Dear Editor,
The COVID-19 pandemic has had a devastating effect on global health, resulting in over 6.2 million deaths worldwide. Continuous emergence of adaptive mutations of SARS-CoV-2 alters its pathogenicity and transmissibility, and renders its resistance to current vaccines and antiviral drugs.1 A new variant named Omicron discovered initially in South Africa has recently been proposed as a variant of concern (VOC) by the World Health Organization, because of its high transmissibility and resistance to current vaccines and therapeutic antibodies.2 Therefore, development of vaccines against prevalent variants including Omicron is urgently needed for COVID-19 prevention.
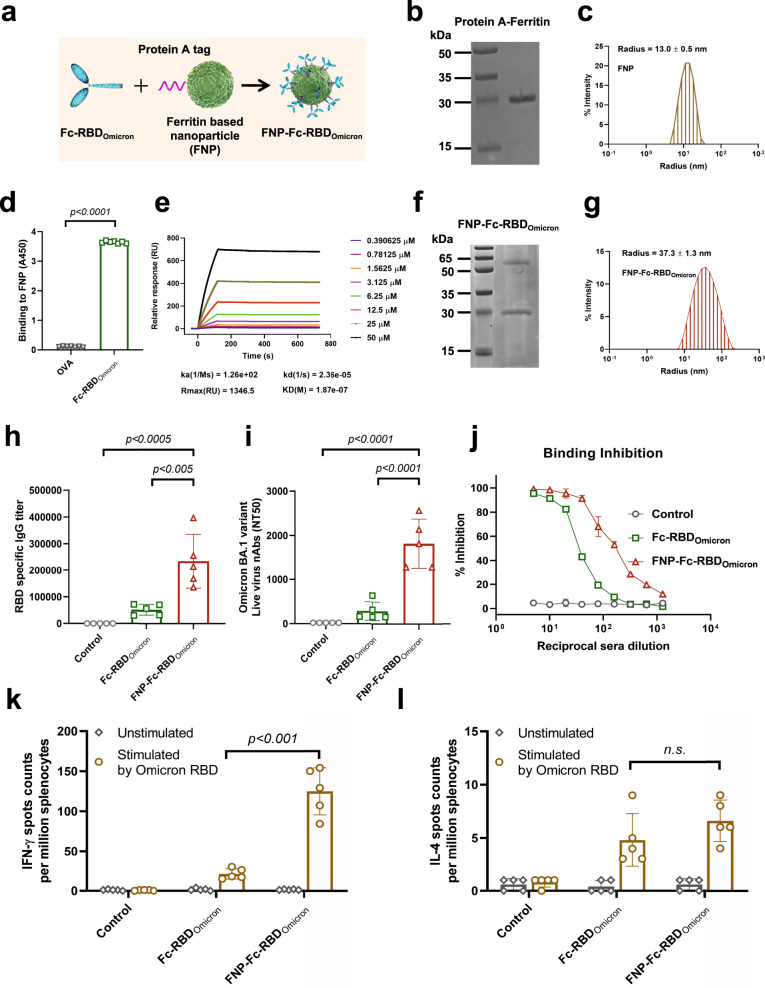
A previous study developed a SARS-CoV-2 vaccine based on a virus-like nanoparticle (VLP) platform, in which sixty copies of a fusion protein including a receptor binding domain (RBD) with a lumazine synthase as the structural scaffold were self-assembled into a nanoparticle.3 Based on this framework, we further designed a self-assembling ferritin-based nanoparticle (FNP) vaccine against the SARS-CoV-2 Omicron variant. In this system, twenty-four copies of ferritin containing an N-terminal protein A tag form a structural scaffold (Fig. 1a). The RBD (residues 331aa-524aa) of the SARS-CoV-2 Omicron spike protein with an Fc tag in the C-terminus (Fc-RBDOmicron) served as an essential immunogen (Fig. 1a).4 The purified Fc-RBDOmicron automatically assembled onto the nanoparticles by the Fc-protein A tag interaction (Fig. 1a). Based on this concept, the antigen of emerging SARS-CoV-2 variants can be assembled onto nanoparticles through a separating preparation and a subsequent Fc-Protein-A-tag-mediated conjugation. Of note, accumulating evidence indicate that the neutralizing potency elicited by a SARS-CoV-2 RBD dimer was much stronger than that by an RBD monomer.5 Therefore, this vaccine strategy may show advantage to stimulate the neutralizing immune responses than the previous design.
Fig. 1.
Development and characterization of the FNP-Fc-RBDOmicron vaccine against SARS-CoV-2 Omicron variant. a Schematic representation of a SARS-CoV-2 Omicron RBD with Fc tag (light green), a ferritin-based 24-meric nanoparticle with N-terminal protein A tag (green), and an FNP-Fc-RBDOmicron complex. b The FNP complex was analyzed by SDS-PAGE. c Size distribution of the FNP complex was detected by DLS. d, e Interaction between Fc-RBDOmicron and FNP was detected by ELISA and SPR. An equal amount of ovalbumin served as a control. The data are presented as mean ± S.E.M. Statistical significance was calculated via ordinary unpaired parametric t test. f The FNP-Fc-RBDOmicron complex was analyzed by SDS-PAGE. g Size distribution of the FNP-Fc-RBDOmicorn was detected by DLS. h, i Measurement of IgG and neutralizing antibodies induced in immunized mice. The mice were immunized via intramuscular (i.m.) prime and boost at 2 weeks (10 μg per mouse, n = 5). Sera at 14 days post-2nd immunization were detected for RBDOmicron-specific IgG antibodies by ELISA. The neutralizing antibodies were assessed by live SARS-CoV-2 Omicron BA.1 virus. The data are presented as mean ± S.E.M. (n = 5). Statistical significance was calculated via one-way ANOVA with multiple comparisons test. j Inhibition potency of immunized sera on SARS-CoV-2 RBD-hACE2 binding in hACE2/HEK293T cells. The inhibition potency was evaluated by flow cytometry. Inhibition percentage (%) was calculated by a relative fluorescence intensity. k, l Splenocytes were stimulated with the RBD protein of Omicron. The IFN-γ and IL-4 secretion condition in splenocytes were detected by an ELISpot assay. Data represented as mean ± S.E.M. (n = 5). Statistical significance was calculated via ordinary unpaired parametric t test
We expressed and purified the ferritin containing an N-terminal protein A tag in Escherichia coli (Supplementary Fig. S1a), and its purity was confirmed by SDS-PAGE (Fig. 1b). The characterization of the self-assembling nanoparticles was analyzed by negative-stain electron microscopy (EM) (Supplementary Fig. S1b) and dynamic light scattering (DLS) (Fig. 1c). The results indicated that the nanoparticles were spherical with a uniform diameter of 13.0 ± 0.5 nm. We next expressed the Fc-RBDOmicron in the FreeStyle 293-F cells (Supplementary Fig. S2a, b). The binding affinity of Fc-RBDOmicron for hACE2 was evaluated by both enzyme linked immunosorbent assay (ELISA) (Supplementary Fig. S3a) and flow cytometry (Supplementary Fig. S3b) with a dose-dependent manner. We next assembled the Fc-RBDOmicron onto the 24-meric FNP by mixing these two components at a 24:1 molar ratio. The Fc-RBDOmicron was capable of tightly interacting with the nanoparticles through the Fc-Protein A, measured by ELISA (Fig. 1d) and surface plasmon resonance technology (SPR) (Fig. 1e). The protein complex was co-eluted and co-purified by gel filtration chromatography, and further evaluated by SDS-PAGE (Fig. 1f). The protein complex was designated as the FNP-Fc-RBDOmicron throughout this investigation. Furthermore, the FNP-Fc-RBDOmicron complex was evaluated by DLS, which confirmed the diameter of FNP-Fc-RBDOmicron being uniformly about 37.3 ± 1.3 nm (Fig. 1g). Altogether, we generated self-assembling ferritin-based nanoparticles to develop a vaccine against the SARS-CoV-2 Omicron variant.
We next evaluated the potency of the FNP-Fc-RBDOmicron to induce immune responses against SARS-CoV-2. To this end, we immunized hACE2-transgenic mice with either FNP-Fc-RBDOmicron or a sole Fc-RBDOmicron. The mice were further boosted with the same dose of immunogens at 2 weeks after the primary immunization. Mouse sera were collected on Day 14 after the second immunization and analyzed for antibody titers and potency to neutralize SARS-CoV-2. The SARS-CoV-2 Omicron RBD-specific IgG titer induced by FNP-Fc-RBDOmicron was 4 times higher than that by Fc-RBDOmicron (Fig. 1h). Subsequently, the neutralizing potency in the sera of immunized animals was assessed by authentic SARS-CoV-2 Omicron virus. The sera showed a higher neutralizing activity in the FNP-Fc-RBDOmicron immunized mice than that of a sole Fc-RBDOmicron immunization (Fig. 1i).
To substantiate the SARS-CoV-2-neutralizing mechanism of vaccine-induced antibodies, we examined the interactions between the SARS-CoV-2 RBD and hACE2 in the presence of the vaccinated mouse sera by flow cytometry. Although the binding of RBDOmicron to hACE2/HKE293T cells was inhibited by either FNP-Fc-RBDOmicron or Fc-RBDOmicron serum effectively in a dose-dependent manner, the former was more potent (Fig. 1j and Supplementary Fig. S4). We examined whether the antibodies induced by FNP-Fc-RBDOmicron immunization could interrupt the entry of HIV pseudotyped with spike of SARS-CoV-2 VOCs. Indeed, FNP-Fc-RBDOmicron vaccinated sera effectively blocked the cellular entry of multiple SARS-CoV-2 VOCs (Supplementary Fig. S5). Nonetheless, the sera showed less neutralizing activity against other SARS-CoV-2 VOCs than that of Omicron, suggesting the diverse antigenicity between Omicron and other SARS-CoV-2 variants (Supplementary Fig. S5). To assess ability and duration of immune protection by FNP-Fc-RBDOmicron vaccination, we measured the RBD specific IgG level and neutralizing activity in the sera of FNP-Fc-RBDOmicron immunized animals on 21 and 42 days after the booster vaccination. The neutralizing potency in the sera maintained a high level on these 2 time points (Supplementary Fig. S6), indicating vaccination of FNP-Fc-RBDOmicron elicited a prolonged immune protection in animals. We next assessed the cellular immune responses in the mice immunized by FNP-Fc-RBDOmicron. The splenocytes were isolated from either immunized or control mice at 45 days after the booster dose. Subsequently, the splenocytes were in vitro stimulated by the purified RBD proteins of Wildtype (Supplementary Fig. S7a, b), Delta (Supplementary Fig. S7c, d) and Omicron BA.1 (Fig. 1k, l) variants. The RBD specific spots numbers of IFN-γ-, but not IL-4-producing T cells, was significantly higher in the FNP-Fc-RBDOmicron immunized animals than that in the Fc-RBDOmicron group, indicating a Th1 biased cellular immune response. Of note, the Wildtype and Delta RBD antigens also induces antigen-specific cellular immune responses in the FNP-Fc-RBDOmicron immunized animals (Supplementary Fig. S7). Overall, immunization of FNP-Fc-RBDOmicron stimulated antigen-specific humoral and cellular responses to multiple SARS-CoV-2 VOCs, thus indicating that the Omicron RBD-based vaccine may serve as a booster against COVID-19.
Overall, these results demonstrate that immunization of a self-assembling ferritin-based nanoparticle vaccine offers a robust humoral immune response against Omicron variant. Herein, a pseudovirus-based neutralization assay confirmed that vaccination with FNP-Fc-RBDOmicron could provide an effective neutralizing potency against both Omicron BA.1 and BA.2 variant infection. This study offers a great potential for the quick response of the emerging SARS-CoV-2 variants and affords versatility to develop vaccines against other emerging and reemerging coronaviruses in future.
Supplementary information
Acknowledgements
This work was supported by the Emergency Key Program of Guangzhou Laboratory (EKPG21-33) to G.C., the National Natural Science Foundation of China (32188101, 32100755, 81730063, 81961160737 and 31825001), the National Key Research and Development Plan of China (2021YFC2300200, 2020YFC1200104 and 2017ZX10304402).
Author contributions
W.T., B.C., S.F., X.Z., J.M., M.P., L.P., Z.Y. and M.T. conducted the study and analyzed the data. G.C. designed and supervised the study, wrote and revised the manuscript.
Data availability
All data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics
All mouse related work was performed strictly in accordance with the guidance and recommendations in the Guide for the Care and Use of Laboratory Animals (National Research Council Institute for Laboratory Animal Research). Experiments were conducted under animal use protocols approved by the Institutional Animal Care and Use Committees at the Shenzhen Bay Laboratory and Tsinghua University. Live virus neutralization assays were conducted in the biosafety level 3 (BSL-3) laboratory.
Footnotes
These authors contributed equally: Wanbo Tai, Benjie Chai, Shengyong Feng, Xinyu Zhuang.
Contributor Information
Mingyao Tian, Email: klwklw@126.com.
Gong Cheng, Email: gongcheng@mail.tsinghua.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41392-022-01041-8.
References
- 1.Johns Hopkins Coronavirus Resource Center. accessed 2022 May. https://coronavirus.jhu.edu/
- 2.Liu, Y., Liu, J. & Shi P. SARS-CoV-2 variants and vaccination. Zoonoses2 (2022). [DOI] [PMC free article] [PubMed]
- 3.Geng Q, et al. Novel virus-like nanoparticle vaccine effectively protects animal model from SARS-CoV-2 infection. PLoS Pathog. 2021;17:e1009897. doi: 10.1371/journal.ppat.1009897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tai W, et al. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell. Mol. Immunol. 2020;17:613–620. doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Z, et al. RBD-Fc-based COVID-19 vaccine candidate induces highly potent SARS-CoV-2 neutralizing antibody response. Signal Transduct. Target. Ther. 2020;5:282. doi: 10.1038/s41392-020-00402-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data and materials that support the findings of this study are available from the corresponding author upon reasonable request.