Abstract
Plant sphingolipids mostly possess 2-hydroxy fatty acids (HFA), the synthesis of which is catalyzed by FA 2-hydroxylases (FAHs). In Arabidopsis (Arabidopsis thaliana), two FAHs (FAH1 and FAH2) have been identified. However, the functions of FAHs and sphingolipids with HFAs (2-hydroxy sphingolipids) are still unknown because of the lack of Arabidopsis lines with the complete deletion of FAH1. In this study, we generated a FAH1 mutant (fah1c) using CRISPR/Cas9-based genome editing. Sphingolipid analysis of fah1c, fah2, and fah1cfah2 mutants revealed that FAH1 hydroxylates very long-chain FAs (VLCFAs), whereas the substrates of FAH2 are VLCFAs and palmitic acid. However, 2-hydroxy sphingolipids are not completely lost in the fah1cfah2 double mutant, suggesting the existence of other enzymes catalyzing the hydroxylation of sphingolipid FAs. Plasma membrane (PM) analysis and molecular dynamics simulations revealed that hydroxyl groups of sphingolipid acyl chains play a crucial role in the organization of nanodomains, which are nanoscale liquid-ordered domains mainly formed by sphingolipids and sterols in the PM, through hydrogen bonds. In the PM of the fah1cfah2 mutant, the expression levels of 26.7% of the proteins, including defense-related proteins such as the pattern recognition receptors (PRRs) brassinosteroid insensitive 1-associated receptor kinase 1 and chitin elicitor receptor kinase 1, NADPH oxidase respiratory burst oxidase homolog D (RBOHD), and heterotrimeric G proteins, were lower than that in the wild-type. In addition, reactive oxygen species (ROS) burst was suppressed in the fah1cfah2 mutant after treatment with the pathogen-associated molecular patterns flg22 and chitin. These results indicated that 2-hydroxy sphingolipids are necessary for the organization of PM nanodomains and ROS burst through RBOHD and PRRs during pattern-triggered immunity.
Fatty acid 2-hydroxylases catalyze the 2-hydroxylation of sphingolipids, which aid in plasma membrane nanodomain organization and reactive oxygen species burst through an NADPH oxidase.
Introduction
Sphingolipids, the major lipids of the plasma membrane (PM), play a role in development, disease resistance, and abiotic stress tolerance in plants (Mamode Cassim et al., 2020). Plant sphingolipids are divided into four groups: free long-chain bases (LCBs); ceramides (Cers), comprising LCBs with fatty acids (FA); glucosylceramides (GlcCers); and glycosil-inositol-phospho-ceramides (GIPCs). GlcCers are conserved between plants and animals, whereas GIPCs are the most abundant in plants and not found in animals. Various modifications of the Cer moiety contribute to the diversity of sphingolipids with varying functions in plants. A representative modification of plant sphingolipids is the 2-hydroxylation of acyl chains (Supplemental Figure S1A). In Arabidopsis thaliana, ∼90% of complex sphingolipids characteristically contain 2-hydroxy FAs (HFA), in which the amide-linked FAs are hydroxylated at the C-2 position (Imai et al., 1995; Markham and Jaworski, 2007). In addition, plant sphingolipids substantially include very long-chain FAs (VLCFAs), which have 20 or more carbons (Haslam and Kunst, 2013).
Sphingolipids with HFAs (2-hydroxy sphingolipids) play important roles in the stress responses of plants. We have shown that 2-hydroxy sphingolipids increase the membrane order and are related to the organization of PM domains in rice (Oryza sativa; Nagano et al., 2016). These are sphingolipid- and sterol-derived nanoscale liquid-ordered (Lo) structures. In plants, there are two types of PM domains, namely nanodomains and microdomains. Nanodomains are submicron structures distributed in a dot-like pattern on the PM surface. They regulate the activity of proteins localized to such domains, whereas microdomains are larger structures which reside at specific sites in the PM to regulate local cellular functions (Ott, 2017; Gronnier et al., 2018, 2019). Since the domains we observed are characterized by their small size and their distribution throughout the PM, they are referred to as nanodomains. In the previous study, we showed that nanodomains formed by 2-hydroxy sphingolipids are essential for resistance to compatible rice blast fungus (Magnaporthe oryzae; Nagano et al., 2016). This occurs through the production of reactive oxygen species (ROS) by NADPH oxidases, respiratory burst homolog B (RBOHB), and RBOHH, which are transiently localized in PM nanodomains during disease responses. In contrast, 2-hydroxy sphingolipids are reported to decrease resistance to Golovinomyces cichoracearum in Arabidopsis (König et al., 2012). Furthermore, 2-hydroxy sphingolipids are related to tolerance of oxidative stress in Arabidopsis (Nagano et al., 2012a).
Sphingolipid FA 2-hydroxylases (FAHs), which are localized in the endoplasmic reticulum membrane and are widely conserved in animals, fungi, and plants, synthesize HFAs (Nagano et al., 2012b; Marquês et al., 2018). In animals and fungi, FAHs possess two functional domains: a C-terminal hydroxylase domain and an N-terminal cytochrome b5 (Cb5)-like domain, which is essential for the activation of the hydroxylase domain (Hama, 2010). In contrast, plant FAHs have defective intramolecular Cb5-like domains, and instead, directly interact with Cb5s for activation (Nagano et al., 2009, 2012a, 2012b). Arabidopsis possesses two homologs of FAH (FAH1 and FAH2; Nagano et al., 2009). We previously established FAH1 knockdown lines (FAH1-KD) by using RNA interference (RNAi) and fah2 T-DNA insertion knockout mutants in Arabidopsis and showed that FAH1 mainly hydroxylates VLCFAs, whereas FAH2 exclusively hydroxylates long-chain FAs (LCFAs), mostly palmitic acid (C16:0) (Nagano et al., 2012a). König et al. (2012) established a fah1 mutant in which a T-DNA insertion in the promoter region of FAH1 considerably, but not completely, decreased its expression. They also generated a fah1fah2 double mutant and revealed that FAH1 and FAH2 are involved in the synthesis of GlcCers and Cers (König et al., 2012). Lenarčič et al. (2017) reported that the content of total GIPCs was reduced in fah1fah2. These findings suggest that FAH1 and FAH2 are essential for the hydroxylation of sphingolipid FAs. However, because of the lack of a fah1 mutant where FAH1 is completely defective, the function of FAH1 in HFA synthesis and the cytological and physiological roles of 2-hydroxy sphingolipids are still unknown. In addition, the effect of FAHs on GIPCs is obscure, although GIPCs account for over 60% of sphingolipids in Arabidopsis.
To elucidate the function of FAH1 and 2-hydroxy sphingolipids in Arabidopsis, we generated a fah1 mutant (fah1c) using CRISPR/Cas9-based genome editing. Sphingolipid analysis revealed the different substrate preferences of FAH1 and FAH2 in the 2-hydroxylation of sphingolipids, including GIPCs, in Arabidopsis. We also demonstrated that the hydroxyl groups of sphingolipid acyl chains are important in the organization of PM nanodomains through lipid analysis, bioimaging, and molecular dynamics (MD) simulations. Furthermore, the localization of PM proteins was markedly affected by 2-hydroxy sphingolipids, and we found a relationship between 2-hydroxy sphingolipids and ROS burst through respiratory burst oxidase homolog D (RBOHD) during disease response.
Results
FAH1 hydroxylates VLCFAs of sphingolipids
To elucidate the function of FAH1 in Arabidopsis, we generated a FAH1 mutant (fah1c) using the CRISPR/Cas9 system. In the fah1c mutant, a thymine was inserted in the third exon of FAH1 (Figure 1Ab Supplemental Figure S1B). Although the full-length FAH1 coding region was slightly expressed in the fah1c mutant without any alternative splicing variants, the mutation was also retained in the FAH1 mRNA (Supplemental Figure 1, C and D). These results suggest that FAH1 in the fah1c mutant has only 44 amino acids, owing to an early stop codon (Figure 1B). Like fah2, fah1c did not show any apparent phenotypes compared to the wild-type (WT; Figure 1, C–F). To examine the 2-hydroxylation ability of FAH1, we analyzed the contents of sphingolipids such as GIPCs, GlcCers, and Cers in whole leaf tissues (Figure 2, A and B; Supplemental Figure S2). The contents of 2-hydroxylated VLCFAs of GIPCs were significantly lower in fah1c than in the WT, whereas those of nonhydroxylated VLCFAs of GIPCs increased, leading to a decrease in the ratio of 2-hydroxylated to total VLCFAs of GIPCs (Supplemental Figure S2, A and D). Although the contents of 2-hydroxylated VLCFAs of GlcCers and Cers did not decrease significantly in fah1c, the contents of nonhydroxylated VLCFAs of GlcCers and Cers were greater than those in fah2 (Supplemental Figure S2, B, C, E, and F). Conversely, the ratio of 2-hydroxylated to total LCFAs of all sphingolipid species decreased to 29.9% in fah2, whereas the amount of 2-hydroxylated LCFAs did not decrease in fah1c (Figure 2, A and B; Supplemental Figure S2). These results suggest that FAH1 mainly hydroxylates VLCFAs of sphingolipids, especially GIPCs, whereas FAH2 preferentially hydroxylates LCFAs.
Figure 1.
Establishment of fah1c and fah1cfah2 mutants. A, Mutated site of FAH1 gene. A thymine was inserted into the third exon of FAH1 using CRISPR/Cas9. B, Comparison of amino acid sequences of FAH1 between WT and fah1c. Mutation by CRISPR/Cas9 resulted in a polypeptide of only 44 amino acids in the fah1c mutant, although full-length FAH1 protein is 237 amino acids. C, Phenotype of 3-week-old WT, fah1c, fah2, fah1cfah2, pFAH1-FAH1/fah1cfah2, and RNAi-FAH1/2 #25 lines. Scale bar = 1 cm. D, Leaf diameter of 3-week-old WT, fah1c (f1), fah2 (f2), fah1cfah2 (f1f2), pFAH1-FAH1/fah1cfah2 (C4 and C5), and RNAi-FAH1/2 (R25, R28, and R39). Data are means ± sd (n = 12). E, Root length of 10-d-old plants. Data are means ± sd (n ≥ 21). F, Stem height of 7-week-old plants. Data are means ± sd (n = 12). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01).
Figure 2.
Sphingolipid analyses in fah1c, fah2, and fah1cfah2 mutants. Total lipids were extracted from 3-week-old leaves, and the sphingolipid amount was analyzed using LC–MS/MS. A, The total sphingolipid content in fah1c and fah2 is shown separately for LCFAs (shorter than C20 FAs), VLCFAs (longer than C20 FAs), HFAs, and nonhydroxy FAs (NFAs). Total sphingolipids include GIPCs, GlcCers, and Cers. Data are presented as mean ± sd (n = 4). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01). B, Relative ratios of HFAs and NFAs in total sphingolipids of fah1c and fah2 are shown separately for LCFAs, VLCFAs, and total FAs. C, The contents of total sphingolipids in fah1cfah2 are shown separately for LCFAs, VLCFAs, HFAs, NFAs, and total FAs. Total sphingolipids include GIPCs, GlcCers, and Cers. Data are presented as mean ± sd (n = 4). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01). D, Relative ratio of HFAs and NFAs in total sphingolipids of fah1cfah2 is shown separately for LCFAs, VLCFAs, and total FAs.
Next, we generated the fah1cfah2 double mutant to elucidate the functions of 2-hydroxy sphingolipids in plants (Supplemental Figure S1B). The leaves, roots, and stems of the fah1cfah2 mutant were smaller than those of the WT (Figure 1, C–F). Exogenous FAH1 controlled by a native promoter (pFAH1::FAH1/fah1cfah2) complemented the growth defect of fah1cfah2 (Figure 1, C–F; Supplemental Figure S1B). Sphingolipid analysis showed that the decrease in the contents of 2-hydroxylated VLCFAs was less in fah1cfah2 than in fah1c, and the increase in the contents of nonhydroxylated LCFAs and VLCFAs was greater in fah1cfah2 than in fah2 and fah1c, respectively (Figure 2, A and C; Supplemental Figures S2–S4). Thus, the ratio of 2-hydroxylated to total LCFAs and VLCFAs decreased to 15.5% and 38.5%, respectively (Figure 2D). These results indicated that both FAH1 and FAH2 catalyze the hydroxylation of LCFAs and VLCFAs, although they have substrate preference. However, 32.8% of the total 2-hydroxy sphingolipids were left even in the fah1cfah2 mutant (Figure 2, C and D), which was also confirmed by gas chromatography–mass spectrometry analysis (Supplemental Figure S5). We also produced Arabidopsis double knockdown lines of FAH1 and FAH2 (RNAi-FAH1/2) by using an RNAi system and obtained similar results for the phenotype and sphingolipid content (Figure 1, C–F; Supplemental Figures S1E and S6).
In plants, α-dioxygenases (α-DOXs) have been thought to be related to the hydroxylation of sphingolipid FAs (König et al., 2012). α-DOXs along with peroxygenases produce HFAs through the generation of unstable 2-hydroperoxy FAs by the incorporation of oxygen at the α-methylene carbon atom of FAs (Bannenberg et al., 2009; Shimada et al., 2014). Hence, we performed sphingolipid analyses in α-dox1, α-dox2, and α-dox1α-dox2. However, the contents of HFAs were comparable to those in the WT (Supplemental Figure S7A). Next, we analyzed the sphingolipid contents in the fah1cfah2α-dox1α-dox2 quadruple mutant, speculating that FAHs may work redundantly. However, the amount of HFAs was not different from that in fah1cfah2 (Supplemental Figure S7B). These results indicate that α-DOXs are not involved in sphingolipid synthesis in Arabidopsis.
2-hydroxy sphingolipids are important for the organization of PM nanodomains
To elucidate the effect of 2-hydroxy sphingolipids in the PM of Arabidopsis, we first analyzed the lipid composition in the PM of the fah1cfah2 mutant. We purified the PM fraction from 8-d-old WT and fah1cfah2 and compared the lipid contents, including sphingolipids, sterols, and phospholipids (Figure 3; Supplemental Figures S8–S10). In the PM of fah1cfah2, almost all 2-hydroxy sphingolipids decreased, whereas nonhydroxy sphingolipids increased (Figure 3, A and B; Supplemental Figures S8 and S9). This result was consistent with that for the sphingolipid content in whole leaf tissues (Figure 2, C and D). The amounts of steryl glycosides (SGs) and acylated SGs (ASGs) were lower in the fah1cfah2 mutant than in the WT (Figure 3C;Supplemental Figure S10). In contrast, the contents of free sterols (FSs), total sterols, and phospholipids were similar in the fah1cfah2 mutant and the WT (Figure 3, C and D;Supplemental Figure S10). To examine the effect of the above change of PM lipid composition on the membrane fluidity, we next examined the PM order in fah1cfah2. The roots grown for 7 d were stained with di-4-ANEPPDHQ, which can detect the membrane order by comparing the intensity of fluorescence between green (Lo; 500–550 nm) and red (liquid disordered [Ld]; 650–750 nm; Sato et al., 2020). The ratio of Lo to Ld was 0.96 in WT, but was significantly decreased to 0.52 in fah1cfah2 (Figure 4, A and B), indicating that the PM of fah1cfah2 is disordered and has higher fluidity. Observation of the PM surface of the cotyledons under higher magnification revealed that highly ordered dot-like structures were distributed in the PM of WT (Figure 4, C and D). In contrast, although dot-like structures were observed in the PM of fah1cfah2, their order and number were much lower than that in the WT (Figure 4, C and D). Moreover, the regions surrounding dot-like structures in the PM surface were less ordered in fah1cfah2 than in WT; however, the difference in order was smaller than that observed in dot-like structures (Figure 4, C and D). These results suggest that 2-hydroxy sphingolipids affect the organization of ordered domains.
Figure 3.
Analysis of the PM lipids in the fah1cfah2 mutant. A, Quantitative analyses of sphingolipids in the PM of the fah1cfah2 mutant. The PM was purified from WT and fah1cfah2, and sphingolipid amount in the PM was analyzed using LC–MS/MS. The contents of total sphingolipids in fah1cfah2 are shown separately for LCFAs (shorter than C20 FAs), VLCFAs (longer than C20 FAs), HFAs, and NFAs. Total sphingolipids include GIPCs, GlcCers, and Cers. Data are mean ± sd (n = 3). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01). B, Relative ratio of HFAs and NFAs in total sphingolipids in the PM fraction of fah1cfah2 is shown separately for LCFAs, VLCFAs, and total FAs. C, Quantitative analyses of sterols (SG, steryl glucoside; ASG, acylated steryl glucoside) in the PM of the fah1cfah2 mutant. Data are mean ± sd (n = 3). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01). D, Quantitative analyses of phospholipids (PE, phosphatidylethanolamine; PS, phosphatidylserine; PI, phosphatidylinositol; PG, phosphatidylglycerol; PA, phosphatidic acid) in the PM of the fah1cfah2 mutant. Data are mean ± sd (n = 3). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01).
Figure 4.
PM order of the fah1cfah2 mutant. A, The roots of 7-d-old Arabidopsis were stained with di-4-ANEPPDHQ, and the fluorescence of Lo phase and Ld phase was observed. Scale bars = 50 µm. B, Quantification of normalized Lo/Ld emission ratios of the PM of the roots. Data are means ± sd (n = 13). Asterisks indicate significant differences compared with the WT (Student’s t test; **P < 0.01). The elements of box plots are as follows; center line, median; box limits, first quartile (25th percentile) and third quartile (75th percentile); whiskers, min and max data points; points, each value. C, The cotyledons of 7-d-old Arabidopsis were stained with di-4-ANEPPDHQ, and the PM surface was observed. Scale bars = 10 µm. D, Quantification of normalized Lo/Ld emission ratios of PM surface of the cotyledons. Dot and Sur indicate dot-like structures and regions surrounding dots in the PM surface, respectively. Data are means ± sd (n = 30). Asterisks indicate significant differences compared with the WT (Student’s t test; **P < 0.01). The elements of box plots are as follows; center line, median; box limits, first quartile (25th percentile) and third quartile (75th percentile); whiskers, min and max data points; points, each value.
To better understand the role of 2-hydroxy sphingolipids in PM organization in Arabidopsis, we attempted to observe the nanodomains in the PM. For imaging the nanodomains in plants, we focused on a cholesterol-binding protein, perfringolysin O (PFO), which is a θ toxin secreted by Clostridium perfringens (Ishitsuka et al., 2011; Maekawa, 2017). The cholesterol-binding domain 4 (D4) of PFO can be fused with a fluorescent protein to visualize cholesterol-derived PM nanodomains in animal cells (Mizuno et al., 2011). However, sterol species are considerably different between animals and plants: plants mainly have β-sitosterols, campesterols, and stigmasterols instead of cholesterols (Valitova et al., 2016). Ohno-Iwashita et al. (2010) reported that the binding ability of PFO to β-sitosterols and stigmasterols was considerably lower than that to cholesterols. We then generated mutated D4 (D4L) by replacing aspartic acid at position 44 with leucine, because this replacement results in the highest binding ability to cholesterol (Farrand et al., 2015). A lipid–protein binding assay showed that AcGFP-D4L had similar binding ability with β-sitosterols and campesterols as with cholesterol, but not with stigmasterols (Figure 5A), suggesting that AcGFP-D4L could bind to β-sitosterol- and campesterol-enriched domains. Next, we generated Arabidopsis transgenic lines expressing sfGFP-D4L with signal peptides (SPs) necessary for the secretion to extracellular spaces, because sterols predominantly localize at the outer leaflet of the PM (Tjellström et al., 2010). We found that 8-day-old Arabidopsis WT cotyledons expressing SP-sfGFP-D4L had a homogeneous distribution of numerous nanoscale dot-like structures on the PM surface (Figure 5B, upper), which disappeared after treatment with methyl-β-cyclodextrin (MβCD), which decreased FSs in the PM (Roche et al., 2008; Figure 5B, middle). These results indicate that SP-sfGFP-D4L can allow the visualization of sterol-derived PM nanodomains in Arabidopsis. The number and brightness of the dot-like structures were lower in fah1cfah2 than in the WT (Figure 5B, bottom). This result indicates that 2-hydroxy sphingolipids play an essential role in the organization of PM nanodomains derived from sterols and sphingolipids.
Figure 5.

Observation of PM nanodomains using GFP-D4L. A, Lipid blot analysis of D4L. PVDF membranes deposited with cholesterol (Chl), β-sitosterol (Sito), campesterol (Cam), and stigmasterol (Stig) were treated with AcGFP-D4L (upper) and AcGFP (bottom), which were expressed in E. coli and purified using affinity chromatography. The α-His antibody was used for the detection of AcGFP-D4L and AcGFP. B, Observation of SP-sfGFP-D4L in the PM surface of 8-day-old cotyledons of WT (upper), WT treated with MβCD (middle), and fah1cfah2 (bottom). Scale bars = 10 µm.
MD simulations
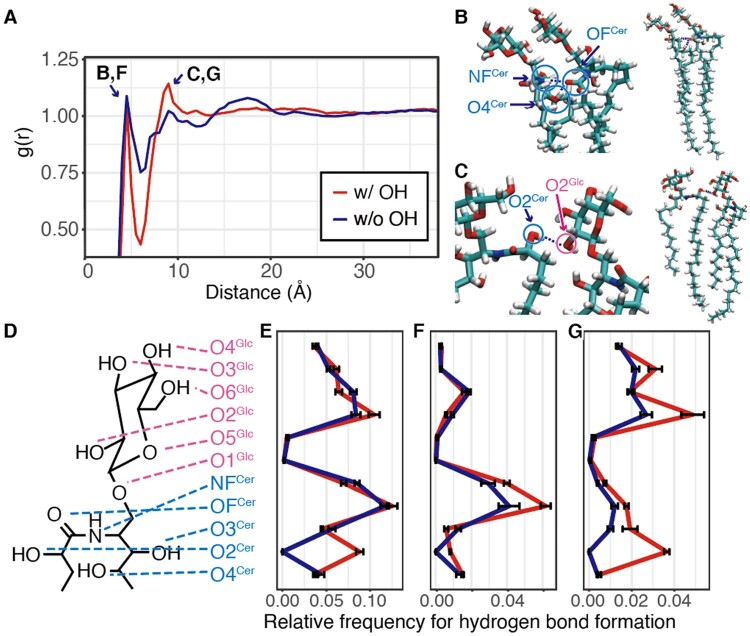
To characterize the physicochemical properties of 2-hydroxy sphingolipids at the atomic level, we performed all-atom MD simulations with three-component lipid bilayer models consisting of 40% phospholipid (1-Palmitoyl-2-oleoylphosphatidylcholine [POPC]), 30% sterol (β-sitosterol), and 30% sphingolipid (GlcCer) (Supplemental Movies S1–S4). The ratio of each species was determined according to the lipid composition of the PM in BY-2 cells reported by Cacas et al. (2016). We chose GlcCer as the sphingolipid model because of the simplicity of its chemical structure. β-sitosterol is reported to condense with sphingolipids, and phosphatidylcholine (PC) is the phospholipid in the outer leaflet of the PM (Fujimoto and Parmryd, 2017; Mamode Cassim et al., 2021). The system size was ca. 8 × 8 × 11 nm, and the membrane, which consisted of 128 lipids in each leaflet, was placed parallel to the x–y plane (Supplemental Figure S11). We simulated the molecular models with and without the hydroxyl group in the GlcCer acyl chain. Five repeats of the 1.0-μs simulation were performed, and the last half of trajectories were analyzed. As a result, the hydroxyl group of the GlcCer acyl chain enhanced the self-assembly of GlcCer (Figure 6A). The distributions of GlcCer–GlcCer distances observed in the simulations showed two peaks of intermolecular distances, implying that two modes of GlcCer–GlcCer interactions exist. The first peak around 4–5 Å indicates tightly packed pairs of GlcCer with contacts of both the acyl chains and LCBs (Figure 6B). In contrast, the second peak around 8–10 Å indicates GlcCer–GlcCer contacts with only a pair of acyl chain and LCB (Figure 6C). Interestingly, removing the hydroxyl group of the GlcCer acyl chain resulted in a drastic reduction of the second peak (Figure 6A). These two interaction modes had distinct hydrogen bonding patterns (Figure 6, D–G), and the second mode primarily included hydrogen bonds between the hydroxyl group of the acyl chain (shown as O2Cer in Figure 6) and sugar (Figure 6, C and G). In contrast, the first mode included the hydrogen bond between an amino group (NFCer) and a carboxyl group (OFCer) (Figure 6, B and F). Additionally, a broad peak was observed around 15–20 Å only for GlcCer without the hydroxyl group of the acyl chain. This peak reflects indirect interactions, for example, the second or third shell of the other peaks. Since the model of GlcCer with the hydroxyl group of acyl chain has two types of interactions, the peak of indirect interactions was not clearly observed. The simulation results suggest that the hydroxyl group of the GlcCer acyl chain contributes to the self-assembly of GlcCer via hydrogen bonding with the sugar group.
Figure 6.
GlcCer–GlcCer interactions observed in the MD simulations. A, Distribution of GlcCer–GlcCer distance. The vertical axis indicates the radial distribution function of the carbon atom next to the glycosidic bond in GlcCer. Distributions in the simulation with and without the hydroxyl group of GlcCer acyl chain are shown in red and blue, respectively. B, A snapshot of a pair of GlcCer with 4–5 Å intermolecular distance. C, A snapshot of a pair of GlcCer with 8–10 Å intermolecular distance. D, Nomenclature of hydrogen bonding donor and acceptor atoms in GlcCer. Atoms in Glc and Cer are shown in pink and cyan, respectively. E, The relative frequency of GlcCer–GlcCer hydrogen bonds in each atom of each GlcCer lipid in each snapshot. F, The relative frequency of GlcCer–GlcCer hydrogen bonds observed in pairs with 4–5 Å distance. G, The relative frequency of GlcCer–GlcCer hydrogen bonds observed in pairs with 8–10 Å distance.
2-hydroxy sphingolipids affect the PM localization of immune proteins
The state of the PM affects the localization and activity of its associated proteins. To reveal the relationship between 2-hydroxy sphingolipids and PM proteins, we performed comparative proteomic analysis of PM fractions obtained from 8-d-old seedlings of WT and fah1cfah2. We analyzed the whole PM proteins using liquid chromatography–tandem mass spectrometry (LC–MS/MS). We detected 611 and 563 proteins in WT and fah1cfah2, respectively (Supplemental Tables S1 and S2). The exponentially modified protein abundance index (emPAI; Ishihama et al., 2005) revealed that 163 proteins, including transporters, cytoskeleton components, membrane traffic-related proteins, and defense-related proteins, were nil or decreased, while 114 proteins were increased (Table 1; Supplemental Tables S3 and S4) in fah1cfah2 compared to that in the WT. In this study, we focused on the proteins which were nil and decreased in fah1cfah2, because the nanodomains were reduced. Several proteins were found to be essential for Arabidopsis immunity, such as pattern recognition receptors (PRRs), NADPH oxidase, and heterotrimeric G proteins (Table 1). To confirm whether these proteins were decreased in the PM of fah1cfah2, we performed immunoblot analyses for PM fractions. Brassinosteroid-insensitive 1-associated receptor kinase 1 (BAK1), chitin elicitor receptor kinase 1 (CERK1), RBOHD, and G protein alpha subunit 1 (GPA1) were found to decrease in the PM of fah1cfah2 (Figure 7A). BAK1 is a co-receptor with flagellin sensing 2 (FLS2) and elongation factor thermos unstable (EF-Tu) receptor 1 (EFR-1), which perceive flagellin and EF-Tu—pathogen-associated molecular patterns—from bacteria, respectively, and induces pattern-triggered immunity (PTI), which is a basic immune response in plants (Yasuda et al., 2017). CERK1 receives chitin oligomers, which are components of the fungal cell wall, with lysin motif-containing receptor-like kinase 5 (LYK5), and also induces PTI (Gong et al., 2020). RBOHD is one of the pivotal NADPH oxidases responsible for the ROS burst in response to pathogen infection (Hu et al., 2020). GPA1 is an α-subunit of heterotrimeric G proteins that are related to immune signaling (Xu et al., 2019). The amounts of BAK1, CERK1, and RBOHD in the total protein and microsomal fractions were equal between WT and fah1cfah2 (Supplemental Figure S12). The expression of BAK1 and RBOHD genes with or without flg22, which is a conserved peptide of bacterial flagellin, was not reduced in fah1cfah2 (Figure 7B). Although CERK1 expression was slightly reduced in fah1cfah2, the difference in its expression between WT and fah1cfah2 was lost after treatment with chitin (Figure 7C). To further confirm the relationship between 2-hydroxy sphingolipids and PM proteins, we observed the fluorescence, and thereby, the localization of eGFP-RBOHD. In the WT, eGFP-RBOHD was localized in the PM, as its fluorescence was clearly merged with that of the PM marker FM4–64 (Figure 7D). But, in fah1cfah2, the fluorescence of eGFP-RBOHD in the PM was very low; instead, it was accumulated in certain spherical aggregated structures. Since the fluorescence of eGFP-RBOHD from these structures was merged with that of FM4–64, we speculated that these structures are derived from the PM. These spherical structures were hardly observed in the WT. These results indicate that 2-hydroxy sphingolipids are important for the localization of immune proteins in the PM.
Table 1.
Candidate defense-related proteins that are more abundant in WT than in fah1cfah2 PM
| emPAI |
Ratio | ||||
|---|---|---|---|---|---|
| Function | Accession No. | Proteins | WT | fah1cfah2 | (WT/fah) |
| Receptor | AT3G21630 | CERK1 | chitin elicitor receptor kinase 1 | 0.12 | – | – |
| AT4G33430 | BAK1 | BRI1-associated receptor kinase | 0.26 | – | – | |
| AT1G21880 | LYM1 | lysm domain GPI-anchored protein 1 | 0.12 | – | – | |
| AT3G24550 | PERK1 | proline extensin-like receptor kinase 1 | 0.12 | – | – | |
| NADPH oxidase | AT5G47910 | RBOHD | respiratory burst oxidase homologue D | 0.04 | – | – |
| GTP protein | AT2G26300 | GPA1 | G protein alpha subunit 1 | 0.19 | – | – |
| AT4G34460 | AGB1 | GTP binding protein beta 1 | 0.94 | 0.33 | 2.85 | |
| AT3G63420 | AGG1 | Ggamma-subunit 1 | 0.41 | – | – | |
| AT3G22942 | AGG2 | G-protein gamma subunit 2 | 0.95 | 0.4 | 2.38 | |
| LRR protein | AT1G51850 | Leucine-rich repeat protein kinase family protein | 0.23 | – | – |
| AT2G01820 | Leucine-rich repeat protein kinase family protein | 0.04 | – | – | |
| AT3G23750 | Leucine-rich repeat protein kinase family protein | 0.17 | 0.04 | 4.25 | |
| Other | AT3G25070 | RIN4 | RPM1 interacting protein 4 | 0.39 | – | – |
| AT5G55850 | RPM1-interacting protein 4 (RIN4) family protein | 1.92 | – | – | |
| AT2G20630 | PIA1 | PP2C induced by AVRRPM1 | 0.29 | – | – | |
| AT5G51570 | Band 7 domain-containing membrane-associated protein family | 0.43 | – | – | |
| AT5G56010 | HSP81-3 | heat shock protein 81-3 | 0.56 | – | – | |
Experiments were repeated twice, and, in both the experiments, the protein level in WT was more than twice that in fah1cfah2. The emPAI score in this table indicates the score in the first set of experiments.
Figure 7.
Analysis of defense-related PM proteins. A, Immunoblot analysis of defense-related proteins in the PM fraction of WT and fah1cfah2 grown in half-strength MS liquid medium for 8 d. B, Expression analysis of BAK1 and RBOHD in 8-d-old seedlings of fah1cfah2 at 6 h after treatment with flg22. Data are means ± sd (n = 3). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01). C, Expression analysis of CERK1 and RBOHD in 8-d-old seedlings of fah1cfah2 6 h after treatment with chitin. Data are means ± sd (n = 3). Asterisks indicate significant differences compared with the WT (Student’s t test; **P < 0.01). D, Imaging of eGFP-RBOHD treated with FM4–64 (PM marker) in 8-d-old cotyledons of WT (upper) and fah1cfah2 (bottom). Scale Bars = 20 µm.
2-hydroxy sphingolipids regulate ROS burst and hypersensitive response during disease responses
The decrease in RBOHD, BAK1, and CERK1 proteins in the PM of fah1cfah2 implies that ROS production is affected during PTI. Thus, we investigated the effects of flg22 and chitin treatment on ROS burst. Although ROS burst was initiated by both flg22 and chitin treatment in WT and fah1cfah2, ROS production was markedly lower in fah1cfah2 than in WT (Figure 8A), indicating that 2-hydroxy sphingolipids regulate the ROS burst during PTI.
Figure 8.
Defense responses in the fah1cfah2 mutant. A, ROS production in WT and fah1cfah2. Three-week-old leaves were treated with flg22 or chitin, and ROS was detected using L-012 reagent. Data are means ± sd (n = 3). B and C, Expression analysis of defense-related genes in 8-d-old seedlings of WT and fah1cfah2 at 6 h after treatment with flg22 (B) or chitin (C). Data are means ± sd (n = 3). D, Bacterial growth assay in the leaves of WT and fah1cfah2 infiltrated with Pto (OD600 = 0.00001) together with 1 µM flg22. The bacterial number at 2 dpi was measured. Data are means ± sd (n = 3). E, Analysis of ion leakage in leaf discs of WT and fah1cfah2 infiltrated with Pto carrying AvrRpt2 (OD600 = 0.1) or mock (10 mM MgCl2). Relative ratios of ion leakage to total ion leakage in 0, 4, 5, 6, 8, 10, and 24 h are shown. Data are means ± sd (n = 4). F, Bacterial growth assay in the leaves of WT and fah1cfah2 infiltrated with Pto carrying AvrRpt2 (OD600 = 0.00005). The bacterial number at 3 dpi was measured. Data are means ± sd (n = 3). Asterisks indicate significant differences compared with the WT (Student’s t test; *P < 0.05, **P < 0.01).
Next, we measured the expression of the defense-related genes flg22-induced receptor-like kinase 1, chitinase, nitrilase 4, WRKY53, WRKY75, and MYB53, which were induced by PAMP treatments (Figure 8, B and C). The expression of almost all defense-related genes was increased in fah1cfah2 by both flg22 and chitin treatment more than that in WT. These results were inconsistent with the data that BAK1 and CERK1 were decreased in the PM of fah1cfah2.
In addition, we tested the resistance to the pathogen, Pseudomonas syringae pv tomato DC3000 (Pto), which is a pathogenic bacterium commonly used for studying Arabidopsis immunity (Katagiri et al., 2002). To reveal the role of 2-hydroxy sphingolipids in PTI triggered by flg22, we infected Arabidopsis leaves with Pto mixed with flg22. Bacterial growth at 2 d after infection was not different between fah1cfah2 and WT, suggesting that 2-hydroxy sphingolipids did not affect flg22-triggered PTI (Figure 8D). In addition, we investigated the relationship between 2-hydroxy sphingolipids and another immunity—effector-triggered immunity (ETI)—which induces stronger immunity responses such as hypersensitive response (HR) in response to effectors from incompatible pathogens (Zhang et al., 2020). To reveal the relationship between 2-hydroxy sphingolipids and HR, we first examined ion leakage, which can detect cell death, induced by AvrRpt2, an effector that is recognized by the resistance protein resistance to pseudomonas syringae 2 (Mackey et al., 2003). When we infected Arabidopsis leaves with Pto carrying AvrRpt2, ion leakage was significantly lower in fah1cfah2 than in WT, from 6 h after the infection (Figure 8E), suggesting that 2-hydroxy sphingolipids affect HR triggered by AvrRpt2. However, the bacterial growth at 3 d after infection with Pto carrying AvrRpt2 was nearly equal between WT and fah1cfah2 (Figure 8F). Taken together, these findings suggest that 2-hydroxy sphingolipids would regulate ROS burst and HR in PTI and ETI, respectively, although they do not affect the resistance to pathogens.
Discussion
FAH1 and FAH2 have different substrate preferences for sphingolipid FAs
In this study, we produced a FAH1 mutant (fah1c) using the CRISPR/Cas9 system to clarify the function of FAH1 and 2-hydroxy sphingolipids. Sphingolipid analyses in fah1c and fah1cfah2 mutants showed that FAH1 predominantly hydroxylates VLCFAs of sphingolipids, whereas the hydroxylation of LCFAs is exclusively catalyzed by FAH2. These findings are supported by our previous FA analysis suggesting that 2-hydroxylated VLCFAs are reduced in the FAH1-knockdown lines, and 2-hydroxylated LCFAs are remarkably decreased in the fah2 mutant (Nagano et al., 2012a). In this study, we found that FAH2 is also related to the hydroxylation of VLCFAs because nonhydroxylated VLCFAs were slightly but significantly increased in the fah2 mutant, and 2-hydroxylated VLCFAs were decreased in the fah1cfah2 double mutant compared to that in the fah1c single mutant. We previously showed that FAH2 synthesized 2-hydroxy hexacosanoic acid (26 h:0) when overexpressed in yeast Δfah1 mutant (Nagano et al., 2012a). Therefore, 2-hydroxylated VLCFAs were considered to be synthesized mainly by FAH1 and moderately by FAH2. Our sphingolipid analyses also showed that FAH1 and FAH2 hydroxylated the FAs of GIPCs, which are the most abundant sphingolipids in plants, indicating that FAHs play a central role in the hydroxylation of sphingolipid acyl chains.
Approximately 30% of 2-hydroxy sphingolipids were left in the fah1cfah2 mutant. 2-hydroxy sphingolipids also remained in the FAH1/2 double knockdown rice lines and the fah1fah2 knockdown Arabidopsis mutant (König et al., 2012; Nagano et al., 2016), and the remaining FAHs in the knockdown lines seemed to synthesize 2-hydroxy sphingolipids. In this study, the fah1cfah2 mutant confirmed that other genes are also related to the hydroxylation of sphingolipid acyl chains. In humans, the depletion of the FAH homolog FA2H reduces only 50% of sphingomyelin with HFAs in fibroblasts and has no impact on the hydroxylation of sphingomyelin FAs in lymphocytes (Dan et al., 2011). Thus, other enzymes are also suggested to exist in animals, but they have not been identified yet. König et al. (2012) suggested that α-DOX1 and α-DOX2 might be the candidates, because α-DOXs are involved in the synthesis of HFAs in plants (Hamberg et al., 2005). In the leaf oil bodies of Arabidopsis, α-DOX1 synthesizes 2-hydroxy octadecatrienoic acid (C18:3) in cooperation with caleosin 3 (Shimada et al., 2014). α-DOX2 has 2-hydroxylation activity against a broad range of FAs from C14:0 to C30:0 (Bannenberg et al., 2009). However, the simultaneous mutations of α-dox1 and α-dox2 did not affect sphingolipid content, indicating that neither α-DOX1 nor α-DOX2 are associated with the 2-hydroxylation of sphingolipid FAs. Further investigation is required to explore unknown enzymes.
2-Hydroxy sphingolipids are important for the organization of PM nanodomains
In the PM fraction of the fah1cfah2 mutant, the HFAs of all sphingolipid species were reduced similar to those in the whole leaf tissues, suggesting that 2-hydroxy sphingolipids synthesized by FAH1 and FAH2 are related to the formation of PM. In contrast, the contents of SG and ASG were reduced in fah1cfah2, although there was no significant effect on the content of total sterols and phospholipids. In tobacco (Nicotiana tabacum) leaves, sphingolipids and sterols including SG and ASG accumulated in the detergent-insoluble membrane fraction, which is considered to biochemically contain PM nanodomains, whereas phospholipids were present in the PM fraction (Cacas et al., 2016). Thus, the change of sphingolipid composition may affect the sterol composition of PM. These lipid changes may result in PM organization, suggesting that 2-hydroxy sphingolipids are largely responsible for forming highly ordered dot-like structures on the PM surface, and are moderately involved in the overall order of the PM. The dot-like structures in Figure 4C were not observed inside the cell and were found not to move within seconds. In addition, the size of the structures was smaller than that of endosomes. Therefore, the dot-like structures are likely to be ordered domains on the PM surface, rather than vesicles in endocytosis. Furthermore, our observation conducted using the sterol-binding marker, sfGFP-D4L, showed that 2-hydroxy sphingolipids have a substantial impact on the formation of PM nanodomains, which are nanoscale ordered domains formed by the predominant assembly between sphingolipids and sterols; they function as a platform for various PM proteins (Mamode Cassim et al., 2019). Since D4 recognizes the hydroxyl group at position 3 of cholesterol (Maekawa, 2017), the decrease in SGs and ASGs not having a hydroxyl group at that position in fah1cfah2 does not seem to affect its binding to sfGFP-D4L. Therefore, the hydroxylation of sphingolipid acyl chains is necessary for the organization of PM nanodomains as it modifies the interaction between sphingolipids and sterols. In addition, the MD simulations using GlcCer, β-sitosterol, and POPC demonstrated that the hydrogen bond formed by the hydroxyl group of GlcCer acyl chain contributes to the close association of GlcCer to each other. The formation of hydrogen bond by the hydroxyl group of sphingolipid acyl chain has been reported in previous biophysical studies (Pascher, 1976; Pascher and Sundell, 1977; Lofgren and Pascher, 1977). Therefore, our result indicates the importance of the hydroxyl group of sphingolipid acyl chain in forming an ordered membrane. On the other hand, our MD simulations also revealed that the hydroxyl group of acyl chain forms hydrogen bond with the hydroxyl group of the glucose molecule of GlcCer at the highest frequency. In this study, GlcCer was used for MD simulations because of its structural simplicity. However, because GIPC is the sphingolipid most involved in the formation of nanodomains in the PM (Mamode Cassim et al., 2021), it would be interesting to examine how hydroxyl groups of acyl chains interact with the head groups of GIPCs such as phosphate and inositol. More than 90% of the sphingolipids in plants contain HFAs (Imai et al., 1995; Markham and Jaworski, 2007), whereas in animals, only a few tissues, such as the nerves and skin, have 2-hydroxy sphingolipids, and that too in lower amounts (Hama, 2010). Thus, 2-hydroxy sphingolipids could be specifically required for the organization of plant nanodomains. Further studies are required to understand the nanodomain organization by 2-hydroxy sphingolipids and the difference in nanodomains between plants and animals.
In this study, we developed sfGFP-D4L to observe plant nanodomains. The D4 of the PFO protein directly binds with cholesterols in animals, and fluorescent protein-fused D4 is applied for the direct imaging of animal rafts (Mizuno et al., 2011; Kishimoto et al., 2016). D4L, the mutated D4, was confirmed to bind with β-sitosterol and campesterol, but not with stigmasterol. Among phytosterols, β-sitosterol has the highest condensation capacity with sphingolipids (Mamode Cassim et al., 2021), and campesterol has a stronger ability to organize ordered membranes than other FSs in the model membrane (Grosjean et al., 2015). Thus, sfGFP-D4L could detect the sterol-derived nanodomains in plants. In fact, sfGFP-D4L allowed the clear and efficient observation of nanodomains in Arabidopsis. We also determined the coordinate interaction between sterols and sphingolipids, because the sterol-derived nanodomains were greatly reduced in the fah1cfah2 mutant with changes in the sphingolipid composition and no change in the total sterol content. The direct observation of composing lipids such as sterols and sphingolipids would be better than the observation of nanodomain-localizing proteins for understanding the actual dynamics of plant nanodomains. sfGFP-D4L would largely contribute to the advancement of the plant nanodomain field.
2-Hydroxy sphingolipids contribute to the ROS burst by maintaining the localization of immune proteins in Arabidopsis
Our proteomic analysis revealed that there was either no or decreased accumulation of 163 proteins in the PM fraction of fah1cfah2 compared to that of the WT, which means that ∼26.7% of proteins identified in the WT were decreased in fah1cfah2. Since PM nanodomains were reduced in fah1cfah2, the decreased proteins might be related to the nanodomains. In this study, among the reduced proteins, we focused on defense-related proteins such as BAK1, CERK1, RBOHD, and GPA1. Since their accumulation in the microsomal fraction and their expression levels in the fah1cfah2 were not affected, 2-hydroxy sphingolipids were considered to be necessary for their correct PM localization. In fact, the observation of eGFP-RBOHD showed abnormal localization of RBOHD in fah1cfah2. When the FLS2-BAK1 receptor complex perceives flagellin from bacteria, it phosphorylates a receptor-like cytoplasmic kinase, Botrytis-induced kinase 1 (BIK1). Activated BIK1 directly interacts with and phosphorylates RBOHD, leading to the ROS burst (Kadota et al., 2014, 2015). GPA1, a canonical Gα, works downstream of the flg22-activated signaling and interacts with RBOHD to enhance ROS burst (Xu et al., 2019). Furthermore, CERK1 is phosphorylated by the perception of chitin oligomers with the co-receptor LYK5, and activated CERK1 also interacts with and phosphorylates BIK1, which is responsible for the activation of RBOHD (Gong et al., 2020). In fah1cfah2, the ROS burst was suppressed after treatment with flg22 and chitin. We previously showed that rice RBOHB and RBOHH, which belong to a clade similar to Arabidopsis RBOHD, were decreased in the PM of FAH-knockdown lines, leading to the loss of ROS burst after treatment with chitin (Nagano et al., 2016). Hao et al. (2014) showed that Arabidopsis RBOHD partly co-localizes with flotillin 1—a PM nanodomain marker protein—and the distribution of RBOHD is affected by sterols, suggesting an association between RBOHD and PM nanodomains. FLS2 also seems to localize to PM nanodomains, because FLS2 co-localizes with remorins, which are plant-specific nanodomain proteins (Bücherl et al., 2017). Therefore, 2-hydroxy sphingolipids would play a crucial role in ROS production during PTI by facilitating the PM (or probably nanodomains) localization of PRRs and RBOH, and this mechanism could be common across plant species.
However, the sensitivity to Pto infection inducing both PTI and ETI did not change in the fah1cfah2 mutant compared to that in the WT. This may be attributed to the modest reduction in the amount of RBOHD and BAK1 in the PM of fah1cfah2. In fact, ROS burst was not completely lost in fah1cfah2 after the treatment with flg22. Thus, the impact of ROS reduction in fah1cfah2 might not be sufficient for the sensitivity to Pto infection. In addition, salicylic acid (SA) has been reported to accumulate in the fah1fah2 mutant (König et al., 2012), and some sphingolipid-related mutants such as accelerated-cell-death11, sphingolipid base hydroxylases (sbh1 and sbh2), and neutral ceramidases (ncer1 and ncer2) show increased expression of defense-related genes (Brodersen et al., 2002; Chen et al., 2008; Zienkiewicz et al., 2020). Thus, the high expression of defense-related genes might counteract the effect of the suppression of ROS burst in the fah1cfah2 mutant. In contrast, in the FAH-knockdown rice lines, the sensitivity to rice blast fungus was higher than that in the WT rice, probably because ROS production was almost completely inhibited even in after treatment with chitin, and SA level was not increased (Nagano et al., 2016). Therefore, the extent to which 2-hydroxy sphingolipids are involved in ROS production and disease resistance may vary among plant species.
In this study, we developed a FAH1-defective mutant and determined the roles of FAH1 and FAH2 in the hydroxylation of sphingolipid FAs. PM analysis revealed the importance of FAHs in the organization of nanodomains and the localization of PM proteins. In addition, we found that 2-hydroxy sphingolipids are necessary for ROS burst during disease resistance.
Materials and methods
Plant materials
Columbia-0 ecotype of Arabidopsis (A. thaliana) (L.) Heynh was used in this study. The T-DNA insertion mutant fah2 was previously reported by Nagano et al. (2012a), and those of α-DOX, namely, α-dox1-1, α-dox1-4, α-dox2-1, α-dox2-2, α-dox1-1α-dox2-2, and α-dox1-4α-dox2-1, were previously reported by Shimada et al. (2014). The pRBOHD-eGFP-RBOHD line, which was previously reported by Hao et al. (2014), was crossed with the fah1cfah2 mutant. To generate the fah1c mutant using the CRISPR/Cas9 system, we introduced a protospacer of FAH1, 5′-GGGCCTTCCTTGGTCGCGAT-3′, into pDe-CAS9 (Fauser et al., 2014) using the Gateway system (Invitrogen, Waltham, MA, USA). To generate pFAH1::FAH1/fah1c lines, we amplified the putative native promotor and gene region of FAH1 using the primer set listed in Supplemental Table S5 and introduced them into pMDC99 plasmids (Curtis and Grossniklaus, 2003) using the Gateway system. To generate RNAi-FAH1/2 lines, we amplified the coding regions of FAH1 and FAH2 using the primer sets for each region (Supplemental Table S5) and combined and introduced them into pANDA35HK (Miki and Shimamoto, 2004) using the Gateway system. To generate SP-sfGFP-D4L lines, we produced mutated D4L by replacing the aspartic acid at position 44 of D4 with leucine using the GeneArt Site-directed Mutagenesis System (Thermo Fisher Scientific, Waltham, MA, USA). The SP sequences of pumpkin 2S albumin (Mitsuhashi et al., 2000), sfGFP, and D4L, amplified using the corresponding primer sets listed in Supplemental Table S5, were combined and introduced into pB2GW7-UBQ10pro, which includes the promoter from Arabidopsis ubiquitin 10 (Ichino et al., 2014), using the Gateway system. Arabidopsis plants were transformed using the floral-dip method (Bechtold and Pelletier, 1998) and cultivated at 22°C under long-day conditions (16-h light and 8-h dark; 60 µmol m−2 s−1).
PM purification
PM was purified from 15 g fresh weight (FW) of 8-d-old Arabidopsis seedlings grown in liquid MS medium, using the two-phase partition method (Uemura et al., 1995). Briefly, the seedlings were homogenized in a buffer comprising 0.5-M sorbitol, 50-mM MOPS-KOH (pH 7.6), 5-mM EDTA, 5-mM EGTA, 1.5% (w/v) PVP-40, 0.5% (w/v) defatted-BSA, 2-mM phenylmethylsulfonyl fluoride, and 2.5-mM DTT. The homogenates were successively centrifuged at 3,000g for 15 min and at 20,000g for 15 min at 4°C. The supernatants were ultracentrifuged at 170,000g for 35 min at 4°C. The pellets (the microsomal fraction) were suspended in a solution of 0.3-M sucrose and 10-mM KH2PO4/K2HPO4 (pH 7.8). The microsomal suspensions were then fractionated with an aqueous, two-polymer, phase-partition system consisting of 5.6% (w/v) PEG 3,350 (Sigma-Aldrich, St Louis, MO, USA) and 5.6% (w/v) dextran T500 (Pharmacia, NJ, USA) in a solution of 0.3-M sucrose, 30-mM NaCl, and 10-mM KH2PO4/K 2HPO4 (pH 7.8). The two-phase partition was repeated 3 times at 4°C. The upper phase of the final two-phase system was ultracentrifuged at 170,000g for 35 min at 4°C. The pellets were suspended in a washing solution containing 0.3-M sucrose, 10-mM Mops/KOH (pH 7.3), 1-mM EGTA, and 2-mM DTT, and re-ultracentrifuged at 170,000g for 35 min at 4°C. The PM-enriched pellet was re-suspended in the washing solution and used for lipid extraction or protein analysis.
Lipid analysis
Lipids were extracted from lyophilized Arabidopsis leaves (50–100 mg FW) grown on soil for 3 weeks or purified PM fractions according to Ishikawa et al. (2018). The leaf extract was subjected to KOH hydrolysis to remove glycerolipids for sphingolipid analysis, while the PM extract was not hydrolyzed for total profiling including phospholipids and acylated sterols.
All lipids were analyzed by the selected multiple reaction monitoring mode using LCMS-8030 system (Shimadzu) equipped with a Shim-pack XR-ODSII column (2.2 µm × 2.0 mm × 75 mm) under the analytical conditions as below: Sphingolipids were analyzed according to Nagano et al. (2014); SG and ASGs were separated with an alternative solvent (acetonitrile/methanol/isopropanol, 1:1:1 by vol, containing 5-mM ammonium acetate) and detected by the MS/MS transitions of [M+CH3CN+Na]+ > [M+Na]+ for SG and [M+NH4]+ > [M−(acyl glucose)+H]+ for ASG; FS was analyzed after picolinyl derivatization according to Honda et al. (2008); Phospholipid was analyzed after permethylation using trimethylsilyldiazomethane according to Cai et al. (2016). FS and phospholipid derivatives were separated using the same solvent system used for sphingolipids. Lipid quantity was calculated by comparison with the corresponding internal standards that were added to each sample prior to the extraction: d18:1-c12:0 Cer (for nCer), d18:1-h12:0 Cer (for hCer), d18:1-c12:0 GlcCer (for n/hGlcCer), ganglioside GM1 (for n/hGIPC), cholestanol (for FS), cholestanol glucoside (for SG and ASG), and synthetic phospholipids (14:0 PC, 14:0 PE, 16:0 PS, 18:0 PG, 18:0 PI, and 18:0 PA). Cholestanol glucoside was synthesized according to Iga et al. (2005), and other standards were purchased from Avanti Polar Lipids.
Determination of membrane order using di-4-ANEPPDHQ
Arabidopsis roots and cotyledons grown in half-strength MS medium for 7 d were treated with 5 µM di-4-ANEPPDHQ (Molecular Probes, Eugene, OR, USA) at 30 min. Fluorescence was excited with a 488 nm Ar laser (laser power 12%), and Lo and Ld images were obtained by measuring fluorescence at 500–550 nm (HV, 600 V; gain, 1.000×; offset 0%) and 650–750 nm (HV, 550 V; gain, 1.000×; offset 0%), respectively, using a confocal laser scanning microscope (FLUOVIEW FV3000; Olympus, Tokyo, Japan). The fluorescence intensity of each image, including each background fluorescence intensity (bk), was measured by ImageJ (http://imagej.nih.gov/ij/). For the roots, we measured the fluorescence intensity at 50-µm length of the PM of the cell in the center of the image. The ratio of Lo/Ld was determined using the (Lo intensity [Lo bk])/(Ld intensity [Ld bk]) equation.
Homemade lipid blot
pCold I-AcGFP-D4L for the expression in Escherichia coli was generated by the mutation of pCold I-AcGFP-D4, which was kindly provided by Dr Toshihide Kobayashi and Dr Reiko Ishitsuka (Riken, Japan). pCold I-AcGFP was generated by the introduction of AcGFP, amplified using the primer set in Supplemental Table S5, into the pCold I vector (Takara, Shiga, Japan). To obtain recombinant AcGFP-D4L and AcGFP proteins, we cultured E. coli BL21 carrying pCold I-AcGFP-D4L or pCold I-AcGFP at 15°C with 1-mM isopropyl-β-d-1-thiogalactopyranoside for 24 h, and total proteins were extracted using BugBuster Master Mix (Merck, Kenilworth, NJ, USA). AcGFP-D4L and AcGFP were purified using AKTA start with HisTrap HP column (Cytiva, Marlborough, MA, USA) and dialyzed in 20-mM Tris–HCl buffer (pH 7.5). Lipid blot analysis was performed according to Cacas et al. (2016). Each sterol (10 µg) dissolved in methanol was deposited on the activated Immobilon-P membrane (Millipore, Burlington, MA, USA). Next, 10 µg/mL of AcGFP-D4L or AcGFP was incubated for 2 h at room temperature. α-His antibody (dilution, 1:1,000; MBL) was used as the first antibody.
Treatment with MβCD and FM4–64
Eight-day-old Arabidopsis cotyledons were treated with 10-mM MβCD (200-mM stock solution prepared in distilled water; Wako Chemicals, Richmond, VA, USA) or FM4–64 (5-mM stock solution prepared in DMSO; Cosmo Bio, Japan) dissolved in half-strength MS liquid medium for 30 min or 2 min, respectively. After treatment, the cotyledons were washed twice with half-strength MS medium.
Confocal microscopy
eGFP-RBOHD and SP-sfGFP-D4L in 8-d-old Arabidopsis cotyledons were observed using FLUOVIEW FV3000 (Olympus). Green fluorescent protein (GFP) was excited using a 488 nm Ar laser (laser power 10%), and the fluorescence was detected at 500–550 nm (HV, 600 V; gain, 1.000×; offset, 0%). FM4–64 was excited using a 561-nm He/Ne laser (laser power 13%), and the fluorescence was detected at 570–620 nm (HV, 900 V; gain, 1.000×; offset, 0%).
Computational methods for MD simulation
The molecular models consisted of a lipid bilayer and 150-mM KCl solution. Each leaflet of the lipid bilayer consisted of 52 molecules of POPC, 38 molecules of GlcCer, and 38 molecules of β-sitosterol. Both the models with and without the hydroxyl group in GlcCer acyl chain were simulated and compared to dissect the effects of the hydroxyl group. The initial structure was generated using CHARMM-GUI (Lee et al., 2018) and by applying an in-house script to modify the chemical structure of GlcCer, which adds hydroxyl groups and moves the position of the unsaturated bond. In the initial structure, the size of the cubic periodic boundary cell was 8.57 nm × 8.57 nm × 10.99 nm, and the membrane normal was along the z-axis. The total number of atoms in the system was 73,412.
Energy minimization and relaxation runs with gradual releasing of position restraining potentials were applied by following the standard protocol of the CHARMM-GUI (Lee et al., 2018). From the final snapshot of the relaxation run, five independent simulations with different random initial atomic velocities were performed. For each simulation lasting over 1 ns, the temperature and pressure were kept at 300 K and 1 atm, respectively, using the Berendsen themostat/barostat. After that, 1 µs simulations at 300 K and 1 atm were performed using the Nosé-Hoover thermostat (Nosé, 1984; Hoover, 1985) and the Parrinello–Rahman barostat (Parrinello, 1981). The trajectory of the first 500 ns in each run was discarded as an equilibrium process. The snapshots were recorded every 100 ps, and the resulting ensemble of 25,000 snapshots was analyzed.
The CHARMM36m forcefield (Huang et al., 2016) and TIP3P model (Jorgensen et al., 1983) were applied for potential energy calculation. The electrostatic potential was calculated using the zero-dipole summation method with the dumping parameter α = 0.0 (Fukuda et al., 2011, 2012). Simulations were performed by using Gromacs 2018 (He et al., 2015).
The trajectories were analyzed using MDAnalysis library (Michaud-Agrawal et al., 2011). Hydrogen bonds were detected with the criterion of donor–hydrogen–acceptor angle ≥120° and donor–acceptor distance ≤3.2 Å.
Proteome analysis
PM proteins were separated using a ready-made 12.5% (w/v) sodium lauryl sulfate (SDS)–polyacrylamide gel (ATTO, NY, USA) and stained using Flamingo (Bio-Rad, Hercules, CA, USA). The gel lane was cut into four slices of equal length. Each gel band was washed twice with HPLC-grade water containing 60% (v/v) acetonitrile (Kanto Chemical, Tokyo, Japan)/50-mM ammonium bicarbonate, and incubated successively in 10-mM DTT/50-mM ammonium bicarbonate for 45 min at 56°C and 55-mM iodoacetamide/50-mM ammonium bicarbonate for 30 min at 25°C. The incubated gel slices were washed twice with HPLC-grade water containing 60% (v/v) acetonitrile/50-mM ammonium bicarbonate and dried in a vacuum concentrator (VC-96W, TAITEC, Saitama, Japan). The dried slices were subsequently treated with 2 µL of 10-ng/µL trypsin (MS grade gold; Promega, Madison, WI, USA)/50-mM ammonium bicarbonate and incubated at 37°C for 16 h. The digested peptides were transferred to a new tube. The gel was then treated twice with 20 µL of 0.2% (v/v) formic acid (Wako)/50% (v/v) acetonitrile, and the three peptide extracts were pooled. The extracts were dried in a vacuum concentrator and dissolved in 0.1% (v/v) formic acid/5% (v/v) acetonitrile. The solution was filtered using an Ultrafree-MS Centrifugal Filter (PVDF 0.45 µm; Millipore) to avoid contamination with gel pieces.
LC–MS/MS analysis was performed using an HTC-PAL/Paradigm MS4 system coupled to an LTQ Orbitrap XL mass spectrometer (Thermo Fisher Scientific). Trypsin-digested peptides were loaded on the L-column (100-µm internal diameter, 15 cm; CERI, Tokyo, Japan) using a Paradigm MS4 HPLC pump (Michrome BioResources, Auburn, CA, USA) and an HTC-PAL autosampler (CTC Analytics, Zwingen, Switzerland). The buffers were 0.1% (v/v) acetic acid and 2% (v/v) acetonitrile in water (Solvent A), and 0.1% (v/v) acetic acid and 90% (v/v) acetonitrile in water (Solvent B). A linear gradient from 5% to 45% buffer B was applied over 26 min, and peptides eluted from the L-column were introduced directly into an LTQ Orbitrap XL mass spectrometer with a flow rate of 500 nL/min and a spray voltage of 2.0 kV. All events for MS scan were controlled and acquired by Xcalibur software version 2.0.7 (Thermo Fisher Scientific). The range of MS scan was m/z 400 to 1,500, and the top three peaks were subjected to MS/MS analysis. The spectra were compared against the TAIR10 protein database using the MASCOT server (version 2.5). The mascot search parameters were as follows: peptide tolerance, 10 ppm; MS/MS tolerance, ±0.6 Da; peptide charge, 2+ or 3+; ≤1 missed cleavage using trypsin; cysteine carbamidomethylation as a fixed modification; and methionine oxidation as a variable modification.
Immunoblotting
The proteins were separated using SDS-PAGE and electrotransferred onto an Immobilon-P membrane (Millipore) for immunoblot detection with each antibody. The α-BAK1, α-CERK1, α-RBOHD, α-GPA1, and α-AGB1 antibodies were purchased from Agrisera (Vannas, Sweden). The α-PIP1s antibody was purchased from Cosmo Bio.
ROS assay
Leaf discs prepared from the Arabidopsis lines grown on soil for 3 weeks were floated in distilled water in white 96-well plates (Greiner Bio-one, Kremsmünster, Austria) and incubated at 22°C overnight in the dark. ROS detection buffers (20-mM L-012 and 1-mg/mL horseradish peroxidase in distilled water) with 1-µM flg22 (Funakoshi, Tokyo, Japan) or 200-µg/mL chitin (Sigma) were added to each well, and chemiluminescence was detected using Amersham Imager 600 (Cytiva). The value was calculated according to Nagano et al. (2016).
Reverse transcription–quantitative PCR analysis
Arabidopsis seedlings grown in half-strength MS medium for 8 d were treated with 1 µM flg22 or 200-µg/mL chitin in half-strength MS liquid medium for 6 h. Total RNA was extracted using a RNeasy Plant Mini Kit (Qiagen, Hilden, Germany). The cDNA was synthesized using ReverTra Ace (Toyobo, Osaka, Japan). Reverse transcription–quantitative PCR analysis was performed using the StepOne Real-Time PCR System (Thermo Fisher Scientific), and the primer sets are shown in Supplemental Table S5.
Bacterial growth assay
To analyze flg22-induced resistance, we co-precipitated Pto (optical density (OD)600 = 0.0001) with 1-µM flg22 into 3-week-old Arabidopsis leaves using a needleless syringe. To analyze AvrRPT2-induced resistance, we infiltrated Pto carrying AvrRpt2 (OD600 = 0.00005) into 3-week-old Arabidopsis leaves using a needleless syringe. Log10-transformed colony-forming units per square centimeter leaf tissue were calculated according to Katagiri et al. (2002).
Ion leakage measurement
Pto carrying AvrRpt2 (OD600 = 0.1) was infiltrated into 3-week-old Arabidopsis leaves using a needleless syringe. Leaf discs were prepared from the infected leaves and floated in distilled water in 24-well plates. Electrolyte leakage was monitored using an electrical conductivity meter (B-173; Horiba, Kyoto, Japan) at several time points and expressed as a relative value against total ion leakage, which was measured in autoclaved samples (Nagano et al., 2012a).
Statistical analyses
Comparisons between the WT and each line were performed using the Student’s t test.
Accession numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: FAH1, At2g34770; FAH2, At4g20870; α-DOX1, At3g01420; α-DOX2, At1g73680; BAK1, At4g33430; CERK1, At3g21630; RBOHD, At5g47910; GPA1, At2g26300; and AGB1, At4g34460.
Supplemental data
The following materials are available in the online version of this article.
Supplemental Figure S1. Establishment of Arabidopsis transgenic lines.
Supplemental Figure S2. Sphingolipid analyses in the fah1c and fah2 mutants.
Supplemental Figure S3. Sphingolipid profiles in the fah1cfah2 mutant.
Supplemental Figure S4. Sphingolipid analyses in the fah1cfah2 mutant.
Supplemental Figure S5. HFA analysis of the fah1cfah2 mutant.
Supplemental Figure S6. Sphingolipid analyses in RNAi-FAH1/2 lines.
Supplemental Figure S7. Sphingolipid analyses in the α-dox mutants.
Supplemental Figure S8. Sphingolipid profiles in the PM of the fah1cfah2 mutant.
Supplemental Figure S9. Sphingolipid analyses in the PM of the fah1cfah2 mutant.
Supplemental Figure S10. Sterol analyses in the PM of the fah1cfah2 mutant.
Supplemental Figure S11. The molecular system with 2-hydroxy GlcCer at the final snapshot of relaxation runs.
Supplemental Figure S12. Immunoblot analysis of defense-related PM proteins in total protein and microsomal fractions.
Supplemental Table S1. Identification of PM-associated proteins in WT.
Supplemental Table S2. Identification of PM-associated proteins in fah1cfah2.
Supplemental Table S3. Identification of PM-associated proteins for which WT > fah1cfah2.
Supplemental Table S4. Identification of PM-associated proteins for which WT < fah1cfah2.
Supplemental Table S5. Primer list used in this study.
Supplemental Movie S1. The top-view movie of a time course of the last 100 ns of a simulation with 2-hydroxy GlcCer.
Supplemental Movie S2. The side-view movie of the same trajectory as that shown in Supplemental Movie S1.
Supplemental Movie S3. The top-view movie of a time course of the last 100 ns of a simulation with nonhydroxy GlcCer.
Supplemental Movie S4. The side-view movie of the same trajectory as that shown in Supplemental Movie S3.
Supplementary Material
Acknowledgments
We are grateful to Drs. Holger Puchta (Karlsruhe Institute of Technology, Germany), Seiichi Toki, Masaki Endo (National Agriculture and Food Research Organization, Japan), and Hidetaka Kaya (Ehime University, Japan) for the pDe-CAS9 vector. The α-dox mutants were provided by Drs. Ikuko Hara-Nishimura (Konan University, Japan) and Takashi L Shimada (Chiba University, Japan). Plasmids including D4 were provided by Drs. Toshihide Kobayashi and Reiko Ishitsuka (RIKEN, Japan). Plasmids including sfGFP were provided by Dr. Yutaka Kodama (Utsunomiya University, Japan). The Arabidopsis pRBOHD-eGFP-RBOHD line was provided by Dr. Jinxing Lin (Beijing Forestry University, China). We appreciate Biomedical Engineering Research Center, Ritsumeikan University, for providing the confocal laser microscope. We thank Drs. Takeshi Ishimizu, Hiroyuki Matsumura, and Atsushi Takeda (Ritsumeikan University, Japan) for letting us use the ultracentrifuge, AKTA start, and fluorescent stereomicroscope, respectively. We also thank Dr. Umechiyo Matsumura (Ritsumeikan University) and Ms. Kaori Tashiro (Saitama University) for their technical support. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research was supported by a Grant-in-Aid by the Japan Society for the Promotion of Science (JSPS), KAKENHI Grant Numbers 17K15412 and 20K05966 to M.N., and 18H02165 and 18K19164 to M.K.
Conflict of interest statement. None declared.
T.U., F.B., T.I., K.K., Y.N., R.I., and K.T. performed the experiments. M.Y., T.T., Y.F., and M.K. supervised the experiments and revised the manuscript. M.N. designed this work and wrote the manuscript.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (https://academic.oup.com/plphys/pages/general-instructions) is Minoru Nagano (mnagano@fc.ritsumei.ac.jp).
References
- Bannenberg G, Martínez M, Rodríguez MJ, López MA, Ponce de León I, Hamberg M, Castresana C (2009) Functional analysis of α-DOX2, an active α-dioxygenase critical for normal development in tomato plants. Plant Physiol 151: 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Pelletier G (1998) In planta Agrobacterium-mediated transformation of adult Arabidopsis thaliana plants by vaccum infiltration. Methods Mol Biol 82: 259–266 [DOI] [PubMed] [Google Scholar]
- Brodersen P, Petersen M, Pike HM, Olszka B, Skov S, Odum N, Jørgensen LB, Brown RE, Mundy J (2002) Knockout of Arabidopsis accelerated-cell-death11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes Dev 16: 490–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C (2017) Plant Immune and growth receptors share common signaling components but localize to distinct plasma membrane nandomains. eLife 6: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacas JL, Buré C, Grosjean K, Gerbeau-Pissot P, Lherminier J, Rombouts Y, Maes E, Bossard C, Gronnier J, Furt F, et al. (2016) Revisiting plant plasma membrane lipids in Tobacco: a focus on sphingolipids. Plant Physiol 170: 364–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T, Shu Q, Liu P, Niu L, Guo X, Ding X, Xue P, Xie Z, Wang J, Zhu N, et al. (2016) Characterization and relative quantification of phospholipids based on methylation and stable isotopic labeling. J Lipid Res 57: 388–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Markham JE, Dietrich CR, Jaworski JG, Cahoon EB (2008) Sphingolipid long-chain base hydroxylation is important for growth and regulation of sphingolipid content and composition in Arabidopsis. Plant Cell 20: 1862–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan P, Edvardson S, Bielawski J, Hama H, Saada A (2011) 2-hydroxylated sphingomyelin profiles in cells from patients with mutated fatty acid 2-hydroxylase. Lipids Health Dis 10: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrand AJ, Hotze EM, Sato TK, Wade KR, Wimley WC, Johnson AE, Tweten RK (2015) The cholesterol-dependent cytolysin membrane-binding interface discriminates lipid environments of cholesterol to support β-barrel pore insertion. J Biol Chem 290: 17733–17744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser F, Schiml S, Puchta H (2014) Both CRISPR/Cas-based nucleases and nickases can be used efficiently for genome engineering in Arabidopsis thaliana. Plant J 79: 348–359 [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Parmryd I (2017) Interleaflet coupling, pinning, and leaflet symmetry- major players in plasma membrane nanodomain formation. Front Cell Dev Biol 4: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda I, Kamiya N, Yonezawa Y, Nakamura H (2012) Simple and accurate scheme to compute electrostatic interaction: zero-dipole summation technique for molecular system and application to bulk water. J Chem Phys 137: 054314. [DOI] [PubMed] [Google Scholar]
- Fukuda I, Yonezawa Y, Nakamura H (2011) Molecular dynamics scheme for precise estimation of electrostatic interaction via zero-dipole summation principle. J Chem Phys 134: 164107. [DOI] [PubMed] [Google Scholar]
- Gong BQ, Wang FZ, Li JF (2020) Hide-and-seek: chitin-triggered plant immunity and fungal counterstrategies. Trends Plant Sci 25: 805–816 [DOI] [PubMed] [Google Scholar]
- Gronnier J, Gerbeau-Pissot P, Germain V, Mongrand S, Simon-Plas F (2018) Divide and rule: plant plasma membrane organization. Trends Plant Sci 23: 899–917 [DOI] [PubMed] [Google Scholar]
- Gronnier J, Legrand A, Loquet A, Habenstein B, Germain V, Mongrand S (2019) Mechanisms governing subcompartmentalization of biological membranes. Curr Opin Plant Biol 52: 114–123 [DOI] [PubMed] [Google Scholar]
- Grosjean K, Mongrand S, Beney L, Simon-Plas F, Gerbeau-Pissot P (2015) Differential effect of plant lipids on membrane organization. J Biol Chem 290: 5810–5825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hama H (2010) Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta 1801: 405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M, Ponce de Leon I, Rodriguez MJ, Castresana C (2005) Alpha-dioxygenases. Biochem Biophys Res Commun 338: 169–174 [DOI] [PubMed] [Google Scholar]
- Haslam TM, Kunst L (2013) Extending the story of very-long-chain fatty acid elongation. Plant Sci 210: 93–107 [DOI] [PubMed] [Google Scholar]
- Hao H, Fan L, Chen T, Li R, Li X, He Q, Botella MA, Lin J (2014) Clathrin and membrane microdomains cooperatively regulate RbohD dynamics and activity in Arabidopsis. Plant Cell 26: 1729–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Tolic A, Bashkin JK, Poon GMK (2015) Heterogeneous dynamics in DNA site discrimination by the structurally homologous DNA-binding domains of ETS-family transcription factors. Nucleic Acids Res 43: 4322–4331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda A, Yamashita K, Miyazaki H, Shirai M, Ikegami T, Xu G, Numazawa M, Hara T, Matsuzaki Y (2008) Highly sensitive analysis of sterol profiles in human serum by LC-ESI-MS/MS. J Lipid Res 49: 2063–2073 [DOI] [PubMed] [Google Scholar]
- Hoover W (1985) Canonical dynamics: equilibrium phase-space distributions. Phys Rev A Gen Phys 31: 1695–1697 [DOI] [PubMed] [Google Scholar]
- Hu CH, Wang PQ, Zhang PP, Nie XM, Li BB, Tai L, Liu WT, Li WQ, Chen KM (2020) NADPH oxidases: the vital performers and center hubs during plant growth and signaling. Cells 9: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Rauscher S, Nawrocki G, Ran T, Feig M, de Groot BL, Grubmüller H, Mackerell AD (2016) CHARMM36m: an improved force for folded and intrinsically disordered proteins. Nat Meth 14: 71–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichino T, Fuji K, Ueda H, Takahashi H, Koumoto Y, Takagi J, Tamura K, Sasaki R, Aoki K, Shimada T, et al. (2014) GFS9/TT9 contributes to intracellular membrane trafficking and flavonoid accumulation in Arabidopsis thaliana. Plant J 80: 410–423 [DOI] [PubMed] [Google Scholar]
- Iga DP, Iga S, Schmidt RR, Buzas MC (2005) Chemical synthesis of cholesteryl β-D-galactofuranoside and –pyranoside. Carbohydr Res 340: 2052–2054 [DOI] [PubMed] [Google Scholar]
- Imai H, Ohnishi M, Kinishita M, Kojima M, Ito S (1995) Structure and distribution of cerebroside containing unsaturated hydroxyl fatty acids in plant leaves. Bios Biotech Biochem 59: 1309–1313 [Google Scholar]
- Ishihama Y, Oda Y, Tabata T, Sato T, Nagasu T, Rappsilber J, Mann M (2005) Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol Cell Proteomics 4: 1265–1272 [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Fang L, Rennie EA, Sechet J, Yan J, Jing B, Moore W, Cahoon EB, Scheller HV, Kawai-Yamada M, et al. (2018) GLUCOSAMINE INOSITOLPHOSPHORYLCERAMIDE TRANSFERASE1 (GINT1) is a GlcNAc-containing glycosylinositol phosphorylceramide glucosyltransferase. Plant Physiol 177: 938–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishitsuka R, Saito T, Osada H, Ohno-Iwashita Y, Kobayashi T (2011) Fluorescence image screening for chemical compound modifying cholesterol metabolism and distribution. J Lipid Res 52: 2084–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) Comparison of simple potential functions for simulating liquid water. J Chem Phys 79: 926–935 [Google Scholar]
- Kadota Y, Sklenar J, Derbyshire P, Stransfeld L, Asai S, Ntoukakis V, Jones JD, Shirasu K, Menke F, Jones A, et al. (2014) Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54(1): 43–55 [DOI] [PubMed] [Google Scholar]
- Kadota Y, Sirasu K, Zipfel C (2015) Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol 56: 1472–1480 [DOI] [PubMed] [Google Scholar]
- Katagiri F, Thilmony R, He SY (2002) The Arabidopsis thaliana-pseudomonas syringae interaction. Arabidopsis Book 1: e0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Ishitsuka R, Kobayashi T (2016) Detectors for evaluating the cellular landscape of sphingomyelin- and cholesterol-rich membrane domains. Biochim Biophys Acta 1861: 812–829 [DOI] [PubMed] [Google Scholar]
- König S, Feussner K, Schwarz M, Kaever A, Iven T, Landesfeind M, Ternes P, Karlovsky P, Lipka V, Feussner I (2012) Arabidopsis mutants of sphingolipid fatty acid α-hydroxylases accumulate ceramides and salicylates. New Phytol 196: 1086–1097 [DOI] [PubMed] [Google Scholar]
- Lee J, Patel DS, Ståhle J, Park SJ, Kern NR, Kim S, Lee J, Cheng X, Valvano MA, Holst O, et al. (2018) CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J Chem Theory Comput 15: 775–786 [DOI] [PubMed] [Google Scholar]
- Lenarčič T, Albert I, Böhm H, Hodnik V, Pirc K, Zavec AB, Podobnik M, Pahovnik D, Žagar E, Pruitt R, et al. (2017) Eudicot plant-specific sphingolipids determine host selectivity of microbial NLP cytolysins. Science 358: 1431–1434 [DOI] [PubMed] [Google Scholar]
- Löfgren H, Pascher I (1977) Molecular arrangements of sphingolipids. The monolayer behaviour of ceramides. Chem Phys Lipids 20: 273–284 [DOI] [PubMed] [Google Scholar]
- Mackey D, Belkhadir Y, Alonso JM, Ecker JR, Dangl JL (2003) Arabidopsis RIN4 is a target of the type III virulence effector AvrRPT2 and modulates RPS2-mediated resistance. Cell 112: 379–389 [DOI] [PubMed] [Google Scholar]
- Maekawa (2017) Domain 4 (D4) of Perfringolysin O to visualize cholesterol in cellular membranes-the update. Sensors 17: 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamode Cassim A, Gouguet P, Gronnier J, Laurent N, Germain V, Grison M, Boutté Y, Gerbeau-Pissot P, Simon-Plas F, Mongrand S (2019) Plant lipids: key players of plasma membrane organization and function. Prog Lipid Res 73: 1–27 [DOI] [PubMed] [Google Scholar]
- Mamode Cassim A, Grison M, Ito Y, Simon-Plas F, Mongrand F, Boutté Y (2020) Sphingolipids in plants: a guidebook on their function in membrane architecture, cellular processes, and environmental or developmental responses. FEBS Lett 594: 3719–37380 [DOI] [PubMed] [Google Scholar]
- Mamode Cassim A, Navon Y, Gao Y, Decossas M, Fouillen L, Grelard A, Nagano M, Lambert O, Bahammou D, Van Delft P, et al. (2021) Biophysical analysis of the plant-specific GIPC sphingolipids reveals multiple modes of membrane regulation. J Biol Chem 296: 100602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JE, Jaworski JG (2007) Rapid measurement of sphingolipids from Arabidopsis thaliana by reversed-phase high-performance liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom 21: 1304–1314 [DOI] [PubMed] [Google Scholar]
- Marquês JT, Marinho HS, de Almeida RFM (2018) Sphingolipid hydroxylation in mammals, yeast and plants – An integrated view. Prog Lipid Res 71: 18–42 [DOI] [PubMed] [Google Scholar]
- Michaud-Agrawal N, Denning EJ, Woolf TB, Bechstein O (2011) MDAnalysis: a toolkit for the analysis of molecular dynamics simulations. J Comput Chem 32: 2319–2327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki D, Shimamoto K (2004) Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant Cell Physiol 45: 490–495 [DOI] [PubMed] [Google Scholar]
- Mitsuhashi N, Shimada T, Mano S, Nishimura M, Hara-Nishimura I (2000) Characterization of organelles in the vacuolar-sorting pathway by visualization with GFP in tobacco BY-2 cells. Plant Cell Physiol 41: 993–1001 [DOI] [PubMed] [Google Scholar]
- Mizuno H, Abe M, Dedecker P, Makino A, Rocha S, Ohno-Iwashita Y, Hofkens J, Kobayashi T, Miyamaki A (2011) Fluorescent probes for superresolution imaging of lipid domains on the plasma membrane. Chem Sci 2: 1548–1553 [Google Scholar]
- Nagano M, Ihara-Ohori Y, Imai H, Inada N, Fujimoto M, Tsutsumi N, Uchimiya H, Kawai-Yamada M (2009) Functional association of cell death suppressor, Arabidopsis Bax inhibitor-1, with fatty acid 2-hydroxylation through cytochrome b5. Plant J 17: 122–134 [DOI] [PubMed] [Google Scholar]
- Nagano M, Ishikawa T, Fujiwara M, Fukao Y, Kawano Y, Kawai-Yamada M, Shimamoto K (2016) Plasma membrane microdomains are essential for Rac1-RbohB/H-mediated immunity in rice. Plant Cell 28: 1966–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Ishikawa T, Ogawa Y, Iwabuchi M, Nakasone A, Shimamoto K, Uchimiya H, Kawai-Yamada M (2014) Arabidopsis Bax inhibitor-1 promotes sphingolipid synthesis during cold stress by interacting with ceramide-modifying enzymes. Planta 240: 77–89 [DOI] [PubMed] [Google Scholar]
- Nagano M, Takahara K, Fujimoto M, Tsutsumi N, Uchimiya H, Kawai-Yamada M (2012a) Arabidopsis sphingolipid fatty acid 2-hydroxylases (AtFAH1 and AtFAH2) are functionally differentiated in fatty acid 2-hydroxylation and stress responses. Plant Physiol 159: 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano M, Uchimiya H, Kawai-Yamada M (2012b) Plant sphingolipid fatty acid 2-hydroxylases have unique characters unlike their animal and fungus counterparts. Plant Signal Behav 7: 1388–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosé S (1984) A molecular dynamics method for simulations in the canonical ensemble. Mol Phys 52: 255–168 [Google Scholar]
- Ohno-Iwashita Y, Shimada Y, Hayashi M, Iwamoto M, Iwashita S, Inomata M (2010) Cholesterol-binding toxins and anti-cholesterol antibodies as structural probes for cholesterol localization. Subcell Biochem 51: 597–621 [DOI] [PubMed] [Google Scholar]
- Ott T (2017) Membrane nanodomains and microdomains in plant-microbe interactions. Curr Opin Plant Biol 40: 82–88 [DOI] [PubMed] [Google Scholar]
- Parrinello M (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52: 7182 [Google Scholar]
- Pascher I (1976) Molecular arrangements in sphingolipids conformation and hydrogen-bonding of ceramide and their implication on membrane stability and permeability. BBA-Biomembranes 455: 433–451 [DOI] [PubMed] [Google Scholar]
- Pascher I, Sundell S (1977) Molecular arrangements in sphingolipids. The crystal structure of cerebroside. Chem Phys Lipids 20: 175–191 [DOI] [PubMed] [Google Scholar]
- Roche Y, Gerbeau-Pissot P, Buhot B, Thomas D, Bonneau L, Gresti L, Mongrand S, Perrier-Cornet JM, Simon-Plas F (2008) Depletion of phytosterols from the plant plasma membrane provides evidence for disruption of lipid rafts. FASEB J 22: 3980–3991 [DOI] [PubMed] [Google Scholar]
- Sato M, Nagano M, Jin S, Miyagi A, Yamaguchi M, Kawai-Yamada M, Ishikawa T (2020) Plant-unique cis/trans isomerism of long-chain base unsaturation is selectively required for aluminum tolerance resulting from glucosylceramide-dependent plasma membrane fluidity. Plants (Basel) 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada TL, Takano Y, Shimada T, Fujiwara M, Fukao Y, Mori M, Okazaki Y, Saito K, Sasaki R, Aoki K, et al. (2014) Leaf oil body functions as a subcellular factory for the production of a phytoalexin in Arabidopsis. Plant Physiol 164: 105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjellström H, Hellgren LI, Wieslander A, Sandelius AS (2010) Lipid asymmetry in plant plasma membranes: phosphate deficiency-induced phospholipid replacement is restricted to the cytosolic leaflet. FASEB J 24: 1128–1138 [DOI] [PubMed] [Google Scholar]
- Uemura M, Joseph RA, Steponkus PL (1995) Cold acclimation of Arabidopsis thaliana (Effect on Plasma membrane lipid composition and freeze-induced lesions). Plant Physiol 109: 15–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valitova JN, Sulkarnayeva AG, Minibayeva FV (2016) Plant sterols: diversity, biosynthesis, and physiological functions. Biochemistry (Mosc) 81: 819–834 [DOI] [PubMed] [Google Scholar]
- Xu L, Yao X, Zhang N, Gong BQ, Li JF (2019) Dynamic G protein alpha signaling in Arabidopsis innate immunity. Biochem Biophys Res Commun 516: 1039–1045 [DOI] [PubMed] [Google Scholar]
- Yasuda S, Okada K, Saijo Y (2017) A look at plant immunity through the window of the multitasking coreceptor BAK1. Curr Opin Plant Biol 38: 10–18 [DOI] [PubMed] [Google Scholar]
- Zhang J, Coaker G, Zhou JM, Dong X (2020) Plant immune mechanism: from reductionistic to holistic points of view. Mol Plant 13: 1358–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienkiewicz A, Gömann J, König S, Herrfurth C, Liu YT, Meldau D, Feussner I (2020) Disruption of Arabidopsis neutral ceramidases 1 and 2 results in specific sphingolipid imbalances triggering different phytohormone-dependent plant cell death programmes. New Phytol 226: 170–188 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.