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien CA, Nakashima T, Takayanagi H. Osteocyte control of osteoclastogenesis. Bone. 2013;54:258–263. doi: 10.1016/j.bone.2012.08.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He F, Bai J, Wang J, Zhai J, Tong L, Zhu G. Irradiation-induced osteocyte damage promotes HMGB1-mediated osteoclastogenesis in vitro. J Cell Physiol. 2019;234:17314–17325. doi: 10.1002/jcp.28351. [DOI] [PubMed] [Google Scholar]
- 21.Willey JS, Lloyd SA, Robbins ME, et al. Early increase in osteoclast number in mice after whole-body irradiation with 2 Gy X rays. Radiat Res. 2008;170:388–392. doi: 10.1667/RR1388.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spiegelberg L, Braks JA, Groeneveldt LC, Djasim UM, van der Wal KG, Wolvius EB. Hyperbaric oxygen therapy as a prevention modality for radiation damage in the mandibles of mice. J Craniomaxillofac Surg. 2015;43:214–219. doi: 10.1016/j.jcms.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Wright LE, Buijs JT, Kim HS, et al. Single-limb irradiation induces local and systemic bone loss in a murine model. J Bone Miner Res. 2015;30:1268–1279. doi: 10.1002/jbmr.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damek-Poprawa M, Both S, Wright AC, Maity A, Akintoye SO. Onset of mandible and tibia osteoradionecrosis: A comparative pilot study in the rat. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:201–211. doi: 10.1016/j.oooo.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhai J, He F, Wang J, Chen J, Tong L, Zhu G. Influence of radiation exposure pattern on the bone injury and osteoclastogenesis in a rat model. Int J Mol Med. 2019;44:2265–2275. doi: 10.3892/ijmm.2019.4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oest ME, Franken V, Kuchera T, Strauss J, Damron TA. Long-term loss of osteoclasts and unopposed cortical mineral apposition following limited field irradiation. J Orthop Res. 2015;33:334–342. doi: 10.1002/jor.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dekker H, Schulten E, van Ruijven L, et al. Bone microarchitecture and turnover in the irradiated human mandible. J Craniomaxillofac Surg. 2020;48:733–740. doi: 10.1016/j.jcms.2020.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Marx RE, Tursun R. Suppurative osteomyelitis, bisphosphonate induced osteonecrosis, osteoradionecrosis: A blinded histopathologic comparison and its implications for the mechanism of each disease. Int J Oral Maxillofac Surg. 2012;41:283–289. doi: 10.1016/j.ijom.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 29.Shuster A, Reiser V, Trejo L, Ianculovici C, Kleinman S, Kaplan I. Comparison of the histopathological characteristics of osteomyelitis, medication-related osteonecrosis of the jaw, and osteoradionecrosis. Int J Oral Maxillofac Surg. 2019;48:17–22. doi: 10.1016/j.ijom.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Svalestad J, Hellem S, Thorsen E, Johannessen AC. Effect of hyperbaric oxygen treatment on irradiated oral mucosa: Microvessel density. Int J Oral Maxillofac Surg. 2015;44:301–307. doi: 10.1016/j.ijom.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 31.Heyboer M, 3rd, Wojcik SM, McCabe JB, et al. Hyperbaric oxygen and dental extractions in irradiated patients: Short- and long-term outcomes. Undersea Hyperb Med. 2013;40:283–288. [PubMed] [Google Scholar]
- 32.Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database Syst Rev. 2012 doi: 10.1002/14651858.CD005005.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Hampson NB, Holm JR, Wreford-Brown CE, Feldmeier J. Prospective assessment of outcomes in 411 patients treated with hyperbaric oxygen for chronic radiation tissue injury. Cancer. 2012;118:3860–3868. doi: 10.1002/cncr.26637. [DOI] [PubMed] [Google Scholar]
- 34.Feldmeier JJ. Hyperbaric oxygen therapy and delayed radiation injuries (soft tissue and bony necrosis): 2012 update. Undersea Hyperb Med. 2012;39:1121–1139. [PubMed] [Google Scholar]
- 35.Law MR, Hackshaw AK. A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: Recognition of a major effect. BMJ. 1997;315:841–846. doi: 10.1136/bmj.315.7112.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med. 2008;121:406–418. doi: 10.1016/j.amjmed.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brugnami F, Then PR, Moroi H, Leone CW. Histologic evaluation of human extraction sockets treated with demineralized freeze-dried bone allograft (DFDBA) and cell occlusive membrane. J Periodontol. 1996;67:821–825. doi: 10.1902/jop.1996.67.8.821. [DOI] [PubMed] [Google Scholar]
- 38.Fenner M, Park J, Schulz N, et al. Validation of histologic changes induced by external irradiation in mandibular bone. An experimental animal model. J Craniomaxillofac Surg. 2010;38:47–53. doi: 10.1016/j.jcms.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 39.Bras J, de Jonge HK, van Merkesteyn JP. Osteoradionecrosis of the mandible: Pathogenesis. Am J Otolaryngol. 1990;11:244–250. doi: 10.1016/0196-0709(90)90084-9. [DOI] [PubMed] [Google Scholar]
- 40.Curi MM, Cardoso CL, de Lima HG, Kowalski LP, Martins MD. Histopathologic and histomorphometric analysis of irradiation injury in bone and the surrounding soft tissues of the jaws. J Oral Maxillofac Surg. 2016;74:190–199. doi: 10.1016/j.joms.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 41.Han B, Yang Z, Nimni M. Effects of gamma irradiation on osteoinduction associated with demineralized bone matrix. J Orthop Res. 2008;26:75–82. doi: 10.1002/jor.20478. [DOI] [PubMed] [Google Scholar]
- 42.Georgiou KR, Hui SK, Xian CJ. Regulatory pathways associated with bone loss and bone marrow adiposity caused by aging, chemotherapy, glucocorticoid therapy and radiotherapy. Am J Stem Cells. 2012;1:205–224. [PMC free article] [PubMed] [Google Scholar]
- 43.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hui SK, Sharkey L, Kidder LS, et al. The influence of therapeutic radiation on the patterns of bone marrow in ovary-intact and ovariectomized mice. PLoS One. 2012;7:e42668. doi: 10.1371/journal.pone.0042668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green DE, Adler BJ, Chan ME, et al. Altered composition of bone as triggered by irradiation facilitates the rapid erosion of the matrix by both cellular and physicochemical processes. PLoS One. 2013;8:e64952. doi: 10.1371/journal.pone.0064952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mostoufi-Moab S, Magland J, Isaacoff EJ, et al. Adverse fat depots and marrow adiposity are associated with skeletal deficits and insulin resistance in long-term survivors of pediatric hematopoietic stem cell transplantation. J Bone Miner Res. 2015;30:1657–1666. doi: 10.1002/jbmr.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Casamassima F, Ruggiero C, Caramella D, Tinacci E, Villari N, Ruggiero M. Hematopoietic bone marrow recovery after radiation therapy: MRI evaluation. Blood. 1989;73:1677–1681. [PubMed] [Google Scholar]
- 48.Carmona R, Pritz J, Bydder M, et al. Fat composition changes in bone marrow during chemotherapy and radiation therapy. Int J Radiat Oncol Biol Phys. 2014;90:155–163. doi: 10.1016/j.ijrobp.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsubara T, Suardita K, Ishii M, et al. Alveolar bone marrow as a cell source for regenerative medicine: Differences between alveolar and iliac bone marrow stromal cells. J Bone Miner Res. 2005;20:399–409. doi: 10.1359/JBMR.041117. [DOI] [PubMed] [Google Scholar]
- 50.Huja SS, Fernandez SA, Hill KJ, Li Y. Remodeling dynamics in the alveolar process in skeletally mature dogs. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:1243–1349. doi: 10.1002/ar.a.20396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chai Y, Maxson RE., Jr Recent advances in craniofacial morphogenesis. Dev Dyn. 2006;235:2353–2375. doi: 10.1002/dvdy.20833. [DOI] [PubMed] [Google Scholar]
- 52.Akintoye SO, Lam T, Shi S, Brahim J, Collins MT, Robey PG. Skeletal site-specific characterization of orofacial and iliac crest human bone marrow stromal cells in same individuals. Bone. 2006;38:758–768. doi: 10.1016/j.bone.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 53.Aghaloo TL, Chaichanasakul T, Bezouglaia O, et al. Osteogenic potential of mandibular vs. long-bone marrow stromal cells. J Dent Res. 2010;89:1293–1298. doi: 10.1177/0022034510378427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamaza T, Ren G, Akiyama K, Chen C, Shi Y, Shi S. Mouse mandible contains distinctive mesenchymal stem cells. J Dent Res. 2011;90:317–324. doi: 10.1177/0022034510387796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vermeer J, Renders G, van Duin MA, Jansen I, Bakker LF, Kroon SA, et al. Bone-site-specific responses to zoledronic acid. Oral Dis. 2017;23:126–133. doi: 10.1111/odi.12587. [DOI] [PubMed] [Google Scholar]
- 56.Vermeer JA, Renders GA, Everts V. Osteonecrosis of the jaw-a bone site-specific effect of bisphosphonates. Curr Osteoporos Rep. 2016;14:219–225. doi: 10.1007/s11914-016-0318-z. [DOI] [PubMed] [Google Scholar]
- 57.Matsuura T, Tokutomi K, Sasaki M, Katafuchi M, Mizumachi E, Sato H. Distinct characteristics of mandibular bone collagen relative to long bone collagen: Relevance to clinical dentistry. Biomed Res Int. 2014;2014 doi: 10.1155/2014/769414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marx RE, Ehler WJ, Tayapongsak P, Pierce LW. Relationship of oxygen dose to angiogenesis induction in irradiated tissue. Am J Surg. 1990;160:519–524. doi: 10.1016/s0002-9610(05)81019-0. [DOI] [PubMed] [Google Scholar]