Abstract
Objective
Sarilumab, as monotherapy or in combination with conventional synthetic DMARDs, such as MTX, has demonstrated improvement in clinical outcomes in patients with RA. The primary objective of this post hoc analysis was to compare the efficacy of sarilumab (200 mg every 2 weeks) monotherapy (MONARCH study) with that of sarilumab and MTX combination therapy (MOBILITY study) at week 24.
Methods
The endpoints assessed were mean change from baseline in the Clinical Disease Activity Index (CDAI), 28-joint Disease Activity using CRP (DAS28-CRP), CRP, haemoglobin (Hb), pain visual analogue scale (VAS) and Functional Assessment of Chronic Illness Therapy (FACIT)–Fatigue. Least square (LS) mean change from baseline (95% CI) at week 24 for all endpoints was compared between the treatment arms for adjusted comparisons.
Results
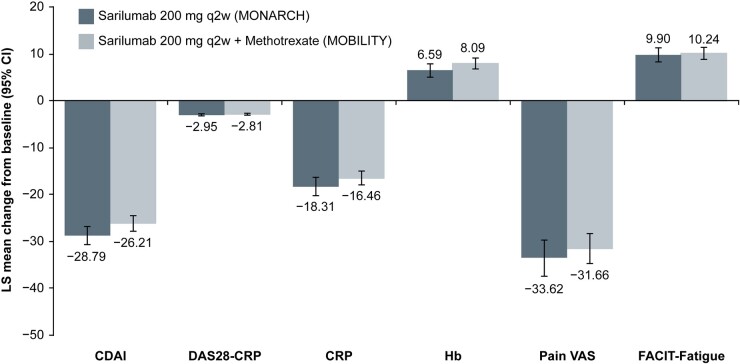
This analysis included 184 patients on sarilumab monotherapy and 399 patients on sarilumab plus MTX. Differences (P < 0.05) were observed in ethnicity, region, body mass index group, rheumatoid factor, anti-cyclic citrullinated peptide antibodies, swollen joint count, CRP, CDAI and oral glucocorticoid use between these treatment groups. After adjusting for these differences in a mixed-effect model repeated measure, LS mean change from baseline for all assessments was similar between the treatment groups with overlapping CIs: CDAI, −28.79 vs −26.21; DAS28-CRP, −2.95 vs −2.81; CRP, −18.31 vs −16.46; Hb, 6.59 vs 8.09; Pain VAS, −33.62 vs −31.66; FACIT-Fatigue, 9.90 vs 10.24.
Conclusion
This analysis demonstrated that the efficacy of sarilumab monotherapy was similar to that of sarilumab and MTX combination therapy.
Keywords: rheumatoid arthritis, sarilumab, IL-6Ri, MONARCH, MOBILITY, monotherapy, combination with MTX
Rheumatology key messages.
Sarilumab, monotherapy or in combination with methotrexate, demonstrated clinical improvements in patients with rheumatoid arthritis.
The efficacy of sarilumab monotherapy was similar to its combination with methotrexate.
Sarilumab monotherapy may be a valuable treatment strategy in patients with a contraindication/intolerance to methotrexate.
Introduction
Treatment guidelines recommend combining biologic and targeted synthetic DMARDs (tsDMARDs) with conventional synthetic DMARDs (csDMARDs), which primarily consists of MTX [1, 2]. A recent real-world study in patients with RA reported suboptimal adherence to MTX citing adverse events as the main reason, which ultimately resulted in poor persistence of MTX [3]. Another systematic review also showed high variability in MTX adherence and persistence in patients with RA [4]. Therefore, there is a need for alternative treatment strategies in patients who are non-adherent to MTX.
IL-6 plays a predominant role in the pathogenesis of RA by regulating a diverse range of activities that drive chronic inflammation associated with RA. IL-6 also mediates various activities that underlie both local and systemic clinical symptoms of RA via cell signalling modulated by membrane-bound and soluble forms of its receptor [5, 6]. IL-6 receptor inhibitors, namely tocilizumab and sarilumab, have shown improvement in clinical outcomes in clinical studies and are approved for use as combination with csDMARDs or as monotherapy in patients with RA [5, 7, 8]. Recent EULAR guidelines recommend that IL-6 pathway inhibitors and tsDMARDs may have some advantages compared with other, biologic DMARDs (bDMARDs) in patients who cannot use csDMARDs as comedication [2].
Sarilumab, an IL-6 receptor-α (IL-6Rα) inhibitor, is a fully human monoclonal antibody which binds soluble and membrane-bound IL-6Rα to inhibit IL-6-mediated signalling [9–11]. In the MONARCH and MOBILITY trials, sarilumab as monotherapy and in combination with MTX, respectively, has demonstrated symptomatic and functional improvements in RA patients with inadequate responses/intolerance to MTX (MTX-IR/INT) [12, 13]. There are no studies that have directly compared the efficacy of sarilumab monotherapy with that of its combination with MTX. In this post hoc analysis, we compared the efficacy of sarilumab monotherapy with sarilumab in combination with MTX using mixed-effect model repeated measure (MMRM) models.
Methods
Patients and study design
This post hoc analysis was performed using data from the MONARCH (NCT02332590 [14]) and MOBILITY (NCT01061736 [15]) phase III trials of sarilumab in patients with active RA. Details of the study design, patient population and outcomes of these trials have been published previously [12, 13]. In the MONARCH trial, MTX-IR/INT patients with RA (enrolled based on the 2010 ACR/EULAR criteria) were randomized to receive subcutaneous (s.c.) sarilumab 200 mg every 2 weeks (q2w) or adalimumab 40 mg q2w in combination with placebo for 24 weeks [12]. In the MOBILITY trial, MTX-IR patients with RA (enrolled based on 1987 ACR revised classification criteria) were randomized to receive s.c. sarilumab 150 mg or 200 mg q2w or placebo in combination with weekly MTX for 52 weeks [13]. Detailed inclusion and exclusion criteria for both the trials were published previously [12, 14–16].
The present post hoc analysis is based on the data collected from MONARCH and MOBILITY studies. Both MONARCH and MOBILITY studies were performed in accordance with the Declaration of Helsinki and the protocols for both the studies were approved by the appropriate ethics committees/institutional review boards for the respective studies and patients gave written consent before participation [12, 13, 17].
Treatment arms
This analysis included all patients who received sarilumab 200 mg q2w in the MONARCH and MOBILITY trials, based on treatment assigned. In the MOBILITY trial, patients received a stable dose of MTX (10–25 mg/week) for a minimum of 6 weeks prior to the screening visit, except patients within the Asia-Pacific region (Taiwan, South Korea, Malaysia, Philippines, Thailand and India) who were allowed to use a stable dose of MTX between 6 and 25 mg/week for a minimum of 6 weeks prior to the screening visit. Patients were to continue the stable dose of MTX for the duration of the study [16].
Endpoints
The endpoints assessed in this analysis included mean change from baseline in Clinical Disease Activity Index (CDAI), 28-joint Disease Activity using CRP (DAS28-CRP), CRP, haemoglobin (Hb), pain visual analogue scale (VAS) and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue. Percentage of responders was analysed for categorical endpoints including CDAI low disease activity (CDAI LDA; CDAI ≤10), DAS28-CRP LDA (DAS28-CRP score <3.2), CRP (mg/l) <10, and minimal clinically important difference (MCID) in Hb (percentage change from baseline in Hb [g/l] >7), pain VAS (change from baseline in pain VAS (mm) ≤−10) and FACIT-Fatigue (change from baseline in FACIT-fatigue ≥4), using observed cases (OC) and intent-to-treat (ITT) population, and was compared between the treatment arms.
Statistical analysis
For adjusted comparisons, continuous changes in endpoints from baseline were set as dependent variables and patient baseline characteristics that differed (P < 0.05) between the two trials were set as covariates in MMRM models; least squares (LS) mean change in endpoints from baseline (95% CI) at week 24 was compared between the treatment arms. Patients with non-missing endpoint values were considered for these comparisons. For unadjusted comparisons of efficacy between monotherapy and combination therapy treatment arms, mean change in endpoints from baseline (95% CI) at week 24 was compared between the treatment arms. Responder analysis was performed using both ITT (patients with missing data imputed as non-responders) and OC (patients with missing data excluded) populations.
Results
Patient baseline characteristics
This analysis included 184 patients in the sarilumab 200 mg q2w monotherapy arm from MONARCH and 399 patients in the sarilumab 200 mg q2w plus MTX combination therapy arm from MOBILITY. Baseline demographic and disease characteristics for patients included in both trials are shown in Table 1. Comparing the baseline characteristics of patients in these two trials, differences (P < 0.05) were observed in ethnicity, region, body mass index group, rheumatoid factor, anti-cyclic citrullinated peptide antibodies, swollen joint count, CRP, CDAI and oral glucocorticoid use between the treatment arms and were selected to be included in the MMRMs (Table 1). Details on the regional distribution of the study patients are provided in Supplementary Data S1, available at Rheumatology online.
Table 1.
Differences in baseline characteristics of patients in the MONARCH and MOBILITY studies
| Parameter | Sarilumab 200 mg q2w (MONARCH; n = 184) | Sarilumab 200 mg q2w + MTX (MOBILITY B; n = 399) | P-value |
|---|---|---|---|
| Agea, mean (s.d.) , years | 50.9 (12.6) | 50.8 (11.8) | 0.9608 |
| Age group (years)b, n (%) | |||
| <65 | 158 (85.9) | 348 (87.2) | 0.6772 |
| ≥65 and <75 | 25 (13.6) | 50 (12.5) | |
| ≥75 | 1 (0.5) | 1 (0.3) | |
| Sexb, n (%) | |||
| Male | 27 (14.7) | 62 (15.5) | 0.7873 |
| Female | 157 (85.3) | 337 (84.5) | |
| Raceb, n (%) | |||
| Caucasian/White | 171 (92.9) | 343 (86.0) | 0.0007 |
| Black | 1 (0.5) | 8 (2.0) | |
| Asian/Oriental | 2 (1.1) | 33 (8.3) | |
| Other | 10 (5.4) | 15 (3.8) | |
| Ethnicityb, n (%) | |||
| Hispanic | 46 (25.0) | 151 (37.8) | 0.0023 |
| Non-Hispanic | 138 (75.0) | 248 (62.2) | |
| Regionb, n (%) | |||
| Region 1 | 61 (33.2) | 75 (18.8) | <0.0001 |
| Region 2 | 36 (19.6) | 155 (38.9) | |
| Region 3 | 87 (47.3) | 169 (42.4) | |
| Weighta,c, mean (s.d.), kg | 72.3 (16.5) | 74.7 (19.7) | 0.1303 |
| Heighta,c, mean (s.d.), cm | 163.3 (9.1) | 161.4 (9.0) | 0.0203 |
| BMIa,c, mean (s.d.), kg/m2 | 27.1 (5.6) | 28.6 (6.7) | 0.0059 |
| BMI group (kg/m2)b,c, n (%) | |||
| <25 | 71 (38.6) | 129 (32.4) | 0.0123 |
| ≥25 and <30 | 70 (38.0) | 127 (31.9) | |
| ≥30 | 43 (23.4) | 142 (35.7) | |
| Duration of RA since diagnosis, mean (s.d.), yearsa | 8.1 (8.1) | 8.6 (7.0) | 0.5051 |
| RA functional classb, n (%) | |||
| I | 29 (15.8) | 42 (10.5) | 0.1488 |
| II | 125 (67.9) | 277 (69.4) | |
| III | 30 (16.3) | 80 (20.1) | |
| IV | 0 | 0 | |
| Rheumatoid factorb,d, n (%) | |||
| Positive | 119 (66.9) | 328 (82.6) | <0.0001 |
| Negative | 59 (33.2) | 69 (17.4) | |
| Anti-CCP antibodyb,d, n (%) | |||
| Positive | 134 (75.3) | 337 (84.9) | 0.0057 |
| Negative | 44 (24.7) | 60 (15.1) | |
| Tender joint count (0–68)a, mean (s.d.) | 28.0 (13.2) | 26.5 (14.5) | 0.2498 |
| Tender joint count (0–28)a, mean (s.d.) | 17.0 (6.1) | 15.5 (6.6) | 0.0102 |
| Swollen joint count (0–66)a, mean (s.d.) | 18.6 (10.7) | 16.8 (9.7) | 0.0418 |
| Swollen joint count (0–28)a, mean (s.d.) | 13.2 (5.7) | 11.9 (5.6) | 0.0106 |
| CRPa, mean (s.d.), mg/l | 17.4 (21.3) | 22.2 (23.8) | 0.0188 |
| HAQ-DI (0–3)a, mean (s.d.) | 1.6 (0.6) | 1.7 (0.6) | 0.3159 |
| DAS28-CRP (>5.1: high disease activity)a, mean (s.d.) | 6.0 (0.9) | 6.0 (0.9) | 0.7433 |
| CDAIa, mean (s.d.) | 43.6 (12.1) | 40.4 (12.3) | 0.0033 |
| Patient’s global assessment of disease activity (0–100 mm)a, mean (s.d.) | 68.0 (17.5) | 66.3 (20.8) | 0.3007 |
| Physician’s global assessment of disease activity (0–100 mm)a, mean (s.d.) | 66.3 (15.7) | 63.5 (17.6) | 0.0643 |
| Pain VAS (0–100 mm)a, mean (s.d.) | 71.6 (18.7) | 66.6 (21.3) | 0.0046 |
| Oral glucocorticoid useb, n (%) | 98 (53.3) | 252 (63.2) | 0.0234 |
Region 1: Western countries; region 2: South America; region 3: rest of world.
P-value was obtained using t-test for equality of variance or Satterthwaite’s t-test.
P-value was obtained using χ2 test or Fisher’s exact test.
n = 398 for the ‘sarilumab plus MTX’ treatment arm.
n = 178 for the ‘sarilumab’ treatment arm and n = 397 for the ‘sarilumab plus MTX’ treatment arm. CDAI: Clinical Disease Activity Index; DAS28-CRP: 28-joint Disease Activity Score using C reactive protein; HAQ-DI: HAQ-Disability Index; n: number of patients assessed; q2w: every 2 weeks.
Efficacy assessments
After adjusting for the selected baseline characteristics in MMRM, LS mean change from baseline at week 24 for all assessments was similar between the treatment arms with overlapping CIs (Fig. 1). Results of unadjusted comparisons were similar to adjusted comparisons (data not shown).
Fig. 1 .
Adjusted comparisons of LS mean change from baseline at week 24 using MMRMa
All values are LS mean change from baseline (95% CI) at week 24. Patients with non-missing endpoint values were considered. aMMRM assuming an unstructured covariance structure with endpoint value at baseline, sarilumab group, visit, sarilumab group-by-visit interaction and selected baseline characteristics (see Results section) as covariates. Anti-CCP: anti-cyclic citrullinated peptide; CDAI: Clinical Disease Activity Index; DAS28-CRP: 28-joint Disease Activity using C reactive protein; FACIT: Functional Assessment of Chronic Illness Therapy; Hb: haemoglobin; LS: least square; MMRM: mixed-effect model repeated measure; q2w: every 2 weeks; SJC: swollen joint count; VAS: visual analogue scale.
Responder analysis
At week 24, there were no discernible differences in the percentage of responders, for all outcomes between the treatment arms. In the ITT population, there were 42% responders in the monotherapy arm vs 43% responders in the combination treatment arm for CDAI LDA; 52% vs 49% for DAS28-CRP LDA; 85% vs 73% for CRP (mg/l) <10; 26% vs 38% for MCID in Hb; 73% vs 64% for MCID in pain VAS; and 66% vs 61% for MCID in FACIT-Fatigue (Supplementary Fig. S1, available at Rheumatology online). A similar trend was observed in responder analyses based on OC (Supplementary Fig. S1, available at Rheumatology online).
Safety
The safety profile of sarilumab has been previously reported, [12, 13] and was not part of this analysis.
Discussion
After 24 weeks of treatment with sarilumab, both monotherapy and combination therapy showed greater clinical improvement in MTX-IR/INT patients with RA in the respective clinical trials. This post hoc analysis showed that for all efficacy assessments, no differences were observed between monotherapy and combination therapy treatment arms suggesting similar effectiveness of these therapies in patients with RA.
Results of the current analysis are in line with the previous findings observed with another IL-6 receptor inhibitor, tocilizumab [18, 19]. In a study that compared two different tocilizumab-based treatment strategies in patients with active RA (ACT-RAY), no clinically relevant superiority was demonstrated with MTX plus tocilizumab add-on strategy compared with tocilizumab monotherapy [18]. Another study that compared tocilizumab monotherapy with its combination with DMARDs in patients with RA and inadequate responses to previous treatments also showed that the monotherapy and combination therapy were similarly effective [19]. However, a recent study reported that TNF inhibitors require comedication with csDMARDs to achieve optimal clinical efficacy [20, 21].
The results of this analysis suggest that sarilumab monotherapy may be a valuable treatment strategy when monotherapy with bDMARDs is recommended in certain patients with RA, specifically those who are MTX-IR/INT, and is in line with the EULAR recommendations for the management of RA [2]. This analysis provides preliminary evidence on similar effectiveness of sarilumab vs its combination with MTX, which might help rheumatologists in making informed treatment decisions, particularly, in MTX-IR/INT patients.
One limitation of this analysis is that the data analysed were obtained from two different study populations. To overcome these differences, adjusted comparisons were made between the treatment arms. Difference in the eligibility criteria did not allow the analyses to be adjusted for prior medication including comparison of the background MTX treatment between monotherapy and combination therapy treatment arms. Another limitation is that radiographic data were not obtained during the MONARCH study due to which it was not possible to account for potential differences in radiographic damage at baseline in this analysis. Moreover, the data included in this analysis were from a relatively short duration (24 weeks), which may not be sufficient to derive longer-term conclusions.
Conclusion
This post hoc analysis in patients with RA, based on the aggregate data from two clinical studies, demonstrated similar efficacy of sarilumab when administered as either monotherapy or in combination with MTX. These data suggest that sarilumab monotherapy may be considered as a potential treatment alternative for patients in whom combination therapy with MTX is not appropriate.
Supplementary Material
Acknowledgements
Medical writing support was provided by Sindhu Doppalapudi (Sanofi) and Nupur Chaubey (Sanofi).
Funding: This analysis was sponsored by Sanofi (Cambridge, MA, USA).
Disclosure statement: G.R.B. has received grant/research support, consulting fees and/or speaker fees/honoraria from AbbVie, Lilly, Merck Sharp & Dohme, Pfizer, Roche, Sanofi Genzyme and UCB; V.P.B. has received grants and/or consulting fees and/or other financial/material support from Amgen, the Cedar Hill Foundation, Gilead, the National Institutes of Health, Pfizer, Sanofi Genzyme/Regeneron and UCB; M.H.B. has received grants from Gilead, Pfizer, Roche and UCB and consulting/honoraria fees from AbbVie, Boehringer-Ingelheim, Eli Lilly and Company, Gilead, Merck, Serono, Pfizer, Sandoz and Sanofi; Y.T. has received speaking fees from Daiichi Sankyo, Inc., Astellas Pharma Inc., Pfizer Inc., Mitsubishi Tanabe Pharma, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd, YL Biologics Ltd, Eli Lilly and Company, Sanofi K.K., Janssen Pharmaceutical K.K. and UCB Japan Co. Ltd and has received research grants from Mitsubishi Tanabe Pharma, Takeda Pharmaceutical Company, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd, Astellas Pharma Inc., AbbVie G.K., MSD K.K., Daiichi Sankyo, Inc., Pfizer Inc., Kyowa Kirin, Inc., Eisai Co., Ltd and Ono Pharmaceutical Co., Ltd; H.K. has received consulting fees, speaking fees and/or honoraria from AbbVie G.K., Asahi Kasei Pharma Co., Ltd, Bristol-Myers Squibb, Chugai Pharmaceutical Co., Ltd, Eli Lilly Japan K.K., Gilead Sciences Inc., Janssen Pharmaceutical K.K., Mitsubishi Tanabe Pharma, Novartis Pharma K.K. and Sanofi K.K. and has received research grants from AbbVie G.K., Asahi Kasei Pharma Co., Ltd, Astellas Pharma Inc., Chugai Pharmaceutical Co., Ltd, Eisai Co., Ltd, Mitsubishi Tanabe Pharma and Novartis Pharma K.K.; A.P. is an employee of Sanofi; H.v.H. is an employee of Sanofi and may hold stock and/or stock options in the company; A.F-N. has received consulting fees from Roche and Stada Nordic ApS, and speaking fees and/or honoraria from Sanofi, Janssen, Pfizer, AbbVie, Novartis, Gedeon Richter, and Lilly; and T.H. has received grants and consultant fees from Ablynx, Bristol-Myers Squibb, Roche and Sanofi.
Data availability statement
Qualified researchers may request access to patient-level data and related documents (including, e.g. the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Contributor Information
Gerd R Burmester, Department of Rheumatology and Clinical Immunology, Charité—University Medicine Berlin, Berlin, Germany.
Vivian P Bykerk, Department of Rheumatology, Inflammatory Arthritis Center, Hospital for Special Surgery, New York, NY, USA.
Maya H Buch, Division of Musculoskeletal & Dermatological Sciences, Centre for Musculoskeletal Research, Faculty of Biology, Medicine & Health, University of Manchester; NIHR Manchester Biomedical Research Centre, Manchester, UK.
Yoshiya Tanaka, The First Department of Internal Medicine, School of Medicine, University of Occupational and Environmental Health, Kitakyushu, Japan.
Hideto Kameda, Division of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Toho University, Tokyo, Japan.
Amy Praestgaard, Department of Biostatistics, Sanofi, Cambridge, MA.
Hubert van Hoogstraten, Department of, Global Medical Affairs, Sanofi, Bridgewater, NJ, USA.
Antonio Fernandez-Nebro, Department of Rheumatology, UGC Rheumatology, Institute of Biomedical Research in Malaga (IBIMA), Regional University Hospital of Malaga, University of Málaga, Málaga, Spain.
Thomas Huizinga, Department of Rheumatology, Leiden University, Leiden, the Netherlands.
Supplementary data
Supplementary data are available at Rheumatology online.
References
- 1. Mian A, Ibrahim F, Scott DL. A systematic review of guidelines for managing rheumatoid arthritis. BMC Rheumatol 2019;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Smolen JS, Landewé RBM, Bijlsma JWJ et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. [DOI] [PubMed] [Google Scholar]
- 3. Michaud K, Vrijens B, Tousset E et al. Real-world adherence to oral methotrexate measured electonically in patients with established rheumatoid arthritis. ACR Open Rheumatol 2019;1:560–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Curtis JR, Bykerk VP, Aassi M, Schiff M. Adherence and persistence with methotrexate in rheumatoid arthritis: a systematic review. J Rheumatol 2016;43:1997–2009. [DOI] [PubMed] [Google Scholar]
- 5. Favalli EG. Understanding the role of interleukin-6 (IL-6) in the joint and beyond: a comprehensive review of IL-6 inhibition for the management of rheumatoid arthritis. Rheumatol Ther 2020;7:473–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hennigan S, Kavanaugh A. Interleukin-6 inhibitors in the treatment of rheumatoid arthritis. Ther Clin Risk Manag 2008;4:767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yip RML, Yim CW. Role of interleukin 6 inhibitors in the management of rheumatoid arthritis. J Clin Rheumatol 2019;doi: 10.1097/RHU.0000000000001293. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KEVZARA (sarilumab) injection, for subcutaneous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761037s000lbl.pdf (8 March 2021, date last accessed).
- 9. Boyce EG, Rogan EL, Vyas D, Prasad N, Mai Y. Sarilumab: review of a second IL-6 receptor antagonist indicated for the treatment of rheumatoid arthritis. Ann Pharmacother 2018;52:780–91. [DOI] [PubMed] [Google Scholar]
- 10. Crotti C, Biggioggero M, Becciolini A, Favalli EG. Sarilumab: patient-reported outcomes in rheumatoid arthritis. Patient Relat Outcome Meas 2018;9:275–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lee EB. A review of sarilumab for the treatment of rheumatoid arthritis. Immunotherapy 2018;10:57–65. [DOI] [PubMed] [Google Scholar]
- 12. Burmester GR, Lin Y, Patel R et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis 2017;76:840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Strand V, Kosinski M, Chen CI et al. Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial. Arthritis Res Ther 2016;18:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Efficacy and safety of sarilumab and adalimumab monotherapy in patients with rheumatoid arthritis (SARIL-RA-MONARCH). https://clinicaltrials.gov/ct2/show/NCT02332590 (8 March 2021, date last accessed).
- 15.Evaluation of Sarilumab (SAR153191/REGN88) on top of methotrexate in rheumatoid arthritis patients (RA-MOBILITY). https://clinicaltrials.gov/ct2/show/NCT01061736 (8 March 2021, date last accessed).
- 16. Genovese MC, Fleischmann R, Kivitz AJ et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 17. Strand V, Gossec L, Proudfoot CWJ et al. Patient-reported outcomes from a randomized phase III trial of sarilumab monotherapy versus adalimumab monotherapy in patients with rheumatoid arthritis. Arthritis Res Ther 2018;20:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dougados M, Kissel K, Sheeran T et al. Adding tocilizumab or switching to tocilizumab monotherapy in methotrexate inadequate responders: 24-week symptomatic and structural results of a 2-year randomised controlled strategy trial in rheumatoid arthritis (ACT-RAY). Ann Rheum Dis 2013;72:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bykerk VP, Östör AJ, Alvaro-Gracia J et al. Comparison of tocilizumab as monotherapy or with add-on disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and inadequate responses to previous treatments: an open-label study close to clinical practice. Clin Rheumatol 2015;34:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Finckh A, Tellenbach C, Herzog L et al. ; on behalf of the physicians and patients of the SCQM. Comparative effectiveness of antitumour necrosis factor agents, biologics with an alternative mode of action and tofacitinib in an observational cohort of patients with rheumatoid arthritis in Switzerland. RMD Open 2020;6:e001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Silvagni E, Bortoluzzi A, Carrara G et al. Comparative effectiveness of first-line biological monotherapy use in rheumatoid arthritis: a retrospective analysis of the RECord-linkage On Rheumatic Diseases study on health care administrative databases. BMJ Open 2018;8:e021447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to patient-level data and related documents (including, e.g. the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications). Patient-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.