Abstract
Thymic stromal lymphopoietin (TSLP) is a pleiotropic cytokine that acts on multiple cell lineages, including dendritic cells, T cells, B cells, neutrophils, mast cells, eosinophils and innate lymphoid cells, affecting their maturation, survival and recruitment. It is best known for its role in promoting type 2 immune responses such as in allergic diseases and, in 2021, a monoclonal antibody targeting TSLP was approved for the treatment of severe asthma. However, it is now clear that TSLP has many other important roles in a variety of settings. Indeed, several genetic variants for TSLP are linked to disease severity, and chromosomal alterations in TSLP are common in certain cancers, indicating important roles of TSLP in disease. In this Review, we discuss recent advances in TSLP biology, highlighting how it regulates the tissue environment not only in allergic disease but also in infectious diseases, inflammatory diseases and cancer. Encouragingly, therapies targeting the TSLP pathway are being actively pursued for several diseases.
Subject terms: Cytokines, Infectious diseases, Tumour immunology, Immunological disorders
The cytokine thymic stromal lymphopoietin (TSLP) has pleiotropic functions beyond allergic diseases and T helper 2-type immune responses. Here, the authors highlight the roles of TSLP — beneficial or deleterious — in infectious disease, chronic inflammatory disease and cancer by acting on many different cell types.
Introduction
The cytokine thymic stromal lymphopoietin (TSLP) was originally detected in the supernatant of a thymic stromal cell line and shown to support the long-term growth of a B cell line and to enhance the proliferation of unfractionated thymocytes responding to stimulation with anti-CD3 antibody1. It was later shown to be a critical mediator of type 2 immune responses and a promoter of T helper 2 (TH2) cell-mediated diseases, including asthma and atopic dermatitis (AD)2–4. However, work since this original definition now shows that TSLP has multiple functions, including in cell maturation, proliferation, survival and recruitment and is involved in various other diseases and host responses. Here, we review how TSLP broadly contributes to pathological conditions such as allergic disease, host defence, cancer and chronic inflammatory disease. Understanding the roles of TSLP has implications for new therapeutic strategies that target this cytokine signalling system.
Targets and sources of TSLP
TSLP is a four α-helical type I cytokine and a paralogue of IL-7 (ref.5) (Box 1). Although first shown to act on B cells1, TSLP was then found to act directly on dendritic cells (DCs) and to indirectly affect T cells based on its effects on DCs6,7; however, TSLP was later shown to also be required for normal CD4+ T cell development and to act directly on CD4+ and CD8+ T cells3,8–11. Furthermore, TSLP has effects on neutrophils, mast cells, basophils, eosinophils, group 2 innate lymphoid cells (ILC2s), natural killer T cells, smooth muscle cells and tumour cells4,12–14. This range of target cells helps to explain the broad functions that can be mediated by this cytokine in both humans and mice.
Epithelial cells and stromal cells in the lungs, skin and gastrointestinal tract are the primary source of TSLP during both homeostatic and inflammatory conditions, although DCs, basophils and mast cells can also produce this cytokine6,15–18 (Fig. 1). TSLP is also produced by hair follicles and, together with IL-7, TSLP contributes to the persistence within skin of ILCs, which tune the skin microbiota by controlling sebaceous gland function14. TSLP production by epithelial cells can be induced by many stimuli, including mechanical injury, ligands for Toll-like receptor 3 (TLR3), TLR2 and NOD2, helminth infection, pro-inflammatory cytokines, and proteases, including trypsin and papain6,19–21. TSLP production in the lungs is also triggered following infection with viruses, including respiratory syncytial virus (RSV), rhinovirus22–24, influenza virus and lymphocytic choriomeningitis virus25. TSLP acts as an alarmin, being released from cells rapidly and inciting further exogenous and endogenous danger signals and exacerbating inflammation.
Fig. 1. Inducers, sources and targets of thymic stromal lymphopoietin.
A variety of environmental agents, including mechanical injury, ligands for Toll-like receptors (TLRs), viruses and cytokines, induce the production of thymic stromal lymphopoietin (TSLP). Epithelial cells are the main source of TSLP production. Fibroblasts, dendritic cells (DCs), basophils and mast cells also produce TSLP following stimulation. TSLP has pleiotropic actions on B cells, T cells, eosinophils, group 2 innate lymphoid cells (ILC2s), natural killer T (NKT) cells, macrophages, smooth muscle cells and nerve cells, and it also has effects on DCs, basophils and mast cells. IFNγ, interferon-γ; LCMV, lymphocytic choriomeningitis virus; TNF, tumour necrosis factor; RSV, respiratory syncytial virus.
TSLP production is positively regulated by the pro-inflammatory TH2-type cytokines IL-4 and IL-13 as well as by tumour necrosis factor (TNF), IL-1β and IL-25, with TNF synergizing with TH2-type cytokines to increase TSLP production. By contrast, interferon-γ (IFNγ) and IL-17 inhibit TSLP release26. β2-Adrenoceptor agonists and glucocorticoids also inhibit TSLP release and synergize to inhibit poly(I:C)-induced release of TSLP27. In the context of tissue injury, macrophage-derived progranulin induces TSLP production by mouse airway epithelial cells, leading to allergic inflammation28,29. In addition, the cross-linking of IgE bound to its high-affinity receptor (FcεRI) induces mast cell production of TSLP6. At the transcriptional level, nuclear factor-κB (NF-κB) and AP1 contribute to TSLP gene expression, and NF-κB binding sites have been identified in the TSLP promoter region30.
Two variants of human TSLP have been described: a long form (lfTSLP) and a short form (sfTSLP)31; the former is also known as TSLP and is the molecule that corresponds to mouse TSLP. Transcription of sfTSLP initiates from a promoter in intron 2 and is thus truncated at the amino-terminus but has the same carboxy-terminus as lfTSLP, having a total of 63 amino acids versus 159 amino acids for lfTSLP31. sfTSLP mRNA is constitutively expressed in keratinocytes, epithelial cells and lung fibroblasts31 and is not upregulated by inflammation, whereas lfTSLP is induced by TLR ligands, including flagellin, and TNF31–35. The distinct regulation of sfTSLP and lfTSLP suggests different roles, with an antibacterial or anti-inflammatory function proposed for sfTSLP and a pro-inflammatory function for lfTSLP35–39. Accordingly, in a mouse asthma model, administration of sfTSLP ameliorated house dust mite-induced asthma, whereas administration of lfTSLP damaged airway barrier function, contributing to the pathogenesis of asthma37. Single nucleotide polymorphisms (SNPs) rs2289276 and rs2289278 in the TSLP promoter confer augmented binding by AP1 and enhanced lfTSLP production, which may explain why these SNPs are associated with increased incidence of childhood atopic disease and adult asthma27. In ovarian and endometrial cancers, sfTSLP is predominantly expressed and promotes tumour growth through the activation of signalling pathways in cancer cells40. Further elucidating the different roles of these isoforms may provide new insights into TSLP biology.
Box 1 Thymic stromal lymphopoietin versus IL-7.
Thymic stromal lymphopoietin (TSLP) and IL-7 are paralogues, likely having risen by a gene duplication event. They signal via a shared IL-7 receptor α-chain (IL-7Rα), which partners with the TSLP receptor (TSLPR) in the case of TSLP, or the common cytokine receptor γ-chain in the case of IL-7. Interestingly, whereas IL-7 binds more robustly to IL-7Rα, TSLP binds more robustly to TSLPR, a situation perhaps analogous to another cytokine pair, IL-4 and IL-13, which share IL-4Rα as a receptor component but where IL-4 dominantly binds to this protein and IL-13 primarily binds to IL-13Rα1 (ref.189). For IL-7 and TSLP, the question is why have both cytokine genes evolved and been retained, especially given that TSLP has a pathological role in several diseases. TSLP and IL-7 indeed share some functions related to lymphoid development but they also have unique functions, with IL-7 primarily contributing to T cell development and homeostasis, and TSLP having roles in allergic disease as well as a broad range of actions on T cells, B cells and other cells (as detailed in this Review and elsewhere3,8–11,30). The sharing of IL-7Rα suggests that these cytokines might also compete for its recruitment and have competing actions. Both IL-7 and TSLP activate STAT5 but IL-7 does so more potently. Thus, what is the in vivo relationship between TSLP and IL-7? When do these cytokines cooperate and when do they potentially compete? Additionally, what is the physiological role of TSLP (that is, long-form TSLP) versus short-form TSLP? These are important questions for the better understanding of the overall biology of TSLP.
TSLP-induced signalling
TSLP signals through a heterodimeric receptor comprising TSLPR, a type I cytokine receptor encoded by Crlf2, and the IL-7 receptor α-chain (IL-7Rα; also known as CD127)41–43 (Fig. 2). This heterodimer is expressed on TSLP target cells such as DCs, mast cells, macrophages, basophils and T cells as well as epithelial cells and neurons13,38,44,45. Unlike its paralogue IL-7, which activates JAK1 and JAK3 via a heterodimeric receptor comprising IL-7Rα and the common cytokine receptor γ-chain, TSLP activates JAK1 (via IL-7Rα) and JAK2 (via TSLPR). JAK1 and JAK2 then primarily activate signal transducer and activator of transcription 5A (STAT5A) and STAT5B and, to a lesser extent, STAT1 and STAT3 (refs45,46), ultimately driving the production of IL-4, IL-5, IL-9 and IL-13 as well as pro-inflammatory effects. Unlike lfTSLP, it is unclear whether sfTSLP signals via the combination of TSLPR and IL-7Rα or potentially has an alternative mechanism of signalling given its truncated form.
Fig. 2. Mechanisms of thymic stromal lymphopoietin-induced signalling.

Thymic stromal lymphopoietin (TSLP) binds to a receptor comprising TSLP receptor (TSLPR) and IL-7 receptor α-chain (IL-7Rα), which are both type 1 membrane receptor proteins. TSLP binding activates JAK1, JAK2 and signal transducer and activator of transcription 5A and 5B (STAT5A and STAT5B) to promote the transcription of target genes, including the type 2 cytokines IL-4, IL-5 and IL-9.
TSLP in allergic diseases
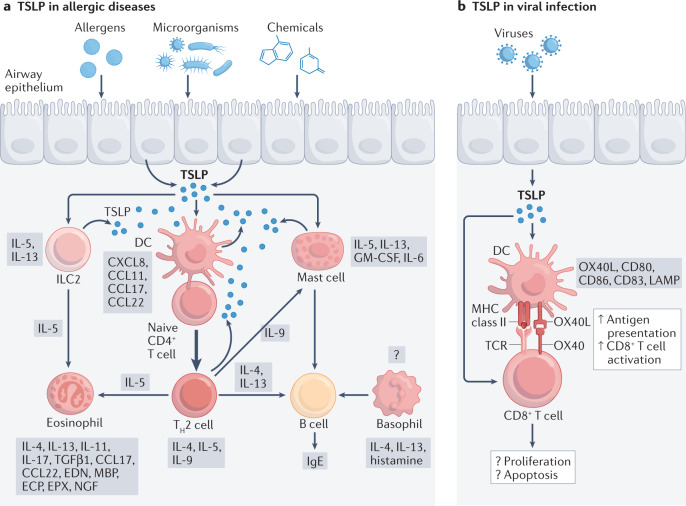
It is well established that TSLP, along with the other epithelial cell-derived cytokines IL-25 and IL-33, play pivotal roles in the development of allergic diseases, including asthma, AD and food hypersensitivity47 (Fig. 3). TSLP was initially shown to promote allergic responses by acting on DCs and inducing their expression of OX40 ligand (OX40L), CD80 and CD86, thereby promoting the differentiation of naive CD4+ T cells into pro-inflammatory TH2 cells that produce IL-4, IL-5, IL-13 and TNF6,7. Subsequently, it was shown that TSLP-activated DCs also stimulate naive CD4+ T cells to differentiate into T follicular helper cells (defined by expression of CXCR5, IL-21, CXCL13 and BCL6), which can induce IgG and IgE secretion by memory B cells48, linking TSLP to IgE production in allergy. TSLP also promotes the release of TH2 cytokines and chemokines by eosinophils, mast cells and macrophages30,49–52. TSLP acting on basophils has been linked to the development of an IgE-independent mouse model of the food allergy-associated inflammatory disease eosinophilic oesophagitis53, although the importance of TSLP in basophil responses remains unclear54,55. A DC–T cell–basophil cascade has been implicated in TSLP-driven type 2 immunity, whereby DCs activated by TSLP prime CD4+ T cells via OX40L to produce IL-3, which then leads to the recruitment of basophils and the production of IL-4 (ref.56). DCs themselves were reported to produce TSLP upon TLR stimulation, suggesting that TSLP might also act in an autocrine manner to amplify the TH2 cell response16.
Fig. 3. TSLP in allergic diseases and viral infection.
a | The release of thymic stromal lymphopoietin (TSLP) is stimulated by epithelial cell exposure to allergens, microorganisms and chemicals. TSLP promotes and amplifies T helper 2 (TH2)-type immunity, which enhances the immune response to antigens or allergens through both adaptive and innate immune mechanisms, leading to the development and/or progression of allergic disease. Whether TSLP induces or enhances the production of histamine, IL-4 and IL-13 by basophils requires further investigation. b | Viral infection also triggers the production of TSLP from epithelial cells. TSLP supports the survival of cytotoxic T cells both directly and indirectly through the activation of dendritic cells (DCs); however, the functional role of TSLP during antiviral immune responses is still controversial in influenza virus infection. CCL, CC-chemokine ligand; ECP, eosinophil cationic protein; EDN, eosinophil‑derived neurotoxin; EPX, eosinophil peroxidase; GM-CSF, granulocyte-macrophage colony-stimulating factor; ILC2, group 2 innate lymphoid cell; LAMP, lysosome-associated membrane protein; MBP, major basic protein; NGF, nerve growth factor; TCR, T cell receptor; TGFβ, transforming growth factor-β.
Besides indirect effects of TSLP on CD4+ T cells, this cytokine also acts directly on CD4+ T cells3,8,57–59 and is required for their full proliferation in response to antigen as well as for the formation of memory TH2 cells and recall responses3,8,60. Mice lacking TSLPR (Crlf2-/- mice) exhibit strong TH1 cell responses associated with high levels of IL-12, IFNγ and IgG2a, but low levels of IL-4, IL-5, IL-10, IL-13 and IgE. Crlf2-/- CD4+ T cells proliferate only weakly to antigen3, and Crlf2-/- mice do not develop ovalbumin (OVA)-induced lung inflammation unless supplemented with wild-type CD4+ T cells3,57. Interestingly, TSLP has different actions in models of airway inflammation depending on whether it is acting on innate or adaptive immune cells. A recent study used cell lineage-specific TSLPR-deficient mice to dissect the cell-intrinsic requirements for TSLP responsiveness in type 2 inflammation in the lungs. In a papain-induced model of airway inflammation, TSLP directly stimulated ILC2s but not basophils to enhance type 2 inflammation, whereas in OVA-induced airway inflammation, TSLP principally acted on DCs and CD4+ T cells, and not basophils or ILC2s, during the sensitization phase61. Thus, TSLP has broad actions related to allergic diseases and, as discussed below, there is genetic predisposition related to TSLP in the development of AD and asthma, with key roles of this cytokine in skin and lung linked to pathogenesis in these diseases.
Atopic dermatitis
AD is a heterogeneous disease with multifactorial pathogenesis, including genetic predisposition and environmental and immunological factors. Genetic variants of TSLP affect the severity and persistence of this disease. For example, homozygosity of the TSLP variant rs1898671 is associated with a reduced risk of AD in children62, whereas the variants rs2289278 and rs1837253 increase the risk for atopic diseases63,64.
TSLP is highly expressed in human AD lesions6,65 and its overexpression in mouse skin results in AD-like disease66. DNA demethylation of a specific region of the TSLP promoter augments expression of TSLP in skin lesions of patients with AD67 and diminishes expression of filaggrin68, a protein in which loss-of-function mutations are associated with epidermal barrier defects and more severe AD62. TSLP expression in human keratinocytes and nasal epithelial cells may also be increased by histamine (a key mediator of allergic diseases) binding to histamine H4 receptor69–71, suggesting a role for histamine in TSLP-dependent atopic disease.
Asthma
The prevalence of asthma varies among ethnic groups72, and 35–80% of asthma may result from genetic variation73,74, with childhood-onset asthma associated with TSLP SNP rs1837253 (refs75,76). In mouse models, overexpression of TSLP in the lungs results in severe airway inflammation and airway hyper-responsiveness (AHR). Additionally, patients with asthma, especially those with severe asthma, have increased levels of TSLP and TH2 cytokines in the airways77,78, with TSLP levels predictive of future asthma exacerbation79. Biopsy sections from individuals with mild atopic asthma show that allergen challenge increases IL-25, IL-33 and TSLP levels in the bronchial epithelium and submucosa, and levels of these cytokines correlate with the extent of airway obstruction80. In addition, patients with eosinophilic asthma have raised levels of IL-4, which not only increases the permeability of airway epithelial cells by reducing expression of filaggrin and adhesion molecules, including E-cadherin, but also increases levels of IL-33 and TSLP, which further enhance the TH2 inflammatory response81.
Individuals with one atopic disease often have other atopic diseases and progress to allergic diseases, including asthma or rhinitis, which is known as the atopic march82. Early therapeutic intervention in individuals with AD who are at risk and a better understanding of the mechanisms triggering asthma may help to prevent asthma and the atopic march. TSLP is involved in both AD and asthma, and recent studies indicate its role in the atopic march83. Consistent with this idea, skin-derived TSLP promotes allergen-sensitive asthma in animal models. Interventions in animal models that induce the systemic release of TSLP, such as keratinocyte-specific deletion of the DNA-binding protein RBP-J (a mediator of Notch signalling important for epidermal differentiation) or topical application of the vitamin D analogue MC903, cause AHR upon allergen challenge in the lungs84,85. These studies are consistent with the hypothesis that skin barrier defects, associated with TSLP production, could trigger systemic atopy. Moreover, when mice are infected with RSV as neonates, upon reinfection, they exhibit enhanced AHR due to TSLP expression, with OX40L expression, lung DC migration and TH2 cell polarization, leading to allergic responses later in life23,86. Correspondingly, more than 40% of infants who have severe bronchitis or respiratory tract infections will develop asthma in childhood87. Persistence of an altered immune phenotype in male mice triggered by early infection of RSV and the associated production of TSLP is consistent with the fact that boys are more vulnerable to RSV infection and the onset of asthma88. Interestingly, TSLP induced by RSV infection alters chromatin structure in DCs and promotes the expression of epigenetic enzymes such as lysine-specific demethylase 6A, which regulates transcriptional programmes mediated by interferon-regulatory factor 4 and STAT3 and leads to a pathogenic gene programme89.
Importantly, the key pathogenic role for TSLP in asthma is supported by the finding that a human monoclonal antibody specific for TSLP, known as tezepelumab (Tezspire), which blocks its binding to TSLPR and its biological actions, reduces eosinophilic inflammation and AHR and lowers disease exacerbation in patients with asthma90. In a phase IIb trial (the PATHWAY trial; NCT02054130), tezepelumab reduced exacerbations by up to 71% and improved lung function, asthma control and health-related quality of life compared with placebo91,92. In a phase III multicentre, randomized, double-blind, placebo-controlled trial (the NAVIGATOR trial; NCT03347279), the rate of asthma exacerbations was significantly lower with tezepelumab than with placebo in patients with severe, uncontrolled asthma, including those with low blood eosinophil counts at baseline. Lung function was improved and exacerbations were reduced, with less hospitalization and emergency room visits for patients treated with tezepelumab93. Accordingly, in 2021, the US FDA approved tezepelumab for the treatment of severe asthma. Several clinical trials with tezepelumab for allergic diseases and chronic inflammatory diseases are ongoing (Table 1). Moreover, a humanized Fc-disabled IgG1 monoclonal antibody against IL-7Rα, which potentially blocks both TSLP and IL-7, is being evaluated for the treatment of autoimmune diseases94. However, in contrast to its effect on asthma, tezepelumab did not achieve a statistically significant improvement in AD in a phase IIa trial (NCT03809663).
Table 1.
Recent and ongoing clinical trials of the TSLP-targeting monoclonal antibody tezepelumab
| Trial | Status | Trial participants | Interventions | Result | Clinical trials identifier and Refs |
|---|---|---|---|---|---|
| Healthy individuals | |||||
| Pharmacokinetics of tezepelumab delivered by APFS, AI, or vial and syringe, phase I | Completed Dec. 2019 | Healthy adult individuals | Tezepelumab | NA | NCT03989544 |
| Tezepelumab pharmacokinetics, phase I | Completed Oct. 2020 | Healthy Chinese individuals | Tezepelumab versus placebo | NA | NCT04362410 |
| Asthma | |||||
| Tezepelumab home use, phase III | Completed Jun. 2020 | Adolescents and adults with severe asthma | Tezepelumab administered by APFS versus AI | APFS and AI were functional and reliable, and performed equally well at home and in the clinic | NCT03968978 (ref.185) |
| Efficacy and safety of tezepelumab in reducing oral corticosteroid use, phase III (SOURCE) | Completed Sep. 2020 | Adults with oral corticosteroid-dependent asthma | Tezepelumab versus placebo | NA | NCT03406078 (ref.186) |
| Efficacy and safety of tezepelumab, phase III (NAVIGATOR) | Completed Nov. 2020 | Adults and adolescents with severe uncontrolled asthma | Tezepelumab versus placebo | Tezepelumab associated with fewer exacerbations and better lung function, asthma control and health-related quality of life than placebo | NCT03347279 (ref.93) |
| Effects of tezepelumab on airway inflammation, phase II (CASCADE) | Completed Nov. 2020 | Adults with uncontrolled asthma and other hypersensitivity airway diseases | Tezepelumab versus placebo | Tezepelumab improved clinical outcomes in patients with asthma, with reduction of eosinophilic airway inflammation; it also reduced hyperresponsiveness to mannitol | NCT03688074 (refs90,187,188) |
| Long-term safety of tezepelumab, phase III | Completed Mar. 2021 | Japanese adults and adolescents with inadequately controlled severe asthma | Tezepelumab | NA | NCT04048343 |
| Pharmacokinetics of tezepelumab, phase I | Recruiting | Children with asthma | Tezepelumab | NA | NCT04673630 |
| Efficacy and safety of tezepelumab, phase III | Recruiting | Adults with severe uncontrolled asthma | Tezepelumab versus placebo | NA | NCT03927157 |
| Extension study on safety and tolerability of tezepelumab, phase III (DESTINATION) | Active, not recruiting | Adults and adolescents with severe, uncontrolled asthma | Tezepelumab versus placebo | NA | NCT03706079 |
| Effect of tezepelumab on the immune response to influenza vaccination, phase III (VECTOR) | Active, not recruiting | Adolescents and young adults with moderate to severe asthma | Tezepelumab versus placebo | NA | NCT05062759 |
| Effect of tezepelumab on airway structure and function, phase III (WAYFINDER) | Not yet recruiting | Adults with uncontrolled moderate-to-severe asthma | Tezepelumab versus placebo | NA | NCT05280418 |
| Efficacy and safety of tezepelumab in reducing oral corticosteroid use, phase III | Not yet recruiting | Adults with severe asthma on high-dose corticosteroids | Tezepelumab | NA | NCT05274815 |
| Other allergic and chronic inflammatory diseases | |||||
| Safety and efficacy of tezepelumab, phase II | Terminated | Patients with moderate-to-severe atopic dermatitis | Tezepelumab versus placebo | Tezepelumab did not reach the targeted efficacy level pre-established for this patient population | NCT03809663 |
| Effect of tezepelumab in COPD exacerbation, phase II | Recruiting | Patients with moderate-to-very-severe COPD | Tezepelumab versus placebo | NA | NCT04039113 |
| Efficacy and safety of tezepelumab, phase II | Recruiting | Adults with chronic spontaneous urticaria | Two doses of tezepelumab versus omalizumab and placebo | NA | NCT04833855 |
| Efficacy and safety of tezepelumab, phase III | Recruiting | Patients with severe chronic rhinosinusitis with nasal polyps | Tezepelumab versus placebo | NA | NCT04851964 |
AI, autoinjector; APFS, accessorized pre-filled syringe; COPD, chronic obstructive pulmonary disease; NA, not available.
TSLP and host defence against infection
Staphylococcus aureus infection
S. aureus can cause serious skin infections in healthy individuals, and these infections are becoming more problematic by the expansion of strains with antibiotic resistance, including methicillin-resistant S. aureus (MRSA). As mentioned, TSLP is highly expressed at barrier surfaces, including skin, and has been shown to enhance neutrophil-mediated killing of MRSA with direct actions of TSLP on neutrophils12. TSLP also enhances the killing of Streptococcus pyogenes, another important cause of skin infections. TSLP mediates its antibacterial effect by directly engaging the complement C5 system to modulate the production of reactive oxygen species by neutrophils and thereby increases MRSA killing in a neutrophil-dependent and complement-dependent manner12.
Helminth infection
TSLP affects the function of immune cells, tissue inflammation and host protective immunity following helminth infection. Use of monoclonal antibody-mediated neutralization of TSLP or deletion of Crlf2 in normally resistant mice showed that TSLP is necessary for the development of protective TH2 cell responses after infection with the helminth Trichuris muris. The absence of TSLP signalling led to increased expression of IL-12p40, IFNγ and IL-17A, leading to severe intestinal inflammation95. Treatment of Crlf2-/- mice with a neutralizing monoclonal antibody to IL-12p40 restored TH2 cytokine production and attenuated IFNγ production, rescuing host protective immunity. It has also been shown that excretory–secretory products from Heligmosomoides polygyrus and Nippostrongylus brasiliensis suppress the production of IL-12p40 by DCs, bypassing the need for TSLP96.
ILC2s are also important in antihelminth immunity through their production of TH2 cytokines. Recent studies show that neurotransmitters and neuropeptides, including catecholamines, nicotine, acetylcholine, neuromedin U, vasoactive intestinal peptide and calcitonin gene-related peptide, can regulate ILC2 responses, highlighting an association between the nervous system and innate immunity at barrier surfaces97–103. Activated ILC2s express increased levels of choline acetyltransferase, the enzyme responsible for the biosynthesis of acetylcholine, after infection with N. brasiliensis or after treatment with alarmins or cytokines, including IL-25, IL-33 and TSLP. Thus, TSLP can stimulate ILC2s to augment the production of choline acetyltransferase as a mechanism for promoting host defence to helminth infection104.
Viral infection
As discussed above, the role of TSLP in TH2-type responses has been extensively studied, but its role in CD8+ T cell responses is less well characterized. Influenza virus infection is a major cause of respiratory disease, with substantial morbidity and mortality, accounting for approximately 500,000 deaths per year. During influenza virus infection or administration of poly(I:C), which mimics viral double-stranded RNA, pulmonary epithelial cells produce pro-inflammatory cytokines, including TSLP, that alter the immune response in the lungs105,106 (Fig. 3). TSLP supports the survival of cytotoxic T cells both directly10 and indirectly via the activation of DCs107,108. However, there are conflicting reports about the effect of TSLP on CD8+ T cells during primary influenza virus infection9,109,110. One study found that IL-7 is necessary for generating robust influenza A virus-specific CD4+ and CD8+ T cell responses but TSLP did not affect the control of primary infection nor viral-specific CD8+ T cell responses109. Another study concluded that TSLP is required for the expansion and activation of virus-specific effector CD8+ T cells in the lungs during primary infection but that this results from TSLP-induced IL-15 production by CD11b+ inflammatory DCs110 rather than from direct effects on CD8+ T cells. Other studies used an adoptive co-transfer model of wild-type and Crlf2-/- T cell receptor-transgenic cells. After infection with influenza virus, selective loss of TSLPR signalling in the antiviral CD8+ T cells decreased their proliferation and accumulation in the respiratory tract, indicating that TSLP enhances primary CD8+ T cell responses9. However, TSLP was also reported to act directly on CD8+ T cells to limit their responses during primary infection, with more virus-specific Crlf2-/- cells than virus-specific wild-type cells25. Conflicting reports on the roles of TSLP in CD8+ T cell responses during primary influenza virus infection may, in part, be owing to differences in experimental models (that is, direct studies in Crlf2-/- mice versus co-transfer models) and different influenza virus strains (that is, X31 versus PR8). Recently, the role of TSLP in memory CD8+ T cells was studied using a competitive adoptive co-transfer model of wild-type and Crlf2-/- P14 T cells (T cell receptor-transgenic CD8+ T cells specific for lymphocytic choriomeningitis virus gp33)25. TSLP did not affect the development or maintenance of memory CD8+ T cells after primary influenza virus infection but it limited memory CD8+ T cell recall responses, with higher responses by Crlf2-/- CD8+ T cells following secondary influenza virus infection.
A recent study revealed a previously unknown pathway in antiviral defence involving TSLP. Influenza virus-induced release of IFNλ can trigger the synthesis of TSLP by airway microfold cells in the upper airway overlying bronchus-associated lymphoid tissue, which stimulates CD103+ migratory DCs and promotes antigen-dependent germinal centre reactions in draining lymph nodes111. The IFNλ–TSLP axis mediated the production of virus-specific IgG1 and IgA after immunization with influenza virus vaccines, leading to enhanced resistance against influenza virus infection. TSLP also suppressed the expression of influenza virus-induced genes related to cell cycle, apoptosis or protection from virus; thus, modulating TSLP might affect the control of influenza virus infection and have therapeutic potential.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), is associated with cytokine release syndrome, which is characterized by TH1 and TH2 cell-associated inflammation. TSLP production is also induced in patients with COVID-19, with high TSLP levels associated with greater severity of disease112,113. Thus, TSLP seems to feed into pathological pathways and may be a useful target for therapeutic strategies to inhibit TH2 cell responses in patients with COVID-19.
Besides respiratory viruses, several other viral infections have been reported to induce TSLP production by epithelial cells, including vesicular stomatitis virus106, hepatitis C virus114,115, immunodeficiency viruses (HIV and SIV)116 and human papillomavirus (HPV)117, highlighting the role of TSLP as an alarmin. Certain strains of HPV cause cervical cancer, the progression of which is associated with a marked increase of serum IgE levels118. Infection with these high-risk HPVs correlated with increased production of TSLP by epithelial cells in cervical cancer, leading to a TH2 cell response and immunosuppressive microenvironment117. More recently, it was shown that expression of HPV oncoprotein in skin drove the onset of AD-like pathology that was associated with the secretion of high levels of TSLP and increased numbers of ILC2s119.
In summary, TSLP functions in a wide range of infectious diseases other than helminth infections, including both bacterial and viral infections. The reported roles of TSLP in viral infection are still controversial given differing results depending on the specific model system used. The roles of TSLP induced by microbial infection may potentially contribute to the exacerbation of allergic diseases, such as asthma, in these settings.
TSLP and cancer
Over the past decade, roles of TSLP in the control and onset of a variety of cancers, both solid tumours and leukaemias, have been elucidated, revealing that TSLP has both pro-tumour and antitumour effects, depending on the context and type of tumour (Fig. 4).
Fig. 4. TSLP in cancer.
a | Thymic stromal lymphopoietin (TSLP) secreted by either cancer-associated fibroblasts or tumour cells has tumour-promoting effects predominantly through the establishment of T helper 2 (TH2)-type inflammation in the tumour microenvironment, mostly through dendritic cell (DC) activation. TH2 cells and eosinophils promote angiogenesis through the production of vascular endothelial growth factor (VEGF) and IL-8. b | TH2 cell-independent mechanisms of TSLP in cancer rely on TSLP-induced signalling in TSLP receptor (TSLPR)-expressing tumour cells or B cell precursors. TSLP signalling in cancer cells can inhibit apoptosis, leading to tumour progression. Regulatory B cells induced by TSLPR signalling impair antitumour immunity in the tumour microenvironment, enabling metastasis. TSLP signalling in T cells prevents accumulation of CD11b+GR1+ myeloid cells that produce WNT ligands activating the WNT–β-catenin pathway in the epithelium, which can lead to carcinogenesis and tumour growth. CTL, cytotoxic T lymphocyte; GM-CSF, granulocyte-macrophage colony-stimulating factor; MDSC, myeloid-derived suppressor cell; PDGF, platelet-derived growth factor; Treg cell, regulatory T cell.
Acute lymphocytic leukaemia
Philadelphia chromosome-like acute lymphoblastic leukaemia (ALL) is commonly associated with genetic alterations affecting CRLF2, which encodes TSLPR120. In a large cohort of patients with T cell ALL, overexpression of CRLF2, causing activation of the JAK–STAT pathway, was associated with poor prognosis121. Indeed, targeting of JAKs, for example, by proteolysis-targeting chimeras (PROTACs) directed against JAKs, has been shown to be a potent treatment for CRLF2-rearranged ALL122. In Down syndrome-associated ALL, in which there is a high rate of rearrangement of CRLF2, TSLP increases binding of the tyrosine phosphatase PTPN11 to RAS, resulting in RAS protein activation, which promotes ALL cell growth123.
Solid tumours
Immune responses in the tumour microenvironment are affected by factors produced by tumour cells and tumour-associated cells, including cancer-associated fibroblasts (CAFs)124. TSLP secretion by CAFs or tumour cells promotes predominantly type 2 inflammation in the tumour microenvironment, mostly via DC activation and upregulation of OX40L, CD80 and CD86 expression125 following TSLPR-induced phosphorylation of STAT5. A detrimental role for TSLP in cancer was first demonstrated in pancreatic cancer126 and breast cancer127,128. Patients with pancreatic cancer in which GATA3+ TH2 cells were dominant had a worse prognosis than patients with T-bet+ TH1 cell infiltrates126. TSLP was secreted by CAFs when activated with tumour-derived pro-inflammatory cytokines, including TNF and IL-1β, and these TSLP-containing supernatants upregulated the expression of TSLPR on myeloid DCs, which secreted TH2 cell-attracting chemokines and promoted TH2 cell polarization of CD4+ T cells126. Interestingly, tumour-released IL-1α, IL-1β and apoptosis-associated speck-like protein containing a CARD (ASC) augment the secretion of TSLP by CAFs, suggesting that targeting these cells might decrease type 2 inflammation and tumour growth129. Moreover, basophil recruitment into draining lymph nodes was associated with TH2 cell polarization in patients with pancreatic cancer, and this was associated with a worse prognosis130.
In breast cancer, cancer cells can produce TSLP, and this is associated with the presence of OX40L+ DCs in primary breast tumour infiltrates128. These DCs promote the development of TH2 cells producing IL-13 and TNF in vitro, and blocking the actions of TSLP or OX40L lowered IL-13 production and reduced tumour growth in a xenograft model128. Interestingly, the release of IL-1β by DCs is necessary for TSLP production by breast cancer cells131. The role of TSLP in the growth and metastasis of breast cancer was also studied in an orthotopic mouse model using 4T1 cells, which are derived from a BALB/c breast ductal carcinoma. 4T1 cells produce TSLP, and the level of TSLP production correlated with the metastatic potential of different 4T1 clones127. Transplantation of 4T1 cells into Crlf2-/- mice was associated with reduced TH2 cytokines and decreased tumour growth132,133. Thus, the delayed tumour development in Crlf2-/- mice was due to defective CD4+ T cell responses127, consistent with TSLP promoting a TH2-type tumour microenvironment that supports the development and growth of metastatic breast cancer.
Conversely, other studies have suggested that TSLP can have tumour-suppressive activity. One study found that genetic or chemical induction of TSLP at a distant site led to robust antitumour immunity against spontaneous breast carcinogenesis in mice, mediated by TH2 cells134. However, in this study, breast cancer-prone PyMttg mice, in which cancer is driven by mammary-specific polyomavirus middle T antigen overexpression, were crossed with mice that overexpress TSLP in their skin, which not only induced TSLP production but also caused systemic inflammation134, making it possible that either TSLP or the inflammatory process or both could contribute to the antitumour effect. Another report found that TSLP was produced in fewer than 10% of breast cancers, with undetectable TSLPR expression on haematopoietic cells or stromal cells within the primary tumour microenvironment135, and another study demonstrated that the expression of TSLP was higher in normal tissue than in breast cancer tissue and that TSLP expression was associated with the increased survival of patients with breast cancer136. Thus, the role of TSLP may be context and/or tumour specific.
Reports regarding the role of TSLP in skin cancer have also differed. Groups have reported an antitumour role for TSLP in skin carcinogenesis in mice with clonal loss of Notch signalling in skin137,138. In this model, high levels of TSLP released by barrier-defective skin caused severe inflammation, resulting in gradual elimination of Notch-deficient epidermal clones and resistance to skin tumorigenesis. CD4+ T cells are required to mediate these effects of TSLP, analogous to the breast cancer models reported above134. Another group reported that cutaneous T cell lymphoma lesions in advanced stages exhibited mainly TH2 cytokines and chemokines139. In vitro and ex vivo cell lines and peripheral blood mononuclear cells from patients with cutaneous T cell lymphoma expressed TSLPR and produced higher levels of IL-4 and IL-13 in response to TSLP. High TSLP expression is a poor prognostic marker for gastric cancer140 and oropharyngeal squamous cell carcinoma141, indicating that TSLP and inflammation can exert pro-tumour activity in these settings.
In addition to the TH2-dependent roles of TSLP in cancer discussed above, TH2 cell-independent effects of TSLP have been reported. Depending on the context, TSLP-induced signalling in tumour cells can lead to apoptosis, proliferation and remodelling of pro-angiogenic gene signatures. In breast cancer, tumour-derived IL-1α can induce expression of TSLP by tumour-infiltrating myeloid cells, which can induce expression of the anti-apoptotic molecules BCL-2 and BCL-xL and promote tumour cell survival44. Consistent with this, TSLP could promote metastasis. Moreover, in another study, tumour cell-derived TSLP increased the invasive and angiogenic gene expression profile of alveolar macrophages, whereas depleting these cells significantly reduced the growth of TSLP-expressing tumour cells142. TSLP could downregulate expression of the bone marrow-retention receptors CXCR4 and VLA4 in B cell precursors, increasing cellular motility, survival and proliferation. These pre-B cells were induced by tumour cells to differentiate into regulatory B cells, which downmodulated antitumour immunity and promoted lung metastases143. Thus, lower TSLP production by cancer cells or lower TSLPR expression by B cells could decrease the accumulation of peripheral pre-B cells and potentially diminish cancer metastasis, suggesting that targeting TSLP might have therapeutic benefit.
A tumour-promoting function for TSLP was also described in lung cancer144. Expression of TSLP protein in tumours was significantly higher than in benign lesions and non-cancer lung tissue, and the prevalence of regulatory T cells in the tumour microenvironment correlated with the expression of TSLP in lung cancer. Furthermore, TSLP and TSLPR are expressed in macrophages purified from the lungs of patients with lung cancer145, with a presumed pro-tumorigenic role for TSLP.
TH2 cell-independent pro-tumour roles of TSLP in cervical cancer, gastric cancer and ovarian cancer have also been reported. TSLP was secreted from cervical cancer cells by hypoxia, inducing the release of chemokine CCL17, which then recruits eosinophils, leading to increased proliferation and diminished apoptosis of tumour cells through the upregulation of Ki-67 and BCL-2, respectively146, and indirectly stimulates angiogenesis by inducing the production of IL-8 and vascular endothelial growth factor147. TSLP also promoted the proliferation and invasion of cervical cancer cells by downregulating microRNA-132, the expression of which was lower in cervical cancer than in non-cancerous tissues148. Human gastric cancer cells also produce TSLP, and its expression correlated with metastasis149. Moreover, in ovarian cancer, higher TSLP expression was associated with worse prognosis150.
Increased expression of TSLP and TSLPR has also been reported in colorectal cancer, and the TSLP SNP rs10043985 was shown to be a biomarker for an increased risk of colorectal cancer in the Saudi population151. Nevertheless, antitumour effects of TSLP in colorectal cancer have also been reported152, with decreased TSLP levels in tumour than in adjacent tissue and TSLP levels negatively correlated with the clinical staging score. In this disease, TSLP was shown to activate JNK and MAPKp38 and promote apoptosis mainly through the extrinsic pathway. Analogous to this antitumour effect in colon carcinoma, TSLP can also prevent skin carcinogenesis through a TH2 cell-independent mechanism138. Using loss-of-function and gain-of-function mouse models for Notch and WNT signalling, it was shown that TSLP-mediated inflammation protects against cutaneous carcinogenesis, and this was mainly mediated by actions of TSLP on T cells. Deleting TSLPR resulted in the accumulation of CD11b+GR1+ myeloid cells that promoted tumour growth by secreting WNT ligands and activating the WNT–β-catenin pathway in the neighbouring epithelium, whereas deleting β-catenin prevented the recruitment of CD11b+GR1+ myeloid cells and carcinogenesis in skin, suggesting that the epithelial population initiates tumour development.
Thus, TSLP has been associated with promoting or reducing cancer in a range of malignancies, suggesting context-dependent effects for this cytokine in malignant disease.
TSLP in fat metabolism
Obesity increases the risk of numerous diseases, including hyperlipidaemia, diabetes mellitus, certain cancers, fatty liver and cardiovascular diseases. It has been shown that chronic, low-grade inflammation of adipose tissue increases type 2 immune cells, including ILC2s and eosinophils153–155. These cells increase the metabolic rate by promoting adipose tissue beiging and upregulating thermogenic energy consumption156–160. Regulatory T cells suppress the inflammatory state of adipose tissue, resulting in improved insulin resistance161,162. Recently, TSLP was reported to play a role in fat metabolism, selectively promoting the loss of white adipose tissue, which protected against obesity both in genetic models and diet-induced obesity as well as in insulin resistance and non-alcoholic steatohepatitis163. Mice with augmented levels of TSLP had greasy hair owing to the excessive loss of lipids through skin as sebum as well as elevated triglycerides, free fatty acids, cholesterol esters, free cholesterol and wax esters, which resulted from the TSLP-mediated induction of sebum and sebum-associated antimicrobial peptide release by skin CD4+ or CD8+ T cells. Ablating TSLPR signalling or deleting T cells diminished the secretion of sebum and antimicrobial peptides with altered skin homeostasis, thus identifying a previously unappreciated role for TSLP in adaptive immunity. Given that ILCs exposed to TSLP in skin negatively regulate sebaceous gland size and lipid content14, it is possible that ILCs and T cells regulated by TSLP might have opposing roles in controlling sebum secretion.
TSLP is also expressed in human adipose tissue and is produced by differentiated adipocytes in response to thyroid-stimulating hormone, IL-1β and TNF164. In humans, the level of TSLP in the serum was related to the basal metabolic index165. Obesity increases the risk of asthma by increasing bronchial hyperreactivity, leading to worse control of asthma, and obese patients with metabolic dysfunction tend to have more severe asthma166. Thus, TSLP produced from more than one source, such as either adipocytes or epithelial cells, can potentially affect the severity of asthma by regulating immune cells, as discussed above.
TSLP in chronic inflammatory diseases
Emerging evidence indicates that TSLP has roles in chronic inflammation and autoimmune diseases that are independent of TH2 cells, highlighting its role beyond the TH2-type response. These findings indicate a wide range of effects for TSLP, potentially in a wide range of human diseases.
Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is generally associated with TH1 cells, macrophages and neutrophils, whereas asthma is primarily associated with TH2 cells, eosinophils and/or mast cells. Despite being a predominantly TH1-associated disease, TSLP mRNA and protein levels were increased in the bronchial epithelium of COPD compared with controls18. Factors known to exacerbate COPD, including respiratory viruses22, double-stranded RNA19,167, cigarette smoke extracts168,169 and pro-inflammatory cytokines that activate NF-κB20,170, stimulate the production of TSLP in patients with COPD, suggesting involvement of TSLP in the development and/or exacerbation of COPD, and thus that TSLP can affect lung pathophysiology in situations beyond TH2-related asthma.
Idiopathic pulmonary fibrosis
Idiopathic pulmonary fibrosis (IPF) is a severe, progressive and ultimately fatal disorder, characterized by interstitial fibrosis of the lungs of unknown aetiology. TSLP and TSLPR are overexpressed in the lungs of patients with IPF33, and TSLP is increased in bronchoalveolar lavage and serum from patients with IPF171,172. The observation that TSLP levels decreased in the lungs of patients treated with anti-fibrotic therapy but not in individuals with progressive disease, supports a possible contribution of TSLP to pro-fibrotic type 2 immune responses in IPF172. However, more studies are required to fully understand the role of TSLP in the aetiology and progression of this disease.
Rheumatoid arthritis
Rheumatoid arthritis (RA) is an autoimmune inflammatory disorder that affects the joints, which are characterized by chronic synovitis — predominantly associated with TH1-type and TH17-type inflammation. Recently, the relationship between TSLP gene polymorphisms and RA susceptibility risk was demonstrated, with SNPs at rs11466749, rs11466750 and rs10073816 of TSLP leading to increased levels of TSLP associated with susceptibility to RA173. TSLP and TNF levels in the synovial fluid and plasma from patients with RA are significantly higher than in control patients173–175. Interestingly, TNF can induce TSLP production by synovial fibroblasts not only from patients with RA but also from control patients, suggesting that TSLP production by synovial fibroblasts is not specific to RA and is augmented by TNF174. Correspondingly, blockade of TSLP activity by anti-TSLP neutralizing antibodies ameliorated TNF-dependent experimental arthritis injury in mice induced by anti-type II collagen antibodies, suggesting a role for TSLP in the pathogenesis of RA. Moreover, in collagen-induced arthritis in mice, TSLP injection significantly exacerbated the severity of arthritis with activation of T cells leading to joint destruction176. Mast cells and macrophages in the RA synovium have been suggested to contribute to TSLP levels in the RA joint177–179. In the collagen-induced arthritis model, which is not a classic TH2 cell-associated disease, the effector inflammatory phase of arthritis depends on TNF, but TSLP also contributes to pro-inflammatory cytokine-dependent inflammation that leads to tissue damage. Collectively, studies suggest that TSLP expression may be a disease marker of RA, and targeting TSLP signalling could represent a rational, new therapeutic strategy for RA.
Ulcerative colitis
Ulcerative colitis (UC) is a severe inflammatory bowel disease characterized by dysregulated immune responses to gut microbiota that can contribute to the development and maintenance of an intestinal inflammatory process. Although the aetiology of UC is not fully elucidated, it is affected by both genetic and environmental factors. Intestinal epithelial cells play an important role in intestinal homeostasis by maintaining and/or controlling barrier function along with innate immune defence and the ability to modulate immune responses in the gut180. Hyperactivation of DCs decreases TSLP production from intestinal epithelial cells, which leads to uncontrolled production of pro-inflammatory cytokines, with the development of intestinal disorders, including inflammatory bowel disease181–183. TSLP mRNA levels are decreased in patients with UC, and TSLP expression was negatively correlated with the severity of UC, suggesting that TSLP has a protective role in UC and that low levels of TSLP promote severe disease184. This protective role of TSLP could be explained by the fact that TSLP can promote both Treg and TH2 cell responses that inhibit TH1 and TH17 cell responses and, consequently, could suppress inflammation in this setting.
Conclusions
TSLP is a pleiotropic cytokine with pleiotropic actions. Although the original role for TSLP as an initiator of type 2 inflammatory responses is well established, the biology and actions of this cytokine extend much further as described in this Review. TSLP is now implicated in viral infections, including influenza virus and SARS-CoV-2 infections, cancer, chronic inflammation and fat metabolism. TSLP appears to often be deleterious (for example, in allergic disease) but, in host defence, it may be protective (for example, in S. aureus or helminth infections) and, in cancer, there are a range of studies that indicate that TSLP can be beneficial or deleterious, depending on the malignancy and biological context. It is possible that TSLP has more than a single effect. Obviously, more studies are needed to rigorously define when the beneficial versus deleterious actions of TSLP occur, including studies and, eventually, clinical trials testing the effect of blocking TSLP. Indeed, the application of anti-TSLP-based therapy may hold promise beyond allergic diseases, an area of ongoing investigation. Moreover, there may be clinical settings in which augmenting, rather than blocking, TSLP might be desirable. Since the discovery of TSLP as a factor that could stimulate B cells, there have been huge advances, but the full significance of this cytokine and the range of therapeutic manipulations are still evolving.
Collectively, the roles of TSLP in a range of diseases and in cellular homeostasis indicate its potential as a predictive marker of disease severity and as a therapeutic target.
Acknowledgements
We thank R. Spolski and J.-X. Lin for critical comments on the manuscript. We acknowledge funding by Division of Intramural Research National Heart, Lung and Blood Institute, National Institutes of Health, Bethesda, MD, USA.
Glossary
- Asthma
A disease characterized by chronic type 2 inflammation, most commonly eosinophilic inflammation, in the airway, with airflow obstruction, hyper-responsiveness and clinical symptoms of wheezing, breathlessness, cough and chest tightness.
- Atopic dermatitis
(AD). The most prevalent inflammatory skin disease, usually developing in childhood; also known as eczema.
- Group 2 innate lymphoid cells
(ILC2s). Innate lymphoid cells characterized by high expression of GATA3 and production of IL-5 and IL-13 that have pathological and protective roles in multiple human diseases such as asthma, atopic dermatitis and infectious diseases.
- Alarmin
Molecules that are released by injured tissue as well as dead or dying cells and activate the host response through the inflammasome.
- Eosinophilic oesophagitis
A chronic eosinophil-mediated inflammatory disease of the oesophagus; affected individuals have oesophageal dysfunction with vomiting, dysphagia or feeding difficulties.
- Filaggrin
A filament-associated protein that binds to keratin fibres in epithelial cells and is essential for normal regulation of epidermal homeostasis; also known as filament aggregating protein.
- Atopic march
The progression of allergic manifestations generally beginning with atopic dermatitis, followed by IgE-mediated food allergy, allergic asthma and allergic rhinitis.
- IFNλ
A type III interferon that, on binding to IFNAR1 and IFNAR2, leads to JAK1 and TYK2 activation and phosphorylation of STAT1 and STAT2, which combine with IRF9 to form the heterotrimeric transcription factor ISGF3.
- Adipose tissue beiging
A process by which white adipose tissue acquires features of beige or brown adipocytes that use extra energy for heat production.
- Chronic obstructive pulmonary disease
(COPD). A chronic lung disease characterized by progressive airflow obstruction in peripheral airways, leading to air trapping, dynamic hyperinflammation and shortness of breath.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Immunology thanks O. Lamiable, F. Ronchese and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
W.J.L. is an inventor on NIH patents related to thymic stromal lymphopoietin (TSLP). R.E-S. declares no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Friend SL, et al. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp. Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 2.Roan F, Obata-Ninomiya K, Ziegler SF. Epithelial cell-derived cytokines: more than just signaling the alarm. J. Clin. Invest. 2019;129:1441–1451. doi: 10.1172/JCI124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J. Exp. Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat. Rev. Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sims JE, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J. Exp. Med. 2000;192:671–680. doi: 10.1084/jem.192.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 7.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J. Exp. Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J. Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 9.Shane HL, Klonowski KD. A direct and nonredundant role for thymic stromal lymphopoietin on antiviral CD8 T cell responses in the respiratory mucosa. J. Immunol. 2014;192:2261–2270. doi: 10.4049/jimmunol.1302085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J. Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4+ T cell development. J. Exp. Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.West EE, et al. A TSLP-complement axis mediates neutrophil killing of methicillin-resistant Staphylococcus aureus. Sci. Immunol. 2016;1:eaaf8471. doi: 10.1126/sciimmunol.aaf8471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corren J, Ziegler SF. TSLP: from allergy to cancer. Nat. Immunol. 2019;20:1603–1609. doi: 10.1038/s41590-019-0524-9. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, et al. Homeostatic control of sebaceous glands by innate lymphoid cells regulates commensal bacteria equilibrium. Cell. 2019;176:982–997.e16. doi: 10.1016/j.cell.2018.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat. Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J. Immunol. 2011;187:1207–1211. doi: 10.4049/jimmunol.1100355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Zhou X, Zhou B. DC-derived TSLP promotes Th2 polarization in LPS-primed allergic airway inflammation. Eur. J. Immunol. 2012;42:1735–1743. doi: 10.1002/eji.201142123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying S, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J. Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J. Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allakhverdi Z, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J. Exp. Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lv J, et al. Airway epithelial TSLP production of TLR2 drives type 2 immunity in allergic airway inflammation. Eur. J. Immunol. 2018;48:1838–1850. doi: 10.1002/eji.201847663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HC, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J. Allergy Clin. Immunol. 2012;130:1187–1196.e5. doi: 10.1016/j.jaci.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han J, et al. Responsiveness to respiratory syncytial virus in neonates is mediated through thymic stromal lymphopoietin and OX40 ligand. J. Allergy Clin. Immunol. 2012;130:1175–1186.e9. doi: 10.1016/j.jaci.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stier MT, et al. Respiratory syncytial virus infection activates IL-13-producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2016;138:814–824.e11. doi: 10.1016/j.jaci.2016.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebina-Shibuya R, et al. Thymic stromal lymphopoietin limits primary and recall CD8+ T-cell anti-viral responses. Elife. 2021;10:e61912. doi: 10.7554/eLife.61912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai T. TSLP expression: cellular sources, triggers, and regulatory mechanisms. Allergol. Int. 2012;61:3–17. doi: 10.2332/allergolint.11-RAI-0395. [DOI] [PubMed] [Google Scholar]
- 27.Harada M, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am. J. Respir. Cell Mol. Biol. 2011;44:787–793. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi JP, et al. Macrophage-derived progranulin promotes allergen-induced airway inflammation. Allergy. 2020;75:1133–1145. doi: 10.1111/all.14129. [DOI] [PubMed] [Google Scholar]
- 29.Jian J, Konopka J, Liu C. Insights into the role of progranulin in immunity, infection, and inflammation. J. Leukoc. Biol. 2013;93:199–208. doi: 10.1189/jlb.0812429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roan F, et al. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J. Leukoc. Biol. 2012;91:877–886. doi: 10.1189/jlb.1211622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harada M, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am. J. Respir. Cell Mol. Biol. 2009;40:368–374. doi: 10.1165/rcmb.2008-0041OC. [DOI] [PubMed] [Google Scholar]
- 32.Xie Y, Takai T, Chen X, Okumura K, Ogawa H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J. Dermatol. Sci. 2012;66:233–237. doi: 10.1016/j.jdermsci.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Datta A, et al. Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. J. Immunol. 2013;191:4867–4879. doi: 10.4049/jimmunol.1300588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin Mena A, et al. The expression of the short isoform of thymic stromal lymphopoietin in the colon is regulated by the nuclear receptor peroxisome proliferator activated receptor-gamma and is impaired during ulcerative colitis. Front. Immunol. 2017;8:1052. doi: 10.3389/fimmu.2017.01052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bjerkan L, et al. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol. 2015;8:49–56. doi: 10.1038/mi.2014.41. [DOI] [PubMed] [Google Scholar]
- 36.Fornasa G, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J. Allergy Clin. Immunol. 2015;136:413–422. doi: 10.1016/j.jaci.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dong H, et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci. Rep. 2016;6:39559. doi: 10.1038/srep39559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varricchi G, et al. Thymic stromal lymphopoietin isoforms, inflammatory disorders, and cancer. Front. Immunol. 2018;9:1595. doi: 10.3389/fimmu.2018.01595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gandolfo S, et al. Thymic stromal lymphopoietin expression from benign lymphoproliferation to malignant B-cell lymphoma in primary Sjogren’s syndrome. Clin. Exp. Rheumatol. 2019;37(Suppl. 118):55–64. [PubMed] [Google Scholar]
- 40.Chan LKY, et al. Short-form thymic stromal lymphopoietin (sfTSLP) is the predominant isoform expressed by gynaecologic cancers and promotes tumour growth. Cancers. 2021;13:980. doi: 10.3390/cancers13050980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pandey A, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat. Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 42.Park LS, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J. Exp. Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verstraete K, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat. Commun. 2017;8:14937. doi: 10.1038/ncomms14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuan EL, Ziegler SF. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat. Immunol. 2018;19:366–374. doi: 10.1038/s41590-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rochman Y, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl Acad. Sci. USA. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu N, et al. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J. Exp. Med. 2009;206:2111–2119. doi: 10.1084/jem.20090153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29–40. doi: 10.1016/j.immuni.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Pattarini L, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J. Exp. Med. 2017;214:1529–1546. doi: 10.1084/jem.20150402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitajima M, Lee HC, Nakayama T, Ziegler SF. TSLP enhances the function of helper type 2 cells. Eur. J. Immunol. 2011;41:1862–1871. doi: 10.1002/eji.201041195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Astrakhan A, et al. Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat. Immunol. 2007;8:522–531. doi: 10.1038/ni1452. [DOI] [PubMed] [Google Scholar]
- 51.Kim BS, et al. TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci. Transl. Med. 2013;5:170ra116. doi: 10.1126/scitranslmed.3005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hams E, Fallon PG. Innate type 2 cells and asthma. Curr. Opin. Pharmacol. 2012;12:503–509. doi: 10.1016/j.coph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Noti M, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat. Med. 2013;19:1005–1013. doi: 10.1038/nm.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Siracusa MC, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salabert-Le Guen N, et al. Thymic stromal lymphopoietin does not activate human basophils. J. Allergy Clin. Immunol. 2018;141:1476–1479.e6. doi: 10.1016/j.jaci.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 56.Leyva-Castillo JM, et al. Skin thymic stromal lymphopoietin initiates Th2 responses through an orchestrated immune cascade. Nat. Commun. 2013;4:2847. doi: 10.1038/ncomms3847. [DOI] [PubMed] [Google Scholar]
- 57.Rochman Y, et al. TSLP signaling in CD4+ T cells programs a pathogenic T helper 2 cell state. Sci. Signal. 2018;11:eaam8858. doi: 10.1126/scisignal.aam8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Dyken SJ, et al. A tissue checkpoint regulates type 2 immunity. Nat. Immunol. 2016;17:1381–1387. doi: 10.1038/ni.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ochiai S, et al. Thymic stromal lymphopoietin drives the development of IL-13+ Th2 cells. Proc. Natl Acad. Sci. USA. 2018;115:1033–1038. doi: 10.1073/pnas.1714348115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Q, Du J, Zhu J, Yang X, Zhou B. Thymic stromal lymphopoietin signaling in CD4+ T cells is required for TH2 memory. J. Allergy Clin. Immunol. 2015;135:781–791.e3. doi: 10.1016/j.jaci.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kabata H, et al. Targeted deletion of the TSLP receptor reveals cellular mechanisms that promote type 2 airway inflammation. Mucosal Immunol. 2020;13:626–636. doi: 10.1038/s41385-020-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chang J, Mitra N, Hoffstad O, Margolis DJ. Association of filaggrin loss of function and thymic stromal lymphopoietin variation with treatment use in pediatric atopic dermatitis. JAMA Dermatol. 2017;153:275–281. doi: 10.1001/jamadermatol.2016.4467. [DOI] [PubMed] [Google Scholar]
- 63.Gao PS, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J. Allergy Clin. Immunol. 2010;125:1403–1407.e4. doi: 10.1016/j.jaci.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang IJ, Wu LS, Lockett GA, Karmaus WJ. TSLP polymorphisms, allergen exposures, and the risk of atopic disorders in children. Ann. Allergy Asthma Immunol. 2016;116:139–145.e1. doi: 10.1016/j.anai.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 65.Clausen ML, Kezic S, Olesen CM, Agner T. Cytokine concentration across the stratum corneum in atopic dermatitis and healthy controls. Sci. Rep. 2020;10:21895. doi: 10.1038/s41598-020-78943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoo J, et al. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005;202:541–549. doi: 10.1084/jem.20041503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luo Y, Zhou B, Zhao M, Tang J, Lu Q. Promoter demethylation contributes to TSLP overexpression in skin lesions of patients with atopic dermatitis. Clin. Exp. Dermatol. 2014;39:48–53. doi: 10.1111/ced.12206. [DOI] [PubMed] [Google Scholar]
- 68.Wallmeyer L, et al. TSLP is a direct trigger for T cell migration in filaggrin-deficient skin equivalents. Sci. Rep. 2017;7:774. doi: 10.1038/s41598-017-00670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schaper K, et al. Stimulation of the histamine 4 receptor upregulates thymic stromal lymphopoietin (TSLP) in human and murine keratinocytes. Pharmacol. Res. 2016;113:209–215. doi: 10.1016/j.phrs.2016.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Schaper-Gerhardt K, et al. The role of the histamine H4 receptor in atopic dermatitis and psoriasis. Br. J. Pharmacol. 2020;177:490–502. doi: 10.1111/bph.14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang WW, Pan YL, Yu HW, Zhang B, Shao SW. Histamine H4 receptor regulates Th2-cytokine profile through thymic stromal lymphopoietin in allergic rhinitis. Eur. Arch. Otorhinolaryngol. 2019;276:1655–1661. doi: 10.1007/s00405-019-05369-w. [DOI] [PubMed] [Google Scholar]
- 72.Moorman JE, et al. National surveillance of asthma: United States, 2001–2010. Vital-. Health Stat. 2012;3:1–58. [PubMed] [Google Scholar]
- 73.Duffy DL, Martin NG, Battistutta D, Hopper JL, Mathews JD. Genetics of asthma and hay fever in Australian twins. Am. Rev. Respir. Dis. 1990;142:1351–1358. doi: 10.1164/ajrccm/142.6_Pt_1.1351. [DOI] [PubMed] [Google Scholar]
- 74.Nieminen MM, Kaprio J, Koskenvuo M. A population-based study of bronchial asthma in adult twin pairs. Chest. 1991;100:70–75. doi: 10.1378/chest.100.1.70. [DOI] [PubMed] [Google Scholar]
- 75.Torgerson DG, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat. Genet. 2011;43:887–892. doi: 10.1038/ng.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hirota T, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat. Genet. 2011;43:893–896. doi: 10.1038/ng.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shikotra A, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J. Allergy Clin. Immunol. 2012;129:104–111. doi: 10.1016/j.jaci.2011.08.031. [DOI] [PubMed] [Google Scholar]
- 78.Murrison LB, et al. TSLP disease-associated genetic variants combined with airway TSLP expression influence asthma risk. J. Allergy Clin. Immunol. 2022;149:79–88. doi: 10.1016/j.jaci.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ko HK, et al. Blood tryptase and thymic stromal lymphopoietin levels predict the risk of exacerbation in severe asthma. Sci. Rep. 2021;11:8425. doi: 10.1038/s41598-021-86179-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang W, et al. Bronchial allergen challenge of patients with atopic asthma triggers an alarmin (IL-33, TSLP, and IL-25) response in the airways epithelium and submucosa. J. Immunol. 2018;201:2221–2231. doi: 10.4049/jimmunol.1800709. [DOI] [PubMed] [Google Scholar]
- 81.Gao W, et al. The pathogenesis of eosinophilic asthma: a positive feedback mechanism that promotes Th2 immune response via filaggrin deficiency. Front. Immunol. 2021;12:672312. doi: 10.3389/fimmu.2021.672312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang L, Fu J, Zhou Y. Research progress in atopic march. Front. Immunol. 2020;11:1907. doi: 10.3389/fimmu.2020.01907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Han H, et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal Immunol. 2012;5:342–351. doi: 10.1038/mi.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demehri S, Morimoto M, Holtzman MJ, Kopan R. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067. doi: 10.1371/journal.pbio.1000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc. Natl Acad. Sci. USA. 2009;106:1536–1541. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tourdot S, et al. Respiratory syncytial virus infection provokes airway remodelling in allergen-exposed mice in absence of prior allergen sensitization. Clin. Exp. Allergy. 2008;38:1016–1024. doi: 10.1111/j.1365-2222.2008.02974.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bacharier LB, et al. Determinants of asthma after severe respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2012;130:91–100.e3. doi: 10.1016/j.jaci.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Malinczak CA, et al. Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol. 2019;12:969–979. doi: 10.1038/s41385-019-0171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malinczak CA, et al. TSLP-driven chromatin remodeling and trained systemic immunity after neonatal respiratory viral infection. J. Immunol. 2021;206:1315–1328. doi: 10.4049/jimmunol.2001205. [DOI] [PubMed] [Google Scholar]
- 90.Diver S, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir. Med. 2021;9:1299–1312. doi: 10.1016/S2213-2600(21)00226-5. [DOI] [PubMed] [Google Scholar]
- 91.Corren J, et al. Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann. Allergy Asthma Immunol. 2021;126:187–193. doi: 10.1016/j.anai.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 92.Corren J, et al. Tezepelumab in adults with uncontrolled asthma. N. Engl. J. Med. 2017;377:936–946. doi: 10.1056/NEJMoa1704064. [DOI] [PubMed] [Google Scholar]
- 93.Menzies-Gow A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N. Engl. J. Med. 2021;384:1800–1809. doi: 10.1056/NEJMoa2034975. [DOI] [PubMed] [Google Scholar]
- 94.Ellis J, et al. Anti-IL-7 receptor alpha monoclonal antibody (GSK2618960) in healthy subjects-a randomized, double-blind, placebo-controlled study. Br. J. Clin. Pharmacol. 2019;85:304–315. doi: 10.1111/bcp.13748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Massacand JC, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl Acad. Sci. USA. 2009;106:13968–13973. doi: 10.1073/pnas.0906367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cardoso V, et al. Neuronal regulation of type 2 innate lymphoid cells via neuromedin U. Nature. 2017;549:277–281. doi: 10.1038/nature23469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klose CSN, et al. The neuropeptide neuromedin U stimulates innate lymphoid cells and type 2 inflammation. Nature. 2017;549:282–286. doi: 10.1038/nature23676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wallrapp A, et al. The neuropeptide NMU amplifies ILC2-driven allergic lung inflammation. Nature. 2017;549:351–356. doi: 10.1038/nature24029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wallrapp A, et al. Calcitonin gene-related peptide negatively regulates alarmin-driven type 2 innate lymphoid cell responses. Immunity. 2019;51:709–723.e6. doi: 10.1016/j.immuni.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Moriyama S, Artis D. Neuronal regulation of group 2 innate lymphoid cells and type 2 inflammation. Adv. Immunol. 2019;143:1–9. doi: 10.1016/bs.ai.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 102.Nagashima H, et al. Neuropeptide CGRP limits group 2 innate lymphoid cell responses and constrains type 2 inflammation. Immunity. 2019;51:682–695.e6. doi: 10.1016/j.immuni.2019.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu H, et al. Transcriptional atlas of intestinal immune cells reveals that neuropeptide alpha-CGRP modulates group 2 innate lymphoid cell responses. Immunity. 2019;51:696–708.e9. doi: 10.1016/j.immuni.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu C, et al. The ChAT-acetylcholine pathway promotes group 2 innate lymphoid cell responses and anti-helminth immunity. Sci. Immunol. 2021;6:eabe3218. doi: 10.1126/sciimmunol.abe3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Brandelius A, et al. dsRNA-induced expression of thymic stromal lymphopoietin (TSLP) in asthmatic epithelial cells is inhibited by a small airway relaxant. Pulm. Pharmacol. Ther. 2011;24:59–66. doi: 10.1016/j.pupt.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 106.Kawasaki J, et al. Viral infection induces thymic stromal lymphopoietin (TSLP) in human keratinocytes. J. Dermatol. Sci. 2011;62:131–134. doi: 10.1016/j.jdermsci.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Gilliet M, et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J. Exp. Med. 2003;197:1059–1063. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sugimoto H, et al. Thymic stromal lymphopoietin plays an adjuvant role in BCG-mediated CD8+ cytotoxic T cell responses through dendritic cell activation. Clin. Immunol. 2010;136:205–216. doi: 10.1016/j.clim.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 109.Plumb AW, et al. Interleukin-7, but not thymic stromal lymphopoietin, plays a key role in the T cell response to influenza A virus. PLoS One. 2012;7:e50199. doi: 10.1371/journal.pone.0050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yadava K, et al. TSLP promotes influenza-specific CD8+ T-cell responses by augmenting local inflammatory dendritic cell function. Mucosal Immunol. 2013;6:83–92. doi: 10.1038/mi.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye L, et al. Interferon-lambda enhances adaptive mucosal immunity by boosting release of thymic stromal lymphopoietin. Nat. Immunol. 2019;20:593–601. doi: 10.1038/s41590-019-0345-x. [DOI] [PubMed] [Google Scholar]
- 112.Choreno-Parra JA, et al. Clinical and immunological factors that distinguish COVID-19 from pandemic influenza A(H1N1) Front. Immunol. 2021;12:593595. doi: 10.3389/fimmu.2021.593595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caterino M, et al. Dysregulation of lipid metabolism and pathological inflammation in patients with COVID-19. Sci. Rep. 2021;11:2941. doi: 10.1038/s41598-021-82426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee HC, et al. Hepatitis C virus promotes T-helper (Th)17 responses through thymic stromal lymphopoietin production by infected hepatocytes. Hepatology. 2013;57:1314–1324. doi: 10.1002/hep.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kowalewska J, et al. Thymic stromal lymphopoietin transgenic mice develop cryoglobulinemia and hepatitis with similarities to human hepatitis C liver disease. Am. J. Pathol. 2007;170:981–989. doi: 10.2353/ajpath.2007.060474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fontenot D, et al. TSLP production by epithelial cells exposed to immunodeficiency virus triggers DC-mediated mucosal infection of CD4+ T cells. Proc. Natl Acad. Sci. USA. 2009;106:16776–16781. doi: 10.1073/pnas.0907347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Feng Q, et al. Th2 type inflammation promotes the gradual progression of HPV-infected cervical cells to cervical carcinoma. Gynecol. Oncol. 2012;127:412–419. doi: 10.1016/j.ygyno.2012.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vijayakumar T, et al. Serum immunoglobulins in patients with carcinoma of the oral cavity, uterine cervix and breast. Cancer Immunol. Immunother. 1986;22:76–79. doi: 10.1007/BF00205721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bergot AS, et al. HPV16 E7 expression in skin induces TSLP secretion, type 2 ILC infiltration and atopic dermatitis-like lesions. Immunol. Cell Biol. 2015;93:540–547. doi: 10.1038/icb.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts KG, et al. Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N. Engl. J. Med. 2014;371:1005–1015. doi: 10.1056/NEJMoa1403088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Palmi C, et al. CRLF2 over-expression is a poor prognostic marker in children with high risk T-cell acute lymphoblastic leukemia. Oncotarget. 2016;7:59260–59272. doi: 10.18632/oncotarget.10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang Y, et al. Degradation of Janus kinases in CRLF2-rearranged acute lymphoblastic leukemia. Blood. 2021;138:2313–2326. doi: 10.1182/blood.2020006846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koschut D, et al. RAS-protein activation but not mutation status is an outcome predictor and unifying therapeutic target for high-risk acute lymphoblastic leukemia. Oncogene. 2021;40:746–762. doi: 10.1038/s41388-020-01567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Binnewies M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu YJ, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu. Rev. Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 126.De Monte L, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Olkhanud PB, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J. Immunol. 2011;186:5656–5662. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pedroza-Gonzalez A, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J. Exp. Med. 2011;208:479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunetto E, et al. The IL-1/IL-1 receptor axis and tumor cell released inflammasome adaptor ASC are key regulators of TSLP secretion by cancer associated fibroblasts in pancreatic cancer. J. Immunother. Cancer. 2019;7:45. doi: 10.1186/s40425-019-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.De Monte L, et al. Basophil recruitment into tumor-draining lymph nodes correlates with Th2 inflammation and reduced survival in pancreatic cancer patients. Cancer Res. 2016;76:1792–1803. doi: 10.1158/0008-5472.CAN-15-1801-T. [DOI] [PubMed] [Google Scholar]
- 131.Wu TC, et al. IL1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Res. 2018;78:5243–5258. doi: 10.1158/0008-5472.CAN-18-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Terabe M, et al. NKT cell-mediated repression of tumor immunosurveillance by IL-13 and the IL-4R-STAT6 pathway. Nat. Immunol. 2000;1:515–520. doi: 10.1038/82771. [DOI] [PubMed] [Google Scholar]
- 133.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 134.Demehri S, et al. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J. Clin. Invest. 2016;126:1458–1470. doi: 10.1172/JCI83724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ghirelli C, et al. No evidence for TSLP pathway activity in human breast cancer. Oncoimmunology. 2016;5:e1178438. doi: 10.1080/2162402X.2016.1178438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ding S, et al. Identification of a novel immune-related prognostic signature associated with tumor microenvironment for breast cancer. Int. Immunopharmacol. 2021;100:108122. doi: 10.1016/j.intimp.2021.108122. [DOI] [PubMed] [Google Scholar]
- 137.Demehri S, et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell. 2012;22:494–505. doi: 10.1016/j.ccr.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell. 2012;22:479–493. doi: 10.1016/j.ccr.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 139.Takahashi N, et al. Thymic stromal chemokine TSLP acts through Th2 cytokine production to induce cutaneous T-cell lymphoma. Cancer Res. 2016;76:6241–6252. doi: 10.1158/0008-5472.CAN-16-0992. [DOI] [PubMed] [Google Scholar]
- 140.Watanabe J, Saito H, Miyatani K, Ikeguchi M, Umekita Y. TSLP expression and high serum TSLP level indicate a poor prognosis in gastric cancer patients. Yonago Acta Med. 2015;58:137–143. [PMC free article] [PubMed] [Google Scholar]
- 141.Lin CM, Lin LW, Chen YW, Ye YL. The expression and prognostic impact of proinflammatory cytokines and their associations with carcinogens in oropharyngeal squamous cell carcinoma. Cancer Immunol. Immunother. 2020;69:549–558. doi: 10.1007/s00262-020-02488-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Burkard-Mandel L, O’Neill R, Colligan S, Seshadri M, Abrams SI. Tumor-derived thymic stromal lymphopoietin enhances lung metastasis through an alveolar macrophage-dependent mechanism. Oncoimmunology. 2018;7:e1419115. doi: 10.1080/2162402X.2017.1419115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ragonnaud E, et al. Tumor-derived thymic stromal lymphopoietin expands bone marrow b-cell precursors in circulation to support metastasis. Cancer Res. 2019;79:5826–5838. doi: 10.1158/0008-5472.CAN-19-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li H, et al. Increased prevalence of regulatory T cells in the lung cancer microenvironment: a role of thymic stromal lymphopoietin. Cancer Immunol. Immunother. 2011;60:1587–1596. doi: 10.1007/s00262-011-1059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Braile M, et al. Human lung-resident macrophages express and are targets of thymic stromal lymphopoietin in the tumor microenvironment. Cells. 2021;10:2012. doi: 10.3390/cells10082012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xie F, et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364:106–117. doi: 10.1016/j.canlet.2015.04.029. [DOI] [PubMed] [Google Scholar]
- 147.Zhang B, et al. TSLP promotes angiogenesis of human umbilical vein endothelial cells by strengthening the crosstalk between cervical cancer cells and eosinophils. Oncol. Lett. 2017;14:7483–7488. doi: 10.3892/ol.2017.7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhou WJ, et al. Human thymic stromal lymphopoietin promotes the proliferation and invasion of cervical cancer cells by downregulating microRNA-132 expression. Oncol. Lett. 2017;14:7910–7916. doi: 10.3892/ol.2017.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Barooei R, Mahmoudian RA, Abbaszadegan MR, Mansouri A, Gholamin M. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med. Oncol. 2015;32:217. doi: 10.1007/s12032-015-0653-4. [DOI] [PubMed] [Google Scholar]
- 150.Xu L, et al. Overexpression of thymic stromal lymphopoietin is correlated with poor prognosis in epithelial ovarian carcinoma. Biosci. Rep. 2019;39:BSR20190116. doi: 10.1042/BSR20190116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Semlali A, et al. Expression and polymorphism of TSLP/TSLP receptors as potential diagnostic markers of colorectal cancer progression. Genes. 2021;12:1386. doi: 10.3390/genes12091386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yue W, et al. Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor growth by promoting apoptosis of tumor cells. Oncotarget. 2016;7:16840–16854. doi: 10.18632/oncotarget.7614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sun K, Kusminski CM, Scherer PE. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cildir G, Akincilar SC, Tergaonkar V. Chronic adipose tissue inflammation: all immune cells on the stage. Trends Mol. Med. 2013;19:487–500. doi: 10.1016/j.molmed.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 155.Cox AJ, West NP, Cripps AW. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015;3:207–215. doi: 10.1016/S2213-8587(14)70134-2. [DOI] [PubMed] [Google Scholar]
- 156.Lee MW, et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell. 2015;160:74–87. doi: 10.1016/j.cell.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Qiu Y, et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell. 2014;157:1292–1308. doi: 10.1016/j.cell.2014.03.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Brestoff JR, et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature. 2015;519:242–246. doi: 10.1038/nature14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu D, et al. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kolodin D, et al. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 2015;21:543–557. doi: 10.1016/j.cmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Choa R, et al. Thymic stromal lymphopoietin induces adipose loss through sebum hypersecretion. Science. 2021;373:eabd2893. doi: 10.1126/science.abd2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma L, Zhen J, Sorisky A. Regulators of thymic stromal lymphopoietin production by human adipocytes. Cytokine. 2020;136:155284. doi: 10.1016/j.cyto.2020.155284. [DOI] [PubMed] [Google Scholar]
- 165.Gluck J, Gluck M, Rogala B, Piecuch J. Epithelial-cell-derived cytokines in patients with obesity before and after bariatric surgery. Int. Arch. Allergy Immunol. 2022;183:566–571. doi: 10.1159/000521456. [DOI] [PubMed] [Google Scholar]
- 166.Barros R, et al. Obesity increases the prevalence and the incidence of asthma and worsens asthma severity. Clin. Nutr. 2017;36:1068–1074. doi: 10.1016/j.clnu.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 167.Calven J, et al. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases. J. Innate Immun. 2012;4:86–99. doi: 10.1159/000329131. [DOI] [PubMed] [Google Scholar]
- 168.Nakamura Y, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J. Allergy Clin. Immunol. 2008;122:1208–1214. doi: 10.1016/j.jaci.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 169.Smelter DF, et al. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J. Immunol. 2010;185:3035–3040. doi: 10.4049/jimmunol.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc. Natl Acad. Sci. USA. 2007;104:914–919. doi: 10.1073/pnas.0607305104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee JU, et al. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm. Med. 2017;17:39. doi: 10.1186/s12890-017-0380-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Majewski S, et al. Epithelial alarmins in serum and exhaled breath in patients with idiopathic pulmonary fibrosis: a prospective one-year follow-up cohort study. J. Clin. Med. 2019;8:1590. doi: 10.3390/jcm8101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang W, et al. The level of thymic stromal lymphopoietin and its gene polymorphism are associated with rheumatoid arthritis. Immunobiology. 2021;226:152055. doi: 10.1016/j.imbio.2021.152055. [DOI] [PubMed] [Google Scholar]
- 174.Koyama K, et al. A possible role for TSLP in inflammatory arthritis. Biochem. Biophys. Res. Commun. 2007;357:99–104. doi: 10.1016/j.bbrc.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 175.Moret FM, et al. Thymic stromal lymphopoietin, a novel proinflammatory mediator in rheumatoid arthritis that potently activates CD1c+ myeloid dendritic cells to attract and stimulate T cells. Arthritis Rheumatol. 2014;66:1176–1184. doi: 10.1002/art.38338. [DOI] [PubMed] [Google Scholar]
- 176.Hartgring SA, et al. Critical proinflammatory role of thymic stromal lymphopoietin and its receptor in experimental autoimmune arthritis. Arthritis Rheum. 2011;63:1878–1887. doi: 10.1002/art.30336. [DOI] [PubMed] [Google Scholar]
- 177.Okayama Y, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur. Respir. J. 2009;34:425–435. doi: 10.1183/09031936.00121008. [DOI] [PubMed] [Google Scholar]
- 178.Jeong HJ, et al. Interleukin-32-induced thymic stromal lymphopoietin plays a critical role in macrophage differentiation through the activation of caspase-1 in vitro. Arthritis Res. Ther. 2012;14:R259. doi: 10.1186/ar4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rivellese F, et al. Mast cells in early rheumatoid arthritis associate with disease severity and support B cell autoantibody production. Ann. Rheum. Dis. 2018;77:1773–1781. doi: 10.1136/annrheumdis-2018-213418. [DOI] [PubMed] [Google Scholar]
- 180.Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 181.Abraham C, Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ordonez F, et al. Pediatric ulcerative colitis associated with autoimmune diseases: a distinct form of inflammatory bowel disease? Inflamm. Bowel Dis. 2012;18:1809–1817. doi: 10.1002/ibd.22864. [DOI] [PubMed] [Google Scholar]
- 183.Ziegler SF, et al. The biology of thymic stromal lymphopoietin (TSLP) Adv. Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tahaghoghi-Hajghorbani S, et al. Protective effect of TSLP and IL-33 cytokines in ulcerative colitis. Auto. Immun. Highlights. 2019;10:1. doi: 10.1186/s13317-019-0110-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Alpizar S, et al. Functionality and performance of an accessorized pre-filled syringe and an autoinjector for at-home administration of tezepelumab in patients with severe, uncontrolled asthma. J. Asthma Allergy. 2021;14:381–392. doi: 10.2147/JAA.S305114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wechsler ME, et al. SOURCE: a phase 3, multicentre, randomized, double-blind, placebo-controlled, parallel group trial to evaluate the efficacy and safety of tezepelumab in reducing oral corticosteroid use in adults with oral corticosteroid dependent asthma. Respir. Res. 2020;21:264. doi: 10.1186/s12931-020-01503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Emson C, et al. CASCADE: a phase 2, randomized, double-blind, placebo-controlled, parallel-group trial to evaluate the effect of tezepelumab on airway inflammation in patients with uncontrolled asthma. Respir. Res. 2020;21:265. doi: 10.1186/s12931-020-01513-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pham TH, et al. Tezepelumab normalizes serum interleukin-5 and -13 levels in patients with severe, uncontrolled asthma. Ann. Allergy Asthma Immunol. 2021;127:689–691. doi: 10.1016/j.anai.2021.08.008. [DOI] [PubMed] [Google Scholar]
- 189.Keegan, A. D. & Leonard, W. J. In Paul Fundamental Immunology 8th edn Ch. 9 (ed. Flajnik, M.) (Wolter Kluwer, 2022).