Abstract
Fragile X syndrome is a genetic condition associated with alterations in brain and subsequent cognitive development. However, due to a milder phenotype relative to males, females with fragile X syndrome are underrepresented in research studies. In the current study, we investigate neuroanatomical differences in young females (age range: 6.03–16.32 years) with fragile X syndrome (N = 46) as compared to age-, sex-, and verbal abilities-matched participants (comparison group; N = 35). Between-group analyses of whole-brain and regional brain volumes were assessed using voxel-based morphometry. Results demonstrate significantly larger total gray and white matter volumes in girls with fragile X syndrome compared to a matched comparison group (Ps < 0.001). In addition, the fragile X group showed significantly larger gray matter volume in a bilateral parieto-occipital cluster and a right parieto-occipital cluster (Ps < 0.001). Conversely, the fragile X group showed significantly smaller gray matter volume in the bilateral gyrus rectus (P < 0.03). Associations between these regional brain volumes and key socio-emotional variables provide insight into gene–brain–behavior relationships underlying the fragile X syndrome phenotype in females. These findings represent the first characterization of a neuroanatomical phenotype in a large sample of girls with fragile X syndrome and expand our knowledge about potential neurodevelopmental mechanisms underlying cognitive–behavioral outcomes in this condition.
Keywords: brain MRI, brain volume, fragile X syndrome, VBM, voxel-based morphometry
Introduction
Fragile X syndrome (FXS) is often cited as the most common heritable cause of intellectual disability and autism symptoms and affects as many as 1 in 2500 males and females (Crawford et al. 2001; Hagerman 2008). FXS results from an expanded CGG repeat region on the X chromosome, resulting in reduced levels of fragile X mental retardation protein (FMRP) (Garber et al. 2008). FMRP is an RNA-binding protein that plays a role in the regulation of local protein synthesis that is critical for neurodevelopment and brain function. Reduced FMRP production is associated with aberrant dendritic spine formation and impairments in synaptic plasticity (Zalfa et al. 2006). Insufficient expression of FMRP underlies the cognitive, behavioral, and social alterations that are commonly associated with FXS (Bagni and Oostra 2013; Budimirovic et al. 2020). Due to the condition being linked to the X chromosome, females with FXS typically have a milder phenotype compared to their male counterparts. Through the process of X chromosome inactivation, FMRP expression in females with FXS is typically about 50% of what is observed in typically developing females or males (Tassone et al. 1999). However, variation of FMRP expression in females with FXS appears to be affected by random selection of the X chromosome that is inactivated early in embryonic development (i.e., with or without the FMR1 mutation), leading to variation in FMRP levels and symptom severity in females with this condition (Tan et al. 1995; Loesch et al. 2004; Di and Disteche 2006; Budimirovic et al. 2020).
Boys with FXS, who are hemizygous for the FMR1 full mutation, typically present with more severe symptoms including significant global developmental delay, intellectual disability, and symptoms of autism spectrum disorder (Bailey et al. 2008). In contrast, females are heterozygous for the full mutation and are often less severely affected due to relatively higher FMRP levels (Loesch et al. 2004; Budimirovic et al. 2020). As a result, females with FXS may present with a phenotype ranging from as severely affected as their male counterparts to outwardly unaffected (Bartholomay et al. 2019). Cognitively, girls with FXS typically fall into the low or low-average range, with relative strengths in their verbal abilities and relative weaknesses in nonverbal and spatial skills (Hessl et al. 2009).
Girls with FXS can also present with symptoms of autism spectrum disorder (ASD), including social deficits and repetitive behaviors. Indeed, approximately 50% of boys and 20% of girls with FXS meet criteria for ASD (Hatton et al. 2006; Hall et al. 2008, Budimirovic et al. 2020). However, inconsistencies in ASD prevalence in clinical diagnoses and research findings suggest that ASD may be underdiagnosed in children with FXS in educational and clinical settings (Klusek et al. 2014). This underdiagnosis of ASD through typical clinical and educational channels is likely further amplified for females with FXS due to the well-publicized sex disparity in ASD diagnoses. Specifically, males are far more likely than females to be diagnosed with and treated for (idiopathic) ASD, though a growing body of evidence suggests that this may be due to historical emphasis on the male presentation of ASD, rather than to sex differences in prevalence of the disorder (Gould 2017). Females with FXS, therefore, may be at risk of overlooked ASD behaviors, which have also been demonstrated to be associated with symptoms of anxiety (Mazzocco et al. 1997).
In addition to ASD, girls with FXS are at greater risk for depression and anxiety and demonstrate shyness and/or social avoidance (Hagerman et al. 1992; Freund et al. 1993; Mazzocco et al. 1997). A national survey of parents found anxiety and depression to be two of the most commonly reported concerns for females with FXS (Bailey et al. 2008), and previous findings have shown that up to 76.9% of females meet criteria for an anxiety disorder, with social phobia and specific phobia being the most frequently diagnosed (Cordeiro et al. 2011). Impairment in social cognition and social functioning associated with FXS may be the driving force behind these outcomes (Turkstra et al. 2014). FMRP dosage has been shown to be related to internalizing problems in females, including social withdrawal and symptoms of anxiety and depression (Hessl et al. 2001). Because of their relatively less affected phenotype, girls are often misdiagnosed and/or undiagnosed with FXS until a close male relative is identified with the condition.
Neuroanatomical variations in FXS have been well documented. Previous studies of adults with FXS demonstrated larger brain volumes in the caudate nucleus and parietal lobes but smaller volumes of the left frontal lobe (Reiss et al. 1995; Guerreiro et al. 1998; Hallahan et al. 2011). Moreover, volume differences in the caudate nucleus and frontostriatal circuitry have been shown to be associated with FXS status (Wilson et al. 2009). Previous studies completed by our group have identified distinct neuroanatomical differences already present in boys with FXS as young as 18 months old (Hoeft et al. 2011; Hazlett et al. 2012). Further, a study of children and adolescents found that the neuroanatomical profile of FXS differs between young girls and boys (Eliez et al. 2001). Another study investigating the neuroanatomical profile of FXS during adolescence found that the caudate nucleus was enlarged compared to controls, while several brain regions including the bilateral medial frontal, gyrus rectus, and superior temporal gyrus were reduced in size (Sandoval et al. 2018). However, these studies have sampled from either all male or mixed-sex participants, leaving it unclear whether brain development in girls with FXS follows the established patterns of either their typically developing peers or of males with FXS. Further research is therefore necessary to assess the association of aberrant brain development in childhood and adolescence with symptoms commonly observed in girls with FXS.
Substantial evidence suggests distinct patterns of brain changes in typically developing children during childhood and adolescence; white matter volume (WMV) increases linearly while gray matter volume (GMV) peaks around 14 years of age for males and 11 years of age for females, with males demonstrating 9% larger total cerebral volume throughout development (Giedd et al. 1996; Lenroot and Giedd 2006). However, it is unclear whether children with FXS follow a similar trajectory of brain development. Dynamic neurodevelopment during childhood is associated with changes in behavioral, cognitive, and emotional development. Adolescence appears to be a period of particularly significant change in the fragile X phenotype. In both girls and boys with FXS, standardized IQ scores decline during adolescence (Quintin et al. 2016), which suggests a slowing in acquiring new cognitive abilities relative to their peers of the same age. Further, delays in the development of executive functioning in girls with FXS appear to become particularly pronounced during this period (Lightbody et al. 2006).
In the current study, we examined neuroanatomical, cognitive, and behavioral profiles in school-age and adolescent girls with FXS. Specifically, using magnetic resonance imaging (MRI) in conjunction with a comprehensive cognitive–behavioral battery, we compared girls with FXS to developmentally matched control participants to provide new insight into the neuroanatomical underpinnings of behavioral and socio-emotional outcomes in girls with this condition. In this first-ever large-scale study focused on girls and early adolescents with FXS, we hypothesized that the group with FXS would demonstrate larger total GMV and WMV and that symptoms related to anxiety, avoidance, and arousal would be associated with specific aspects of aberrant brain development.
Materials and Methods
Participants
The FXS group comprised 46 young females with a confirmed genetic diagnosis (>200 repeats) of FMR1 full mutation FXS (mean age = 10.70 years, range = 6.03–16.32, standard deviation [SD] = 2.71). Participants with FXS were recruited through the National Fragile X Foundation, regional FXS organizations (Community Support Networks), the Fragile X Online Registry With Accessible Research Database (FORWARD), the current Stanford FXS family registry, and various website and social media announcements. FXS diagnoses were confirmed by molecular genetic testing (provided by caregivers upon study enrollment) as having more than 200 CGG repeats in the FMR1 gene. The comparison group consisted of 35 females (mean age = 10.63 years, range = 6.86–15.23, SD = 2.50) who were matched for gender; age t (79) = 0.19, P = 0.85; and verbal cognitive skills t (79) = −1.55, P = 0.12. Comparison participants were recruited through advertisements to community and advocacy groups, California State Regional Centers, and educational settings throughout the state such as parent organizations and schools for individuals with learning disabilities. Individuals in either group were excluded from the study if they were born very preterm (<30 weeks), had MRI contraindications, exhibited a major sensory deficit (vision/hearing), or had evidence of current or past major neurological or major psychiatric diagnoses (e.g., seizure disorder, psychosis, bipolar disorder, and/or head trauma with loss of consciousness). All study procedures were collected in accordance with the latest guidelines in the Declaration of Helsinki and approved by the Stanford Institutional Review Board for human subjects’ research. Consents and assents were obtained from all caregivers and participants.
Measurements
IQ for each participant was assessed using the age appropriate version of the Differential Ability Scales, Second Edition (DAS-II), which provides subscales for verbal, nonverbal, and spatial reasoning ability and an overall composite score (Elliott 2007). In particular, we chose to use the DAS-II to assess IQ as it has been shown to demonstrate greater sensitivity to cognitive differences in this population (Hessl et al. 2009). The Autism Diagnostic Observation Scheduled-Second Edition (ADOS-2; Lord et al. 2012), a semi-structured interactive behavioral observation, was administered directly to the participants by a trained examiner to assess social, communicative, play and ritualistic/receptive behaviors of each participant. All participants received Module 3 of the ADOS-2 (for children/adolescents with fluent speech). In addition to direct assessments, questionnaires were also completed by participants’ caregivers, including the Social Responsiveness Scales-2 (SRS-2; Constantino and Gruber 2012) and the Anxiety, Depression, and Mood Scale (ADAMS; Esbensen et al. 2003). The SRS-2 assesses social awareness, social information processing, capacity for reciprocal social response, social anxiety/avoidance, and characteristic autistic preoccupations/traits. The ADAMS assesses mood and anxiety disorder symptoms in individuals with intellectual disability and has been observed to be particularly relevant for the FXS population (Cordeiro et al. 2011). These assessments are standardized and well-accepted measures of cognition, behavior, and emotion skills designed for the age range and developmental level of the participants in this study.
MRI Preparation
All MRI scans were completed without sedation through behavioral training based on established MRI preparatory procedures in young children (Barnea-Goraly et al. 2014). Prior to the scan, participants were shown a video about the MRI procedure including the MRI sounds and tips on how to stay still during the scan. Then, all participants were introduced to a mock MRI environment to desensitize them to the sights and sounds of the MRI. Each participant completed at least one MRI training session prior to the scan, and further sessions were completed as needed until they were able to remain lying in the supine position in a mock scanner with minimal motion as measured by an accelerometer worn on the forehead (motion spikes <1 mm) for at least 7 min.
MR Image Acquisition
MRI was performed on a GE 3T SIGNA Premier whole-body MR system using a standard 48-channel head coil (GE Medical Systems, Milwaukee, WI). Axial T1 images of the brain were acquired using a 3D magnetization-prepared rapid gradient echo pulse sequence with the following parameters: repetition time = 1985 ms, echo time = 2.8 ms, inversion time = 900 ms, flip angle = 8°, slice thickness = 1.2 mm, field of view = 24.0 cm, acquisition matrix = 240 × 240, voxel size = 1.2 × 1.0 × 1.0 mm, and duration = 4:22. Visual inspection of the image quality was performed at the time of acquisition. Scans were repeated, if necessary, to ensure the collection of high-quality data.
Image Preprocessing
Imaging data were visually inspected for head motion artifacts and then manually aligned onto the axis of the anterior and posterior commissures (Talairach and Tournoux 1988). Voxel-based morphometry was performed using Statistical Parametric Mapping software (SPM12) in the MATLAB 2018a computing environment (Ashburner and Friston 2000). Data were segmented into GM, WM, and cerebrospinal fluid (Ashburner and Friston 2005). High-dimensional registration was then performed by generating a cohort-specific template using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra toolbox (Ashburner 2007). Finally, images were warped and modulated into Montreal Neurological Institute space, downsampled to 1.5 × 1.5 × 1.5 mm voxels, and spatially smoothed using a 3-dimensional 8 mm full-width at half-maximum Gaussian smoothing kernel.
Statistical Analysis: Voxel-Wise Brain Volume
Regional differences in GMV and WMV between participants in the FXS and comparison groups were analyzed using a voxel-wise general linear model, covarying for total GMV or WMV, age, and verbal IQ, centered on the total group mean. Clusters with voxels having a height threshold exceeding P < 0.001 (uncorrected) and at a cluster extent threshold of P < 0.05 (corrected for family-wise error) are reported.
Statistical Analysis: Exploratory Associations between GMV, IQ, Age, and Behavior
A linear regression model was constructed in SPSS software (version 22.0, spss.com) to explore associations between age-adjusted GMV and WMV in regions significantly different between the 2 groups and cognitive and behavioral variables. Correlations were conducted separately within each group between regional GM volume extracted from each significant cluster and total scores on the SRS-2 and the ADAMS. The ADOS-2 was used to assess how the groups were matched on ASD characteristics and cutoff criteria for ASD, while symptom profiles measured by parent report on the SRS-2 were utilized for assessing brain–behavior correlations due to its well-balanced sensitivity and specificity for social and communication deficits typical for ASD. Exploratory associations between GMV, WMV, and age were visually plotted for each group.
Data Availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to confidentiality of participants’ health information.
Results
Group Comparisons and Volumetric Brain Differences
No significant differences were found between the groups for age, DAS-II verbal abilities, or ADOS-2 scores (P’s > 0.05; Table 1). The FXS group showed significantly larger total GMV and WMV compared to the comparison group (P’s = 0.0001; Table 1). We also compared our data to a separate normative sample of 325 children aged 4.5–18 years (Ball et al. 2012) to assess whether the GMV differences were a function of atypically low GMV in the comparison group or a function of elevated GMV in the FXS group. The results showed that girls with FXS in the present study had a larger total GMV than the typically developing population-based sample of females (t = 4.95, P < 0.0001), while the comparison group in the present study showed no significant difference to the typically developing population in this separate study (t = 0.44, P = 0.66). For the regional analysis (covarying by total GM or WM), the FXS group showed disproportionately larger GMV in 2 brain regions: 1) a bilateral parieto-occipital cluster that included the precuneus, superior parietal lobule, and occipital gyri and 2) a right parieto-occipital cluster that included the right occipital gyrus, lingual gyrus, and calcarine sulcus (P = 0.001). Conversely, the FXS group showed disproportionately smaller GMV in a bilateral frontal cluster that included the gyrus rectus and orbital gyri (P < 0.05; Table 2, Fig. 1). There were no significant group differences in regional WMV. Associations between age and total GMV and WMV are shown in Figure 2A,B.
Table 1.
Demographics, IQ, ADOS-2, and volumetric measures
| FXS (n = 46) | Control (n = 35) | P-value (2-tailed) | |
|---|---|---|---|
| Age, mean (SD) | 10.74 (2.71) | 10.63 (2.50) | 0.85 |
| GMV (L), mean (SD)a | 0.81 (0.05) | 0.76 (0.07) | <0.001* |
| WMV (L), mean (SD)a | 0.40 (0.04) | 0.37 (0.04) | <0.001* |
| DAS-II Verbal, mean (SD) | 81.83 (16.83) | 87.94 (18.47) | 0.12 |
| DAS-II Nonverbal, mean (SD) | 74.80 (17.50) | 82.86 (18.02) | 0.05** |
| DAS-II Spatial, mean (SD) | 80.74 (17.18) | 87.86 (13.26) | 0.05** |
| DAS-II GCA, mean (SD) | 76.78 (16.62) | 84.26 (15.46) | 0.04** |
| ADOS-2 Social Affect Total, mean (SD) | 6.02 (4.25) | 4.31 (4.05) | 0.07 |
| ADOS-2 RRB, mean (SD) | 0.74 (1.06) | 0.77 (1.09) | 0.89 |
| ADOS-2 Comparison Score, mean (SD) | 3.96 (2.62) | 3.17 (2.64) | 0.19 |
| ADOS-2, overall, mean (SD) | 6.76 (4.61) | 5.09 (4.60) | 0.11 |
Note: GCA, General Conceptual Ability; RRB, Restricted and Repetitive Behavior.
aAdjusted for age.
*Correlation is significant at the 0.01 level (2-tailed).
**Correlation is significant at the 0.05 level (2-tailed).
Table 2.
Clusters displaying significant group GMV differences at whole-brain level
| Cluster | Brain areas | FWE-corrected P-value (cluster-level) | Cluster size (k) | T-score (maximum) | MNI coordinates | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| FXS > Comparison | |||||||
| Bilateral parieto-occipital cluster | Precuneus, superior parietal lobule, occipital gyri | <0.001 | 3018 | 4.94 | 30 | −62 | 60 |
| Right parieto-occipital cluster | Right occipital gyrus, lingual gyrus, calcarine sulcus | 0.001 | 1777 | 4.32 | 34 | −92 | −8 |
| FXS < Comparison | |||||||
| Gyrus rectus cluster | Gyrus rectus, orbital gyri | 0.030 | 875 | 4.39 | 10 | 15 | −22 |
Note: FWE, family-wise error; MNI, Montreal Neurological Institute.
Figure 1.
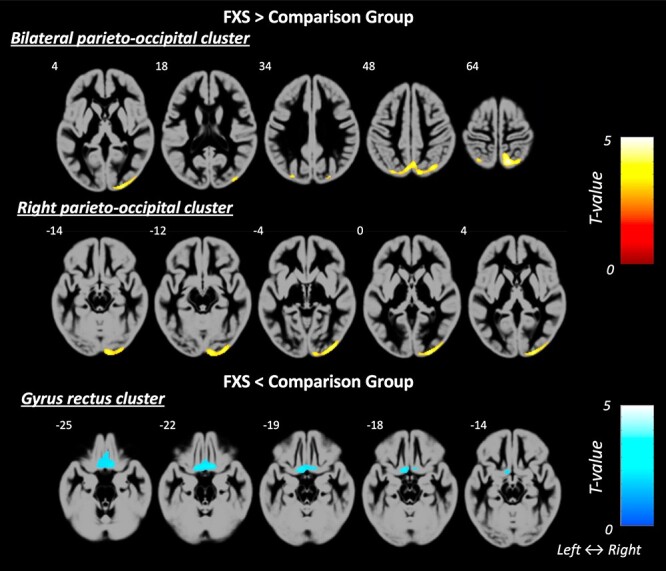
GMV was found to be significantly different between the groups in 3 brain regions, with larger GMV in the FXS group relative to comparison group in 1) a bilateral parieto-occipital cluster and 2) a right parieto-occipital cluster (both shown in warm colors), and smaller GMV in the FXS group relatively to the comparison group in 3) the gyrus rectus cluster (shown in cold colors).
Figure 2.
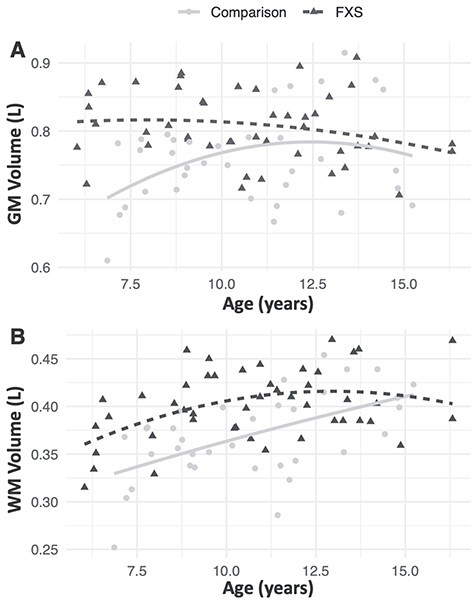
Visual representation of association between age and (A) total GMV and (B) total WMV.
Exploratory Associations between Voxel-Wise GMV/WMV and Behavioral Variables
No significant correlations were found in either group for the bilateral parieto-occipital cluster or the right parieto-occipital cluster. Exploratory correlation analyses indicated that GMV in the gyrus rectus cluster was negatively correlated with total scores on the SRS-2 and ADAMS in the FXS group (P’s < 0.02, uncorrected for multiple comparisons; Table 3) but not the comparison group. Post hoc analyses indicated that all subscales in the SRS-2 showed significant negative correlations within the FXS group, though correlation strength was significantly different between the 2 groups only for the social communication domain of the SRS-2, z = −2.41, P = 0.03, uncorrected for multiple comparisons (Table 3). In the FXS group, gyrus rectus GMV was significantly negatively correlated with 4 out of 5 domains of the ADAMS, including depressed mood, social avoidance, general anxiety, and obsessive compulsive (P’s < 0.01). Correlations were only significantly different between the groups for the compulsive behavior domain z = −2.22, P = 0.03, uncorrected for multiple comparisons (Table 3, Figures 3 and 4).
Table 3.
Correlations between SRS-2, ADAMS, and gyrus rectus cluster, significant at P < 0.05, uncorrected for multiple comparisons
| Name of assessments | Correlations within FXS (n = 46) | Correlations within control (n = 35) | Group difference z-score (Fisher r-to-z transformation) | Group difference P value (2-tailed) |
|---|---|---|---|---|
| SRS-2 Subscales | ||||
| Social Awareness | −0.39* | −0.15 | −1.12 | 0.263 |
| Social Cognition | −0.31** | −0.04 | −1.20 | 0.230 |
| Social Communication | −0.43* | 0.04 | −2.14 | 0.032** |
| Social Motivation | −0.32** | −0.02 | −1.33 | 0.184 |
| Social Communication and Interaction (SCI) | −0.41* | −0.02 | −1.78 | 0.075 |
| Restricted Interests and Repetitive Behavior (RRB) | −0.38* | −0.01 | −1.76 | 0.078 |
| SRS Total Score | −0.41* | −0.02 | −1.78 | 0.075 |
| ADAMS Subscales | ||||
| Depressed Mood | −0.35** | 0.02 | −1.65 | 0.099 |
| Social Avoidance | −0.41* | 0.02 | −1.95 | 0.051 |
| General Anxiety | −0.37** | −0.11 | −1.19 | 0.234 |
| Compulsive Behavior | −0.36** | 0.14 | −2.22 | 0.026*** |
*Correlation is significant at the 0.01 level (2-tailed).
**Correlation is significant at the 0.05 level (2-tailed).
***Significant difference in correlation strength between groups.
Figure 3.
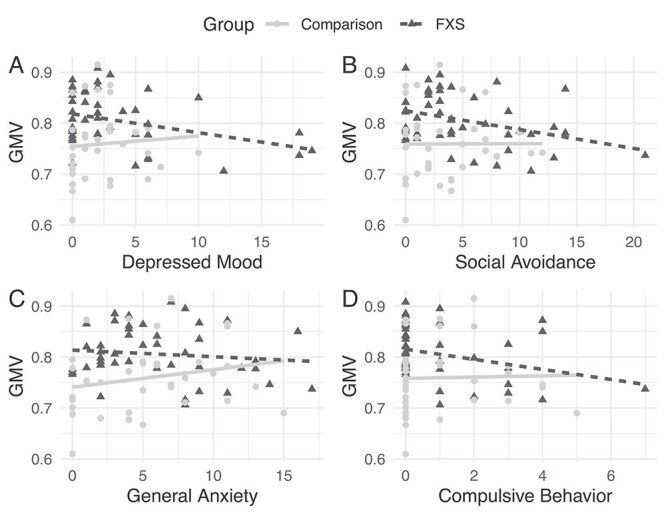
Scatterplots of significant correlations between average GMV in the gyrus rectus cluster and the following ADAMS Domains (A) Depressed Mood (B) Social Avoidance (C) General Anxiety (D) Compulsive Behavior.
Figure 4.
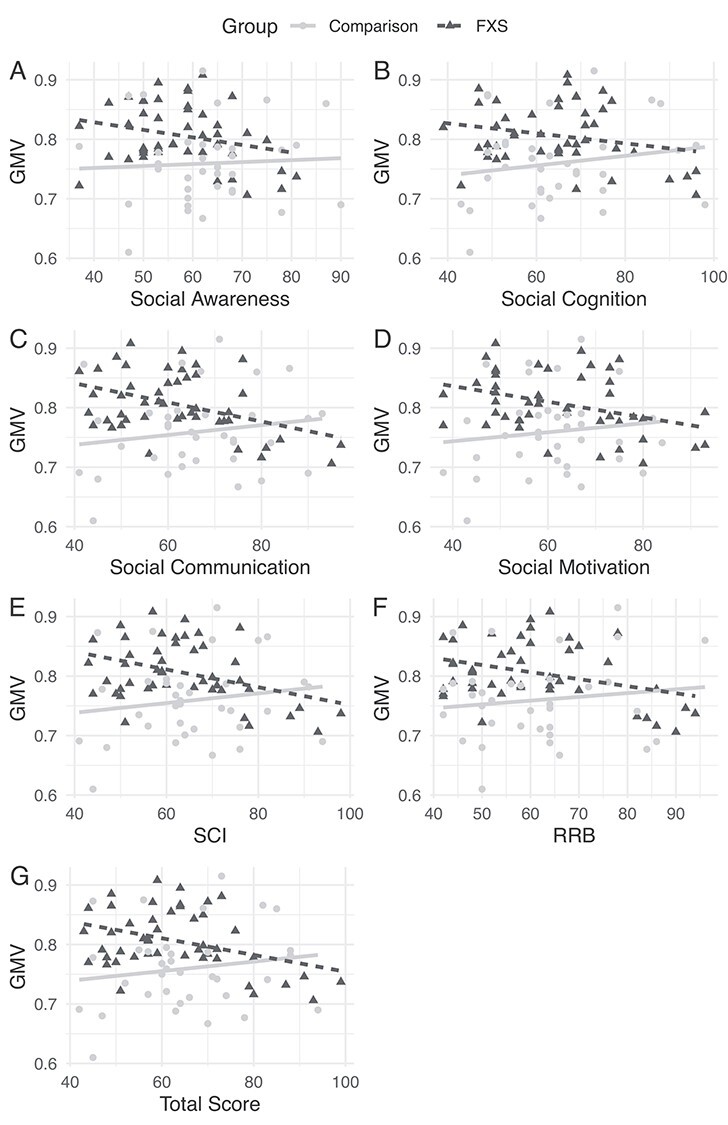
Scatterplots of significant correlations between average GMV in the gyrus rectus cluster and the following SRS-2 Domains (A) Social Awareness (B) Social Cognition (C) Social Communication (D) Social Motivation (E) Social Communication and Interaction (F) Restricted Interests and Repetitive Behaviors and (G) Total SRS-2 Score.
Discussion
In the present study, neuroanatomical differences were investigated comparing young females with FXS and an age and developmentally matched comparison group during a critical developmental period: middle childhood and adolescence. A particular strength of this study involves the use of a comparison group that is matched on sex, age, and verbal IQ, potentially allowing for an assessment of neuroanatomical differences specific to FXS-associated genetic factors rather than due to developmental delays or impaired cognitive abilities. Results demonstrated that girls with FXS exhibit larger total GMV and WMV than comparison participants (see Table 1), a finding that is consistent with neuroimaging investigations of young boys with FXS (Hazlett et al. 2012). After covarying by total GMV, regional GMV was found to be significantly different between the groups in 3 brain areas, with larger GMV in the FXS group relative to controls in a bilateral parieto-occipital cluster and a right parieto-occipital cluster, and smaller GMV in the FXS group relative to controls in the gyrus rectus. Correlations with brain volumes in these regions and social and mood variables were explored. Examination of neuroanatomical differences and their association with cognitive and socio-emotional functioning provides potential insight into the neural phenotype of females with FXS, a historically underrepresented group in research.
Girls with FXS in this study demonstrated significantly larger GMV than the control group. However, the control group was composed of a heterogeneous group of children with a wide range of disabilities and developmental delays. A typically developing group was not included in the study design. In order to assess whether the GMV differences were a function of atypically low GMV in the control group or a function of elevated GMV in the FXS group, we compared our data to a separate normative sample of 325 children aged 4.5–18 years (Ball et al. 2012). This comparison revealed that girls with FXS in the present study had a larger total GMV than the typically developing population-based sample of females, while the control group in the present study had similar total GMV and WMV relative to the typically developing population in this separate study. This post hoc analysis indicates that the larger total brain volume observed in FXS can likely be attributed to influences of the FMR1 full mutation itself rather than abnormally reduced volumes in the comparison group. FMRP plays a critical role in dendritic spine remodeling, pruning of axon branches, and translational regulation (Laggerbauer et al. 2001; Antar et al. 2003; Sears and Broadie 2018). Taken with the results of the current study, these findings suggest that larger WMV and GMV in girls with FXS may be attributable to reduced FMRP leading to a reduction of typical synaptic remodeling and pruning in the developing brain, resulting in excess brain matter that is not fully optimized.
Previous studies of child and adolescent neurodevelopment have demonstrated an inverted U-shaped relationship between GMV and age, with GMV peaking around 11 years of age (Lenroot and Giedd 2006). While cross-sectional findings in our comparison group were consistent with this established trajectory, the FXS cohort deviated from this pattern, with GMV peaking before 6 years of age (when the youngest children were seen; Fig. 2). Research on FXS has historically been limited by the availability of objective biomarkers and endpoints (Budimirovic et al. 2017), but this finding may provide insight into the age at which therapeutics and interventions might have maximal impact on normalizing aberrant brain development among girls with FXS. However, this result should be interpreted with caution as the current study is cross-sectional in nature and therefore cannot fully describe the developmental trajectory of individuals with FXS. Additional longitudinal data will be needed to more specifically examine age-related changes between specific neurodevelopmental features and cognition over time in girls with FXS.
The spatially distinct regions that showed statistically different GMV between groups in the voxel-wise analyses may provide insight into the specific neuroanatomical correlates of the socio-emotional profile of girls with FXS. The bilateral parieto-occipital region, for example, including the bilateral precuneus, superior parietal lobule, and occipital gyri, demonstrated significantly larger GMV for girls with FXS relative to the comparison group. This region has been shown to be significantly associated with social deficits and ASD symptoms (Via et al. 2011), behavioral inhibition and executive functioning, and social anxiety (Fuentes et al. 2012; Tükel et al. 2015; Wang et al. 2018). These behavioral features are each hallmark characteristics of the FXS phenotype, particularly among females (Bailey et al. 2001; Bennetto et al. 2001; Lightbody et al. 2006; Bartholomay et al. 2019). However, at present, it is unknown if larger volume in this region directly contributes to or is a correlate of deficits in social skills and social anxiety associated with FXS.
Disproportionately larger GMV in the FXS group was also found in the right parieto-occipital cluster, including the right occipital gyrus, right lingual gyrus, and calcarine sulcus (Table 2). These regions have been demonstrated to exhibit atypical activation in functional imaging studies of the participants with FXS (Garrett et al. 2004; Hoeft et al. 2007; Lightbody and Reiss 2009). In one study of a male cohort, for example, the right lingual gyrus showed lower activation in the FXS group compared to controls during a Go/NoGo task, while the left lingual gyrus showed greater activation (Hoeft et al. 2007). Another functional imaging study found that females with FXS had significantly lower activation in the right lingual gyrus while completing a face and gaze processing task (Garrett et al. 2004). The reasons for a coupling underlying reduced functioning in areas of larger volume are unclear, as are the precise molecular–cellular mechanisms leading to larger regional GMV. Extant research however suggests that lack of synaptic remodeling due to reduced FMRP levels could result in larger brain volumes as nonoptimized brain matter (Tessier and Broadie 2008; Sears and Broadie 2018) that may result in a less efficient pathways for cognitive–behavioral function.
Lastly, we observed disproportionately reduced GMV in FXS relative to the comparison group in a ventral prefrontal cluster, including the bilateral gyrus rectus and superior orbital gyrus (Table 2). This finding is consistent with previous research on adolescents with FXS from our group (Sandoval et al. 2018). The gyrus rectus is thought to be involved in a resting-state network known as the self-referential network (SRN), and aberrant functional connectivity of the SRN has been shown to be correlated with higher rates of social anxiety disorder (Liao et al. 2010). Aberrant regulation of the SRN has also been demonstrated in patients with major depressive disorder (Sheline et al. 2009). Additionally, the gyrus rectus is part of the orbital frontal cortex (OFC), a key hub in an expansive neural circuitry of sociality (Nestor et al. 2012). Abnormality in the OFC has been reported in many psychiatric disorders, namely schizophrenia, mood disorder, anxiety disorders, personality disorder, and drug addiction (Jackowski et al. 2012). While the correlations in the present study were not all significantly different “between” groups, this region was significantly correlated with several domains of the ADAMS, including symptoms of depression and anxiety, and the SRS-2, indicating social skills deficits, “within” the FXS group (Table 3). These data suggest that atypical brain development in this region may be related to the socio-emotional symptoms commonly seen in girls with FXS. To date, there has been a paucity of objective biomarkers and endpoints in FXS clinical trials (Budimirovic et al. 2017), and this region may therefore provide a promising and much needed target for future study and intervention.
Limitations
The current study has several notable limitations. First, we did not assess FMRP levels for either our control or FXS group. While FMRP levels in the comparison group are expected to be 100%, reduced FMRP levels have been shown to be correlated with many features of FXS. Though advanced methodologies have recently enabled FMRP analysis from both blood and buccal samples (Budimirovic et al. 2020), FMRP level testing has historically been assessed from blood, not the central nervous system. As FMRP in the CNS is not necessarily highly correlated with FMRP in the blood (Hinds et al. 1993), FMRP testing may not be accurate in terms of levels predicting neuroanatomical changes. Second, although we speculate on neurodevelopmental trajectories of children in our sample, the current study was cross-sectional in nature. Thus, future studies, involving repeated assessment of the same cohort over time, are needed. Finally, although it may be considered a unique strength of our study, our control group comprised a heterogeneous group of girls who were matched to our study FXS cohort for verbal abilities and adaptive behavior. Thus, they are neither a homogeneous comparison population nor a typically developing control group. For this reason, it is difficult to draw conclusions about the comparison group or girls with verbal abilities in this range as a whole.
Conclusion
FXS is the most common heritable cause of intellectual disability and ASD. Due to its presence on a region of the X chromosome that undergoes inactivation, females with FXS often have milder phenotypes compared to their male counterparts. As a result, females with FXS are underrepresented in research studies, particularly in recent years. The current study represents the first large scale study which investigates the phenotype and neuroanatomical profile of the condition in young females. The FXS group was found to have significantly larger whole brain GMV and WMV relative to a developmentally matched comparison group. Relative to the comparison group, girls with FXS showed disproportionately elevated brain volumes in a bilateral parieto-occipital cluster and a right parieto-occipital cluster and disproportionately reduced volume in the gyrus rectus. The significant associations that we observed between these regions and symptoms of anxiety, avoidance, and arousal provide potential insight into gene–brain–behavior relationships underlying the phenotype of females with FXS. Future longitudinal studies that examine these alterations in the context of providing potential targets for future therapeutics and interventions from a precision medicine perspective are warranted.
Contributor Information
Cindy H Lee, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Kristi L Bartholomay, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Matthew J Marzelli, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Jonas G Miller, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Jennifer L Bruno, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Amy A Lightbody, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA.
Allan L Reiss, Department of Psychiatry & Behavioral Sciences, Stanford University, Stanford, CA 94305, USA; Department of Radiology, Stanford University, Stanford, CA 94305, USA; Department of Pediatrics, Stanford University, Palo Alto, CA 94304, USA.
Funding
National Institute of Mental Health (grant numbers R01MH050047 and T32MH019908). Additional funding was provided by the Lynda and Scott Canel Fund for Fragile X Research, the Jesse Hough Fund for Pediatric Autism Research, and the Kelvin Foundation.
Notes
We thank the families who participated in this study, many members of the Center for Interdisciplinary Brain Sciences Research who assisted with this project, and the Richard M. Lucas Center for Imaging at Stanford University. Conflict of Interest: The authors declare no competing interests. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Antar LN, Bassell GJ, Donnell O. 2003. Sunrise at the synapse : the FMRP mRNP shaping the synaptic Interface. Neuron. 37:555–558. [DOI] [PubMed] [Google Scholar]
- Ashburner J. 2007. A fast diffeomorphic image registration algorithm. Neuroimage. 38:95–113. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2000. Voxel-based morphometry-the methods. Neuroimage. 11:805–821. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. 2005. Unified segmentation. Neuroimage. 26:839–851. [DOI] [PubMed] [Google Scholar]
- Bagni C, Oostra BA. 2013. Fragile X syndrome: from protein function to therapy. Am J Med Genet Part A. 161:2809–2821. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Hatton DD, Skinner M, Mesibov G. 2001. Autistic behavior, FMR1 protein, and developmental trajectories in young males with fragile X syndrome. J Autism Dev Disord. 3:165–174. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. 2008. Co-occurring conditions associated with FMR1 gene variations: findings from a national parent survey. Am J Med Genet Part A. 146:2060–2069. [DOI] [PubMed] [Google Scholar]
- Ball WS, Byars AW, Schapiro M, Bommer W, Carr A, German A, Dunn S, Rivkin MJ, Waber D, Mulkern R, et al. 2012. Total and regional brain volumes in a population-based normative sample from 4 to 18 years: the NIH MRI study of normal brain development. Cereb Cortex. 22:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnea-Goraly N, Weinzimer SA, Ruedy KJ, Mauras N, Beck RW, Marzelli MJ, Mazaika PK, Aye T, White NH, Tsalikian E, et al. 2014. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner-the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol. 44:181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomay KL, Lee CH, Bruno JL, Lightbody AA, Reiss AL. 2019. Closing the gender gap in fragile X syndrome: review on females with FXS and preliminary research findings. Brain Sci. 9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetto L, Taylor AK, Pennington BF, Porter D, Hagerman RJ. 2001. Profile of cognitive functioning in women with the fragile X mutation. Neuropsychology. 15:290–299. [PubMed] [Google Scholar]
- Budimirovic DB, Berry-Kravis E, Erickson CA, Hall SS, Hessl D, Reiss AL, King MK, Abbeduto L, Kaufmann WE. 2017. Updated report on tools to measure outcomes of clinical trials in fragile X syndrome. J Neurodev Disord. 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Schlageter A, Filipovic-Sadic S, Protic DD, Bram E, Mahone EM, Nicholson K, Culp K, Javanmardi K, Kemppainen J, et al. 2020. A genotype-phenotype study of high-resolution FMR1 nucleic acid and protein analyses in fragile X patients with neurobehavioral assessments. Brain Sci. 10:694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Gruber CP. 2012. Social responsiveness scale (SRS). 2nd ed. Los Angeles (CA): Western Psychological Services. [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. 2011. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. J Neurodev Disord. 3:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. 2001. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med. 3:359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di KN, Disteche CM. 2006. Dosage compensation of the active X chromosome in mammals. Nat Genet. 38:47–53. [DOI] [PubMed] [Google Scholar]
- Eliez S, Blasey CM, Freund LS, Hastie T, Reiss AL. 2001. Brain anatomy, gender and IQ in children and adolescents with fragile X syndrome. Brain. 124:1610–1618. [DOI] [PubMed] [Google Scholar]
- Elliott CD. 2007. Differential Ability Scales. 2nd ed. Minneapolis (MN): NCS Pearson, Inc. [Google Scholar]
- Esbensen AJ, Rojahn J, Aman MG, Ruedrich S. 2003. Reliability and validity of an assessment instrument for anxiety, depression, and mood among individuals with mental retardation. J Autism Dev Disord. 33:617–629. [DOI] [PubMed] [Google Scholar]
- Freund LS, Reiss AL, Abrams MT. 1993. Psychiatric disorders associated with fragile X in the young female. Pediatrics. 91:321–329. [PubMed] [Google Scholar]
- Fuentes P, Barrós-Loscertales A, Bustamante JC, Rosell P, Costumero V, Ávila C. 2012. Individual differences in the behavioral inhibition system are associated with orbitofrontal cortex and precuneus gray matter volume. Cogn Affect Behav Neurosci. 12:491–498. [DOI] [PubMed] [Google Scholar]
- Garber KB, Visootsak J, Warren ST. 2008. Fragile X syndrome. Eur J Hum Genet. 16:666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Menon V, Mackenzie K, Reiss AL. 2004. Here’s looking at you, kid: neural systems underlying face and gaze processing in fragile X syndrome. Arch Gen Psychiatry. 61:281–288. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, et al. 1996. Quantitative magnetic resonance imaging of human brain development: ages 4-18. Cereb Cortex. 6:551–560. [DOI] [PubMed] [Google Scholar]
- Gould J. 2017. Towards understanding the under-recognition of girls and women on the autism spectrum. Autism. 21:703–705. [DOI] [PubMed] [Google Scholar]
- Guerreiro MM, Camargo EE, Kato M, Marques-De-Faria AP, Ciasca SM, Guerreiro CAM, Menezes Netto JR, Moura-Ribeiro MVL. 1998. Fragile X syndrome: clinical, electroencephalographic and neuroimaging characteristics. Arq Neuropsiquiatr. 56:18–23. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ. 2008. The fragile X prevalence paradox. J Med Genet. 45:498–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Jackson C, Amiri K, O’Connor R, Sobesky W, Silverman AC. 1992. Girls with fragile X syndrome: physical and neurocognitive status and outcome. Pediatrics. 89:395–400. [PubMed] [Google Scholar]
- Hall SS, Lightbody AA, Reiss AL. 2008. Compulsive, self-injurious, and autistic behavior in children and adolescents with fragile X syndrome. Am J Ment Retard. 113:44–53. [DOI] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, Robertson D, Murphy KC, Murphy DGM. 2011. In vivo brain anatomy of adult males with fragile X syndrome: an MRI study. Neuroimage. 54:16–24. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. 2006. Autistic behavior in children with fragile X syndrome: prevalence, stability, and the impact of FMRP. Am J Med Genet Part A. 140:1804–1813. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Styner M, MacFall JR, Reiss AL, Piven J. 2012. Trajectories of early brain volume development in fragile X syndrome and autism. J Am Acad Child Adolesc Psychiatry. 51:921–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessl D, Dyer-Friedman J, Glaser B, Wisbeck J, Barajas RG, Taylor A, Reiss AL. 2001. The influence of environmental and genetic factors on behavior problems and autistic symptoms in boys and girls with fragile X syndrome. Pediatrics. 108:e88. [DOI] [PubMed] [Google Scholar]
- Hessl D, Nguyen DV, Green C, Chavez A, Tassone F, Hagerman RJ, Senturk D, Schneider A, Lightbody A, Reiss AL, et al. 2009. A solution to limitations of cognitive testing in children with intellectual disabilities: the case of fragile X syndrome. J Neurodev Disord. 1:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds HL, Ashley CT, Sutcliffe JS, Nelson DL, Warren ST, Housman DE, Schalling M. 1993. Tissue specific expression of FMR–1 provides evidence for a functional role in fragile X syndrome. Nat Genet. 3:36–43. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, Parthasarathy S, Watson CL, Hall SS, Reiss AL. 2007. Fronto-striatal dysfunction and potential compensatory mechanisms in male adolescents with fragile X syndrome. Hum Brain Mapp. 28:543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, Reiss AL. 2011. Neuroanatomical differences in toddler boys with fragile X syndrome and idiopathic autism. Arch Gen Psychiatry. 68:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski AP, de Araújo Filho GM, de Almeida AG, de Araújo CM, Reis M, Nery F, Batista IR, Silva I, Lacerda ALT. 2012. The involvement of the orbitofrontal cortex in psychiatric disorders: an update of neuroimaging findings. Rev Bras Psiquiatr. 34(2):207–212. [DOI] [PubMed] [Google Scholar]
- Klusek J, Martin GE, Losh M. 2014. Consistency between research and clinical diagnoses of autism among boys and girls with fragile X syndrome. J Intellect Disabil Res. 58:940–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel E, Ostareck-lederer A, Fischer U. 2001. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 10:329–338. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. 2006. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neurosci Biobehav Rev. 30:718–729. [DOI] [PubMed] [Google Scholar]
- Liao W, Chen H, Feng Y, Mantini D, Gentili C, Pan Z, Ding J, Duan X, Qiu C, Lui S, et al. 2010. NeuroImage selective aberrant functional connectivity of resting state networks in social anxiety disorder. Neuroimage. 52:1549–1558. [DOI] [PubMed] [Google Scholar]
- Lightbody AA, Hall SS, Reiss AL. 2006. Chronological age, but not FMRP levels, predicts neuropsychological performance in girls with fragile X syndrome. Am J Med Genet Part B Neuropsychiatr Genet. 141:468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightbody AA, Reiss AL. 2009. Gene, brain, and behavior relationships in fragile X syndrome: evidence from neuroimaging studies. Dev Disabil Res Rev. 15:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Huggins RM, Hagerman RJ. 2004. Phenotypic variation and FMRP levels in fragile X. Ment Retard Dev Disabil Res Rev. 10:31–41. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. 2012. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Manual (Part I): modules 1–4. Torrance (CA): Western Psychological Services. [Google Scholar]
- Mazzocco MMM, Kates WR, Baumgardner TL, Freund LS, Reiss AL. 1997. Autistic behaviors among girls with fragile X syndrome. J Autism Dev Disord. 27:415–435. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Nakamura M, Niznikiewicz M, Thompson E, Levitt JJ, Choate V, Shenton ME, McCarley RW. 2012. In search of the functional neuroanatomy of sociality: MRI subdivisions of orbital frontal cortex and social cognition. Soc Cogn Affect Neurosci. 8(4):460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin EM, Jo B, Hall SS, Bruno JL, Chromik LC, Raman MM, Lightbody AA, Martin A, Reiss AL. 2016. The cognitive developmental profile associated with fragile X syndrome: a longitudinal investigation of cognitive strengths and weaknesses through childhood and adolescence. Dev Psychopathol. 28:1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. 1995. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med. 1:159–167. [DOI] [PubMed] [Google Scholar]
- Sandoval GM, Shim S, Hong DS, Garrett AS, Quintin EM, Marzelli MJ, Patnaik S, Lightbody AA, Reiss AL. 2018. Neuroanatomical abnormalities in fragile X syndrome during the adolescent and young adult years. J Psychiatr Res. 107:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears JC, Broadie K. 2018. Fragile X mental retardation protein regulates activity-dependent membrane trafficking and trans-synaptic signaling mediating synaptic remodeling. Front Mol Neurosci. 10:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, Mintun MA, Wang S, Coalson RS, Raichle ME. 2009. The default mode network and self-referential processes in depression. Proc Natl Acad Sci. 106:1942–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J. 1988. Co-planar stereotaxic atlas of the human brain-3-dimensional proportional system. An approach to cerebral imaging. Vol 1988. [Google Scholar]
- Tan SS, Faulkner-Jones B, Breen SJ, Walsh M, Bertram JF, Reese BE. 1995. Cell dispersion patterns in different cortical regions studied with an X-inactivated transgenic marker. Development. 121:1029–1039. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Iklé DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. 1999. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 84:250–261. [PubMed] [Google Scholar]
- Tessier CR, Broadie K. 2008. Drosophila fragile X mental retardation protein developmentally regulates activity-dependent axon pruning. Development. 135:1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tükel R, Aydin K, Yüksel Ç, Ertekin E, Koyuncu A, Taş C. 2015. Gray matter abnormalities in patients with social anxiety disorder: a voxel-based morphometry study. Psychiatry Res Neuroimaging. 234:106–112. [DOI] [PubMed] [Google Scholar]
- Turkstra LS, Abbeduto L, Meulenbroek P. 2014. Social cognition in adolescent girls with fragile X syndrome. Am J Intellect Dev Disabil. 119:319–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via E, Radua J, Cardoner N, Happé F, Mataix-Cols D. 2011. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Arch Gen Psychiatry. 68:409–418. [DOI] [PubMed] [Google Scholar]
- Wang X, Cheng B, Luo Q, Qiu L, Wang S. 2018. Gray matter structural alterations in social anxiety disorder: a voxel-based meta-analysis. Front Psych. 9:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Tregellas JR, Hagerman RJ, Rogers SJ, Rojas DC. 2009. A voxel-based morphometry comparison of regional gray matter between fragile X syndrome and autism. Psychiatry Res Neuroimaging. 174:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Achsel T, Bagni C. 2006. mRNPs, polysomes or granules: FMRP in neuronal protein synthesis. Curr Opin Neurobiol. 16:265–269. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to confidentiality of participants’ health information.


