Abstract
A novel dehalogenating/transhalogenating enzyme, halomethane:bisulfide/halide ion methyltransferase, has been isolated from the facultatively methylotrophic bacterium strain CC495, which uses chloromethane (CH3Cl) as the sole carbon source. Purification of the enzyme to homogeneity was achieved in high yield by anion-exchange chromatography and gel filtration. The methyltransferase was composed of a 67-kDa protein with a corrinoid-bound cobalt atom. The purified enzyme was inactive but was activated by preincubation with 5 mM dithiothreitol and 0.5 mM CH3Cl; then it catalyzed methyl transfer from CH3Cl, CH3Br, or CH3I to the following acceptor ions (in order of decreasing efficacy): I−, HS−, Cl−, Br−, NO2−, CN−, and SCN−. Spectral analysis indicated that cobalt in the native enzyme existed as cob(II)alamin, which upon activation was reduced to the cob(I)alamin state and then was oxidized to methyl cob(III)alamin. During catalysis, the enzyme shuttles between the methyl cob(III)alamin and cob(I)alamin states, being alternately demethylated by the acceptor ion and remethylated by halomethane. Mechanistically the methyltransferase shows features in common with cobalamin-dependent methionine synthase from Escherichia coli. However, the failure of specific inhibitors of methionine synthase such as propyl iodide, N2O, and Hg2+ to affect the methyltransferase suggests significant differences. During CH3Cl degradation by strain CC495, the physiological acceptor ion for the enzyme is probably HS−, a hypothesis supported by the detection in cell extracts of methanethiol oxidase and formaldehyde dehydrogenase activities which provide a metabolic route to formate. 16S rRNA sequence analysis indicated that strain CC495 clusters with Rhizobium spp. in the alpha subdivision of the Proteobacteria and is closely related to strain IMB-1, a recently isolated CH3Br-degrading bacterium (T. L. Connell Hancock, A. M. Costello, M. E. Lidstrom, and R. S. Oremland, Appl. Environ. Microbiol. 64:2899–2905, 1998). The presence of this methyltransferase in bacterial populations in soil and sediments, if widespread, has important environmental implications.
Chloromethane (CH3Cl) is the most abundant volatile halocarbon present in the atmosphere; the atmospheric concentration of about 600 parts per 1012 (by volume) represents a total tropospheric burden of 5 million metric tons. Of chlorine-catalyzed ozone destruction in the stratosphere, 15 to 20% can be attributed to CH3Cl (35, 40). Atmospheric CH3Cl appears to be predominantly of natural origin, derived from biological and chemical processes in both marine and terrestrial environments. Important sources identified to date include oceanic emissions (27, 36), release by wood-rotting fungi (20, 22, 23, 59), and biomass burning (1, 31). The major chemical sink for atmospheric CH3Cl is reaction with hydroxyl radicals in the troposphere, although photolysis and hydrolysis may also play minor roles (12). Studies of biological sinks for CH3Cl have been largely neglected until very recently, despite the observation that biodegradation of bromomethane (CH3Br) by soil bacteria is a significant route for the destruction of atmospheric CH3Br (25, 47). On the basis of measurements of soil-atmosphere exchange in a Brazilian forest and in arctic grasslands and tundra, Khalil and Rasmussen (28) have estimated that the annual global uptake of CH3Cl by soil is of the order of 0.5 million metric tons.
Cometabolism of CH3Cl by several microorganisms has been observed (26, 43, 48), but there are few reports of the isolation of organisms that can use it as a sole carbon and energy source. A methylotrophic homoacetogenic bacterium which is capable of growing on CH3Cl (2% in the gas phase) as the sole carbon source under strictly anaerobic conditions has been isolated from sewage sludge (55). The organism contained an inducible CH3Cl dehalogenase which transferred the methyl group of CH3Cl to tetrahydrofolate, releasing inorganic chloride (33). The enzyme has yet to be purified and characterized, but preliminary studies indicate that activity apparently is dependent on the presence of ATP (32). An aerobic methylotrophic Hyphomicrobium sp. capable of growth on CH3Cl was isolated from industrial sewage (24), and although the mechanism of dehalogenation was not elucidated, it apparently did not involve hydrolysis to methanol or oxidation by methane monooxygenase.
A number of aerobic methylotrophic bacteria able to use CH3Cl as the sole C and energy source have been isolated (10, 11), and all contained an inducible enzyme system catalyzing the conversion of CH3Cl to Cl− and formaldehyde, with the latter compound undergoing either oxidation via formate to CO2 or assimilation via the serine pathway. One of these organisms, Methylobacterium sp. strain CM4, isolated from soil at a petrochemical plant, has been subjected to more detailed physiological and genetic investigations (58). The observed growth yields with CH3Cl as substrate, and also the properties of transposon Tn5 insertion mutants with altered growth characteristics on CH3Cl and other C1 substrates, suggested that it was unlikely that the degradative pathway involved an oxygenase or hydrolase. It was postulated that the bacterium metabolized CH3Cl by initial dehalogenation via a methyltransferase reaction followed by a series of dehydrogenase-catalyzed oxidations which differed from those involved in the metabolism of methanol or methylamine by the strain. However, no CH3Cl dehalogenase activity was obtained in cell extracts of strain CM4. Recently, a facultative methylotroph, strain IMB-1, possibly related to strain CM4, was isolated independently by Connell Hancock et al. (9) by enrichment culture on CH3Br. Strain IMB-1 also grew on CH3Cl and CH3I, but no investigation of the enzymology of halomethane degradation has been reported.
It is noteworthy that the anaerobic CH3Cl utilizer studied (32, 33) and also the aerobic utilizers investigated (24, 58) were obtained either from sewage sludge or from soil at industrially polluted sites. We have adopted an alternative approach to isolating CH3Cl-degrading microorganisms by screening soil samples from pristine sites where natural production of CH3Cl might be expected to occur. The microorganism used in the investigation described here was isolated from the litter layer of a woodland soil, an environment where biological release of CH3Cl by wood-rotting fungi is likely (59). We describe a study of the degradation of CH3Cl by this organism and the purification and characterization of the unusual corrinoid methyltransferase enzyme involved in its dehalogenation.
MATERIALS AND METHODS
Isolation and culture of the organism.
A methylotrophic microorganism designated strain CC495 was isolated from the top 5 cm of soil in a beech wood in County Down, Northern Ireland, by enrichment culture using CH3Cl as the sole carbon and energy source (1 g of soil/100 ml of minimal medium in 500-ml flasks containing 0.125 g of CH3Cl). For enrichment culture and growth experiments, strain CC495 was grown in 500-ml screw-cap conical flasks, each fitted with a polytetrafluoroethylene (PTFE) septum and a Mininert valve and containing minimal medium (100 ml) with the following composition (in grams per liter): KH2PO4 (4.5), K2HPO4 (10.5), MgSO4 · 7H2O (0.15), and NH4NO3 (1.5). The pH of the medium was adjusted to 7.2 with 6 M NaOH, and the medium was supplemented with a trace element solution (10 ml liter−1) containing (in milligrams per liter) H3BO3 (500), CuSO4 · 5H2O (40), KI (100), FeSO4 · 7H2O (200), MnSO4 · 7H2O (400), (NH4)6Mo7O24 · 4H2O (200), and ZnSO4 (400). In culture of the pure organism, the medium was further supplemented with a vitamin solution (5 ml liter−1) containing (in milligrams per liter) folic acid (4), p-aminobenzoic acid (200), and cyanocobalamin (200). CH3Cl (0.15 g) was added as an aqueous solution to give a concentration in the culture medium, after equilibration of the gaseous and aqueous phases, of 11.8 mM (30 mM if partitioning is neglected and the total CH3Cl present is expressed as a concentration in the aqueous phase). During production of large quantities of cells for preparation of cell extracts, cultures were grown in 2-liter conical flasks fitted with PTFE-coated rubber stoppers and containing 500 ml of medium with a CH3Cl concentration of 6.9 mM in the aqueous phase after equilibration. Cultures were incubated at 25°C on a rotary shaker (140 rpm). CH3Br, CH3I, and CH3SH were tested as growth substrates by replacing CH3Cl in the medium by an equivalent molar concentration of each compound. These substrates were added either as a single supplement or as a series of discrete 1-mmol additions; each injection was made after the consumption of the previous pulse so as to avoid inhibition by high initial concentrations of the compounds (9, 34). Other carbon sources examined as growth substrates were methylamine (0.2%, wt/vol), methanol (0.1%, wt/vol), formaldehyde (0.03%, wt/vol), sodium formate (0.1%), glucose (0.2%), glycerol (0.2%, wt/vol), sodium pyruvate (0.2%), veratric acid (0.1%), and syringic acid (0.1%).
Preparation of cell extracts.
Bacterial cells from CH3Cl-grown cultures (20 liters) in the late-exponential phase were harvested by centrifugation for 1 h at 10,000 × g and washed with 50 mM phosphate buffer (pH 7.2). Cells (15 g) were suspended in 20 mM phosphate buffer (pH 7.2) (14 ml) containing 0.5 mM dithiothreitol (DTT) and disrupted by sonication for a total duration of 9 min with an MSE 150-W ultrasonic disintegrator at maximum amplitude. Cooling in an ice bath ensured that the temperature did not rise above 10°C. The resulting homogenate was centrifuged for 20 min at 30,000 × g to remove cell debris.
Measurement of oxygen uptake by cell suspensions.
Cells were harvested as described above, and oxygen consumption by washed cell suspensions was recorded at 25°C with a Clark-type glass oxygen electrode (4 ml). Incubation mixtures contained 50 mM phosphate buffer (pH 7.8), the cell suspension (100 mg [wet wt]), and 2 mM substrate in a total volume of 3 ml.
Purification of enzyme.
Column chromatography was performed at 4°C on a Pharmacia fast protein liquid chromatography (FPLC) system. Cell extract (2 ml) prepared as described above was applied to a Pharmacia FPLC Mono Q 10/10 anion-exchange column equilibrated with 20 mM phosphate buffer (pH 6.5) containing 0.5 mM DTT. The column was washed with this buffer (16 ml, 2 ml/min) and then with a linear gradient of 0 to 0.5 M NaCl in 20 mM phosphate buffer (pH 6.5) containing 0.5 mM DTT (50 ml, 2 ml/min), and fractions (2 ml) were collected. Each fraction was dialyzed against 5 liters of 20 mM phosphate buffer (pH 7.2) containing 0.5 mM DTT for 12 h. Enzyme activity emerged as a single discrete peak confined to the fractions eluted at NaCl concentrations between 0.20 and 0.25 M. An aliquot (200 μl) of the fraction with the highest specific activity was applied to a Pharmacia FPLC Superose 12 column equilibrated with 20 mM phosphate buffer (pH 7.2) containing 0.5 mM DTT. The column was eluted with this buffer, and fractions (0.5 ml) were collected. The enzyme eluted from the column at an elution volume of 1.67 relative to the void volume of the column.
Activation of enzyme.
For experiments on the characterization and kinetics of the pure enzyme, activation was routinely conducted by preincubating the enzyme preparation in 50 mM phosphate buffer, pH 7.0 (1.7 ml, 0.5 mg of protein/ml), for 60 min at 25°C in the presence of 5 mM DTT and 0.5 mM (aqueous-phase concentration) CH3Cl in a 20-ml crimped-cap vial. The vial was then uncapped and allowed to stand at 25°C for 60 min prior to experimental use of the enzyme so as to ensure that any residual CH3Cl diffused from the solution. Gas chromatographic analysis of the activated enzyme preparation detected no CH3Cl in the solution after this period. In experiments where the effect of reductants other than DTT on activation was examined, the above procedure was used, with the reductant replacing DTT in the preincubation mixture. The enzyme was activated enzymically by replacing DTT with 0.2 mg (0.01 U) of NADH-flavin mononucleotide (FMN) oxidoreductase, 1 mM NADH, and 50 μM FMN. During initial purification of the enzyme, no preassay activation was conducted. Consequently, activation occurred during the assay, resulting in a lag period during which the reaction rate became progressively faster, but ultimately it became linear with respect to time. In these experiments reaction rates were measured only after linearity was achieved.
Enzyme and protein assays.
Methyltransferase activity was routinely assayed by measuring the production of CH3I when the activated enzyme preparation was incubated with KI and CH3Cl. The standard assay was conducted at 25°C in duplicate 20-ml crimped-cap vials sealed with silicone-PTFE discs and containing (in a total volume of 1 ml) phosphate buffer (50 mM; pH 7.0), KI (3 mM), CH3Cl (0.5 mM in the aqueous phase), and 0.1 ml of the enzyme preparation (corresponding to ∼0.02 mg of enzyme protein). After incubation for 1.5 to 6 h, the CH3I level was determined by gas chromatography. Glutathione-dependent formaldehyde dehydrogenase, serine hydroxymethyltransferase, hydroxypyruvate reductase, and 3-hexulose phosphate synthase in cell extracts were assayed by methods described previously (4, 8, 30, 44). Methanethiol oxidase was assayed by measuring the formation of NADH at 340 nm in the presence of NAD, CH3SH, and formaldehyde dehydrogenase (50). Formate dehydrogenase was assayed by measuring the formation of NADH at 340 nm in the presence of 50 mM formate (13). Protein was determined with a Coomassie Protein Assay Kit (Pierce Chemical Co., Rockford, Ill.) by using bovine serum albumin as a standard.
Determination of halomethanes and methanethiol.
A modification of the gas chromatographic method of Harper and Kennedy (22) was used. Samples of headspace (2 ml) were withdrawn from vials and injected into a Hewlett-Packard (HP) 6890 gas chromatograph that was fitted with a flame ionization detector and a glass column (1.5 m by 2 mm) packed with Tenax TA 60-80 and was operated at a N2 gas flow of 20 ml min−1. The oven temperature was programmed from 60 to 120°C at 24°C min−1. Under these conditions, CH3Cl, CH3Br, CH3I, and CH3SH eluted at retention times of 1.35, 2.18, 3.47, and 2.18 min, respectively. For the assay of CH3F, the oven temperature was programmed at a rate of 24°C min−1 between 40 and 80°C, and CH3F eluted at 0.25 min. Calibration was against samples of the headspace above standard solutions (1 ml) of known concentrations equilibrated at 25°C. The identities of eluting peaks were initially confirmed by gas chromatography-mass spectrometry (GC-MS). Lower limits of detection per vial were 0.15 nmol for CH3F, 0.20 nmol for CH3Cl, 0.35 nmol for CH3Br, 0.35 nmol for CH3I, and 0.30 nmol for CH3SH.
Determination of methylated products by GC-MS.
In studies of the substrate specificity of the methyltransferase, a Hewlett-Packard 5890 series II gas chromatograph linked to an HP 5971 mass selective detector was used to identify and quantify various methylated products. For the determination of CH3CN, CH3NO2, and CH3SCN, the gas chromatograph was fitted with a Poraplot-Q capillary column (10 m by 0.32 mm). Helium (1 ml min−1) was used as the carrier gas. After splitless injection of a sample (2 ml) of headspace, the oven temperature was held at 30°C for 1 min, then programmed at 10°C min−1 to 220°C. For identification of compounds, ion currents were monitored at m/e 38, 40, and 41 (molecular ion, M+) for CH3CN, at m/e 46 and 61 (M+) for CH3NO2, and at m/e 45, 72, and 73 (M+) for CH3SCN. For quantification of these compounds, the ion current of the molecular ion at the retention time of the compound was compared with that given by headspace above standard solutions of the authentic compound.
For determination of CH337Cl, a technique similar to the above was used except that headspace (0.1 ml) was injected with a split ratio of 50:1 and ion currents were monitored at m/e 35, 37, 50, and 52. The total amount of CH3Cl present was quantified by comparing the ion current at m/e 50 and 52 with that given by headspace above standard solutions of CH3Cl. The ratio of ion currents at m/e 35 and 37 was then used to calculate the proportion of CH335Cl to CH337Cl in the sample.
For identification of C2H5Cl or CH3F in samples, conditions were as described above except that headspace (20 ml) was applied to the column by using a Harvard Apparatus 22 syringe pump programmed at 1 ml min−1. Injection was splitless, and volatiles were initially focused on the first 10 cm of the column by cooling this section in liquid N2. The mass spectrometer was operated in the scanning mode, and ion currents between m/e 30 and 350 were monitored.
Determination of formate and identification of formaldehyde.
Formate in cell extracts was determined by GC-MS after derivatization as the anilide (29) by using H13COOH as an internal standard. Conditions were as described above, except that the gas chromatograph was fitted with an Ultra 1 capillary column (12 m by 0.2 mm), and after splitless injection of the derivatized sample (1 μl), the oven temperature was held at 66°C for 1 min and then programmed at 10°C min−1 to 300°C. Ion currents at m/e 66, 93, 121 (M+), and 122 were monitored. The molecular ion was used for quantification.
Formaldehyde in cell extracts was identified after derivatization as the 2,4-dinitrophenylhydrazine (29) by GC-MS under the conditions described above by operating the mass spectrometer in scanning mode.
SDS-PAGE.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with a Pharmacia Phastsystem gel electrophoresis unit by using commercially prepared gels of 12.5% polyacrylamide stained with Coomassie blue R. For molecular weight determination, the following proteins were used in calibration (with the molecular mass of the subunit in parentheses): rabbit muscle myosin (205 kDa), Escherichia coli β-galactosidase (116 kDa), rabbit muscle phosphorylase b (97 kDa), bovine serum albumin (66 kDa), ovalbumin (45 kDa), rabbit glyceraldehyde-3-phosphate dehydrogenase (36 kDa), bovine erythrocyte carbonic anhydrase (29 kDa), bovine pancrease trypsinogen (24 kDa), soybean trypsin inhibitor (20 kDa), and bovine milk α-lactalbumin (14 kDa).
Isoelectric focusing.
Isoelectric focusing on a polyacrylamide gel (Phastgel IEF [pH 4 to 6.5], 0.35 mm thick) was conducted by using a Pharmacia Phastsystem. Enzyme protein was dissolved in 20 mM phosphate buffer (pH 7.2), and an aliquot (1 μl, containing approximately 0.2 μg of protein) was run alongside a calibrating mixture of proteins of known pI values. Protein bands were located by staining with Coomassie blue R. Subsequently, gels were silver stained with a Bio-Rad kit to identify any minor protein components.
N-terminal amino acid sequencing.
The purified enzyme was separated by electrophoresis on an SDS–12% polyacrylamide gel and electroblotted onto a nitrocellulose membrane. N-terminal sequencing was carried out by Edman degradation with an Applied Biosystems model 477A automated sequencing instrument equipped with on-line phenylthiohydantoin analysis and using a pulsed liquid delivery.
Molecular weight determination.
The molecular weight of the enzyme was determined by both gel filtration and SDS-PAGE. Gel filtration of the purified enzyme was conducted on a Pharmacia Superose 12 column eluted with 20 mM phosphate buffer (pH 7.2) containing 0.5 mM DTT. The column was calibrated with the following reference proteins (with the molecular mass in parentheses): β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), bovine serum albumin (67 kDa), and peroxidase (40 kDa). The molecular weight of the enzyme was also estimated by comparing the mobility of the purified enzyme with those of standard reference proteins by SDS-PAGE as described above.
UV-visible light spectroscopy.
The absorption spectrum of the purified enzyme between 250 and 750 nm was recorded on a Pye Unicam SP8-200 UV-visible light spectrophotometer at room temperature in a quartz cuvette with a 1-cm light path. The spectrum of the enzyme as isolated was initially obtained, and the cuvette was scanned again after addition of 5 mM DTT and then after subsequent addition of 10 mM CH3Cl.
Metal determinations.
Cobalt, copper, iron, magnesium, manganese, nickel, and zinc contents of purified enzyme preparations were measured by flameless atomic absorption spectroscopy on a Varian Spectra AA 300 instrument equipped with a graphite furnace and a Zeeman background corrector. All values were corrected for the metal content of the buffer in which the sample was dissolved.
Chloride analysis.
Chloride was determined coulometrically by the Volhard procedure by using a Corning Chloride Analyzer 926.
Chemicals.
Sodium [37Cl]chloride (95 atom% 37Cl) and [13C]formic acid (99 atom% 13C) were obtained from Sigma Aldrich Co. Ltd., Poole, Dorset, United Kingdom. Nitromethane, methyl isothiocyanate, ethyl chloride, and methanethiol were also obtained from Sigma Aldrich. Titanium(III) citrate was prepared from titanium(III) chloride as described by Zehnder and Wuhrmann (61). NADH-FMN oxidoreductase (EC 1.6.99.3) from Vibrio harveyii was obtained from Sigma Aldrich, as was formaldehyde dehydrogenase (EC 1.2.46) from Pseudomonas putida.
Determination of 16S rRNA gene sequence.
Total DNA was isolated from CC495 cells grown in 50 ml of 2YT medium (45) to an optical density at 590 nm of 0.8 to 1.0. An almost-complete 16S rRNA gene was amplified from total DNA preparations by PCR using the following universal primers (38): forward, 5′-AGAGTTTGATCCTGGCTCAG (positions 8 to 27 [E. coli numbering]); reverse, 5′-AAGGAGGTGATCCAGCCGCA (positions 1541 to 1522). Amplification of the CC495 16S rRNA gene was conducted with Taq+ DNA polymerase (Stratagene) in a buffer supplied by the manufacturer. Reactions were carried out in volumes of 25 μl with deoxynucleoside triphosphates at 200 μM concentrations, 0.15 μM each primer, DNA at 100 to 200 ng, and Taq+ at 0.5 U per reaction. The following temperature profile was used: denaturation at 95°C for 3 min, followed by 30 cycles of 94°C for 40 s, 60°C for 30 s, and 72°C for 1 min. The amplification reactions were performed with a Perkin-Elmer DNA Thermal Cycler 480. The PCR products were purified by using GFX PCR DNA and a Gel Band Purification Kit (Pharmacia Biotech).
Purified PCR products were used in sequencing reactions with the Taq Dye-Deoxy Terminator Cycle Sequencing Kit (Applied Biosystems). The primers used for PCR amplification were also used for sequencing, and more primers were designed after the initial sequencing information was obtained. The nucleotide sequences of both strands were determined by using an automatic sequencer (Applied Biosystems, model 373A). Editing and initial analysis of the sequences were performed with the DNASIS (Hitachi) software package. Searches for nucleotide and amino acid sequence similarities were carried out by using the FASTA and BLAST programs (39) and the EMBL and GenBank databases.
Alignments of the sequences were performed with the ClustalW program (53). Phylogenetic analysis of the alignment was accomplished by using the PHYLIP (version 3.57c) package (15) and the TREECON program (56). For the PHYLIP analysis, bootstraps were obtained with the SEQBOOT program (100 data sets were generated). Parsimony analyses were conducted with the DNAPARS programs by using ordinary parsimony and a randomized input order of sequences. For the analyses with the TREECON program, Tajima and Nei correction (51) was used and trees were generated by neighbor joining.
Nucleotide sequence accession number.
The nucleotide sequence of the 16S rRNA gene of strain CC495 has been assigned GenBank accession no. AF107722.
RESULTS
Characterization and growth of strain CC495.
Cells of strain CC495 were gram-negative rods which formed round, shiny, smooth, and faintly pink colonies when grown on agar plates composed of mineral medium supplemented with vitamins and incubated in an atmosphere of CH3Cl (2%, vol/vol). Strain CC495 was able to use glucose, glycerol, pyruvate, and methylamine as growth substrates, but not methane, methanol, formaldehyde, formate, veratrate, or syringate. When first isolated, strain CC495 could be cultured on CH3Cl only as part of a stable consortium with another microorganism. However, supplementation of the culture medium with cyanocobalamin (1 mg liter−1) allowed the organism to be grown in pure culture on CH3Cl. In the absence of cyanocobalamin or when an equimolar concentration of inorganic cobalt was substituted for cyanocobalamin, no growth was observed. Cyanocobalamin supplementation of the medium was not required for the growth of the organism on methylamine.
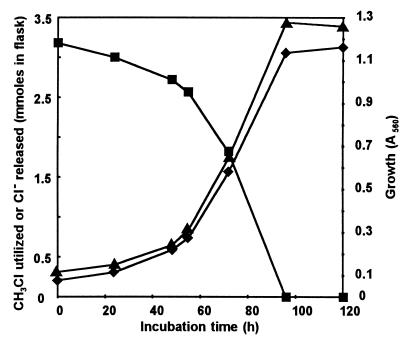
During the growth of strain CC495 on CH3Cl, the disappearance of the halocarbon was accompanied by a stoichiometric release of chloride (Fig. 1). If a further addition of CH3Cl (3 mmol/flask) was made to the culture toward the end of the logarithmic phase, growth continued until all the CH3Cl was utilized. However, a fall in the pH of the culture medium restricted further growth upon additional supplementation with CH3Cl. No growth occurred on dichloromethane or on CH3Br, CH3I, or CH3SH when the compound was added to the culture medium at a molar concentration similar to that of CH3Cl. However, growth on CH3Br, but not on CH3I or CH3SH, did occur when pulsed additions of small quantities of the compound were made over an extended period so that the concentration of the substrate in the medium did not rise above 0.3 mM.
FIG. 1.
Growth of strain CC495 on chloromethane. Symbols: ■, chloromethane (measured in millimoles); ⧫, chloride (in millimoles); ▴, growth measured as absorbance at 560 nm.
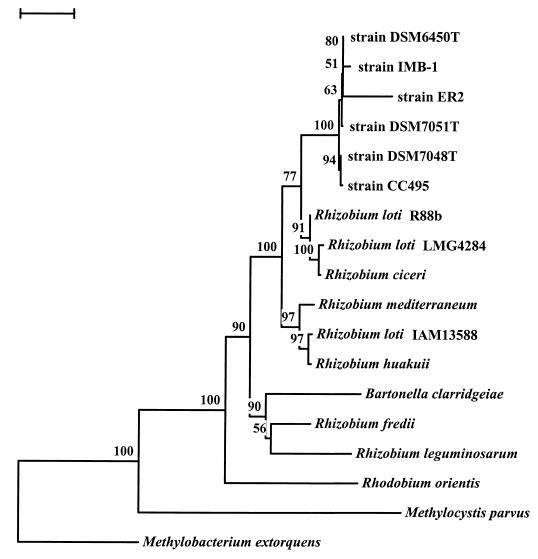
Phylogenetic analysis of the 16S rRNA gene sequence of strain CC495 places the bacterium in the alpha subdivision of the Proteobacteria (Fig. 2). It appears to be closely associated with the nitrogen-fixing bacteria of the genus Rhizobium and in particular to two facultative methylotrophs (strains ER2 and IMB-1) isolated from agricultural soils. Strain ER2 is a serine pathway methylotroph which can use the N-methyl group of carbofuran and other methylcarbamate insecticides as its sole carbon source (54). Strain IMB-1 is a methylotroph capable of growth or CH3Br, CH3I, CH3Cl, and various methylamines (9). These three strains are very closely related to three other novel strains assigned to a new genus, Pseudoaminobacter. Together these strains appear to represent a novel group of methylotrophic bacteria related to, but phylogenetically distinct from, Rhizobium spp.
FIG. 2.
Phylogenetic analysis of the 16S rRNA gene from strain CC495. The tree was obtained by the neighbor-joining approach using the TREECON program. Similar phylogenesis was obtained when parsimony analysis of the same data was conducted. Bootstrap values (in percentages) are given at the nodes. Bar, 0.02 base substitutions per site. The 16S rRNA gene sequence from Methylocystis parvus OBBp was used as the outgroup. The GenBank accession numbers of the organisms are as follows: strain CC495, AF107722; strain DSM 7048T, AF011759; strain DSM 7051T, AF011760; strain DSM 6450T, AF011762; strain ER2, L20802; strain IMB-1, AF034798; Rhizobium loti R88b, U50165; R. loti LMG4284, X67230; Rhizobium ciceri UPM-Ca7, U07934; Rhizobium mediterraneum UPM-Ca36, L38825; R. loti IAM13588, D12791; Rhizobium huakuii IAM14158, D12797; Bartonella clarridgeiae CIP104772, X97822; Rhizobium fredii LMG6217, X67321; Rhizobium leguminosarum USDA2370, U29386; Rhodobium orientis MB312, D30792; M. extorquens, M29027; M. parvus OBBp, M29026.
Induction of CH3Cl-degrading activity and pathway of carbon assimilation.
Resting cells of strain CC495 grown on CH3Cl rapidly oxidized CH3Cl, CH3Br, CH3I, and formaldehyde. Formate and CH3SH were also oxidized, although comparatively slowly, but CH3F, chloroethane, methanol, and methylamine were not (Table 1). These observations suggest that oxidation of CH3Cl occurred via formaldehyde but did not involve an initial hydrolytic cleavage to form methanol and chloride. Cells consumed 1.48 ± 0.07 nmol of O2 for each nanomole of CH3Cl degraded, indicating complete oxidation of the compound to CO2 and Cl−. The low rate of formate utilization could signify that oxidation of formaldehyde does not proceed via the linear route through formate to CO2. However, hexulose-6-phosphate activity was not detected in cell extracts, implying that oxidation did not occur by the cyclic ribulose monophosphate pathway, which many non-methane-utilizing methylotrophs use (2). Moreover, substantial glutathione-dependent formaldehyde dehydrogenase activity (19.5 nmol/min/mg) and significant formate dehydrogenase activity (1.9 nmol/min/mg) were present in cell extracts. It therefore seems probable that the sluggish oxidation of formate by whole cells may reflect the high Km (∼15 mM) of bacterial formate dehydrogenases isolated to date (3, 13).
TABLE 1.
Oxygen uptake by CH3Cl-grown resting cells of strain CC495 on various substrates (2 mM)
| Substrate | O2 uptakea (nmol/min/mg [wet wt]) |
|---|---|
| CH3F | 0 |
| CH3Cl | 0.62 ± 0.07 |
| CH3Br | 0.61 ± 0.03 |
| CH3I | 0.46 ± 0.01 |
| CH3SH | 0.09 ± 0.03 |
| CH3OH | 0 |
| HCHO | 0.60 ± 0.07 |
| HCOOH | 0.04 ± 0 |
| CH3NH2 | 0 |
| CH2Cl2 | 0 |
| C2H5Cl | 0 |
Initial rates of O2 uptake were determined as described in Materials and Methods. Values are corrected for an endogenous respiration rate of 0.22 nmol/min/mg (wet weight) and are the means of three replicates ± standard deviations.
Cells grown on methylamine were unable to oxidize CH3Cl, demonstrating that the CH3Cl-degrading system was inducible. Interestingly, a growth yield of 12.1 g (dry weight) per mol of C was observed with CH3Cl as the growth substrate, but only 5.1 g (dry weight) per mol of C was observed during growth on methylamine, implying that there was a much lower assimilation efficiency during metabolism of the latter compound. Both hydroxypyruvate reductase activity (7.2 nmol/mg/min) and serine hydroxymethyl transferase activity (16.6 nmol/mg/min) were detected in cell extracts from CH3Cl-grown cells, indicating that the serine pathway for formaldehyde assimilation was operating. The growth yield on CH3Cl was at the upper end of the range of values (9.8 to 13.1 g [dry weight] per mol of C) reported for bacteria growing on methanol utilizing the serine pathway (19) and suggests that the conversion of CH3Cl to formaldehyde may be energy yielding.
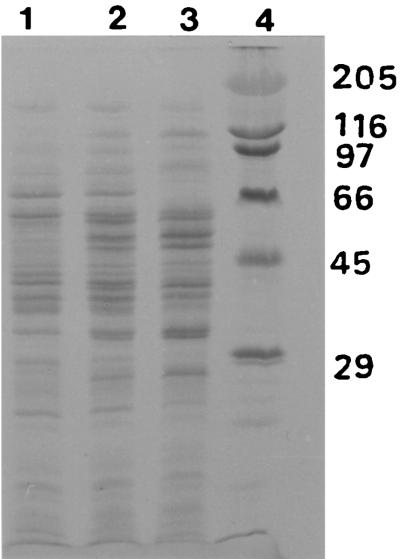
The protein pattern in crude extracts of CH3Cl-grown cells is compared in Fig. 3 with those for cells grown on CH3Cl-methylamine and methylamine alone. In both CH3Cl-grown and CH3Cl-methylamine-grown cells, at least two additional proteins not present in methylamine-grown cells were induced. The most prominent of these bands corresponded to a molecular mass of 68 kDa, while a fainter band was clearly visible at 29 kDa.
FIG. 3.
Coomassie blue-stained gels after SDS-PAGE of cell extracts of strain CC495 grown on CH3Cl, CH3Cl-methylamine, or methylamine. Lanes 1, 2, and 3 contain 2.5 μg protein of extracts from cells grown on CH3Cl, CH3Cl-methylamine, and methylamine, respectively. Lane 4 contains protein standards with molecular masses (in kilodaltons) as indicated.
Degradation of CH3Cl in cell extracts.
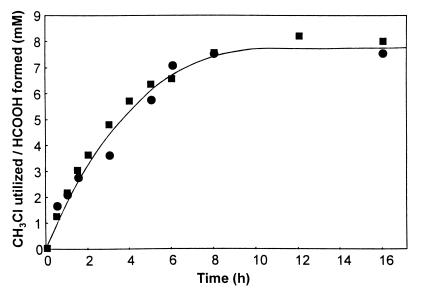
When cell extracts of strain CC495 were incubated with 1.4 mM CH3Cl in 50 mM phosphate buffer (pH 7.8) in the presence of 1 mM NADH and 0.5 mM DTT, the halocarbon was consumed at a rate of 2.2 nmol/mg/min for 3 h, after which the rate of utilization slowly declined. In the absence of supplemental NADH, degradation of CH3Cl proceeded at a rate ca. 40% of that exhibited in the presence of the cofactor, but only after an initial lag period of approximately 1 h. In both instances, disappearance of CH3Cl was accompanied by stoichiometric release of Cl−. Although formaldehyde could not be detected by GC-MS in cell extracts after incubation with CH3Cl, formation of stoichiometric quantities of formate was demonstrated (Fig. 4). Over 10 h, approximately 50% conversion of 17 mM CH3Cl to formate was observed. After dialysis of cell extracts against 5 liters of 20 mM phosphate buffer (pH 7.8) containing 0.5 mM DTT, CH3Cl-degrading activity in the presence of 1 mM NADH showed an increase to 5.7 nmol/mg/min, while activity in the absence of NADH was similarly enhanced.
FIG. 4.
CH3Cl utilization and formate formation by cell extracts of strain CC495. Cell extracts were incubated at 25°C with 50 mM phosphate buffer (pH 7.8) containing 1 mM NADH, 0.5 mM DTT, and 17.2 mM CH3Cl. Formate concentrations (■) were determined by GC-MS, and CH3Cl concentrations (●) were determined by gas chromatography.
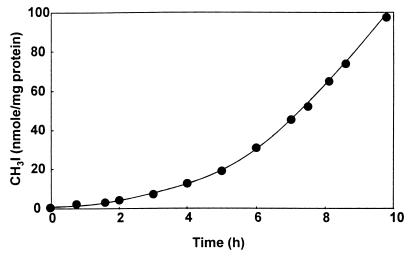
Initial attempts at purification of the enzyme responsible for CH3Cl degradation from cell extracts by using ion-exchange chromatography on a Mono Q column led to a complete loss of activity, even if all eluting fractions were recombined and concentrated by ultrafiltration. To investigate whether this lack of activity was due to the high concentration of Cl− (up to 500 mM) used in gradient elution, the effects of Cl− and other anions on CH3Cl-degrading activity in dialyzed cell extracts were examined (Table 2). Preliminary experiments had established that phosphate buffer did not inhibit the enzyme reaction at concentrations below 100 mM. All the anions tested, including many which were not halide or pseudohalide ions, showed significant inhibition of CH3Cl degradation at 20 mM, and all displayed substantial inhibition of activity at 100 mM. To shed light on this phenomenon, dialyzed cell extracts were incubated with 3 mM I−, 0.5 mM DTT, and 0.5 mM CH3Cl in 50 mM phosphate buffer, and the headspace was monitored by gas chromatography. The formation of substantial quantities of a compound with a retention time similar to that of CH3I was observed, and its identity was confirmed by GC-MS. After an initial lag period of about 7 h, during which the rate of CH3I formation gradually increased, the production of CH3I became linear with respect to time (Fig. 5). The addition of 1 mM NADH increased the lag period slightly but did not significantly affect the maximal rate of CH3I formation. CH3I was not detectable in control samples to which boiled cell extract had been added. The behavior of the CH3Cl degradation system as a transhalogenation system in the presence of halide ions allowed the development of a much more sensitive assay for the enzyme based on the methylation of I− in the presence of CH3Cl rather than on CH3Cl disappearance. By measuring the rate of CH3I formation after the initial lag period (which presumably represents the period required for activation), enzyme activity was monitored where indicated during the purification of the CH3Cl-degrading system described below.
TABLE 2.
Effects of various anions on CH3Cl-degrading activity in dialyzed cell extracts
| Anion | Relative activitya at the indicated concn of the anion
|
|
|---|---|---|
| 20 mM | 100 mM | |
| F− | 89 | 51 |
| Cl− | 69 | 14 |
| Br− | 52 | 11 |
| I− | 0 | 0 |
| SCN− | 0 | 0 |
| CN− | 0 | 0 |
| HS− | 17 | 13 |
| NO2− | 0 | 0 |
| NO3− | 0 | 0 |
| SO42− | 94 | 37 |
| HCOO− | 5 | 0 |
The dialyzed cell extract was incubated in 20 mM phosphate buffer (pH 7.8) with 1 mM NADH, 0.5 mM DTT, 1.38 mM CH3Cl, and the anion at the concentration indicated. The relative activity of the extract in the absence of added ion is assigned a value of 100. The values are means of duplicates. The average standard deviation was ±5.
FIG. 5.
CH3I formation from CH3Cl and I− by dialyzed cell extracts of strain CC495. Dialyzed extracts were incubated at 25°C with 3 mM I−, 0.5 mM DTT, and 0.5 mM CH3Cl in 50 mM phosphate buffer (pH 7.8). CH3I formation was determined by gas chromatography.
Purification of enzyme.
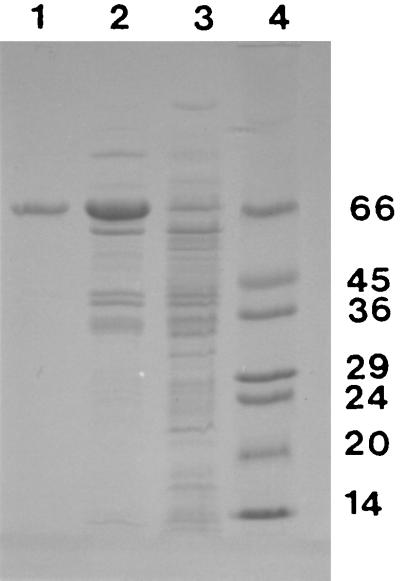
The results of a typical enzyme purification are summarized in Table 3, and results of SDS-PAGE of the fractions obtained at each purification stage are shown in Fig. 6. Purification by this procedure was 20-fold, and the overall yield was 46%. SDS-PAGE of the purified enzyme yielded a single band, indicating the apparent homogeneity of the preparation (Fig. 6).
TABLE 3.
Purification of halomethane:bisulfide/halide ion methyltransferase from cell extracts of the methylotroph strain CC495
| Stage of purification | Vol (ml) | Total enzyme activity (U)a | Yield (%) | Protein (mg/ml) | Sp act (U/mg of protein) |
|---|---|---|---|---|---|
| Cell extract | 2 | 267b | 100 | 46.3 | 2.88b |
| Anion-exchange chromatography and dialysis | 2 | 209 | 78.3 | 4.4 | 23.7 |
| Gel filtration on Superose 12 | 5 | 124 | 46.4 | 0.42 | 58.9 |
One unit of enzyme activity is defined as the amount of fully activated enzyme required to catalyze the formation of 1 nmol of CH3I min−1 at 25°C in 50 mM phosphate buffer (pH 7.0) with 3 mM KI, 0.5 mM DTT, and 0.5 mM CH3Cl.
Enzyme activity measured after dialysis against 50 mM phosphate buffer (pH 7.0) containing 0.5 mM DTT.
FIG. 6.
SDS-PAGE of protein fractions from stages in the purification of methyltransferase from strain CC495. The gel was stained with Coomassie blue. Lane 1, 0.55 μg of protein from the gel filtration stage; lane 2, 0.69 μg of protein from the anion-exchange stage; lane 3, 0.46 μg of protein from crude cell extracts; lane 4, protein standards with molecular masses (in kilodaltons) as indicated.
N-terminal amino acid sequence.
The N-terminal amino acid sequence of the purified enzyme was identified as H2N- Ala-Thr-Val-Gly-Lys-Met-Thr-Ser-Arg-Glu-Arg-Met-Phe-Ala-Ala-Val-Thr-Met. A search of the EMBL and SwissProt databases using the FASTA and BLITZ programs revealed no significant homology with other cobalamin-containing enzymes for which sequences are known or with any other reported sequence.
Enzyme activation.
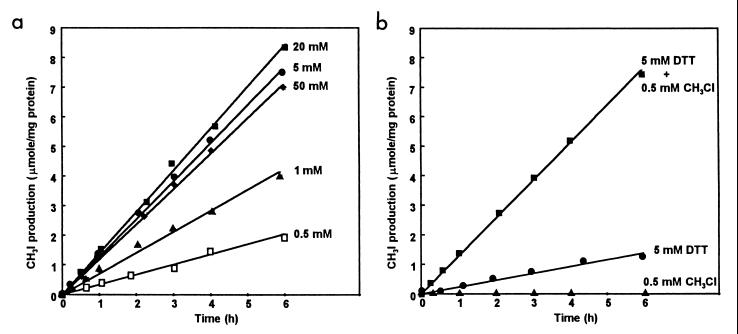
As cell extracts passed through the various stages of purification, the initial lag period displayed by the enzyme during the assay became progressively shorter, signifying faster activation of the enzyme. Pure enzyme required a period of about 1 h before the rate of enzyme reaction became linear with respect to time when it was assayed without preincubation under standard assay conditions in the presence of 0.5 mM DTT. The effects of preincubation of pure enzyme with CH3Cl and various concentrations of DTT on the duration of the lag period and on the extent of enzyme activation were investigated by using pure enzyme previously dialyzed against 20 mM phosphate buffer (pH 7.0) (Fig. 7).
FIG. 7.
Effects of preincubation with DTT and CH3Cl on the activation of methyltransferase. (a) Effect of preincubation for 60 min at 25°C with 0.5 mM CH3Cl and different concentrations of DTT in 50 mM phosphate buffer (pH 7.0) on the activity of methyltransferase in the standard assay. (b) Effect of preincubation for 60 min at 25°C in 50 mM phosphate buffer (pH 7.0) with 0.5 mM CH3Cl, 5 mM DTT, or 0.5 mM CH3Cl plus 5 mM DTT on the activity of methyltransferase in the standard assay.
In the presence of 0.5 mM CH3Cl, the lag period was abolished by preincubation with all concentrations of DTT tested but maximum enzyme activity was exhibited with 20 mM DTT (Fig. 7a). Because ca. 90% of maximal activation was obtained with 5 mM DTT, this concentration was routinely used for preincubation of enzyme prior to the standard assay. Omission of CH3Cl from the preincubation solution resulted in enzyme activity being reduced to 19% of that observed in its presence. No activity was detected when the enzyme was preincubated with 0.5 mM CH3Cl alone (Fig. 7b). The effects of substituting equimolar concentrations of various other electron donors for DTT in the preincubation mixture were also examined. Enzyme activities after preincubation with 5 mM mercaptoethanol or reduced glutathione were, respectively, 83 and 37% of that with 5 mM DTT. However, neither titanium citrate nor NADH alone produced detectable enzyme activation, but preincubation of the enzyme with NADH-FMN oxidoreductase, 1 mM NADH, 50 μM FMN, and 0.5 M CH3Cl together yielded 62% of the activity shown after preincubation with DTT as the reductant. Supplementation of the preincubation mixture with 10 μM aquocobalamin in addition to DTT did not increase the level of enzyme activation above that observed with DTT alone. When activation of the enzyme by preincubation with 5 mM DTT and 0.5 mM CH3Cl was conducted under N2, enzyme activity was only 67% of that shown when preincubation was performed under air. Preincubation in darkness instead of under normal laboratory lighting had no significant effect on the activation process. Replacement of 0.5 mM CH3Cl by 1 mM S-adenosylmethionine during preincubation did not result in enzyme activation above that of the enzyme assayed without preincubation.
Stability of the enzyme.
Preparations of the purified enzyme had a half-life of 90 h in 50 mM phosphate buffer (pH 7.0) containing 0.5 mM DTT at 4°C and could be frozen at −70°C for 3 months without significant loss of activity. Normal laboratory lighting did not affect the stability of the enzyme.
Influence of enzyme concentration.
Under standard assay conditions with 3 mM I− as the substrate, the initial velocity of the reaction was directly proportional to the enzyme concentration at protein concentrations from 15 to 120 μg ml−1. The rate of CH3I formation was linear with respect to time over approximately 6 h at 25°C.
Molecular weight.
Upon gel filtration of the purified enzyme, activity emerged as a single discrete peak at a relative elution volume corresponding to a molecular weight of 68,000. SDS-PAGE of the pure enzyme yielded a single band corresponding to a molecular weight of 66,000, indicating that the enzyme was monomeric (Fig. 6).
Metal content, spectral properties, and isoelectric point of the enzyme.
Purified enzyme preparations were analyzed for various metal ions by atomic absorption spectroscopy. The mean cobalt content of several preparations was 13.7 ± 0.6 nmol mg−1 of protein. Based on a molecular weight of 67,000 for the enzyme, a molar ratio of cobalt to enzyme of 0.92 was calculated. No iron, zinc, manganese, copper, nickel, or magnesium was detected in enzyme preparations.
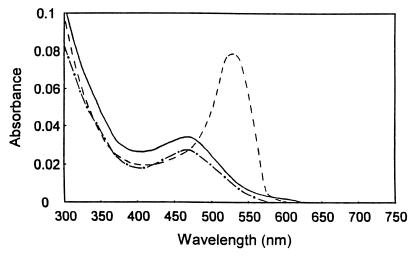
The unactivated enzyme was straw-colored, and the visible absorption spectrum exhibited a maximum at 465 nm (Fig. 8), typical of a cob(II)alamin (18, 41). Upon the addition of 5 mM DTT, the wavelength of maximum absorption remained unchanged but the absorption decreased slightly in intensity. Upon complete activation of the enzyme with 5 mM DTT and 10 mM CH3Cl, the maximum shifted to 530 nm and increased markedly in intensity. This spectral change is consistent with the generation of a methylated cob(III)alamin possessing a coordinated (base-on) dimethylbenzimidazole substituent, which characteristically displays a peak at ca. 520 nm (5, 41). A UV absorption maximum at 280 nm, exhibited by the unactivated enzyme, increased 15-fold upon the addition of 5 mM DTT and returned to its original intensity upon complete activation of the enzyme with 5 mM DTT and 10 mM CH3Cl, suggesting the intermediary formation of a cob(I)alamin state (41). By using an extinction coefficient at 470 nm of 11,000 M−1 cm−1 for cob(II)alamin (18), measurement of the absorbance of the unactivated enzyme gave a molar ratio of cobalt to enzyme of 0.83, a value consistent with that obtained by atomic absorption.
FIG. 8.
Absorbance spectra of methyltransferase in unactivated and activated states: purified enzyme (7.5 μM) in 50 mM phosphate buffer (pH 7.0) (solid line), after addition of 5 mM DTT (dashed-and-dotted line), and after addition of 5 mM DTT and 10 mM CH3Cl (dashed line).
Analytical isoelectric focusing of the unactivated enzyme followed by staining with Coomassie blue yielded a single band with a pI of 4.90, which presumably corresponded to the cob(II)alamin form of the enzyme. However, with the more sensitive silver-staining technique, two other minor but distinct bands were apparent at pIs of 4.75 and 5.15; these were tentatively ascribed to the presence of trace amounts of forms of the enzyme containing, respectively, cob(I)alamin and cob(III)alamin [possibly aquocob(III)alamin]. Activation of the enzyme with 5 mM DTT and 10 mM CH3Cl prior to isoelectric focusing yielded an additional prominent band at a pI of 5.00, presumably corresponding to the methylcob(III)alamin form of the enzyme.
Influence of pH and temperature.
The effect of pH on enzyme activity was measured under standard conditions in 50 mM 2,2-dimethylsuccinate, phosphate, Tris, and glycine buffers spanning a pH range from 5 to 10. The activated enzyme showed maximal methyltransferase activity between pH 6.0 and 7.0, with the velocity of the reaction falling rapidly below pH 6.0 and above pH 8.5. When assayed under standard conditions for 2 h, the optimum temperature for the enzyme reaction was 45°C. Rapid denaturation of the enzyme occurred at 60°C. The activation energy of the enzyme reaction between 15 and 40°C, determined by an Arrhenuis plot, was 58.4 kJ mol−1.
Substrate range.
The relative rates of methylation of a variety of monovalent anions by the enzyme were measured by using CH3Cl as the methyl donor and 10 mM acceptor ion (Table 4). Under these conditions the best substrate was I−, but methyl transfer to the other halide ions, Br− and Cl−, also occurred readily. However, there was no detectable formation of CH3F from F−, even at concentrations of 50 mM F−. The pseudohalide ions, CN− and SCN−, were poor substrates; the latter ion showed detectable methylation only at a concentration of 50 mM. Some enzymic conversion of NO2− to CH3NO2 was also observed. Most significantly in terms of the metabolic role of the enzyme, methyl transfer to HS− occurred readily, resulting in the formation of CH3SH.
TABLE 4.
Substrate anion range of methyltransferase
| Substrate anion | Ratea |
|---|---|
| I− | 100 |
| F− | <0.1 |
| Cl− | 28 ± 0.6b |
| Br− | 17 ± 0.8 |
| HS− | 22 ± 5.3 |
| NO2− | 4.1 ± 0.3 |
| CN− | 3.6 ± 0.8 |
| SCN− | <0.4 (3.6 ± 0.3)c |
Rate of methylation by the activated enzyme on a molar basis relative to that with I− as the substrate anion in 50 mM phosphate buffer (pH 7.0) with 0.5 mM CH3Cl as the methyl donor and 10 mM substrate anion as the sodium salt at 25°C. With each anion the rate is corrected for any chemical conversion observed. Values are means of three replicates ± standard deviations.
Determined by using 10 mM 37Cl− as the anion as described in Materials and Methods.
Rate of methylation with 50 mM SCN−.
Only CH3Cl, CH3Br, and CH3I would act as methyl donors for the enzyme. No detectable enzyme-catalyzed methyl group transfer to any acceptor anion occurred from CH3F, CH3SH, CH3OH, or CH3NO2, nor was transfer of an ethyl group from C2H5Cl observed. Neither CH2Cl2, CHCl3, nor CCl4 was a substrate for the methyltransferase.
Kinetic parameters.
The steady-state kinetics of the methyltransferase reaction were investigated by using Cl−, Br−, I−, and HS− as acceptor ions and CH3Cl, CH3Br, and CH3I as methyl donors. Normal Michaelis-Menten kinetics were observed with all substrate combinations except at concentrations of I− and HS− above 3 and 0.2 mM, respectively. Under these conditions significant departures from linearity were noted, indicating some substrate inhibition at higher concentrations. In the case of HS−, inhibition was apparently due to deactivation of the enzyme, and this could be greatly reduced by increasing the concentration of DTT to 20 mM in the enzyme assay. This modification allowed normal Michaelis-Menten kinetics to be exhibited up to 1 mM HS−. Lineweaver-Burk treatment of the data yielded apparent Km and Vmax values for methyl donors and acceptor ions (Table 5). Experiments were not conducted with CH3SH as the methyl donor because no detectable methyl transfer from this compound to any acceptor ion was observed (Fig. 9). The apparent Km and Vmax values of the enzyme for CH3Br and CH3Cl as methyl donors were measured in the presence of 3 mM I− as the acceptor ion, while the kinetic parameters for CH3I were measured by using 120 mM Cl− as the acceptor ion. The apparent Km and Vmax values of the enzyme for Cl−, Br−, and HS− as acceptor ions were determined in the presence of 0.4 mM CH3I as the methyl donor, while these parameters for I− were measured with 0.5 mM CH3Br as the methyl donor.
TABLE 5.
Kinetic parameters of halomethane:bisulfide/halide ion methyltransferase
| Methyl donor | Kinetic parametera
|
Acceptor anion | Kinetic parametera
|
||||
|---|---|---|---|---|---|---|---|
| App. Km (μM) | App. Vmax (U/mg) | Vmax/Km (U/mg/μM) | App. Km (mM) | App. Vmax (U/mg) | Vmax/Km (U/mg/μM) | ||
| CH3Clb | 7 | 42.6 | 6.1 | Cl−c | 11.6 | 105 | 9.1 |
| CH3Brb | 30 | 49.4 | 1.7 | Br−c | 5.1 | 45.9 | 9.0 |
| CH3Id | 36 | 87.0 | 2.4 | I−e | 0.37 | 80.1 | 216 |
| CH3SH | —f | —f | HS−c | 1.7 | 241 | 142 | |
The apparent (App.) Km and Vmax values are the means of duplicate experiments. The relative standard deviations for Km and Vmax were 6.3 and 4.7%, respectively.
Acceptor anion, 3 mM I−.
Methyl donor, 0.4 mM CH3I.
Acceptor anion, 120 mM Cl−.
Methyl donor, 0.5 mM CH3Br.
—, CH3SH does not act as a methyl donor with any acceptor ion.
FIG. 9.
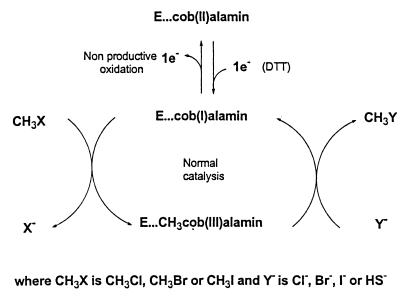
Scheme showing postulated mechanism of activation and catalysis for halomethane:bisulfide/halide ion methyltransferase.
The difference in acceptor ions renders the apparent Vmax values obtained for CH3Cl and CH3Br as methyl donors not strictly comparable to that obtained for CH3I. Similarly, the apparent Vmax values obtained for Cl−, Br−, and HS− as acceptor ions are not strictly analogous to that obtained for I− because of the difference in methyl donors. Nevertheless, on mechanistic grounds it seems unlikely that the apparent Vmax for CH3I with I− as the acceptor ion will diverge substantially from the value given in Table 5 for the apparent Vmax for CH3I with Cl− as the acceptor ion. One measure of the internal consistency of results in Table 5 can be obtained by a comparison of the apparent Vmax for CH3I with Cl− as the acceptor ion with the apparent Vmax of Cl− with CH3I as the methyl donor. The level of agreement appears reasonable, considering that concentrations of the acceptor ion and methyl donor, while high (∼10 Km), were not sufficient to completely saturate the enzyme. Additional confirmation that the kinetic parameters obtained for a given acceptor ion can be considered largely independent of the methyl donor is provided by the very similar Km and Vmax values for Cl− obtained with CH3Br as a methyl donor (11.1 mM and 120 U/mg, respectively) compared with those obtained with CH3I as a methyl donor, which are shown in Table 5.
Effect of inhibitors.
Methylating activity was examined in the presence of a variety of possible inhibitors. The purified enzyme was activated in the presence of 5 mM DTT and 0.5 mM CH3Cl and then preincubated with the putative inhibitor for 20 min before the addition of substrate. In the presence of DTT, propyl iodide is a specific inhibitor of most cobalamin-dependent enzymes, blocking enzyme action by forming a propylcob(III)alamin derivative of the enzyme. The inhibition can normally be reversed by photolysis of the propyl-cobalt bond with light (52). However, no inhibition of methyl transfer by propyl iodide at a final concentration of 0.05 or 0.5 mM was observed either in the light or in the dark. Incubation of unactivated enzyme with this compound prior to normal activation also failed to produce detectable inhibition.
A number of substrate analogues of the methyl donor (CH3F, CH3OH, CH2Cl2, CHCl3, CCl4, and CH3CH2Cl) which had previously been shown to be inactive as substrates for the methyltransferase were tested at a concentration of 0.5 mM as possible competitive inhibitors, but all failed to display significant inhibition of methyl transfer. The monovalent ions F− and NO3−, neither of which would serve as acceptor ion substrates for the methyltransferase, were also not effective as competitive inhibitors at a concentration of 1 mM. The chelating agent EDTA (1 mM) did not inhibit the enzyme, nor did Hg2+ (0.1 mM), which is known to demethylate methylcob-(III)alamin (42). Enzyme activity was, however, completely abolished by 6 M urea and could not be restored upon dialysis and addition of aquocobalamin or methylcobalamin. When activated enzyme was incubated in the standard assay under an atmosphere of N2O, a specific inhibitor of cobalamin-dependent methionine synthase (6), no decrease in the rate of enzyme reaction was observed.
Metabolism of the product of the methyltransferase reaction in vivo.
The irreversible formation of methanethiol from CH3Cl and HS− catalyzed by the methyltransferase suggests that the metabolism of CH3Cl in vivo by strain CC495 may proceed via this compound. A methanethiol oxidase has previously been purified and characterized from a Hyphomicrobium sp. and has been shown to catalyze the formation of formaldehyde, sulfide, and hydrogen peroxide from methanethiol in the presence of oxygen (50). Extracts of cells of strain CC495 in the late-exponential phase were therefore assayed for the presence of methanethiol oxidizing activity by measuring formaldehyde formation from methanethiol by a coupled assay with formaldehyde dehydrogenase. A methanethiol oxidase activity of 1.6 nmol/mg of protein/min was found.
DISCUSSION
The facultative methylotroph strain CC495 is the first aerobic CH3Cl-degrading organism from which a specific dehalogenating enzyme has been isolated. Phylogenetic analysis (Fig. 2) indicates that the organism is closely related to two other facultative methylotrophs recently isolated: strain ER2, which utilizes the methyl groups of N-methylcarbamates as its sole carbon source (54), and strain IMB-1, which is capable of growth on CH3Cl, CH3Br, and CH3I (9). Like both of these organisms, strain CC495 is incapable of growth on methanol and formate but can use methylamine as its sole carbon source. However, during growth on C1 substrates, neither strain ER2 nor strain IMB-1 has the exceedingly high requirement for cyanocobalamin exhibited by strain CC495 during growth on CH3Cl. These strains, together with three other recently isolated novel strains assigned to a new genus, Pseudoaminobacter, form a distinct clade within the Rhizobium cluster of the alpha subdivision of the Proteobacteria (Fig. 2). The Rhizobium genus consists of aerobes capable of fixing atmospheric nitrogen, often in symbiotic associations with plant hosts. Strain CC495 does not appear to have a close phylogenetic relationship to Methylobacterium extorquens CM4, an organism, isolated from Russian soil, which is capable of growth on CH3Cl but, unlike strain CC495, can utilize methanol as a carbon source (10, 11, 58).
CH3Cl degradation in strain CC495 was clearly inducible. Although CH3Br degradation by strain IMB-1 initially appeared constitutive (9), it has now been shown to be induced by very low concentrations of CH3Br (46). Growth of strain CC495 on CH3Cl caused induction of at least two additional proteins not present in methylamine-grown cells (Fig. 3). It is perhaps significant that the molecular masses of these proteins, 67 and 29 kDa, are very similar to those of two proteins with masses of 65 and 35 kDa induced by growth of M. extorquens CM4 on CH3Cl but not on methanol (55). On the basis of the latter evidence, inter alia, it was proposed that a methyltransferase reaction, rather than a hydrolytic cleavage to yield methanol, was involved in the initial dehalogenation of CH3Cl by M. extorquens CM4. Whole-cell studies with strain CC495 also suggested that the metabolism of CH3Cl, although proceeding via formaldehyde, did not involve an initial hydrolysis to methanol (Table 1). Cell extracts from strain CC495 showed rapid and quantitative conversion of CH3Cl to formate when incubated in the presence of NADH and DTT (Fig. 4). Attempts to purify the CH3Cl-degrading activity by anion-exchange chromatography were initially unsuccessful because of the inhibitory effects of high concentrations of anions on CH3Cl degradation (Table 2). This phenomenon is attributable in the case of Cl− to the ability of the ion to act as an acceptor for methyl transfer from methylcobalamin, leading to resynthesis of CH3Cl. Inhibition by the pseudohalide ions SCN− and CN− and also NO2− is probably due to these rather poor substrates for the methyltransferase blocking the active site of the enzyme and effectively acting as competitive inhibitors. Inhibition by Br− and I− is more difficult to explain but may be related to these halide ions or the halomethanes formed from them behaving as inhibitors of a subsequent stage in enzymic processing of the methyl group, e.g., methanethiol oxidase.
In the absence of information regarding the identity of the normal acceptor ion substrate for the methyltransferase enzyme in vivo, the use of I− as an acceptor ion enabled assay of the enzyme during purification by measurement of CH3I formation in the presence of CH3Cl. The purified enzyme displayed considerable stability, and in contrast to many cobalamin-containing enzymes (49, 52), catalysis was not affected by light in the visible range. As isolated the enzyme was inactive and exhibited a lag period in the standard assay during which activation of the enzyme occurred (Fig. 5). In order to eliminate this lag period, activation of the enzyme before assay was necessary by preincubation with 5 mM DTT and a methyl donor (normally 0.5 mM CH3Cl). Other thiols such as mercaptoethanol and reduced glutathione could also be used as reductants but were less effective than DTT in the activation process. Aquocobalamin, which promotes the activation of methionine synthase by DTT (52), did not stimulate DTT-mediated activation of the methyltransferase. Neither NADH nor titanium citrate, which is required in reductive activation of some corrinoid-dependent methyl transfer reactions (49), produced measurable activation of the purified enzyme. Spectral analysis showed that in the unactivated enzyme the cobalt of the prosthetic group existed as cob(II)alamin, which upon activation by treatment with DTT and CH3Cl, was first reduced to the cob(I)alamin state and then oxidized to methylcob(III)alamin. This change in the valence of the cobalt atom upon activation was reflected in the observed change in the isoelectric point of the protein.
The substrate range of the enzyme as regards methyl donors appears restricted to CH3Cl, CH3Br, and CH3I, with CH3Cl, on the basis of the relative specificity constant Vmax/Km, acting as the best substrate (Table 5). A variety of monovalent anions behave as acceptor substrates for the enzyme, of which I− and HS− are by far the best in terms of Vmax/Km, but CI− and Br− were also rapidly methylated (Table 5). The enzyme catalyzed significant methylation, albeit at a comparatively low level, of the pseudohalide ions, CN− and SCN−, and in addition NO2− behaved as an acceptor ion, but F− did not (Table 4).
Both the reductive methylation process involved in enzyme activation and the nature of the reaction catalyzed by the methyltransferase have features in common with those of the cobalamin-dependent methionine synthase from E. coli (5, 6, 16, 52). Methionine synthase catalyzes the transfer of a methyl group from methyltetrahydrofolate to homocysteine, generating tetrahydrofolate and methionine. Methionine synthase requires initial reductive methylation in which a reductant such as a thiol converts the cob(II)alamin enzyme to a cob(I)alamin intermediate, which is then methylated by S-adenosylmethionine to form the catalytically active methylcob(III)alamin enzyme. During catalysis the enzyme then shuttles between the methyl cob(III)alamin and cob(I)alamin states, being alternatively demethylated by homocysteine and remethylated by methyltetrahydrofolate. A broadly similar model for the mechanism of the methyltransferase reaction can be proposed, differing principally in that the methyl donor substrate (CH3X, where X is Cl, Br, or I) also acts as the methyl donor in the reductive activation process. Nevertheless, as with methionine synthase, reductive activation of the enzyme can be accomplished not only by chemical reductants such as DTT but also by physiological reducing systems such as NADH-FMN oxidoreductase. This observation probably accounts for the stimulatory effects of NADH on CH3Cl degradation in unpurified cell extracts. A major difference between methionine synthase and the methyltransferase is that with the latter enzyme, the methylated product CH3Y (where Y is Cl, Br, or I) can be recycled as the methyl donor substrate (Fig. 9). If the acceptor ion is HS−, the reaction proceeds in one direction only, because CH3SH cannot act as a methyl donor substrate. However, if the halogen of the acceptor ion differs from that of the halomethane initially present as the methyl donor, the relative proportions of each halomethane and each halide ion at equilibrium will vary depending on the initial concentration of each substrate and the kinetic parameters associated with each. Consequently, the methyltransferase effectively behaves as a transhalogenation system which can be driven in a given direction by manipulating the concentrations of each substrate.
The failure of the specific methionine synthase inhibitor, propyl iodide, and various substrate analogues, such as ethyl chloride, CH2Cl2, and CHCl3, to cause detectable inhibition of the methyltransferase betokens a highly specific and sterically constrained binding site on the enzyme for the methyl donor substrate. The surprising observation that Hg2+, which is known to demethylate methylcob(III)alamin in a nonphysiological reaction (42), did not affect enzyme activity could imply that the cobalamin prosthetic group is in a site in the protein that is inaccessible to cations of this size. Such a protected location would also help to explain the unusual stability of this corrinoid enzyme to both light and oxygen. However, the complete insensitivity of the methyltransferase to the specific methionine synthase inhibitor, N2O, is difficult to rationalize.
An important point with regard to the functioning of the methyltransferase in vivo is the identity of the acceptor ion. The ready catalysis of the reaction of HS− with CH3Cl to form CH3SH seems to implicate HS− as the physiological acceptor ion, particularly since the reaction is highly exergonic (7) and hence is unlikely to be reversible under the conditions of enzyme catalysis. Nevertheless, the rather high Km of the enzyme with HS− and the low turnover number of the enzyme (15 min−1 with HS− as the substrate) tends to militate against such a role. However, the use of DTT as a chemical activation system for the enzyme may be partly responsible for the low turnover number observed. Studies on the methionine synthase from E. coli have demonstrated that the use of an endogenous reduced triphosphopyridine nucleotide-dependent flavoprotein system for activation rather than DTT doubled the turnover number of the enzyme (17). Also, the methyltransferase constitutes a comparatively high proportion (∼5%) of the total soluble protein of the cells, which may compensate for its relative inefficiency as a catalyst. Moreover, cell extracts contain significant methanethiol oxidase activity, providing a metabolic route from CH3SH to formaldehyde within the organism. Although compelling, this evidence is not conclusive, and further investigations are clearly necessary to establish unequivocally that HS− is the physiological acceptor for the enzyme during CH3Cl degradation. A demonstration that the 29-kDa protein that is coinduced with the methyltransferase by the growth of strain CC495 on CH3Cl is involved in the metabolism of methanethiol would provide strong support for this hypothesis.
It seems quite feasible that the other two CH3Cl-degrading strains recently isolated, M. extorquens CM4 (58) and strain IMB-1 (9), share a methyltransferase pathway for metabolic degradation of CH3Cl similar to that of strain CC495. The molecular weights of the two proteins induced by the growth of M. extorquens CM4 on CH3Cl are very similar to those induced by the growth of strain CC495 on CH3Cl. Moreover, in a very recent report (57) it has been concluded, on the basis of Tn5 mutagenesis and subsequent DNA sequence analysis of the genes, that catabolism of CH3Cl by strain CM4 probably involves a corrinoid-dependent methyltransferase enzyme. However, studies with cell extracts suggest that in this organism, unlike strain CC495, the methyl group is apparently transferred from CH3Cl to tetrahydrofolate. The failure of CH3F to act either as a substrate or as a competitive inhibitor for the methyltransferase of strain CC495 is also significant in the light of the observations that CH3F was not a growth substrate for IMB-1 and did not affect its ability to grow on or oxidize CH3Br.
It is interesting to speculate on the possible ecological niches occupied by such CH3Cl-degrading bacteria and the environmental implications of the widespread distribution in microorganisms of methyltransferase enzymes of the type described in this paper. It is difficult to envisage an organism utilizing atmospheric CH3Cl as a major carbon and energy source, given the low background concentrations of the gas in the troposphere. However, strain CC495 was isolated from an environment where, as a result of CH3Cl release by wood-rotting fungi (59), the concentrations of CH3Cl were probably locally enhanced sufficiently to allow the induction of the CH3Cl-degrading system and the utilization of the halocarbon as a significant source of carbon and energy for the organism. In view of the high vitamin B12 requirement by strain CC495 during CH3Cl metabolism, such degradation would have to occur within a consortium of two or more microorganisms which could satisfy the demand for this coenzyme. A variety of microhabitats where biological CH3Cl production can lead to sufficiently elevated concentrations of the gas to permit CH3Cl-dependent growth probably exist both in the soil and on the surfaces of plants. Investigations by Harper et al. (21) have suggested the presence of colonies of CH3Cl-metabolizing microorganisms in the lenticels of potato tubers. These pores represent the main points of efflux of CH3Cl generated within the tuber.
If the methyltransferase found in strain CC495 occurs widely in the bacterial populations of soil and sediments, several environmentally significant biotransformations could be mediated. We postulate that, under aerobic conditions, the methyltransferase will simply convert CH3Cl to CH3SH, which will be oxidized by the organism via HCHO with concomitant HS− release. HS− will then be recycled to act as an acceptor ion for further degradation of CH3Cl or, alternatively, for degradation of CH3Br and CH3I, since these halomethanes are also enzyme substrates even if they are not necessarily inducers of enzyme expression. By contrast, under low O2 tensions or in the presence of high concentrations of sulfide, where the limiting degradative step is oxidation of CH3SH, the methyltransferase provides a mechanism for the conversion of CH3Cl, CH3Br, and CH3I to CH3SH as the end product. Such conditions are likely to exist in anoxic sediments. Oremland et al. (37) showed that the conversion of CH3Br to CH3SH could take place abiotically in salt marsh sediments, which consequently may behave as sinks for CH3Br. Little abiotic transformation of CH3Cl was recorded under such conditions by these workers, but if bacterial populations with the methyltransferase activity of strain CC495 are present in these environments, they could represent an important biological sink for CH3Cl. Preliminary, as yet unpublished studies by Coulter et al. (9a) with CH3Cl-grown resting-cell suspensions of strain CC495 incubated in 4 mM CH3Cl and 2 mM H2S demonstrate almost stoichiometric conversion of HS− to CH3SH within 2 h even under fully aerobic conditions. The transhalogenating potential of the methyltransferase in the presence of halide ions raises the possibility that in marine environments, where Cl− concentrations will normally be of the order of 0.5 M, rapid conversion of CH3Br and CH3I to CH3Cl may also occur, particularly under anoxic conditions. Unpublished studies in this laboratory with CH3Cl-grown resting-cell suspensions of strain CC495, incubated under N2 in seawater diluted 1:4, indicate quantitative conversion of CH3Br and CH3I to CH3Cl within 2 h; even under aerobic conditions, conversion exceeded 70%. Considered in the context of the fact that chemical reaction of CH3I and CH3Br with Cl− at average surface temperatures in the sea has a half-life of about 3 weeks (14, 60), these observations suggest that the implications for atmospheric halomethane budgets of the presence of bacteria possessing the methyltransferase enzyme in the marine environment are of some significance.
One possible application for strain CC495 is to enhance the biodegradation of CH3Br after it is used for agricultural fumigation of soils in the field and under glass. Connell Hancock et al. (9) have argued that seeding soils with live cells of mass-cultured strain IMB-1 might be a viable option for reducing the postfumigation release of CH3Br to the atmosphere, thereby minimizing the undesirable effects of the use of the bromocarbon on stratospheric ozone.
ACKNOWLEDGMENT
This study was supported by EC Environment and Climate Research Programme contract ENV4-CT95-0086.
REFERENCES
- 1.Andreae M O. Biomass burning: its history, use and distribution and its impact on environmental quality and global climate. In: Levine J S, editor. Global biomass burning. Cambridge, Mass: MIT Press; 1991. pp. 3–21. [Google Scholar]
- 2.Anthony C. Assimilation of carbon by methylotrophs. In: Goldberg I, Rokem J S, editors. Biology of methylotrophs. Stoneham, Mass: Butterworth-Heinemann; 1991. pp. 79–109. [DOI] [PubMed] [Google Scholar]
- 3.Asano Y, Sekigawa T, Inukai H, Nakazawa A. Preparation and properties of formate dehydrogenase from Moraxella sp. strain C-1. J Bacteriol. 1988;170:3189–3193. doi: 10.1128/jb.170.7.3189-3193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Attwood M M. Formaldehyde dehydrogenases from methylotrophs. Methods Enzymol. 1990;188:314–327. [Google Scholar]
- 5.Banerjee R V, Harder S R, Ragsdale S W, Matthews R G. Mechanism of reductive activation of cobalamin-dependent methionine synthase: an electron paramagnetic resonance spectroelectrochemical study. Biochemistry. 1990;29:1129–1135. doi: 10.1021/bi00457a005. [DOI] [PubMed] [Google Scholar]
- 6.Banerjee R V, Matthews R G. Cobalamin-dependent methionine synthase. FASEB J. 1990;4:1450–1459. doi: 10.1096/fasebj.4.5.2407589. [DOI] [PubMed] [Google Scholar]
- 7.Braus-Stromeyer S A, Cook A M, Leisinger T. Biotransformation of chloromethane to methanethiol. Environ Sci Technol. 1993;27:1577–1579. [Google Scholar]
- 8.Cinder K, Lidstrom M E. Hydroxypyruvate reductase from Methylobacterium extorquens AMI. Methods Enzymol. 1990;188:373–378. [Google Scholar]
- 9.Connell Hancock T L, Costello A M, Lidstrom M E, Oremland R S. Strain IMB-1, a novel bacterium for the removal of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1998;64:2899–2905. doi: 10.1128/aem.64.8.2899-2905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9a.Coulter, C., M. J. Larkin, and D. B. Harper. Unpublished data.
- 10.Doronina N V, Sokolov A P, Trotsenko Y A. Isolation and initial characterization of aerobic chloromethane-utilizing bacteria. FEMS Microbiol Lett. 1996;142:179–183. [Google Scholar]
- 11.Doronina N V, Trotsenko Y A. Isolation and characterization of aerobic degraders of methyl chloride. Mikrobiologiya. 1997;66:70–77. [Google Scholar]
- 12.Edwards P R, Campbell I, Milne G S. The impact of chloromethanes on the environment. Part 2. Methyl chloride and methylene chloride. Chem Ind. 1982;1982:619–622. [Google Scholar]
- 13.Egorov A M, Avilova T V, Dikov M, Popov V O, Rodionov Y V, Berezin I V. NAD-dependent formate dehydrogenase from methylotrophic bacterium, strain 1. Eur J Biochem. 1979;99:569–576. doi: 10.1111/j.1432-1033.1979.tb13289.x. [DOI] [PubMed] [Google Scholar]
- 14.Elliott S, Rowland F S. Nucleophilic substitution rates and solubilities for methyl halides in seawater. Geophys Res Lett. 1993;20:1043–1046. [Google Scholar]
- 15.Felsenstein J. PHYLIP (phylogeny inference package), version 3.57c. Seattle: Department of Genetics, University of Washington; 1997. [Google Scholar]
- 16.Frasca V, Banerjee R V, Durham W R, Sands R H, Matthews R W. Cobalamin-dependent methionine synthase from Escherichia coli B: electron paramagnetic resonance spectra of the inactive form and the active methylated form of the enzyme. Biochemistry. 1988;27:8458–8465. doi: 10.1021/bi00422a025. [DOI] [PubMed] [Google Scholar]
- 17.Fujii K, Huennekens F M. Activation of methionine synthetase by a reduced triphosphopyridine nucleotide-dependent flavoprotein system. J Biol Chem. 1974;249:6745–6753. [PubMed] [Google Scholar]
- 18.Fujii K, Huennekens F M. Methionine synthetase: characterization of protein components and mechanisms for activation and catalysis. In: Yogi K, editor. Biochemical aspects of nutrition. Tokyo: Japan Scientific Societies Press; 1979. pp. 173–184. [Google Scholar]
- 19.Goldberg I, Rock J S, Ben-Bassat A, Mateles R I. Bacterial yields on methanol, methylamine, formaldehyde and formate. Biotechnol Bioeng. 1976;18:1657–1668. doi: 10.1002/bit.260181202. [DOI] [PubMed] [Google Scholar]
- 20.Harper D B. Halomethane from halide ion—a highly efficient fungal conversion of environmental significance. Nature. 1985;315:55–57. [Google Scholar]
- 21.Harper D B, Harvey B M R, Jeffers M R, Kennedy J T. Emissions, biogenesis and metabolic utilization of chloromethane by tubers of the potato (Solanum tuberosum) New Phytol. 1999;142:5–17. [Google Scholar]
- 22.Harper D B, Kennedy J T. Effect of growth conditions on halomethane production by Phellinus species: biological and environmental implications. J Gen Microbiol. 1986;132:1231–1246. [Google Scholar]
- 23.Harper D B, Kennedy J T, Hamilton J T G. Chloromethane biosynthesis in poroid fungi. Phytochemistry. 1988;27:3147–3153. [Google Scholar]
- 24.Hartmans S, Schmuckle A, Cook A M, Leisinger T. Methyl chloride: naturally occurring toxicant and C-1 growth substrate. J Gen Microbiol. 1986;132:1139–1142. [Google Scholar]
- 25.Hines M E, Crill P M, Varner R K, Talbot R W, Shorter J H, Kolb C E, Harriss R C. Rapid consumption of low concentrations of methyl bromide by soil bacteria. Appl Environ Microbiol. 1998;64:1864–1870. doi: 10.1128/aem.64.5.1864-1870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keuning S, Janssen D B, Witholt B. Purification and characterization of hydrolytic haloalkane dehalogenase from Xanthobacter autotrophicus GJ10. J Bacteriol. 1985;163:635–639. doi: 10.1128/jb.163.2.635-639.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil M A K, Moore R M, Harper D B, Lobert J M, Erickson D J, Koropolov V, Sturges W T, Keene W C. Natural emissions of chlorine-containing gases: Reactive Chlorine Emissions Inventory. J Geophys Res. 1999;104:8333–8346. [Google Scholar]
- 28.Khalil M A K, Rasmussen R A. The exchange of methyl chloride and chloroform between the atmosphere and soils. Report 05–98. Portland, Oreg: Department of Physics., Portland State University; 1998. [Google Scholar]
- 29.Knapp D R. Handbook of analytical derivatization reactions. J. New York, N.Y: Wiley and Sons; 1979. [Google Scholar]
- 30.Lidstrom M E. Serine hydroxymethyl transferases from Methylobacterium organophilum XX. Methods Enzymol. 1990;188:365–372. [Google Scholar]
- 31.Lobert J M, Keene W C, Logan J A, Yevich R. Global chlorine emissions from biomass burning: Reactive Chlorine Emissions Inventory. J Geophys Res. 1999;104:8373–8389. [Google Scholar]
- 32.Messmer M, Reinhardt S, Wohlfarth G, Diekert G. Studies on methyl chloride dehalogenase and O-demethylase in cell extracts of the homoacetogen strain MC based on a newly developed coupled enzyme assay. Arch Microbiol. 1996;165:18–25. [Google Scholar]
- 33.Messmer M, Wohlfarth G, Diekert G. Methyl chloride metabolism of the strictly anaerobic, methyl chloride-utilizing homoacetogen strain MC. Arch Microbiol. 1993;160:383–387. [Google Scholar]
- 34.Miller L G, Connell T L, Guidetti J R, Oremland R S. Bacterial oxidation of methyl bromide in fumigated agricultural soils. Appl Environ Microbiol. 1997;63:4346–4354. doi: 10.1128/aem.63.11.4346-4354.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montzka S A, Butler J H, Myers R C, Thompson T M, Swanson T H, Clark A D, Lock L T, Elkins J W. Decline in the tropospheric abundance of halogen from halocarbons: implications for stratospheric ozone depletion. Science. 1996;272:1318–1322. doi: 10.1126/science.272.5266.1318. [DOI] [PubMed] [Google Scholar]
- 36.Moore R M, Groszko W, Niven S. Ocean-atmospheric exchange of methyl chloride: results from N.W. Atlantic and Pacific Ocean studies. J Geophys Res. 1996;101:28529–28538. [Google Scholar]
- 37.Oremland R S, Miller L G, Cuthbertson C W, Connell T L, Jahnke L. Degradation of methyl bromide by methanotrophic bacteria in cell suspensions and soils. Appl Environ Microbiol. 1994;60:3640–3646. doi: 10.1128/aem.60.10.3640-3646.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pascual C, Lawson P A, Farrow J A E, Gimenez M N, Collins M D. Phylogenetic analysis of the genus Corynebacterium based on 16S rRNA gene sequences. Int J Syst Bacteriol. 1995;45:724–728. doi: 10.1099/00207713-45-4-724. [DOI] [PubMed] [Google Scholar]
- 39.Pearson W R, Lipman D J. Improved tools for biological sequence comparison. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prather M J, Watson R T. Stratospheric ozone depletion and future trends of atmospheric chlorine and bromine. Nature. 1990;344:729–734. [Google Scholar]
- 41.Pratt J M. Inorganic chemistry of vitamin B12. London, United Kingdom: Academic Press; 1972. [Google Scholar]
- 42.Pratt J M. Making and breaking the Co-alkyl bond in B12 derivatives. Met Ions Biol Syst. 1993;29:229–286. [Google Scholar]
- 43.Rasche M E, Hicks E R, Hyman M R, Arp D J. Oxidation of monohalogenated ethanes and n-chlorinated alkanes by whole cells of Nitrosomonas europaea. J Bacteriol. 1990;172:5368–5373. doi: 10.1128/jb.172.9.5368-5373.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahm H, Schutte H, Kula M R. 3-Hexulosephosphate synthase from Methylomonas M15. Methods Enzymol. 1982;90:319–323. doi: 10.1016/s0076-6879(82)90148-3. [DOI] [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 46.Schaefer, J. K., and R. S. Oremland. Oxidation of methyl halides by the facultative methylotroph, strain IMB-1. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 47.Shorter J H, Kolb C E, Crill P M, Kerwin R A, Talbot R W, Hines M E, Harriss R C. Rapid degradation of atmospheric methyl bromide in soils. Nature. 1995;377:717–719. [Google Scholar]
- 48.Stirling D I, Dalton H. The fortuitous oxidation and cometabolism of various carbon compounds by whole cell suspensions of Methylococcus capsulatus (Bath) FEMS Microbiol Lett. 1979;5:315–318. [Google Scholar]
- 49.Stupperich E, Konle R. Corrinoid-dependent methyl transfer reactions are involved in methanol and 3,4-dimethyoxybenzoate metabolism by Sporomusa ovata. Appl Environ Microbiol. 1993;59:3110–3116. doi: 10.1128/aem.59.9.3110-3116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suylen G M H, Large P J, Van Dijken J P, Kuenen J G. Methyl mercaptan oxidase, a key enzyme in the metabolism of methylated sulphur compounds by Hyphomicrobium EG. J Gen Microbiol. 1987;133:2989–2997. [Google Scholar]
- 51.Tajima F, Nei M. Estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol. 1984;1:269–285. doi: 10.1093/oxfordjournals.molbev.a040317. [DOI] [PubMed] [Google Scholar]
- 52.Taylor R T. B12-dependent methionine biosynthesis. In: Dolphin D, editor. B12. J. New York, N.Y: Wiley & Sons; 1982. pp. 307–355. [Google Scholar]
- 53.Thompson J D, Higgins D G, Gibson T J. Clustal W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Topp E, Hanson R S, Ringelberg D B, White D C, Wheatcroft R. Isolation and characterization of an N-methylcarbamate insecticide-degrading methylotrophic bacterium. Appl Environ Microbiol. 1993;59:3339–3349. doi: 10.1128/aem.59.10.3339-3349.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Traunecker J, Preuß A, Diekert G. Isolation and characterization of a methyl chloride utilizing, strictly anaerobic bacterium. Arch Microbiol. 1991;156:416–421. [Google Scholar]
- 56.Van de Peer Y, De Wachter R. TREE CON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci. 1994;10:569–570. doi: 10.1093/bioinformatics/10.5.569. [DOI] [PubMed] [Google Scholar]
- 57.Vannelli T, Messmer M, Studer A, Vuilleumier S, Leisinger T. A corrinoid-dependent catabolic pathway for growth of a Methylobacterium strain with chloromethane. Proc Natl Acad Sci USA. 1999;96:4615–4620. doi: 10.1073/pnas.96.8.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vannelli T, Studer A, Kertesz M, Leisinger T. Chloromethane metabolism by Methylobacterium sp. strain CM4. Appl Environ Microbiol. 1998;64:1933–1936. doi: 10.1128/aem.64.5.1933-1936.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watling R, Harper D B. Chloromethane production by wood-rotting fungi and an estimate of the global flux to the atmosphere. Mycol Res. 1998;102:769–787. [Google Scholar]
- 60.Zafiriou O C. Reaction of methyl halides with seawater and marine aerosols. J Mar Res. 1975;33:75–81. [Google Scholar]
- 61.Zehnder A J B, Wuhrmann K. Titanium (III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes. Science. 1976;194:1165–1166. doi: 10.1126/science.793008. [DOI] [PubMed] [Google Scholar]