Summary
Abundant immune cells reside in barrier tissues. Understanding the regulation of these cells can yield insights on their roles in tissue homeostasis and inflammation. Here, we report that the chemokine CCL27 is critical for establishment of resident lymphocytes and immune homeostasis in barrier tissues. CCL27 expression is associated with normal skin and hair follicle development independent of commensal bacterial stimulation, indicative of a homeostatic role for the chemokine. Accordingly, in the skin of CCL27-knockout mice, there is a reduced presence and dysregulated localization of T cells that express CCR10, the cognate receptor to CCL27. Besides, CCL27-knockout mice have overreactive skin inflammatory responses in an imiquimod-induced model of psoriasis. Beyond the skin, CCL27-knockout mice have increased infiltration of CCR10+ T cells into lungs and reproductive tracts, the latter of which also exhibit spontaneous inflammation. Our findings demonstrate that CCL27 is critical for immune homeostasis across barrier tissues.
Subject areas: Biological sciences, Immunology, Immune response
Graphical abstract
Highlights
-
•
The creation of a line of total CCL27-knockout (KO) mice
-
•
CCL27-KO mice are impaired in establishment of skin-resident Treg and Teff cells
-
•
Imiquimod-treated CCL27-KO mice have increased skin IL-17+ T cells and inflammation
-
•
CCL27-KO mice have impaired T cell homeostasis in lungs and reproductive tracts
Biological sciences; Immunology; Immune response
Introduction
Barrier tissues, including the skin and mucosa, harbor a diverse complement of immune cells. These tissue-resident immune cells protect against various assaults and help tissue development and function. Understanding molecular mechanisms regulating their migration and maintenance of tissue-resident immune cells may yield insight into their contributions to tissue homeostasis and inflammation.
Chemokines are small chemoattractive proteins that, together with their cognate receptors, regulate immune cell localization and function. CCL27 (also called ESkine, ALP, ILC, or CTACK) is a chemokine predominantly expressed by skin keratinocytes (Baird et al., 1999; Homey et al., 2000b; Hromas et al., 1999; Ishikawa-Mochizuki et al., 1999; Morales et al., 1999). The majority of skin-resident lymphocytes, including T cells and innate lymphoid cells (ILCs), express CCR10, the only known receptor for CCL27 (Homey et al., 2000b; Jarmin et al., 2000; Xiong et al., 2012; Yang et al., 2016). The CCL27/CCR10 axis was initially suggested to regulate inflammatory T cell infiltration into the skin (Homey et al., 2002). However, studies using CCR10-knockout (KO) mice found that CCR10 is dispensable for the migration of T cells into inflamed skin (Tubo et al., 2011; Xia et al., 2014). Our lab found that CCR10-KO mice have dysregulated presence of regulatory T (Treg) cells and effector T (Teff) cells in the skin and have increased inflammatory responses in several induced skin disease models, suggesting that the CCL27/CCR10 axis primarily functions in helping establishment of tissue-resident lymphocytes and maintenance of skin immune homeostasis (Fu et al., 2016; Jin et al., 2010; Li et al., 2021; Xia et al., 2014; Yang et al., 2016, 2020).
Consistent with the suggestion that the CCL27/CCR10 axis is primarily involved in skin immune homeostasis, CCL27 expression is associated with normal skin development and keratinocyte differentiation (Mildner et al., 2014). In the healthy skin, CCL27 expression was predominantly detected in keratinocytes of the epidermis and hair follicles (HF) in both humans and mice (Homey et al., 2002; Joost et al., 2016; Mildner et al., 2014; Simonetti et al., 2004). CCL27 expression is suppressed in the lesional skin of various skin inflammatory diseases such as psoriasis, hidradenitis suppurativa (HS), and alopecia areata (Fletcher et al., 2020; Gudjonsson et al., 2010; Hotz et al., 2016; Kelly et al., 2015; Quaranta et al., 2014; Riis et al., 2011a; Simonetti et al., 2004). We recently found that trans-epidermal injection of exogenous CCL27 into the skin reduces imiquimod (IMQ)-induced skin inflammation in mice, suggesting a role of CCL27 in skin homeostatic regulation (Li et al., 2021). Previous studies found that cytokines, such as TNFα, IL-1β, IFNγ, and IL-17A, differentially regulate CCL27 expression in cultured keratinocytes, consistent with clinical observations that CCL27 expression is affected by inflammatory conditions (Kanda et al., 2005; Karakawa et al., 2014; Morales et al., 1999; Riis et al., 2011b). Poly-I:C and flagellin were also shown to induce CCL27 expression in cultured keratinocytes, suggesting that bacteria or viruses drive CCL27 expression (Lebre et al., 2007). However, the in vivo function of CCL27 in the skin is unclear up to date.
CCL27 has also been detected in mucosal sites of lungs and reproductive tracts (Menzies et al., 2020; Qiu et al., 2008; Sennepin et al., 2017; Wick et al., 2008). Lungs harbor a small fraction of CCR10-expressing T cells and ILCs (Weston et al., 2019), whereas some other mucosal sites, such as intestines, do not have CCR10+ T cells (Kunkel et al., 2003; Zhao et al., 2020). Instead, IgA antibody-secreting plasma cells are the major type of immune cells that express CCR10 in the mucosal sites (Hu and Xiong, 2013; Kunkel et al., 2003). The migration of these CCR10+ plasma cells into mucosal tissues is mostly mediated by the chemokine CCL28, another ligand for CCR10 preferentially expressed by mucosal tissues (Burkhardt et al., 2019; Lazarus et al., 2003; Matsuo et al., 2018; Wang et al., 2000). The functional importance of CCL27 within mucosal tissues is unknown.
Studying CCL27 has largely been impeded by the lack of in vivo models. The generation of CCL27-knockout (KO) mice is complicated by the existence of gene duplications in commonly used inbred strains of mice. An early study identified CCL27a and CCL27b genes in C57BL/6 mice (Nakano and Gunn, 2001), and genomic sequencing of C57BL/6 mice revealed presence of the third CCL27 gene, GM13306 (https://www.ncbi.nlm.nih.gov/gene/100039863) (Shibata et al., 2013). It is not clear whether the CCL27 gene duplicates are functional. In addition, high levels of CCL27 in the skin limit the efficacy of antibody neutralization and mouse models of skin inflammatory disease utilizing this technique have yielded inconsistent results (Homey et al., 2002; Mirshahpanah et al., 2008; Reiss et al., 2001).
In this report, we found that CCL27 expression in the skin is independent of commensal bacterial stimulation. Using a line of mice with all CCL27 genes knocked-out, we provided direct evidence that CCL27 is important in the establishment of CCR10+ resident Treg and Teff cells in the skin. Beyond the skin, we found that the CCL27-KO mice had increased infiltration of CCR10+ T cells in lungs and reproductive tracts, the latter of which displayed signs of increased inflammation. These findings establish CCL27 as a critical regulator of immune homeostasis in the skin and mucosal tissues.
Results
Expression of CCL27 by skin keratinocytes is developmentally programmed
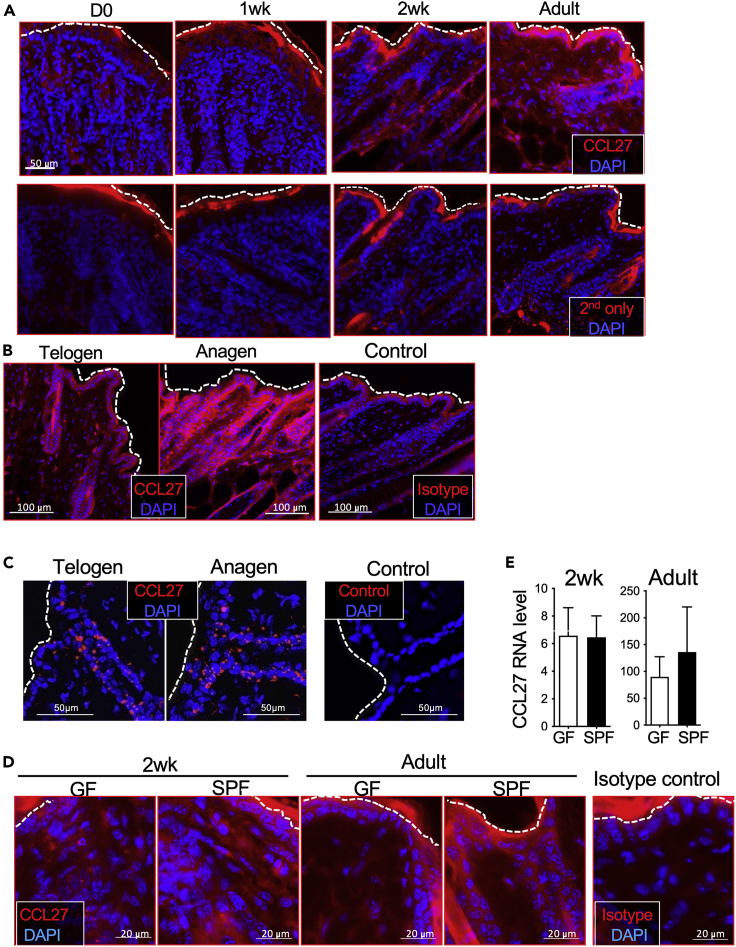
In line with an age-associated increase of CCL27 expression in the human skin (Mildner et al., 2014), immunofluorescent staining with anti-CCL27 antibodies showed an increase of CCL27 expression in the skin of mice following birth, with significant staining at 2 weeks (Figures 1A and S1A). CCL27 was found in the epidermis and HF (Figures 1A and S1A), consistent with previous reports (Homey et al., 2002; Joost et al., 2016; Mildner et al., 2014; Simonetti et al., 2004). In adult mice, CCL27 expression in the HF was higher at the growth phase (anagen) than at the resting phase (telogen) (Figure 1B). Because CCL27 is a secreted protein, the anti-CCL27 immunofluorescent staining displayed a diffused pattern. To confirm that epidermal and HF keratinocytes expressed CCL27, we performed in situ hybridization staining of skin sections with an anti-sense CCL27-specific RNA probe. Keratinocytes of the epidermis and HF, particularly the upper HF, expressed CCL27 transcripts based on the in situ hybridization analysis (Figures 1C and S1B), consistent with a reported single cell RNA-seq (scRNA-seq) study (Joost et al., 2016) (http://linnarssonlab.org/epidermis/).
Figure 1.
Preferential localization of CCR10+ lymphocytes around growing hair follicles correlates with high CCL27 expression by follicular keratinocytes
(A) Representative immunofluorescent skin sections (14μm) stained with anti-CCL27 antibody. The dashed lines mark the epidermal surface of the skin. N = 7 mice for D0, 1 week, and adult ages, six mice for 2 weeks.
(B) Immunofluorescent microscopic images of anti-CCL27 antibody-stained skin sections (14μm) at resting (telogen) and growing (anagen) phases of HF cycling in adult mice. N = 3 mice for each phase.
(C) Fluorescent images (7μm) representative of skin at telogen and anagen phases of hair follicle cycling stained by in situ hybridization with an antisense CCL27 RNA probe or a nonspecific RNA probe as the control. The control probe recognizes the DapB gene (accession # EF191515) of a soil bacterial strain Bacillus subtilis SMY. N = 3 mice per HF cycle phase. N = 3 mice for each phase.
(D) Anti-CCL27 antibody immunofluorescent staining images of 14μm skin sections of SPF and GF mice.
(E) Real-time RT-PCR analysis of CCL27 expression in the skin of SPF and GF mice, normalized to β-actin. N = 5 mice for 2-week-old GF, seven for 2-week-old SPF, seven for adult GF, and five for adult SPF. Unpaired one-tailed t-test.
Although CCL27 expression is associated with the normal skin and HF development, it has been reported that stimulation of TLRs with bacterial components upregulates CCL27 expression in cultured keratinocytes (Lebre et al., 2007). To investigate whether CCL27 expression in the skin was dependent upon commensal bacterial stimulation, we compared CCL27 expression in the skin specific pathogen-free (SPF) versus germ-free (GF) mice and found that they had similar levels of the CCL27 expression at both young and adult ages (Figures 1D and 1E). These results indicate CCL27 expression is developmentally driven independent of commensal bacterial stimulation.
CCL27a-knockout had little effect on establishment of CCR10+ lymphocytes in the skin
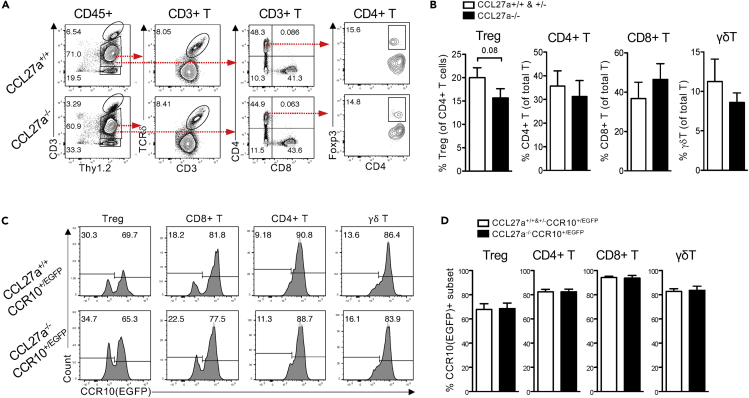
To test whether CCL27 was integral to the establishment of the skin-resident lymphocyte repertoire by attracting CCR10+ lymphocytes, we analyzed CCL27a-knockout (CCL27a−/−) mice, in which the coding sequence for the CCL27a gene is deleted, whereas its duplicate genes CCL27b and Gm13306 remain intact (Figures S2A and S2B). There was no significant difference in percentages of CD4+, CD8+, or γδT cells. There was marginal reduction of Treg cells in the skin of CCL27a−/− mice compared to CCL27a+/+ or CCL27a+/− mice (Figures 2A and 2B). To determine the effect of CCL27a-KO on CCR10+ lymphocytes, we crossed CCL27a-KO mice to a CCR10-EGFP reporter mouse line (CCL27a−/−CCR10+/EGFP) (Jin et al., 2010). However, there was no significant difference in percentages of CCR10(EGFP)+ Treg or other T cell subsets in the skin of CCL27a−/−CCR10+/EGFP and CCL27a-sufficient control mice (Figures 2C and 2D). Immunofluorescent staining and real-time RT-PCR analyses still detected a significant level of CCL27 expression in the skin of CCL27a−/− mice as a result of CCL27b and/or Gm13306 expression (Figures S2C and S2D). These results show that CCL27a-knockout has little effect on establishment of skin lymphocytes, likely because CCL27b and/or Gm13306 genes are still expressed.
Figure 2.
CCL27a-knockout has little effect on the establishment of CCR10+ lymphocytes in the skin
(A) Representative FC analysis of gated skin CD45+ immune cells for T cell subsets and ILCs in adult CCL27a−/− and CCL27a+/+ mice.
(B) Percentages of T cell and ILC populations from CCL27a−/− versus CCL27a+/+ or CCL27a+/− adult mouse skin. N = 7 mice each for CCL27a−/− and control groups. Unpaired one-tailed Student’s t-test.
(C) Representative FC analysis of skin T cell subsets and ILCs from adult CCL27−/−CCR10+/EGFP and CCL27a+/+CCR10+/EGFP mice gated for their CCR10(EGFP)+ subpopulations. CD8+ and CD4+ T cells are gated on CD8+ and CD4+ CD45+Thy1.2+CD3int+ populations respectively. ILCs are CD45+Th1.2+CD3−. γδT cells are CD45+Thy1.2+CD3int+TCRδ+ (excluding CD3high dendritic epidermal γδT cells).
(D) Comparative percentages of CCR10+ subsets of indicated skin lymphocyte populations from CCL27a−/−CCR10+/EGFP versus CCL27a+/+CCR10+/EGFP or CCL27a+/−CCR10+/EGFP adult mice. N = 6 mice each for CCL27a−/− and control group. Unpaired one-tailed Student’s t-test.
Severely impaired migration of CCR10+ lymphocytes into the skin of total CCL27-knockout mice
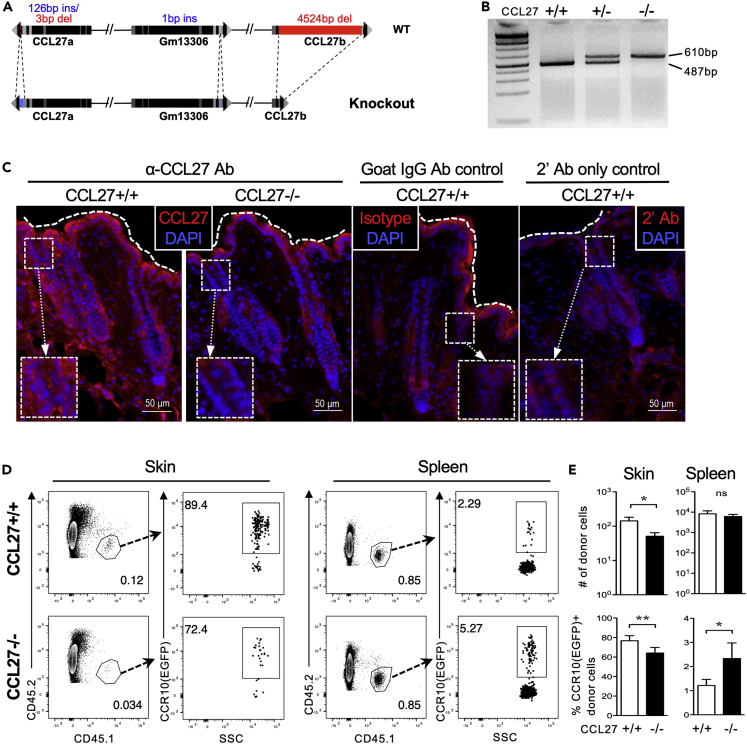
We then generated total CCL27-KO (CCL27−/−) mice using CRISPR to target the exon II shared by all three CCL27 genes. CCL27−/− mice had a 126bp insertion and a 3bp deletion in CCL27a, a 1bp insertion in Gm13306, and a 4524bp deletion in CCL27b at their respective exon II regions (Figures 3A and 3B). CCL27−/− mice had no specific antibody staining for CCL27 in the skin, confirming that CCL27 protein was not expressed (Figure 3C). To determine whether the lack of CCL27 impaired migration of CCR10+ lymphocytes, we transferred cells from the skin-draining lymph nodes (sLN) of CCR10+/EGFP mice, which contained a small fraction of CCR10(EGFP)+ lymphocytes, into CCL27−/− and WT littermates and analyzed the donor cells in the skin two days after transfer. Most donor cells in the skin of recipient mice expressed CCR10(EGFP), whereas few donor cells in the spleen of recipients did, suggesting that only CCR10+ donor cells migrate into the skin (Figure 3D). There were significantly fewer donor cells in the skin of CCL27−/− recipients than of WT recipients (Figures 3D and 3E), demonstrating that CCL27 is critical for efficient migration of CCR10+ lymphocytes into the skin.
Figure 3.
Severely impaired migration of CCR10+ lymphocytes into the skin of total CCL27-knockout mice
(A) Diagram of CRISPR targeting in total CCL27-knockout mice.
(B) Genomic PCR identifying wild-type (+/+), heterozygous (+/−), and homozygous (−/−) total CCL27-KO mice. Band sizes of wild-type and knockout CCL27 alleles are 484 and 607bp, respectively.
(C) Immunofluorescent α-CCL27 antibody staining images on 10μm skin sections from CCL27+/+ and CCL27−/− mice. Staining with a nonspecific goat IgG antibody or the secondary antibody (2′ Ab) only is included as controls. The dashed lines mark the surface of the skin. N = 3 mice for each genotype.
(D) FC analysis of CD45+ cells from the skin and spleen of CCL27+/+ and CCL27−/− recipient mice two days after receiving transfer of sLN cells of CCR10+/EGFP mice. Donor-derived CCR10(EGFP)+ lymphocytes are gated on CD45.1+ cells. N = 6 mice for each group.
(E) Total numbers (#) of donor cells and percentages (%) of donor cells that express CCR10(EGFP) in the skin and spleen of CCL27+/+ and CCL27−/− recipient mice. N = 6 mice for each group. ∗p < 0.05; ∗∗p < 0.01; ns: no significant difference. Paired one-tailed t-test.
Impaired establishment and dysregulated localization of CCR10+ lymphocytes in the skin of CCL27−/− mice
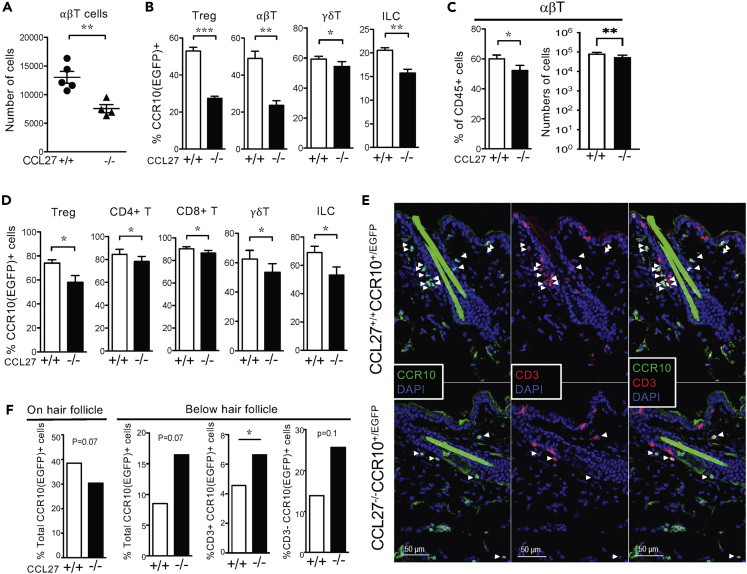
We then analyzed the skin CCR10+ lymphocyte repertoire in detail in CCL27−/−CCR10+/EGFP mice. Compared to CCL27+/+CCR10+/EGFP littermates, two week-old CCL27−/−CCR10+/EGFP mice had significantly reduced numbers of αβT cells in the skin (Figures 4A and S3A), whereas their numbers of skin γδT cells or innate lymphoid cells (ILCs) were not statistically different (Figures S3A and S3B). However, percentages of CCR10(EGFP)+ Treg, αβT, γδT cells, and ILCs were all reduced in the skin of two week-old CCL27−/−CCR10+/EGFP mice compared to their corresponding CCL27+/+CCR10+/EGFP controls (Figure 4B). Similarly, adult CCL27−/−CCR10+/EGFP mice had significantly reduced numbers of αβT cells but not γδT or ILCs in the skin compared to their WT controls (Figures 4C, S3C, and S3D). However, percentages of CCR10(EGFP)+ Treg, CD8+ T, CD4+ T, γδT, and ILCs were also all reduced in CCL27−/−CCR10+/EGFP mice compared to CCL27+/+CCR10+/EGFP controls (Figure 4D). Associated with this, numbers of CCR10+ CD4+ and CD8+ T cells were also significantly reduced in CCL27−/−CCR10+/EGFP mice (Figure S3E). These results demonstrate that CCL27-KO predominantly impairs establishment of CCR10+ T cells and ILCs in the skin.
Figure 4.
Impaired establishment and dysregulated localization of CCR10+ lymphocytes in the skin of CCL27−/− mice
(A and B) Numbers of αβT cells (A) and percentages of CCR10(EGFP)+ Treg, αβT, γδT, cells and ILCs (B) in the skin of two-week-old CCL27+/+CCR10+/EGFP and CCL27−/−CCR10+/EGFP mice. N = 5 mice for CCL27+/+ and four for CCL27−/− samples. The cell number is calculated from FC analysis of cell preparations of the whole trunk skin.
(C) Percentages and cell counts of αβT cells of skin CD45+ cells from adult CCL27+/+CCR10+/EGFP and CCL27−/−CCR10+/EGFP mice. N = 11 mice for CCL27+/+CCR10+/EGFP and 10 for CCL27−/−CCR10+/EGFP samples.
(D) Percentages of CCR10(EGFP)+ subsets of different skin lymphocyte populations in adult CCL27+/+CCR10+/EGFP and CCL27−/−CCR10+/EGFP mice. N = 5 mice of each genotype for the CD8+, CD4+ and γδ T cell data and three each for the ILC data.
(E) Immunofluorescent images of skin sections of 6-week-old CCL27+/+CCR10+/EGFP and CCL27−/−CCR10+/EGFP mice for CCR10(EGFP)+ lymphocytes and T cells. Arrowheads identify CCR10(EGFP)+ cells. The green long rod-like structures are autofluorescent hairs. Super-bright CD3+ cells are DETCs.
(F) Relative percentages of CCR10(EGFP)+ lymphocytes and T cells close to or below hair follicle structures in CCL27+/+CCR10+/EGFP versus CCL27−/−CCR10+/EGFP mice, based on calculation of total 1078 and 943 CCR10+ cells, respectively. Representative of four CCL27+/+CCR10+/EGFP and CCL27−/−CCR10+/EGFP gender-matched littermate adult mice with 20 images analyzed per mouse. One tailed paired t-test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Because CCL27 is preferentially expressed in the upper HF of the skin (Figure 1), we assessed whether CCL27-KO also affected the localization of CCR10+ lymphocytes to this region. Compared to CCL27+/+CCR10+/EGFP mice, CCL27−/−CCR10+/EGFP mice had a lower frequency of CCR10(EGFP)+ innate lymphocytes and T cells localized close to hair follicles but a higher percentage of them localized below hair follicles (Figures 4E and 4F), indicating that CCL27 is critical for efficient migration and localization of CCR10+ lymphocytes into specific niches of the skin microstructures.
We further assessed effects of CCL27-KO on the cytokine secretion profiles by CCR10+ skin T cells and ILCs. There were no significant difference in production of IL-17A or IFNγ by total or CCR10(EGFP)+ skin ILCs, CD8+, CD4+, and γδ T cells of CCL27−/−CCR10+/EGFP and control CCL27+/+CCR10+/EGFP mice, whereas there was a small (<5%) increase in the percentage of IL-10-expressing skin CD4+ T cells in CCL27−/−CCR10+/EGFP mice (Figures S4A–S4F). These results suggest that CCL27-KO had little effect on cytokine production profiles of skin-resident lymphocytes under homeostatic conditions.
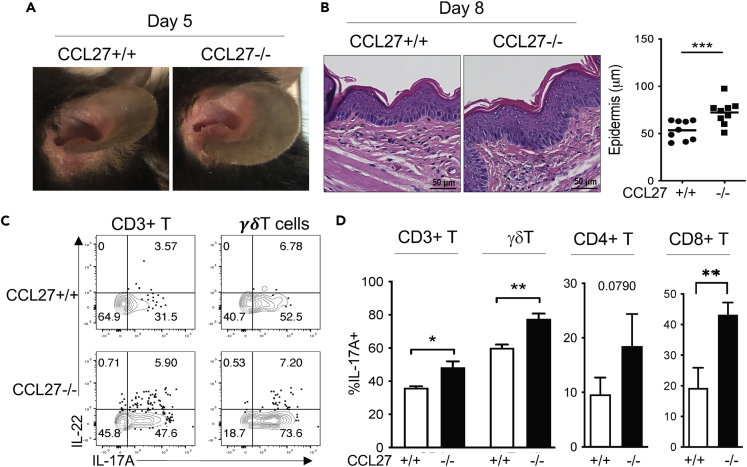
Increased skin inflammation in CCL27−/− mice in response to topical imiquimod stimulation
To further investigate how the dysregulated migration and localization of skin-resident lymphocytes in CCL27−/− mice affected skin immune function, we treated these mice topically with imiquimod (IMQ) to induce psoriasis-like skin inflammation (van der Fits et al., 2009). In the IMQ-induced model, IL-17-producing γδT (γδT17) cells are the major pathogenic T cells responsible for the skin inflammation (Cai et al., 2011; Gray et al., 2011; Sandrock et al., 2018; van der Fits et al., 2009), and their activation is not restricted by Treg cells (Stockenhuber et al., 2018). Compared to IMQ-treated WT (CCL27+/+) littermate controls, IMQ-treated ears of CCL27−/− mice visibly displayed more inflammation (Figure 5A) and increased epidermal thickness (Figure 5B). FC analysis found that frequencies of IL-17A+ total, γδ, CD8+, and CD4+ T cells in the IMQ-treated ears of CCL27−/− mice were all increased compared to corresponding WT controls (Figures 5C and 5D). These results show that CCL27−/− mice had overactivation of IL-17-producing skin T cells in response to the topical IMQ stimulation, resulting in severe skin inflammation.
Figure 5.
Increased skin inflammation in CCL27−/− mice in response to topical imiquimod stimulation
(A) Images of ears 5 days after topical treatment with IMQ.
(B) Microscopic images of H&E-stained ear sections of CCL27+/+ and CCL27−/− mice 8 days after the IMQ treatment. The average epidermal thickness of IMQ-treated ears of CCL27+/+ and CCL27−/− mice was shown in the graph on the right. One dot is of one mouse.
(C) FC analysis of gated CD45+CD3int+ and CD45+CD3int+ γδTCR+ skin T cells of IMQ-treated (day 8) mice for IL-17A and IL-22 expression.
(D) Percentages of CD3+, γδ, CD4+, and CD8+ T cells that express IL-17A in IMQ-treated skin of CCL27+/+ and CCL27−/− mice. N = 5 mice for both CCL27+/+ and CCL27−/− categories. Paired one tailed t-test. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
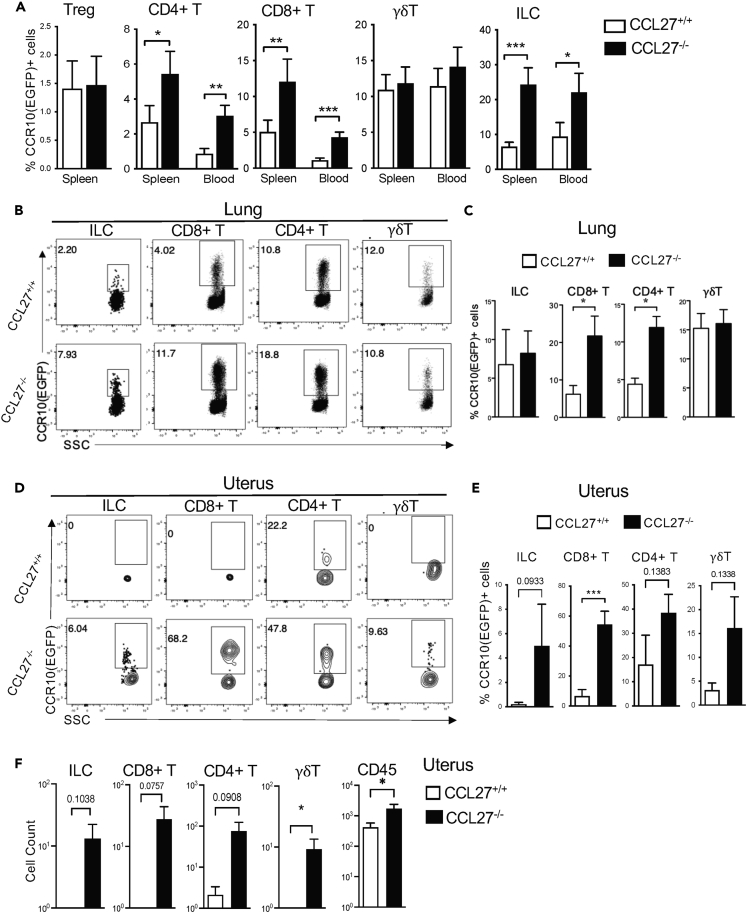
Increased accumulation of CCR10+ T cells and ILCs in the blood and spleens of CCL27-KO mice
We then assessed whether dysregulation of skin-resident CCR10+ lymphocytes in CCL27-KO mice had systemic effects on immune cell compositions in circulation and within the spleen. There were significantly higher percentages of CCR10(EGFP)+ CD4+, CD8+ αβT cells, and ILCs in the spleen or blood of CCL27−/−CCR10+/EGFP mice than of CCL27+/+CCR10+/EGFP mice (Figures 6A, S5A, and S5B), suggesting that in absence of CCL27, CCR10+ lymphocytes cannot effectively migrate into the skin and instead continue to circulate in the blood and accumulate in the spleen. There were very few CCR10(EGFP)+ Treg cells in spleens of either CCL27−/−CCR10+/EGFP or CCL27+/+CCR10+/EGFP mice (Figures 6A and S5B), suggesting that they did not accumulate outside of the skin. On the other hand, the percentage of CCR10(EGFP)+ γδT cells in the blood and spleens of CCL27−/−CCR10+/EGFP mice was not significantly increased (Figures 6A and S5B).
Figure 6.
Increased infiltration of CCR10+ T cells in mucosal sites of CCL27−/− mice
All CCL27−/− and control CCL27+/+ littermate mice carry a CCR10-KO/EGFP-KI allele (CCR10+/EGFP) for the purpose of reporting CCR10 expression with EGFP.
(A) Percentages of CCR10(EGFP)+ subsets of T cells and ILCs in the spleen and blood of adult CCL27+/+ and CCL27−/− mice. N = 9 mice of each genotype for blood data, 12 for the spleen ILCs, CD8+, CD4+, γδ T cell data and seven for the spleen Treg data.
(B and C) FC analysis of gated populations of T cells and ILCs for their CCR10(EGFP) expression (B) and average percentages of CCR10(EGFP)+ subsets of T cells and ILCs (C) in lungs of CCL27+/+ and CCL27−/− mice. N = 4 each.
(D and E) FC analysis of gated populations of T cells and ILCs for their CCR10(EGFP) expression (D) and average percentages of CCR10(EGFP)+ subsets of T cells and ILCs (E) in uterus of female CCL27+/+ and CCL27−/− mice.
(F) Total numbers of CD45+ immune cells and different subsets of CCR10(EGFP)+ T cells and ILCs recovered from the uterus of female WT and CCL27−/− mice. N = 8 CCL27+/+ and seven CCL27−/− mice for the panels D-E. Statistical analysis by one tailed paired t-test. Significance: ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
Increased infiltration of CCR10+ T cells and ILCs and immune dysregulation in lungs and female reproductive tracts of CCL27-KO mice
CCL27 has been detected in mucosal tissues including lungs and reproductive tracts (Menzies et al., 2020; Qiu et al., 2008; Sennepin et al., 2017; Weston et al., 2019; Wick et al., 2008), although CCL28 is suggested as the dominant CCR10 ligand expressed in the mucosal tissues (Burkhardt et al., 2019; Lazarus et al., 2003; Matsuo et al., 2018; Wang et al., 2000). We assessed whether CCL27-KO affected the CCL28 expression and compositions of CCR10+ lymphocytes in lungs and female reproductive tracts. Compared to WT controls, lungs and female reproductive tracts of CCL27−/− mice had little or no significant increases in the CCL28 expression (Figure S5C). Surprisingly, CCL27−/− mice had significantly increased percentages of CCR10(EGFP)+ CD4+ and CD8+ αβT cells in lungs and uteri compared to control WT mice, whereas frequencies of CCR10(EGFP)+ ILCs and γδT cells were mildly increased or unaltered in lungs and uteri of CCL27−/− mice (Figures 6B–6E). Particularly, in the uteri of CCL27−/− mice, a large fraction (40–50%) of CD4+ and CD8+ αβT cells expressed CCR10(EGFP) (Figures 6D and 6E). This increase of CCR10+ T cells was paralleled with a significant increase in the absolute number of CD45+ immune cells in the uteri of CCL27−/− mice, indicating immune dysregulation in the tissue (Figure 6F). Consistent with the notion, uteri in some CCL27−/− mice had visible inflammation compared to their WT littermates (Figure S5D). Together, these results demonstrate that CCL27 deficiency leads to increased infiltration of CCR10+ lymphocytes into these mucosal tissues, which could lead to local immune homeostatic dysregulation and inflammation, particularly in the reproductive tract.
Discussion
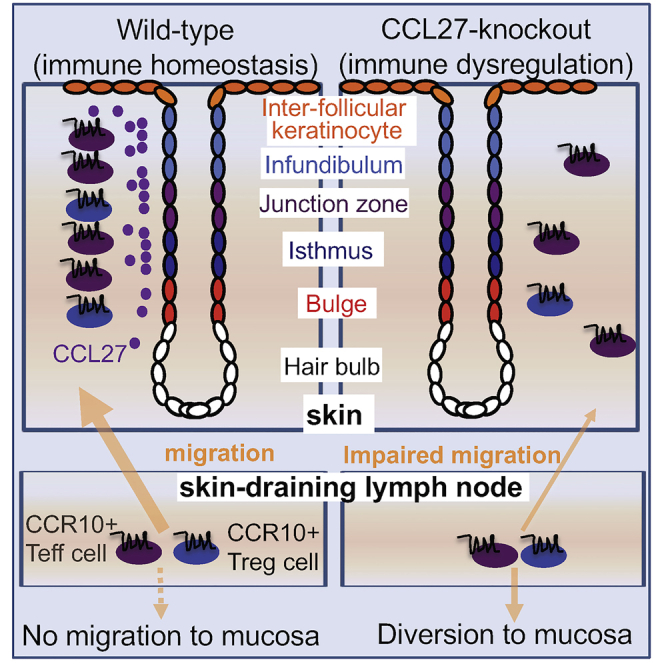
CCL27 is a chemokine predominantly expressed in the skin, although it has also been detected in other barrier tissues such as lungs and reproductive tracts (Baird et al., 1999; Homey et al., 2000b; Hromas et al., 1999; Ishikawa-Mochizuki et al., 1999; Menzies et al., 2020; Morales et al., 1999; Qiu et al., 2008; Sennepin et al., 2017; Wick et al., 2008). The precise role of CCL27 in regulating immune cells within barrier tissues is not fully understood. In this study, we assessed the in vivo function of CCL27 using two strains of newly generated CCL27-knockout mice. Knockout of the presumptive dominant CCL27 gene, CCL27a, resulted in only a slight change of the skin lymphocyte composition because of the continued expression of the other two duplicated CCL27 genes CCL27b and/or Gm13306. Knockout of all the CCL27 genes resulted in significantly reduced frequencies of CCR10+ lymphocytes in the skin, particularly within the Treg and CD8+ T cell populations. Total CCL27-KO mice displayed increased inflammatory responses to topical IMQ treatment, indicating CCL27-regulated homeostatic establishment and maintenance of skin-resident CCR10+ T cells and ILCs is crucial for the local tissue homeostasis. The similar phenotypes in the skin of CCL27-KO and CCR10-KO mice solidify CCL27 as the major ligand regulating the localization and function of CCR10+ resident lymphocytes in the skin to help the local immune homeostatic maintenance (Fu et al., 2016; Li et al., 2021; Xia et al., 2014; Yang et al., 2016).
We also found that expression of CCL27 in the skin is associated with HF cycling, revealing the underlying mechanism of preferential localization of CCR10+ lymphocytes to specific niches within the skin for local homeostatic regulation. The hair follicle is known to harbor HFSCs and is considered as an immune-privileged site (Fuchs et al., 2004; Paus et al., 2018; Westgate et al., 1991). However, the HF is also a site of extensive interaction between foreign environmental agents and local epithelial cells (Schneider and Paus, 2014). Tightly regulated immune responses are required to maintain the immune homeostasis throughout the dynamic change of HF cycling. Follicular epithelial cells express various chemokines that direct localization of immune cells to this region. It was reported that keratinocytes of the infundibulum and isthmus produce CCL2 and CCL20 to recruit myeloid cells in response to external stress (Nagao et al., 2012). Expression of CCL20 by follicular cells was also reportedly induced by microbiota to attract CCR6+ Treg cells to this niche in neonatal mice (Scharschmidt et al., 2017), and the preferential localization of Treg cells to the HF facilitates stem cell differentiation (Ali et al., 2017). The association of CCL27 expression with HF cycling suggests its role in directing localization of CCR10+ lymphocytes into the skin under non-inflammatory settings for tissue homeostatic regulation. As both CCL27−/− and CCR10−/− mice have dysregulated skin immune homeostasis (Fu et al., 2016; Li et al., 2021; Xia et al., 2014; Yang et al., 2016), it will be interesting to test how impaired localization of CCR10+ lymphocytes affects HF cycling in CCL27-KO and CCR10-KO mice.
Our findings help explain the expression pattern of CCL27 in pathogenesis of skin inflammatory diseases. For example, in psoriasis, increased CCL27 expression has been reported in the perilesional skin, whereas its expression in lesional skin is decreased (Gudjonsson et al., 2010; Karakawa et al., 2014; Quaranta et al., 2014; Riis et al., 2011a; Sahmatova et al., 2017). As CCL27 is critical for skin immune homeostasis, an increase of CCL27 in perilesional skin could be a mechanism to direct CCR10+ regulatory cells to the impacted skin, whereas its downregulation in lesional skin could lead to a loss of this regulatory axis and the progression of inflammatory symptoms. CCL27 expression is also severely suppressed in the lesional skin of patients with hidradenitis suppurativa (HS), a defective hair follicle-associated inflammatory disease (Hotz et al., 2016; Zouboulis et al., 2020). Lesional skin of HS patients has dysregulated Treg and Teff cells (Melnik et al., 2018). The loss of CCL27-mediated immune regulation might contribute to the T cell dysregulation and inflammatory pathology of the HS. CCL27 expression is also downregulated in HFs of patients with alopecia areata (Simonetti et al., 2004), suggesting that its dysregulation might be involved in hair loss. Our CCL27-KO mice may prove to be a useful model in assessing the role of CCL27 in different skin inflammatory diseases.
Besides CCR10, other chemokine receptors such as CCR6 and CCR4 are also expressed on skin-homing or resident T cells to regulate their localization and function under homeostatic and inflammatory conditions (Andrew et al., 2001; Baekkevold et al., 2005; Cai et al., 2011; Gray et al., 2011; Jiang et al., 2010) (Campbell et al., 2007; Casciano et al., 2020; Matsuo et al., 2021; Puig et al., 2022). These chemokine receptors could have redundant functions in directing localization of CCR10+ T cells into the skin in absence of CCL27. Notably, however, roles of CCR6 and CCR4 and their ligands in regulation of IL-17-producing T cell activation in the skin inflammation are opposite from those of CCR10 and CCL27. Mice deficient of CCR6 or its ligand CCL20 have reduced skin inflammation in response to the IMQ stimulation (Campbell et al., 2017; Cochez et al., 2017; Robert et al., 2017; Shi et al., 2021; Yu et al., 2019), suggesting that in contrast to the CCR10/CCL27 axis, the CCR6/CCL20 axis promotes the skin γδT17 cell activation and tissue inflammation. CCR4 is preferentially expressed in skin Th17 but not γδT17 cells and CCR4-deficient mice also have reduced skin inflammation in response to topical IMQ stimulation (Matsuo et al., 2021). Corroborating with these mouse studies, clinical observations found that expression of CCL17, the ligand of CCR4, and CCL20 is increased, whereas CCL27 is decreased in the lesional skin of psoriatic patients (Gudjonsson et al., 2010; Homey et al., 2000a; Kim et al., 2014; Quaranta et al., 2014; Sahmatova et al., 2017). Mechanisms underlying unique roles of the CCL27/CCR10 axis in the skin immune homeostasis need further investigation.
In contrast to the skin, lungs and reproductive tracts of CCL27-KO mice had markedly increased CCR10+ T cells, particularly CD4+ and CD8+ αβT cells. These results indicate that CCL27 is not required for promoting localization of CCR10+ T cells into these mucosal sites. The reproductive tracts of some CCL27−/− mice have spontaneous inflammation, suggesting that increased infiltration of CCR10+ T cells into the tissue leads to dysregulated local immune homeostasis. These findings demonstrate that CCL27 is a critical regulator of immune homeostasis across barrier tissues, suggesting that properly regulated localization of T cells into the skin by the CCL27-CCR10 axis is not only important within the skin but also for prevention of unintended pathological consequences in other tissues. Impairment of these regulatory mechanisms, such as the downregulation of CCL27 in skin inflammatory diseases, could potentially lead to diversion of skin-homing T cells and ILCs into the circulation and other barrier tissues, leading to disruption of local immune homeostasis of various tissues and organs. Supporting this notion, skin inflammatory diseases such as psoriasis and hidradenitis suppurativa are associated with complications in mucosal tissues and blood vessels (Boehncke, 2018; Korman, 2020; Masson et al., 2020; Pescitelli et al., 2018; Sabat et al., 2020).
The majority of CCR10+ skin-resident αβT cells express markers of tissue-resident memory T cells and contribute to the tissue-resident memory T cell repertoire (Fu et al., 2016; Xia et al., 2014). Skin immunization is commonly used in vaccination against mucosa-associated infectious diseases. Our finding that CCL27-KO mice have decreased localization of CCR10+ T cells in the skin and increased accumulation in mucosal tissues suggests that targeting CCL27 during skin immunization could potentially direct CCR10+ skin-homing memory T cells to mucosal sites for better protection against mucosal infections. Our newly developed CCL27-KO mice will be a useful tool to test this strategy in the future.
Limitations of the study
Although our study of CCL27-KO mice provides the first definite evidence that CCL27 is a critical chemokine in maintenance of skin homeostasis through regulation of CCR10+ T cells, molecular mechanisms of the CCL27/CCR10 axis in promoting Treg cells and suppressing pathogenic γδT17 cells in the healthy and imiquimod-induced inflammatory skin need further study. Because Treg cells primarily function to restrict type 1 interferon-induced CD8+ T cell responses in the IMQ-induced skin inflammation (Stockenhuber et al., 2018), roles of the CCL27-CCR10 axis in promoting Treg cells and suppressing pathogenic IL-17-producing T cells might represent two independent mechanisms to help maintain the local tissue immune homeostasis. In future, it will be helpful to test CCL27-KO, CCR10-KO, or CCL27/CCR10-double KO mice in various models of inflammation and infection to determine their independent and collaborative functions in regulation of different subsets of skin-resident T cells to help the skin homeostasis. Our study also reveals that in contrast to the prediction that CCL27 helps recruitment of CCR10+ T cells into mucosal sites (Menzies et al., 2020; Qiu et al., 2008; Sennepin et al., 2017; Weston et al., 2019; Wick et al., 2008), CCL27-KO mice have increased CCR10+ T cells in lungs, and particularly, reproductive tracts that could lead to local immune dysregulation. However, mechanisms of the increased accumulation of CCR10+ T cells in the mucosal tissues and its effects on local immune homeostasis in CCL27-KO mice are yet to be determined. Our findings of reduced CCR10+ T cells in the skin and increased CCR10+ T cells in the mucosal sites of CCL27-KO mice support the notion that CCR10+ T cells could not efficiently migrate into the skin in absence of CCL27, leading to their increased accumulation in the circulation and diversion into the mucosal sites. How the CCL28/CCR10 axis and other mucosal-homing chemokine receptors such as CCR4 are involved in migration of CCR10+ T cells into the mucosal tissues needs further study.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD45 | Biolegend | 30-F11 |
| anti-mouse TCRδ | Biolegend | Clone GL3 |
| anti-mouse CD3, | Biolegend | 17A2 |
| anti-mouse CD4 | Biolegend | GK1.5 |
| anti-mouse CD8 | Biolegend | 53-6.7 |
| anti-mouse Thy1.2 | Biolegend | 30-H12 |
| anti-mouse IL-17A | Biolegend | TC11-18H10.1 |
| anti-mouse TCRβ | Biolegend | H57-597 |
| anti-mouse CD45.2 | Biolegend | 104 |
| anti-Sca1 | Biolegend | E13-161.7 |
| anti-EpCAM | Biolegend | G8.8 |
| anti-I-A/I-E | Biolegend | M5/114.15.2 |
| anti-CD45 | Biolegend | 30-F11 |
| and anti-mouse CD45.1 | Biolegend | A20 |
| Anti-mouse/human IL-22 | eBioscience | IL22JOP |
| anti-mouse FoxP3 | eBioscience | FJK-16s |
| Anti-mouse CD34 | BD Biosciences | RAM34 |
| goat anti-mouse CCL27 antibodies | R&D systems | AF725 |
| Control goat IgG antibody | R&D systems | AB-108-C |
| Alexa Fluor 647 chicken anti-goat antibody | Invitrogen | A-21469 |
| Chemicals, peptides, and recombinant proteins | ||
| Imiquimod | Perrigo | NDC 45802-368-00 |
| DAPI | Biolegend | 422801 |
| Prolong Diamond Antifade | Invitrogen | P36961 |
| Critical commercial assays | ||
| BaseScope Reagent Kit-Red | Advanced Cell Diagnostics, INC | Cat #: CAT NO: 323600 |
| Experimental models: Organisms/strains | ||
| CCL27a-KO mice | UC-Davis KOMP Repository | C57BL/6N-CCL27a tm1(KOMP)Vlcg |
| Total CCL27-KO mice | Generated in lab with help of the Gene Modification Facility of the Albert Einstein College of Medicine. | None |
| CCR10-KO/EGFP-KI mice | Generated in lab | None |
| Oligonucleotides | ||
| CCL27 RNA probe: 5′TTGCTTCTGAGCCCGGC TCCTGAAGCAGCCTTGCCTCTGCCCTCCAGC |
Advanced Cell Diagnostics, INC | None |
| Primers for qRT-PCR of total CCL27 mRNA: (F)5′GA TGGGGACTGTCACCTCCAG, (R)5′CCTTGGAGCC TTTTCCCTTGGCGT |
IDT | None |
| Primers for qRT-PCR of CCL27a mRNA: (F)5′CACA TGGAACTGCAGGAGGCC, (R)5′TGTAGTACCAGA TTTAAACTGGGTACAGTC |
IDT | None |
| Primers for qRT-PCR of CCL27b mRNA: (F)5′CACA TGGAACTGCAGGAGGCT, (R)5′TGTAGTACCAGA TTTAAACTGGGTACAGTT |
IDT | None |
| Primers for qRT-PCR of CCL28 mRNA: (F)5′CAGCC CGCACAATCGTACT, (R)5′ACGTTTTCTCTGCCAT TCTTCTTT |
IDT | None |
| Primers for qRT-PCR of β-actin mRNA: (F)5′CT GTCGAGTCGCGTCCA, (R)5′CACGATGGAG GGGAATACAGC |
IDT | None |
| Primers for qRT-PCR of GAPDH mRNA: (F)5′A GGTCGGTGTGAACGGATTTG, (R)5′TGTAGA CCATGTAGTTGAGGTCA |
IDT | None |
| Primers for PCR genotyping of total CCL27-KO mice: (F)5′GCCAAGAGTTAGAGCTCAGCTTC, (R)5′GGTGACAGTCCCCATCGG | IDT | None |
| Primers for PCR genotyping of CCL27a-WT allele: (F)5′CTTTAGCGCCGCAGCCGCCT, (R)5′CTATAGAAAGGACCTTGGACCCTCA | IDT | None |
| Primers for PCR genotyping CCL27a-KO allele: (F)5′ACTTGCTTTAAAAAACCTC CCACA, (R)5′CTGTGGATGGAGGTCTCAGCTGA |
IDT | None |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Na Xiong (xiongn@uthscsa.edu).
Materials availability
A new line of CCL27-knockout mice is generated and is available upon request of reagent from lead contact.
Experimental model and subject details
Mice
CCL27a-KO (C57BL/6N-CCL27a tm1(KOMP)Vlcg) mice were obtained from the UC-Davis KOMP Repository (https://www.komp.org/geneinfo.php?project=VG14813). Total CCL27-KO mice were generated with the help of the Gene Modification Facility of the Albert Einstein College of Medicine. CCR10-knockout(KO)/EGFP-knockin (KI) CCR10+/EGFP mice were previously described (Jin et al., 2010). In some experiments, CCL27a-KO and total CCL27-KO mice were crossed with CCR10-KO/EGFP-KI mice to generate CCL27a−/−CCR10+/EGFP and CCL27−/−CCR10+/EGFP mice for the purpose of reporting CCR10 expression with EGFP. All mice were on the C57BL/6 genetic background. Sex- and age-matched male and female mice were used. Littermates were used when possible. Mice from newborn (day 0) to adult stages (2–3 months) were used and were indicated in legends. All mouse experiments were performed in accordance with protocols approved by Institutional Animal Care and Use Committees of Pennsylvania State University and University of Texas Health Science Center at San Antonio.
Method details
Preparation, immunofluorescent staining and imaging analysis of skin sections
Mouse skin was washed in PBS and fixed in 4% PFA overnight at 4°C. Fixed skin was incubated in 10%, 20% and 30% sucrose solutions (4–12 h, 4°C) and frozen in OCT. Frozen skin was sectioned and placed on slides. For immunofluorescent staining, frozen skin slides were warmed at 60°C for 1h and then rehydrated for 10min. Slides were blocked in a PBS buffer containing 5% FBS and 0.3% Triton X-100 for 1h, and then incubated with unlabeled polyclonal goat anti-mouse CCL27 antibodies (R&D systems; AF725) or normal goat IgG control antibody (R&D systems; AB-108-C) in PBS containing 1% BSA and 0.3% Triton X-100 for 1h. Slides were then incubated with fluorescently labeled Alexa Fluor 647 chicken anti-goat secondary antibody (Invitrogen; A-21469) for 30min and counterstained with DAPI (Biolegend; 422801) for 3min. Alternatively, skin sections were stained with polyclonal goat anti-mouse CCL27 antibodies conjugated to nanoparticles that were labeled by DNA monomers, followed by the DNA-initiated polarization of fluorescently labeled DNA probes for increased sensitivities (Chen et al., 2013). Between each step, slides were washed with PBS 2-3 times. Stained slides were mounted with Prolong Diamond Antifade (Invitrogen; P36961) and cured overnight. Stained sections were analyzed with a Zeiss AXIO imager M1m or a Keyence BZ-X800 microscope equipped with BZ-X800 Analyzer software. CCL27 staining intensity was calculated by ImageJ software, taking the mean gray value of a randomly gated area of the upper dermis.
Analysis of localization of CCR10+ lymphocytes within skin hair follicles
Skin sections of mice carrying the CCR10-EGFP reporter were stained for CD3 and analyzed on a Keyence BZ-X800 microscope equipped with BZ-X800 Analyzer software. 20 images with hair follicles in view were taken for each sample at a 20X magnification. CCR10+ lymphocytes were identified as CD45+EGFP+DAPI+ cells. Cells were considered to be on the hair follicle if they were located within or touching the hair follicle structure. Cells were considered to be near the hair follicle if they were localized within 15μm of the edge of a hair follicle.
In situ hybridization with an antisense CCL27 RNA probe
In-situ hybridization staining to detect CCL27 transcripts in skin sections was carried out per the manufacturer’s instruction (Advanced Cell Diagnostics, INC; BaseScope Reagent Kit-Red). The anti-sense CCL27 RNA probe used recognizes the mRNA sequence that spans the signal peptide-coding exon 1b and exon II of the CCL27 gene. A probe recognizing the DapB gene (accession # EF191515) of a soil bacterial strain Bacillus subtilis SMY is used as a negative control.
RNA preparation for real-time RT-PCR analysis
RNA were isolated from skin or uterus tissues using TRIzol for the real-time RT-PCR analysis. Two or three replicates were tested for each RT-PCR analysis.
Isolation of lymphocytes, immunofluorescent staining, and flow cytometry
The skin, lungs and female reproductive tracts were digested in DMEM medium containing collagenase-1, collagenase-4, hyaluronidase and DNase I (Xia et al., 2014). Digested single cell suspensions were enriched for mononucleocytes by centrifugation through 40% and 80% percoll gradients. The trunk skin was used to isolate skin immune cells except in the IMQ experiments in which ears are treated and analyzed. For staining of surface molecules, cells were incubated with properly fluorescently labeled antibodies in a PBS solution containing 3% FBS for 30–45 min at 4°C. For intracellular Foxp3 staining, cells were first stained for surface molecules, then fixed with 4% paraformaldehyde, permeabilized with Foxp3/Transcription Factor Staining Buffer (eBioscience) and stained with anti-Foxp3 antibodies. For intracellular cytokine staining, cells were stimulated with PMA, ionomycin and Brefeldin A for 4hrs, followed by staining for surface molecule and then intracellular staining for cytokines. Stained cells were analyzed on BD LSRII or BD LSRFortessa (BD Biosciences, San Jose, CA). Data were analyzed with FlowJo software (BD Biosciences).
In vivo migration assay
Equal numbers of lymphocytes from skin-draining lymph nodes of CCR10+/EGFP (CD45.1+) mice were injected retro-orbitally into sex-matched CCL27+/+ and CCL27−/− (CD45.2+) mice. The skin and spleen of recipient mice were analyzed for donor cells two days post injection.
Topical application of imiquimod
The experiment procedure was adapted from the previously described protocol (van der Fits et al., 2009). Approximately 62.5μg of imiquimod (IMQ) cream were applied to the ears of adult mice (6–8 weeks old) for 7 consecutive days. Mice were observed for ear redness and thickness at the endpoint.
Hematoxylin and eosin (H&E) staining
Tissue was fixed in formalin, embedded in paraffin, sectioned and H&E stained. Stained tissue sections were viewed on a Keyence BZ-X800 microscope equipped with BZ-X800 Analyzer software.
Quantification and statistical analysis
Graphs were created and analyzed using Prism software. Statistical difference was determined by paired student t-test unless otherwise indicated. The data was presented as mean ± standard error of mean (SEM). The p value < 0.05 is considered significant.
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Numbers R01AR070887 and AR064831 (to N.X) and R01AR073364 (to Y.W.). The content is solely the responsibility of the authors and does not necessarily represent official views of the funding agencies. We thank Veronika Weaver for assistance. We thank the University of Texas Health Science Center of San Antonio Histology and Immunohistochemistry core facility and the Flow Cytometry Core Facility, the Pennsylvania State University Huck Institutes of the Life Sciences Flow Cytometry Facility, and the Microscopy Facility and Albert Einstein College of Medicine Gene Modification Facility for service and technical support.
Author contributions
M.L.D. designed and performed the experiments, analyzed the data, and wrote the manuscript. M.X., C.H., E.H.S., E.G., and L.J. performed the experiments. Y.W. and M.T.C. provided critical reagents. N.X. designed the experiments, analyzed the data, wrote the manuscript, and supervised the study.
Declaration of interests
Na Xiong has an US patent (# 10,588,941) that is related to this work. All the other authors declare no competing interests.
Published: June 17, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.104426.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Ali N., Zirak B., Rodriguez R.S., Pauli M.L., Truong H.A., Lai K., Ahn R., Corbin K., Lowe M.M., Scharschmidt T.C., et al. Regulatory T cells in skin facilitate epithelial stem cell differentiation. Cell. 2017;169:1119–1129.e11. doi: 10.1016/j.cell.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew D.P., Ruffing N., Kim C.H., Miao W., Heath H., Li Y., Murphy K., Campbell J.J., Butcher E.C., Wu L. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J. Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- Baekkevold E.S., Wurbel M.A., Kivisakk P., Wain C.M., Power C.A., Haraldsen G., Campbell J.J. A role for CCR4 in development of mature circulating cutaneous T helper memory cell populations. J. Exp. Med. 2005;201:1045–1051. doi: 10.1084/jem.20041059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird J.W., Nibbs R.J., Komai-Koma M., Connolly J.A., Ottersbach K., Clark-Lewis I., Liew F.Y., Graham G.J. ESkine, a novel beta-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J. Biol. Chem. 1999;274:33496–33503. doi: 10.1074/jbc.274.47.33496. [DOI] [PubMed] [Google Scholar]
- Boehncke W.H. Systemic inflammation and cardiovascular comorbidity in psoriasis patients: causes and consequences. Front. Immunol. 2018;9:579. doi: 10.3389/fimmu.2018.00579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt A.M., Perez-Lopez A., Ushach I., Catalan-Dibene J., Nuccio S.P., Chung L.K., Hernandez-Ruiz M., Carnevale C., Raffatellu M., Zlotnik A. CCL28 is involved in mucosal IgA responses, olfaction, and resistance to enteric infections. J. Interferon Cytokine Res. 2019;39:214–223. doi: 10.1089/jir.2018.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Shen X., Ding C., Qi C., Li K., Li X., Jala V.R., Zhang H.G., Wang T., Zheng J., Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., O'Connell D.J., Wurbel M.A. Cutting Edge: chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J. Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J.J., Ebsworth K., Ertl L.S., McMahon J.P., Newland D., Wang Y., Liu S., Miao Z., Dang T., Zhang P., et al. IL-17–Secreting γδ T cells are completely dependent upon CCR6 for homing to inflamed skin. J. Immunol. 2017;199:3129–3136. doi: 10.4049/jimmunol.1700826. [DOI] [PubMed] [Google Scholar]
- Casciano F., Diani M., Altomare A., Granucci F., Secchiero P., Banfi G., Reali E. CCR4(+) skin-tropic phenotype as a feature of central memory CD8(+) T cells in healthy subjects and psoriasis patients. Front. Immunol. 2020;11:529. doi: 10.3389/fimmu.2020.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Li S., Battig M.R., Wang Y. Programmable imaging amplification via nanoparticle-initiated DNA polymerization. Small. 2013;9:3944–3949. doi: 10.1002/smll.201300806. [DOI] [PubMed] [Google Scholar]
- Cochez P.M., Michiels C., Hendrickx E., Dauguet N., Warnier G., Renauld J.C., Dumoutier L. Ccr6 is dispensable for the development of skin lesions induced by imiquimod despite its effect on epidermal homing of IL-22-producing cells. J. Invest. Dermatol. 2017;137:1094–1103. doi: 10.1016/j.jid.2016.12.023. [DOI] [PubMed] [Google Scholar]
- Fletcher J.M., Moran B., Petrasca A., Smith C.M. IL-17 in inflammatory skin diseases psoriasis and hidradenitis suppurativa. Clin. Exp. Immunol. 2020;201:121–134. doi: 10.1111/cei.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Yang J., Xiong N. Cutting edge: skin CCR10+ CD8+ T cells support resident regulatory T cells through the B7.2/receptor Axis to regulate local immune homeostasis and response. J. Immunol. 2016;196:4859–4864. doi: 10.4049/jimmunol.1502662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Tumbar T., Guasch G. Socializing with the neighbors: stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gray E.E., Suzuki K., Cyster J.G. Cutting edge: identification of a motile IL-17–producing γδ T cell population in the dermis. J. Immunol. 2011;186:6091–6095. doi: 10.4049/jimmunol.1100427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson J.E., Ding J., Johnston A., Tejasvi T., Guzman A.M., Nair R.P., Voorhees J.J., Abecasis G.R., Elder J.T. Assessment of the psoriatic transcriptome in a large sample: additional regulated genes and comparisons with in vitro models. J. Invest. Dermatol. 2010;130:1829–1840. doi: 10.1038/jid.2010.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homey B., Dieu-Nosjean M.C., Wiesenborn A., Massacrier C., Pin J.J., Oldham E., Catron D., Buchanan M.E., Muller A., deWaal Malefyt R., et al. Up-regulation of macrophage inflammatory protein-3α/CCL20 and CC chemokine receptor 6 in psoriasis. J. Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- Homey B., Wang W., Soto H., Buchanan M.E., Wiesenborn A., Catron D., Muller A., McClanahan T.K., Dieu-Nosjean M.C., Orozco R., et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC) J. Immunol. 2000;164:3465–3470. doi: 10.4049/jimmunol.164.7.3465. [DOI] [PubMed] [Google Scholar]
- Homey B., Alenius H., Muller A., Soto H., Bowman E.P., Yuan W., McEvoy L., Lauerma A.I., Assmann T., Bunemann E., et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat. Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Hotz C., Boniotto M., Guguin A., Surenaud M., Jean-Louis F., Tisserand P., Ortonne N., Hersant B., Bosc R., Poli F., et al. Intrinsic defect in keratinocyte function leads to inflammation in hidradenitis suppurativa. J. Invest. Dermatol. 2016;136:1768–1780. doi: 10.1016/j.jid.2016.04.036. [DOI] [PubMed] [Google Scholar]
- Hromas R., Broxmeyer H.E., Kim C., Christopherson K., 2nd, Hou Y.H. Isolation of ALP, a novel divergent murine CC chemokine with a unique carboxy terminal extension. Biochem. Biophys. Res. Commun. 1999;258:737–740. doi: 10.1006/bbrc.1999.0507. [DOI] [PubMed] [Google Scholar]
- Hu S., Xiong N. Programmed downregulation of CCR6 is important for establishment of epidermal γδT cells by regulating their thymic egress and epidermal location. J. Immunol. 2013;190:3267–3275. doi: 10.4049/jimmunol.1202261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa-Mochizuki I., Kitaura M., Baba M., Nakayama T., Izawa D., Imai T., Yamada H., Hieshima K., Suzuki R., Nomiyama H., Yoshie O. Molecular cloning of a novel CC chemokine, interleukin-11 receptor alpha-locus chemokine (ILC), which is located on chromosome 9p13 and a potential homologue of a CC chemokine encoded by molluscum contagiosum virus. FEBS Lett. 1999;460:544–548. doi: 10.1016/s0014-5793(99)01406-4. [DOI] [PubMed] [Google Scholar]
- Jarmin D.I., Rits M., Bota D., Gerard N.P., Graham G.J., Clark-Lewis I., Gerard C. Cutting edge: identification of the orphan receptor G-protein-coupled receptor 2 as CCR10, a specific receptor for the chemokine ESkine. J. Immunol. 2000;164:3460–3464. doi: 10.4049/jimmunol.164.7.3460. [DOI] [PubMed] [Google Scholar]
- Jiang X., Campbell J.J., Kupper T.S. Embryonic trafficking of γδ T cells to skin is dependent on E/P selectin ligands and CCR4. Proc. Natl. Acad. Sci. U S A. 2010;107:7443–7448. doi: 10.1073/pnas.0912943107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y., Xia M., Sun A., Saylor C.M., Xiong N. CCR10 is important for the development of skin-specific γδT cells by regulating their migration and location. J. Immunol. 2010;185:5723–5731. doi: 10.4049/jimmunol.1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joost S., Zeisel A., Jacob T., Sun X., La Manno G., Lonnerberg P., Linnarsson S., Kasper M. Single-cell transcriptomics reveals that differentiation and spatial signatures shape epidermal and hair follicle heterogeneity. Cell Syst. 2016;3:221–237.e9. doi: 10.1016/j.cels.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N., Koike S., Watanabe S. IL-17 suppresses TNF-alpha-induced CCL27 production through induction of COX-2 in human keratinocytes. J. Allergy Clin. Immunol. 2005;116:1144–1150. doi: 10.1016/j.jaci.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Karakawa M., Komine M., Hanakawa Y., Tsuda H., Sayama K., Tamaki K., Ohtsuki M. CCL27 is downregulated by interferon gamma via epidermal growth factor receptor in normal human epidermal keratinocytes. J. Cell. Physiol. 2014;229:1935–1945. doi: 10.1002/jcp.24643. [DOI] [PubMed] [Google Scholar]
- Kelly G., Hughes R., McGarry T., van den Born M., Adamzik K., Fitzgerald R., Lawlor C., Tobin A.M., Sweeney C.M., Kirby B. Dysregulated cytokine expression in lesional and nonlesional skin in hidradenitis suppurativa. Br. J. Dermatol. 2015;173:1431–1439. doi: 10.1111/bjd.14075. [DOI] [PubMed] [Google Scholar]
- Kim T.G., Jee H., Fuentes-Duculan J., Wu W.H., Byamba D., Kim D.S., Kim D.Y., Lew D.H., Yang W.I., Krueger J.G., Lee M.G. Dermal clusters of mature dendritic cells and T cells are associated with the CCL20/CCR6 chemokine system in chronic psoriasis. J. Invest. Dermatol. 2014;134:1462–1465. doi: 10.1038/jid.2013.534. [DOI] [PubMed] [Google Scholar]
- Korman N.J. Management of psoriasis as a systemic disease: what is the evidence? Br. J. Dermatol. 2020;182:840–848. doi: 10.1111/bjd.18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel E.J., Kim C.H., Lazarus N.H., Vierra M.A., Soler D., Bowman E.P., Butcher E.C. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J. Clin. Invest. 2003;111:1001–1010. doi: 10.1172/jci17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus N.H., Kunkel E.J., Johnston B., Wilson E., Youngman K.R., Butcher E.C. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J. Immunol. 2003;170:3799–3805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- Lebre M.C., van der Aar A.M., van Baarsen L., van Capel T.M., Schuitemaker J.H., Kapsenberg M.L., de Jong E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Invest. Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- Li C., Xu M., Coyne J., Wang W.B., Davila M.L., Wang Y., Xiong N. Psoriasis-associated impairment of CCL27/CCR10-derived regulation leads to IL-17A/IL-22-producing skin T-cell overactivation. J. Allergy Clin. Immunol. 2021;147:759–763.e9. doi: 10.1016/j.jaci.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson W., Lobo M., Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv. Ther. 2020;37:2017–2033. doi: 10.1007/s12325-020-01346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K., Nagakubo D., Yamamoto S., Shigeta A., Tomida S., Fujita M., Hirata T., Tsunoda I., Nakayama T., Yoshie O. CCL28-Deficient mice have reduced IgA antibody-secreting cells and an altered microbiota in the colon. J. Immunol. 2018;200:800–809. doi: 10.4049/jimmunol.1700037. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Kitahata K., Kaibori Y., Arima Y., Iwama A., Ito M., Hara Y., Nagakubo D., Quan Y.S., Kamiyama F., et al. CCR4 involvement in the expansion of T helper type 17 cells in a mouse model of psoriasis. J. Invest. Dermatol. 2021;141:1985–1994. doi: 10.1016/j.jid.2020.12.034. [DOI] [PubMed] [Google Scholar]
- Melnik B.C., John S.M., Chen W., Plewig G. T helper 17 cell/regulatory T-cell imbalance in hidradenitis suppurativa/acne inversa: the link to hair follicle dissection, obesity, smoking and autoimmune comorbidities. Br. J. Dermatol. 2018;179:260–272. doi: 10.1111/bjd.16561. [DOI] [PubMed] [Google Scholar]
- Menzies F.M., Oldham R.S., Waddell C., Nelson S.M., Nibbs R.J.B. A comprehensive profile of chemokine gene expression in the tissues of the female reproductive tract in mice. Immunol. Invest. 2020;49:264–286. doi: 10.1080/08820139.2019.1655573. [DOI] [PubMed] [Google Scholar]
- Mildner M., Prior M., Gschwandtner M., Schuster C., Tschachler E., Elbe-Burger A. Epidermal CCL27 expression is regulated during skin development and keratinocyte differentiation. J. Invest. Dermatol. 2014;134:855–858. doi: 10.1038/jid.2013.394. [DOI] [PubMed] [Google Scholar]
- Mirshahpanah P., Li Y.Y.Y., Burkhardt N., Asadullah K., Zollner T.M. CCR4 and CCR10 ligands play additive roles in mouse contact hypersensitivity. Exp. Dermatol. 2008;17:30–34. doi: 10.1111/j.1600-0625.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- Morales J., Homey B., Vicari A.P., Hudak S., Oldham E., Hedrick J., Orozco R., Copeland N.G., Jenkins N.A., McEvoy L.M., Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc. Natl. Acad. Sci. U S A. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagao K., Kobayashi T., Moro K., Ohyama M., Adachi T., Kitashima D.Y., Ueha S., Horiuchi K., Tanizaki H., Kabashima K., et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat. Immunol. 2012;13:744–752. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H., Gunn M.D. Gene duplications at the chemokine locus on mouse chromosome 4: multiple strain-specific haplotypes and the deletion of secondary lymphoid-organ chemokine and EBI-1 ligand chemokine genes in the plt mutation. J. Immunol. 2001;166:361–369. doi: 10.4049/jimmunol.166.1.361. [DOI] [PubMed] [Google Scholar]
- Paus R., Bulfone-Paus S., Bertolini M. Hair follicle immune privilege revisited: the key to alopecia areata management. J. Investig. Dermatol. Symp. Proc. 2018;19:S12–S17. doi: 10.1016/j.jisp.2017.10.014. [DOI] [PubMed] [Google Scholar]
- Pescitelli L., Ricceri F., Prignano F. Hidradenitis suppurativa and associated diseases. G. Ital. Dermatol. Venereol. 2018;153:8–17. doi: 10.23736/S0392-0488.17.05772-8. [DOI] [PubMed] [Google Scholar]
- Puig L., Costanzo A., Munoz-Elias E.J., Jazra M., Wegner S., Paul C.F., Conrad C. The biological basis of disease recurrence in psoriasis: a historical perspective and current models. Br. J. Dermatol. 2022;186:773–781. doi: 10.1111/bjd.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L., Huang D., Chen C.Y., Wang R., Shen L., Shen Y., Hunt R., Estep J., Haynes B.F., Jacobs W., Jr., et al. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1α, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-β, TIM1,and TLR2 but low antigen-specific cellular responses. J. Infect. Dis. 2008;198:1514–1519. doi: 10.1086/592448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaranta M., Knapp B., Garzorz N., Mattii M., Pullabhatla V., Pennino D., Andres C., Traidl-Hoffmann C., Cavani A., Theis F.J., et al. Intraindividual genome expression analysis reveals a specific molecular signature of psoriasis and eczema. Sci. Transl. Med. 2014;6:244ra290. doi: 10.1126/scitranslmed.3008946. [DOI] [PubMed] [Google Scholar]
- Reiss Y., Proudfoot A.E., Power C.A., Campbell J.J., Butcher E.C. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J. Exp. Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riis J.L., Johansen C., Vestergaard C., Bech R., Kragballe K., Iversen L. Kinetics and differential expression of the skin-related chemokines CCL27 and CCL17 in psoriasis, atopic dermatitis and allergic contact dermatitis. Exp. Dermatol. 2011;20:789–794. doi: 10.1111/j.1600-0625.2011.01323.x. [DOI] [PubMed] [Google Scholar]
- Riis J.L., Johansen C., Vestergaard C., Otkjaer K., Kragballe K., Iversen L. CCL27 expression is regulated by both p38 MAPK and IKKβ signalling pathways. Cytokine. 2011;56:699–707. doi: 10.1016/j.cyto.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Robert R., Ang C., Sun G., Juglair L., Lim E.X., Mason L.J., Payne N.L., Bernard C.C., Mackay C.R. Essential role for CCR6 in certain inflammatory diseases demonstrated using specific antagonist and knockin mice. JCI Insight. 2017;2:e94821. doi: 10.1172/jci.insight.94821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabat R., Jemec G.B.E., Matusiak L., Kimball A.B., Prens E., Wolk K. Hidradenitis suppurativa. Nat. Rev. Dis. Primers. 2020;6:18. doi: 10.1038/s41572-020-0149-1. [DOI] [PubMed] [Google Scholar]
- Sahmatova L., Sugis E., Sunina M., Hermann H., Prans E., Pihlap M., Abram K., Rebane A., Peterson H., Peterson P., et al. Signs of innate immune activation and premature immunosenescence in psoriasis patients. Sci. Rep. 2017;7:7553. doi: 10.1038/s41598-017-07975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock I., Reinhardt A., Ravens S., Binz C., Wilharm A., Martins J., Oberdorfer L., Tan L., Lienenklaus S., Zhang B., et al. Genetic models reveal origin, persistence and non-redundant functions of IL-17–producing γδ T cells. J. Exp. Med. 2018;215:3006–3018. doi: 10.1084/jem.20181439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharschmidt T.C., Vasquez K.S., Pauli M.L., Leitner E.G., Chu K., Truong H.A., Lowe M.M., Sanchez Rodriguez R., Ali N., Laszik Z.G., et al. Commensal microbes and hair follicle morphogenesis coordinately drive Treg migration into neonatal skin. Cell Host Microbe. 2017;21:467–477.e5. doi: 10.1016/j.chom.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M.R., Paus R. Deciphering the functions of the hair follicle infundibulum in skin physiology and disease. Cell Tissue Res. 2014;358:697–704. doi: 10.1007/s00441-014-1999-1. [DOI] [PubMed] [Google Scholar]
- Sennepin A., Real F., Duvivier M., Ganor Y., Henry S., Damotte D., Revol M., Cristofari S., Bomsel M. The human penis is a genuine immunological effector site. Front. Immunol. 2017;8:1732. doi: 10.3389/fimmu.2017.01732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Wu X., Wu C.Y., Singh S.P., Law T., Yamada D., Huynh M., Liakos W., Yang G., Farber J.M., et al. Bile acids improve psoriasiform dermatitis through inhibition of IL-17A expression and CCL20-CCR6-mediated trafficking of T cells. J. Invest. Dermatol. 2021;142:1381–1390.e11. doi: 10.1016/j.jid.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K., Nomiyama H., Yoshie O., Tanase S. Genome diversification mechanism of rodent and Lagomorpha chemokine genes. BioMed Res. Int. 2013;2013:1–9. doi: 10.1155/2013/856265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonetti O., Lucarini G., Bernardini M.L., Simoncini C., Biagini G., Offidani A. Expression of vascular endothelial growth factor, apoptosis inhibitors (survivin and p16) and CCL27 in alopecia areata before and after diphencyprone treatment: an immunohistochemical study. Br. J. Dermatol. 2004;150:940–948. doi: 10.1111/j.1365-2133.2004.05881.x. [DOI] [PubMed] [Google Scholar]
- Stockenhuber K., Hegazy A.N., West N.R., Ilott N.E., Stockenhuber A., Bullers S.J., Thornton E.E., Arnold I.C., Tucci A., Waldmann H., et al. Foxp3(+) T reg cells control psoriasiform inflammation by restraining an IFN-I-driven CD8(+) T cell response. J. Exp. Med. 2018;215:1987–1998. doi: 10.1084/jem.20172094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tubo N.J., McLachlan J.B., Campbell J.J. Chemokine receptor requirements for epidermal T-cell trafficking. Am. J. Pathol. 2011;178:2496–2503. doi: 10.1016/j.ajpath.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Fits L., Mourits S., Voerman J.S.A., Kant M., Boon L., Laman J.D., Cornelissen F., Mus A.M., Florencia E., Prens E.P., Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- Wang W., Soto H., Oldham E.R., Buchanan M.E., Homey B., Catron D., Jenkins N., Copeland N.G., Gilbert D.J., Nguyen N., et al. Identification of a novel chemokine (CCL28), which binds CCR10 (GPR2) J. Biol. Chem. 2000;275:22313–22323. doi: 10.1074/jbc.m001461200. [DOI] [PubMed] [Google Scholar]
- Westgate G.E., Craggs R.I., Gibson W.T. Immune privilege in hair growth. J. Invest. Dermatol. 1991;97:417–420. doi: 10.1111/1523-1747.ep12481002. [DOI] [PubMed] [Google Scholar]
- Weston C.A., Rana B.M.J., Cousins D.J. Differential expression of functional chemokine receptors on human blood and lung group 2 innate lymphoid cells. J. Allergy Clin. Immunol. 2019;143:410–413.e9. doi: 10.1016/j.jaci.2018.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick N., Haluza D., Gurnhofer E., Raab I., Kasimir M.T., Prinz M., Steiner C.W., Reinisch C., Howorka A., Giovanoli P., et al. Lymphatic precollectors contain a novel, specialized subpopulation of podoplanin low, CCL27-expressing lymphatic endothelial cells. Am. J. Pathol. 2008;173:1202–1209. doi: 10.2353/ajpath.2008.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia M., Hu S., Fu Y., Jin W., Yi Q., Matsui Y., Yang J., McDowell M.A., Sarkar S., Kalia V., Xiong N. CCR10 regulates balanced maintenance and function of resident regulatory and effector T cells to promote immune homeostasis in the skin. J. Allergy Clin. Immunol. 2014;134:634–644.e10. doi: 10.1016/j.jaci.2014.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong N., Fu Y., Hu S., Xia M., Yang J. CCR10 and its ligands in regulation of epithelial immunity and diseases. Protein Cell. 2012;3:571–580. doi: 10.1007/s13238-012-2927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Hu S., Zhao L., Kaplan D.H., Perdew G.H., Xiong N. Selective programming of CCR10(+) innate lymphoid cells in skin-draining lymph nodes for cutaneous homeostatic regulation. Nat. Immunol. 2016;17:48–56. doi: 10.1038/ni.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Restori K.H., Xu M., Song E.H., Zhao L., Hu S., Lyu P., Wang W.B., Xiong N. Preferential perinatal development of skin-homing NK1.1(+) innate lymphoid cells for regulation of cutaneous microbiota colonization. iScience. 2020;23:101014. doi: 10.1016/j.isci.2020.101014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S., Wu X., Zhou Y., Han D., Anderson L.S., Simon S.I., Hwang S.T., Imai Y. Is CCR6 required for the development of psoriasiform dermatitis in mice? J. Invest. Dermatol. 2019;139:485–488. doi: 10.1016/j.jid.2018.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L., Hu S., Davila M.L., Yang J., Lin Y.D., Albanese J.M., Lo Y., Wang Y., Kennett M.J., Liu Q., Xiong N. Coordinated co-migration of CCR10(+) antibody-producing B cells with helper T cells for colonic homeostatic regulation. Mucosal Immunol. 2020;14:420–430. doi: 10.1038/s41385-020-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zouboulis C.C., Benhadou F., Byrd A.S., Chandran N.S., Giamarellos-Bourboulis E.J., Fabbrocini G., Frew J.W., Fujita H., Gonzalez-Lopez M.A., Guillem P., et al. What causes hidradenitis suppurativa ?-15 years after. Exp. Dermatol. 2020;29:1154–1170. doi: 10.1111/exd.14214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.