Highlights
-
•
This systematic review and meta-analysis of 103 studies comprising 948 217 patients found that NAFLD is associated with a significantly higher risk of HCC as compared to no NAFLD.
-
•
In addition, NAFLD nonsignificantly increased the risk of HCC-related mortality but not of recurrence or overall mortality.
-
•
Given the higher risk of HCC in patients with NAFLD, general health interventions and screening should be implemented for high-risk cases (eg, those with steatohepatitis and fibrosis).
Keywords: Nonalcoholic fatty liver disease, Steatosis, NASH, Hepatocellular carcinoma, Meta-analysis
Abstract
Background and aims
Hepatic steatosis of nonalcoholic etiology (nonalcoholic fatty liver disease; NAFLD) is an emergent condition that may lead to hepatic cirrhosis and finally to liver cancer. We evaluate the risk of developing hepatocellular carcinoma (HCC) and quantify the prognosis in terms of recurrence (DFS) as well as HCC-specific and overall survival (CSS and OS) of patients with and without NAFLD.
Methods
We searched published articles that evaluated the risk and outcomes of HCC in patients with steatosis/steatohepatitis from inception to July 2021 were identified by searching the PubMed, EMBASE, and Cochrane Library databases. Prospective cohort, case-control, or retrospective studies were selected that were published in English and provided incidence and survival rates of HCC patients with NAFLD. A random-effects model was created to estimate the pooled effect size. The primary outcome of interest was HCC incidence. The secondary endpoints were DFS, CSS, and OS.
Results
In total, 948 217 patients with NAFLD were analyzed, from n = 103 observational studies. NAFLD significantly increased the risk of HCC (HR = 1.88 [95% CI, 1.46-2.42]; P < .01] but not risk of recurrence (HR = 0.99 [95% CI, 0.85-1.15]; P = .9) or overall mortality (HR = 1.04 [95% CI, 0.88-1.24]; P = 0.64). Conversely, NAFLD increased HCC-related mortality risk (HR = 2.16 [95% CI, 0.85-5.5]; P = .1). Risk of HCC was increased in Western countries but not in Asian countries.
Conclusions
Patients with NAFLD have an increased risk of HCC as compared to patients without NAFLD. NAFLD also increases liver cancer (HCC) mortality. These results justify applying general measures to patients with proven NAFLD and monitoring patients with NASH and fibrosis.
Introduction
Hepatic steatosis of nonalcoholic etiology (nonalcoholic fatty liver disease or NAFLD) is an emergent condition that may lead to hepatic cirrhosis and finally to liver cancer. To define NAFLD, there must be evidence of hepatic steatosis and an absence of secondary causes of fat accumulation in the liver (eg, alcohol consumption). In most cases, NAFLD is commonly associated with metabolic comorbidities such as obesity, diabetes mellitus, and dyslipidemia. NAFLD comprises simple steatosis or steatohepatitis (NASH), where steatosis is associated with liver inflammation, with or without liver fibrosis [1]. The global incidence of NAFLD is rising in both Western and Asian countries due to the metabolic increase of etiological factors (eg, diabetes and obesity) [2], [3].
There is no specific therapy for NAFLD or screening method for at-risk patients; general health suggestions (eg, weight loss) are the only possible way to avoid or reduce the risk of NAFLD progression in fibrosis patients. An estimated 20% of patients with NASH will develop cirrhosis, and NASH is predicted to become the leading indication for liver transplants in the United States.
Nonalcoholic fatty liver disease with associated cirrhosis is a risk factor for the development of HCC. In a previous systematic review of 61 studies of patients with steatosis or NASH, the risk of HCC among those without and with cirrhosis ranged from 0.03 to 3.78 × 100 000 person-years [4]. Among subjects without cirrhosis, the risk of mortality from HCC was 0%-3% after longer observation. Furthermore, NASH is associated with liver-associated and overall mortality [5].
We performed an updated meta-analysis to verify the correlation and the prognostic significance of NAFLD in patients with HCC.
Materials and Methods
To comprehensively calculate the cumulative incidence and prognosis of HCC in patients with NAFLD, a systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Eligibility Criteria for the Studies
All articles reporting the risk of HCC as a complication of NAFLD were included. NAFLD cases were defined by a positive biopsy for steatosis or by a suspect radiology examination of the liver. All cross-sectional, retrospective, and prospective studies that included patients with NAFLD and reported incidence of HCC were considered eligible. Case reports with fewer than 10 patients and case series, including all editorials, reviews, and commentaries, were excluded. Studies targeting special populations such as pregnant women, children, and other groups, were excluded. Only articles written in English were included.
Search Strategy
Three bibliographical databases (PubMed, EMBASE, and the Cochrane Library) were searched to identify potential articles (as of July 31, 2021). The search criteria were as follows: ((("liver fatty"[All Fields] OR ("naflds"[All Fields] OR "non alcoholic fatty liver disease"[MeSH Terms] OR ("non alcoholic"[All Fields] AND "fatty"[All Fields] AND "liver"[All Fields] AND "disease"[All Fields]) OR "non alcoholic fatty liver disease"[All Fields] OR "nafld"[All Fields]) OR ("fatty liver"[MeSH Terms] OR ("fatty"[All Fields] AND "liver"[All Fields]) OR "fatty liver"[All Fields] OR "steatohepatitis"[All Fields]) OR ("fatty liver"[MeSH Terms] OR ("fatty"[All Fields] AND "liver"[All Fields]) OR "fatty liver"[All Fields] OR "steatosis"[All Fields]) OR "nash"[All Fields]) AND ("hcc"[All Fields] OR "HEPATOCELLULAR"[All Fields]) AND ("cancer s"[All Fields] OR "cancerated"[All Fields] OR "canceration"[All Fields] OR "cancerization"[All Fields] OR "cancerized"[All Fields] OR "cancerous"[All Fields] OR "neoplasms"[MeSH Terms] OR "neoplasms"[All Fields] OR "cancer"[All Fields] OR "cancers"[All Fields] OR ("carcinoma"[MeSH Terms] OR "carcinoma"[All Fields] OR "carcinomas"[All Fields] OR "carcinoma s"[All Fields])) AND "english"[Language])
Data Extraction and Inclusion Criteria
Data were extracted from the articles and supplementary materials. Reference lists from the eligible articles were retrieved to obtain further relevant studies. Duplicates between the databases were removed. To identify eligible studies, the retrieved articles were screened based on their title and abstract. Then, the potentially eligible studies were fully reviewed by 2 authors (AG and FP). Information was collected on the study characteristics, country, study design, follow-up, number of patients with NAFLD, number of patients with HCC, and NAFLD characteristics (such as type, diagnosis other than HCC stage, and outcome).
Endpoints and Statistical Analysis
The primary endpoints were (a) the global incidence of HCC in NAFLD patients and (b) the association of NAFLD with the risk of HCC. The secondary endpoints were the associations of HCC with relapse (DFS), cancer mortality (CSM), and all-cause mortality (OS).
Critical assessment was conducted of the study setting and SARS-CoV-2 diagnosis to reduce the bias. The Newcastle–Ottawa scale (NOS) was used as a critical appraisal tool with which to assess the quality of the eligible studies.
The cumulative incidence rate of HCC was calculated for NAFLD cases by dividing the number of NAFLD cases with HCC by the total number of NAFLD cases, which was expressed as a percentage (%) with 95% confidence intervals (95% CI). Pooled odds ratios (HRs) and 95% CIs were calculated to assess the association of NAFLD with the occurrence of HCC, as compared to non-NAFLD subjects. Similarly, HRs for DFS, CSM, and OS were calculated to correlate HCC with the outcome. The pooled HRs and 95% CIs are presented in a forest plot.
Metaregression analyses were also performed for the primary analysis according to steatosis/NASH, cirrhosis, and hepatitis B/C rate among patients as well as race, duration of follow-up, and type of study.
Z tests were performed to assess the association between HCC and the presence of NAFLD (P < .05 was considered statistically significant). Q tests were used to evaluate the heterogeneity among studies, and the data with heterogeneity were analyzed using a random effects model. The publication bias was assessed using Egger's test and a funnel plot (P < 0.05 for Begg's test was considered having potential for publication bias). The data were analyzed using Review Manager, version 5.3.
Results
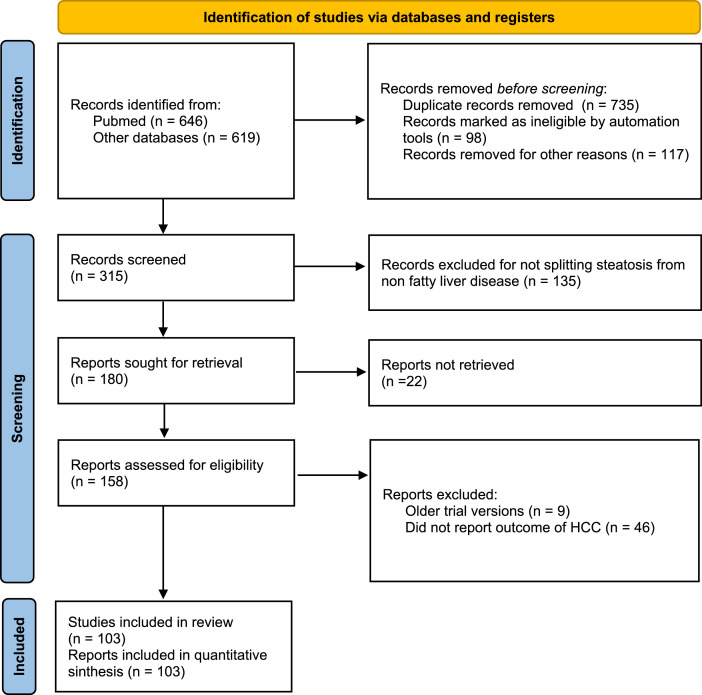
A total of 1265 citations were identified. Overall, 103 studies were eligible for inclusion in the present meta-analysis (Figure 1; Table 1; Suppl. File 1). Thus, a total of 948 217 participants with NAFLD were evaluated between 1992 and 2021.
Fig. 1.
flow diagram of included studies.
Table 1.
Characteristics of included studies.
| Author/year | Type of study | Country | Median follow up (months) | N° pts with NAFLD (all pts) | Steatosis only % | Steatohepatitis (NASH) % | Cirrosis % | Hepatitis B/C % | Diagnosis (radiological) % | Diagnosis: biopsy % |
|---|---|---|---|---|---|---|---|---|---|---|
| Aigelsreiter/2016 | Retrospective | Germany | 141 | 47 | 36.7 | 15.6 | - | - | - | 100 |
| Alexander/2019 | Retrospective | Europe | 39.3 | 136703 | 68.3 | 2 | 0.4 | - | - | - |
| Alvarez/2020 | Retrospective | US | 324 | 4355 | - | - | - | - | 100 | - |
| Amarapurkar/2008 | Prospective | India | - | 585 | - | 7 | 17.8 | 19/14.2 | 100 | - |
| Ampuero/2015 | Cross sectional | Spain | - | 34 | 23.5 | 76.5 | 70.5 | - | - | 100 |
| Arase/2012 | Retrospective | Japan | 98.4 | 1600 | - | - | - | 0 | 100 | - |
| Asahina/2013 | Retrospective | Japan | 73.2 | 431* | - | - | - | 100 | - | 100 |
| Ascha/2010 | Retrospective | Lebanon | 32.4 | 195 | 100 | 100 | 100 | 12.8 | - | 100 |
| Asfari/2020 | Cross sectional | US | - | 218950 | 100 | 100 | 8.2 | 2.6 | - | - |
| Bengtsson/2019 | Retrospective | Sweden | 16.2 | 225 | - | - | 63 | 0 | - | - |
| Best/2020 | Prospective | Japan | 167 | 392 | - | - | - | - | - | - |
| Beste/2015 | Retrospective | US | - | 1029 | - | - | - | - | 100 | - |
| Bhala/2011 | Prospective | UK | 85.6 | 247 | - | - | 100 | - | - | - |
| Carr/2018 | Retrospective | Italy | - | 61 | - | - | 80 | 0 | - | 100 |
| Chan/2017 | Retrospective | China | 79.9 | 107 | 100 | - | - | 100 | - | 100 |
| Chen CL/2014 | Case control | Taiwan | - | 50 | - | - | - | 100 | 100 | - |
| Cho/2011 | Retrospective | Korea | - | 54 | - | - | - | 50 | 100 | - |
| Choi/2020 | Retrospective | Canada | 120 | 185 | - | 100 | 93 | 100 | - | 100 |
| Chuma/2008 | Retrospective | Japan | 122 | 75 | 100 | - | - | 100 | - | 100 |
| Cotrim/2011 | Retrospective | Brazil | - | 1280 | 42 | 58 | 27 | 0 | - | 100 |
| D'Ambrosio/2018 | Prospective | Italy | 120 | 5 | 100 | - | - | 100 | - | 100 |
| Dal Bello/2010 | Retrospective | Italy | 36 | 33** | - | - | - | - | - | 100 |
| Doycheva/2019 | Retrospective | US | - | 1925 | 100 | 100 | 0 | 0 | - | - |
| Dugum/2015 | Retrospective | US | 40 | 838 | 100 | 100 | 0 | 0 | 0 | 100 |
| Dunn/2013 | Retrospective | US | - | 233 | 100 | 9 | 4 | 0 | 0 | 100 |
| Ekstedt/2015 | Retrospective | Sweden | 396 | 229 | - | 100 | 10 | 0 | 68 | 32 |
| El-derany/2020 | Prospective | Egypt | - | 134 | 100 | 100 | 0 | 0 | 0 | 100 |
| Ertle/2011 | Retrospective | Germany | - | 36 | 100 | 100 | 49 | 0 | 0 | 100 |
| Grimaudo/2020 | Prospective | Italy | 64.6 | 471 | 100 | 76.2 | 34.3 | 0 | 0 | 100 |
| Hamoir/2021 | Prospective | Belgium | 13 | 16 | 100 | - | 100 | 100 | 0 | 100 |
| Hashimoto/2009 | Prospective | Japan | 40.3 | 382 | 100 | 100 | 100 | 0 | 0 | 100 |
| Hayashi/2016 | Retrospective | Japan | 52.7 | 544 | 22.7 | - | 38.2 | - | - | 100 |
| Hernandez-Alejandro/2012 | Retrospective | Canada | - | 17 | - | 100 | - | - | 100 | 100 |
| Hester/2019 | Cross sectional | US | - | 2820 | - | - | 46.9 | 28.9 | - | - |
| Hsiang/2014 | Retrospective | New Zeland | 47 | 122 | - | - | 100 | 59.6 | 22.1 | 21.2 |
| Huang MY/2017 | Retrospective | Taiwan | 72 | 263 | - | - | 2.4 | 2.1 | - | - |
| Huang Y/2020 | Retrospective | Australia | 54 | 1597 | - | - | . | 70 | - | - |
| Hui/2003 | Prospective cohort | Australia | 60 | 23 | - | 100 | - | - | - | 100 |
| Ioannou/2019 | Retrospective | US | 44 | 7068 | - | - | 100 | 0 | - | - |
| Jain/2012 | Retrospective | India | - | 47 | - | - | 100 | - | - | 100 |
| Ji/2021 | Prospective | China | 48 | 1241 | 25.5 | - | 100 | 100 | - | 100 |
| Kai/2017 | Retrospective | Japan | 67 | 10 | - | - | - | 0 | - | 100 |
| Kanwal/2018 | Retrospective cohort | US | 108 | 296707 | - | - | 1.4 | - | - | - |
| Kaplan/2019 | Retrospective | US | 30 | 11306 | - | - | - | - | - | - |
| Kawamara/2011 | Retrospective | Japan | 68 | 6508 | - | - | - | 0 | 100 | - |
| Kim/2018 | Retrospective | Korea | 12 | 8721 | - | - | - | 0 | 100 | 100 |
| Kodama/2013 | Prospective | Japan | 50 | 72 | - | 100 | 100 | 0 | 100 | 100 |
| Kumar.2005 | Prospective | Australia | 26.2 | 25 | 76 | - | 25 | 100 | 100 | 100 |
| Kurosaki/2010 | Pospective | Japan | 54 | 1279 | 100 | - | - | 100 | 100 | 100 |
| Lee/2016 | Prospective | Korea | 45.2 | 24 | 100 | - | - | 100 | 100 | 100 |
| Li/2021 | Prospective | US | 140 | 1079 | 100 | 2.5 | 100 | 100 | 100 | |
| Lim/2020 | Retrospective | Singapore | 111 | 185 | 100 | - | 10.3 | 100 | 100 | 100 |
| Lin/2021 | Retrospective | Taiwan | 65 | 369 | 100 | - | - | 100 | - | 100 |
| Malik/2009 | Retrospective | US | 60 | 98 | 77.6 | 22.4 | 0 | - | 72.4 | |
| Marot/2017 | Retrospective | Switzerland/Belgium | - | 78 | - | - | - | - | - | - |
| Mittal/2015 | Retrospective | USA | - | 120 | 100 | 0 | 58.3 | - | - | 53.4 |
| Nakajima/2011 | Retrospective | Japan | - | 92 | 34.8 | 59.8 | 5.4 | 0 | 100 | 100 |
| Nirei/2017 | Retrospective | Japan | - | 170 | 100 | - | 100 | 100 | 0 | 100 |
| Nkontchou/2011 | Retrospective | France | 66 | 340 | 100 | - | 100 | 100 | - | 100 |
| Ogawa/2020 | Retrospective | Japan | 60 | 290 | 0 | 100 | 76 | 100 | - | 100 |
| Ohata/2003 | Retrospective | Japan | 76.5 | 90 | 76 | 100 | 100 | 100 | - | 100 |
| Paradis/2009 | Retrospective | France | - | 60 | - | - | - | - | - | 100 |
| Pekow/2006 | Retrospective | US | - | 23 | 100 | 0 | 100 | 100 | - | 100 |
| Peleg/2019 | Retrospective | Israel | 72 | 241 | 100 | 0 | 19.3 | 100 | - | 100 |
| Petit/2013 | Retrospective | France | NA | 141 | - | - | 100 | - | - | - |
| Phan/2019 | Retrospective | US | - | 28 | - | - | 89 | - | - | 100 |
| Pinyopornpanish/2021 | Retrospective | US | 13.8 | 346 | - | - | 14 | - | - | - |
| Reddy/2012 | Retrospective | US | 50 | 52 | - | 100 | - | - | - | 100 |
| Sadler/2017 | Retrospective | US/Canada | 56.1 | 60 | 0 | 100 | 0 | 0 | 100 | - |
| Safcak/2021 | Retrospective | Slovakia | - | 54 | - | - | 85.2 | 0 | 100 | - |
| Sanyal/2010 | Retrospective | US | - | 3933 | - | - | - | 4.5/31.1 | - | - |
| Schutte/2014 | Retrospective | Germany | - | 43 | - | 100 | - | - | - | - |
| Sharma/2018 | Retrospective | UK/Canada | - | 111 | - | - | 100 | - | - | - |
| Shibahara/2014 | Retrospective | Japan | - | 106 | - | 38.7 | 36.8 | 11.3/45.3 | - | 100 |
| Shimomura/2017 | Prospective observational | Japan | - | 69 | 14 | 55 | - | 0 | - | 100 |
| Shingina/2019 | Retrospective | US | - | 182368 | - | 9 | - | 38 | - | - |
| Simon/2021 | Retrospective | Sweden | - | 10568 | 67.2 | 27.2 | 5.6 | 0 | - | 100 |
| Su/2015 | Retrospective | China | 69.8 | 74 | - | - | - | 93 | - | 100 |
| Takahashi/2011 | Prospective cohort | Japan | - | 13 | 100 | - | - | 100 | - | 100 |
| Takuma/2007 | Retrospective | Japan | 45.1 | 25 | 100 | - | - | 65.9 | - | - |
| Tanaka/2013 | Retrospective | Japan | - | 49 | 26.5 | 73.5 | - | 0 | - | 100 |
| Tateishi/2015 | Retrospective | Japan | 31 | 596 | - | - | 61.7 | 26.7 | - | - |
| Thuluvath/2018 | Retrospective | US | - | 11302 | - | 100 | - | 100 | - | - |
| Tokushige/2010 | Prospective observational | Japan | 35.4 | 34 | - | 100 | - | 0 | - | 61.7 |
| Tokushige/2013 | Retrospective | Japan | - | 292 | - | - | 72 | 83 | - | 100 |
| Van meer/2015 | Retrospective | The Netherlands | 11 | 176 | - | - | 97 | 37 | - | 100 |
| Van Meer/2016 | Retrospective | The Netherlands | 12 | 181 | - | - | 81 | 38 | - | 100 |
| Viganò/2015 | Retrospective | Italy | 44.6 | 96 | 45.8 | 25 | 22.9 | 0 | - | 100 |
| Wakai/2011 | Retrospective | Japan | 87 | 17 | - | 47 | 75 | 92 | - | 100 |
| Walker/2016 | Retrospective | US | - | 204 | - | - | 100 | 74 | - | 100 |
| Wang /2021 | Retrospective | China | - | 17528 | - | - | - | - | 100 | - |
| Wild/2018 | Retrospective | UK | 56.4 | 1452 | - | - | - | - | - | 19 |
| Wong/2019 | Retrospective | US | - | 138 | 0 | 100 | 0 | 64 | - | 100 |
| Yatsuji/2008 | Prospective | Japan | - | 68 | - | 100 | - | - | - | 100 |
| Wu/2011 | Retrospective | China | 53.1 | 355 | 100 | - | - | 91.9 | 100 | 100 |
| Wu/2018 | Retrospective | US/Asia | - | 113 | - | 100 | - | - | 100 | 100 |
| Yang 2016 | Retrospective | US | 38 | 173 | - | - | 100 | 44 | 100 | 100 |
| Yen/2017 | Retrospective | China | 97.3 | 140 | 100 | - | 100 | 100 | 100 | 100 |
| Yoon/2020 | Prospective | Korea | 74.9 | 88 | - | 100 | 39.8 | 100 | 100 | 100 |
| Younossi/2019 | Retrospective | US | - | 2690 | - | 100 | - | - | 100 | 100 |
| Yu/2008 | Prospective | Taiwan (China) | 176.4 | 1850 | - | - | 22.1 | 100 | 100 | 100 |
| Zhang/2016 | Prospective | China | - | 7 | - | - | 75.3 | 100 | 100 | 100 |
| Zheng/2017 | Retrospective | US | 23 | 141 | 100 | - | 25 | 45 | 100 | 100 |
NAFLD, non-alcoholic fatty liver disease; NASH, non alcoholic steato-hepatitis; *. severe steatosis only; °. grade 2-3 only; **. grade 3 only; °°. higher fibrosis only
Characteristics of the Included Studies
All of the studies were observational and not intervention studies, 77 were retrospective series, 22 were prospective studies, 1 was a case control-study, and 3 were cross-sectional studies. Among the included studies, 42 were conducted in Asia, with the remaining having been conducted in Europe, Australia, or the United States. The median follow-up ranged from 11 to 396 months (mean 85). The mean NOS score was 6.4. A total of 209 110 cases of HCC were described, for a pooled incidence of 22%.
NAFLD and HCC Risk
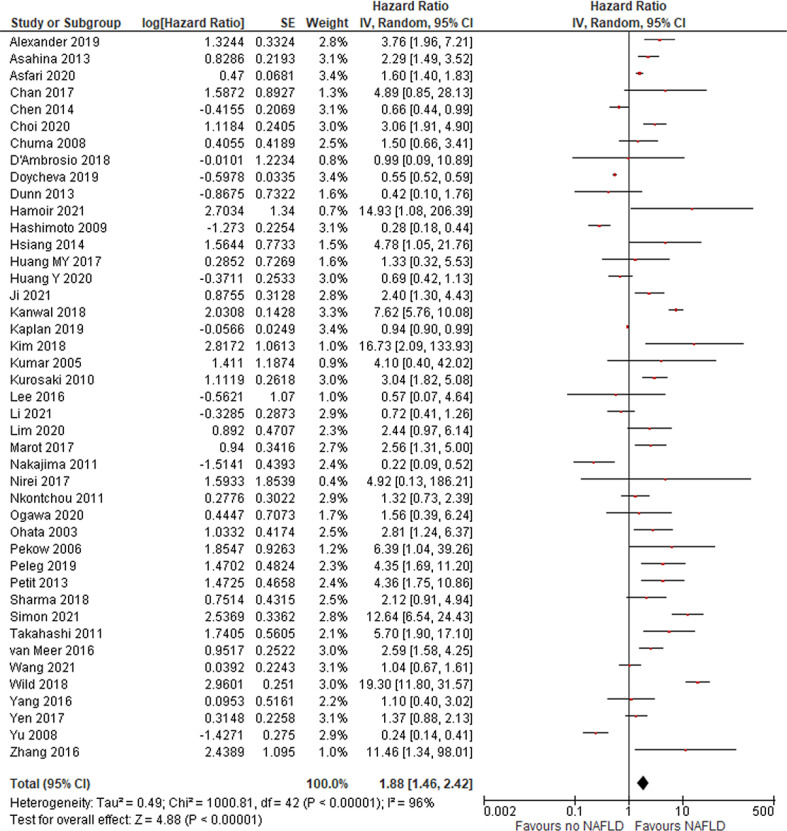
A total of 43 papers evaluated the risk of HCC over time in patients with steatosis/NASH with or without cirrhosis. The risk of HCC was 1.88 (95% CI, 1.46, 2.42), P < .01 (Figure 1). After excluding 5 studies in which HRs were calculated according to univariate analysis, the risk was even higher (HR = 2.19 [95% CI, 1.67, 2.87]; P < .01). This means that NAFLD is an independent risk factor for HCC development. The risk was unchanged after meta-regression analysis was performed according to the rate of steatosis, NASH, cirrhosis, viral hepatitis, and the follow-up duration. Regarding ethnicity, results were not significant for the studies conducted in Asian countries (HR = 1.43 [95% CI, 0.94-2.17]; P = .1) but were for studies conducted in Western countries (HR = 2.63 [95% CI, 1.79-3.87]; P < .01). In papers with poor- to moderate-quality NOS scores, the risk of HCC was not significant, but the risk was significant in good-quality papers (with longer/known follow-up periods; HR = 3.18 [95% CI, 1.98-5.11]; P < .01).
HCC Prognosis According to Steatosis
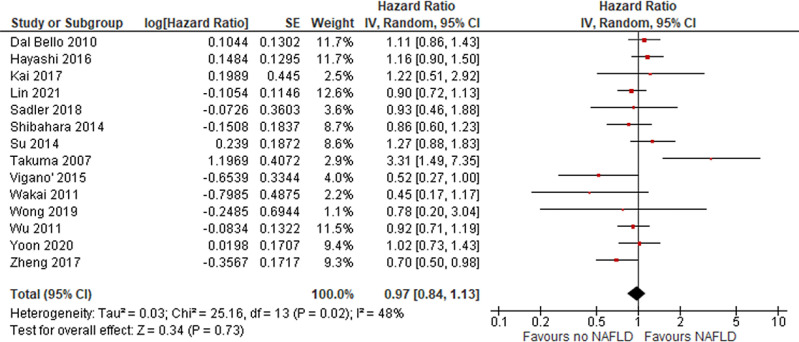
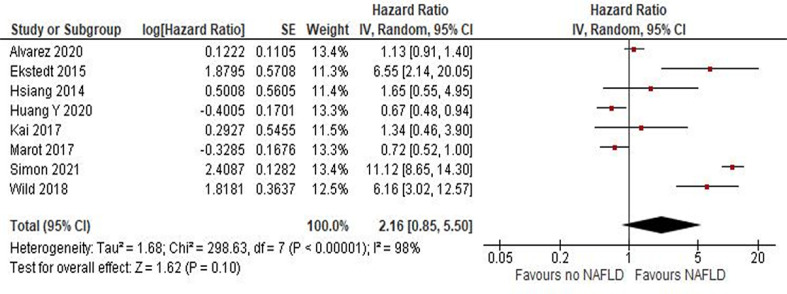
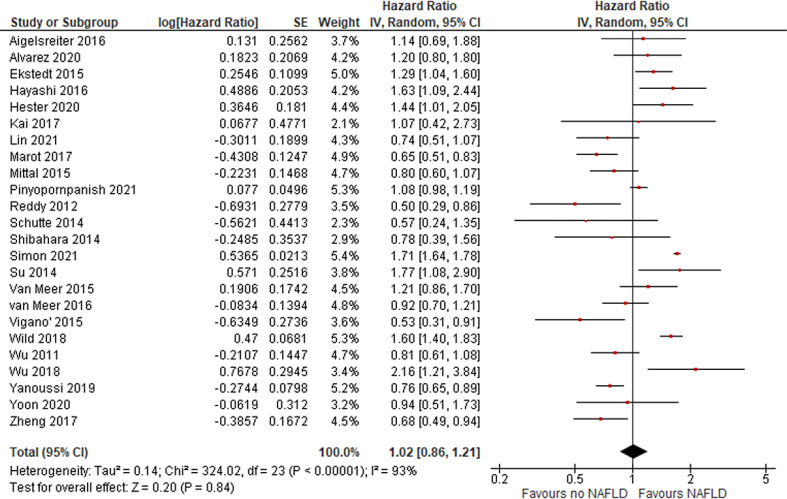
In total, 14, 8, and 24 studies evaluated HCC prognosis according to NAFLD state in terms of DFS, CSM, and OS, respectively. Overall, no difference was found for recurrence (DFS HR = 0.97 [95% CI, 0.84, 1.13]; = P = .73), CSM (HR = 2.16 [95% CI, 0.85, 5.5]; P = .1; Figure 2), or OS (HR = 1.02 [95% CI, 0.86, 1.21]; P = .84; Figure 3), revealing that outcome of HCC is not more unfavorable in patients with NAFLD.
Fig. 2.
risk of HCC in patients with NAFLD.
Fig. 3.
DFS in patients with HCC and NAFLD.
Publication Bias
Evidence of publication bias regarding risk of HCC meta-analysis was observed based on the results of a funnel plot (P < .01) but not with Egger's test (P = .18).
Discussion
Through a meta-analysis of published literature, we evaluated the risk of HCC in patients with NAFLD in the general population. We confirmed that NAFLD was independently associated with an 88% increased risk of HCC, as compared to no NAFLD, in a series of 103 studies published across 3 decades. In a similar meta-analysis published in 2018, Stine et al. found that the risk of HCC was significantly increased only in patients with noncirrhotic NASH but not in the whole NASH population (with or without cirrhosis) [6]. However, a meta-regression analysis adjusted for the rate of steatosis/NASH and fibrosis did not confirm these findings. Steatohepatitis was also associated with an increased risk of intrahepatic cholangiocarcinoma and colorectal cancer [7],[8]. Similar causative factors such as diabetes, overweight, or hepatitis C may be responsible for this association. In fact, approximately 30% to 40% of incident HCC cases are associated with metabolic syndrome. Type 2 diabetes is also a risk factor for NAFLD and increases HCC incidence [9].
NAFLD mouse models showed altered compositions of their gut microbiome. NAFLD HCC patients had increased levels of IL-13, which can activate myeloid-derived suppressor cells and promote tumor progression by inhibiting cancer immunity [10].
Another mechanism of NAFLD-associated HCC is PNPLA3 polymorphisms, which are associated with general NAFLD progression, by enhancing inflammatory signals, including in the IL-6/STAT3 and CCL5 pathways [11].
Nonalcoholic fatty liver disease (and NASH-related cirrhosis in particular) is an emerging risk factor for HCC in Western countries. However, risk of HCC was increased in Western populations but not Asian populations in subgroup analysis. The indication for liver transplant is increased more than 11-fold worldwide [12]. It is rare, however, to observe HCC in the absence of liver inflammation or cirrhosis [13]. In the present meta-analysis, in fact, the papers included almost all subjects with NASH/cirrhosis, with or without viral hepatitis. We found that steatosis/NASH was an independent risk factor for HCC, as compared to no steatosis/no NASH (more than doubling the risk). Conversely, NAFLD-associated HCC was not linked with a poorer prognosis, as compared to non-NAFLD-related cancers. It appears that steatosis or NASH may exert a somewhat protective effect on the HCC course. Even in the general population, NASH patients without or with minimal fibrosis, but not those with higher levels of fibrosis, have a better prognosis in terms of overall mortality [14], [15]. Even in the NHANES cohort, patients with NASH but not advanced fibrosis had a lower risk of death [16]. However, in our review, HCC-related mortality but not overall mortality was (not significantly) higher in patients with NAFLD. This may be due to the high rates of fibrosis and viral hepatitis C in our cohorts.
These observations highlight that patients with NASH with or without initial fibrosis may need intensive surveillance and treatment to slow or revert fibrosis evolution and cancer transformation. In patients with NAFLD and cirrhosis, in fact, the management is similar to that for cirrhosis due to other causes and includes screening for hepatocellular carcinoma, lifestyle interventions, and evaluation for liver transplantation, for patients with decompensated cirrhosis or HCC.
Treatment of NAFLD-associated HCC is not different from that of HCC related to other causes. Instead, the role of immunotherapy, which provides a better outcome for advanced HCC than antiangiogenetic drugs do, has been questioned in NASH patients. In preclinical models of NASH-induced HCC, the delivery of immunotherapy-targeting programmed death-1 (PD1), in fact, expanded activated CD8+PD1+ T-cells within tumors but did not lead to tumor regression, indicating that tumor immune surveillance was impaired. These observations seem to confirm that NASH-associated HCC might be less responsive to immunotherapy, probably due to NASH-related aberrant T-cell activation causing tissue damage that leads to impaired immune surveillance [17].
Our meta-analysis suffers several limitations, including regarding its inclusion criteria (patients with both steatosis or NASH and various degrees of fibrosis), confounders due to viral hepatitis coinfection, duration of follow-up, race, and NAFLD diagnosis. However, this is the largest meta-analysis to have been performed on studies on how NAFLD affects the risk and prognosis of HCC.
We can conclude that NAFLD, with or without fibrosis, is a major risk factor for the development of HCC. Despite this, overall mortality and CSM are not significantly increased when HCC is diagnosed. Despite the heterogeneity of the included literature and the degree of NAFLD of our populations, these subjects deserve similar follow-up and management like patients with other chronic liver diseases receive.
Fig. 4.
CSM in patients with HCC and NAFLD.
Fig. 5.
OS in patients with HCC and NAFLD.
Table 2.
Frequence and outcome of HCC in patients with NAFLD.
| Author/year | HCC n/% | HCC risk: HR or OR (95%CI) | Type of analysis | HCC DFS: HR (95%CI) | Type of analysis | Cancer mortality HR (95%CI) | Type of analysis | HCC OS: HR (95%CI) | Type of an alysis | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Aigelsreiter/2016 | - | - | - | 1.07 (0.67-1.69) | UVA | - | - | 1.14 (0.69-1.89) | UVA | 8 |
| Alexander/2019 | 176/0.1 | 3.76 (1.96-7.20)° | MVA | - | - | - | - | - | - | 6 |
| Alvarez/2020 | - | - | - | - | - | 1.13 (0.91-1.39) | MVA | 1.2 (0.8-1.34) | MVA | 9 |
| Amarapurkar/2008 | 54/9.2 | - | - | - | - | - | - | - | - | 5 |
| Ampuera/2015 | 7/20.6 | - | - | - | - | - | - | - | - | 5 |
| Arase/2012 | 10/6 | - | - | - | - | - | - | - | - | 8 |
| Asahina/2013 | - | 2.29 (1.49-3.50)^ | MVA | - | - | - | - | - | - | 8 |
| Ascha/2010 | 25/12.8 | - | - | - | - | - | - | - | - | 7 |
| Asfari/2020 | 10947/0.5 | 1.6 (1.4-1.9)° | MVA | - | - | - | - | - | - | 6 |
| Bengtsson/2019 | 225/14.4 | - | - | - | - | - | - | - | - | 6 |
| Best/2020 | 29/7.1 | - | - | - | - | - | - | - | - | 8 |
| Beste/2015 | 1029/- | - | - | - | - | - | - | - | - | 9 |
| Bhala/2011 | 6/2.4 | - | - | - | - | - | - | - | - | 8 |
| Carr/2081 | 16/- | - | - | - | - | - | - | - | - | 5 |
| Chan/2017 | 11/4.1 | 6.58 (0.9-46.8)* 3.2 (0.8-12.3)° | UVA | - | - | - | - | - | - | 9 |
| Chen/2014 | 50/- | 0.66 (0.44–0.99) | MVA | - | - | - | - | - | - | 5 |
| Cho/2011 | 54/- | - | - | - | - | - | - | - | - | 5 |
| Choi/2020 | 16/8.6 | 3.06 (1.91-4.91)° | UVA | - | - | - | - | - | - | 9 |
| Chuma/2008 | 35/33.7 | 1.5 (0.66–3.4)^ | MVA | - | - | - | - | - | - | 9 |
| Cotrim/2011 | 3/0.2 | - | - | - | - | - | - | - | - | 5 |
| D'Ambrosio/2018 | 5/- | 0.99 (0.09-10.89) | UVA | - | - | - | - | - | - | 9 |
| Dal Bello/2010 | 207/- | - | - | 1.11 (0.86-1.42) | UVA | - | - | - | - | 6 |
| Doycheva/2019 | 1925/- | 0.55 (0.52-0.59) | MVA | - | - | - | - | - | - | 5 |
| Dugum/2015 | 838/- | - | - | - | - | - | - | - | - | 7 |
| Dunn/2013 | 2/1 | 0.42 [0.10, 1.76] | UVA | - | - | - | - | - | - | 5 |
| Ekstedt/2015 | - | - | - | - | - | 6.55 (2.14-20) | UVA | 1.24 (1.04-1.59) | UVA | 9 |
| El-derany/2020 | 55/- | - | - | - | - | - | - | - | - | 5 |
| Ertle/2011 | 36/- | - | - | - | - | - | - | - | - | 5 |
| Grimaudo/2020 | 13/2.7 | - | - | - | - | - | - | - | - | 8 |
| Hamoir/2021 | 3/18.7 | 14.93 (1.08-206.39) | MVA | - | - | - | - | - | - | 6 |
| Hashimoto/2009 | 34/8.9 | 0.28 (0.18-0.45) | UVA | - | - | - | - | - | - | 6 |
| Hayashi/2016 | 544/- | - | - | 1.17 (0.90-1.53) | MVA | - | - | 1.63 (1.09-2.52) | MVA | 7 |
| Hernandez-Alejandro/2012 | 17/- | - | - | - | - | - | - | - | - | 5 |
| Hester/2019 | 2820/- | - | - | - | - | - | - | 1.44 (1.01-2.07) | MVA | 8 |
| Hsiang/2014 | - | 4.78 (1.05-21.79) | MVA | - | - | 1.11 (1.01-1-13) | MVA | - | - | 6 |
| Huang/2017 | - | 1.33 (0.32-5.53) | MVA | - | - | - | - | - | - | 5 |
| Huang/2020 | 226/2 | 1.69 (1.43-2) | MVA | - | - | 0.67 (0.48-0.95) | MVA | - | - | 7 |
| Hui/2003 | 0/0 | - | - | - | - | - | - | - | - | 7 |
| Ioannou/2019 | 690/54 | - | - | - | - | - | - | - | - | 5 |
| Jain/2012 | 8/17 | - | - | - | - | - | - | - | - | |
| Ji/2021 | 54/4.3 | 2.4 (1.3-4-2) | MVA | - | - | - | - | - | - | 6 |
| Kai/2017 | 83/100 | - | - | 1.22 (0.51-2.89) | UVA | - | - | 1.07 (0.42-2.73) | UVA | 6 |
| Kanwal/2018 | 367/0.12 | 7.62 (5.76-10.09) | MVA | - | - | - | - | - | - | 9 |
| Kaplan/2019 | - | 0.94 (0.90-0.99) | MVA | - | - | - | - | - | - | 6 |
| Kawamura/2011 | 16/0.25 | - | - | - | - | - | - | - | - | 5 |
| Kim/2018 | 13/8721 | 16.73 (2.09-133.85) | MVA | - | - | - | - | - | - | 6 |
| Kodama/2013 | 16/- | - | - | - | - | - | - | - | - | 7 |
| Kumar/2005 | 25/- | 4.1 (0.4-39)* | MVA | - | - | - | - | - | - | 8 |
| Kurosaki/2010 | 68/- | 3.04 (1.82-5.06) | MVA | - | - | - | - | - | - | 7 |
| Lee/2016 | - | 0.57 (0.07-4.74) | UVA | - | - | - | - | - | - | 7 |
| Li/2021 | 40/3.74 | 0.72 (0.41-1.30) | MVA | - | - | - | - | - | - | 9 |
| Lim/2020 | 27/289 | 2.44 (0.97-6.1) | MVA | - | - | - | - | - | - | 9 |
| Lin/2021 | 369/- | - | - | 0.9 (0.72-0.13) | UVA | 0.74 (0.51-1.07) | UVA | - | - | 8 |
| Malik/2009 | 17/17.3 | - | - | - | - | - | - | - | - | 7 |
| Marot/2017 | 12/15 | 2.56 (1.31-5.00) | MVA | - | - | - | - | - | - | 5 |
| Mittal/2015 | 120/- | - | - | - | - | - | - | 0.8 (0.6-1.0) | MVA | 5 |
| Nakajima/2011 | 14/15.2 | 0.22 (0.09-0.61) | UVA | - | - | - | - | - | - | 5 |
| Nirei/2017 | 12/7 | 4.92 (0.13-186)* | MVA | - | - | - | - | - | - | 5 |
| Nkontchou/2011 | 96/28 | 1.32 (0.73-2.39)* | - | - | - | - | - | - | - | 7 |
| Ogawa/2020 | 16/0.5 | 1.56 (0.39-6.24)* | UVA | - | - | - | - | - | - | 7 |
| Ohata/2003 | - | 2.81 (1.24-6.37)^ | MVA | - | - | - | - | - | - | 8 |
| Paradis/2009 | 60/- | - | - | - | - | - | - | - | - | 5 |
| Pekow/2006 | 32/- | 6.39 (1.04-39.3)* | MVA | - | - | - | - | - | - | 5 |
| Peleg/2019 | 14/5.8 | 4.35 (1.69-11.2) | MVA | - | - | - | - | - | - | 8 |
| Phan/2019 | 3/- | - | - | - | - | - | - | - | - | 5 |
| Pinyopornpanish/2021 | 346/- | - | - | - | - | - | - | 1.08 (0.98-1.28) | MVA | 6 |
| Reddy/2012 | 52/- | - | - | - | - | - | - | 0.50 (0.29-0.88) | MVA | 6 |
| Sadler/2017 | 60/- | - | - | 0.93 (0.45-1.92) | UVA | - | - | - | - | 8 |
| Safcak/2021 | 54/- | - | - | - | - | - | - | - | - | 5 |
| Sanyal/2010 | 2578/58.5 | - | - | - | - | - | - | - | - | 5 |
| Schutte/2014 | 43/- | - | - | - | - | - | - | 0.57 (0.24-1.34) | UVA | 5 |
| Sharma/2018 | 8/3.5 | 2.12 (0.91-4.92) | MVA | - | - | - | - | - | - | 5 |
| Shibahara/2014 | 106/- | - | - | 0.87 (0.62-1.23)^0.83 (0.55-1.25)° | UVA | - | - | 0.80 (0.41-1.56)^0.75 (0.34-1.64)° | UVA | 5 |
| Shimomura/2017 | - | - | - | - | - | - | - | - | - | 6 |
| Shingina/2019 | 2181/13 | - | - | - | - | - | - | - | - | 6 |
| Simon/2021 | 186/- | - | - | - | - | - | - | - | - | 6 |
| Su/2015 | 74/- | - | MVA | - | - | - | - | - | - | 7 |
| Takahashi/2011 | 6/46.2 | 5.7 (1.9-17.1) | MVA | - | - | - | - | - | - | 7 |
| Takuma/2007 | 25/- | - | - | 3.31 (1.49-7.41) | MVA | - | - | - | - | 7 |
| Tanaka/2013 | 6/16.7 | - | - | - | - | - | - | - | - | 5 |
| Tateishi/2015 | 596/- | - | - | - | - | - | - | - | - | 6 |
| Thuluvath/2018 | 2166/19 | - | - | - | - | - | - | - | - | 5 |
| Tokushige/2010 | 34/- | - | - | - | - | - | - | - | - | 7 |
| Tokushige/2013 | 292/- | - | - | - | - | - | - | - | - | 5 |
| Van Meer/2015 | 176/- | - | - | - | - | - | - | - | - | 6 |
| Van Meer/2016 | 181/- | 2.59 (1.58-4.26) | MVA | - | - | - | - | - | - | 6 |
| Viganò/2015 | 96/- | - | - | 0.55 (0.36-0.85) | MVA | - | - | 0.53 (031-0.91) | MVA | 7 |
| Wakai/2011 | 17/- | 0.45 (0.17-1.17) | MVA | - | - | - | - | - | - | 8 |
| Walker/2016 | 204/- | - | - | - | - | - | - | - | - | 5 |
| Wang/2021 | 39/0.2 | 1.07 (0.73-1.58) | UVA | - | - | - | - | - | - | 5 |
| Wild/2018 | 19/- | 19.3 (11.8-31.4) | - | - | - | 6.16 (3.02-12.6) | MVA | - | - | 7 |
| Wong/2019 | 138/- | 0.78 (0.20-3.03) | UVA | - | - | - | - | - | - | 5 |
| Yatsuji/2008 | 7/10 | - | - | - | - | - | - | - | - | 5 |
| Wu/2011 | 355/- | - | - | 0.92 (0.71-1.19) | MVA | - | - | 0.81 (0.61-1.08) | MVA | 6 |
| Wu/2018 | 113/- | - | - | - | - | - | - | 2.16 (1.21-3.84) | - | 5 |
| Yang/2016 | - | 1.10 (0.40-3.02) | MVA | - | - | - | - | - | - | 6 |
| Yen/2017 | 140/14.36 | 1.37 (0.88-2.13) | MVA | - | - | - | - | - | - | 8 |
| Yoon/2020 | 196/50 | - | - | 1.02 (0.73-1.43) | MVA | - | - | 0.94 (0.51-1.73) | MVA | 8 |
| Younossi/2019 | 2690/- | - | - | - | - | 4.17 (3.81-4.56) | MVA | 0.76 (0.65-0.89) | MVA | 5 |
| Yu/2008 | - | 0.24 (0.14-0.41) | MVA | - | - | - | - | - | - | 9 |
| Zhang/2016 | 6/1.38 | 11.46 (1.34-98.01) | MVA | - | - | - | - | - | - | 5 |
| Zheng/2017 | 141/- | - | - | 0.70 (0.50-0.98) | MVA | - | - | 0.68 (0.49-0.94) | MVA | 6 |
HCC, hepatocellular carcinoma; HR, hazard ratio; OR, odds ratio; CI, confidence interval; DFS, disease-free survival; OS, overall survival; UVA, univariate analysis; MVA, multivariate analysis; *, grade 2-3 vs 0; °, steatohepatitis; ^, steatosis grade 1-3 vs 0, **, composite outcome of cancer incidence and mortality.
Declaration of Competing Interest
None to declare
Funding
None to declare
Footnotes
Author contributions: all authors contributed equally
References
- 1.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018 doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016 doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Zhou J, Wang W, et al. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology. 2019 doi: 10.1002/hep.30702. [DOI] [PubMed] [Google Scholar]
- 4.Orci LA, Sanduzzi-Zamparelli M, Caballol B, et al. Incidence of Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review, Meta-analysis, and Meta-regression. Clin Gastroenterol Hepatol. 2021 doi: 10.1016/j.cgh.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Sheka AC, Adeyi O, Thompson J, Hameed B, Crawford PA, Ikramuddin S. Nonalcoholic Steatohepatitis: A Review. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.2298. [DOI] [PubMed] [Google Scholar]
- 6.Stine JG, Wentworth BJ, Zimmet A, et al. Systematic review with meta-analysis: risk of hepatocellular carcinoma in non-alcoholic steatohepatitis without cirrhosis compared to other liver diseases. Aliment Pharmacol Ther. 2018 doi: 10.1111/apt.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corrao S, Natoli G, Argano C. Nonalcoholic fatty liver disease is associated with intrahepatic cholangiocarcinoma and not with extrahepatic form: Definitive evidence from meta-analysis and trial sequential analysis. Eur J Gastroenterol Hepatol. 2020 doi: 10.1097/MEG.0000000000001684. [DOI] [PubMed] [Google Scholar]
- 8.Lin XL, You FM, Liu H, Fang Y, Jin SG, Wang QL. Site-specific risk of colorectal neoplasms in patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis. PLoS One. 2021 doi: 10.1371/journal.pone.0245921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massarweh NN, El-Serag HB. Epidemiology of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Cancer Control. 2017 doi: 10.1177/1073274817729245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ponziani FR, Bhoori S, Castelli C, et al. Hepatocellular Carcinoma Is Associated With Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology. 2019 doi: 10.1002/hep.30036. [DOI] [PubMed] [Google Scholar]
- 11.Liu YL, Patman GL, Leathart JBS, et al. Carriage of the PNPLA3 rs738409 C >g polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014 doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin Gastroenterol Hepatol. 2019 doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 13.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular Carcinoma in the Absence of Cirrhosis in United States Veterans Is Associated With Nonalcoholic Fatty Liver Disease. Clin Gastroenterol Hepatol. 2016 doi: 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015 doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 15.Wattacheril J, Chalasani N. Nonalcoholic fatty liver disease (NAFLD): Is it really a serious condition? Hepatology. 2012 doi: 10.1002/hep.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le MH, Devaki P, Ha NB, et al. Prevalence of non-Alcoholic fatty liver disease and risk factors for advanced fibrosis and mortality in the United States. PLoS One. 2017 doi: 10.1371/journal.pone.0173499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfister D, Núñez NG, Pinyol R, et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature. 2021 doi: 10.1038/s41586-021-03362-0. [DOI] [PMC free article] [PubMed] [Google Scholar]