Keywords: MDR, ABC transporter, P-glycoprotein, BCRP, MRP-1, retinoids
Abstract
Multidrug resistance (MDR) means that tumour cells become unresponsive during or after the course of treatment to one or more of chemotherapeutic drugs. Chemotherapeutic resistance critically limits the treatment outcomes and remains a key challenge for clinicians. The alternation in intracellular drug concentration through the modulation of its transport across the plasma membrane is the major cause for MDR and is adopted by various mediators, including ATP-requiring enzymes (ATPases). Among these ATPases, ABC transporters have been extensively studied, and found to be highly implicated in tumorigenesis and MDR. The present review sheds light on the documented effects of retinoids on ABC enzymes to understand their mechanism in combating cancer cell resistance. This would open the gate to test the mechanism and applicability of different new synthetic retinoids in literature and market as modulators of ATP-dependent efflux pumping activity, and promote their applicability in diminishing anti-cancer drug resistance.
1. Introduction
Multidrug resistance (MDR) occurs when cancer cells become progressively unresponsive to anti-cancer drugs independently of their structures and/or mechanisms of action [1]. MDR might arise due to alteration in drug target molecules, interrupted access to target cells, genetic responses, enhanced DNA repair mechanisms, counteracting growth factors, metabolic effects or altered transport of the chemotherapeutic agent across the plasma membrane [1–4]. The latter mechanism is mediated by a wide range of ATP-requiring enzymes (ATPases). ATPase family members are indispensable enzymes for both normal and cancer cells [5]. They are widely distributed within cells and differ considerably in structures and biological activities. They share the ability to hydrolyse the phosphate γ–β bond of ATP to release free energy that is harnessed subsequently by the enzyme to perform its biological functions [6]. ATPase superfamily comprises ATP-binding cassette (ABC) transporters, P-type ATPases, V-type ATPases, kinesins, helicases, heat-shock proteins as well as ATPases associated with different cellular activities (AAA-ATPases) [5,7]. Of special interest, ABC transport systems that have been extensively studied as mediators of MDR in various types of cancer [8].
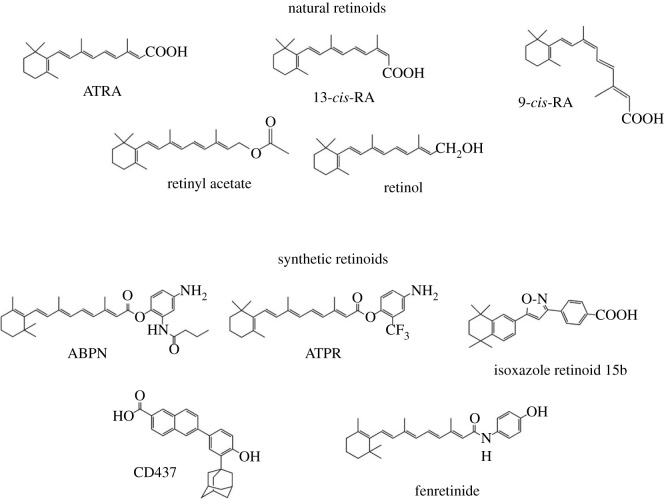
Although ABC transport systems are constitutively expressed in normal and cancer cells, their expression is also modulated by external factors, like retinoids. In the last few decades, it has become increasingly evident that retinoids, alone or in combinations, are promising anti-cancer compounds with considerable potency [9]. Significant correlation has been described between retinoids and ATPase transporters in cancer [10–12]. Besides the deregulation of ATPase gene expression after treatment with retinoids, ATRA and its analogues were found to be substrates for the MDR transporters and thus exposed to variations in their intracellular concentration leading to pharmacokinetic disturbances and variable anti-cancer response [13,14]. Figure 1 and table 1 summarizes the chemical structures and possible activity of retinoids by either re-sensitizing multiple MDR cell lines to chemotherapy or inducing direct growth inhibition of these MDR cells. Interestingly, the concentrations of retinoids needed for the demise of 50% of cultured cells growth (IC50) were found to be at, or slightly lower than, the micromolar scale [9,66,73,74]. Given this potency, more deep insights into the interplay between retinoids and MDR-conferring ATPases are still needed.
Figure 1.
The chemical structures of multidrug resistance (MDR)-combating retinoids (natural and synthetic).
Table 1.
The chemo-sensitizing effect of natural and synthetic retinoids on MDR cell lines.
| retinoid | class | resistant cancer cell line | cancer origin | resistance to chemotherapeutic agent(s) | IC50 (µM) | Ref. |
|---|---|---|---|---|---|---|
| ATRA | retinoic acid receptor (RAR) Pan-agonist | MDA-MB-231 | breast | paclitaxel (PTX) and 5-fluorouracil (5-FU) | 34.1b,c | [15,16] |
| LoVo/MDR | colon | doxorubicin (Dox) | NAd | [17] | ||
| HEN-16-2/CDDP | cervical | cisplatin (CDDP) | NAd | [18] | ||
| L1210/VCR | mouse lymphocytic leukaemia | vincristine (VCR) | NAd | [19,20] | ||
| mS-0.5 | melanoma | colchicine | NAd | [21] | ||
| J82-NVB | bladder | navelbine | NAd | [22] | ||
| HL60/DNR | acute promyelocytic leukaemia (APL) | daunorubicin | NAd | [23] | ||
| retinol | — | SW620 | colorectal | etoposide | NAd | [24] |
| isoxazole retinoid 15b | RAR pan-agonist | HL60R | APL | ATRA | 1.4b,c | [25] |
| K562 | leukaemia | ATRA | 2.3b,c | [25] | ||
| HUT78 | T-cell lymphoma | ATRA | 0.8b,c | [25] | ||
| fenretinide or 4-HPR | RAR-β selective agonist | Bel-7402 | HCC | Dox and VCR | 13.1a | [26,27] |
| MDA-MB-231 | breast | PTX and 5-FU | 6.5b,c | [28] | ||
| CHLA-119 | neuroblastoma | ABT-737 (small-molecule BH3- mimetic) alone | NAd | [29] | ||
| ABPN (or CBG41) | RAR pan-agonist | MDA-MB-231 | breast | PTX and 5-FU | 3.3b,c | [28] |
| ATPR | RAR pan-agonist | MDA-MB-231 | breast | PTX and 5-FU | 18.1b,c | [15] |
aIC50 (concentration of the compound caused 50% reduction in comparison to untreated cells) was calculated after 72 h of treatment.
bIC50 was calculated after 48 h of treatment.
cIC50 was calculated after 24 h of treatment.
dNA = not available.
2. The interplay between ABC transporters and MDR
Among the currently known ABC genes in the human genome, perturbations in the expression levels of some of these transporters are implicated in various human diseases, including cancer [30]. Furthermore, ABC transporters are highly associated with MDR [31–35]. Members of the ABCB, ABCC and ABCG subfamilies are major determinants for the emergence of MDR [36], Most importantly, P-gp, BCRP and multidrug resistance-associated protein-1 (MRP-1 or ABCC-1) are the best characterized [31–35]. The induction of the expression of these ATP-requiring proteins leads to significant changes in the signalling of many ions and molecules promoting tumorigenesis, including metal ions, vitamins and carbohydrates [5,37,38]. More profoundly, these efflux transporters pump a wide range of structurally diverse anti-cancer compounds outside the cell, reducing their bioavailability and therapeutic potential [1,39]. The overexpression of P-gp, BCRP and/or MRP-1 confers significant resistance to various neutral and cationic hydrophobic chemotherapeutic compounds [40–42]. These observations highlight the intimate link between disturbances in ABC transporters and conferred drug resistance, leading subsequently to increased tumour burden and reduced treatment outcomes.
As expected, inhibition of the pumping activity of the ABC transporter enzymes often leads to an increased cellular concentration of the cytotoxic drugs, and thus greater anti-cancer activity and reduced MDR [43]. Despite their structural differences, P-gp and BCRP share several common ligands that are transported across cell membrane [44–46]. These translocated ligands are collectively called allocrites [47]. Although they are functionally similar, P-gp and BCRP share only about 20% protein sequence identity in the NBDs with no significant sequence identity in the TMDs [48–51]. Nevertheless, both share various anti-cancer allocrites giving rise to MDR. The structural insights into the interactions between ABC transporters and their ligands show clearly that the hydrophobic nature of allocrites, including the retinoic acid and its analogues (or retinoids), is one of the major determinants of their ability to communicate with the transporters [48,52–58]. Finding chemosensitizers that are both effective and safe and could help rescue the emerging resistance to standard chemotherapeutic compounds in cancer is still needed.
3. Natural retinoids induce alternations in ABC transporters on different levels including expression, activity and binding interaction
The data available in the literature showed some examples of natural retinoids that proved their ability to modulate ABC transporters in cancer types on different levels and reverse MDR. For instance, retinol caused significant reduction in P-gp expression in colorectal carcinoma cells (CRC) leading to enhanced anti-tumor efficacy of etoposide [24,59]. In leukaemia, cell line L1210, ATRA caused transcriptional repression of P-gp enhancing the activity of verapamil substrate [19]. Interestingly, the latter effect was not attributed to the direct binding of RAR merely to the ABCB1 promoter; instead, it appears to be mediated by RXRα sequestration after RAR-RXRα heterodimer formation [19]. As a result, fewer RXRα may be available to mediate the binding of ABCB1-activating progesterone-X-receptor (PXR) to ABCB1 promoter. These results suggest that retinoic acid and related isomers attenuate the ABC transporter through modulation of mRNA expression levels.
On the level of ATPase activity, Spodoptera frugiperda (Sf9) membrane preparations expressing P-gp and BCRP was used to investigate the effects of some natural retinoids with verapamil and quercetin as their substrates respectively [10,11]. The study showed retinol and 13-cis-RA could significantly inhibit both the basal and the substrate-stimulated ATPase activity while ATRA, 9-cis-RA, retinyl-propionate and retinyl-palmitate did not have these effects. [11]. The ATPase inhibitory effect of retinoids observed in these experiments might be rooted in the hypothesis of retinoid-induced allosteric inhibition in activity of the transporters, related either to the competitive inhibition caused by direct interaction of retinoids with the substrate-binding site (s), or the membrane structural changes induced by retinoids.
On the level of binding interaction, studies revealed the interaction of ATPases with retinoic acid analogues to be stereospecific [11,53,60]. For example, 13-cis-RA inhibited both P-gp and BCRP transporters, while its stereoisomers ATRA and 9-cis-RA did not influence the enzymatic activity. Beside the stereo-selective binding of retinoids to P-gp and BCRP that occurs primarily at the level of the drug binding sites (allosteric sites) of the transporters, there is another level of binding at the plasma membrane itself from where the substrates and modulators probably interact with the drug binding site(s) [52]. The latter observation was confirmed by Fluorescence anisotropy assay using fluorescent membrane probe 1,6-diphenyl-1,3,5-hexatriene (DPH). Retinyl-acetate, 13-cis-RA, and retinol selectively increase the membrane viscosity and packing density in the depth of the membrane while, ATRA and 9-cis-RA did not have similar effects [11].
Calculating the kinetic parameters (Km and Vmax) of the substrate-stimulated ATPase activity with or without retinoids showed retinol with higher Km and lower Vmax values of both transporters, suggesting mixed-type inhibition of P-gp and BCRP. Although 13-cis-RA showed mixed-type transporter inhibition of BCRP too, it caused a reduction of Vmax with no significant increment of Km value in the case of P-gp, emphasizing the non-competitive mode of inhibition of P-gp [11]. All these observations imply that natural retinoids with different stereoisomers have distinct modes of interaction and binding affinity with MDR-related ATPases, and suggest that minute differences in their structure might substantially influence the ATPase enzymatic activity.
4. The MDR-reversing activity of synthetic retinoids
The main obstacles of using formulations delivering natural retinoids into the systemic circulation are in vitro photo-instability [61–66] and in vivo enzymatic catabolism [67–70]. Therefore, there was urgent need for development of synthetic retinoic acid analogues, which mimic the biological actions and physico-chemical characteristics of natural ones, and to reverse MDR induced by cancer cells [9,71–76]. A heterocycle-containing retinoid called isoxazole retinoid 15b (figure 1) was synthesized and used to reverse the MDR activity of an acute promyelocytic leukaemia ATRA-resistant cell line called HL60R (table 1) [25]. This synthetic retinoid 15b rendered the cells more prone to the growth-inhibitory activity of ATRA and reactivated the cellular apoptosis pathway. Fenretinide [28], ABPN [28] and ATPR [15] (figure 1) are further examples of synthetic retinoids that were able to sensitize multi-drug resistant triple-negative breast cancer to paclitaxel and 5-fluorouracil. Nevertheless, these promising in vitro results need to be confirmed in vivo using chemo-resistant cancer animal models exposed to standard chemotherapeutics plus synthetic retinoids.
Despite this MDR-reversing activity in various cancers, an early report showed the cross-resistance to CD437 (a synthetic RARγ-selective agonist; figure 1) in paclitaxel-resistant human ovarian cancer cells which are overexpressing P-gp [77]. Others claimed that N-(4-hydroxyphenyl) retinamide (4HPR, aka fenretinide; figure 1) could potentiate the cytotoxicity of cisplatin in ovarian [78], breast [79] and lung [80] cancers. The underlying cause of this chemo-sensitization can be explained in light of the dose perspective point of view. Active doses from natural retinoids in blood are few in nanomolar range (1–20 nanomolar) [81] compared to the stable synthetic retinoids that can be taken through either parenteral or oral administration with relatively sufficient high local retinoid concentrations in the blood. The available doses of synthetic retinoids were able to subsequently block P-gp and BCRP expressed at the surface of resistant cancer cells [82–85].
5. Concluding remarks
Understanding of cancer resistance has evolved over the past few decades, and cancer resistance is suggested to be related to loss of retinoid-ABC transporter signalling. Also, emerging evidence sheds light on the development of MDR and the roles played by ATPases in chemotherapy resistance. Unfortunately, current chemotherapy regimens lead to limited efficacy and an upsurge in the number of cells with high levels of expression of ABC transporters. Various chemical compounds have been identified and tested to modulate or inhibit the transport function of ABC transporters, including P-gp and BCRP, and thus chemo-sensitize multidrug-resistant cancer cells. Nevertheless, ABC-modulatory compounds showing great potential on the bench frequently failed to prove efficiency in the clinic. Therefore, this presents a formidable challenge to medicinal chemists and structural biologists in defining P-gp and BCRP substrates with new structural diversity to modulate P-gp- and BCRP-mediated drug transport including retinoids. This requires precise knowledge of their structural domains and the exact mechanisms of interactions. Given that modulation of the ABC transporters might influence the pharmacokinetics of other co-administered chemotherapeutic drugs, more care should be taken upon the combination of retinoids with other anti-cancer drugs to avoid drug–drug interactions occurring at the level of the membrane transporters, P-gp and BCRP.
Considering the anti-cancer potency of synthetic retinoids, future research should focus on unravelling the impact of these compounds on the expression and activity of efflux pumps and other drug transporters. This could pave the way for recruiting synthetic retinoids as chemosensitizers that specifically target MDR-promoting transporters and could help in fighting the battle against chemoresistance.
Data accessibility
This article has no additional data.
Authors' contributions
M.R.A.: conceptualization, methodology, validation, writing—original draft, writing—review and editing; H.H.: conceptualization, investigation, methodology, project administration, supervision, writing—original draft, writing—review and editing.
Both authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare that we have no competing interests.
Funding
This work was supported by the Egyptian Academy of Scientific Research and Technology (ASRT) under the grant call ‘National Program for Research and Innovation in Health and Biomedical Sciences' (ID: PRISM _ 5173), 2020–2022.
References
- 1.Bukowski K, Kciuk M, Kontek R. 2020. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21, 3233. ( 10.3390/ijms21093233) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikolaou M, Pavlopoulou A, Georgakilas AG, Kyrodimos E. 2018. The challenge of drug resistance in cancer treatment: a current overview. Clin. Exp. Metastasis. 35, 309-318. ( 10.1007/s10585-018-9903-0) [DOI] [PubMed] [Google Scholar]
- 3.Avendaño C, Menéndez JC. 2002. Inhibitors of multidrug resistance to antitumor agents (MDR). Curr. Med. Chem. 9, 159-193. ( 10.2174/0929867023371175) [DOI] [PubMed] [Google Scholar]
- 4.Luqmani YA. 2005. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 14(Suppl 1), 35-48. ( 10.1159/000086183) [DOI] [PubMed] [Google Scholar]
- 5.Chène P. 2003. The ATPases: a new family for a family-based drug design approach. Expert Opin. Ther. Targets. 7, 453-461. ( 10.1517/14728222.7.3.453) [DOI] [PubMed] [Google Scholar]
- 6.Zhang S, Mao Y. 2020. AAA+ ATPases in protein degradation: structures, functions and mechanisms. Biomolecules 10, 629. ( 10.3390/biom10040629) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas C, et al. 2020. Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett. 594, 3767-3775. ( 10.1002/1873-3468.13935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu SW, et al. 2020. SERCA and P-glycoprotein inhibition and ATP depletion are necessary for celastrol-induced autophagic cell death and collateral sensitivity in multidrug-resistant tumor cells. Pharmacol. Res. 153, 104660. ( 10.1016/j.phrs.2020.104660) [DOI] [PubMed] [Google Scholar]
- 9.Abdelaal MR, Soror SH, Elnagar MR, Haffez H. 2021. Revealing the potential application of EC-synthetic retinoid analogues in anticancer therapy. Molecules 26, 506. ( 10.3390/molecules26020506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkadi B, Price EM, Boucher RC, Germann UA, Scarborough GA. 1992. Expression of the human multidrug resistance cDNA in insect cells generates a high activity drug-stimulated membrane ATPase. J. Biol. Chem. 267, 4854-4858. ( 10.1016/S0021-9258(18)42909-2) [DOI] [PubMed] [Google Scholar]
- 11.Tarapcsák S, et al. 2017. Interactions of retinoids with the ABC transporters P-glycoprotein and breast cancer resistance protein. Sci. Rep. 7, 41376. ( 10.1038/srep41376) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Launay S, et al. 1999. Lineage-specific modulation of calcium pump expression during myeloid differentiation. Blood 93, 4395-4405. ( 10.1182/blood.V93.12.4395) [DOI] [PubMed] [Google Scholar]
- 13.Tzimas G, Collins MD, Bürgin H, Hummler H, Nau H. 1996. Embryotoxic doses of vitamin A to rabbits result in low plasma but high embryonic concentrations of all-trans-retinoic acid: risk of vitamin A exposure in humans. J. Nutr. 126, 2159-2171. ( 10.1093/jn/126.9.2159) [DOI] [PubMed] [Google Scholar]
- 14.Iqbal M, Audette MC, Petropoulos S, Gibb W, Matthews SG. 2012. Placental drug transporters and their role in fetal protection. Placenta 33, 137-142. ( 10.1016/j.placenta.2012.01.008) [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Yan Y, Zhou J, Zhou Q, Gui S, Wang Y. 2013. A novel all-trans retinoid acid derivatives inhibits the migration of breast cancer cell lines MDA-MB-231 via myosin light chain kinase involving p38-MAPK pathway. Biomed. Pharmacother. 67, 357-362. ( 10.1016/j.biopha.2013.03.016) [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Lu L, Feng Y, Wang H, Dai L, Li Y, Zhang P. 2011. PKD2 mediates multi-drug resistance in breast cancer cells through modulation of P-glycoprotein expression. Cancer Lett. 300, 48-56. ( 10.1016/j.canlet.2010.09.005) [DOI] [PubMed] [Google Scholar]
- 17.Bartolini G, Orlandi M, Papi A, Ammar K, Guerra F, Ferreri AM, Rocchi P. 2006. A search for multidrug resistance modulators: the effects of retinoids in human colon carcinoma cells. In Vivo 20, 729-733. [PubMed] [Google Scholar]
- 18.Ding Z, Green AG, Yang X, Chernenko G, Tang SC, Pater A. 2002. Retinoic acid inhibits telomerase activity and downregulates expression but does not affect splicing of hTERT: correlation with cell growth rate inhibition in an in vitro cervical carcinogenesis/multidrug-resistance model. Exp. Cell Res. 272, 185-191. ( 10.1006/excr.2001.5412) [DOI] [PubMed] [Google Scholar]
- 19.Breier A, Stetka J, Bohacova V, Macejova D, Brtko J, Sulova Z. 2014. Effect of 9-cis retinoic acid and all-trans retinoic acid in combination with verapamil on P-glycoprotein expression in L1210 cells. Neoplasma 61, 553-565. ( 10.4149/neo_2014_068) [DOI] [PubMed] [Google Scholar]
- 20.Sulová Z, Macejová D, Seres M, Sedlák J, Brtko J, Breier A. 2008. Combined treatment of P-gp-positive L1210/VCR cells by verapamil and all-trans retinoic acid induces down-regulation of P-glycoprotein expression and transport activity. Toxicol. In Vitro 22, 96-105. ( 10.1016/j.tiv.2007.08.011) [DOI] [PubMed] [Google Scholar]
- 21.Stromskaya TP, Filippova NA, Rybalkina E, Egudina SV, Shtil AA, Eliseenkova AV, Stavrovskaya AA. 1995. Alterations of melanin synthesis in human melanoma cells selected in vitro for multidrug resistance. Exp. Toxicol. Pathol. 47, 157-166. ( 10.1016/s0940-2993(11)80308-8) [DOI] [PubMed] [Google Scholar]
- 22.Debal V, Breillout F, Manfait M. 1997. Concomitant decrease of resistance and modifications of the cytoskeleton after all-trans retinoic acid and phorbol ester treatments in a navelbine-resistant bladder carcinoma cell line. Anticancer Res. 17, 1147-1154. ( 10.1038/bjc.1994.458) [DOI] [PubMed] [Google Scholar]
- 23.Zhou DC, Marie JP, Maisonneuve L, Faussat-Suberville AM, Zittoun R. 1993. Effect of differentiating agents on modulation of MDR1 gene expression in multidrug-resistant hematopoietic HL60/DNR cell line. Exp. Hematol. 21, 779-784. [PubMed] [Google Scholar]
- 24.Klamt F, Passos DT, Castro MA, Gelain DP, Grivicich I, Moreira JC. 2008. Inhibition of MDR1 expression by retinol treatment increases sensitivity to etoposide (VP16) in human neoplasic cell line. Toxicol. In Vitro 22, 873-878. ( 10.1016/j.tiv.2008.01.004) [DOI] [PubMed] [Google Scholar]
- 25.Simoni D, et al. 2001. Heterocycle-containing retinoids. Discovery of a novel isoxazole arotinoid possessing potent apoptotic activity in multidrug and drug-induced apoptosis-resistant cells. J. Med. Chem. 44, 2308-2318. ( 10.1021/jm0010320) [DOI] [PubMed] [Google Scholar]
- 26.Shentu J, Zhang B, Fan L, He Q, Yang B, Chen Z. 2007. Anti-proliferative activity of fenretinide in human hepatoma cells in vitro and in vivo. Anticancer Drugs 18, 47-53. ( 10.1097/CAD.0b013e32800feeb5) [DOI] [PubMed] [Google Scholar]
- 27.Huang M, Liu G. 1999. The study of innate drug resistance of human hepatocellular carcinoma Bel7402 cell line. Cancer Lett. 135, 97-105. ( 10.1016/s0304-3835(98)00280-8) [DOI] [PubMed] [Google Scholar]
- 28.Um SJ, Han HS, Kwon YJ, Park SH, Rho YS, Sin HS, Park JS. 2003. Novel retinoic acid derivative ABPN has potent inhibitory activity on cell growth and apoptosis in cancer cells. Int. J. Cancer. 107, 1038-1046. ( 10.1002/ijc.11489) [DOI] [PubMed] [Google Scholar]
- 29.Fang H, Harned TM, Kalous O, Maldonado V, DeClerck YA, Reynolds CP. 2011. Synergistic activity of fenretinide and the Bcl-2 family protein inhibitor ABT-737 against human neuroblastoma. Clin. Cancer Res. 17, 7093-7104. ( 10.1158/1078-0432.ccr-11-0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkens S. 2015. Structure and mechanism of ABC transporters. F1000Prime Rep. 7, 14. ( 10.12703/P7-14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshizawa K, Nozaki S, Kitahara H, Ohara T, Kato K, Kawashiri S, Yamamoto E. 2007. Copper efflux transporter (ATP7B) contributes to the acquisition of cisplatin-resistance in human oral squamous cell lines. Oncol. Rep. 18, 987-991. ( 10.3892/or.18.4.987) [DOI] [PubMed] [Google Scholar]
- 32.Robey RW, Polgar O, Deeken J, To KW, Bates SE. 2007. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 26, 39-57. ( 10.1007/s10555-007-9042-6) [DOI] [PubMed] [Google Scholar]
- 33.Sharom FJ. 2011. The P-glycoprotein multidrug transporter. Essays Biochem. 50, 161-178. ( 10.1042/bse0500161) [DOI] [PubMed] [Google Scholar]
- 34.Borst P, Elferink RO. 2002. Mammalian ABC transporters in health and disease. Annu. Rev. Biochem. 71, 537-592. ( 10.1146/annurev.biochem.71.102301.093055) [DOI] [PubMed] [Google Scholar]
- 35.Efferth T. 2001. The human ATP-binding cassette transporter genes: from the bench to the bedside. Curr. Mol. Med. 1, 45-65. ( 10.2174/1566524013364194) [DOI] [PubMed] [Google Scholar]
- 36.Amawi H, Sim HM, Tiwari AK, Ambudkar SV, Shukla S. 2019. ABC Transporter-mediated multidrug-resistant cancer. Adv. Exp. Med. Biol. 1141, 549-580. ( 10.1007/978-981-13-7647-4_12) [DOI] [PubMed] [Google Scholar]
- 37.Dean M, Rzhetsky A, Allikmets R. 2001. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11, 1156-1166. ( 10.1101/gr.184901) [DOI] [PubMed] [Google Scholar]
- 38.Saier MH Jr. 2000. A functional-phylogenetic classification system for transmembrane solute transporters. Microbiol. Mol. Biol. Rev. 64, 354-411. ( 10.1128/mmbr.64.2.354-411.2000) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lehne G. 2000. P-glycoprotein as a drug target in the treatment of multidrug resistant cancer. Curr. Drug Targets. 1, 85-99. ( 10.2174/1389450003349443) [DOI] [PubMed] [Google Scholar]
- 40.Tiwari AK, Sodani K, Dai CL, Ashby CR Jr, Chen ZS. 2011. Revisiting the ABCs of multidrug resistance in cancer chemotherapy. Curr. Pharm. Biotechnol. 12, 570-594. ( 10.2174/138920111795164048) [DOI] [PubMed] [Google Scholar]
- 41.Washio I, Nakanishi T, Ishiguro N, Bister B, Tamai I. 2019. Effect of endogenous multidrug resistance 1 and P-glycoprotein expression on anticancer drug resistance in colon cancer cell lines. Biopharm. Drug Dispos. 40, 32-43. ( 10.1002/bdd.2167) [DOI] [PubMed] [Google Scholar]
- 42.Bugde P, Biswas R, Merien F, Lu J, Liu DX, Chen M, Zhou S, Li Y. 2017. The therapeutic potential of targeting ABC transporters to combat multi-drug resistance. Expert Opin. Ther. Targets. 21, 511-530. ( 10.1080/14728222.2017.1310841) [DOI] [PubMed] [Google Scholar]
- 43.Cheung BB, Marshall GM. 2011. Targeting ATP7A to increase the sensitivity of neuroblastoma cells to retinoid therapy. Curr. Cancer Drug Targets. 11, 826-836. ( 10.2174/156800911796798968) [DOI] [PubMed] [Google Scholar]
- 44.Kodaira H, Kusuhara H, Ushiki J, Fuse E, Sugiyama Y. 2010. Kinetic analysis of the cooperation of P-glycoprotein (P-gp/Abcb1) and breast cancer resistance protein (Bcrp/Abcg2) in limiting the brain and testis penetration of erlotinib, flavopiridol, and mitoxantrone. J. Pharmacol. Exp. Ther. 333, 788-796. ( 10.1124/jpet.109.162321) [DOI] [PubMed] [Google Scholar]
- 45.Polli JW, Olson KL, Chism JP, John-Williams LS, Yeager RL, Woodard SM, Otto V, Castellino S, Demby VE. 2009. An unexpected synergist role of P-glycoprotein and breast cancer resistance protein on the central nervous system penetration of the tyrosine kinase inhibitor lapatinib (N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine; GW572016). Drug Metab. Dispos. 37, 439-442. ( 10.1124/dmd.108.024646) [DOI] [PubMed] [Google Scholar]
- 46.Egido E, Müller R, Li-Blatter X, Merino G, Seelig A. 2015. Predicting activators and inhibitors of the breast cancer resistance protein (ABCG2) and P-Glycoprotein (ABCB1) based on mechanistic considerations. Mol. Pharm. 12, 4026-4037. ( 10.1021/acs.molpharmaceut.5b00463) [DOI] [PubMed] [Google Scholar]
- 47.Holland IB, Blight MA. 1999. ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J. Mol. Biol. 293, 381-399. ( 10.1006/jmbi.1999.2993) [DOI] [PubMed] [Google Scholar]
- 48.Ni Z, Mark ME, Cai X, Mao Q. 2010. Fluorescence resonance energy transfer (FRET) analysis demonstrates dimer/oligomer formation of the human breast cancer resistance protein (BCRP/ABCG2) in intact cells. Int. J. Biochem. Mol. Biol. 1, 1-11. [PMC free article] [PubMed] [Google Scholar]
- 49.Homolya L, Holló Z, Germann UA, Pastan I, Gottesman MM, Sarkadi B. 1993. Fluorescent cellular indicators are extruded by the multidrug resistance protein. J. Biol. Chem. 268, 21 493-21 496. ( 10.1016/s0021-9258(20)80566-3) [DOI] [PubMed] [Google Scholar]
- 50.Gottesman MM, Pastan I. 1993. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu. Rev. Biochem. 62, 385-427. ( 10.1146/annurev.bi.62.070193.002125) [DOI] [PubMed] [Google Scholar]
- 51.Li-Blatter X, Beck A, Seelig A. 2012. P-glycoprotein-ATPase modulation: the molecular mechanisms. Biophys. J. 102, 1383-1393. ( 10.1016/j.bpj.2012.02.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu Y, Egido E, Li-Blatter X, Müller R, Merino G, Bernèche S, Seelig A. 2015. Allocrite sensing and binding by the breast cancer resistance protein (ABCG2) and P-glycoprotein (ABCB1). Biochemistry 54, 6195-6206. ( 10.1021/acs.biochem.5b00649) [DOI] [PubMed] [Google Scholar]
- 53.Aller SG, et al. 2009. Structure of P-glycoprotein reveals a molecular basis for poly-specific drug binding. Science 323, 1718-1722. ( 10.1126/science.1168750) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ni Z, Bikadi Z, Rosenberg MF, Mao Q. 2010. Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr. Drug Metab. 11, 603-617. ( 10.2174/138920010792927325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu J, Peng H, Chen Q, Liu Y, Dong Z, Zhang JT. 2007. Oligomerization domain of the multidrug resistance-associated transporter ABCG2 and its dominant inhibitory activity. Cancer Res. 67, 4373-4381. ( 10.1158/0008-5472.can-06-3169) [DOI] [PubMed] [Google Scholar]
- 56.McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. 2006. Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure 14, 1623-1632. ( 10.1016/j.str.2006.08.014) [DOI] [PubMed] [Google Scholar]
- 57.Li J, Jaimes KF, Aller SG. 2014. Refined structures of mouse P-glycoprotein. Protein Sci. 23, 34-46. ( 10.1002/pro.2387) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosenberg MF, Bikadi Z, Chan J, Liu X, Ni Z, Cai X, Ford RC, Mao Q. 2010. The human breast cancer resistance protein (BCRP/ABCG2) shows conformational changes with mitoxantrone. Structure 18, 482-493. ( 10.1016/j.str.2010.01.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shalinsky DR, Bischoff ED, Gregory ML, Lamph WW, Heyman RA, Hayes JS, Thomazy V, Davies PJ. 1996. Enhanced antitumor efficacy of cisplatin in combination with ALRT1057 (9-cis retinoic acid) in human oral squamous carcinoma xenografts in nude mice. Clin. Cancer Res. 2, 511-520. [PubMed] [Google Scholar]
- 60.Dey S, Hafkemeyer P, Pastan I, Gottesman MM. 1999. A single amino acid residue contributes to distinct mechanisms of inhibition of the human multidrug transporter by stereoisomers of the dopamine receptor antagonist flupentixol. Biochemistry 38, 6630-6639. ( 10.1021/bi983038l) [DOI] [PubMed] [Google Scholar]
- 61.Murayama A, Suzuki T, Matsui M. 1997. Photoisomerization of retinoic acids in ethanol under room light: a warning for cell biological study of geometrical isomers of retinoids. J. Nutr. Sci. Vitaminol. 43, 167-176. ( 10.3177/jnsv.43.167) [DOI] [PubMed] [Google Scholar]
- 62.Bempong DK, Honigberg IL, Meltzer NM. 1995. Normal phase LC-MS determination of retinoic acid degradation products. J. Pharm. Biomed. Anal. 13, 285-291. ( 10.1016/0731-7085(95)01270-U) [DOI] [PubMed] [Google Scholar]
- 63.Suzuki T, Kunchala SRAO, Matsui M, Murayama A. 1998. Molecular Flexibility of Retinoic Acid under White Fluorescent Light. J. Nutr. Sci. Vitaminol. 44, 729-736. ( 10.3177/jnsv.44.729) [DOI] [PubMed] [Google Scholar]
- 64.Kunchala SR, Suzuki T, Murayama A. 2000. Photoisomerization of retinoic acid and its influence on regulation of human keratinocyte growth and differentiation. Indian J. Biochem. Biophys. 37, 71-76. [PubMed] [Google Scholar]
- 65.Christie VB, et al. 2008. Synthesis and evaluation of synthetic retinoid derivatives as inducers of stem cell differentiation. Org. Biomol. Chem. 6, 3497-3507. ( 10.1039/b808574a) [DOI] [PubMed] [Google Scholar]
- 66.Haffez H, Chisholm DR, Valentine R, Pohl E, Redfern C, Whiting A. 2017. The molecular basis of the interactions between synthetic retinoic acid analogues and the retinoic acid receptors. MedChemComm 8, 578-592. ( 10.1039/c6md00680a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adedoyin A, Stiff DD, Smith DC, Romkes M, Bahnson RC, Day R, Hofacker J, Branch RA, Trump DL. 1998. All-trans-retinoic acid modulation of drug-metabolizing enzyme activities: investigation with selective metabolic drug probes. Cancer Chemother. Pharmacol. 41, 133-139. ( 10.1007/s002800050719) [DOI] [PubMed] [Google Scholar]
- 68.Muindi J, Frankel SR, Miller WH Jr, Jakubowski A, Scheinberg DA, Young CW, Dmitrovsky E, Warrell RP Jr. 1992. Continuous treatment with all-trans retinoic acid causes a progressive reduction in plasma drug concentrations: implications for relapse and retinoid ‘resistance’ in patients with acute promyelocytic leukemia. Blood 79, 299-303. ( 10.1182/blood.v79.2.299.bloodjournal792299) [DOI] [PubMed] [Google Scholar]
- 69.Muindi JR, Frankel SR, Huselton C, DeGrazia F, Garland WA, Young CW, Warrell RP Jr. 1992. Clinical pharmacology of oral all-trans retinoic acid in patients with acute promyelocytic leukemia. Cancer Res. 52, 2138-2142. ( 10.1016/0753-3322(92)90256-7) [DOI] [PubMed] [Google Scholar]
- 70.Trump DL, Smith DC, Stiff D, Adedoyin A, Day R, Bahnson RR, Hofacker J, Branch RA. 1997. A phase II trial of all-trans-retinoic acid in hormone-refractory prostate cancer: a clinical trial with detailed pharmacokinetic analysis. Cancer Chemother. Pharmacol. 39, 349-356. ( 10.1007/s002800050582) [DOI] [PubMed] [Google Scholar]
- 71.Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. 2007. RAR and RXR modulation in cancer and metabolic disease. Nature Rev. Drug Discov. 6, 793-810. ( 10.1038/nrd2397) [DOI] [PubMed] [Google Scholar]
- 72.Di Masi A, Leboffe L, De Marinis E, Pagano F, Cicconi L, Rochette-Egly C, Lo-Coco F, Ascenzi P, Nervi C. 2015. Retinoic acid receptors: from molecular mechanisms to cancer therapy. Mol. Aspects Med. 41, 1-115. ( 10.1016/j.mam.2014.12.003) [DOI] [PubMed] [Google Scholar]
- 73.Haffez H, Khatib T, McCaffery P, Przyborski S, Redfern C, Whiting A. 2018. Neurogenesis in response to synthetic retinoids at different temporal scales. Mol. Neurobiol. 55, 1942-1950. ( 10.1007/s12035-017-0440-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haffez H, Chisholm DR, Tatum NJ, Valentine R, Redfern C, Pohl E, Whiting A, Przyborski S. 2018. Probing biological activity through structural modelling of ligand-receptor interactions of 2,4-disubstituted thiazole retinoids. Bioorg. Med. Chem. 26, 1560-1572. ( 10.1016/j.bmc.2018.02.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun S-Y, Lotan R. 2002. Retinoids and their receptors in cancer development and chemoprevention. Crit. Rev. Oncol./Hematol. 41, 41-55. ( 10.1016/s1040-8428(01)00144-5) [DOI] [PubMed] [Google Scholar]
- 76.Crowe DL. 2002. Receptor selective synthetic retinoids as potential cancer chemotherapy agents. Curr. Cancer Drug Targets. 2, 77-86. ( 10.2174/1568009023333935) [DOI] [PubMed] [Google Scholar]
- 77.Kumar A, Soprano DR, Parekh HK. 2001. Cross-resistance to the synthetic retinoid CD437 in.a paclitaxel-resistant human ovarian carcinoma cell line is independent of the overexpression of retinoic acid receptor-gamma. Cancer Res. 61, 7552-7555. [PubMed] [Google Scholar]
- 78.Formelli F, Cleris L. 1993. Synthetic retinoid fenretinide is effective against a human ovarian carcinoma xenograft and potentiates cisplatin activity. Cancer Res. 53, 5374-5376. [PubMed] [Google Scholar]
- 79.Grunt Th W, Dittrich E, Offterdinger M, Schneider SM, Dittrich C, Huber H. 1998. Effects of retinoic acid and fenretinide on the c-erbB-2 expression, growth and cisplatin sensitivity of breast cancer cells. Br. J. Cancer. 78, 79-87. ( 10.1038/bjc.1998.446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalemkerian GP, Ou X. 1999. Activity of fenretinide plus chemotherapeutic agents in small-cell lung cancer cell lines. Cancer Chemother. Pharmacol. 43, 145-150. ( 10.1007/s002800050875) [DOI] [PubMed] [Google Scholar]
- 81.Arnold SL, Amory JK, Walsh TJ, Isoherranen N. 2012. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J. Lipid Res. 53, 587-598. ( 10.1194/jlr.D019745) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wise EM, Graber EM. 2011. Clinical pearl: comedone extraction for persistent macrocomedones while on isotretinoin therapy. J. Clin. Aesthet. Dermatol. 4, 20-21. [PMC free article] [PubMed] [Google Scholar]
- 83.Skeel RT, et al. 2003. A phase II study of 13-cis retinoic acid plus interferon alpha-2a in advanced stage penile carcinoma: an Eastern Cooperative Oncology Group study (E3893). Cancer Invest. 21, 41-46. ( 10.1081/cnv-120016402) [DOI] [PubMed] [Google Scholar]
- 84.Veal GJ, Errington J, Rowbotham SE, Illingworth NA, Malik G, Cole M, Daly AK, Pearson AD, Boddy AV. 2013. Adaptive dosing approaches to the individualization of 13-cis-retinoic acid (isotretinoin) treatment for children with high-risk neuroblastoma. Clin. Cancer Res. 19, 469-479. ( 10.1158/1078-0432.ccr-12-2225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reynolds CP, Matthay KK, Villablanca JG, Maurer BJ. 2003. Retinoid therapy of high-risk neuroblastoma. Cancer Lett. 197, 185-192. ( 10.1016/s0304-3835(03)00108-3) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This article has no additional data.