Abstract
Major depressive disorder (MDD), bipolar disorder (BD) and schizophrenia-spectrum disorders (SSD) are heterogeneous psychiatric disorders, which place significant burden on patient's well-being and global health. Disruptions in the gut-microbiome may play a role in these psychiatric disorders. This review presents current data on composition of the human gastrointestinal microbiota, and its interaction mechanisms in the gut–brain axis in MDD, BD and SSD. Diversity metrics and microbial relative abundance differed across studies. More studies reported inconsistent findings (n = 7) or no differences (n = 8) than studies who reported lower α-diversity in these psychiatric disorders (n = 5). The most consistent findings across studies were higher relative abundances of the genera Streptococcus, Lactobacillus, and Eggerthella and lower relative abundance of the butyrate producing Faecalibacterium in patients with psychiatric disorders. All three increased genera were associated with higher symptom severity. Confounders, such as medication use and life style have not been accounted for. So far, the results of probiotics trials have been inconsistent. Most traditional and widely used probiotics (consisting of Bifidobacterium spp. and Lactobacillus spp.) are safe, however, they do not correct potential microbiota disbalances in these disorders. Findings on prebiotics and faecal microbiota transplantation (FMT) are too limited to draw definitive conclusions. Disease-specific pro/prebiotic treatment or even FMT could be auspicious interventions for prevention and therapy for psychiatric disorders and should be investigated in future trials.
Key words: Bipolar disorder, gastrointestinal permeability, gut-microbiome, major depressive disorder, probiotics, schizophrenia-spectrum disorders
Introduction
The human gut microbiome (GM), comprised of trillions of microorganisms, bacteria, viruses, fungi and other life forms, has been implicated in numerous aspects of human health and disease (Lai, Gao, & Zhang, 2020). The GM is diverse, personalized, dynamic and can be influenced, especially early in life, by factors such as vaginal/caesarean birth, diet, sleep, contact with other humans and stress (Gacesa et al., 2022; Szeligowski, Yun, Lennox, & Burnet, 2020). However, genetic factors, especially immunological background also determine a person's microbiome composition (Thaiss, Zmora, Levy, & Elinav, 2016). The GM partakes in a bidirectional communication with the central nervous system (CNS), called the gut–brain axis (Cryan et al., 2019). Disruptions to the GM have been associated with several neuropsychiatric disorders, including major depressive disorder (MDD), bipolar disorder (BD) and schizophrenia-spectrum disorders (e.g. schizophrenia, schizo-affective disorder and schizophreniform disorder, SSD) (Bastiaanssen et al., 2020; Genedi, Janmaat, Haarman, & Sommer, 2019; Nguyen, Hathaway, Kosciolek, Knight, & Jeste, 2021; Szeligowski et al., 2020).
MDD, BD and SSD are heterogeneous psychiatric disorders, which place significant burden on a patient's well-being and global health (WHO, 2006). Worldwide, MDD, BD and SSD affect 264 million, 45 million and 20 million people, respectively (James et al., 2018). Besides the overlap in psychiatric symptomatology, cognitive and biological functions, there is also a large genetic overlap between MDD, BD and SSD (Anttila et al., 2018). Nowadays it is generally assumed that SSD, BD and MDD are disorders that are not entirely separated, but represent different stages of a continuum of clinical pictures (Haarman, Riemersma-Van der Lek, Burger, Drexhage, & Nolen, 2016).
Research into the role of the GM in MDD, BD and SSD is still in its infancy. This review is a narrative review which we have approached in a structured way and it presents data on the development and composition of the human gastrointestinal microbiota, and its interaction mechanisms in the gut–brain axis in MDD, BD and SSD. Furthermore, the therapeutic potential of pre/probiotics, diet and faecal microbiota transplantation (FMT) in these disorders will also be discussed. We begin by showing the different routes between the gut and brain and will then discuss the microbiome, after that we will discuss gut permeability and the GM in the psychiatric disorders. Finally, we will discuss potential mechanisms on how to adapt the GM to improve the clinical situation of patients.
Gut–brain axis
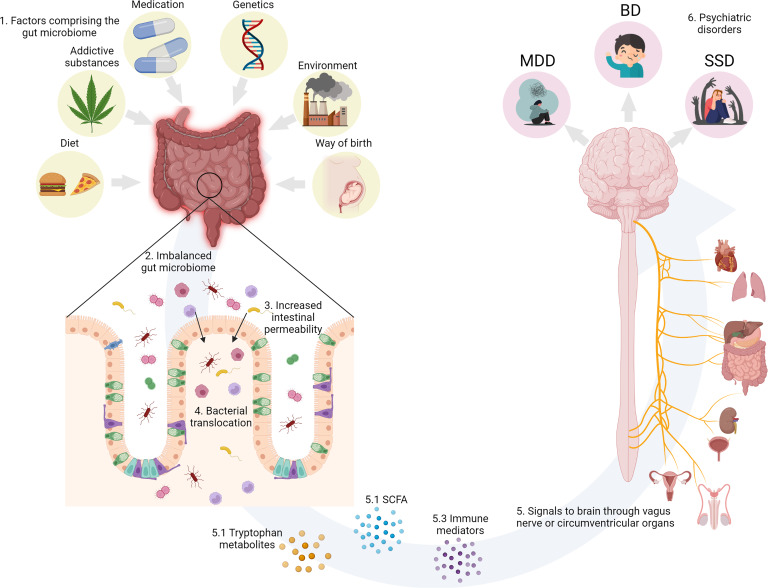
The gut and the brain communicate bidirectionally via several routes, including the vagal nerve, hypothalamic–pituitary–adrenal (HPA) axis, the production of bacterial metabolites, such as short-chain fatty acids (SCFAs), immune mediators and entero-endocrine signalling (Cryan et al., 2019; Golofast & Vales, 2020) (Fig. 1).
Fig. 1.
(1) Environmental factors known to impinge on the human GM. (2) GM dysbiosis impairs intestinal permeability. (3) Increased intestinal permeability causes translocation of luminal components and reactivity of the intestinal immune system. (4) Bacterial translocation activates the gut–brain axis. (5) The gut and the brain communicate bidirectionally via several routes, including the vagal nerve, the HPA axis, immune mediators such as cytokines, and the production of bacterial metabolites, such as SCFAs. (6) The environmental factors, GM dysbiosis and increased permeability separately and in concert could contribute the development of psychiatric disorders. Created with BioRender.com.
Vagal nerve
The most direct route between the gut and the brain is the vagal nerve, roughly translated as the wandering nerve, and also known as the 10th cranial nerve. In reports from 1953 and 1961, ablation of the vagal nerve, formerly used for the treatment of peptic ulcers, resulted in an increase in the incidence of psychiatric disorders (Browning & Houseworth, 1953; Whitlock, 1961). Interestingly, this procedure recently turned out to reduce the incidence of Parkinson's disease (Svensson et al., 2015). In animals, Sgritta et al. (2019) observed that the effects of Lactobacillus reuteri on social behaviour were dissipated after vagotomy in a genetic mouse model of autism (Shank3B−/− mouse).
Short-chain fatty acids
SCFAs are capable of signalling to the brain indirectly via nerve activation and can therefore influence behaviour (Sherwin, Sandhu, Dinan, & Cryan, 2016). Ninety five per cent of SCFAs consist of acetate, propionate and butyrate. The primary source of SCFAs is microbial fermentation of dietary fibres in the gut, which is supported by the fact that germ-free animals and antibiotic treatment results in low SCFA levels (Cryan et al., 2019). SCFAs are capable of modulating neurotransmission. For example, propionic acid increases tryptophan hydroxylase expression which can reduce indoleamine serotonin, thereby influencing serotonergic neurotransmission (El-Ansary, Bacha, & Kotb, 2012; Nankova, Agarwal, MacFabe, & La Gamma, 2014). So far, only a few studies have investigated relations between neuropsychiatric disorders and SCFAs. Skonieczna-żydecka et al. (2018) reported lower median content of acetate and higher isocaproic acid concentration in depressed women as compared to healthy women. Negative correlations were yielded between acetic, propionic and isocaproic acids and severity of depression as assessed with the Beck's Depression Inventory (BDI) scores. Szczesniak, Hestad, Hanssen, and Rudi (2016) reported significantly higher isovaleric levels in the depressed patients as compared to healthy subjects. However, Kelly et al. (2016) found no differences in acetate, propionate, isobutyrate and butyrate between depressed patients and healthy controls. The limited sample sizes in all three studies could explain these inconsistent results. In animal models of mania, sodium butyrate reversed mania-like, e.g. behavioural hyperactivity, and depressive-like behaviour (Moretti et al., 2011; Resende et al., 2013; Valvassori et al., 2015)
Immune mediators
Immune mediators are important intermediaries between the gut microbiota and the brain. Cytokines can signal to the brain from the periphery via the vagal nerve or via the circumventricular organs (Sherwin et al., 2016). In the blood, bacteria or fragments from bacteria can bind lipopolysaccharide (LPS) binding protein (LBP), which is then connected to TLR-4, expressed on monocytes, macrophages and microglia, by soluble cluster of differentiation 14 (sCD14) (Kiecolt-Glaser et al., 2018; Kitchens & Thompson, 2005; Lim, Chang, & Wu, 2019), which can lead to nuclear factor kappa-light-chain enhancer of activated B cells (NFκB) activation, inducing pro-inflammatory cytokine production (Genedi et al., 2019; Sherwin et al., 2016). Thus, the presence of bacteria or small parts of bacteria can activate the immune system which also affects the brain. Blood levels of LBP, sCD14 and NFκB can reflect activity of this route. The group of Robert Yolken showed that these levels are increased in patients with BD and SSD, when patients had comorbid gastro-intestinal complaints (Severance et al., 2013; Severance, Dickerson, & Yolken, 2020). Moreover, Foster, Baker, and Dursun (2021) recently reviewed the relation of the GM and the immune system in MDD, where they draw attention to the importance of the immune system as an important player in the neurobiology combined with the GM in subtypes of depression.
Tryptophan metabolites
Along with influencing other metabolites, gut-bacteria influence tryptophan metabolism (Carlessi, Borba, Zugno, Quevedo, & Réus, 2021). Tryptophan is an essential amino acid, which is metabolized by two main pathways, namely, the serotonin (5-HT) pathway and the kynurenine pathway. According to a meta-analysis of 101 studies tryptophan and kynurenine are decreased across MDD, BD and SSD (Marx et al., 2020). Conventional antidepressants enhance levels of central serotonin to produce a therapeutic effect. Most of the tryptophan is metabolized into kynurenine in the liver by tryptophan-2,3-dioxygenase for energy production, or following an inflammatory stimulus by indoleamine-2,3-dioxygenase. The presence of gut bacteria such as Clostridium perfringens can modulate gut production of 5-HT (Beaver & Wostmann, 1962; Yano et al., 2015). Studies in rats have shown that probiotic treatment can influence tryptophan, kynurenine and serotonin levels (Sherwin et al., 2016). Rats treated with Bifidobacterium infantis 35624 had increased circulating tryptophan levels (Desbonnet, Garrett, Clarke, Bienenstock, & Dinan, 2009). Furthermore, rats treated with Lactobacillus johnsonii had lower circulating kynurenine levels with increased serotonin levels in the ileum and in the circulation (Valladares et al., 2013). Moreover, one study in MDD patients found decreases in the kynurenine/tryptophan ratio after treatment with probiotics (Lactobacillus helveticus and Bifidobacterium longum) (Kazemi, Noorbala, Azam, Eskandari, & Djafarian, 2019). A meta-analysis studying the effect of probiotic supplementation on the tryptophan–kynurenine pathway observed significantly lower serum kynurenine and a decreased kynurenine/tryptophan ratio after treatment (Purton et al., 2021). The results so far provide preliminary evidence that probiotics can modulate the tryptophan–kynurenine pathway.
Gut microbiome
The GM contains 1014 microorganisms, 2–20 million unique genes and over 1000 unique bacterial species. The GM is a perplexing genomic structure with many more genes than the human genome (Golofast & Vales, 2020). In contrast to the human genome, which is unalterable over lifetime, the GM is highly adaptable (Nguyen et al., 2021). The GM is already influenced by the environment at the day of birth. Vaginally delivered neonates' microbiota resembles the maternal vaginal and faecal bacteria, whereas for infants born by caesarean section, their microbiota resembles the maternal skin and hospital environment (Bäckhed et al., 2015; Korpela et al., 2020; Mitchell et al., 2020). In 2002 Cannon, Jones and Murray already identified unplanned caesarean section as a risk factor for schizophrenia in their seminal meta-analysis. One epidemiological study found a weak association between birth by planned caesarean section, but not by unplanned emergency caesarean section and the risk of developing psychosis or BD (O'Neill et al., 2016). However, after correcting for matched siblings, this effect was no longer significant. The Finnish birth register data also showed an odds ratio (OR) of 2.5 for BD for birth by caesarean section (Chudal et al., 2014).
Associations between antibiotic exposure and psychiatric disorders have been found in multiple large population-based studies (Köhler et al., 2017; Liang et al., 2021; Lurie, Yang, Hayne, Mamtani, & Boursi, 2015). Lurie et al. (2015) found that treatment with a single antibiotic course was associated with higher risk for depression (n = 202,974) compared to a healthy control group (n = 803,961), with adjusted ORs of 1.23 [95% confidence level (CL) 1.18–1.29] for penicillin's and 1.25 (95% CL 1.15–1.35) for quinolones. Köhler et al. (2017) performed a large-scale study in individuals born in Denmark in 1985–2002 (n = 1,015,447), of which 5759 individuals were diagnosed with schizophrenia. The association of infections treated with anti-infective agents and the subsequent risk of schizophrenia and affective disorders during 1995–2013 was studied. Antibiotics, with a hazard rate ratio (HRR) of 1.44 (95% CL 1.25–1.66), and especially broad spectrum antibiotics (HRR = 1.53; 95% CL 1.32–1.71) were associated with increased risk for schizophrenia. However, Lurie et al. (2015) did not find an association between antibiotic use and psychosis (n = 8487). Liang et al. (2021) observed positive associations between long-term antibiotic use during early life (defined by the UK Biobank as child or teenager) and anxiety and depression. Lastly, there are also studies showing that antibiotic use can induce hypomania or mania, also known as antibiomania (Abouesh, Stone, & Hobbs, 2002). In a review of 47 published cases, clarithromycin, a broad spectrum antibiotic, was related to (hypo)mania in 16 out of the 47 cases (Lambrichts, Van Oudenhove, & Sienaert, 2017). One explanation could be that the administration of antibiotics can result in changes in the microbiome which could in turn increases the risk of (hypo)mania, for example by lowering the bacterial production of gamma-amino-butyric acid (Dickerson, Severance, & Yolken, 2017).
Gastrointestinal permeability in patients with severe psychiatric disorders
Gastrointestinal barrier
The gastrointestinal barrier is a dynamic, multilayer system which consists of a physical barrier and a biochemical barrier. The main components of the physical barrier are the epithelial cells sealed by tight junctions and the gut mucosa. The biochemical barrier consists of the gut microbiota and the mucosal immune system. Its main function is to keep pathogens out of the host's internal milieu, while at the same time facilitate the absorption of nutrients, water and electrolytes (Bischoff et al., 2014). For the homoeostasis of the organism, the permeability of the gastrointestinal barrier needs to be maintained within a narrow equilibrium.
Diet and lifestyle factors such as energy-dense food and alcohol can disturb gut permeability and lead to translocation of luminal components and reactivity of the intestinal immune system (Bischoff et al., 2014). Increased intestinal permeability can also be a result of changes in gut microbiota, infections or reduced perfusion of the gut (Bischoff et al., 2014).
Gastrointestinal permeability and brain structure and function
Gastrointestinal permeability and brain structure and function are governed by a bidirectional interaction. On one hand, pre-clinical studies (Keita, Söderholm, & Ericson, 2010; Kiliaan et al., 1998; Söderholm et al., 2002; Vicario et al., 2010) and studies in healthy volunteers (Vanuytsel et al., 2014) suggest a causal effect of psychosocial stress on gut permeability, likely through corticotropin-releasing hormone-mediated mast cell activation and decreased blood flow to the gut during stressful periods. On the other hand, it is hypothesized that increased gut permeability and abnormal influx of food- and bacteria-derived antigens drives systemic low-grade immune dysregulation, which in turn affects brain structure and function (Genedi et al., 2019). Lastly, psychiatric comorbidity is common in diseases with known structural and functional abnormalities of the gastrointestinal barrier, such as Crohn's disease and colitis ulcerosa (Bennett, Tennant, Piesse, Badcock, & Kellow, 1998; Faresjö et al., 2007; Nicholl et al., 2008). It is therefore hypothesized that abnormal intestinal permeability is involved both as an effect and as a cause of severe psychiatric disorder, yet, this is still a research area in its infancy.
Evidence of gastrointestinal barrier dysfunction in MDD, BD and SSD
In MDD, BD and SSD, gastrointestinal permeability has been assessed with markers of structural barrier integrity and paracellular permeability such as zonulin and intestinal-type fatty acid-binding protein (I-FABP) (Table 1). In a study in MDD or anxiety disorder, authors reported significantly higher levels of zonulin and I-FABP in patients compared to controls and this was associated with gut dysbiosis (Stevens et al., 2018). Alvarez-Mon et al. (2019) also reported significantly higher levels of I-FABP in MDD patients compared to controls but no significant difference was observed for zonulin. In another study, patients of different psychiatric diagnoses who had recently attempted suicide had higher levels of I-FABP but lower levels of zonulin compared to both MDD patients without a history of suicide attempt and healthy controls (Ohlsson et al., 2019). In BD, patients in full remission showed higher zonulin and claudin-5 compared to controls, while there were no differences between active manic episode and remission (Kılıç, Işık, Demirdaş, Doğuç, & Bozkurt, 2020). Interestingly, this observation might suggest that zonulin and claudin-5 are ‘trait’ rather than ‘state’ markers of BD. However, the small sample size of this study precludes firm conclusions, before the findings are confirmed in larger populations. Moreover, there is a certain level of uncertainty surrounding the measurement of zonulin with commercially available ELISA kits. The results should be interpreted with caution until an in depth understanding of the biomarker is acquired (Massier, Chakaroun, Kovacs, & Heiker, 2021). In schizophrenia, Maes, Kanchanatawan, Sirivichayakul, and Carvalho (2019a) and Maes, Sirivichayakul, Kanchanatawan, and Vodjani (2019b) showed that immunoglobulin M (IgM) responses to zonulin were higher in patients compared to controls, while IgM response to occludin was significantly associated with deficit schizophrenia. Authors also reported an increased ratio of IgM towards components of the paracellular route (zonulin + occludin)/components of the transcellular route (talin + actin + vinculin) in deficit v. non-deficit schizophrenia and in schizophrenia v. controls. This ratio was significantly associated with increased IgA responses to Gram-negative bacteria. Schizophrenia patients with deficit syndrome (i.e. severe negative and cognitive symptoms) can be expected to have unhealthy dietary habits, which could explain the association with IgM response to occludin. Barber et al. (2019) showed that 42.9% of the patients had higher levels of zonulin than the cut-off for elevated levels (>2.33 mg/dl).
Table 1.
Main findings of studies assessing gut permeability in MDD, BD and SSD
| Measure of gastrointestinal permeability | Reference | Diagnosis | Sample size | Main findings |
|---|---|---|---|---|
| Markers of paracellular and transcellular disruption | ||||
| I-FABP Zonulin LPS |
Stevens et al. (2018) | MDD or anxiety disorder | MDD or anxiety disorder: n = 22 HC: n = 28 |
↑plasma levels of LPS, zonulin and I-FABP in patients compared to controls Gut dysbiosis in patients, not in controls |
| LBP I-FABP Zonulin |
Alvarez-Mon et al. (2019) | MDD | MDD: n = 22 HC: n = 14 |
↑levels of circulating LBP, and I-FABP in patients compared to controls No significant differences in levels of zonulin |
| I-FABP Zonulin sCD14 |
Ohlsson et al. (2019) | Different psychiatric diagnosis with recent suicide attempt (rSA) and MDD without history of suicide attempt (nsMDD) | rSA: n = 54 nsMDD: n = 13 HC: n = 17 |
↑I-FABP and ↓zonulin levels in the group with recent suicide attempters compared to both the MDD group without history of suicide attempt and the healthy controls |
| Zonulin Claudin-5 |
Kılıç et al. (2020) | BD | BD: n = 41 HC: n = 41 |
↑zonulin and claudin-5 levels in patients compared to healthy controls. No difference in zonulin and claudın-5 levels between patients with manic episodes and patients in remission |
| IgM against zonulin, occluding, talin, actin and vinculin IgA against Gram-(−) bacteria |
Maes, Sirivichayakul, Kanchanatawan, and Vodjani (2019b) | SSD | SSD: n = 78 HC: n = 40 |
↑ratio of IgM to zonulin + occludin/talin + actin + vinculin (PARA/TRANS) in deficit v. non-deficit schizophrenia and in schizophrenia v. controls PARA/TRANS ratio significantly associated with increased IgA responses to Gram-(−) bacteria IgM to zonulin positively associated with schizophrenia (v. controls) |
| Zonulin | Barber et al. (2019) | SSD | SSD: n = 98 | 42.9% of the patients had higher levels of zonulin than the cut-off for elevated levels (>2.33 mg/dl) |
| Markers of bacterial translocation | ||||
| sCD14 LBP |
Severance et al. (2013) | BD SSD |
BD: n = 75 SSD: n = 141 HC: n = 78 |
↑sCD14 in BD and SSD compared to HC ↑LBP in SCZ compared to BD |
| IgM/IgA against LPS of Gram-(−) bacteria | Maes et al. (2013) | MDD | MDD: n = 113 HC: n = 28 |
↑IgM/IgA against the LPS of Gram-(−) bacteria in MDD compared to controls |
| sCD14 | Tanaka et al. (2017) | BD SCZ HC |
BD: n = 32 SCZ: n = 28 HC: n = 60 |
↑sCD14 in BD and SCZ compared to HC |
| sCD14 | Morch et al. (2019) | SSD HC |
SSD: n = 675 HC: n = 647 |
sCD14 was not significantly different between SSD and HC |
| IgM/IgA against LPS of Gram-(−) bacteria | Simeonova et al. (2020) | MDD BD |
MDD: n = 44 BD: n = 66 |
IgA/IgM against LPS of gut commensal bacteria were positively associated with MDD and BD as compared to healthy controls |
| IgM/IgA against Gram-(−) bacteria | Maes et al. (2019a, 2019b) | SSD | SSD: n = 80 HCL: n = 38 |
IgM/IgA against gut commensal bacteria were associated with negative symptoms, neurocognitive impairments and the deficit phenotype of SSD |
| sCD14 | Dzikowski et al. (2020) | SCZ HC |
SCZ: n = 50 HC: n = 60 |
↑sCD14 in SCZ compared to HC |
sCD14, soluble cluster of differentiation 14; LBP, lipopolysaccharide (LPS) binding protein; I-FABP, fatty acid-binding protein.
Markers of bacteria translocation can also reflect abnormal gastrointestinal permeability. LPS and LBP were up-regulated in MDD or anxiety disorder (Alvarez-Mon et al., 2019; Stevens et al., 2018). In BD, sCD14, a co-receptor for the LBP, used as a marker for bacterial translocation was significantly higher in patients compared to controls (Severance et al., 2013). In SSD, sCD14 was significantly higher in multiple studies (Dzikowski et al., 2020; Severance et al., 2013; Tanaka et al., 2017; Weber et al., 2018) but not all (Morch et al., 2019). In regard to antibodies against gut commensal bacteria, in MDD, Maes et al. (2013) showed increased IgM/IgA against Gram-negative bacteria. More recently, Simeonova et al. (2020) showed increased immune responses to Gram-negative bacteria in both MDD and BD, especially in the presence of melancholia, compared to healthy controls. Interestingly, IgA/IgM response profiles differ among the two diagnosis, as well as between BD I and II subtypes and patients with melancholia. This might reflect a common underlying disruption of the gut barrier across diagnoses, accompanied however by distinct microbiome profiles and immune susceptibilities, though this remains to be confirmed in future studies. In SSD IgM/IgA against gut commensal bacteria were associated with negative symptoms, neurocognitive impairments and the deficit phenotype of SSD (Maes et al., 2019b).
GM in three major psychiatric disorders
Alpha and beta diversity
α-Diversity is the mean species diversity in sites or habitats at a local scale (Whittaker, 1972). α-Diversity is often measured by the Fisher, Ace, Chao, Simpson and/or Shannon indices. β-Diversity shows differentiation among habitats (Whittaker, 1972). Multiple studies analysed diversity metrics; the findings however were mixed.
For MDD studies (Table 2), α-diversity was not different from controls in three studies (Chung et al., 2019; Zheng et al., 2016, 2020). Two studies found lower α-diversity in MDD (Huang et al., 2018; Liu et al., 2016). Four studies found inconsistent findings across indices (Jiang et al., 2015; Kelly et al., 2016; Lai et al., 2021; Rong et al., 2019). β-Diversity was significantly different between MDD and controls in four studies (Huang et al., 2018; Kelly et al., 2016; Lai et al., 2021; Zheng et al., 2016) and not different in three studies (Chung et al., 2019; Jiang et al., 2015; Rong et al., 2019).
Table 2.
Studies of the GM in major depressive disorder
| Study design | Alpha and beta diversity | Significantly more abundant taxa in the MDD group | Significantly more abundant taxa in the control group | Association with clinical features |
|---|---|---|---|---|
|
AStudy group: HC (n = 63); MDD (n = 58) Mean age (s.d.): HC: 41.8 (12.3); MDD: 40.6 (11.7) Sex (F/M): HC: 40/23; MDD: 36/22 HDRS Scores (s.d.): HC: 0.3 (0.7); MDD: 22 (2.4) Experimental method: 16S rRNA gene sequencing from faecal samples |
α: No differences (observed species, Shannon index, phylogenetic diversity and Simpson) β: Different (unweighted and weighted UniFrac analysis) |
Phyla: Actinobacteria | Phyla: Bacteroidetes | |
|
BStudy group: HC (n = 30); MDD (n = 29) Mean age (s.d.): HC: 26.8 (5.4); MDD: 25.3 (5.4) Sex (F/M): HC: 15/15; MDD: 11/18 MADRS scores (s.d.): MDD: 27.4 (8.5) HAMDS scores (s.d.): MDD: 29.8 (7.6) Experimental method: 16S rRNA gene 454 sequencing (Roche) from faecal samples |
α: One index was significantly higher in the MDD group (Shannon index), three other measurements were not different (Ace, Chao 1 and Simpson index) β: Not different (UniFrac analysis) |
Phylum: Fusobacteria, Proteobacteria, Bacteroidetes Family: Acidaminococcaceae, Enterobacteriaceae, Fusobacteriaceae, Porphyromonadaceae, Rikenellaceae Genus: Alistipes, Blautia, Clostridium XIX, Lachnospiraceae incertae sedis, Megamonas, Parabacteroides, Parasutterella, Phascolarctobacterium, Oscillibacter, Roseburia |
Phylum: Actinobacteria, Firmicutes Family: Bacteroidaceae, Erysipelotrichaceae, Lachnospiraceae, Prevotellaceae, Ruminococcaceae, Veillonellaceae Genus: Bacteroides, Dialister, Faecalibacterium, Prevotella, Ruminococcus |
Negative relationships between the relative abundance of Faecalibacterium and depressive symptom severity in MADRS and HAMDS |
|
CStudy group: HC (n = 33); MDD (n = 34) Mean age (s.d.): HC: 45.8 (11.9); MDD: 45.8 (11.5) Sex (F/M): HC: 14/19; MDD: 13/21 Beck Depression (s.d.): MDD: 32.4 (9.92) HAMD 17 median (range): MDD: 19.5 (14) Experimental method: 16S rRNA gene sequencing (Illumina MiSeq platform) from faecal samples |
α: Chao 1, total observed species and phylogenetic diversity were decreased in the MDD group. Shannon index was not significantly different. Richness: β: Different (Bray–Curtis, unweighted and weighted UniFrac) |
Family: Thermoanaerobacteriaceae Genus: Eggerthella, Holdemania, Gelria, Turicibacter, Paraprevotella, Anaefilum |
Family: Prevotellaceae Genus: Prevotella, Dialister |
|
|
DStudy group: HC (n = 37); MDD (n = 36) Mean age (s.d.): HC: 41.19 (12.73); MDD: 45.83 (14.08) Sex (F/M): HC: 23/14; MDD: 28/8 Beck Depression (s.d.): HC: 4.54 (4.85), MDD: 19.18 (12.47) Experimental method: 16S rRNA gene sequencing (Illumina MiSeq platform) from faecal samples |
α: No differences (Shannon index) β: No differences (weighted and unweighted UniFrac) |
Phylum: Firmicutes, Actinobacteria Family: Peptostreptococcaceae, Porphyromonadaceae, Streptococcaceae, Bifidobacteriaceae, Lachnospiraceae Genus: Clostridium XI, Holdemania, Adlercreutzia, Eggerthella, Parabacteroides, Streptococcus, Ruminococcus, Bifidobacterium, Blautia |
Phylum: Proteobacteria, Bacteroidetes Family: Alcaligenaceae, Prevotellaceae Genus: Megamonas, Sutterella, Prevotella |
Families Peptostreptococcaceae and Porphyromonadaceae show positive correlations with BDI scores. Families Prevotellaceae and Alcaligenaceae show negative correlations with BDI scores. At the genus level Blautia, Ruminococcus, Parabacteroides, Eggerthella and Clostridium XI show positive correlations with BDI scores. Prevotella and Sutterella show negative correlations with BDI scores. |
|
EStudy group: HC (n = 27); MDD (n = 27) Mean age (s.d.): HC: 42.3 (14.1); MDD: 48.7 (12.8) Sex (F/M): HC: 20/7; MDD: 20/7 Experimental method: 16S rRNA gene sequencing (Illumina HiSeq2500) from faecal samples |
α: Significantly lower in the MDD group (ACE, Chao 1, Shannon and Faith's phylogenetic diversity) β: Different (weighted and unweighted UniFrac) |
Genus: Oxalobacter, Parvimonas, Bulleidia, Pseudomonas, Peptostreptococcus, Gemella |
Phylum: Firmicutes Family: Lachnospiraceae, Ruminococcaceae, Clostridiaceae, Genus: Blautia, Faecalibacterium, Dorea, Coprococcus |
|
|
FStudy group: HC (n = 29); MDD (n = 26) Mean age (s.d.): HC: 39.41 (10.96); MDD: 43.73 (11.46) Sex (F/M): HC: 16/13; MDD: 18/8 HAMD (s.d.): 19.81 (2.95) Experimental method: Shotgun metagenomics – Illumina HiSeq2500 |
α: Fischer index was significantly lower in the MDD group. Shannon index showed no differences between the groups. β: Different (PCoA plots, Bray–Curtis index) |
Phylum: Actinobacteria Family: Bifidobacteriaceae, Micrococcaceae, Atopobiaceae, Eggerthellaceae, Enterococcaceae, Oscillospiraceae, Peptococcaceae, Acidaminococcaceae, Veillonellaceae Genus: Slackia, Eggerthella, Coriobacterium, Lactobacillaceae, Olsenella, Atopobium, Rothia, Bifidobacterium, Enterococcus, Lactobacillus, Streptococcus, Heliobacterium, Lachnoclostridium, Oscillibacter, Desulfitobacterium, Acidaminococcus, Megaspheara, Sphaerotheata Species: Bifidobacterium adolescentis, Bifidobacterium longum, Bifidobacterium dentium, Bifidobacterium bifidum, Bifidobacterium breve m breve |
Phylum: Bacteroidetes Family: Bacteroidaceae, Cytophagaceae, Flavobacteriaceae, Sphingobacteriaceae Genus: Bacteroides, Sphingobacterium |
|
|
GStudy group: HC (n = 20); MDD (n = 15) Mean age (s.d.): HC: 43.9 (11.2); MDD: 44.8 (14.9) Sex (F/M): HC: 13/7; MDD: 11/4 HAMD (s.d.): 19.81 (2.95) Experimental method: 16S rRNA gene 454 sequencing (Roche) from faecal samples |
α: Significantly lower in MDD group (Shannon index) β: Not reported |
Genus: Prevotella, Bacteroides, Paraprevotella, Dialister, Veillonella, Haemophilus | Genus: Bifidobacterium, Barnesiella, Odoribacter, Butyricimonas, Alistipes, Parabacteroides, Faecalibacterium, Lachnospiracea_incertae_sedis, Blautia, Coprococcus, Ruminococcus, Mituokella, Megamonas, Clostridium XI, Oscillibacter, Clostridium sensu stricto, Clostridium IV, Roseburia, Acetivibrio, Fusobacterium, Escherichia/Shigella, Gemmiger, Comamonas, Sutterella, Vampirovibrio | |
|
HStudy group: HC (n = 171); MDD (n = 122) Mean age (s.d.): HC: 26.85 (5.48); MDD: 26.54 (4.07) Sex (F/M): HC: 100/71; MDD: 77/45 HAMD (s.d.): 22.65 (5.5) Experimental method: 16S rRNA gene sequencing from stool samples |
α: No differences (ACE, Chao, inverse Simpson and Shannon) β: Significantly different with PC2 PLS-DA analysis, but not with PC1 PLS-DA analysis |
Phylum: Bacteroidetes Family: Bacteroidaceae, Bifidobacteriaceae |
Family: Enterobacteriaceae | Peptostreptococcaceae OTU901 was negatively correlated with the HAMD |
|
IStudy group: HC (n = 30); MDD (n = 31) Mean age (s.d.): HC: 39.47 (10.22); MDD: 41.58 (10.40) Sex (F/M): HC: 22/9; MDD: 22/9 HAMD (s.d.): 20.23 (3.11) HCL-32: 6.68 (7.15) Experimental method: Shotgun metagenomics Illumina HiSeq2500 |
α: Shannon index and the inverse Simpson index were not significantly different between groups Gm coefficient and Chao 1 were significantly lower in the MDD group. β: No differences (Bray–Curtis index) |
Phylum: Firmicutes, Actinobacteria Genera: Clostridium, Bifidobacterium, Oscillibacter, Streptococcus, Selenomonas, Megasphaera, Acidaminococcus, Treponema, Lactobacillus, Ethanoligenens, Enterococcus, Cellulosilyticum, Eggerthella, Desulfovibrio, Desulfitobacterium, Sphaerochaeta, Desulfotomaculum, Heliobacterium Species: Eubacterium rectale ATCC 33656, Enterobacteriaceae, Prevotella, Clostridium saccharolyticum WM1, Escherichia coli, Megasphaera elsdenii DSM 20460, Oscillibacter valericigenes Sjm18-20, Bifidobacterium adolescentis ATCC 15703, Eubacterium rectale, Prevotella melaninogenica ATCC 25845, Prevotella intermedia 17, Prevotella denticola F0289, Bifidobacterium, Selenomonas ruminantium subsp. Lactilytica TAM6421, Akkermansia muciniphila ATCC BAA-835, Bifidobacterium longum, Selenomonas sputigena ATCC 35185, Acidaminococcus intestine RyC-MR95, Bifidobacterium dentium_Bd1 |
Phylum: Bacteroidetes Genera: Bacteroides, Odoribacter, Tannerella, Veillonella, Haemophilus, Porphyromonas, Paludibacter Species: Haemophilus parainfluenzae T3T1 |
|
|
JStudy group: HC (n = 47); MDD (n = 43) Mean age (s.d.): HC: 22.1 (1.8); MDD: 21.9 (2.1) Sex (F/M): HC: 34/13; MDD: 38/5 PROMIS Depression Score (s.d.): HC: 9.3 (1.4); MDD: 25 (6.9) Experimental method: 16S rRNA gene sequencing from stool samples |
α: Faith's phylogenetic diversity was significantly lower in the MDD group. Shannon index was significantly different. β: Significantly different (Bray–Curtis index and UniFrac distance) |
Phylum: Bacteroidetes Class: Bacteroidia, Gammaproteobacteria Order: Bacteroidales Family: Enterococcaceae Genera: Flavonifractor, Sellimonas, Enterococcus |
Phylum: Firmicutes Class: Clostridia Order: Clostridiales, Rhodospirillales Family: Ruminococcaceae, Christensenellaceae, Barnesiellaceae Genera: Faecalibacterium, Subdoligranulum, Coprostanoligenes group, Ruminococcus 1, Fusicatenibacter, Tyzzerella 3, Ventrosium group, Barnesiella, Desulfovibrio |
Phylum Firmicutes, class Clostridia, order Clostridiales and phyla Ruminococcaceae, Faecalibacterium and Coprostanoligenes group were more reduced in patients with more severe symptoms. Phylum Bacteroidetes, class Bacteroidia and Gammaproteobacterial, order Bacteroidales and genera Flavinofractor and Sellimonas were more increased in patients with more severe symptoms. |
HC, healthy controls; MDD, major depressive disorder; PCoA, principal coordinates analysis; PLS-DA, partial least-squares discriminant analysis; HDRS, Hamilton Depression Rating Scale; MADRS, Montgomery–Åsberg Depression Rating Scale; HAMDS, Hamilton's Depression Scale; PROMIS Depression Score: Patient-Reported Outcomes Measurement Information System Depression Score; OTU, operational taxonomic unit.
For α-diversity in the BD studies (Table 3), three had inconsistent findings (Hu et al., 2019; Painold et al., 2018; Rong et al., 2019) and one found no differences (Zheng et al., 2020). β-Diversity was different in two studies (Hu et al., 2019; Zheng et al., 2020) and two found no differences (Painold et al., 2018; Rong et al., 2019).
Table 3.
Studies of the GM in individuals with BD
| Study design | Microbial richness/diversity | Significantly more abundant taxa in the BD group | Significantly more abundant taxa in the control group | Association with clinical features |
|---|---|---|---|---|
|
TStudy group: HC (n = 45); BD (n = 52) Mean age (s.d.): HC: 36.29 (12.22); BD: 24.15 (9.5) Sex (F/M): HC: 22/23; BD: 25/27 MADRS (s.d.): 28.15 (8.85) HDRS-17 (s.d.): 30.15 (8.31) YMRS (s.d.): 1.87 (1.43) Experimental method: 16S rRNA gene sequencing from stool samples |
α: Greater α-diversity in HC when measured with Obs, Chao 1 and incidence-based coverage estimators. However, no differences when measured with Shannon, Simpson or inverse Simpson indices. β: Different (PCoA) |
Phylum: Bacteroidetes Class: Flavobacteria, Bacteroidia Order: Oceanospirillales, Flavobacteriales, Bacteroidales Family: Halomonadaceae, Flavobacteriaceae, Porphyromonadaceae, Bacteroidaceae Genera: Weissella, Anaerofustis, Halomonas, Parabacteroides, Bacteroides |
Phylum: Firmicutes Class: Clostridia Order: Clostridiales, Rhizobiales Family: Lachnospiraceae, Hyphomicrobiaceae Genera: Roseburia, Faecalibacterium, Ruminococcus, Gemmiger, Parasutterella, Coprococcus |
MADRS scores were negatively correlated with Acetanaerobacterium, Stenotrophomonas, Anaerotruncus and Raoultella, but positively correlated with Acinetobacter and Cronobacter |
|
UStudy group: HC (n = 171); BD (n = 169) Mean age (s.d.): HC: 26.85 (5.48); BD: 25.59 (8.41) Sex (F/M): HC: 100/71; BD: 84/85 HAMD (s.d.): 26.13 (9.79) YMRS (s.d.): 3.24 (4.43) Experimental method: 16S rRNA gene sequencing from stool samples |
α: Greater α-diversity in HC when measured with Ace and Chao 1. However, no differences when measured with inverse Simpson and Shannon indices β: Different (PLS-DA, PC1 PLS-DA, PC2 PLS-DA) |
Phylum: Proteobacteria, Fusobacteria, Saccharibacteria, Synergistetes Family: Pseudomonadaceae |
Phylum: Bacteroidetes | Not reported |
|
VStudy group: HC (n = 10); BD (n = 32) Mean age (s.d.): HC: 31.4 (7.61); BD: 41.31 (17.73) Sex (F/M): HC: 6/4; MDD: 14/18 HAMD (s.d.): 6.94 (4.37) BDI (s.d.): 16.45 (11.41) Experimental method: 16S rRNA gene sequencing from stool samples |
α: No differences (observed species, Chao 1, Shannon and Simpson indices) β: No differences (weighted PCoA UniFrac and unweighted PCoA UniFrac) |
Phylum: Actinobacteria Class: Coriobacteria Family: Coriobacteriaceae Order: Coriobacteriaceae |
Family: Ruminococcaceae Order: Faecalibacterium |
No significant association of microbial diversity with depression levels was found |
|
WStudy group: HC (n = 30); BD (n = 30) Mean age (s.d.): HC: 39.47 (10.22); BD: 38.4 (8.33) Sex (F/M): HC: 22/9; MDD: 15/15 HAMD (s.d.): 20.37 (3.41) HCL-32: 20.23 (4.58) Experimental method: Shotgun metagenomics Illumina HiSeq2500 |
α: Gm coefficient was significantly lower in the BD group. Chao, Shannon and inverse Simpson indices did not differ. β: No differences (Bray–Curtis) |
Phylum:
Firmicutes, Proteobacteria, Actinobacteria Genera: Escherichia, Clostridium, Bifidobacterium, Oscillibacter, Klebsiella, Streptococcus, Selenomonas, Megasphaera, Acidaminococcus, Veillonella, Treponema, Lactobacillus, Ethanoligenens, Enterococcus, Cellulosilyticum, Eggerthella, Enterobacter, Desulfovibrio, Shigella, Desulfitobacterium, Sphaerochaeta, Salmonella, Desulfotomaculum, Heliobacterium Species: Enterobacteriaceae, Eubacterium rectale ATCC 33656, clostridium saccharolyticum WM1, Escherichia coli, Oscillibacter valericigenes Sjm18-20, Bifidobacterium adolescentis ATCC 15703, Akkermansia muciniphila ATCC BAA-835, Megasphaera elsdenii DSM 20460, Klebsiella, Bifidobacterium, Selenomonas ruminantium subsp. Lactilytica TAM6421, Ethanoligenens harbinense YUAN-3, Acidaminococcus fermentans DSM 20731, Acaidaminococcus intensi RyC MR95, Selenomonas sputigena ATCC 35185 |
Phylum: Bacteroidetes Genera: Bacteroides, Odoribacter, Tannerella, Haemophilus, Porphyromonas, Paludibacter Species: Bacteroides helcogenes p 36–108, Bacteroidetes helcogenes, Bacteroidetes, Haemophilus parainfluenzae T3T1, Klebsiella pneumoniae |
Not reported |
HC, healthy controls; BD, bipolar disorder; PLS-DA, partial least-squares discriminant analysis; PCoA, principal coordinates analysis; HCL-32, Hypomania Check List-32; HAMD, Hamilton's Depression Scale; BDI, Beck Depression Inventory; MADRS, Montgomery–Åsberg Depression Rating Scale; HDRS-17, 17-item Hamilton Depression Rating Scale.
In SSD (Table 4), three studies reported lower α-diversity in the SSD groups compared to healthy controls (Ma et al., 2020; Xu et al., 2020; Zheng et al., 2019), four reported no differences (He et al., 2018; Li et al., 2020; Nguyen et al., 2018; Shen et al., 2018), two did not report on α-diversity (Schwarz et al., 2018; Yuan et al., 2018) and one reported higher α-diversity in the SSD group (Zhu et al., 2020b). β-Diversity was different between groups in five studies (He et al., 2018; Nguyen et al., 2021; Shen et al., 2018; Xu et al., 2020; Zheng et al., 2019), from those five, two studies reported tighter clustering in the healthy control group (Nguyen et al., 2018; Shen et al., 2018).
Table 4.
Studies of the GM in schizophrenia-spectrum disorder
| Study design | Alpha and beta diversity | Significantly more abundant taxa in the SSD group | Significantly more abundant taxa in the control group | Association with clinical features |
|---|---|---|---|---|
|
KStudy group: Schizophrenia patients (n = 63) and healthy controls (n = 69) Mean age (s.d.): HC: 39.99 (1.62); SSD: 43.49 (1.68) Sex (F/M): HC: 33/36; SSD: 21/42 PANSS (s.d.): 71.87 (1.85) Experimental method: 16S rRNA gene sequencing from faecal samples |
α: Shannon and Chao indices were significantly lower in the SSD group β: Different at the OTU level (PLS-DA) |
Families: Veillonellaceae, Prevotellaceae, Bacteroidaceae, Coriobacteriaceae | Families: Lachnospiraceae, Ruminococcaceae, Enterobacteriaceae | Bacteroidaceae OTU172, Streptococcaceae OTU834, Ruminococcaceae OTU181 and two Lachnospiraceae OTU477 and 629 were positively correlated with PANSS. Veillonellaceae OTU191 and Ruminococcaceae OTU725 were negatively correlated with PANSS |
|
LStudy group: Schizophrenia patients (n = 84) and healthy controls (n = 84) Mean age (s.d.) shotgun group: SSD: 35 (11); HC: 34(9) Mean age (s.d.) 16S rRNA group: SSD: 35 (11); HC: 35(11) Sex (M/F) shotgun group: SSD: 20/20; HC: 20/20 Sex (M/F) 16S rRNA group: SSD: 28/16; HC: 21/16 PANSS (s.d.): 71.87 (1.85) Experimental method: shotgun metagenome sequencing or 16S rRNA gene sequencing from faecal samples |
α: Chao 1 was significantly lower in the SSD group β: Different at the species level (NMDS) |
Phylum: Actinobacteria Class: Deltaproteobacteria Order: Actinomycetales, Sphingomonadales Families: Sphingomonadaceae Genus: Eggerthella, Megasphaera Species: Akkermansia muciniphila, Bifidobacterium adolescentis, Clostridium perfingens, Lactobacillus gasseri, Megasphaera elsdenii |
Order: Rhodocyclales Families: Alcaligenaceae, Enterococcaceae, Leuconostocaceae, Rhodocyclaceae, Rikenellaceae Genus: Enterococcus |
Not reported |
|
MStudy group: Schizophrenia patients (n = 25) and healthy controls (n = 25) Mean age (s.d.): HC: 54.7 (10.7); SSD: 52.9 (11.2) Sex (F/M): HC: 15/10; SSD: 14/11 SAPS (s.d.) and SANS (s.d.): 4.56 (3.3) and 4.16(5.1) Experimental method: 16S rRNA gene sequencing from faecal samples |
α: No differences (Shannon index) β: Different (unweighted UniFrac, Bray–Curtis dissimilarity), with tighter clustering in HC (unweighted UniFrac, Bray–Curtis dissimilarity) |
Genera: Anaerococcus |
Phylum: Proteobacteria Genera: Haemophilus, Sutterella, Clostridium |
Bacteroides was correlated with greater severity of depressive symptoms (r = 0.70, p = 0.0002). Decreased abundance of Ruminococcaceae was associated with increased negative symptoms (r = −0.74, p = 0.0002) |
|
NStudy group: Schizophrenia patients (n = 64) and healthy controls (n = 53) Mean age (s.d.): HC: 39 (14); SSD: 42 (11) Sex (F/M): HC: 18/35; SSD: 28/36 Experimental method: 16S rRNA gene sequencing from faecal samples |
α: No differences (Shannon index, Simpson, ACE, Chao 1) β: Different (unweighted UniFrac, PCoA), with tighter clustering in HC (unweighted UniFrac) |
Phyla: Proteobacteria Classes: Gammaproteobacteria Orders: Aeromonadales, Fusobacteriales Families: Veillonellaceae, Prevotellaceae, Enterobacteriaceae, Succinivibrionaceae, Fusobacteriaceae, Lactobacillaceae Genera: Succinivibrio, Megasphaera, Collinsella, Clostridium, Klebsiella, Methanobrevibacter, Prevotella, Lactobacillus, Fusobacterium, Citrobacter, Acidaminococcus, Desulfovibrio, Phascolarctobacterium Species: Collinsella aerofaciens, Bacteroides fragilis, Prevotella stercorea, Lactobacillus mucosae, Bifidobacterium adolescentis |
Phyla: Firmicutes Classes: Clostridia Orders: Clostridiales Families: Lachnospiraceae, Alcaligenaceae Genera: Blautia, Coprococcus, Roseburia, Streptococcus Species: Roseburia faecis, Blautia producta, Collinsella plebeius, Bacteroides eggerthii |
Not reported |
|
OStudy group: Schizophrenia patients (n = 19) and healthy controls (n = 69) Mean age (s.d.): HC: 23.13 (3.89); SSD: 20.47 (4.57) Sex (F/M): HC: 32/37; SSD: 4/15 Positive symptom score (s.d.): 11.47 (6.76) Negative symptom score (s.d.): 10.26 (5.13) Disorganized symptom score (s.d.): 4.89 (4.48) General symptom score (s.d.): 4.37 (3.52) Experimental method: 16S rRNA gene sequencing from faecal samples |
α: No differences (observed OTUs, Shannon index) β: Different at the OTU level (PCoA) |
Orders: Clostridiales, Lactobacillales, Bacteroidales Genera: Lactobacillus, Prevotella Species: Lactobacillus ruminis |
Not reported | Not reported |
|
PStudy group: HC (n = 80); SSD (n = 80) Mean age (s.d.): HC: 41.03 (14.34); SSD: 42.15 (13.13) Sex (F/M): HC: 41/39; SSD: 36/46 PANSS (s.d.): 59.12 (18.18) Experimental method: 16S rRNA gene sequencing from faecal samples |
α: No differences (Faith's phylogenetic diversity, observed species, Shannon index) β: Different (PCoA) |
Phylum: Actinobacteria Genera: Collinsella, Corynebacterium, Lactobacillus, Mogibacterium, Succinivibrio, Undefined Eubacterium, Undefined Ruminococcus |
Phylum: Firmicutes Genera: Adlercreutzia, Faecalibacterium, Ruminococcus |
PANSS scores were positively correlated with the abundance of the genus Succinivibrio. Increased negative symptoms were negatively correlated with the abundance of the genus Corynebacterium |
|
QStudy group: First psychotic episode patients (n = 41) and healthy controls (n = 41) Mean age (s.d.): HC: 24.7 (6.7); FPE: 23.1 (8.0) Sex (F/M): HC: 21/20; FPE: 18/23 PANSS-positive (s.d.): 22.4 (6.3) PANSS-negative (s.d.): 22.3 (6.3) PANSS-general (s.d.): 37.6 (7.4) PANSS-total (s.d.): 82.3 (12.7) Experimental method: 16S rRNA gene sequencing from faecal samples |
Not reported | Species: Clostridium coccoides |
Genera: Bifidobacterium, Lactobacillus Species: Escherichia coli |
Not reported |
|
RStudy group: Antipsychotic-free first episode patients (n = 90) and healthy controls (n = 81) Mean age (s.d.): HC: 32.8 (12.3); FPE: 28.6 (9.54) Sex (F/M): HC: 40/41; FPE: 44/46 Experimental method: shotgun metagenome sequencing |
α: Greater in the SSD group at the genus level (Shannon index) β: Higher at the genus level in the SSD group (Bray–Curtis dissimilarity index) |
Genera: Acidaminococcus, Akkermansia, Anaerotruncus, Bifidobacterium, Citrobacter, Clavibacter, Comamonas, Coprobacillus, Cryptobacterium, Dialister, Enterococcus, Lactobacillus, Methanobrevibacter, Peptoniphilus, Pseudoflavonifractor, Veillonella | Genera: Butyrivibrio, Gemella | Not reported |
|
SStudy group: First psychotic episode patients (n = 28) and healthy controls (n = 16) Mean age (s.d.): HC: 27.8 (6); FPE: 25.9 (5.5) Sex (F/M): HC: 8/8; FPE: 16/12 Experimental method: 16S rRNA gene sequencing from faecal samples |
Not reported |
Families:
Lactobacillaceae, Halothiobacillaceae, Brucellaceae, Micrococcineae Genera: Lactobacillus, Tropheryma, Halothiobacillus, Saccharophagus, Ochrobactrum, Deferribacter, Halorubrum |
Families: Veillonellaceae Genera: Anabaena, Nitrosospira, Gallionella |
Lachnospiraceae (r = 0.38, p = 0.048), Bacteroides spp. (r = 0.38, p = 0.049) and Lactobacillus group (r = 0.48, p = 0.009) had positive correlations witch BPRS total scores. Lactobacillus group correlated positively with positive symptoms (r = 0.47, p = 0.012) Lachnospiraceae (r = 0.49, p = 0.008), Ruminococcaceae (r = 0.47, p = 0.011) and predominant bacteria (r = 0.42, p = 0.027) had positive correlations with negative symptoms |
|
XStudy group: Antipsychotic-free first episode patients (FSCZ, n = 40); chronically antipsychotic-treated patients (TSCZ, n = 85), healthy controls (n = 69) Mean age (s.d.): HC: 23.14 (3.20); FSCZ and TSCZ: 24.19 (6.18) Sex (F/M): HC: 32/37; FSCZ and TSCZ: 57/68 Experimental method: 16S rRNA gene sequencing from faecal samples |
α: Richness (Chao) and diversity (Shannon index) was significantly higher in the HC compared to the TSCZ group. No differences between HC and FSCZ β: Unweighted UniFrac analysis demonstrated compositional differences across groups, weighted UniFrac analysis did not |
FSCZ – HC Family: Christensenellaceae, Enterobacteriaceae, Victivallaceae Genera: Escherichia TSCZ – HC Phyla: Proteobacteria Family: Christensenellaceae, Enterobacteriaceae, Enterococcaceae, Lactobacillaceae Genera: Escherichia, Bulleidia, Coprococcus, Trabulsiella, Enterococcus, Lactobacillus, Shigella, Streptococcus, Veillonella |
FSCZ – HC Family: Pasteurellaceae, Turicibacteraceae, Peptostreptococcaceae, Veillonellaceae, Succinivibrionaceae Genera : Actinobacillus, Fusobacterium, Megasphaera, SMB53 TSCZ – HC Phyla: Cyanobacteria Family: Pasteurellaceae, Turicibacteraceae Genera: Bacteroides, Parabacteroides, Turicibacter |
Not reported |
HC, healthy controls; FPE, first psychotic episode patients; SSD, schizophrenia-spectrum disorder patients; FSCZ, antipsychotic-free first episode patients; TSCZ, chronically antipsychotic-treated patients; OTU, operational taxonomic unit; PLS-DA, partial least-squares discriminant analysis; NMDS, non-metric multidimensional scaling; PCoA, principal coordinates analysis; BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Score.
In the present review more studies reported inconsistent findings (n = 7) or no differences (n = 8) than studies who reported lower α-diversity in the psychiatric disorders (n = 5). These results are in line with other studies (Sanada et al., 2020; Simpson et al., 2021), suggesting that host–microbe interactions are more complex than can be modelled by α-diversity.
Findings at different taxonomic levels
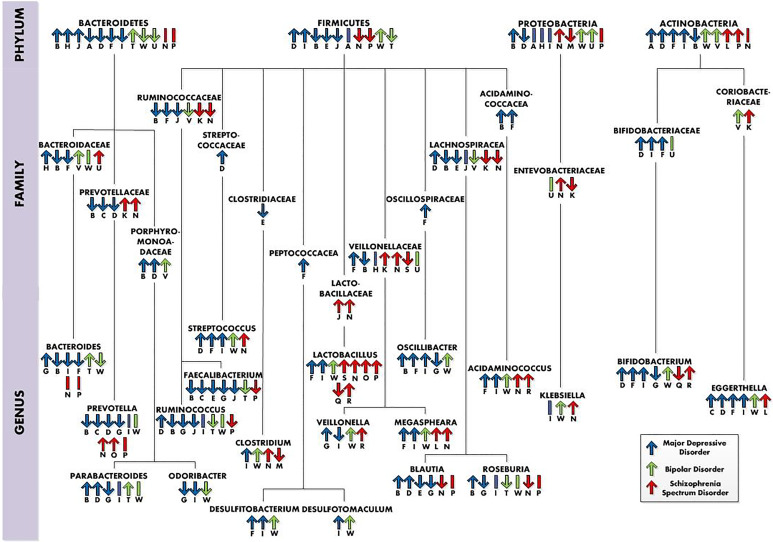
A large number of bacterial taxa were significantly different in their relative abundance between control and psychiatric groups [MDD groups (Table 2), BD groups (Table 3) and SSD groups (Table 4)]. Interestingly, multiple bacterial taxa abundances were similar between psychiatric disorders (Fig. 2). All investigations reported taxonomic differences between the neuropsychiatric disorders and healthy control groups; in the next paragraphs the most important differences, similarities and findings are discussed.
Fig. 2.
Taxonomic differences in neuropsychiatric disorders (at the phylum, family and genus levels), whereby ‘↑’ = higher relative abundance in the neuropsychiatric disorder group, ‘↓’ = lower relative abundance in the neuropsychiatric group and ‘I’ = no differences in abundance. The letters below the arrows refer to the studies the information was retrieved from and also can be connected to the letters in the tables. Studies: AZheng et al. (2016); BJiang et al. (2015); CKelly et al. (2016); DChung et al. (2019); EHuang et al. (2018); FLai et al. (2021); GLiu et al. (2016); HZheng et al. (2020); IRong et al. (2019); JLiu et al. (2020); KZheng et al. (2019); LXu et al. (2020); MNguyen et al. (2018); NShen et al. (2018); OHe et al. (2018); PLi et al. (2020); QRong et al. (2019); RPainold et al. (2018); SSchwarz et al. (2018); THu et al. (2019); UZheng et al. (2020); VPainold et al. (2018); WRong et al. (2019).
Firmicutes
The most abundant phylum in the human gut, Firmicutes, showed multiple inconsistencies at the phylum, family and genus levels (Fig. 2). At the genus level, divergent findings were reported for Ruminococcus, which was higher in one MDD study (Chung et al., 2019), but lower in three other MDD studies (Jiang et al., 2015; Liu et al., 2020, 2016), one BD study (Hu et al., 2019) and one SSD study (Li et al., 2020). In line with these results, inconsistent findings were also reported in relation to clinical features. One study reported that decreased relative abundance of Ruminococcaceae was associated with an increase in negative symptoms (Nguyen et al., 2018). Another study reported Ruminococcaceae OTU725 to be negatively correlated with symptom severity in SSD (Zheng et al., 2019). In contrast, one study found a positive correlation of Ruminococcaceae with negative symptoms (Schwarz et al., 2018). One MDD study found a positive correlation between Ruminococcus and symptom severity scores (Chung et al., 2019). Another MDD study found a negative association of Ruminococcaceae with symptom severity (Liu et al., 2020).
Moreover, at the genus level Faecalibacterium were decreased in five MDD studies (Huang et al., 2018; Jiang et al., 2015; Kelly et al., 2016; Liu et al., 2020, 2016), one BD study (Hu et al., 2019) and one SSD study (Li et al., 2020). In line with these results Jiang et al. (2015) found a negative correlation between Faecalibacterium and depressive symptom severity. Furthermore, Liu et al. (2020) observed reduced Faecalibacterium in MDD patients with more severe symptoms. Moreover, the recently published systematic review and meta-analysis of Nikolova et al. (2021) observed depleted levels of Faecalibacterium in BD, MDD and schizophrenia as well. Faecalibacterium, especially species Faecalibacterium prausnitzii, is usually considered a ‘good’ gut bacterium and is associated with positive healthy outcomes, and its depletion with negative healthy outcomes (Ferreira-Halder, de Faria, & Andrade, 2017; Gacesa et al., 2022). Furthermore, F. prausnitzii was negatively associated with MDD (Gacesa et al., 2022).
The genus Streptococcus was reported to be higher in three MDD studies (Chung et al., 2019; Lai et al., 2021; Rong et al., 2019), one BD study (Rong et al., 2019) and one SSD study (Shen et al., 2018). In line with these results, Zheng et al. (2019) reported a positive correlation between Streptococcaceae OTU834 and symptoms severity. At the family level, the relative abundance of Lactobacillaceae was reported to be higher in two SSD studies (Schwarz et al., 2018; Shen et al., 2018). Consistently, at the genus level the relative abundance of Lactobacillus was significantly higher in two MDD studies (Lai et al., 2021; Rong et al., 2019), in one BD study (Rong et al., 2019) and in four SSD studies (He et al., 2018; Li et al., 2020; Schwarz et al., 2018; Shen et al., 2018). In line with these results, general symptom severity and positive symptom severity were positively correlated with Lactobacillus (Schwarz et al., 2018). These are interesting results since specific strains of Lactobacillus are commonly used in probiotics (Simpson et al., 2021). However, other studies have observed increased Lactobacillus as well in other disorders, like inflammatory bowel disease, indicating specific strains may have inflammatory potential (Wang et al., 2014). Rocha-Ramírez et al. (2017) found that several Lactobacillus species increased proinflammatory cytokines such as interleukin-8 (IL-8), tumour necrosis factor-α, IL-12p70 and IL-6. Moreover, Zhu et al. (2020b) reported increases of subspecies of Lactobacillus not typically present in the healthy gut in schizophrenia patients. It remains to be determined which species of Lactobacillus genus are increased in psychiatric disorders.
Actinobacteria
At the family level in the phylum Actinobacteria, Coriobacteriaceae were relatively more abundant in one BD study (Painold et al., 2018) and one SSD study (Zheng et al., 2019). From Coriobacteriaceae, the abundance of Eggerthella was relatively higher in four MDD studies (Chung et al., 2019; Kelly et al., 2016; Lai et al., 2021; Rong et al., 2019), in one BD study (Rong et al., 2019) and one SSD study (Xu et al., 2020). In line with these results, Eggerthella correlated positively with depression, anxiety and stress scores (Chung et al., 2019). Gacesa et al. (2022) found a positive association of the species Eggerthella lenta with BD. Moreover, Rekdal, Bess, Bisanz, Turnbaugh, and Balskus (2019) suggest that E. lenta is capable of selectively removing the para-hydroxyl group of dopamine, thereby decreasing dopamine levels. This suggestion of Rekdal et al. (2019) could explain the positive correlation found between Eggerthella and depression scores. Moreover, the BD patients in the study of Rong et al. (2019) were at the time of the study in a major depressive episode. Eggerthella could be related to depression in multiple disorders and could therefore be a target for depression. Unfortunately, Eggerthella and E. lenta, previously known as Eubacterium lentum, have been underrecognized due to historical difficulties with phenotypic identification, therefore not much information is available about the bacterial species.
Only a few studies have investigated microbiome difference among subtypes of psychiatric disorders. Hu et al. (2019) compared the GM of BD type 1 to BD type 2 patients with each other. They observed relatively higher relative abundance of the families Streptococcaceae and Erysipelotrichaceae, genera Streptococcus, Bacilli and Veillonella and lower relative abundance of the genus Ruminococcus in the BD type 1 group compared to the BD type 2 group. Zheng et al. (2020) compared unipolar depression to bipolar depression and found Bacteroidaceae and Veilonellaceae to be higher and Enterobacteriaceae and Pseudomonadaceae lower in MDD v. BD. Studying the GM of subtypes of psychiatric disorders has the potential to be of great value in understanding differences between these subtypes.
Inconsistencies across studies may be attributable partly to the heterogeneity in sample characteristics across studies. Most studies used small sample sizes; only one study used a sample size of more than 100 patients (Zheng et al., 2020). In addition, dietary habits may change among sites of study and socio-economic class of the participants. Then, psychiatric medication probably affects the microbiome. Methods used for obtaining the taxonomic profiles were not consistent. Moreover, statistical analysis used to compare GM composition between groups was quite heterogeneous across studies. Microbiota composition differences between groups were analysed by using several statistical methods, namely, analysis of composition of microbiomes, linear discriminant analysis effect size, Wilcoxon rank-sum test, Mann–Whitney U test, Kruskal–Wallis test and Welch's t test.
The most consistent findings across studies were higher relative abundances of the genera Streptococcus, Lactobacillus and Eggerthella and lower relative abundances of the genus Faecalibacterium in the neuropsychiatric disorders (Fig. 2), perhaps most interesting in the relationship between the psychiatric disorders and lower levels of Faecalibacterium. F. prausnitzii has been shown to have anti-inflammatory effects, to produce the SCFA butyrate and has been associated with improving the intestinal barrier by increasing levels of tight junction proteins occludin and E-cadherin (Carlsson et al., 2013; He, Zhao, & Li, 2021; Laval et al., 2015; Martín et al., 2015). Next to that, F. prausnitzii has been associated with smoking, which is in line with the fact that the percentage of smokers is higher in psychiatric disorders (Gacesa et al., 2022; Lê Cook et al., 2014). Multiple of the findings, like higher relative abundances of Actinobacteria and lower abundancy of Prevotella in MDD have been associated unhealthy dietary patterns. Higher relative abundances of Actinobacteria have been associated with high-fat and animal protein diet (Fritsch et al., 2021). Low carbohydrate intake has been associated with reduced Prevotellaceae (Kang et al., 2013). However, the majority of the studies did not control for diet, making it difficult to relate the findings to possible unhealthy diet patterns in the disorders.
Treatments targeting the GM
Understanding how our GM can be targeted could lead to the development of microbiota-based therapies.
Probiotics
Probiotics are defined as live organisms that exert a health benefit when ingested in an adequate amount. Probiotics contain living beneficial bacteria, traditionally from genera Lactobacilli and Bifidobacteria.
In MDD, Kazemi et al. (2019) conducted an 8 week randomized, double-blind, placebo-controlled study, in which 110 depressed patients were randomly assigned to receive probiotic (L. helveticus and B. longum), prebiotic (galactooligosaccharide) or placebo treatment. Probiotic supplementation resulted in a significant decrease in symptom severity compared to both the prebiotic and the placebo groups. Additionally, the serum kynurenine/tryptophan ratio was significantly decreased in the probiotic group compared to the placebo group. Another randomized, double-blind, placebo-controlled 8 weeks probiotics study by Akkasheh et al. (2016) studied a probiotic (Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum) in 40 MDD patients. As a result symptom severity decreased significantly in the probiotic group. In addition, serum insulin levels, insulin resistance and serum high-sensitivity C-reactive protein concentrations were decreased.
In BD, Dickerson et al. (2018) found that the adjunctive probiotic treatment (Lactobacillus rhamnosus strain GG and Bifidobacterium animalis subsp. lactis strain Bb12) for 24 weeks prevented rehospitalization in patients with acute mania (n = 66). Probiotic treatment also resulted in fewer days of hospitalization. Another study (n = 38) found no effects of probiotics compared to placebo on symptom severity of both depression and mania (Shahrbabaki et al., 2020). In another longitudinal cohort study 20 euthymic individuals with BD received a probiotic called ‘OMNi-BiOTiC® Stress Repair’ (B. bifidum W23, B. lactis W51, B. lactis W52, L. acidophilus W22, L. casei W56, Lactobacillus paracasei W20, L. plantarum W62, L. salivarius W24, L. lactis W19) over a time period of 3 months (Reininghaus et al., 2020). Cognition concerning attention and psychomotor processing speed was improved as well as executive functioning.
Three studies investigated the effect of probiotics in SSD patients. One studied the combination three bacteria (L. acidophilus, B. bifidum, L. reuteri and L. fermentum) and vitamin D in a randomized placebo-controlled trial (n = 60) (Ghaderi et al., 2019). The combination was associated with significant improvement in symptom severity and had beneficial effects on metabolic profiles in reducing fasting plasma glucose levels, insulin concentrations, triglycerides and total cholesterol levels. Another study investigated a probiotic (L. acidophilus, B. lactis, B. bifidum and B. longum) and selenium co-supplementation in a randomized placebo-controlled trial with 60 people with chronic schizophrenia (Jamilian & Ghaderi, 2021). The probiotic and selenium co-supplementation resulted in a significant improvement in symptom severity compared to the placebo group. However, in both studies it is uncertain which component of the intervention was responsible for the changes. Only one study studied the effect of a probiotic alone (Dickerson et al., 2014). Their 65 participants were subjected to a double-blind adjunctive probiotic (L. rhamnosus strain GG and B. animalis subsp. lactis strain Bb12) or placebo therapy for 14 weeks. No significant differences were found in symptom severity between probiotic and placebo supplementation.
Prebiotics
Prebiotics are non-digestible fibres that are selectively metabolized by the intestinal microbiome. Prebiotics mostly include fructans and glucans. So far, only one study has studied the effects of prebiotics in SSD, namely, by using the purified prebiotic Bimuno™ galactooligosaccharides (B-GOS®) formulation. Thirty-nine non-hospitalized participants with non-affective psychosis received either B-GOS® or a placebo for 24 weeks. Participants performed the Brief Assessment of Cognition in Schizophrenia (BACS, Keefe et al., 2004) and the B-GOS® group had significantly improved global cognitive performance, compared to the placebo group (Kao et al., 2019). While interesting, this study requires replication in larger studies.
Faecal microbiota transplantation
Faecal transplants from depressed patients to germ-free rats show interesting results. Kelly et al. (2016) showed that FMT from depressed patients to microbiota-depleted rats can induce behavioural and physiological features characteristic of MDD in the receiving animals, including anhedonia and anxiety-like behaviour, as well as an increased plasma kynurenine/tryptophan ratio. In two studies, FMT from schizophrenia patients to germ-free mice led to hyperactivity and reduced anxiety in the open-field test (Zheng et al., 2019; Zhu et al., 2020a). Zhu et al. (2020a) also found that mice that received FMT from schizophrenia patients displayed impaired spatial learning and memory. Remarkable, however, was the heightened amount of social interaction these mice had compared to the control mice. In human, FMT has shown initial positive effects on autism spectrum disorder patients (Kang et al., 2019, 2017).
The most traditional and widely used probiotics (consisting of Bifidobacterium spp. and Lactobacillus spp.) are safe, yet they are not disease-specific. Although different Lactobacillus and Bifidobacterium strains were used in the probiotic studies mentioned above, most of the GM studies in the psychiatric disorders already showed heightened Lactobacillus and Bifidobacterium levels compared to their control groups. Rather, F. prausnitzii could be a promising target for therapeutic purpose in psychiatric patients (Verhoog et al., 2019). Research in rats showed preventive and therapeutic effects of F. prausnitzii on chronic unpredictable mild stress-induced depression-like and anxiety-like behaviour (Hao, Wang, Guo, & Liu, 2019). Supplementation of prebiotics consisting of inulin-oligofructose or inulin-type fructans and fructo-oligosaccharides have been demonstrated to increase F. prausnitzii levels (Dewulf et al., 2013; Hustoft et al., 2017; Ramirez-Fariaz et al., 2009), although this is not a consistent finding (Majid, Cole, Emery, & Whelan, 2014).
Conclusions
The GM revolution has opened new frontiers for examining the relation between the brain and the GM in the context of understanding and treating/preventing psychiatric disorders. This paper provides a detailed overview of current findings regarding alterations of the GM in MDD, BD and SSD patients. All the reviewed studies reported alterations of the GM in the psychiatric disorders. Alterations may partly be caused by medication use, and other lifestyle factors like smoking, diet and alcohol use. Diversity metrics and microbial relative abundance reported to be anomalous across articles varied. The most consistent findings across studies were higher relative abundances of the genera Streptococcus, Lactobacillus and Eggerthella and lower relative abundance of the anti-inflammatory butyrate-producing genus Faecalibacterium in the psychiatric disorders. All three increased genera were reported to be associated with higher symptom severity. The similarities found between the disorders in the relative abundances and the associations of certain genera and symptoms suggest overlap in MDD, BD and SSD. So far, the results of probiotics trials have been highly discrepant, though few have shown promising results. Findings on prebiotics and FMT are too limited to draw definitive conclusions. There is a need of expanding our knowledge on bacterial species in bigger populations with psychiatric disorders, including the influence of medication and dietary habits. Information about differences in specific bacterial strains could lead to the use of disease-specific pro/prebiotics. In the end, study of the GM could lead to a new strategy of treating psychiatric disorders.
Financial support
The research was supported by grants from the Stanley Medical Research Institute (grant number 18T-004) and ZonMW (Netherlands Organisation for Health Research and Development; grant number 636320010).
Conflict of interest
None.
References
- Abouesh, A., Stone, C., & Hobbs, W. R. (2002). Antimicrobial-induced mania (antibiomania): A review of spontaneous reports. Journal of Clinical Pharmacology, 22(1), 71–81. 10.1097/00004714-200202000-00012. [DOI] [PubMed] [Google Scholar]
- Akkasheh, G., Kashani-Poor, Z., Tajabadi-Ebrahimi, M., Jafari, P., Akbari, H., Taghizadeh, M., … Esmaillzadeh, A. (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition, 32(3), 315–320. 10.1016/j.nut.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Mon, M. A., Gómez, A. M., Orozco, A., Lahera, G., Sosa, M. D., Diaz, D., … Alvarez-Mon, M. (2019). Abnormal distribution and function of circulating monocytes and enhanced bacterial translocation in major depressive disorder. Frontiers in Psychiatry, 10(812), 1–12. 10.3389/fpsyt.2019.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anttila, V., Bulik-Sullivan, B., Finucane, H. K., Walters, R. K., Bras, J., Duncan, L., … Neale, B. M. (2018). Analysis of shared heritability in common disorders of the brain. Science, 360(6395), 1–40. 10.1126/science.aap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., … Jun, W. (2015). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host and Microbe, 17(5), 690–703. 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Barber, G. S., Sturgeon, C., Fasano, A., Gascella, N. G., Eaton, W. W., McMahon, R. P., & Kelly, D. L. (2019). Elevated zonulin, a measure of tight-junction permeability, may be implicated in schizophrenia. Schizophrenia Research, 211, 111–112. 10.1016/j.schres.2019.07.006. [DOI] [PubMed] [Google Scholar]
- Bastiaanssen, T. F. S., Cussotto, S., Claesson, M. J., Clarke, G., Dinan, T. G., & Cryan, J. F. (2020). Gutted! Unraveling the role of the microbiome in major depressive disorder. Harvard Review of Psychiatry, 28(1), 26–39. 10.1097/HRP.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver, M. H., & Wostmann, B. S. (1962). Histamine and 5-hydroxytryptamine in the intestinal tract of germ-free animals, animals harbouring one microbial species and conventional animals. British Journal of Pharmacology and Chemotherapy, 19(3), 385–393. 10.1111/j.1476-5381.1962.tb01443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett, E. J., Tennant, C. C., Piesse, C., Badcock, C. A., & Kellow, J. E. (1998). Level of chronic life stress predicts clinical outcome in irritable bowel syndrome. Gut, 43(2), 256–261. 10.1136/gut.43.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff, S. C., Barbara, G., Buurman, W., Ockhuizen, T., Schulzke, J.-D., Serino, M., … Wells, J. M. (2014). Intestinal permeability – A new target for disease prevention and therapy. BMC Gastroenterology, 14(1), 189. 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning, J. S., & Houseworth, J. H. (1953). Development of new symptoms following medical and surgical treatment for duodenal ulcer. Psychosomatic Medicine, 15(4), 328–336. 10.1097/00006842-195307000-00006. [DOI] [PubMed] [Google Scholar]
- Cannon, M., Jones, P. B., & Murray, R. M. (2002). Obstetric complications and schizophrenia: Historical and meta-analytic review. American Journal of Psychiatry, 159(7), 1080–1092. 10.1176/appi.ajp.159.7.1080. [DOI] [PubMed] [Google Scholar]
- Carlessi, A. S., Borba, L. A., Zugno, A. I., Quevedo, J., & Réus, G. Z. (2021). Gut microbiota–brain axis in depression: The role of neuroinflammation. European Journal of Neuroscience, 53, 222–235. 10.1111/ejn.14631. [DOI] [PubMed] [Google Scholar]
- Carlsson, A. H., Yakymenko, O., Olivier, I., Håkansson, F., Postma, E., Keita, Å. V., & Söderholm, J. D. (2013). Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scandinavian Journal of Gastroenterology, 48(10), 1136–1144. 10.3109/00365521.2013.828773. [DOI] [PubMed] [Google Scholar]
- Chudal, R., Sourander, A., Polo-Kantola, P., Hinkka-Yli-Salomäki, S., Lehti, V., Sucksdorff, D., … Brown, A. S. (2014). Perinatal factors and the risk of bipolar disorder in Finland. Journal of Affective Disorders, 155(1), 75–80. 10.1016/j.jad.2013.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, Y. C. E., Chen, H. C., Chou, H. C. L., Chen, I. M., Lee, M. S., Chuang, L. C., … Kuo, P. H. (2019). Exploration of microbiota targets for major depressive disorder and mood related traits. Journal of Psychiatric Research, 111(17), 74–82. 10.1016/j.jpsychires.2019.01.016. [DOI] [PubMed] [Google Scholar]
- Cryan, J. F., O'riordan, K. J., Cowan, C. S. M., Sandhu, K. V., Bastiaanssen, T. F. S., Boehme, M., … Dinan, T. G. (2019). The microbiota–gut–brain axis. Physiological Reviews, 99(4), 1877–2013. 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- Desbonnet, L., Garrett, L., Clarke, G., Bienenstock, J., & Dinan, T. G. (2009). The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. Journal of Psychiatric Research, 43(2), 164–174. 10.1016/j.jpsychires.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Dewulf, E. M., Cani, P. D., Claus, S. P., Fuentes, S., Puylaert, P. G. B., Neyrinck, A. M., … Delzenne, N. M. (2013). Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut, 62(8), 1112–1121. 10.1136/gutjnl-2012-303304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, F., Adamos, M., Katsafanas, E., Khushalani, S., Origoni, A., Savage, C., … Yolken, R. H. (2018). Adjunctive probiotic microorganisms to prevent rehospitalization in patients with acute mania: A randomized controlled trial. Bipolar Disorders, 20(7), 614–621. 10.1111/bdi.12652. [DOI] [PubMed] [Google Scholar]
- Dickerson, F., Severance, E., & Yolken, R. (2017). The microbiome, immunity, and schizophrenia and bipolar disorder. Brain, Behavior, and Immunity, 62, 46–52. 10.1016/j.bbi.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson, F. B., Stallings, C., Origoni, A., Katsafanas, E., Savage, C. L. G., Schweinfurth, L. A. B., … Yolken, R. H. (2014). Effect of probiotic supplementation on schizophrenia symptoms and association with gastrointestinal functioning. The Primary Care Companion for CNS Disorders, 16(1). 10.4088/PCC.13m01579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzikowski, M., Juchnowicz, D., Dzikowska, I., Rog, J., Próchnicki, M., Kozioł, M., … Karakula-Juchnowicz, H. (2020). The differences between gluten sensitivity, intestinal biomarkers and immune biomarkers in patients with first-episode and chronic schizophrenia. Journal of Clinical Medicine, 9(11), 1–13. 10.3390/jcm9113707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary, A. K., Bacha, A. B., & Kotb, M. (2012). Etiology of autistic features: The persisting neurotoxic effects of propionic acid. Journal of Neuroinflammation, 9, 1–14. 10.1186/1742-2094-9-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faresjö, Å., Grodzinsky, E., Johansson, S., Wallander, M. A., Timpka, T., & Åkerlind, I. (2007). Psychosocial factors at work and in every day life are associated with irritable bowel syndrome. European Journal of Epidemiology, 22(7), 473–480. 10.1007/s10654-007-9133-2. [DOI] [PubMed] [Google Scholar]
- Ferreira-Halder, C. V., de Faria, A. V. S., & Andrade, S. S. (2017). Action and function of Faecalibacterium prausnitzii in health and disease. Best Practice and Research: Clinical Gastroenterology, 31(6), 643–648. 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- Foster, J. A., Baker, G. B., & Dursun, S. M. (2021). The relationship between the gut microbiome–immune system–brain axis and major depressive disorder. Frontiers in Neurology, 12, 1–9. 10.3389/fneur.2021.721126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch, J., Garces, L., Quintero, M. A., Pignac-Kobinger, J., Santander, A. M., Fernández, I., … Abreu, M. T. (2021). Low-fat, high-fiber diet reduces markers of inflammation and dysbiosis and improves quality of life in patients with ulcerative colitis. Clinical Gastroenterology and Hepatology, 19(6), 1189–1199.e30. 10.1016/j.cgh.2020.05.026. [DOI] [PubMed] [Google Scholar]
- Gacesa, R., Kurilshikov, A., Vich Vila, A., Sinha, T., Klaassen, M. A. Y., Bolte, L. A., … Weersma, R. K. (2022). Environmental factors shaping the gut microbiome in a Dutch population. Nature, 604, 733–739. 10.1101/2020.11.27.401125. [DOI] [PubMed] [Google Scholar]
- Genedi, M., Janmaat, I. E., Haarman, B. C. M., & Sommer, I. E. C. (2019). Dysregulation of the gut–brain axis in schizophrenia and bipolar disorder: Probiotic supplementation as a supportive treatment in psychiatric disorders. Current Opinion in Psychiatry, 32(3), 185–195. 10.1097/YCO.0000000000000499. [DOI] [PubMed] [Google Scholar]
- Ghaderi, A., Banafshe, H. R., Mirhosseini, N., Moradi, M., Karimi, M. A., Mehrzad, F., … Asemi, Z. (2019). Clinical and metabolic response to vitamin D plus probiotic in schizophrenia patients. BMC Psychiatry, 19(1), 1–10. 10.1186/s12888-019-2059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golofast, B., & Vales, K. (2020). The connection between microbiome and schizophrenia. Neuroscience and Biobehavioral Reviews, 108(August 2019), 712–731. 10.1016/j.neubiorev.2019.12.011. [DOI] [PubMed] [Google Scholar]
- Haarman, B. C. (Benno), Riemersma-Van der Lek, R. F., Burger, H., Drexhage, H. A., & Nolen, W. A. (2016). The dysregulated brain: Consequences of spatial and temporal brain complexity for bipolar disorder pathophysiology and diagnosis. Bipolar Disorders, 18(8), 696–701. 10.1111/bdi.12454. [DOI] [PubMed] [Google Scholar]
- Hao, Z., Wang, W., Guo, R., & Liu, H. (2019). Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology, 104, 132–142. 10.1016/j.psyneuen.2019.02.025. [DOI] [PubMed] [Google Scholar]
- He, X., Zhao, S., & Li, Y. (2021). Faecalibacterium prausnitzii: A next-generation probiotic in gut disease improvement. Canadian Journal of Infectious Diseases and Medical Microbiology, 2021, 1–10. 10.1155/2021/6666114. [DOI] [Google Scholar]
- He, Y., Kosciolek, T., Tang, J., Zhou, Y., Li, Z., Ma, X., … Chen, X. (2018). Gut microbiome and magnetic resonance spectroscopy study of subjects at ultra-high risk for psychosis may support the membrane hypothesis. European Psychiatry, 53(2018), 37–45. 10.1016/j.eurpsy.2018.05.011. [DOI] [PubMed] [Google Scholar]
- Hu, S., Li, A., Huang, T., Lai, J., Li, J., Sublette, M. E., … Xu, Y. (2019). Gut microbiota changes in patients with bipolar depression. Advanced Science, 6(14), 1–11. 10.1002/advs.201900752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y., Shi, X., Li, Z., Shen, Y., Shi, X., Wang, L., … Liang, Y. (2018). Possible association of firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatric Disease and Treatment, 14, 3329–3337. 10.2147/NDT.S188340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustoft, T. N., Hausken, T., Ystad, S. O., Valeur, J., Brokstad, K., Hatlebakk, J. G., & Lied, G. A. (2017). Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microenvironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterology and Motility, 29(4), 1–9. 10.1111/nmo.12969. [DOI] [PubMed] [Google Scholar]
- James, S. L., Abate, D., Abate, K. H., Abay, S. M., Abbafati, C., Abbasi, N., … Murray, C. J. L. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. The Lancet, 392(10159), 1789–1858. 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamilian, H., & Ghaderi, A. (2021). The effects of probiotic and selenium co-supplementation on clinical and metabolic scales in chronic schizophrenia: A randomized, double-blind, placebo-controlled trial. Biological Trace Element Research, 199(12), 4430–4438. 10.1007/s12011-020-02572-3. [DOI] [PubMed] [Google Scholar]
- Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., … Ruan, B. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Kang, D. W., Adams, J. B., Coleman, D. M., Pollard, E. L., Maldonado, J., McDonough-Means, S., … Krajmalnik-Brown, R. (2019). Long-term benefit of microbiota transfer therapy on autism symptoms and gut microbiota. Scientific Reports, 9(1), 1–9. 10.1038/s41598-019-42183-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., … Krajmalnik-Brown, R. (2017). Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome, 5(1), 1–16. 10.1186/s40168-016-0225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, D. W., Park, J. G., Ilhan, Z. E., Wallstrom, G., LaBaer, J., Adams, J. B., … Krajmalnik-Brown, R. (2013). Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE, 8(7), 1–13. 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao, A. C. C., Safarikova, J., Marquardt, T., Mullins, B., Lennox, B. R., & Burnet, P. W. J. (2019). Pro-cognitive effect of a prebiotic in psychosis: A double blind placebo controlled cross-over study. Schizophrenia Research, 208, 460–461. 10.1016/j.schres.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Kazemi, A., Noorbala, A. A., Azam, K., Eskandari, M. H., & Djafarian, K. (2019). Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clinical Nutrition, 38(2), 522–528. 10.1016/j.clnu.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Keefe, R. S. E., Goldberg, T. E., Harvey, P. D., Gold, J. M., Poe, M. P., & Coughenour, L. (2004). The brief assessment of cognition in schizophrenia: Reliability, sensitivity, and comparison with a standard neurocognitive battery. Schizophrenia Research, 68, 283–297. 10.1016/j.schres.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Keita, Å. V., Söderholm, J. D., & Ericson, A. C. (2010). Stress-induced barrier disruption of rat follicle-associated epithelium involves corticotropin-releasing hormone, acetylcholine, substance P, and mast cells. Neurogastroenterology and Motility, 22(7), 770–778. 10.1111/j.1365-2982.2010.01471.x. [DOI] [PubMed] [Google Scholar]
- Kelly, J. R., Borre, Y., O’ Brien, C., Patterson, E., El Aidy, S., Deane, J., … Dinan, T. G. (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research, 82, 109–118. 10.1016/j.jpsychires.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser, J. K., Wilson, S. J., Bailey, M. L., Andridge, R., Peng, J., Jaremka, L. M., … Belury, M. A. (2018). Marital distress, depression, and a leaky gut: Translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology, 98, 52–60. 10.1016/j.psyneuen.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiliaan, A. J., Saunders, P. R., Bijlsma, P. B., Cecilia Berin, M., Taminiau, J. A., Groot, J. A., & Perdue, M. H. (1998). Stress stimulates transepithelial macromolecular uptake in rat jejunum. American Journal of Physiology – Gastrointestinal and Liver Physiology, 275(5), 1037–1044. 10.1152/ajpgi.1998.275.5.g1037. [DOI] [PubMed] [Google Scholar]
- Kitchens, R. L., & Thompson, P. A. (2005). Modulatory effects of sCD14 and LBP on LPS–host cell interactions. Journal of Endotoxin Research, 11(4), 225–229. 10.1179/096805105X46565. [DOI] [PubMed] [Google Scholar]
- Kılıç, F., Işık, Ü., Demirdaş, A., Doğuç, D. K., & Bozkurt, M. (2020). Serum zonulin and claudin-5 levels in patients with bipolar disorder. Journal of Affective Disorders, 266, 37–42. 10.1016/j.jad.2020.01.117. [DOI] [PubMed] [Google Scholar]
- Köhler, O., Petersen, L., Mors, O., Mortensen, P. B., Yolken, R. H., Gasse, C., & Benros, M. E. (2017). Infections and exposure to anti-infective agents and the risk of severe mental disorders: A nationwide study. Acta Psychiatrica Scandinavica, 135(2), 97–105. 10.1111/acps.12671. [DOI] [PubMed] [Google Scholar]
- Korpela, K., Helve, O., Kolho, K. L., Saisto, T., Skogberg, K., Dikareva, E., … de Vos, W. M. (2020). Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: A proof-of-concept study. Cell, 183(2), 324–334. 10.1016/j.cell.2020.08.047. [DOI] [PubMed] [Google Scholar]
- Lai, J., Gao, X., & Zhang, D. (2020). Gut microbial clues to bipolar disorder: State-of-the-art review of current findings and future directions. Clinical and Translational Medicine, 10, 1–14. 10.1002/ctm2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, W. T., Deng, W. F., Xu, S. X., Zhao, J., Xu, D., Liu, Y. H., … Rong, H. (2021). Shotgun metagenomics reveals both taxonomic and tryptophan pathway differences of gut microbiota in major depressive disorder patients. Psychological Medicine, 51(1), 90–101. 10.1017/S0033291719003027. [DOI] [PubMed] [Google Scholar]
- Lambrichts, S., Van Oudenhove, L., & Sienaert, P. (2017). Antibiotics and mania: A systematic review. Journal of Affective Disorders, 219, 149–156. 10.1016/j.jad.2017.05.029. [DOI] [PubMed] [Google Scholar]
- Laval, L., Martin, R., Natividad, J. N., Chain F, F. C., Miquel, S., Desclée de Maredsous, C., … Langella, P. (2015). Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice. Gut Microbes, 6(1), 1–9. 10.4161/19490976.2014.990784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê Cook, B., Wayne, G. F., Kafali, E. N., Liu, Z., Shu, C., & Flores, M. (2014). Trends in smoking among adults with mental illness and association between mental health treatment and smoking cessation. JAMA – Journal of the American Medical Association, 311(2), 172–182. 10.1001/jama.2013.284985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, S., Zhuo, M., Huang, X., Huang, Y., Zhou, J., Xiong, D., … Wu, K. (2020). Altered gut microbiota associated with symptom severity in schizophrenia. PeerJ, 8, 1–22. 10.7717/peerj.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, X., Ye, J., Wen, Y., Li, P., Cheng, B., Cheng, S., … Zhang, F. (2021). Long-term antibiotic use during early life and risks to mental traits: An observational study and gene–environment-wide interaction study in UK Biobank cohort. Neuropsychopharmacology, 46(6), 1086–1092. 10.1038/s41386-020-00798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, P. S., Chang, Y. K., & Wu, T. K. (2019). Serum lipopolysaccharide-binding protein is associated with chronic inflammation and metabolic syndrome in hemodialysis patients. Blood Purification, 47, 28–36. 10.1159/000492778. [DOI] [PubMed] [Google Scholar]
- Liu, R. T., Rowan-Nash, A. D., Sheehan, A. E., Walsh, R. F. L., Sanzari, C. M., Korry, B. J., & Belenky, P. (2020). Reductions in anti-inflammatory gut bacteria are associated with depression in a sample of young adults. Brain, Behavior, and Immunity, 88, 308–324. 10.1016/j.bbi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Y., Zhang, L., Wang, X., Wang, Z., Zhang, J., Jiang, R., … Duan, L. (2016). Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clinical Gastroenterology and Hepatology, 14(11), 1602–1611. 10.1016/j.cgh.2016.05.033. [DOI] [PubMed] [Google Scholar]
- Lurie, I., Yang, Y.-X., Hayne, K., Mamtani, R., & Boursi, B. (2015). Antibiotic exposure and the risk for depression, anxiety, or psychosis: A neste case-control study. The Journal of Clinical Psychiatry, 76(11), 1522–1528. 10.4088/JCP.15m09961. [DOI] [PubMed] [Google Scholar]
- Ma, X., Asif, H., Dai, L., He, Y., Zheng, W., Wang, D., … Chen, X. (2020). Alteration of the gut microbiome in first-episode drug-naïve and chronic medicated schizophrenia correlate with regional brain volumes. Journal of Psychiatric Research, 123, 136–144. 10.1016/j.jpsychires.2020.02.005. [DOI] [PubMed] [Google Scholar]
- Maes, M., Kanchanatawan, B., Sirivichayakul, S., & Carvalho, A. F. (2019a). In schizophrenia, increased plasma IgM/IgA responses to gut commensal bacteria are associated with negative symptoms, neurocognitive impairments, and the deficit phenotype. Neurotoxicity Research, 35(3), 684–698. 10.1007/s12640-018-9987-y. [DOI] [PubMed] [Google Scholar]
- Maes, M., Kubera, M., Leunis, J. C., Berk, M., Geffard, M., & Bosmans, E. (2013). In depression, bacterial translocation may drive inflammatory responses, oxidative and nitrosative stress (O&NS), and autoimmune responses directed against O&NS-damaged neoepitopes. Acta Psychiatrica Scandinavica, 127(5), 344–354. 10.1111/j.1600-0447.2012.01908.x. [DOI] [PubMed] [Google Scholar]
- Maes, M., Sirivichayakul, S., Kanchanatawan, B., & Vodjani, A. (2019b). Upregulation of the intestinal paracellular pathway with breakdown of tight and adherens junctions in deficit schizophrenia. Molecular Neurobiology, 56(10), 7056–7073. 10.1007/s12035-019-1578-2. [DOI] [PubMed] [Google Scholar]
- Majid, H. A., Cole, J., Emery, P. W., & Whelan, K. (2014). Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: A randomised controlled trial. Clinical Nutrition, 33(6), 966–972. 10.1016/j.clnu.2013.11.008. [DOI] [PubMed] [Google Scholar]
- Martín, R., Miquel, S., Chain, F., Natividad, J. M., Jury, J., Lu, J., … Bermúdez-Humarán, L. G. (2015). Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiology, 15(1), 1–12. 10.1186/s12866-015-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx, W., McGuinness, A. J., Rocks, T., Ruusunen, A., Cleminson, J., Walker, A. J., … Fernandes, B. S. (2020). The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: A meta-analysis of 101 studies. Molecular Psychiatry, 26(8), 4158–4178. 10.1038/s41380-020-00951-9. [DOI] [PubMed] [Google Scholar]
- Massier, L., Chakaroun, R., Kovacs, P., & Heiker, J. T. (2021). Blurring the picture in leaky gut research: How shortcomings of zonulin as a biomarker mislead the field of intestinal permeability. Gut, 70(9), 1801–1802. 10.1136/gutjnl-2020-323026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell, C. M., Mazzoni, C., Hogstrom, L., Bryant, A., Bergerat, A., Cher, A., … Yassour, M. (2020). Delivery mode affects stability of early infant gut microbiota. Cell Reports Medicine, 1(9), 1–9. 10.1016/j.xcrm.2020.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morch, R. H., Dieset, I., Færden, A., Reponen, E. J., Hope, S., Hoseth, E. Z., … Andreassen, O. A. (2019). Inflammatory markers are altered in severe mental disorders independent of comorbid cardiometabolic disease risk factors. Psychological Medicine, 49(10), 1749–4757. 10.1017/S0033291719000291. [DOI] [PubMed] [Google Scholar]
- Moretti, M., Valvassori, S. S., Varela, R. B., Ferreira, C. L., Rochi, N., Benedet, J., … Quevedo, J. (2011). Behavioral and neurochemical effects of sodium butyrate in an animal model of mania. Behavioural Pharmacology, 22(8), 766–772. 10.1097/FBP.0b013e32834d0f1b. [DOI] [PubMed] [Google Scholar]
- Nankova, B. B., Agarwal, R., MacFabe, D. F., & La Gamma, E. F. (2014). Enteric bacterial metabolites propionic and butyric acid modulate gene expression, including CREB-dependent catecholaminergic neurotransmission, in PC12 cells – Possible relevance to autism spectrum disorders. PLoS ONE, 9(8), 1–16. 10.1371/journal.pone.0103740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T., Hathaway, H., Kosciolek, T., Knight, R., & Jeste, D. V. (2021). Gut microbiome in serious mental illnesses: A systematic review and critical evaluation. Schizophrenia Research, 234, 24–40. 10.1016/j.schres.2019.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. T., Kosciolek, T., Maldonado, Y., Daly, R. E., Martin, A. S., McDonald, D., … Jeste, D. V. (2018). Differences in gut microbiome composition between persons with chronic schizophrenia and healthy comparison subjects. Schizophrenia Research, 204, 23–29. 10.1016/j.schres.2018.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholl, B. I., Halder, S. L., Macfarlane, G. J., Thompson, D. G., O'Brien, S., Musleh, M., & McBeth, J. (2008). Psychosocial risk markers for new onset irritable bowel syndrome – Results of a large prospective population-based study. Pain, 137(1), 147–155. 10.1016/j.pain.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova, V. L., Hall, M. R. B., Hall, L. J., Cleare, A. J., Stone, J. M., & Young, A. H. (2021). Perturbations in gut microbiota composition in psychiatric disorders: A review and meta-analysis. JAMA Psychiatry, 78(12), 1343–1354. 10.1001/jamapsychiatry.2021.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson, L., Gustafsson, A., Lavant, E., Suneson, K., Brundin, L., Westrin, … Lindqvist, D. (2019). Leaky gut biomarkers in depression and suicidal behavior. Acta Psychiatrica Scandinavica, 139(2), 185–193. 10.1111/acps.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill, S. M., Curran, E. A., Dalman, C., Kenny, L. C., Kearney, P. M., Clarke, G., … Khashan, A. S. (2016). Birth by caesarean section and the risk of adult psychosis: A population-based cohort study. Schizophrenia Bulletin, 42(3), 633–641. 10.1093/schbul/sbv152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painold, A., Mörkl, S., Kashofer, K., Halwachs, B., Dalkner, N., Bengesser, S., … Reininghaus, E. Z. (2018). A step ahead: Exploring the gut microbiota in inpatients with bipolar disorder during a depressive episode. Bipolar Disorders, 21, 40–49. 10.1111/bdi.12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purton, T., Staskova, L., Lane, M. M., Dawson, S. L., West, M., Firth, J., … Marx, W. (2021). Prebiotic and probiotic supplementation and the tryptophan–kynurenine pathway: A systematic review and meta analysis. Neuroscience and Biobehavioral Reviews, 123, 1–13. 10.1016/j.neubiorev.2020.12.026. [DOI] [PubMed] [Google Scholar]
- Ramirez-Fariaz, C., Slezak, K., Fuller, Z., Duncan, A., Holtrop, G., & Louis, P. (2009). Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. British Journal of Nutrition, 101(4), 533–540. 10.1017/S0007114508019880. [DOI] [PubMed] [Google Scholar]
- Reininghaus, E. Z., Wetzlmair, L. C., Fellendorf, F. T., Platzer, M., Queissner, R., Birner, A., … Dalkner, N. (2020). The impact of probiotic supplements on cognitive parameters in euthymic individuals with bipolar disorder: A pilot study. Neuropsychobiology, 79(1), 63–70. 10.1159/000492537. [DOI] [PubMed] [Google Scholar]
- Rekdal, V. M., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J., & Balskus, E. P. (2019). Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science, 364(6445), 1–8. 10.1126/science.aau6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resende, W. R., Valvassori, S. S., Réus, G. Z., Varela, R. B., Arent, C. O., Ribeiro, K. F., … Quevedoa, J. (2013). Effects of sodium butyrate in animal models of mania and depression: Implications as a new mood stabilizer. Behavioural Pharmacology, 24(7), 569–579. 10.1097/FBP.0b013e32836546fc. [DOI] [PubMed] [Google Scholar]
- Rocha-Ramírez, L. M., Pérez-Solano, R. A., Castañón-Alonso, S. L., Moreno Guerrero, S. S., Ramírez Pacheco, A., García Garibay, M., … Eslava, C. (2017). Probiotic lactobacillus strains stimulate the inflammatory response and activate human macrophages. Journal of Immunology Research, 2017, 1–14. 10.1155/2017/4607491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong, H., Xie, X. H., Zhao, J., Lai, W. T., Wang, M. B., Xu, D., … Liu, T. B. (2019). Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. Journal of Psychiatric Research, 113, 90–99. 10.1016/j.jpsychires.2019.03.017. [DOI] [PubMed] [Google Scholar]
- Sanada, K., Nakajima, S., Kurokawa, S., Barceló-Soler, A., Ikuse, D., Hirata, A., … Kishimoto, T. (2020). Gut microbiota and major depressive disorder: A systematic review and meta-analysis. Journal of Affective Disorders, 266, 1–13. 10.1016/j.jad.2020.01.102. [DOI] [PubMed] [Google Scholar]
- Schwarz, E., Maukonen, J., Hyytiäinen, T., Kieseppä, T., Orešič, M., Sabunciyan, S., … Suvisaari, J. (2018). Analysis of microbiota in first episode psychosis identifies preliminary associations with symptom severity and treatment response. Schizophrenia Research, 192, 398–403. 10.1016/j.schres.2017.04.017. [DOI] [PubMed] [Google Scholar]
- Severance, E. G., Dickerson, F., & Yolken, R. H. (2020). Complex gastrointestinal and endocrine sources of inflammation in schizophrenia. Frontiers in Psychiatry, 11, 1–9. 10.3389/fpsyt.2020.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severance, E. G., Gressitt, K. L., Stallings, C. R., Origoni, A. E., Khushalani, S., Leweke, F. M., … Yolken, R. H. (2013). Discordant patterns of bacterial translocation markers and implications for innate immune imbalances in schizophrenia. Schizophrenia Research, 148(1–3), 130–137. 10.1016/j.schres.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgritta, M., Dooling, S. W., Buffington, S. A., Momin, E. N., Francis, M. B., Britton, R. A., & Costa-Mattioli, M. (2019). Mechanisms underlying microbial-bediated changes in social behavior in mouse models of autism spectrum disorder. Neuron, 101(2), 246–259. 10.1016/j.neuron.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrbabaki, M. E., Sabouri, S., Sabahi, A., Barfeh, D., Divsalar, P., Esmailzadeh, M., & Ahmadi, A. (2020). The efficacy of probiotics for treatment of bipolar disorder-type 1: A randomized, double-blind, placebo controlled trial. Iranian Journal of Psychiatry, 15(1), 10–16. 10.18502/ijps.v15i1.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Y., Xu, J., Li, Z., Huang, Y., Yuan, Y., Wang, J., … Liang, Y. (2018). Analysis of gut microbiota diversity and auxiliary diagnosis as a biomarker in patients with schizophrenia: A cross-sectional study. Schizophrenia Research, 197, 470–477. 10.1016/j.schres.2018.01.002. [DOI] [PubMed] [Google Scholar]
- Sherwin, E., Sandhu, K. V., Dinan, T. G., & Cryan, J. F. (2016). May the force be with you: The light and dark sides of the microbiota–gut–brain axis in neuropsychiatry. CNS Drugs, 30(11), 1019–1041. 10.1007/s40263-016-0370-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeonova, D., Stoyanov, D., Leunis, J. -C, Carvalho, A. F., Kubera, M., Murdjeva, M., & Maes, M. (2020). Increased serum immunoglobulin responses to gut commensal Gram-negative bacteria in unipolar major depression and bipolar disorder type 1, especially when melancholia is present. Neurotoxicity Research, 37(2), 338–348. 10.1007/s12640-019-00126-7. [DOI] [PubMed] [Google Scholar]
- Simpson, C. A., Diaz-Arteche, C., Eliby, D., Schwartz, O. S., Simmons, J. G., & Cowan, C. S. M. (2021). The gut microbiota in anxiety and depression – A systematic review. Clinical Psychology Review, 83(101943), 1–18. 10.1016/j.cpr.2020.101943. [DOI] [PubMed] [Google Scholar]
- Skonieczna-żydecka, K., Grochans, E., Maciejewska, D., Szkup, M., Schneider-Matyka, D., Jurczak, A., … Stachowska, E. (2018). Faecal short chain fatty acids profile is changed in Polish depressive women. Nutrients, 10(12), 1–14. 10.3390/nu10121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Söderholm, J. D., Yang, P. C., Ceponis, P., Vohra, A., Riddell, R., Sherman, P. M., & Perdue, M. H. (2002). Chronic stress induces mast cell-dependent bacterial adherence and initiates mucosal inflammation in rat intestine. Gastroenterology, 123(4), 1099–1108. 10.1053/gast.2002.36019. [DOI] [PubMed] [Google Scholar]
- Stevens, B. R., Goel, R., Seungbum, K., Richards, E. M., Holbert, R. C., Pepine, C. J., … Genomics, F. (2018). Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut, 67(8), 1555–1557. 10.1136/gutjnl-2017-314759.Increased. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson, E., Horváth-Puhó, E., Thomsen, R. W., Djurhuus, J. C., Pedersen, L., Borghammer, P., & Sørensen, H. T. (2015). Vagotomy and subsequent risk of Parkinson's disease. Annals of Neurology, 78(4), 522–529. 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- Szczesniak, O., Hestad, K. A., Hanssen, J. F., & Rudi, K. (2016). Isovaleric acid in stool correlates with human depression. Nutritional Neuroscience, 19(7), 279–283. 10.1179/1476830515Y.0000000007. [DOI] [PubMed] [Google Scholar]
- Szeligowski, T., Yun, A. L., Lennox, B. R., & Burnet, P. W. J. (2020). The gut microbiome and schizophrenia: The current state of the field and clinical applications. Frontiers in Psychiatry, 11(156), 1–10. 10.3389/fpsyt.2020.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, T., Matsuda, T., Hayes, L. N., Yang, S., Rodriguez, K., Severance, E. G., … Eaton, W. W. (2017). Infection and inflammation in schizophrenia and bipolar disorder. Neuroscience Research, 115, 59–63. 10.1016/j.neures.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Thaiss, C. A., Zmora, N., Levy, M., & Elinav, E. (2016). The microbiome and innate immunity. Nature, 535(7610), 65–74. 10.1038/nature18847. [DOI] [PubMed] [Google Scholar]
- Valladares, R., Bojilova, L., Potts, A. H., Cameron, E., Gardner, C., Lorca, G., & Gonzalez, C. F. (2013). Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB Journal, 27(4), 1711–1720. 10.1096/fj.12-223339. [DOI] [PubMed] [Google Scholar]
- Valvassori, S., Resende, W., Budni, J., Dal-Pont, G., Bavaresco, D., Reus, G., … Quevedo, J. (2015). Sodium butyrate, a histone deacetylase inhibitor, reverses behavioral and mitochondrial alterations in animal models of depression induced by early- or late-life stress. Current Neurovascular Research, 12(4), 312–320. 10.2174/1567202612666150728121121. [DOI] [PubMed] [Google Scholar]
- Vanuytsel, T., Van Wanrooy, S., Vanheel, H., Vanormelingen, C., Verschueren, S., Houben, E., … Tack, J. (2014). Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut, 63(8), 1293–1299. 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- Verhoog, S., Taneri, P. E., Díaz, Z. M. R., Marques-Vidal, P., Troup, J. P., Bally, L., … Muka, T. (2019). Dietary factors and modulation of bacteria strains of akkermansia muciniphila and faecalibacterium prausnitzii: A systematic review. Nutrients, 11(7), 1–20. 10.3390/nu11071565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario, M., Guilarte, M., Alonso, C., Yang, P., Martínez, C., Ramos, L., … Santos, J. (2010). Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain, Behavior, and Immunity, 24(7), 1166–1175. 10.1016/j.bbi.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Wang, W., Chen, L., Zhou, R., Wang, X., Song, L., Huang, S., … Xia, B. (2014). Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate-producing bacteria in inflammatory bowel disease. Journal of Clinical Microbiology, 52(2), 398–406. 10.1128/JCM.01500-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, N. S., Gressitt, K. L., Cowan, D. N., Niebuhr, D. W., Yolken, R. H., & Severance, E. G. (2018). Monocyte activation detected prior to a diagnosis of schizophrenia in the US Military New Onset Psychosis Project (MNOPP). Schizophrenia Research, 197, 465–469. 10.1016/j.schres.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, F. A. (1961). Some psychiatric consequences of gastrectomy. British Medical Journal, 1(5242), 1560–1564. 10.1136/bmj.1.5242.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon, 21, 213–251. [Google Scholar]
- World Health Organization (2006). The world health report : 2006 : working together for health. World Health Organization. https://apps.who.int/iris/handle/10665/43432.
- Xu, R., Wu, B., Liang, J., He, F., Gu, W., Li, K., … Wang, M. (2020). Altered gut microbiota and mucosal immunity in patients with schizophrenia. Brain, Behavior, and Immunity, 85, 120–127. 10.1016/j.bbi.2019.06.039. [DOI] [PubMed] [Google Scholar]
- Yano, J. M., Yu, K., Donaldson, G. P., Shastri, G. G., Ann, P., Ma, L., … Hsiao, E. Y. (2015). Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell, 161(2), 264–276. 10.1016/j.cell.2015.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, X., Zhang, P., Wang, Y., Liu, Y., Li, X., Kumar, B. U., … Song, X. (2018). Changes in metabolism and microbiota after 24-week risperidone treatment in drug naïve, normal weight patients with first episode schizophrenia. Schizophrenia Research, 201, 299–306. 10.1016/j.schres.2018.05.017. [DOI] [PubMed] [Google Scholar]
- Zheng, P., Yang, J., Li, Y., Wu, J., Liang, W., Yin, B., … Wang, G. (2020). Gut microbial signatures can discriminate unipolar from bipolar depression. Advanced Science, 7(7), 1–11. 10.1002/advs.201902862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, P., Zeng, B., Liu, M., Chen, J., Pan, J., Han, Y., … Xie, P. (2019). The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Science Advances, 5(2), 1–11. 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., … Fan, S. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host's metabolism. Molecular Psychiatry, 21, 786–796. 10.1038/mp.2016.44. [DOI] [PubMed] [Google Scholar]
- Zhu, F., Guo, R., Wang, W., Ju, Y., Wang, Q., Ma, Q., … Ma, X. (2020a). Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Molecular Psychiatry, 25(11), 2905–2918. 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- Zhu, F., Ju, Y., Wang, W., Wang, Q., Guo, R., Ma, Q., … Ma, X. (2020b). Metagenome-wide association of gut microbiome features for schizophrenia. Nature Communications, 11(1), 1–10. 10.1038/s41467-020-15457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]