Abstract
The coronavirus disease 2019 (COVID-19) can disrupt various brain functions. Over a one-year period, we aimed to assess brain activity and cognitive function in 53 COVID-19 patients and 30 individuals without COVID-19 (or asymptomatic). The Montreal Cognitive Assessment, Trail Making Test Parts A and B (TMT-A and B), and Digit Span Test were used to assess cognitive function. Cognitive variables and electroencephalography (EEG) data (activity, mobility, and complexity) were compared between the groups at rest and during cognitive demand (F3-F7, Fz-F3, Fz-F4, and F4-F8). There was a reduction in F3-F7 activity during the TMT-B in the COVID-19 group at 6-12 months compared to the controls (p = 0.01) at baseline (p = 0.03), a reduction in signal complexity at F3-F7 at rest in the COVID-19 group at baseline and 6-12 months compared to the controls (p < 0.001), and a reduction in Fz-F4 activity at rest from 6-12 months in the post-COVID group compared to baseline (p = 0.02) and 3-6 months (p = 0.04). At 6-12 months, there was a time increase in TMT-A in the COVID-19 group compared to that in the controls (p = 0.04). Some correlations were found between EEG data and cognitive test in both groups. In conclusion, there was a reduction in brain activity at rest in the Fz-F4 areas and during high cognitive demands in the F3-F7 areas. A reduction in signal complexity in F3-F7 at rest was found in the COVID-19 group at 6-12 months after acute infection. Furthermore, individuals with COVID-19 experience long-term changes in cognitive function.
Keywords: SARS-CoV-2, COVID-19, electroencephalography, cognition
Introduction
The coronavirus disease 2019 (COVID-19) outbreak in China has rapidly spread, resulting in a large number of cases and deaths worldwide.1 The most common symptoms of viral infection are fever, dry cough, and fatigue.2 However, some studies have found links between severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and neurological dysfunction both in the early stages3 and the long term (long COVID).4 According to recent studies, 36.4%3 and 57.4%5 of patients had early neurological manifestations such as headache, dizziness, acute cerebrovascular diseases, and a decreased level of consciousness, in addition to long cognitive deficits in executive function, attention, language, and delayed recall.6
The interaction of SARS-CoV-2 and angiotensin-converting enzyme-2 receptor (ACE-2)-expressing neuronal/glial cells may facilitate virus entry into the brain by circulation, cranial nerve I, or the olfactory bulb.7–9 ACE-2 is expressed in various brain regions, such as the substantia nigra, ventricles, middle temporal gyrus, posterior cingulate cortex, and olfactory bulb.10 The virus can move into cerebral circulation, and once inside the brain, it can infect neural cells, astrocytes, and microglia.11 These cells express ACE-2, which initiates the viral budding cycle, followed by neuronal damage and inflammation.12 Furthermore, a cytokine storm is observed in some patients with COVID-19, and some cytokines can cross the blood-brain barrier and activate the brain’s immune cells to produce neural cytokines, leading to brain dysfunction.8
Several authors have observed that COVID-19 can change brain activity and connectivity,13–15 and a pronounced reduction in gray matter thickness in the orbitofrontal cortex,16 causing cognitive dysfunction for months after the infection has resolved.17 In addition, there are increasingly frequent reports of memory impairment, concentration difficulties, and long-term neuropsychiatric symptoms.18 In a UK Biobank imaging study, multimodal brain imaging data were obtained, and the authors showed modifications in some brain regions in milder cases of SARS-COV-2.19 On the other hand, there are other cost-effective ways to analyze brain activity in mild to moderate COVID-19, such as electroencephalography (EEG). EEG is one of the few techniques that allow the noninvasive study of brain functioning with good time resolution.20
EEG and cognitive tests can be simple and effective in assessing brain activity and the evolution of cognitive performance in the long term after COVID-19.21 Several studies have developed EEG-based measures of cognitive function.22 In particular, time domain Hjorth parameters, such as, activity, mobility, and complexity are useful to detect the abnormal brain functions based on nonlinear EEG features.23 Theses parameter have achieved successful results, including detection of epilepsy,24 depressive status25 and neurodegenerative disorders.26 In addition, activity, mobility, and complexity27 are considered good indicators of the functional states of the brain,28 and are associated with brain metabolic changes29 and mild cognitive impairment.30 Therefore, nonlinear EEG features are also a good tool for cognitive processes research in neurological and psychiatric disorders of our interest.
Graham et al emphasized the importance of long-term studies to assess the cognitive impact of SARS-CoV-2 infection in non-hospitalized individuals, who comprise the majority of COVID-19 patients.31 EEG changes following COVID-19 may be independent of the severity of the acute infection and suggest a link with ongoing neuropsychiatric symptoms. More follow-up data are needed to confirm COVID-19's reversibility and/or evolution.32 There is much evidence of brain-related abnormalities in COVID-19; however, it remains unknown whether the impact of SARS-CoV-2 infection can be detected in milder and moderate cases. Therefore, a standardized investigation of EEG and cognitive symptoms during the long COVID-19 is required to better understand the evolution of the disease, outline appropriate treatment procedures, and clarify the evolution and impact of SARS-CoV-2.
Two questions must be answered in this context. (1) Do EEG changes occur during the evolution of COVID-19? (2) Are there any long-term cognitive effects of COVID-19? This study aimed to examine changes in electrical brain activity and cognitive function in people with mild to moderate COVID-19 over the course of a year. This study hypothesized that electrical brain activity and cognitive functions would be reduced in the first months compared to non-COVID-19 individuals, with progressive changes over the year.
Materials and Methods
Study Design, Setting, and Participants
This 12-month prospective cohort study included patients with and without COVID-19. This research was performed at XXX between September 2020 and September 2021. We prospectively analyzed 83 individuals (53 individuals with COVID-19 and 30 healthy volunteers without COVID-19). The COVID-19 diagnosis was confirmed using SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) of nasopharyngeal swabs. The control group criteria were that they should be negative for COVID-19 at the time of evaluation and should not have had a positive diagnosis of COVID-19 or be asymptomatic since the beginning of the pandemic.
Individuals were recruited via radio television, and digital media (Instagram, Facebook, Twitter, and WhatsApp), and also referred through the Uberaba municipal health department. This study was approved by our institutional review board (CAAE: 30684820.4.0000.5154).
Eligibility Criteria
Individuals with mild to moderate COVID-19 symptoms who met the COVID-19 diagnostic standard, had an education level greater than nine years (completed at least a junior middle school level of education), and could complete the tests independently were included. Based on the imaging examination, mild COVID-19 was defined as mild clinical symptoms without pneumonia. Moderate COVID-19 was defined as fever and respiratory symptoms with pneumonia without ICU admission or mechanical ventilation.33
Individuals without COVID-19 infection who were 18 years or older, able to understand the tests, had visual acuity (VA ≥ 0.8) in the Snellen test,34 normal auditory acuity in the Weber and Rinne test,34 and had retained cognitive ability comprised the control group. Patients with severe and critical COVID-19; a history of mental disorders; current treatment for mental illnesses, such as antipsychotics, antidepressants, mood stabilizers, antiepileptics, benzodiazepines, and other drugs that may interfere with the assessment; severe physical illnesses that may interfere with the assessment; a history of drug abuse or drug dependence due to interference in cortical excitability; serious suicidal thoughts; pregnant or lactating women; and others were excluded. During follow-up, individuals were excluded from the study if they did not complete the proposed tests at the time of collection, did not attend reassessments, were exposed to a new COVID-19 infection, or had a neurological or psychiatric disease unrelated to COVID-19 infection during follow-up were excluded from the study.
Procedures
All participants filled out an electronic form regarding their health and were later referred for screening to confirm the diagnosis of COVID-19. Individuals were directed to the study evaluation protocol after confirmation by RT-PCR. The principal investigator and research team performed all tests three times within a year of COVID-19 diagnosis: (a) baseline (15 days after RT-PCR + ), (b) 3-6 months after acute infection, and (c) 6-12 months after acute infection. The control group underwent only one test, and all individuals tested negative for RT-PCR. All study investigators were instructed on each protocol item (Supplemental File 1).
Variables
Outcomes
Features (activity, mobility, and complexity) were estimated from electrical brain activity measured by EEG and cognitive functions measured by the Montreal Cognitive Assessment (MoCA), Trail Making Test (TMT), Part A and Part B), and digit span (DS) tests.
Exposures
Individuals with SARS-CoV-2 laboratory-positive (baseline; 3-6 months; and 6-12 months) and 30 laboratory-negative individuals with SARS-CoV-2 infection.
Potential Confounders
Demographic and clinical variables (age, sex, race, BMI, schooling), pre-existing comorbidities, hospital anxiety and depression scale (HADS), Nottingham sensory assessment (NSA), Medical Research Council (MRC) scale, and post-COVID-19 functional status (PCFS) scale.
Data Sources/Measurement
a) Demographic and clinical variables
Age, race, ethnicity, socioeconomic status, and formal education were reported by patients or their legal representatives in an interview. Medical history, including main comorbidities and neurological evaluation, was determined during the participants’ screening. Neurological evaluation included anxiety and depression evaluation by HADS (values greater than 9 points individuals had anxiety or depression),35 somatosensory function by Nottingham sensory assessment (NSA) (higher values indicate better somatosensory function),36 and muscle strength by the Medical Research Council (MRC) scale (total score ranges from zero to 60, with higher values indicating better global muscle strength).37
b) Post-COVID-19 functional status (PCFS) scale
This scale was used to assess the level of impairment in the functional status of patients with COVID-19.38 The PCFS scale stratification is composed of five grades: grade 0 (no functional limitations), grade 1 (negligible functional limitations), grade 2 (slight functional limitations), grade 3 (moderate functional limitations), and grade 4 (severe functional limitations).
c) Cognitive function evaluation
Cognitive screening was performed using three tests: 1) MoCA; 2) TMT; and 3) DS. The MoCA tests ten cognitive domains using rapid, sensitive, and easy-to-administer cognitive tasks to evaluate global cognitive function. The total possible score was 30 points, with a score of 26 or above considered normal.39 The TMT consists of 25 circles distributed on a sheet of paper. In Part A, the circles were numbered 1-25, and the participant drew lines to connect the numbers in ascending order. In Part B, the circles included two numbers (1-13) and letters (A-L). The participant was then instructed to connect the circles as quickly as possible without lifting the pen or pencil from the paper, marking the time that the individual took to connect the circles using a chronometer.40 The difference between the raw scores for the TMT-A and TMT-B was computed as TMT-B minus TMT-A. Subtracting the time of TMT-A from that of TMT-B is important for analyzing more complex executive functions such as cognitive flexibility.34 The DS test consisted of eight series for forward order (DSF) and seven for backward order (DSB), with a gradual increase in the number of digits in each series. The test started with three numbers, and direct order was applied first. This was followed by the inverse, which was administered independently if the participant completely failed in the direct order. Each item consisted of two sets of digits, constituting two attempts, both of which were applied. The maximum possible score on the test was 30 points. A researcher recorded the test numbers such that the same was applied to all participants to avoid bias in the speed, intensity, and pitch of the voice.41
d) Brain electrical activity
This outcome was assessed using electroencephalography (EEG CGX Dev Kit, CGX A Cognionics Company, San Diego, CA) using the international 10-20 system for electrode placement.42 Individuals’ EEG data were collected during three tasks: 1) at rest, 2) while performing the TMT-A (low cognitive demand), and 3) while performing the TMT-B (high cognitive demand). For the experimental protocol, the patients were instructed to refrain from engaging in strenuous activities and consuming beverages containing caffeine, alcohol, or tobacco for 24 h before the test.
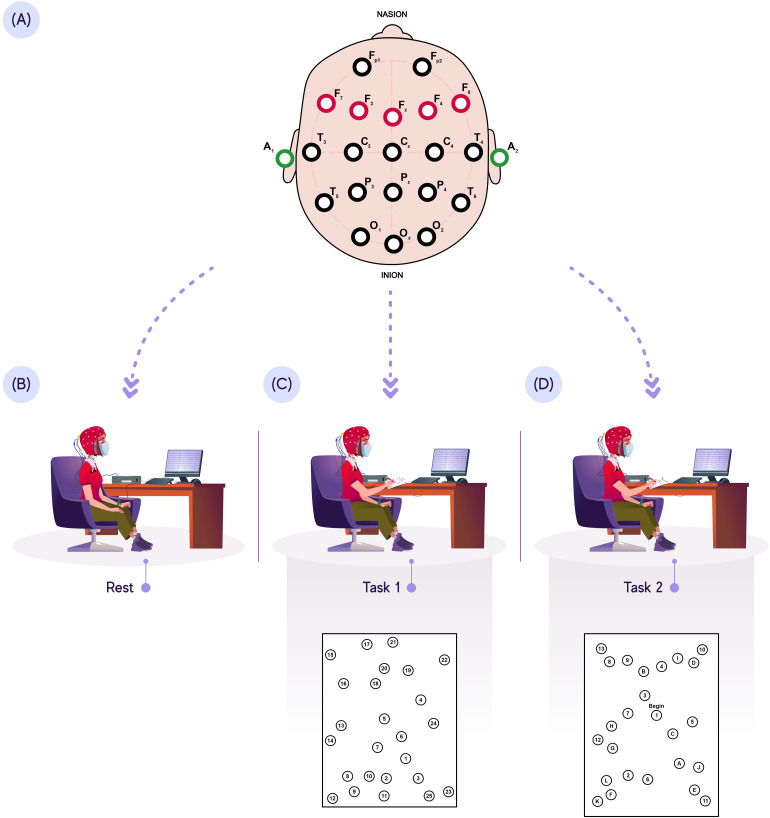
Individuals were then evaluated in a room with few external influences (noise, lamps, or electromagnetic waves). Thus, the signals for each task were collected while the participants were comfortably seated in a chair, and the hydroflex electrodes were placed on the scalp using an EEG cap (Kandel Medical, São Paulo, Brazil) (Figure 1). The impedance was kept below 5 kΩ for all participants. In addition to skin abrasion, hydroflex electrodes were used with the application of conductive gel to reduce the impedance.
Figure 1.
EEG protocol. (A): PFC EEG acquisition; (B) EEG acquisition at rest; (C) EEG acquisition during TMT-A (low cognitive demand); (D) EEG acquisition during TMT-B (high cognitive demand).
The acquisition system in use had four differential channels in a bipolar configuration with dry electrodes positioned over these areas (F3-F7, Fz-F3, Fz-F4, and F4-F8) and grounding electrodes on the earlobe (A1 and A2). The bipolar, ie, differential EEG montage was used to deliberately differencing potentials between spatially adjacent locations as this may lead to improved signal-to-noise ratio of the collected signal. This type of configuration is also known as longitudinal configuration and widely employed in clinical practice.43 For hardware conditioning of the EEG signals, the captured signals were amplified by an RHD200 (Intan Technologies) amplifier and filtered with second-order Butterworth low-pass and high-pass filters with cutoff frequencies of 70 and 1 Hz, respectively.
To reduce potential power line interference, a notch filter with a cutoff frequency of 60 Hz was used. Furthermore, signals were filtered in both the forward and reverse directions using zero-phase digital filtering to remove the direct-current voltage (DC) level. All the signals were captured at 1 kHz and transferred to a laptop computer via a USB cable. EEG signals were recorded in the traditional Intan file format for further analysis.
e) EEG signal processing
Signal processing was performed using R project for statistical computing (version 4.1.0). The main steps were as follows:
e.1) Signal visualization
Signal visualization is an important step in signal processing. This step allows us to observe how the waveforms of the EEG signals differ between channels. It can also display changes in amplitude over time as well as the order of magnitude of the signals.
e.2) Resampling
EEG signals were recorded at a sampling rate of 1 kHz. The collected data were resampled at a sampling frequency of 200 Hz to reduce the computational cost of processing, without compromising the waveform and behavior of the EEG signals.
e.3) EEG signal decomposition
Complete ensemble empirical mode decomposition with adaptive noise (CEEMDAN) was used to decompose pre-processed EEG signals. To obtain information regarding the fundamental components that compose each EEG signal, 11 intrinsic mode functions (IMFs) and a residual component were estimated. IMFs are components that describe oscillation modes embedded in a signal. The residual component is a nonoscillatory mode, defined as the trend mode.
CEEMDAN is a variation of the ensemble empirical mode decomposition (EMD) algorithm, which provides an exact reconstruction of the original signal with improved IMF spectral separation. This is owing to the inclusion of Gaussian noise components in the sifting process of each IMF to address the EMD decomposition scale inconsistency. Complete ensemble EMD with adaptive noise (CEEMDAN) is a variation of Empirical Mode Decomposition (EMD) that improves the process of estimating basic oscillations from EEG signals.44 The benefit of decomposing the signal is that no assumptions about its frequency content are made, as is the case with standard band-pass band filters (eg, a Butterworth filter). Another advantage of decomposition is that it allows the estimation of features from each component, providing a relevant way to capture the underlying information in the signal.
e.4) Signal windowing
Signal windowing, such as changes in amplitude, variability, or frequency, is commonly used to capture changes in the signal over time. Windowing allows the estimation of the characteristics to capture the local behavior of the signal over time. Each IMF was windowed using a rectangular window of 1 s with 50% overlap so that features could be extracted from each window.
e.5) Feature extraction
The activity, mobility, and complexity are estimated for each window of the estimated IMF. These features are the parameters proposed by Hjorth for a quantitative description of EEG. In general, the Hjorth method allows the interpretation of EEG signals in the frequency domain through features estimated in the time domain. This is because the Hjorth parameters are based on spectral moments.27–29
The activity (), as defined in (1), is the squared standard deviation of the amplitude, which is the variance of the signal. In the time domain, it is a measure of variability in the amplitude of the EEG signal. That is, the variance measures the form of data distribution in relation to the mean. On the other hand, activity refers to the mean power of the signal in the frequency domain.
| (1) |
where N is the number of samples of the signal, is the i − tℎ data sample, and corresponds to the mean of the signal.
Mobility (mob) is defined as in (2)
| (2) |
where corresponds to the standard deviation of the first derivative of the signal and is the signal variance (ie, the square root of activity). In the frequency domain, the Hjorth parameter can be interpreted as the mean frequency of the signal.
Complexity (comp) is defined as the relationship between the mobility of the first derivative and that of the signal, as expressed in (3). This feature describes the degree of irregularity of the signal with reference to the pure sine wave, and consequently, represents the changes in the frequency of the signal.
| (3) |
where corresponds to the standard deviation of the second derivative of the signal.
The median of each feature was calculated by considering the individual values of each feature in the signal window. Essentially, the median is a measure of the central tendency that describes typical data behavior. Unlike mean, this statistic is less sensitive to outliers and skewed data. As a result, this procedure was carried out to eliminate the contribution of features extracted from signal windows with potential ocular artifacts (such as eye blinks and movements) inherent in the test execution (TMT-A and TMT-B).
Signal windowing makes the characteristics sensitive to changes in signal behavior over time because these artifacts typically have a higher amplitude than the EEG signal. Consequently, even if the participant blinks, the median can accurately describe the typical behavior of the estimated characteristics for each segment.
Statistical Methods
Data normality was analyzed using the Shapiro-Wilk test. Continuous demographic data are described as medians and ranges, and categorical data are described as percentages. Continuous demographic data were compared using the Mann-Whitney U test, and categorical data were compared using the chi-square test. The Mann-Whitney U test for numerical variables and chi-square or Fisher’s exact test for categorical variables were used to identify potential confounders.
For EEG analysis, we used three multivariate analyses of variance (MANOVAs) with dependent variables: three EEG characteristics and independent variables: channels, tasks, and groups. Group variables (between effects) were considered: 1) control group; 2) COVID-19 at 0-3 months or COVID-19 at 3-6 months or COVID-19 at 6-12 months. In addition, another MANOVA was conducted, with dependent variables: three EEG characteristics, and independent variables: channels, tasks, and evaluation time (within effects). The evaluation time variables were as follows: 1) COVID-19 at 0-3 months; 2) COVID-19 at 3-6 months; and 3) COVID-19 at 6-12 months.
For cognitive analysis, we used three MANOVAs with dependent variables (MoCA, TMT-A, TMT-B, DSTFN, DSTBN, DSTFL, and DSTBL) and independent variables (groups). Group variables (between effects) were considered: 1) control group; 2) COVID-19 at 0-3 months or COVID-19 at 3-6 months or COVID-19 at 6-12 months. In addition, another MANOVA was conducted, with dependent variables: MoCA, TMT-A, TMT-B, DSTFN, DSTBN, DSTFL, and DSTBL; and independent variables: evaluation time (within effects). The evaluation time variables were as follows: 1) COVID-19 at 0-3 months; 2) COVID-19 at 3-6 months; and 3) COVID-19 at 6-12 months. A Bonferroni correction was conducted for each comparison.
In addition, there was performed Spearman’s rank correlation test between EEG parameters (activity, mobility, and complexity) and cognitive tests in both groups. Data were analyzed using the GraphPad Prism software (version 8.0). Statistical significance was set at P < 0.05.
Results
Demographic and Clinical Data
We screened 135 individuals over the course of a year. Fifty-two patients did not meet the criteria for study inclusion due to: severe COVID-19 (n = 20), education level less than nine years (n = 11); use of antidepressants or mood stabilizers (n = 8), history of alcohol abuse (n = 2), pregnant women (n = 2), and non-attendance for reassessments (n = 9). Of the 83 patients included in this analysis, 53 were infected with COVID-19 and 30 were controls. In the COVID-19 group, the mean age was 42.3 years, 69.8% were women, and 79.2% were white. And, In the control group, the mean age was 37.9 years, 73.3% were female, and 80.0% were white. The clinical and demographic profiles of both the groups are summarized in Table 1.
Table 1.
Clinical and Demographic Profile of Both Groups.
| COVID-19 (n = 53) | Control (n = 30) | P-value | |
|---|---|---|---|
| Age, year (median [IQR])1 | 42.3 (25 - 69) | 37.9 (21 - 55) | 0.78 |
| Sex, n (%)2 | |||
| Males | 16 (30.2) | 8 (26.7) | |
| Females | 37 (69.8) | 22 (73.3) | 0.80 |
| Race, n (%)2 | |||
| White | 42 (79.2) | 24 (80.0) | 0.99 |
| Black | 9 (16.9) | 5 (16.7) | |
| Asian | 2 (3.7) | 1 (6.7) | |
| BMI, kg/m2 (median [IQR])1 | 27.8 (19.2 - 46.0) | 26.4 (20.8 - 34.8) | 0.37 |
| Years of studying, (median [IQR])1 | 14.3 (11 - 21) | 14.8 (10 - 22) | 0.78 |
| Any pre-existing comorbidities n (%)2 | |||
| Cardiovascular disease3 | 16 (37.2) | 12 (40.0) | 0.47 |
| Lung disease4 | 12 (22.6) | 7 (23.3) | 0.99 |
| Type 2 Diabetes | 15 (28.3) | 9 (30.0) | 0.99 |
| Hematological disease | 4 (7.5) | 2 (6.7) | 0.99 |
| Hepatic disease | 2 (3.7) | 0 (0) | 0.53 |
| Renal disease | 1 (1.9) | 1 (6.7) | 0.99 |
| Socio-economic status | |||
| Family income ($)(median [IQR])1 | 412.0 (0 - 986.0) | 421.7 (0 - 986.0) | 0.68 |
| Class I, n (%) | 9 (16.9) | 6 (20.0) | 0.77 |
| Class II, n (%) | 12 (22.6) | 8 (26.7) | 0.79 |
| Class IIIa, n (%) | 10 (18.9) | 5 (16.6) | >0.99 |
| Class IIIb, n (%) | 14 (26.4) | 6 (20.0) | >0.99 |
| Class IVabc, n (%) | 8 (15.1) | 5 (16.6) | >0.99 |
| Neurological evaluation | |||
| HADS (median [IQR])1 | 10.0 (1.0 - 18,0) | 9.0 (2.0 - 21.0) | 0.45 |
| NSA (median [IQR])1 | 241.8 (224 - 241) | 242.9 (233 - 244) | 0.89 |
| MRC (median [IQR])1 | 56 (52 - 60) | 60 (58 - 60) | 0.47 |
| PCFS (median [IQR])1 | 1.5 (0 - 3) | 0 |
IQR: interquartile range; Class I: professionals, administrators and managers, higher-grade; Class II: professionals, lower-grade, and higher-degree technicians; Class IIIa: routine non-manual employees, higher-grade; Class IIIb: routine non-manual employees, lower-grade; Class IVabc: small proprietors and employers, and self-employed workers; BMI: body mass index; HADS: Hospital Anxiety and Depression Scale; PCFS: post-COVID-19 functional status scale; NSA: Nottingham sensory assessment; MRC: Medical Research Council scale; 1: Mann-Whitney U test; 2: X2 test. 3 – COVID-19 group: hypertension (n = 12), congestive heart failure (n = 1), and atrial fibrillation (n = 3). Control group: Hypertension (n = 9), congestive heart failure (n = 1), and atrial fibrillation (n = 2). 4 - COVID-19 group: obstructive sleep apnea (n = 6), asthma (n = 4), and chronic obstructive pulmonary disease (n = 2); control group: obstructive sleep apnea (n = 4), asthma (n = 2), and chronic obstructive pulmonary disease (n = 1).
None of the patients had acute COVID-19 symptoms during cognitive and EEG evaluations. However, during the first evaluation, the individuals after COVID-19 presented the following clinical manifestation: anosmia (n = 31), dysgeusia (n = 28), muscle weakness (n = 21), dizziness (n = 16), mental confusion (n = 13), irritability (n = 10), brain fog (n = 9), headache (n = 8), walking problems (n = 8), arthralgia (n = 7), and myalgia (n = 7). The second evaluation (3-6 months) revealed hyposmia (n = 18), dysgeusia (n = 13), muscle weakness (n = 12), brain fog (n = 15), and fatigue (n = 16). And at the last evaluation (n = 6-12 months), hyposmia (n = 12), dysgeusia (n = 8), brain fog (n = 17), and fatigue (n = 16) were observed.
Cognitive Function Over one Year
There was a statistically significant difference in the fixed-effect model for cognitive function (F [DFn, DFd] [F (1767, 22.97) = 1.52]; p = 0.03). In the Bonferroni correction, an increase in execution time was observed during TMT-A in the COVID-19 group at 6-12 months compared to the baseline values in control group (MD: −8.1; 95% CI −16.6 a −0.08; p = 0.04). The median and range scores of the cognitive tests are reported in Table 2.
Table 2.
Cognitive Changes of the Control Group and During the one Year of Follow-up in the COVID-19 Group.
| Control12 | COVID-19 | |||
|---|---|---|---|---|
| Reference values | 0-3 months | 3-6 months | 6-12 months | |
| MoCA | 25.0 (17.0 - 30.0) | 25.0 (20.0 - 30.0) | 26.0 (23.0 - 30.0) | 26.0 (11.0 - 29.0) |
| TMT-A (s) | 26.8 (17.6 - 47.1)a | 30.8 (18.1 - 48.7) | 28.3 (17.8 - 53.7) | 32.7 (20.6 - 120.7)a |
| TMT-B (s) | 64.6 (36.7 - 155.2) | 64.4 (48.1 - 114.1) | 67.7 (45.4 - 188.6) | 76.5 (37.3 - 207.5) |
| TMT (B-A) | 32.6 (-1.7 - 49.9) | 38.1 (19.9 - 72.3) | 36.3 (23.1 - 62.2) | 43.9 (16.0 - 86.8) |
| DSFN | 29.0 (11.0 - 49.0) | 36.0 (10.0 - 75.0) | 28.5 (13.0 - 64.0) | 41.5 (14.0 - 72.0) |
| DSBN | 17.0 (8.0 - 34.0) | 21.0 (2.0 - 41.0) | 17.0 (8.0 - 33.0) | 17.5 (14.0 - 2.0) |
| DSFL | 7.0 (4.0 - 10.0) | 9.0 (3.0 - 13.0) | 7.0 (3.0 -.0) | 9.0 (4.0 - 13.0) |
| DSBL | 5.0 (3.0 - 8.0) | 6.0 (1.0 - 9.0) | 5.0 (3.0 - 9.0) | 5.0 (1.0 - 8.0) |
a = Statistically significant difference.
MoCA = Montreal cognitive assessment; TMT-A = trail making test part A; TMT-B = trail making test part B. TMT (B-A): differences between trail making test parts B and A; DSFN = digit span forward numbers. DSBN = digit span backward number, DSFL = digit span forward line. DSBL = digit-span backward line.
Brain Electrical Activity Over one Year
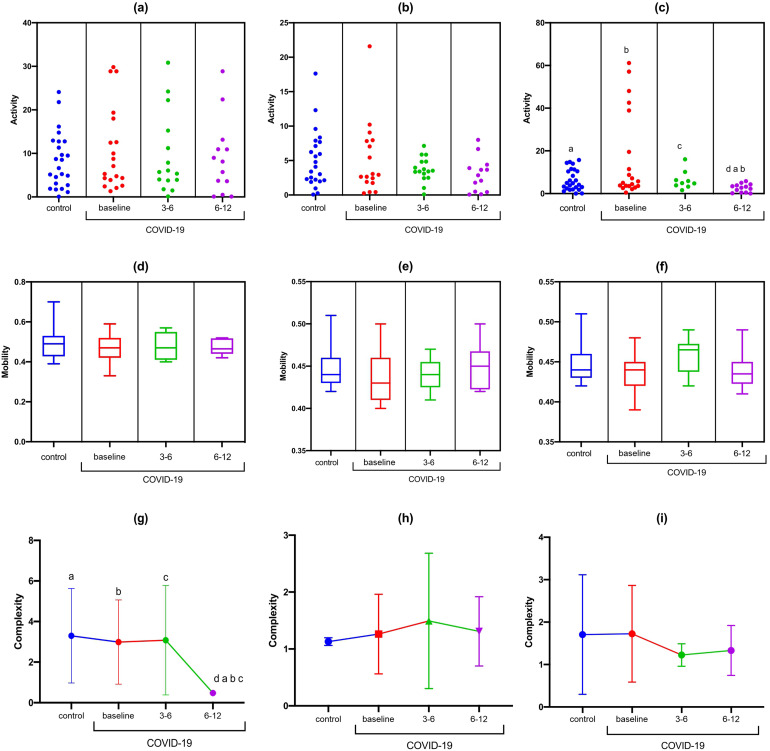
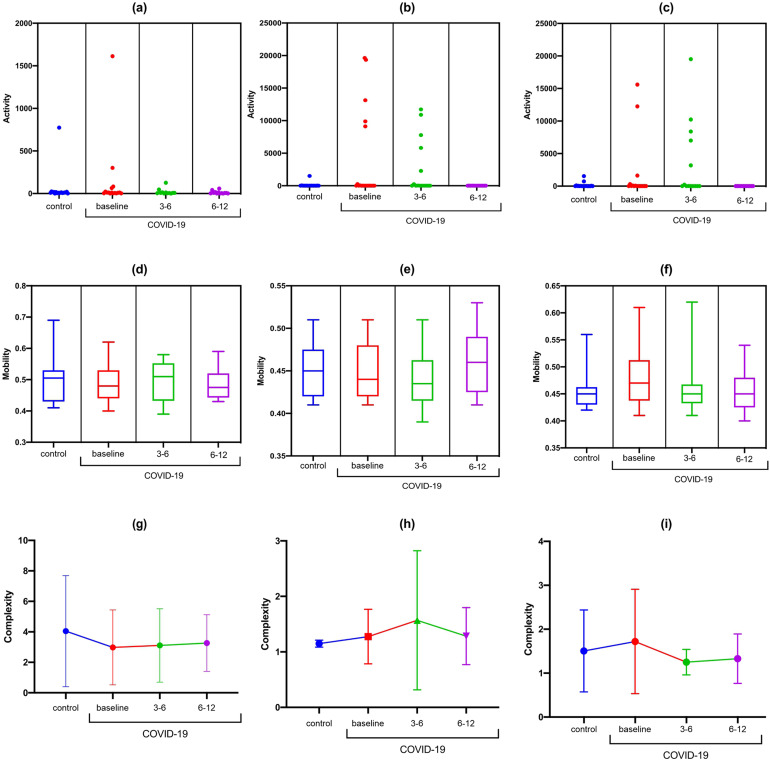
For the F3-F7 channel, a statistically significant difference was observed in the activity data during the high cognitive demand with the TMT-B (F [DFn, DFd] [F [3.0, 23.61] = 5.89); p = 0.003) and complexity at rest (F [DFn, DFd] [F [3.0, 34.03] = 7.01); p = 0.0008). In the Bonferroni correction, there was a reduction in F3-F7 activity observed during TMT-B in the COVID-19 group at 6-12 months compared to the baseline values from control group (MD: 3.8; 95% CI 0.32 to 7.27; p = 0.01) and compared to the COVID-19 group at baseline (MD: 14.04; 95% CI −0.71, 28.8; p = 0.03). There was a reduction in signal complexity at F3-F7 at rest in the COVID-19 group at 6-12 months compared to the baseline values in control group (MD: 2.82; 95% CI 1.35 to 4.29; p < 0.001) and the COVID-19 group at baseline (MD: 2.51; 95%CI 1.09 to 3.98; p < 0.001) (Figure 2).
Figure 2.
Statistical properties used in signal processing in the time domain of the F3-F7 channel in the control and COVID-19 groups. (A) F3-F7 activity at rest; (B) F3-F7 activity during TMT-A; (C) F3-F7 activity during TMT-B; (D) F3-F7 mobility at rest; (E) F3-F7 mobility during TMT-A; (F) F3-F7 mobility during TMT-B; (G) F3-F7 complexity at rest; (H) F3-F7 complexity during TMT-A; (I) F3-F7 complexity during TMT-A.
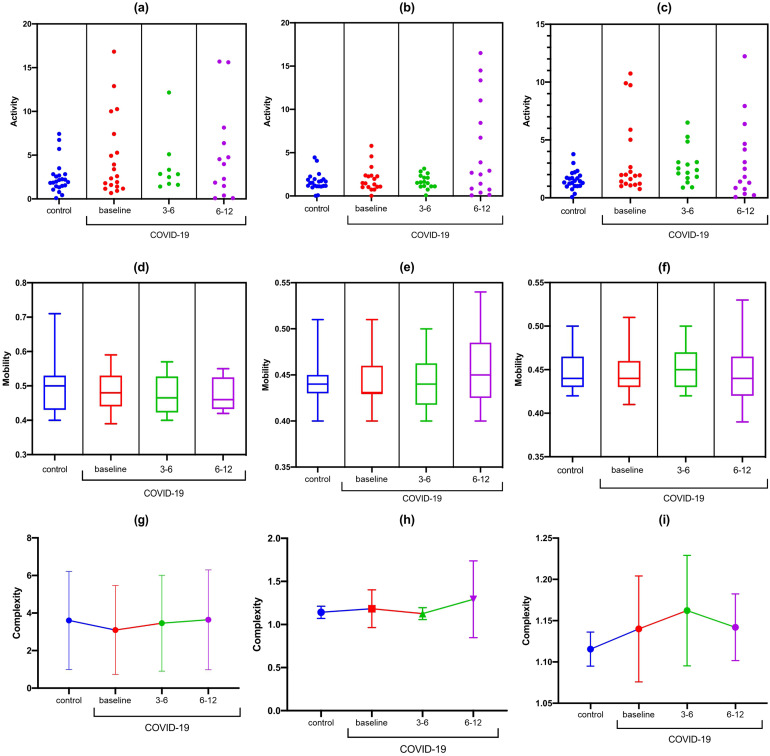
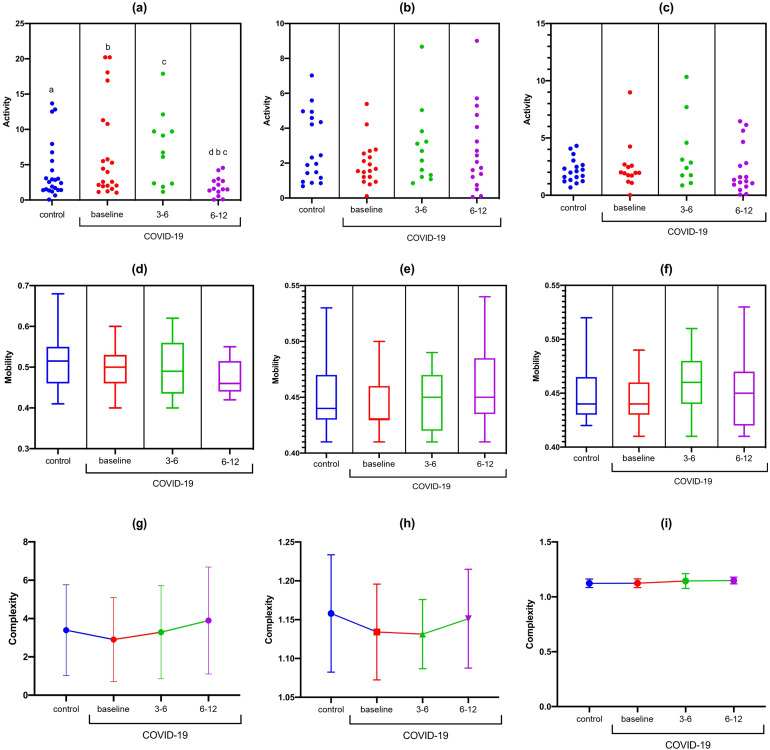
There were no differences in activity, mobility, and complexity at rest or during cognitive tasks (TMT-A and TMT-B) in the Fz-F3 channels (Figure 3). There was a statistically significant difference in the Fz-F4 activity at rest (F [DFn, DFd] [F (3, 65) = 3.737; p <0.0001). In the Bonferroni correction, a reduction in Fz-F4 activity was observed to occur at rest from 6-12 months in the post-COVID-19 group compared with baseline (MD: 4.26; 95% CI 0.38 to 8.21; p = 0.02) and 3-6 months (MD: 5.25; 95% CI 0.08 to 10.43; p = 0.04) (Figure 4). There were no differences in activity, mobility, and complexity at rest and during cognitive tasks (TMT-A and TMT-B) in the F4-F8 channels (Figure 5).
Figure 3.
Statistical properties used in signal processing in the time domain of the Fz-F3 channel in the control and COVID-19 groups. (A) Fz-F3 activity at rest; (B) Fz-F3 activity during TMT-A; (C) Fz-F3 activity during TMT-B; (D) Fz-F3 mobility at rest; (E) Fz-F3 mobility during TMT-A; (F) Fz-F3 mobility during TMT-B; (G) Fz-F3 complexity at rest; (H) Fz-F3 complexity during TMT-A; (I) Fz-F3 complexity TMT-A.
Figure 4.
Statistical properties used in signal processing in the time domain of the Fz-F4 channel in the control and COVID-19 groups. (A) Fz-F4 activity at rest; (B) Fz-F4 activity during TMT-A; (C) Fz-F4 activity during TMT-B; (D) Fz-F4 mobility at rest; (E) Fz-F4 mobility during TMT-A; (f) Fz-F4 mobility during TMT-B; (G) Fz-F4 complexity at rest; (H) Fz-F4 complexity during TMT-A; (I) Fz-F4 complexity during TMT-A.
Figure 5.
Statistical properties used in time domain signal processing of the F4-F8 channel in the control and COVID-19 groups. (A) F4-F8 activity at rest; (b) F4-F8 activity during TMT-A; (c) F4-F8 activity during TMT-B; (d) F4-F8 mobility at rest; (e) F4-F8 mobility during TMT-A; (f) F4-F8 mobility during TMT-B; (g) F4-F8 complexity at rest; (h) F4-F8 complexity during TMT-A; (i) F4-F8 complexity during TMT-A.
A graphical illustration of the EEG spectra for each electrode channel after EEG processing is shown in Supplemental File 2.
Correlation Between EEG Parameters and Cognitive Tests
In the control group, a negative correlation was found between EEG complexity during TMTA with TMTB test (r = −0.41; p = 0.043) in F3-F7. A positive correlation was found between EEG complexity during TMTB with DSF test (r = 0.58; p = 0.004) and EEG complexity during TMTB with DSB test (r = 0.51; p = 0.014) in Fz-F4.
In the COVID-19 group (0-3 months), a negative correlation was found between EEG activity during TMTA with TMTA test (r = −0.40; p = 0.040) in Fz-F3; positive correlation was found between EEG complexity during TMTB with DSB test (r = 0.62; p = 0.034) in Fz-F4; and negative correlation was found between EEG activity during TMTB with TMTB test (r = −0.71; p = 0.016) in F4-F8. In the COVID-19 group (3-6 months), a negative correlation was found between EEG complexity during TMTA with TMTA test (r = −0.67; p = 0.029) in F3-F7; and positive correlation was found between EEG activity during TMTA with MOCA (r = 0.78; p = 0.007) in Fz-F3. In the COVID-19 group (6-12 months), a positive correlation was found between EEG complexity during TMTA with MOCA (r = 0,80; p = 0024) in F3-F7.
Discussion
This study found changes in brain activity and cognitive function after post-COVID-19 over a one-year period. A decline in cognitive function was observed within 6-12 months after COVID-19. During the same period, brain electrical activity was reduced at rest in the Fz-F4 areas and during high cognitive demands in the F3-F7 areas. A reduction in signal complexity in F3-F7 at rest was found in the COVID-19 group at 6-12 months after acute infection.
Our study observed a reduction in brain electrical activity in Fz-F4 at rest and in F3-F7 during tasks with high cognitive demand for 6-12 months after COVID-19 infection. Abnormalities in EEG after COVID-19 have been extensively investigated. Many studies have reported that SARS-CoV-2 spike proteins can be detected in the brain.45,46 Some studies have observed changes in EEG function, such as the distribution of EEG bands, structure of spectral entropy, and hemispheric connectivity.25,26 Vellieux et al45 showed that frontal EEG abnormalities could emphasize the hypothesis that SARS-CoV-2 enters the brain through olfactory structures and then spreads to the central nervous system via the frontal lobes.
Another changed EEG parameter was the reduction of complexity in F3-F7 at rest 6-12 months after COVID-19 acute infection. EEG abnormalities, such as reduced EEG complexity, are associated with cognitive deterioration.47,48 Many authors have found an association between EEG complexity and the presence of neuropsychological impairments in the long term.49–51 Jeong highlights that the reduction in complexity can be explained by the deficiency of neurotransmitters, such as acetylcholine, and/or loss of connectivity of local neuronal networks.49 We did not observe changes in the mean frequency (mobility) over the one-year period, and there were no significant differences between the COVID-19 and control groups. Other studies have shown that mobility could be altered in some neurological conditions, such as dementia, and in the cerebral activity of subjects with "forgetfulness”.51–53 However, mild-to-moderate COVID-19 did not cause changes in the neuronal networks to the point of altering EEG mobility.
Several studies have demonstrated the utility of these features in the analysis of EEG signals. For instance, they have been used in brain-computer interface (BCI) control and EEG-controlled robotic navigation,54 as well as in the assessment of mild cognitive impairment,44,55 attention deficit hyperactivity disorder,56 neurodegenerative disorders such as Alzheimer's disease,26 and extraction of information from different biomedical signals.57–59
We also observed a decline in cognitive function 6-12 months after acute COVID-19 infection, as demonstrated by TMT-A. The COVID-19 patients also showed an increase in TMT time (parts B and B-A), with high variability of execution times in the tests over the one-year period after infection; however, this difference was not statistically significant. Similarly, Douaud et al found a significantly greater increase in the time taken to complete Trails A (numeric) and B (alphanumeric) of the Trail Making Test in the mild SARS-CoV-2 group.19 The TMT assesses the aspects of sustained attention, alternating attention, mental flexibility, visual processing speed, and motor function. It also assesses the ability to search through visual scanning and inhibition.60 It is considered a useful tool in research and clinical practice because of its sensitivity to frontal lobe damage and dementia.61,62
Other studies have also observed long-term cognitive and neuropsychiatric alterations after COVID-19.63,64 This study provides evidence for substantial neurological and psychiatric impairments six months after COVID-19 infection, and these changes were not limited to patients with severe COVID-19.65 Al-Aly et al showed that beyond the first 30 days of illness, people with COVID-19 exhibit sequelae in the respiratory system as well as several other sequelae, including nervous system and neurocognitive disorders.56 In addition, Graham et al demonstrated disturbances in processing speed, attention, executive function, and working memory 6-12 months after COVID-19 infection. In addition, our findings highlight a progressive alteration in both cognitive and EEG functions over time, which may suggest brain function deterioration.
Some authors have reported both direct and indirect mechanisms effects involved in long-term cognitive changes in patients after COVID-19.66,67 Could there be long-term viral reactivation or immune system hyperactivity?66 Would there also be associated extrinsic aspects, such as environmental changes, social isolation, personal and economic factors, and lifestyle changes that could later modify neurological and neuropsychiatric function?66–68 A better understanding of the direct and indirect effects is required in addition to the biological mechanisms and epidemiological determinants of the long-term consequences of COVID-19 to improve the rehabilitation process.66
In our study we found important correlations between the nonlinear EEG features and cognitive tests in both groups. In the control group, we observed that the reduction in EEG activity during the cognitive task is associated to the increase in the time of TMT-A and TMT-B. In addition, reduction in EEG complexity during cognitive task was associated with worsened in digit span test. In the COVID-19 group we also find that the reduction in EEG activity and complexity during the cognitive task was associated with the worsening of cognitive tests in all COVID-19 phases. The associations between EEG characteristics and cognitive tasks demonstrate the importance of this method of analysis for understanding executive function and monitoring patients with possible long-term cognitive deterioration.
Study Limitations
This study had several limitations. First, and most importantly, the small sample size prevented us from analyzing the relationship between the demographic and clinical characteristics of COVID-19 patients and EEG variables. Second, brain imaging was not performed at baseline to exclude brain lesions. Third, we have a limited number of EEG channels, and we believe that, in addition to the analyzed areas, neural networks and other brain circuits may also be affected and deserve attention in future research. Fourth, the control group was only investigated once without any re-testing, which limits the interpretation of the long-term results. Fourth, we evaluated the brain function of participants while performing only two tasks. Many cognitive tasks exist, including processing systems, perceptual domains, and difficulties. Therefore, it would be highly speculative to extrapolate the results from a trail-making task to other cognitive tasks. Future research should address how changes in brain complexity are modulated by other cognitive factors. Furthermore, an associated neuroimaging method could affirm if there is impairment of brain areas in patients with mild to moderate COVID-19 in the long term, as EEG can show associated neuronal networks and does not always specifically show the region of the corresponding electrode. We also suggest another control group with episodic infection-provoking pneumonia not due to SARS-CoV-2 virus or non-psychiatric chronic infection to compare EEG findings and cognitive functions in the long term.
Clinical Implications
Based on the observed EEG changes, can SARS-CoV-2 cause neurological damage to the point of decreasing brain activity and cognitive performance in the first year after COVID-19? Can EEG be a resource to diagnose early alterations or post-COVID syndrome, considering the signal pattern? Is EEG a potential predictor of the progression of cognitive loss in long COVID-19?
Our findings have important clinical implications for the acute phase of COVID-19. EEG is a simple, low-cost method that can be used as a diagnostic method for post-COVID-19 syndrome. Techniques such as neurofeedback or noninvasive brain stimulation can be used to reorganize EEG changes and improve cognitive function. In addition, these results will help plan and develop multidisciplinary care strategies to improve brain activity and cognitive performance among individuals with COVID-19.
Conclusion
In conclusion, there was a reduction in brain activity at rest in the Fz-F4 areas and during high cognitive demands in the F3-F7 areas. A reduction in signal complexity in F3-F7 at rest was found in the COVID-19 group at 6-12 months after acute infection. During the same period, the cognitive function worsened. Correlations between the nonlinear EEG features and cognitive tests were also observed in both groups.
Supplemental Material
Supplemental material, sj-tiff-1-eeg-10.1177_15500594221103834 for Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A one-Year Prospective Study After Acute Infection by Pablo Andrei Appelt, Angélica Taciana Sisconetto, Kelly Savana Minaré Baldo Sucupira, Eduardo de Moura Neto, Tatiane de Jesus Chagas, Rodrigo Bazan, Ariana Moura Cabral, Adriano de Oliveira Andrade, Luciane Aparecida Pascucci Sande de Souza and Gustavo José Luvizutto in Clinical EEG and Neuroscience
Supplemental material, sj-docx-2-eeg-10.1177_15500594221103834 for Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A one-Year Prospective Study After Acute Infection by Pablo Andrei Appelt, Angélica Taciana Sisconetto, Kelly Savana Minaré Baldo Sucupira, Eduardo de Moura Neto, Tatiane de Jesus Chagas, Rodrigo Bazan, Ariana Moura Cabral, Adriano de Oliveira Andrade, Luciane Aparecida Pascucci Sande de Souza and Gustavo José Luvizutto in Clinical EEG and Neuroscience
Acknowledgments
This study was conducted with the support of the National Council for Scientific and Technological Development (CNPq), Coordination for the Improvement of Higher Education Personnel, the Ministry of Science, Technology, Innovations, and Communications (MCTIC), and the Foundation for Research Support of the State of Minas Gerais. In addition, we would like to thank Matias Djalma Appelt and Tobias Rafael Appelt for helping with data processing and all participants and researchers who helped in the development of this study.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Desenvolvimento Científico e Tecnológico, (grant number 88881.370894/2019-01, 88887.159028/2017-00, 304818/2018-6, 401192/2020-2, 58524/2020-9).
Ethical Approval: Not applicable, because this article does not contain any studies with human or animal subjects.
ORCID iDs: Adriano de Oliveira Andrade https://orcid.org/0000-0002-5689-6606
Gustavo José Luvizutto https://orcid.org/0000-0002-6914-7225
Supplemental material: Supplemental material for this article is available online.
References
- 1.Li Q, Guan X, Wu Pet al. Early transmission dynamicsinWuhan,China,ofnovelcoronavirus- infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li Xet al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao L, Jin H, Wang Met al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. https://.doi:10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crook H, Raza S, Nowell J, Young M, Edison P. Long COVID - mechanisms, risk factors, and management. Br Med J. 2021;37(4):n1648. 10.1136/bmj.n1648 [DOI] [PubMed] [Google Scholar]
- 5.Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz Eet al. Neurologic manifestations in hospitalized patients with COVID-19: the ALBACOVID registry. Neurology. 2020;95(8):e1060–e1070. doi: 10.1212/WNL.0000000000009937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ermis U, Rust MI, Bungenberg Jet al. Neurological symptoms in COVID-19: a cross-sectional monocentric study of hospitalized patients. Neurol Res Pract. 2021;3(1):17. 10.1186/s42466-021-00116-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107 (6):1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakhmola S, Indari O, Chatterjee S, Jha HC. SARS-CoV-2, an underestimated pathogen of the nervous system. SN Compr Clin Med. 2020;2:2137–2146. doi: 10.1007/s42399-020-00522-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiappelli F. Towards neuro-CoViD-19. Bioinformation. 2020;16(4):288–292. doi: 10.6026/97320630016288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267:2179–2184. doi: 10.1007/s00415-020-09929-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- 12.Verdecchia P, Cavallini C, Spanevello A, Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edison P. Brain connectivity and COVID-19. Brain Connect. 2021;11(4):251–252. doi: 10.1089/brain.2021.29022.ped [DOI] [PubMed] [Google Scholar]
- 14.Edison P. Brain connectivity and neurological sequalae in COVID-19. Brain Connect. 2021;11(5):331–332. doi: 10.1089/brain.2021.29023.ped [DOI] [PubMed] [Google Scholar]
- 15.Fu Z, Tu Y, Calhoun VDet al. et al. Dynamic functional network connectivity associated with post-traumatic stress symptoms in COVID-19 survivors. Neurobiol Stress. 2021 Nov;15:100377. doi: 10.1016/j.ynstr.2021.100377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Douaud G, Lee S, Alfaro-Almagro Fet al. Brain imaging before and after COVID-19. UK Biobank MedRxiv. 2021;604:1–55. doi: 10.1101/2021.06.11.21258690 [DOI] [Google Scholar]
- 17.Hampshire A, Trender W, Chamberlain SRet al. Cognitive deficits in people who have recovered from COVID-19. EClinicalmedicine. 2021 Jul 23;39:101044. doi: 10.1016/j.eclinm.2021.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Y, Li X, Geng Det al. Cerebral micro-structural changes in COVID-19 patients - an MRI-based 3 month follow-up study. EClinicalmedicine. 2020;25:100484. 10.1016/j.eclinm.2020.100484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douaud G, Lee S, Alfaro-Almagro Fet al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697–707. doi: 10.1038/s41586-022-04569-5. Epub ahead of print. PMID: 35255491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burle B, Spieser L, Roger C, Casini L, Hasbroucq T, Vidal F. Spatial and temporal resolutions of EEG: is it really black and white? A scalp current density view. Int J Psychophysiol. 2015;97(3):210–220. doi: 10.1016/j.ijpsycho.2015.05.004] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galanopoulou AS, Ferastraoaru V, Correa DJet al. EEG Findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5(2):314–324. doi: 10.1002/epi4.12399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman N, Fekete T, Gal K, Shriki O. EEG-based prediction of cognitive load in intelligence tests. Front Hum Neurosci. 2019;13:191. doi: 10.3389/fnhum.2019.00191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehmood RM, Lee HJ. Towards emotion recognition of EEG brain signals using Hjorth parameters and SVM. Adv Sci Technol Lett Biosci Med Res. 2015;91:24–27. [Google Scholar]
- 24.Cecchin T, Ranta R, Koessler L, Caspary O, Vespignani H, Maillard L. Seizure lateralization in scalp EEG using Hjorth parameters. Clin Neurophysiol. 2010;121(3):290–300. [DOI] [PubMed] [Google Scholar]
- 25.Pezard L, Nandrino J, Renault B, et al. Depression as a dynamical disease. Biol Psychiatry. 1996;39(12):991–999. [DOI] [PubMed] [Google Scholar]
- 26.Safi MS, Safi SMM. Early detection of Alzheimer’s disease from EEG signals using Hjorth parameters. Biomed Signal Process Control. 2021;65(11):102338. 10.1016/j.bspc.2020.102338 [DOI] [Google Scholar]
- 27.Hjorth B. EEG Analysis based on time domain properties. Electroencephalogr Clin Neurophysiol. 1970;29(3):306–310. doi: 10.1016/0013-4694(70)90143-4 [DOI] [PubMed] [Google Scholar]
- 28.Hjorth B. Physical aspects of EEG data as a basis for topographic mapping. In: Duffy f H topographic mapping of brain electrical activity. Butterworths; 1986:175–193. [Google Scholar]
- 29.Hjorth B. Time domain descriptors quantify EEG changes related to hypothyroidism. Electroencephalogr Clin Neurophysiol. 1975;38(2):208–216. [Google Scholar]
- 30.Li Y, Xiao S, Li Y, Li Y, Yang B. 2020. Classification of Mild Cognitive Impairment from multi-domain features of resting-state EEG. Engineering in Medicine & Biology Society (EMBC) 42nd Annual International Conference of the IEEE, pp. 256-259. doi:10.1109/EMBC44109.2020.9176053.
- 31.Graham EL, Clark JR, Orban ZSet al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann Clin Transl Neurol. 2021;8(5):1073–1085. https://.doi:10.1002/acn3.51350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agosta F, Vabanesi M, Canu Eet al. Prospective EEG evaluation in patients with recent COVID-19 and cognitive disturbances. Eur J Neurol. 2021;28(suppl 1):250. [Google Scholar]
- 33.Zhai W, Luo Z, Zheng Yet al. Moderate vs. mild cases of overseas-imported COVID-19 in Beijing: a retrospective cohort study. Sci Rep. 2021;11:6483. doi: 10.1038/s41598-021-85869-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell WW. De Jonǵs The Neurologic Examination. Lippincott, Williams & Wilkins. 6th ed. 2005. [Google Scholar]
- 35.Botega NJ, Pereira WAB, Bio MR, Garcia Júnior C, Zomignani MA. Psychiatric morbidity among medical in-patients: a standardized assessment (GHQ-12 and CIS-R) using ‘lay’ interviewers in a Brazilian hospital. Soc Psychiatry Psychiatr Epidemiol. 1995;30(3):127–131. doi: 10.1007/BF00802041 [DOI] [PubMed] [Google Scholar]
- 36.Lima DH, Queiroz AP, De Salvo G, Yoneyama SM, Oberg TD, Lima NM. Brazilian version of the Nottingham sensory assessment: validity, agreement and reliability. Rev Bras Fisioter. 2010 Mar-Apr;14(2):166–174. doi: 10.1590/S1413-35552010005000006 [DOI] [PubMed] [Google Scholar]
- 37.Turan Z, Topaloglu M, Ozyemisci Taskiran O. Medical research council-sumscore: a tool for evaluating muscle weakness in patients with post-intensive care syndrome. Crit Care. 2020;24(1):562. DOI: 10.1186/s13054-020-03282-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Machado FVC, Meys R, Delbressine JMet al. Construct validity of the post-COVID-19 functional Status scale in adult subjects with COVID-19. Health Qual Life Outcomes. 2021;19(1):40. 10.1186/s12955-021-01691-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nasreddine ZS, Phillips NA, Bédirian Vet al. The Montreal cognitive assessmente, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 40.Gaudino EA, Geisler MW, Squires NK. Construct validity in the trail making test: what makes part B harder? J Clin Exp Neuropsychol. 1995;17(4):529–535. doi: 10.1080/01688639508405143 [DOI] [PubMed] [Google Scholar]
- 41.Reynolds CR. Forward and backward memory span should not be combined for clinical analysis. Arch Clin Neuropsychol. 1997;12(1):29–40. [PubMed] [Google Scholar]
- 42.Jasper HH. Localized analyses of human brain function by the electroencephalogram. J Nerv Ment Disord. 1936;84:679–683. [Google Scholar]
- 43.Kutluay E, Kalamangalam GP. Montages for noninvasive EEG recording. J Clin Neurophysiol. 2019;36(5):330–336. doi: 10.1097/WNP.0000000000000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney-Reed CM, Nasuto SJ, Vieira MF, Andrade AO. Empirical mode decomposition and its extensions applied to EEG analysis: a review. Advances in Data Science and Adaptive Analysis. 2018;1(2):1840001. 10.1142/S2424922X18400016 [DOI] [Google Scholar]
- 45.Vellieux G, Sonneville R, Vledouts S, Jaquet P, Rouvel-Tallec A, d’Ortho MP. COVID-19-Associated neurological manifestations: an emerging electroencephalographic literature. Feb. Front Physiol. 2020;11:622466. doi: 10.3389/fphys.2020.622466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fullard JF, Lee HC, Voloudakis Get al. Single-nucleus transcriptome analysis of human brain immune response in patients with severe COVID-19. Genome Med. 2021 Jul 19;13(1):118. doi: 10.1186/s13073-021-00933-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pati S, Toth E, Chaitanya G. Quantitative EEG markers to prognosticate critically ill patients with COVID-19: a retrospective cohort study. Clin Neurophysiol. 2020;131(8):1824–1826. doi: 10.1016/j.clinph.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiter-Gasser U, Gasser T, Ziegler P. Quantitative EEG analysis in early onset Alzheimer’s disease: correlations with severity, clinical characteristics, visual EEG and CCT. Electroencephalogr Clin Neurophysiol. 1994;90(4):267–272. doi: 10.1016/0013-4694(94)90144-9 [DOI] [PubMed] [Google Scholar]
- 49.Jeong J. EEG Dynamics in patients with Alzheimer disease. Clin Neurophysiol. 2004;115(7):1490–1505. doi: 10.1016/j.clinph.2004.01.001 [DOI] [PubMed] [Google Scholar]
- 50.Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG Synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2003;108(2):90–96. doi: 10.1034/j.1600-0404.2003.02067.x [DOI] [PubMed] [Google Scholar]
- 51.Al-Nuaimi AHH, Jammeh E, Sun L, Ifeachor E. Complexity measures for quantifying changes in electroencephalogram in Alzheimer’s disease. Complexity. 2018;2018:1–12. doi: 10.1155/2018/8915079 [DOI] [Google Scholar]
- 52.Ibáñez-Molina AJ, Lozano V, Soriano MF, Aznarte JI, Gómez-Ariza CJ, Bajo MT. EEG Multiscale complexity in schizophrenia during picture naming. Front Physiol. 2018;9:1213. doi: 10.3389/fphys.2018.01213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin-Loeches M, Garcia-Trapero J, Gil P, Rubia FJ. Topography of mobility and complexity parameters of the EEG in Alzheimer’s disease. Biol Psychiatry. 1991 Dec 1;30(11):1111–1121. doi: 10.1016/0006-3223(91)90181-k. PMID: 1777528. [DOI] [PubMed] [Google Scholar]
- 54.Francis MJN, Keran MP, Chetan R, Krupa BN. EEG-Controlled Robot navigation using Hjorth parameters and Welch-PSD. International Journal of Intelligent Engineering and Systems. 2021;14(4):231–240. doi: 10.22266/ijies2021.0831.21 [DOI] [Google Scholar]
- 55.Youssef N, Xiao S, Liu Met al. et al. Functional brain networks in mild cognitive impairment based on resting electroencephalography signals. Front Comput Neurosci. 2021;15:698386. doi: 10.3389/fncom.2021.698386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chow JC, Ouyang CS, Chiang CTet al. et al. Novel method using Hjorth mobility analysis for diagnosing attention-deficit hyperactivity disorder in girls. Brain Dev. 2019 Apr;41(4):334–340. doi: 10.1016/j.braindev.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 57.Mouzé-Amady M, Horwat F. Evaluation of Hjorth parameters in forearm surface EMG analysis during an occupational repetitive task. Electroencephalogr Clin Neurophysiol. 1996 Apr;101(2):181–183. doi: 10.1016/0924-980x(96)00316-5 [DOI] [PubMed] [Google Scholar]
- 58.Rizal A, Hadiyoso S. ECG Signal classification using Hjorth descriptor. 2015 International Conference on Automation, Cognitive Science, Optics, Micro Electro-Mechanical System, and Information Technology (ICACOMIT). 2015(2):87–90. doi: 10.1109/ICACOMIT.2015.7440181 [DOI] [Google Scholar]
- 59.Liu Y, Lan Z, Khoo HHG, Li KHH, Sourina O, Mueller-Wittig W. EEG-based Evaluation of mental fatigue using machine learning algorithms. 2018 International Conference on Cyberworlds (CW). 2018:276–279. doi: 10.1109/CW.2018.00056 [DOI] [Google Scholar]
- 60.Alves FO, Zaninotto ALC, Mioto EC, de Lucia MCS, Scaff M. Evaluation of alternating and sustained attention in a sample of healthy adults with high level of schooling. Psicol Hosp. (São Paulo). 2010;8(2):89–105. [Google Scholar]
- 61.Amieva H, Lafont S, Auriacombe Set al. et al. Analysis of error types in the trail making test evidences an inhibitory deficit in dementia of the Alzheimer type. J Clin Exp Neuropsychol. 1998;20(2):280–285. doi: 10.1076/jcen.20.2.280.1161 [DOI] [PubMed] [Google Scholar]
- 62.MacPherson SE, Della Sala S, Cox SR, Girardi A, Iveson MH. Handbook of frontal lobe assessment. OUP; 2015. [Google Scholar]
- 63.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month Neurological and psychiatric outcomes in 236379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–427. doi: 10.1093/med:psych/9780199669523.001.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594(5):259–264. doi: 10.1038/s41586-021-03553-9 [DOI] [PubMed] [Google Scholar]
- 65.Figueroa JD, Brennan PM, Theodoratou Eet al. Distinguishing between direct and indirect consequences of COVID-19. Br Med J. 2020;369(7862):m2377. doi: 10.1136/bmj.m2377 [DOI] [PubMed] [Google Scholar]
- 66.Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA. 2020;324:1723–1724. doi: 10.1001/jama.2020.19719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Knipe D, Evans H, Marchant A, Gunnell D, John A. Mapping population mental health concerns related to COVID-19 and the consequences of physical distancing: a Google trends analysis. Wellcome Open Res. 2020;5(17):82. doi: 10.12688/wellcomeopenres.15870.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahase E. COVID-19: mental health consequences of pandemic need urgent research, paper advises. Br Med J. 2020;369:m1515. doi: 10.1136/bmj.m1515 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tiff-1-eeg-10.1177_15500594221103834 for Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A one-Year Prospective Study After Acute Infection by Pablo Andrei Appelt, Angélica Taciana Sisconetto, Kelly Savana Minaré Baldo Sucupira, Eduardo de Moura Neto, Tatiane de Jesus Chagas, Rodrigo Bazan, Ariana Moura Cabral, Adriano de Oliveira Andrade, Luciane Aparecida Pascucci Sande de Souza and Gustavo José Luvizutto in Clinical EEG and Neuroscience
Supplemental material, sj-docx-2-eeg-10.1177_15500594221103834 for Changes in Electrical Brain Activity and Cognitive Functions Following Mild to Moderate COVID-19: A one-Year Prospective Study After Acute Infection by Pablo Andrei Appelt, Angélica Taciana Sisconetto, Kelly Savana Minaré Baldo Sucupira, Eduardo de Moura Neto, Tatiane de Jesus Chagas, Rodrigo Bazan, Ariana Moura Cabral, Adriano de Oliveira Andrade, Luciane Aparecida Pascucci Sande de Souza and Gustavo José Luvizutto in Clinical EEG and Neuroscience