Abstract
Background
Coronavirus disease 19 (COVID-19) is a recently described infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Iran was the first country where the SARS-Cov-2 was detected in the Middle East. In the current study, we aimed to evaluate the clinical, radiological and laboratory findings in hospitalized COVID-19 confirmed cases in Iran.
Methods
The clinical manifestations, radiological data, laboratory findings, and the underlying diseases of the patients with COVID-19 were obtained from electronic medical records. Next, this information was compared in discharged and dead patients.
Results
Overall, 4028 patients with COVID-19 including 3088 discharged, 778 dead, and 162 still hospitalized patients were enrolled in this study. The highest percentage of people who recovered (55%) was between 30 and 60 years old and the highest percentage of deaths (74.4%) was more than 60 years old. Based on demographic data, 50.05% were female and 49.95% were male. Clinical evaluations revealed that dyspnea (56.9%), cough (31.4%) and fever (17.8%) were the most manifestations. Comorbidities were significantly higher in the dead group. Laboratory analysis revealed abnormalities in lymphocyte count (LYM), erythrocyte sedimentation rate (ESR), and inflammatory biomarkers such as C-reactive protein (CRP). The most prevalent computed tomography (CT) scan data were ground-glass opacity (GGO) (30.5%) and consolidation (9.4%).
Conclusions
Laboratory parameters and clinical and radiological findings help to evaluate the follow-up of the disease in patients. Age and comorbidities are factors that predispose people to COVID-19. Further research is needed to evaluate the effects of various factors on the progression of COVID-19 infection.
Keywords: COVID-19, Iran, Clinical signs, SARS-CoV-2, Laboratory findings
Abstract
Antecedentes
La enfermedad por coronavirus de 19 (COVID-19) es una enfermedad infecciosa recientemente descrita causada por el síndrome respiratorio agudo severo por coronavirus 2 (SARS-CoV-2). Irán fue el primer país de Oriente Medio donde se detectó SARS-Cov-2. En el estudio actual, nuestro objetivo fue evaluar los hallazgos clínicos, radiológicos y de laboratorio en pacientes hospitalizados con confirmación de COVID-19 en Irán.
Métodos
Se obtuvieron las manifestaciones clínicas, los datos radiológicos, los hallazgos de laboratorio y las enfermedades subyacentes de los registros clínicos electrónicos. Seguidamente, se comparó esta información con los pacientes dados de alta y fallecidos.
Resultados
A nivel global, se incluyó en este estudio a 4.028 pacientes con COVID-19, de los cuales 3.088 habían recibido el alta, 778 habían fallecido, y 162 seguían hospitalizados. El mayor porcentaje de recuperaciones (55%) se produjo entre las personas de 30 a 60 años, y el mayor porcentaje de muertes (74,4%) se dio en los mayores de 60 años. Sobre la base de los datos demográficos, el 50,05% fueron mujeres y el 49,95% varones. Las evaluaciones clínicas revelaron que la disnea (56,9%), la tos (31,4%) y la fiebre (17,8%) fueron las manifestaciones más prevalentes. Las comorbilidades fueron significativamente más elevadas en el grupo de fallecidos. Las analíticas revelaron anomalías en cuanto a recuento linfocitario, tasa de sedimentación eritrocitaria (ESR), y biomarcadores inflamatorios tales como proteína C reactiva (PCR). Los datos procedentes de la tomografía computarizada (TC) fueron opacidad en vidrio esmerilado (GGO) (30,5%) y consolidación (9,4%).
Conclusiones
Los parámetros de laboratorio y los hallazgos clínicos y radiológicos ayudan a evaluar el seguimiento de la enfermedad en los pacientes. La edad y las comorbilidades son factores que predisponen a las personas a la COVID-19. Es necesaria más investigación para evaluar los efectos de los diversos factores en la progresión de la infección por COVID-19.
Palabras clave: COVID-19, Irán, Signos clínicos, SARS-CoV-2, Hallazgos de laboratorio
Introduction
Coronavirus disease 19 (COVID-19), initially appeared in Wuhan City of China in late 2019, is a recently described infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 , 2 COVID-19 represents a particularly aggressive form3; however, for other coronaviruses, at least two epidemics have been reported over the last two decades.4 As stated by the World Health Organization (WHO), COVID-19 outbreak is a public health emergency of international concern.5 , 6 The mortality rate of critically ill COVID-19 patients is extremely high.7 Age and comorbidities, such as hypertension (HTN) and chronic cardiac disease, were the main risk factors for the mortality of COVID-19 patients.8 , 9
SARS-CoV-2 infection is asymptomatic or mild, but in most cases, it resembles the common cold.10 , 11 In more severe cases, the infection may clinically manifest as interstitial pneumonia with fever, cough, dyspnea, and bilateral infiltrates on chest imaging.12 , 13 It also may progress to acute respiratory distress syndrome, multiple-organ failure, and even death, likely as a consequence of excessive activation of the immune system that leads to a cytokine storm.14 According to the clinical classification method, patients are divided into four (ordinary, mild, severe, and critically ill) types considering the severity of the disease.15 In some situations, asymptomatic carriers with positive reverse transcription-polymerase chain reaction (RT-PCR) tend to have normal health conditions during the laboratory examination, and others show abnormality. Therefore, discriminating their health status from other infected patients complicates the diagnóstico.16 Significant elevation in the hepatic enzymes and serum creatinine (Cr) and also reduction in lymphocytes with high C-reactive protein (CRPs) are important markers for the severity. The most common blood test abnormalities include increased CRP (87%), decreased lymphocyte count (LYM) (68%), and enhanced lactate dehydrogenase (LDH) (69%). In addition, abnormal laboratory findings entail decreased albumin (43%), increased aspartate aminotransferase (AST) (47%), and elevated Cr (10%).17
A chest computed tomography (CT) can help determine the stage of temporary illness and severity of COVID-19 pneumonia. In the early stage of viral replication (days 0–4), turbidity diffuse of the ground-glass opacity (GGO) is predominant. In the progressive stage (days 5–8), crazy-paving patterns show a raise in the uptake of inflammatory cells to the lung interstitium. The peak stage (days 10–13) is characterized by consolidation with fibrosis and diffuse alveolar damage. These radiologic lesions are also observed in other viral pneumonia and noninfectious inflammatory lung diseases, but in a pandemic context, they may have diagnostic potential for infection with SARS-CoV-2.18
The purpose of this study was to compare demographic, clinical, laboratory and radiological findings of fatal and recovered COVID-19 cases in Iran.
Material and methods
Study design
The present single-center retrospective descriptive investigation was performed on COVID-19 cases admitted to Vasei Hospital, Sabzevar, Iran. The approach to the disease was in conformity with the national health instructions, adapted from the WHO guidelines, as well as based on the latest studies on COVID-19.19
Inclusion/exclusion criteria
The inclusion criteria included all patients who were hospitalized for COVID-19 from 20 February 2020 to 21 September 2021, cases whose clinical, laboratory, and radiological information were accessible in the Registration Center, and those who were tested positive for RT-PCR. Patients whose demographic information, laboratory tests, clinical signs, and/or radiological findings were not available in the Registration System were excluded from the study. Also, patients with hematological disorders were not included in the study.
Ethical considerations
The current study was approved by the Ethics Committee of Sabzevar University of Medical Sciences, Sabzevar, Iran (code of ethics: IR.MEDSAB.REC.1400.114). The patients' information was kept confidential.
Clinical assessment
Cases with fever, rhinorrhea, sore throat, cough, and respiratory distress were considered as patients suspected of COVID-19. The disease was diagnosed based on clinical and chest examination, laboratory findings, and RT-PCR test.20 The clinical diagnóstico was determined by radiographic features of the lung, and COVID-19 diagnóstico was confirmed using RT-PCR with throat and nose swab from the upper respiratory tract.
Laboratory assessment
Peripheral venous blood samples were collected at the time of admission. Routine blood tests, including the counts of red and white blood cells (RBC and WBC), leukocyte subtypes, hematocrit (HCT), hemoglobin (Hb), and platelet (PLT), were performed using an automated hematology analyzer (Sysmex Corporation, Kobe, Japan).16 Collected laboratory data included PLT, LYM, and neutrophil counts (NEU), serum urea, creatinine (Cr) and albumin levels, erythrocyte sedimentation rate (ESR), and CRP.
CT image acquisition
Radiological evaluations were performed according to CT images. The CT scan was performed for the COVID-19 patients who had respiratory problems. Two expert radiologists assessed the presence of any radiological abnormality based on the evidence or explanations in the medical records and finally re-examined the results.
Statistical analysis
Descriptive statistics (e.g. mean, frequency tables, standard deviation, and variance), and analytical tests (Chi square, Pierson correlation coefficient test, and ANOVA) were calculated using SPSS version 26. The probability level (p) of ≤ 0.05 was considered statistically significant.
Results
Overall, 4028 patients with COVID-19 including 3088 discharged, 778 dead, and 162 still hospitalized patients were enrolled in this study. Among these patients, the gender distribution was 50% male and 49% female. Moreover, 103 (78%) cases were adults, and 29 (22%) cases were the elderly. The patients were partitioned into diverse age groups: 579 (74.4%) of deaths were > 60 years old, and 191 (24.6%) of deaths were between 30 and 60 years. Table 1 provides detailed information on the demographic characteristics of the two groups.
Table 1.
Demographic information of patients with COVID-19.
| Variable | Total (%) N(4028) |
Death (%) N(778) |
Live (%) N(3088) |
P-value | |
|---|---|---|---|---|---|
| Age | 30 > 30–60 > 60 |
230(5.8) 1956(48.5) 1842(45.7) |
8(1.0) 191(24.6) 579(74.4) |
207(6.7) 1698(55.0) 1183(38.3) |
< 0.001 |
| Gender | Female Male |
2016(50.05) 2012(49.95) |
355(45.6) 423(54.4) |
1583(51.3) 1505(48.7) |
0.016 |
| Marital status | Married Single Deceased wife |
3563(88.3) 218(5.5) 247(6.2) |
646(83.0) 32(4.1) 100(12.9) |
2781(90.1) 174(5.6) 133(4.3) |
< 0.001 |
| Job | Workless Student Employed Not defined |
2505(62.2) 27(0.7) 1450(36.0) 46(1.1) |
553(71.1) 1(0.1) 211(27.1) 13(1.7) |
1847(59.8) 24(0.8) 1185(38.4) 32(1.0) |
0.001 |
| Education | Unlettered High school or less University Not defined |
754(18.7) 1202(29.85) 281(7.0) 1791(44.45) |
227(29.2) 172(22.1) 21(2.7) 358(46.0) |
490(15.9) 988(32.0) 240(7.8) 1370(44.4) |
< 0.001 |
| Location | Villager Townspeople |
1206(29.94) 2820(69.96) |
235(30.2) 543(69.8) |
925(30.0) 2163(70.0) |
0.012 |
| CRP | Negative Positive Not defined |
296(7.35) 2016(50.05) 1716(42.6) |
54(6.9) 360(46.3) 364(46.8) |
229(7.4) 1567(50.7) 1292(41.8) |
0.020 |
Abbreviations: CRP: C reactive protein; N: number.
Table 2 outlines the primary manifestations related to the disease. Dyspnea (56.9%), cough (31.4%), and fever (17.8%) were the most frequent symptoms reported by the patients and physician. These manifestations were significantly differed in death and live groups (p < 0.05). Less common symptoms detected in patients included myalgia (8.3%), sore throat (0.8%), chills (5.8%), anorexia (4.8%), weakness (23.6%), nausea (7.8%), headache (3.6%), chest pain (1.8%), diarrhea (1.9%), vomiting (2.9%), and confusion (1.5%). All of the patients confirmed a decrease in the saturation of peripheral oxygen (SpO2) level. The vital signs of patients with COVID-19 are summarized in Table 3 . The data on the underlying diseases, including diabetes, HTN, hyperlipidemia (HLP), heart failure, lung diseases, surgery, cancer, addiction, and pregnancy are depicted in Table 4 . In total, 1079 (26.8%) and 726 (18%) cases had HTN and diabetes, respectively.
Table 2.
Clinical features of patients with COVID-19.
| Variable | Total (%) N(4028) |
Death (%) N(778) |
Live (%) N(3088) |
P-value | |
|---|---|---|---|---|---|
| Dyspnea | Yes No |
2294(56.9) 1734(43.1) |
491(63.1) 287(36.9) |
1700(55.1) 1388(44.9) |
< 0.001 |
| Cough | Yes No |
1266(31.43) 2762(68.57) |
141(18.1) 637(81.9) |
1085(35.1) 2003(64.9) |
< 0.001 |
| Fever | Yes No |
718(17.8) 3310(82.2) |
128(16.5) 650(83.5) |
563(18.2) 2525(81.8) |
0.011 |
| Myalgia | Yes No |
336(8.34) 3692(91.56) |
32(4.1) 746(95.9) |
288(9.3) 2800(90.7) |
0.005 |
| Sore throat | Yes No |
33(0.8) 3995(99.2) |
3(0.4) 775(99.6) |
28(0.9) 3060(99.1) |
< 0.001 |
| Chills | Yes NO |
232(5.8) 3796(94.2) |
30(3.9) 748(96.1) |
191(6.2) 2897(93.8) |
0.002 |
| Anorexia | Yes No |
194(4.8) 3834(95.2) |
24(3.1) 754(96.9) |
165(5.3) 2923(94.7) |
< 0.001 |
| Weakness | Yes NO |
952(23.6) 3076(76.4) |
182(23.4) 596(76.6) |
739(23.9) 2349(76.1) |
0.011 |
| Nausea | Yes NO |
313(7.8) 3715(92.2) |
27(3.5) 751(96.5) |
274(8.9) 2814(91.1) |
< 0.001 |
| Headache | Yes NO |
145(3.6) 3883(96.4) |
7(0.9) 771(99.1) |
132(4.3) 2956(95.7) |
< 0.001 |
| Chest pain | Yes No |
72(1.8) 3956(98.1) |
4(0.5) 774(99.5) |
64(2.1) 3024(97.9) |
0.004 |
| Diarrhea | Yes No |
77(1.9) 3951(98.0) |
13(1.7) 765(98.3) |
60(1.9) 3028(98.1) |
0.003 |
| Vomiting | Yes No |
116(2.9) 3912(97.1) |
14(1.8) 764(98.2) |
98(3.2) 2990(96.8) |
< 0.001 |
| Confusion | Yes No |
59(1.5) 3969(98.5) |
4(0.5) 774(99.5) |
51(1.7) 3037(98.3) |
< 0.001 |
| Consciousness | Yes No |
3468(86.1) 560(13.9) |
554(71.2) 224(28.8) |
2774(89.8) 314(10.2) |
0.015 |
Table 3.
Vital signs of patients with COVID-19.
| Finding | Total mean(± SD) N(4028) |
Death mean(± SD) N(778) |
Live mean(± SD) N(3088) |
P-value | |
|---|---|---|---|---|---|
| Blood Pressure | Upper lower |
123.07(± 0.29) 79.0(0.037) |
139.50(± 0.04) 86.0(0.13) |
110.89(± 0.17) 73.0(0.07) |
0.111 |
| Pulse Rate | 96.43(± 0.47) | 98.49(± 1.56) | 95.56(± 0.33) | < 0.001 | |
| Respiratory Rate | 22.45(± 0.45) | 24.87(± 1.66) | 21.60(± 0.19) | 0.021 | |
| Temperature | 37.37(± 0.04) | 37.51(± 0.11) | 37.34(± 0.05) | < 0.001 | |
| SpO2 | 88.88(± 0.44) | 79.11(± 0.54) | 91.40(± 0.54) | < 0.001 |
SpO2: oxygen saturation; N: number; SD; standard deviation.
Table 4.
Underlying diseases of patients with COVID-19.
| Variable | Total (%) N(4028) |
Death (%) N(778) |
Live (%) N(3088) |
P-value | |
|---|---|---|---|---|---|
| Diabetes | Yes No |
726(18.0) 3302(82.0) |
184(23.7) 594(76.3) |
507(16.4) 2581(83.6) |
< 0.001 |
| HTN | Yes No |
1079(26.8) 2949(73.2) |
295(37.9) 483(62.1) |
741(24.0) 2347(76.0) |
< 0.001 |
| HLP | Yes No |
473(11.7) 3555(88.1) |
110(14.1) 668(85.9) |
343(11.1) 2745(88.9) |
0.011 |
| Heart failure | Yes No |
430(10.7) 3598(89.2) |
131(16.8) 647(83.2) |
280(9.1) 2808(90.9) |
0.002 |
| Lung disease | Yes No |
254(6.3) 3774(93.7) |
75(9.6) 703(90.4) |
165(5.3) 2923(94.7) |
0.015 |
| Surgery | Yes No |
502(12.5) 3526(87.5) |
109(14.0) 669(86.0) |
377(12.2) 2711(87.8) |
0.006 |
| Cancer | Yes No |
46(1.1) 3982(98.9) |
16(2.1) 762(97.9) |
29(0.9) 3059(99.1) |
< 0.001 |
| Addiction | Yes NO |
245(6.1) 3783(93.9) |
71(9.1) 707(90.9) |
159(5.1) 2929(94.9) |
0.003 |
| Pregnancy | Yes No |
71(1.8) 3957(98.2) |
1(0.1) 777(99.9) |
66(2.2) 3022(97.8) |
0.003 |
| Other past medical | Yes No |
793(19.7) 3235(80.3) |
191(24.6) 586(75.3) |
567(18.4) 2455(79.5) |
< 0.001 |
Abbreviations: HTN: Hypertension; HLP: hyperlipidemia; N: number.
The details of laboratory data are demonstrated in Table 5 . There was a significant difference in parameters such as WBC, Hb, PLT, urea, Cr, sodium (Na +), potassium(K), and ESR between alive and dead groups (p < 0.05). Lymphocyte count was significantly lower in death group (13.12 ± 0.44) than in live group (18.77 ± 0.22).
Table 5.
Laboratory findings of patients with COVID-19.
| Findings | Mean Total(± SD) N(4028) |
Mean Death(± SD) N(778) |
Mean Live(± SD) N(3088) |
P-value |
|---|---|---|---|---|
| WBC (103) | 7.53(± 0.08) | 9.67(± 0.25) | 6.98(± 0.08) | 0.001 |
| NEU (%) | 80.22(± 0.20) | 84.85(± 0.46) | 78.98(± 0.23) | 0.001 |
| LYM (%) | 17.58(± 0.19) | 13.12(± 0.44) | 18.77(± 0.22) | < 0.001 |
| Hb | 13.44(± 0.05) | 12.9(± 0.14) | 13.58(± 0.06) | 0.002 |
| PLT | 200.71(± 1.88) | 189.13(± 3.94) | 202.85(± 2.08) | < 0.001 |
| Urea | 47.64(± 0.66) | 75.73(± 2.19) | 40.74(± 0.53) | < 0.001 |
| Cr | 1.26(± 0.01) | 1.75(± 0.06) | 1.14(± 0.14) | < 0.001 |
| Na | 137.85(± 0.11) | 138.34(± 0.26) | 137.73(± 0.12) | 0.016 |
| K | 4.45(± 0.05) | 4.6(± 0.06) | 4.41(± 0.06) | 0.002 |
| ESR | 46.48(± 0.52) | 50.51(± 1.23) | 45.41(± 0.57) | < 0.001 |
Abbreviations,WBC: white blood cell; NEU: neutrophil count; LYM: lymphocyte count; Hb: hemoglobin; PLT: platelet count; Cr: creatinine; Na: sodium; K: potassium; ESR: erythrocyte sedimentation rate; N: number; SD; standard deviation.
Table 6 shows the radiologic data which were collected from all the patients with COVID-19. CT images were classified according to the type and size of pathological findings. GGO (30.5%), consolidation (9.4%), crazy paving (9.1%), and the reticular patterns (5.2%) were the most frequent radiologic findings (Fig 1, Fig. 2 ).
Table 6.
Radiologic findings of patients with COVID-19.
| Variable | Total (%) N(4028) |
Death (%) N(778) |
Live (%) N(3088) | P-value | |
|---|---|---|---|---|---|
| Ground-glass opacity | Yes No |
1232(30.6) 2796(69.4) |
328(42.2) 450(57.8) |
853(27.6) 2235(72.4) |
0.002 |
| Crazy paving | Yes No |
369(9.2) 3659(90.8) |
118(15.2) 660(84.8) |
234(7.6) 2854(92.4) |
0.002 |
| Halo | Yes No |
14(0.4) 4014(99.6) |
4(0.5) 774(99.5) |
10(0.3) 3078(99.7) |
< 0.001 |
| Reticular | Yes No |
210(5.2) 3818(94.8) |
82(10.5) 696(89.5) |
114(3.7) 2974(96.3) |
< 0.001 |
| Consolidation | Yes No |
378(9.4) 3650(90.6) |
142(18.3) 636(81.7) |
215(7.0) 2873(93.0) |
< 0.001 |
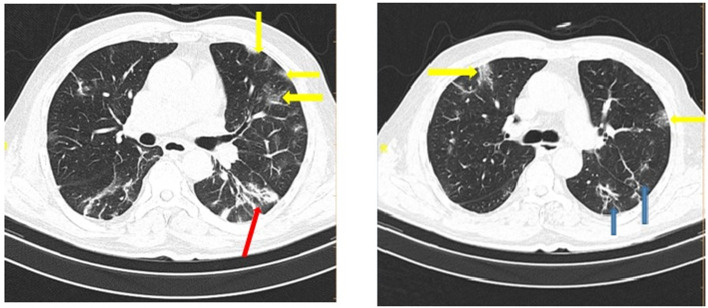
Fig 1.
COVID-19 pneumonia: CT scan shows bilateral ground-glass opacity (yellow arrows), consolidation (red arrow), and reticular opacities (blue arrows). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
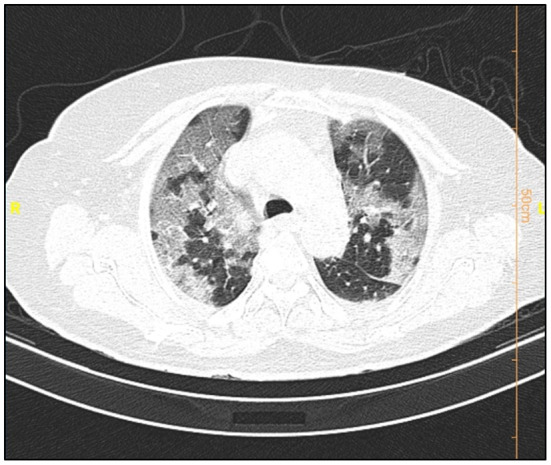
COVID-19 pneumonia: CT scan shows crazy paving pattern.
Discussion
When new infections emerge, clinical, laboratory, and imaging findings are important data that needs to be carefully investigated.21., 22., 23. In this context, the present descriptive retrospective study was conducted to evaluate the clinical features of 4028 patients in Vasei Hospital.
The middle age group (30–60) had the highest rate of infection. This finding was consistent with the age reports of infected individuals in previous studies.24 , 25 Of note, SARS-CoV-2 can infect all ages. Women were almost more infected than men, but the death rate was found to be greater in males than females. An earlier survey has also emphasized that the incidence of SARS-CoV-2 infection is approximately 15% higher in premenopausal women than men in some age ranges. However, COVID-19 mortality among men is significantly higher than in women of all ages,26 but this outcome was reverse in some other studies.27., 28., 29.
In this study, dyspnea, cough, and fever were the most common symptoms in COVID-19 patients, which affirms the findings of previous studies on this matter.27 , 30., 31., 32. Some other investigations reported fever and cough as the most prevalent clinical signs.29 , 33 Symptoms of the upper respiratory tract such as sore throat were less common in patients. Unlike SARS, patients with SARS-CoV-2 rarely had gastrointestinal manifestations, including vomiting and diarrhea. In our study, most patients were asymptomatic related to the symptoms of COVID-19. This maximum probably means that all sufferers with SARS-CoV-2 viral contamination can be admitted irrespective of symptoms. Comorbidities can enhance the severity of COVID-19 disease. Cardiovascular diseases (CVDs), diabetes, and chronic obstructive pulmonary disease have been attributed to the poor outcomes of COVID-19.34 The dead groups had a high rate of diabetes, HTN, and heart and lung diseases compared to the live groups. Patients with COVID-19 and type 2 diabetes require multiple interventions, and the risk of intensive care unit (ICU) admissions is higher than non-diabetic individuals. It has been proven that people with low blood glucose manage aggravation conditions and increase mortality.35 , 36 CVD was strongly associated with another coronavirus. Similarly, the increased prevalence of CVD was observed in patients with COVID-19, particularly those with severe signs and symptoms.36 The high threat of COVID-19 in CVD sufferers possibly arises from ACE-2 receptors presence on cardiomyocytes. Atherosclerosis, procoagulant activation, and hemodynamic instability are the main consequences of inflammatory cytokine release in COVID-19.37 Patients with HTN are more prone to serious complications from COVID-19. The invasion of SARS-CoV-2 into cells involves ACE2, an important enzyme in blood pressure homeostasis. Therefore, changes in the renin-angiotensin system can affect the occurrence and progression of COVID-19.38
Our study found abnormalities in inflammatory biomarkers such as CRP and ESR. It turned out that most COVID-19 patients had exceeded the CRP and ESR levels, and the higher level was detected in the dead group. CRP has been regarded as an essential predictor of disease severity in SARS.39 , 40 Data from patients were associated with lymphopenia and increased urea levels, especially in the dead group. Lymphopenia suggests that COVID-19 may affect lymphocytes, especially T lymphocytes.28 Viral particles spread throughout the airway mucosa and first use the ACE2 receptors of ciliary bronchial epithelial cells to infect other cells, triggering cytokine storms in the body and producing various immune responses, peripheral leukocytes, and immune cells that cause alterations in lymphocytes and so forth.41 , 42 Other data, including biochemical parameters such as serum Na+ and K+ were almost normal in all cases.
Data from the patients' CT scan demonstrated that the most common lesion was a ground-glass appearance, which supports the results achieved from Bao et al.'s and Qaisieh et al.'s research works.43 , 44 Well-known diagnostic imaging features of early CT in COVID-19 cases include bilateral GGO with peripheral or posterior distribution (or both), predominantly in the lower lobe, but less commonly in the right middle lobe.45 In our study, GGO with consolidation was found to be 9.4% in the general and 18.3% in the dead groups. It has also been indicated that there is a relationship between the pattern of CT findings and the progression of the disease.46 This study has several limitations. First, the period of follow-up was short. Second, some of the patients were still hospitalized and we could not place them in one of the dead or deceased groups. Third, patients with insufficient data were excluded from the study. Fourth, false-positive and false-negative results may occur during the detection of virus via PCR test. Finally, this study was limited to a single center and it is better to interpret it more carefully.
Conclusion
In summary, we studied the medical information of 778 sufferers who died from COVID-19 contamination, and 3088 sufferers who recovered. Patients in the dead group revealed greater pre-present comorbidities, including dyspnea, oxygen saturation decrease, reduced lymphocytes, and extended CRP levels. Thus, it is counseled that clinician examine the elements in the preliminary prognosis of this contamination and additionally in the course of the remedy of the contamination.
Conflict of interests
The authors declare that no competing interest exists.
Authors' contribution
Mahbobe Jafari, Maryam Akbari, Maryam Navidkia, Shirin Dashtbin, Faezeh Mmousavi, Mohsen Heidary, and Saeed Khoshnood contributed in revising and final approval of the version to be published. All authors agreed and confirmed the manuscript for publication.
Acknowledgments
This study was approved by the Student Research Committee and Ethics Committee of Sabzevar University of Medical Sciences, Sabzevar, Iran (code of ethics: IR.MEDSAB.REC.1400.114). The research team expresses gratitude to the medical staff of Sabzevar Vasei Hospital.
References
- 1.Lau S.K., Luk H.K., Wong A.C., Li K.S., Zhu L., He Z., et al. Possible bat origin of severe acute respiratory syndrome coronavirus 2. Emerg Infect Dis. 2020;26(7):1542. doi: 10.3201/eid2607.200092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoshnood S., Arshadi M., Akrami S., Koupaei M., Ghahramanpour H., Shariati A., et al. An overview on inactivated and live-attenuated SARS-CoV-2 vaccines. J Clin Lab Anal. 2022;36(5) doi: 10.1002/jcla.24418. e24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contini C., Di Nuzzo M., Barp N., Bonazza A., De Giorgio R., Tognon M., et al. The novel zoonotic COVID-19 pandemic: an expected global health concern. J Infect Develop Countries. 2020;14(03):254–264. doi: 10.3855/jidc.12671. [DOI] [PubMed] [Google Scholar]
- 4.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Team EE Note from the editors: World Health Organization declares novel coronavirus (2019-nCoV) sixth public health emergency of international concern. Eurosurveillance. 2020;25(5):200131e. doi: 10.2807/1560-7917.ES.2020.25.5.200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koupaei M., Mohamadi M.H., Yashmi I., Shahabi A.H., Shabani A.H., Heidary M., et al. Clinical manifestations, treatment options, and comorbidities in COVID-19 relapse patients: A systematic review. J Clin Lab Anal. 2022;36(5) doi: 10.1002/jcla.24402. e24402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan X., Zhang S., Xu J., Zhou M., Huang Q., Duan L., et al. Comparison of clinical characteristics among younger and elderly deceased patients with COVID-19: a retrospective study. Aging (Albany NY) 2021;13(1):16. doi: 10.18632/aging.202139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naimi A., Yashmi I., Jebeleh R., Imani Mofrad M., Azimian Abhar S., Jannesar Y., et al. Comorbidities and mortality rate in COVID-19 patients with hematological malignancies: A systematic review and meta-analysis. J Clin Lab Anal. 2022;36(5) doi: 10.1002/jcla.24387. e24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frater J.L., Zini G., d'Onofrio G., Rogers H.J. COVID-19and the clinical hematology laboratory. Int J Lab Hematol. 2020;42:11–18. doi: 10.1111/ijlh.13229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahdizade Ari M., Mohamadi M.H., Shadab Mehr N., Abbasimoghaddam S., Shekartabar A., Heidary M., et al. Neurological manifestations in patients with COVID-19: A systematic review and meta-analysis. J Clin Lab Anal. 2022;36(5) doi: 10.1002/jcla.24403. e24403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heidary M., Asadi A., Noorbakhsh N., Dashtbin S., Asadollahi P., Dranbandi A., et al. COVID-19 in HIV-positive patients: A systematic review of case reports and case series. J Clin Lab Anal. 2022;36(4) doi: 10.1002/jcla.24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Q., Wang B., Mao J. The pathogenesis and treatment of theCytokine Storm'in COVID-19. J Inf Secur. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong, W., Chen, Q., Qian, S., Basharat, Z., Zimmer, V., Wang, Y., Zippi, M., Pan, J., Critically Ill vs. Non-Critically Ill Patients With COVID-19 Pneumonia: Clinical Features, Laboratory Findings, and Prediction. Frontiers in cellular and infection microbiology. 2021. [DOI] [PMC free article] [PubMed]
- 16.Albahri A.S., Hamid R.A., Albahri O.S., Zaidan A. Detection-based prioritisation: framework of multi-laboratory characteristics for asymptomatic COVID-19 carriers based on integrated Entropy–TOPSIS methods. Artif Intell Med. 2021;111 doi: 10.1016/j.artmed.2020.101983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu J., Wang Y. The clinical characteristics and risk factors of severe COVID-19. Gerontology. 2021;67(3):255–266. doi: 10.1159/000513400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Smet K., De Smet D., Ryckaert T., Laridon E., Heremans B., Vandenbulcke R., et al. Diagnostic performance of chest CT for SARS-CoV-2 infection in individuals with or without COVID-19 symptoms. Radiology. 2021;298(1) doi: 10.1148/radiol.2020202708. E30-E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization WH . 2021. Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected Interim guidance M. [Google Scholar]
- 20.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuzan T.Y., Altıntoprak K.M., Çiftçi H.Ö., Ergül U., Özdemir N.B.Ü., Bulut M., et al. A comparison of clinical, laboratory and chest CT findings of laboratory-confirmed and clinically diagnosed COVID-19 patients at first admission. Diagn Interv Radiol. 2021;27(3):336. doi: 10.5152/dir.2020.20270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koupaei M., Shadab Mehr N., Mohamadi M.H., Asadi A., Abbasimoghaddam S., Shekartabar A., et al. Clinical symptoms, diagnosis, treatment, and outcome of COVID-19-associated encephalitis: a systematic review of case reports and case series. J Clin Lab Anal. 2022;36(5) doi: 10.1002/jcla.24426. e24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koupaei M., Naimi A., Moafi N., Mohammadi P., Tabatabaei F.S., Ghazizadeh S., et al. Clinical characteristics, diagnosis, treatment, and mortality Rate of TB/COVID-19 coinfectetd patients: a systematic review. Front Med. 2021;2188 doi: 10.3389/fmed.2021.740593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y., Ling Y., Bai T., Xie Y., Huang J., Li J., et al. COVID-19 with different severities: a multicenter study of clinical features. Am J Respir Crit Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newson L., Manyonda I., Lewis R., Preissner R., Preissner S., Seeland U. Sensitive to infection but strong in defense—female sex and the power of oestradiol in the COVID-19 pandemic. Frontiers in Global. Women Health. 2021;22 doi: 10.3389/fgwh.2021.651752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K., Chen Y., Lin R., Han K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J Inf Secur. 2020;80(6) doi: 10.1016/j.jinf.2020.03.005. e14-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lq Li, Huang T., Yq Wang, Zp Wang, Liang Y., Tb Huang, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lake M.A. What we know so far: COVID-19 current clinical knowledge and research. Clin Med. 2020;20(2):124. doi: 10.7861/clinmed.2019-coron. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak–an update on the status. Mil Med Res. 2020;7(1):1–10. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin Y.-H., Cai L., Cheng Z.-S., Cheng H., Deng T., Fan Y.-P., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7(1):1–23. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E., Villamizar-Peña R., Holguin-Rivera Y., Escalera-Antezana J.P., et al. Clinical, laboratory and imaging features of COVID-19: A systematic review and meta-analysis. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Comprehens Clin Med. 2020;2(8):1069–1076. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu L, She Z-G, Cheng X, Qin J-J, Zhang X-J, Cai J, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31(6):1068–77. e3. [DOI] [PMC free article] [PubMed]
- 36.Ejaz H., Alsrhani A., Zafar A., Javed H., Junaid K., Abdalla A.E., et al. COVID-19 and comorbidities: Deleterious impact on infected patients. J Infect Public Health. 2020;13(12):1833–1839. doi: 10.1016/j.jiph.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonow R.O., Fonarow G.C., O'Gara P.T., Yancy C.W. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. 2020;5(7):751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 38.Muhamad S.-A., Ugusman A., Kumar J., Skiba D., Hamid A.A., Aminuddin A. COVID-19 and hypertension: the what, the why, and the how. Front Physiol. 2021;12:589. doi: 10.3389/fphys.2021.665064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jang T.-N., Yeh D., Shen S.-H., Huang C.-H., Jiang J.-S., Kao S.-J. Severe acute respiratory syndrome in Taiwan: analysis of epidemiological characteristics in 29 cases. J Inf Secur. 2004;48(1):23–31. doi: 10.1016/j.jinf.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leong H.-N., Earnest A., Lim H.-H., Chin C.-F., Tan C.S., Puhaindran M.E., et al. SARS in Singapore-predictors of disease severity. Annals-Acad Med Singapore. 2006;35(5):326. [PubMed] [Google Scholar]
- 41.Rodríguez-Morales A.J., MacGregor K., Kanagarajah S., Patel D., Schlagenhauf P. Going global–Travel and the 2019 novel coronavirus. Travel Med Infect Dis. 2020;33 doi: 10.1016/j.tmaid.2020.101578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qaisieh R., Al-Tamimi M., El-Hammuri N., Shalabi M., Kilani M.M., Taha H., et al. Clinical, laboratory, and imaging features of COVID-19 in a Cohort of patients: cross-sectional comparative study. JMIR Public Health Surveill. 2021;7(9) doi: 10.2196/28005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao C., Liu X., Zhang H., Li Y., Liu J. Coronavirus disease 2019 (COVID-19) CT findings: a systematic review and meta-analysis. J Am Coll Radiol. 2020;17(6):701–709. doi: 10.1016/j.jacr.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salehi S., Abedi A., Balakrishnan S., Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020;215(1):87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 46.Kanne J.P. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiol Soc North Am. 2020:16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]