Abstract
Objectives
Tocilizumab, an anti-IL-6 receptor antibody, was investigated in patients with refractory Takayasu arteritis (TAK) in a phase 3 randomized controlled trial. In this post hoc analysis, we investigated whether tocilizumab treatment inhibited the progression of vascular lesions caused by TAK in these patients.
Methods
Included patients received at least one dose of tocilizumab and underwent CT at baseline and at week 48 after tocilizumab initiation. Three radiologists not involved in the original trial independently evaluated the CT images. Twenty-two arteries from each patient were assessed for change from baseline in wall thickness (primary endpoint), dilatation/aneurysm, stenosis/occlusion or wall enhancement for at least 96 weeks after tocilizumab initiation. Patient-level assessments were also conducted.
Results
In 28 patients, 86.7% of 22 arteries had improved or stable wall thickness at week 96. Proportions of patients with improved or stable, partially progressed or newly progressed lesions were 57.1%, 10.7% and 28.6%, respectively, for wall thickness; proportions with improved or stable lesions were 92.9% for dilatation/aneurysm, and 85.7% for stenosis/occlusion. Patients with newly progressed lesions, reflecting more refractory disease, were prescribed glucocorticoids at dosages that could not be reduced below 0.1 mg/kg/day at week 96.
Conclusions
Approximately 60% of patients with TAK did not experience progression in wall thickness within 96 weeks after initiation of tocilizumab treatment. Few patients experienced progressed dilatation/aneurysm, or stenosis/occlusion. Wall thickness progression likely resulted from refractory TAK. Patients who experience this should be monitored regularly by imaging, and additional glucocorticoid or immunosuppressive treatment should be considered to avoid vascular progression.
Trial registration
Japan Pharmaceutical Information Centre number, JapicCTI-142616.
Keywords: biological therapies, CT scanning, cardiovascular, Takayasu’s disease, vasculitis
Rheumatology key messages.
Fifty-seven percent of patients with Takayasu arteritis receiving tocilizumab experienced no progression in artery wall thickness.
Few patients with refractory Takayasu arteritis receiving tocilizumab experienced progression in dilatation/aneurysm or stenosis/occlusion.
Patients with refractory disease should be monitored regularly by imaging to avoid vascular progression.
Introduction
Takayasu arteritis (TAK) is a rare large-vessel vasculitis characterized by inflammation of the aorta and its main branches, and of the pulmonary arteries. TAK most often manifests in women at ∼20 years of age [1–4]. Infiltration of inflammatory cells into large vessels and release of proinflammatory cytokines, including IFN-γ, TNF-α, IL-6, IL-8, IL-17A and IL-18, and acute-phase reactants such as CRP, occur during the pathogenesis of TAK. Elevated levels of these cytokines are detected in the sera of patients with TAK [5–8], levels of IL-6 correlate with disease activity [9] and elevated CRP levels are associated with relapse [10]. Arterial inflammation can result in arterial wall thickening and remodelling, leading to fibrosis, stenosis or dilatation, aneurysm and thrombus formation [11, 12].
Classification criteria have been developed based on clinical and imaging features [13], but measurement of acute-phase reactants, such as CRP and ESR, might not adequately reflect TAK disease activity. Guidelines state that patients with suspected TAK should be referred for large-vessel imaging. The use of imaging modalities, such as ultrasonography, MRI, CT and [18F]-fluorodeoxyglucose PET, has become central to the diagnosis and management of TAK [4, 11, 14–17].
Tocilizumab is an anti-IL-6 receptor mAb that blocks IL-6 signalling and reduces inflammation [18]. The TAKT study was a randomized, placebo-controlled, double-blind, phase 3 trial that investigated the efficacy and safety of tocilizumab for the treatment of refractory TAK. The trial enrolled 36 patients with refractory TAK and included a double-blind period that lasted until 19 patients experienced relapse and a subsequent open-label extension period of at least 96 weeks’ duration [19, 20]. The primary endpoint was not met in this trial; the hazard ratio for time to relapse after the induction of remission with glucocorticoids was 0.41 (95.41% CI 0.15, 1.10; P = 0.0596) [19]. Although the primary endpoint was not met, the results suggested a benefit of tocilizumab over placebo in the time to relapse. After 96 weeks of treatment, the median glucocorticoid dosage was reduced to 0.105 (interquartile range 0.039–0.153) mg/kg/day, which was less than half that administered at the time of relapse before study entry [20]. Patients in the TAKT trial also reported clinically relevant improvements in patient-reported outcome measures with tocilizumab treatment [20]. In addition, imaging evaluation (CT or MRI performed by the investigating physician at each study site) suggested a favourable effect of tocilizumab in the open-label period (85.7% of patients had improved or stable condition after 96 weeks compared with baseline) [20]. Although several case reports have been published on vascular outcomes in patients with TAK treated with tocilizumab [21, 22], evaluation of vascular progression in a larger patient population is needed.
The imaging evaluation conducted in the original TAKT trial and the long-term extension was performed by the investigator’s assessment, but additional details were not collected and evaluated. Therefore, we performed a post hoc analysis of the TAKT trial to investigate whether tocilizumab can inhibit the progression of vascular lesions in patients with refractory TAK.
Methods
Patients and study design
The TAKT study was a double-blind, placebo-controlled, multicentre trial conducted in Japan (Japan Pharmaceutical Information Centre number, JapicCTI-142616). Patients at least 12 years of age with a diagnosis of TAK [23] were enrolled in the TAKT trial between 2 October 2014 and 31 August 2015 [19]. Patients had to have experienced a relapse of TAK within the 12 weeks before enrolment despite having received glucocorticoids (prednisone-equivalent dosage at least 0.2 mg/kg/day). Relapse was determined by the investigator and required signs in at least two of five categories (objective systemic symptoms, subjective systemic symptoms, elevated inflammation markers, vascular signs and symptoms, ischaemic symptoms), with TAK confirmed as the cause [19].
Patients who experienced a relapse of TAK were induced into remission by treatment with glucocorticoids at a dose at least twice that received at the time of relapse. After remission (defined as published [19]) of at least 1-week duration was achieved, patients were randomly assigned (1:1) to receive weekly s.c. injections of tocilizumab 162 mg or placebo in combination with scheduled glucocorticoid tapering (10% per week from week 4 to a minimum of 0.1 mg/kg/day). Thirty-six patients with refractory TAK were enrolled, and the double-blind period of the trial continued until 19 patients experienced a first relapse of TAK. Patients who completed the double-blind period were followed up during open-label treatment with tocilizumab 162 mg/week for at least 96 weeks. Oral glucocorticoid doses could be tapered during the open-label period at the investigator’s discretion according to clinical data and patient symptoms.
All patients gave written informed consent to participate in the study, which was conducted in accordance with the declaration of Helsinki and Good Clinical Practice, and was approved by the Institutional Review Board of Osaka University Hospital and other appropriate institutional review boards (listed in Supplementary Data S1, available at Rheumatology online).
Imaging assessments
This post hoc analysis was planned and conducted after the results of the TAKT trial and the long-term extension became available. Images included in this analysis were obtained from patients randomly assigned in the TAKT trial who had contrast CT images taken at baseline and at week 48 after tocilizumab initiation. Images at each timepoint were selected for evaluation according to the following predefined criteria: for the tocilizumab group, images obtained before the first double-blind dose, 48 weeks after the first double-blind dose and every 48 weeks thereafter, and at occurrence of any signs of relapse in the double-blind or open-label period; for the placebo group, images obtained before the first double-blind dose, before the first dose of open-label tocilizumab, 48 weeks after the first double-blind dose and every 48 weeks thereafter, and at occurrence of any signs of relapse (excluding relapse that was ongoing from the double-blind period) during the open-label period. CT imaging protocols have already been published [19].
The selected images were evaluated by three independent readers: two first readers (M.Y. and A.H.) and one second reader (H.H.) (supplementary Table S1, available at Rheumatology online). If the assessments of the first readers agreed, the result was used as the final assessment. If the assessments of the first readers differed, independent assessment was performed by the second reader; if the second reader agreed with one of the first readers, the independent assessment was used as the final assessment. If all three readers made different assessments, the median assessment was used as the final assessment; for example, if the three results were improved, stable and progressed, the final assessment was stable. If one of the readers considered the images unevaluable, an independent assessment was performed by the second reader. If the second reader agreed with the other first reader, this assessment was used as the result. If, however, the second reader considered the images to be unevaluable or disagreed with the other first reader, the final result was considered to be unevaluable.
Twenty-two arteries of interest were assessed in each CT image—right and left carotid arteries, right and left vertebral arteries, brachiocephalic artery, right and left subclavian arteries, right and left axillary arteries, ascending aorta, aortic arch, descending aorta, abdominal aorta, coeliac artery, superior mesenteric artery, right and left renal arteries, right and left iliac arteries, pulmonary trunk, and right and left pulmonary arteries—based on modified methods [24]. Assessments included wall thickness, dilatation or aneurysm, stenosis (<50% and at least 50% to <99%), occlusion and wall enhancement (assessed by uptake of contrast dye in contrast CT images) (additional details in supplementary Table S2, available at Rheumatology online).
Endpoints
The primary endpoint in this post hoc analysis was the change from baseline (defined as before the first dose of double-blind study medication) in wall thickness at week 96 (i.e. 96 weeks after the first dose of tocilizumab) assessed for each artery by central radiological assessment. Secondary endpoints assessed for each artery and each patient were wall thickness, stenosis/occlusion, dilatation/aneurysm, wall enhancement over time and change from baseline. Aggregate assessment criteria were used to evaluate each artery or each patient as improved, stable, partially progressed or newly progressed (supplementary Table S3, available at Rheumatology online).
Statistical analysis
Analyses were performed in all patients from the TAKT study who had available CT images. Partially progressed and newly progressed endpoints for each artery and all endpoints for each patient were exploratory analyses defined after data collection. Assessments were conducted in all patients, even if some arteries were unevaluable. If all arteries were unevaluable, the final result for the patient was assigned as unevaluable. Analysis by artery involved counting the number of arteries that met prespecified criteria among the 22 arteries analysed (supplementary Table S3, available at Rheumatology online). Analysis by patient involved counting the number of patients who met prespecified criteria. CIs for response rates were calculated as descriptive statistics using the Pearson–Clopper exact method. SAS software version 9.2 was used for all analyses.
Results
Patients and baseline imaging
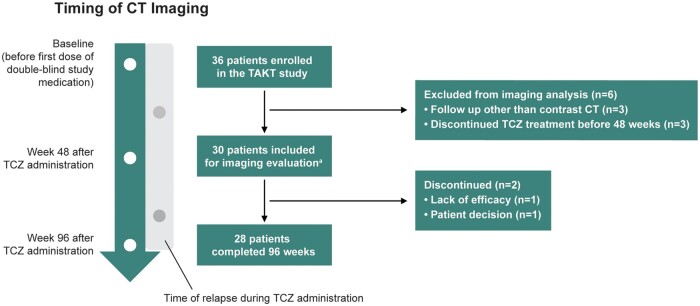
Overall, 36 patients were enrolled in the TAKT study. Six patients were excluded from the current analysis, three because they were followed up with imaging other than contrast CT and three because they discontinued tocilizumab treatment before week 48. Among 30 patients who received at least one dose of tocilizumab in the TAKT study, 28 were evaluated by central radiological imaging over the 96-week study period (Fig. 1). One patient withdrew from the study 48 weeks after the first tocilizumab dose because of a lack of efficacy, and another withdrew 82 weeks after the first tocilizumab dose based on a personal decision. Baseline demographics and disease characteristics were comparable among the 30 patients evaluated in this analysis (supplementary Table S4, available at Rheumatology online) and in the entire TAKT study population [19, 20].
Fig. 1.
Patient disposition
aPatients who had contrast CT images evaluable before and after administration of tocilizumab for ≥48 weeks. TCZ: tocilizumab.
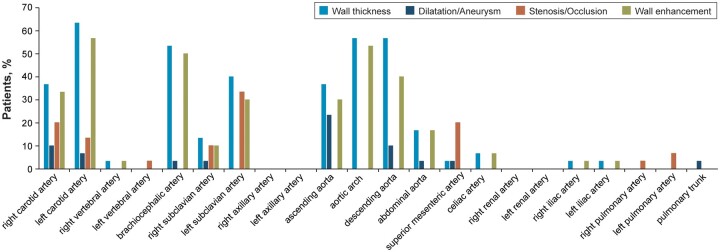
Among the 22 arteries assessed, there was a mean (s.d.) involvement of 3.9 (2.6) arteries with increased wall thickness, 0.7 (1.0) with dilatation or aneurysm, 1.1 (1.1) with stenosis or occlusion, and 3.4 (2.7) with wall enhancement at baseline for each patient by artery (Table 1). The proportion of patients with increased wall thickness at baseline exceeded 30% in the left and right carotid arteries, brachiocephalic artery, left subclavian artery, ascending aorta, aortic arch and descending aorta (Fig. 2). The left vertebral artery, right and left axillary arteries, right and left renal arteries, right and left pulmonary arteries, and pulmonary trunk did not show any wall thickness at baseline. Almost all patients (29/30; 96.7%) had more than one lesion of increased wall thickness in any artery at baseline; 13 (43.3%) had dilatation or aneurysm, 21 (70.0%) had stenosis or occlusion and 25 (83.3%) had wall enhancement, by patient (supplementary Table S4, available at Rheumatology online).
Table 1.
Assessment of arteries at baselinea
| Arteries, mean (s.d.) (%) | Normal | Arterial lesions | Unevaluable |
|---|---|---|---|
| Wall thickness | 16.8 (4.2) (76.2) | 3.9 (2.6) (17.9) | 1.3 (4.0) (5.9) |
| Dilatation/aneurysm | 20.8 (1.3) (94.5) | 0.7 (1.0) (3.0) | 0.5 (0.8) (2.4) |
| Stenosis/occlusion | 19.7 (3.9) (89.5) | 1.1 (1.1) (5.0) | 1.2 (4.0) (5.5) |
| Wall enhancement | 16.7 (4.2) (76.1) | 3.4 (2.7) (15.3) | 1.9 (4.1) (8.6) |
Data are shown for all patients with central radiological assessment (N = 30). aPercentages are based on 22 arteries assessed in each patient.
Fig. 2.
Assessment of arteries at baseline
Imaging evaluation by artery after tocilizumab treatment
The primary endpoint—change from baseline to week 96 in wall thickness assessed per artery—was assessed in the 28 patients who received tocilizumab; 86.7% of arteries had improved (2.1%) or stable (84.6%) wall thickness lesions at week 96 [Table 2; measurement for stable arteries includes normal and abnormal arteries at baseline (71.3% + 13.3% = 84.6%)]. The mean (s.d.) number of improved arteries at week 96 was 0.5 (0.9), whereas 18.6 (4.2) were stable, 0.5 (1.0) showed partial progression and 0.8 (1.5) showed new progression {1.6 (4.1) were unevaluable; measurement for stable arteries includes normal and abnormal arteries at baseline [15.7 (4.2) + 2.9 (2.5) = 18.6 (4.2)]}.
Table 2.
Assessment of arteries at week 96 in patients who received tocilizumaba
| Arteries, mean (s.d.) (%) |
Improved | Stable | Partially progressed | Newly progressed | Unevaluable | ||
|---|---|---|---|---|---|---|---|
| Baseline condition | Abnormal | Total | Abnormal | Normal | Abnormal | Normal | NA |
| Wall thickness |
0.5 (0.9) (2.1) |
18.6 (4.2) (84.6) |
2.9 (2.6) (13.3) |
15.7 (4.3) (71.3) |
0.5 (1.0) (2.4) |
0.8 (1.5) (3.6) |
1.6 (4.1) (7.3) |
| Dilatation/aneurysm |
0.0 0 |
21.2 (1.0) (96.3) |
0.6 (1.0) (2.9) |
20.5 (1.4) (93.3) |
0.1 (0.3) (0.3) |
0.1 (0.6) (0.5) |
0.6 (0.8) (2.9) |
| Stenosis/occlusion |
0.1 (0.4) (0.6) |
20.3 (4.1) (92.2) |
1.0 (1.0) (4.4) |
19.3 (4.0) (87.8) |
0.0 (0.0) |
0.2 (0.8) (1.0) |
1.4 (4.1) (6.2) |
| Wall enhancement |
0.0 (0.2) (0.2) |
19.0 (4.4) (86.5) |
3.4 (2.8) (15.3) |
15.7 (4.1) (71.3) |
0.0 (0.0) |
0.6 (1.3) (2.6) |
2.4 (4.3) (10.7) |
Data are shown for all patients with central radiological assessment who received at least one dose of tocilizumab (N = 28). aPercentages are based on 22 arteries assessed in each patient. Imaging data were evaluated at 96 weeks (day 673; range, days 505–841). If multiple scans were available during the time window, the scan conducted closest to the scheduled date was used. NA, not applicable.
Imaging evaluation by patient after tocilizumab treatment
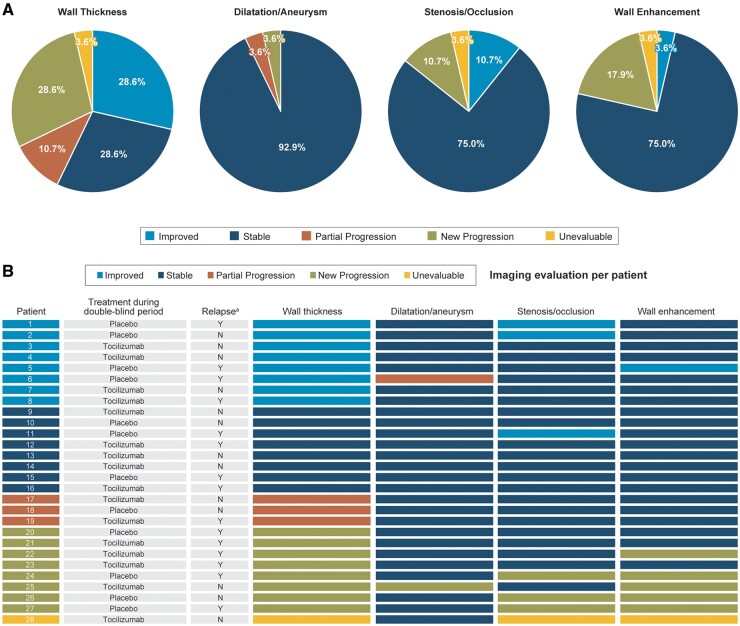
Secondary endpoints—changes from baseline in wall thickness, stenosis/occlusion, and dilatation/aneurysm lesions at week 96—were assessed for each of the 28 patients who received tocilizumab. Improved (n = 8; 28.6%) or stable (n = 8; 28.6%) wall thickness lesions were observed in 57.1% of patients, partial progression in 10.7% (n = 3) and new progression in 28.6% (n = 8) (Fig. 3A).
Fig. 3.
Assessment of imaging evaluation by patient after tocilizumab treatment for 96 weeks (N = 28)
(A) Change from baseline in the condition of arteries in patients treated with tocilizumab for 96 weeks. (B) Outcomes for wall thickness, dilatation/aneurysm, stenosis/occlusion, and wall enhancement after 96 weeks of tocilizumab treatment. aPatients who experienced relapse of Takayasu arteritis during 96 weeks of tocilizumab treatment: Y: yes; N: no. Imaging data were evaluated at 96 weeks (day 673; range, days 505–841). If multiple scans were available during the time window, the scan conducted closest to the scheduled date was used.
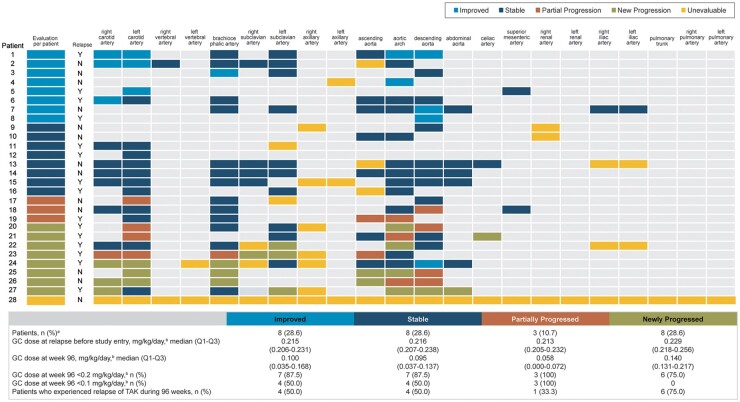
Among six of eight patients with newly progressed lesions, the median glucocorticoid dosage at week 96 (0.140 mg/kg/day; interquartile range 0.131–0.217) was less than the dosage of >0.2 mg/kg/day received at relapse before study entry (Fig. 4). No patients with newly progressed lesions achieved a dosage of <0.1 mg/kg/day, whereas 50% of patients with improved or stable lesions achieved a dosage of <0.1 mg/kg/day. The rate of relapse from TAK in the eight patients with newly progressed lesions was 75.0% (n = 6), which was slightly higher than the rate among patients who had improved (50.0%) and stable (50.0%) lesions. Three of these eight patients with newly progressed lesions withdrew from tocilizumab treatment during the study period because of adverse events [two infections (furuncle and pneumonia caused by Haemophilus parainfluenza), one pulmonary infarction] [20], and three patients experienced relapse of TAK after tocilizumab treatment was interrupted.
Fig. 4.
Change from baseline to week 96 in wall thickness and glucocorticoid doses (N = 28)
aOne patient was unevaluable for wall thickness. bPrednisone equivalent. Data are shown for all patients with central radiological assessment who received at least one dose of tocilizumab. Light grey spaces in the grid indicate no abnormal arteries were detected either at baseline or at week 96, and the patient’s condition was evaluated as stable. Imaging data were evaluated at 96 weeks (day 673; range, days 505–841). If multiple scans were available during the time window, the scan conducted closest to the scheduled date was used. Y: yes; N: no; GC: glucocorticoid; TAK: Takayasu arteritis.
Patients who had arteries with improved or newly progressed wall thickness tended to be younger and to have shorter durations of disease than patients who had stable or partially progressed wall thickness (supplementary Table S5, available at Rheumatology online).
Three patients (10.7%) had partially progressed wall thickness; one had a long placebo treatment period (56 weeks), two experienced partial progression in one artery and one experienced partial progression in two arteries. Glucocorticoid dosages were <0.1 mg/kg/day at week 96 in all three patients, and only one of them experienced relapse of TAK during mandatory glucocorticoid tapering in the double-blind period (Fig. 4).
Stable dilatation/aneurysm was observed in 92.9% of patients; no patients had improved dilatation/aneurysm, and 7.1% of patients had partial lesions (n = 1; 3.6%) or newly progressed lesions (n = 1; 3.6%) of dilatation/aneurysm (Fig. 3A; supplementary Fig. S1A, available at Rheumatology online). Improved or stable stenosis/occlusion was observed in 24 patients (85.7%) [21 (75.0%) stable and three (10.7%) improved], and new progression of lesions of stenosis/occlusion was observed in three patients (10.7%) (Fig. 3A; supplementary Fig. S1B, available at Rheumatology online). Seventy-five percent of patients had stable wall enhancement, 3.6% had improved wall enhancement and 17.9% had newly progressed wall enhancement (Fig. 3A; supplementary Fig. S1C, available at Rheumatology online). All patients with newly progressed lesions of dilatation/aneurysm, stenosis/occlusion and wall enhancement also had newly progressed wall thickness (Fig. 3B).
Assessment of change from baseline in the condition of arteries assessed per patient at week 48, at the time of relapse and at week 96 showed that among all patients in the analysis, the proportion who had arteries with improved wall thickness was numerically higher at week 96 (8/28; 28.6%) than at week 48 (4/30; 13.3%). No patients had improved dilatation/aneurysm at week 48 or 96; five patients had improved stenosis/occlusion, two at week 48 and three at week 96; and one patient had improved wall enhancement at weeks 48 and 96 (supplementary Table S6, available at Rheumatology online).
Among 10 patients who experienced relapse during the study and had imaging results of relapse that occurred during 96 weeks of tocilizumab treatment, one (10.0%) had partially progressed wall thickness and four (40.0%) had newly progressed wall thickness compared with baseline (supplementary Table S7, available at Rheumatology online).
Agreement between readers
Agreement between the first readers in assessment of the change from baseline results was good, with an observed agreement rate of 0.79 (95% CI 0.77, 0.81) and a Cohen’s kappa coefficient of 0.31 (95% CI 0.26, 0.36) for wall thickness, 0.97 (95% CI 0.96, 0.98) and 0.50 (95% CI 0.38, 0.61), respectively, for dilatation or aneurysm, 0.92 (95% Cl: 0.91, 0.94) and 0.31 (95% CI 0.23, 0.40), respectively, for stenosis or occlusion, and 0.86 (95% CI 0.84, 0.87) and 0.24 (95% CI 0.18, 0.30), respectively, for wall enhancement (supplementary Table S8, available at Rheumatology online).
Discussion
Assessment of disease activity in TAK is complex and challenging; therefore, vascular imaging at regular intervals is essential to detect improvement in or progression of vascular lesions in patients with TAK [25]. This study provides the first in-depth analysis of vascular imaging evaluation in patients with TAK who received long-term treatment with tocilizumab in a clinical trial. In this analysis, imaging evaluation was assessed by up to three independent readers who were not involved in the original TAKT study. Twenty-two arteries were comprehensively evaluated for lesions of wall thickness, dilatation/aneurysm, stenosis/occlusion, and wall enhancement according to modified methods [24]. These evaluations provided a robust and balanced assessment of the effect of tocilizumab on TAK vascular lesions for ≤96 weeks of treatment.
The results of this study show inhibition of progression of vascular lesions in the arteries of patients with TAK who were able to continue tocilizumab treatment for 96 weeks. Among patients who received tocilizumab in the TAKT trial, a mean of 0.5 arteries were improved and 18.6 were stable compared with baseline for the primary endpoint of wall thickness. Compared with baseline, 57.1% of patients had improved or stable wall thickness, 92.9% had stable dilatation/aneurysm, 85.7% had improved or stable stenosis/occlusion, and 75.0% had stable wall enhancement. These results suggest that tocilizumab treatment was effective in inhibiting vascular lesion progression in these patients.
Partial progression of wall thickness was observed in three patients (10.7%) in the current analysis; however, TAK-mediated inflammation appeared to be controlled in these patients because two experienced partial progression in only one artery, the other experienced partial progression in two arteries and all three were receiving glucocorticoid dosages of <0.1 mg/kg/day at week 96. Furthermore, one patient had a 56-week placebo treatment period and therefore experienced partial progression in wall thickness before receiving tocilizumab. It remains to be determined whether additional treatment is needed for patients who experience progression only in wall thickness.
Newly progressed wall thickness was observed in eight patients (28.6%), suggesting that some had inadequate response to tocilizumab treatment. Patients with newly progressed lesions in wall thickness were able to decrease their glucocorticoid dosage compared with the dosage received at the time of relapse before study entry, but not to <0.1 mg/kg/day. In addition, six of these patients experienced relapse during tocilizumab treatment in the study, suggesting that they might have had more refractory TAK. Reports from a retrospective analysis of patients with TAK treated with prednisone and MTX showed that 75% of patients developed new vascular lesions [26], which is higher than the rate observed in our study. Overall, in our study, 10.7% of patients had partial or newly progressed stenosis/occlusion and 7.1% had partial or newly progressed dilatation/aneurysm. Furthermore, the patients who had newly progressed dilatation/aneurysm, stenosis/occlusion and wall enhancement also had newly progressed wall thickness. Additional treatment should be considered for patients who experience progression of dilatation/aneurysm, and stenosis/occlusion, because this type of vascular damage is irreversible and progression of aneurysm would necessitate surgery. Newly progressed wall thickness is likely a result of refractory TAK; patients who experience this should be monitored regularly by imaging assessment and should receive an increase in glucocorticoid dose or additional immunosuppressive treatment or they should consider switching to TNF inhibitors to avoid vascular progression.
Previous work suggests that wall enhancement (imaged by MRI) may not be a reliable index of arteries [27]. However, five of the eight patients in our study who had new progression of wall thickness also demonstrated new progression of wall enhancement, suggesting that evaluation of wall thickness and wall enhancement is important in assessing vascular abnormalities in TAK.
Although the results presented here were based on a small number of patients, younger age and shorter disease duration were observed in patients with improved or newly progressed wall thickness than in those who had stable lesions or partial progression, suggesting that these patients should be monitored carefully by vascular imaging during tocilizumab treatment.
During tocilizumab treatment, TAK relapse compared with baseline was indicated by the observation of newly progressed wall thickness in four of 10 patients (40.0%). Patients whose imaging evaluation shows progression of wall thickness after TAK relapse should undergo imaging tests to monitor for vascular lesions.
Higher proportions of patients in this study had improved wall thickness at week 96 than at week 48, which reflects the slow nature of the disease and suggests that long-term tocilizumab treatment might be required to improve artery wall thickness in patients with refractory TAK. Long-term tocilizumab treatment might also allow patients to reduce their glucocorticoid dose given that 46.4% of patients tapered their dosage to <0.1 mg/kg/day by week 96 of the TAKT study [20].
Study limitations
This study was a post hoc analysis of data from a published trial [19, 20]; however, the analysis was conducted using readers who were not involved in the original analysis and therefore provided an in-depth and independent analysis of the CT scans obtained during the trial. Patients who were randomly assigned to receive placebo in the double-blind period did not have their changes assessed from the first dose of open-label tocilizumab but rather from the time of randomization to the placebo arm [20]. Therefore, the results of this study did not take into account the effects of the double-blind period in the placebo group. The follow-up period of the TAKT study was limited to 96 weeks, and data collected at randomization were used as the baseline from which changes in outcomes were assessed. Hence, longer follow-up and real-world data might be required to evaluate vascular imaging in patients with TAK treated with tocilizumab over a longer time period [22].
Conclusions
Approximately 60% of patients with refractory TAK treated with tocilizumab did not experience progression of wall thickness. Few patients experienced progressed dilatation/aneurysm, or stenosis/occlusion. The observation of progressed wall thickness is likely a result of refractory TAK, and patients who experience this should be monitored regularly by imaging assessment; furthermore, an increased glucocorticoid dose or additional immunosuppressive treatment should be considered to avoid vascular progression.
Acknowledgements
The authors thank the investigators who provided imaging data from the TAKT study (Supplementary Data, available at Rheumatology online). Third-party medical writing assistance was provided by ApotheCom (San Francisco, CA, USA) and was funded by Chugai Pharmaceutical Co., Ltd (Tokyo, Japan). All authors contributed to the conception and design of study.
Y.N., M.Y., A.H., K.Y., N.O., S.Y. and H.H. carried out analysis of data.
Interpretation of data was done by all authors.
Y.N., K.Y., N.O. and S.Y. drafted the manuscript.
All authors revised the manuscript critically for important intellectual content, and gave final approval of the manuscript to be submitted.
All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by Chugai Pharmaceutical Co., Ltd. The funder of the study had a role in the study design, provision of study drugs, protocol development, safety monitoring, data collection, data analysis, data interpretation and writing of the report, in collaboration with the study authors. All authors had access to study data and had final responsibility for the decision to submit for publication.
Disclosure statement: Y.N. reports consulting fees or honoraria from Chugai Pharmaceutical Co., Ltd (Chugai) related to the submitted work and consultancy fees from AbbVie; grants from Chugai, Bayer, Daiichi-Sankyo, Takeda, Astellas and Tanabe-Mitsubishi; and payment for lectures or speaker bureaus from Chugai, Takeda, Japan Blood Products Organization and Actelion outside the submitted work. M.Y. reports consulting fees or honoraria from Chugai related to the submitted work and travel/accommodation/meeting expenses from Canon Medical Systems outside the submitted work. A.H. reports consulting fees or honoraria from Chugai related to the submitted work. K.Y., N.O. and S.Y. are employees of Chugai. H.H. reports consulting fees or honoraria from Chugai related to the submitted work. D.J. reports board membership for Aurinia Pharmaceuticals; consultancy fees from AstraZeneca, Boehringer Ingelheim, ChemoCentryx, Chugai, CSL, GlaxoSmithKline, Infla-RX, Insmed, Novartis, Takeda and Vifor; and grants from ChemoCentryx, GlaxoSmithKline, Roche/Genentech and Sanofi-Genzyme outside the submitted work.
Data availability statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). For further details on Chugai’s Data Sharing Policy and how to request access to related clinical study documents, see www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html.
Supplementary data
Supplementary data are available at Rheumatology online.
Supplementary Material
Contributor Information
Yoshikazu Nakaoka, Department of Vascular Physiology, National Cerebral and Cardiovascular Centre Research Institute, Suita; Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine.
Masahiro Yanagawa, Department of Diagnostic and Interventional Radiology, Osaka University Graduate School of Medicine, Osaka.
Akinori Hata, Department of Diagnostic and Interventional Radiology, Osaka University Graduate School of Medicine, Osaka.
Katsuhisa Yamashita, Medical Science Department, Medical Affairs.
Norihiro Okada, Biometrics Department.
Shinji Yamakido, Department of Primary Clinical Development, Chugai Pharmaceutical Co., Ltd, Tokyo.
Hiromitsu Hayashi, Department of Radiology, Nippon Medical School, Tokyo, Japan.
David Jayne, Department of Medicine, University of Cambridge, Cambridge, UK.
References
- 1. Mason JC. Takayasu arteritis—advances in diagnosis and management. Nat Rev Rheumatol 2010;6:406–15. [DOI] [PubMed] [Google Scholar]
- 2. Chatterjee S, Flamm SD, Tan CD, Rodriguez ER. Clinical diagnosis and management of large vessel vasculitis: Takayasu arteritis. Curr Cardiol Rep 2014;16:499. [DOI] [PubMed] [Google Scholar]
- 3. Watanabe Y, Miyata T, Tanemoto K. Current clinical features of new patients with Takayasu arteritis observed from cross-country research in Japan: age and sex specificity. Circulation 2015;132:1701–9. [DOI] [PubMed] [Google Scholar]
- 4. Isobe M, Amano K, Arimura Y et al. ; JCS Joint Working Group. JCS 2017 guideline on management of vasculitis syndrome—digest version. Circ J 2020;84:299–359. [DOI] [PubMed] [Google Scholar]
- 5. Park MC, Lee SW, Park YB, Lee SK. Serum cytokine profiles and their correlations with disease activity in Takayasu’s arteritis. Rheumatology (Oxford) 2006;45:545–8. [DOI] [PubMed] [Google Scholar]
- 6. Alibaz-Oner F, Yentur SP, Saruhan-Direskeneli G, Direskeneli H. Serum cytokine profiles in Takayasu’s arteritis: search for biomarkers. Clin Exp Rheumatol 2015;33:S-32-5. [PubMed] [Google Scholar]
- 7. Saadoun D, Garrido M, Comarmond C et al. Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol 2015;67:1353–60. [DOI] [PubMed] [Google Scholar]
- 8. Kong X, Sun Y, Ma L et al. The critical role of IL-6 in the pathogenesis of Takayasu arteritis. Clin Exp Rheumatol 2016;34(Suppl 97):S21–7. [PubMed] [Google Scholar]
- 9. Noris M, Daina E, Gamba S, Bonazzola S, Remuzzi G. Interleukin-6 and RANTES in Takayasu arteritis: a guide for therapeutic decisions? Circulation 1999;100:55–60. [DOI] [PubMed] [Google Scholar]
- 10. Comarmond C, Biard L, Lambert M et al. ; French Takayasu Network. Long-term outcomes and prognostic factors of complications in Takayasu arteritis: a multicenter study of 318 patients. Circulation 2017;136:1114–22. [DOI] [PubMed] [Google Scholar]
- 11. Tombetti E, Mason JC. Takayasu arteritis: advanced understanding is leading to new horizons. Rheumatology (Oxford) 2019;58:206–19. [DOI] [PubMed] [Google Scholar]
- 12. Yoshifuji H. Pathophysiology of large vessel vasculitis and utility of interleukin-6 inhibition therapy. Mod Rheumatol 2019;29:287–93. [DOI] [PubMed] [Google Scholar]
- 13. Arend WP, Michel BA, Bloch DA et al. The American College of Rheumatology 1990 criteria for the classification of Takayasu arteritis. Arthritis Rheum 1990;33:1129–34. [DOI] [PubMed] [Google Scholar]
- 14. Dejaco C, Ramiro S, Duftner C et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Ann Rheum Dis 2018;77:636–43. [DOI] [PubMed] [Google Scholar]
- 15. Hellmich B, Agueda A, Monti S et al. 2018 Update of the EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis 2020;79:19–30. [DOI] [PubMed] [Google Scholar]
- 16. Bardi M, Diamantopoulos AP. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice summary. Radiol Med 2019;124:965–72. [DOI] [PubMed] [Google Scholar]
- 17. Uy CP, Tarkin JM, Gopalan D et al. The impact of integrated noninvasive imaging in the management of Takayasu arteritis. JACC Cardiovasc Imaging 2021;14:495–500. [DOI] [PubMed] [Google Scholar]
- 18. Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol 2008;181:151–60. [DOI] [PubMed] [Google Scholar]
- 19. Nakaoka Y, Isobe M, Takei S et al. Efficacy and safety of tocilizumab in patients with refractory Takayasu arteritis: results from a randomised, double-blind, placebo-controlled, phase 3 trial in Japan (the TAKT study). Ann Rheum Dis 2018;77:348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nakaoka Y, Isobe M, Tanaka Y et al. Long-term efficacy and safety of tocilizumab in refractory Takayasu arteritis: final results of the randomized controlled phase 3 TAKT study. Rheumatology (Oxford) 2020;59:2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muratore F, Salvarani C. Aortic dilatation in a patient with Takayasu arteritis treated with tocilizumab [correspondence]. Ann Rheum Dis 2021;80:e121. [DOI] [PubMed] [Google Scholar]
- 22. Nakaoka Y, Higuchi K, Arita Y et al. Tocilizumab for the treatment of patients with refractory Takayasu arteritis. Int Heart J 2013;54:405–11. [DOI] [PubMed] [Google Scholar]
- 23.JCS Joint Working Group. Guideline for management of vasculitis syndrome (JCS 2008). Japanese Circulation Society. Circ J 2011;75:474–503. [DOI] [PubMed] [Google Scholar]
- 24. Nakagomi D, Cousins C, Sznajd J et al. Development of a score for assessment of radiologic damage in large-vessel vasculitis (Combined Arteritis Damage Score, CARDS). Clin Exp Rheumatol 2017;35(Suppl 103):139–45. [PubMed] [Google Scholar]
- 25. Nakagomi D, Jayne D. Outcome assessment in Takayasu arteritis. Rheumatology (Oxford) 2016;55:1159–71. [DOI] [PubMed] [Google Scholar]
- 26. Freitas DS, Camargo CZ, Mariz HA, Arraes AE, de Souza AW. Takayasu arteritis: assessment of response to medical therapy based on clinical activity criteria and imaging techniques. Rheumatol Int 2012;32:703–9. [DOI] [PubMed] [Google Scholar]
- 27. Tso E, Flamm SD, White RD et al. Takayasu arteritis: utility and limitations of magnetic resonance imaging in diagnosis and treatment. Arthritis Rheum 2002;46:1634–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Qualified researchers may request access to individual patient-level data through the clinical study data request platform (www.clinicalstudydatarequest.com). For further details on Chugai’s Data Sharing Policy and how to request access to related clinical study documents, see www.chugai-pharm.co.jp/english/profile/rd/ctds_request.html.