Abstract
Introduction
Survival gaps in acute heart failure (AHF) continue to expand globally. Multinational heart failure (HF) registries have highlighted variations between countries. Whether discrepancies in HF practice and outcomes occur across different health systems (ie, private, public or universal healthcare) within a city or between countries remain unclear. Insight into organisational care is also scarce. With increasing public scrutiny of health inequalities, a study to address these limitations is timely.
Method
KOLCOV-HF study prospectively compared patients with AHF in public (Nil Ratan Sircar Hospital (NRS)) versus private (Apollo Gleneagles Hospital (AGH)) hospitals of Kolkata, India, and one with universal health coverage in a socioeconomically comparable city of Coventry, England (University Hospitals Coventry & Warwickshire (UHCW)). Data variables were adapted from UK’s National HF Audit programme, collected over 24 months. Predictors of in-hospital mortality and length of hospitalisation were assessed for each centre.
Results
Among 1652 patients, in-hospital mortality was highest in government-funded NRS (11.9%) while 3 miles north, AGH had significantly lower mortality (7.5%, p=0.034), similar to UHCW (8%). This could be attributed to distinct HF phenotypes and differences in clinical and organisational care. As expected, low blood pressure was associated with a significantly greater risk of death in patients served by public hospitals UHCW and NRS.
Conclusion
Marked differences in HF characteristics, management and outcomes exist intra-regionally, and between low–middle versus high-income countries across private, public and universal healthcare systems. Physicians and policymakers should take caution when applying country-level data locally when developing strategies to address local evidence-practice gaps in HF.
Keywords: Delivery of Health Care, Health Services, Heart Failure
WHAT IS ALREADY KNOWN ON THIS TOPIC
Survival gaps in acute heart failure (HF) continue to expand globally. Multinational HF registries have highlighted variations between countries.
WHAT THIS STUDY ADDS
Major variations in HF practice can exist within cities with a mixed health infrastructure which is not highlighted in multinational HF registries. For example, in-hospital death in public hospital of Kolkov was 1.58 times greater than that in the private hospital within the same city, 3 miles apart.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND/OR POLICY
Physicians and policymakers should take caution when applying country-level data locally when developing strategies to address local evidence-practice gaps in HF.
Introduction
With over 35 million cases worldwide, heart failure (HF) constitutes a substantial burden of premature mortality and hospitalisations.1 2 Despite remarkable advances in HF treatment over the past 25 years, disparities in survival and evidence-based practice continue to expand, with disproportionately higher mortality inflicted on low-income and middle-income countries.3–7 In fact, it has already reached epidemic proportions in India, which faces a double burden from its indigenous rheumatic heart disease and rising prevalence of risk factors for HF, for example, ischaemic heart disease (IHD) and diabetes.5
To help reduce this gap, multinational registries such as the recent ASIAN-HF3 and REPORT-HF6 have comprehensively highlighted variations in phenotype, socioeconomic factors and management of acute HF (AHF). However, they are not without limitations. As these registries typically compare between countries, generalisability of its data becomes equivocal when comparing at regional level, hence inaccurate conclusions may be reached. For instance, cities such as Kolkata, India, consist of a mixture of public and private hospitals, each serving a distinct HF population with independent health policies. Clinical outcomes may thus be considerably different within the same geographical area. Even in England where healthcare is universal with discretionary insurance-based systems, private payers had significantly better outcomes after cardiac surgery than public users of the National Health Service (NHS) in a recent study.8 Corresponding data for HF is lacking and with increasing public scrutiny of health inequalities, an HF registry centred around this theme is timely.
A further limitation arises when classifying countries according to economic status and ethnicity. Although England is classed as a high-income country, its Government’s Index of Deprivation (based on multiple domains including health, education and living standards) acknowledges the varying levels of poverty between cities.9 Coventry, UK, is ranked 38th out of 326 cities (1=most deprived), indicating a relatively deprived area. Moreover, Coventry has one of the largest concentrations of South Asian residents (16.3%) in the UK, with a sizeable proportion of its HF population being first-generation Indians. Whereas only <2% of Scotland’s population are of South Asian ethnicity.10 11 Such economic and ethnic disparities within a country are rarely acknowledged in international HF registries.
Another shortcoming of current registries is the lack of data on organisational care which can influence the clinical outcomes of HF. It has been shown that managing HF on a cardiology ward or access to specialist input including HF nurse specialists (HFNS) affords better survival for patients with AHF; they are more likely to receive up-to-date echocardiography and for HF with reduced ejection fraction (HFrEF), more likely to receive disease-modifying therapies.12 13 Organisational care forms the benchmark of the UK National HF Audit (NHFA) under the National Institute of Cardiovascular Outcomes Research (NICOR). Through its efforts in driving such measures, UK has seen an 18% relative reduction in HF mortality over the past decade, despite an ageing population and stalled progress in AHF treatment.13 For these reasons, it will be insightful for HF registries to collect this information, beyond the usual data on clinical care.
KOLCOV is designed to address these limitations with two key objectives. First, we aim to determine the clinical profile and major determinants of mortality between Indian and British HF groups, uniquely choosing cities with comparable socioeconomic status served by different healthcare models. As the three hospitals are likely to serve different HF populations, we aim to quantify these differences in demographic features between countries and within a country and investigate their contribution to HF outcomes. This data may prove useful when planning targeted strategies in tackling evidence-practice gaps. Second, the overarching aim is to examine not only clinical but also, system-level factors that may sway the clinical course of AHF. This is essential in providing high-quality care to this growing population.
Methodology
Study design and setting
This was a multicentre observational registry that prospectively recruited patients with AHF over 24 months (2014–2016) in two hospitals of Kolkata, West Bengal, India, and contemporaneously compared with a UK hospital in Coventry, West Midlands, using its prospective NICOR database. Located at the heart of Kolkata are Apollo Gleneagles Hospital (AGH), a private hospital with over 100 coronary (CCU) and intensive care unit (ICU) beds, and Nil Ratan Sircar Medical College & Hospital (NRS), a government-funded hospital with about one-third equivalent beds. University Hospitals Coventry and Warwickshire (UHCW) is a tertiary public hospital with similar CCU/ICU capacities as NRS. From published epidemiological trends,10 11 14 both cities offer a fair representation of the respective country’s HF case mix. Despite the inevitable city-independent structural and organisational differences, we believe Coventry is a suitable city to compare its HF trend and universal healthcare against the mixed health infrastructure of Kolkata. At the same time, we can examine how the innate structural differences may impact on HF outcomes. The study was approved by local research ethics committee15 and was conducted in accordance with the Declaration of Helsinki.
Study population
Screening all patients admitted through emergency departments and acute medicine, consecutive patients (≥18 years) admitted with a primary diagnosis of AHF (de novo or worsening chronic HF) were recruited after informed consent. As the UK NICOR database was established for quality improvement as part of the national HF audit, Section 251 of the NHS Act 2006 granted a waiver for informed consent to collect anonymised data for the UHCW population.
Data collection
A shared database, adapted from the NICOR HF audit, standardised data collection. This was performed by cardiologists and experienced HFNS who verified the diagnosis of HF according to the 2016 European Society of Cardiology HF guidelines.16 Left ventricular ejection fraction (LVEF) <40% defined HFrEF. For patients with multiple hospitalisations, we selected the first admission as index. Key data included demography, comorbidities, HF characteristics, guideline-directed medical therapies (GDMT) at discharge, specialty input and place of care. Comorbidities relate to traditional risk factors for HF. GDMT refers to a combination of beta-blockers, renin-angiotensin-aldosterone system inhibitor (RAASi) and mineralocorticoid receptor antagonist. As reflected in UK NICOR HF audits,13 it is not possible to accurately ascertain the aetiology of HF during an acute admission and thus, this variable was not included in the data collection. Primary outcome was all-cause in-hospital mortality and length of hospital stay (LOHS) was secondary. Automated data check (on Microsoft Excel) prevented duplicate entries.
Statistical analysis
With a few missing data on comorbidities, multivariate models were fitted after imputation where absence of comorbidity was assumed for missing values.17 Patients without outcomes available were removed. A significant number of echocardiography reports at UHCW contained either qualitative assessment (eg, mild/moderate/severely impaired) or wide estimated ranges, for example, LVEF <40%. Hence, mean LVEF was unavailable for UHCW.
Categorical data were summarised by counts (percentages) and compared between hospitals with χ2 test. Continuous data reported as mean (SD) were assessed using t-test or Mann-Whitney U test depending on normality distribution. To adjust for three pairwise comparisons among the three hospitals, a p value<0.05/3 (Bonferroni correction) was considered statistically significant. Predictors of outcome were assessed separately for in-hospital mortality and LOHS. Likewise, data for each site were analysed separately since age markedly differed between hospitals. For in-hospital mortality, adjusted multivariable logistic regression analysis was performed. OR with 95% CIs were reported. For LOHS, a negative binomial model was fitted, providing rate ratios. To mitigate overfitting in multivariate analysis, a composite variable was computed from pre-defined comorbidities, categorising patients into ≤1 comorbidity versus ≥2 comorbidities. A two-sided p value<0.05 was considered statistically significant. The multivariable models included 10 variables that had data collected in all three hospitals. There were four deaths per predictor in NRS. In order to adjust for confounding, this is better than reducing the number of predictors in the multivariable models.18
Results
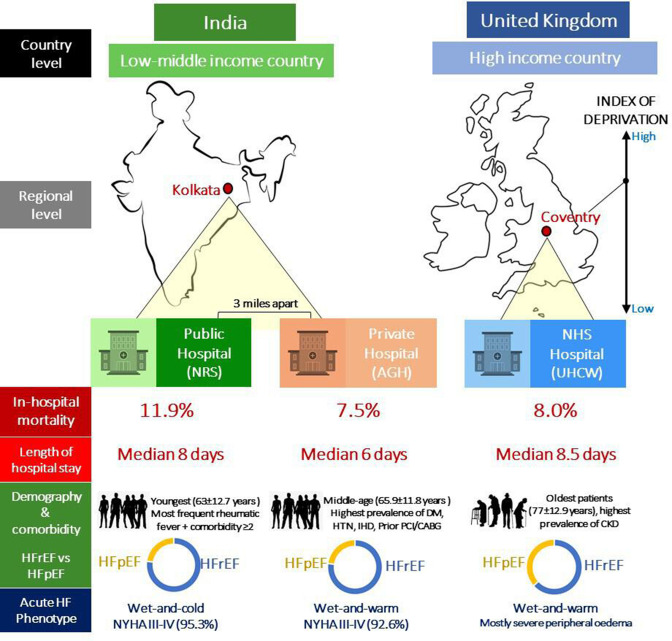
After excluding 15 patients (AGH=4 and NRS=11) without outcomes recorded, a total of 1652 patients with AHF were analysed (51% from Coventry and 49% Kolkata). Outcomes are presented in table 1. Government-funded NRS had the highest in-hospital mortality (11.9%) while only 3 miles north from it, the privately-run AGH had lower rates (7.5%, p=0.034), very similar to the universal health system of UHCW (8%). LOHS was significantly longer for those admitted to public hospitals (NRS 8 days and UHCW 8.5 days vs AGH 6 days, p<0.001). A detailed analysis below presents some interesting insight into these important differences.
Table 1.
Comparison of baseline characteristics, treatment and outcomes between hospitals of patients admitted with acute heart failure
| Characteristics | Kolkata (India) | UHCW (UK) (n=842) |
P value (accounting for multiplicity, difference statistically significant if p<0.05/3) | |||
| AGH (private) (n=508) |
NRS (public) (n=302) |
NRS–AGH | UHCW–AGH | UHCW–NRS | ||
| Demographics and medical history | ||||||
| Age - years, mean (SD) | 65.9 (11.8) | 63.0 (12.7) | 77.0 (12.9) | 0.001 | <0.001 | <0.001 |
| Female, n (%) | 171 (34) | 106 (35) | 363 (43) | 0.677 | 0.001 | 0.015 |
| Number of comorbidities, n (%)* | ||||||
| ≤1 2–8 |
416 (81.9) 92 (18.1) |
159 (52.6) 143 (47.4) |
509 (60.5) 333 (39.5) |
<0.001 | <0.001 | <0.001 |
| IHD, n (%): | 328 (66.1) | 93 (31.0) | 367 (44.3) | <0.001 | <0.001 | <0.001 |
| Valve disease, n (%): | 33 (6.6) | 53 (17.5) | 163 (19.6) | <0.001 | <0.001 | 0.453 |
| Hypertension, n (%): | 340 (67.9) | 128 (42.7) | 349 (41.9) | <0.001 | <0.001 | 0.829 |
| Diabetes, n (%): | 289 (57.1) | 114 (37.7) | 282 (33.8) | <0.001 | <0.001 | 0.219 |
| COPD/asthma, n (%): | 77 (15.3) | 16 (5.3) | 52 (6.2) | <0.001 | <0.001 | 0.562 |
| CKD, n (%): | 108 (21.4) | 32 (10.6) | 256 (36.0) | <0.001 | <0.001 | <0.001 |
| Revascularisation (PCI/CABG), n (%): | 159 (31.9) | 33 (10.9) | 142 (19.9) | <0.001 | <0.001 | 0.001 |
| Rheumatic fever, n (%): | 7 (1.4) | 28 (9.5) | 0 (0) | <0.001 | 0.002 | <0.001 |
| LVEF - mean (SD) | 40.8 (10.9) | 43.1 (14.8) | N/A | 0.01 | N/A | N/A |
| Classification of HF n (%) | ||||||
| HF with reduced ejection fraction HF with preserved ejection fraction No inpatient echocardiography available |
388 (76.4) 115 (22.6) 5 (1) |
216 (71.5) 58 (19.2) 28 (9.3) |
480 (57) 275 (32.7) 87 (10.3) |
<0.002 | <0.001 | <0.001 |
| Clinical and laboratory characteristics on admission | ||||||
| Weight - kg, mean (SD) | ||||||
| On admission On discharge |
65.7 (10.8) 57.3 (21.6) |
62.2 (14.1) 51.2 (24.4) |
78.9 (21.2) 57.4 (38.2) |
0.015 0.018 |
<0.001 1 |
<0.001 0.01 |
| Breathlessness, n (%) | ||||||
| No limitation of physical activity Slight limitation Marked limitation Symptoms at rest |
0 (0) 37 (7.4) 84 (16.8) 380 (75.8) |
0 (0) 14 (4.7) 84 (27.9) 203 (67.4) |
28 (4.2) 72 (10.8) 443 (66.2) 126 (18.8) |
0.001 | <0.001 | <0.001 |
| Peripheral oedema, n (%): | ||||||
| No Mild Moderate Severe |
355 (71.0) 32 (6.4) 103 (20.6) 10 (2.0) |
88 (29.2) 105 (34.9) 84 (27.9) 24 (8.0) |
80 (13.7) 118 (20.2) 260 (44.6) 125 (21.4) |
<0.001 | <0.001 | <0.001 |
| Systolic blood pressure | ||||||
| Mean (SD) Median (IQR) |
144 (32.6) 140 (120–170) |
117 (31.2) 110 (90–140) |
132 (26.4) 130 (115–150) |
<0.001 | <0.001 | <0.001 |
| Heart rate: | 32–170 | 30–180 | 40–174 | |||
| Mean (SD) Median (IQR) |
98 (23.3) 96 (82–113) |
120 (24.7) 120 (110–132) |
84 (20.2) 80 (70–96) |
<0.001 | <0.001 | <0.001 |
| Clinical and organisational care | ||||||
| Disease-modifying drugs, n (%)† | ||||||
| ACEi/ARB only Beta-blocker only ACEi /ARB and beta-blocker ACEi/ARB, beta-blocker and MRA No ACEi/ARB, beta-blocker and MRA Contraindication to ACE inhibitor/ARB Loop diuretic and/or thiazide diuretic |
47 (9.3) 65 (12.8) 27 (5.3) 42 (8.3) 138 (27.2) 51 (10) 394 (77.6) |
5 (1.6) 33 (10.6) 13 (4.2) 39 (12.9) 29 (9.6) 32 (16.8) 279 (89.4) |
89 (10.6) 154 (18.3) 239 (28.4) 127 (15.1) 157 (18.6) 22 (2.6) 672 (79.8) |
<0.001 0.43 0.52 0.03 <0.001 0.8 <0.001 |
<0.44 0.007 <0.001 <0.001 <0.001 <0.001 0.33 |
<0.001 0.003 <0.001 0.36 <0.001 <0.001 <0.001 |
| Device therapy, n (%)‡ | ||||||
| None Cardiac re-synchronisation therapy Implantable cardioverter-defibrillator Permanent pacemaker |
437 (86.9) 16 (3.2) 9 (1.8) 41 (8.1) |
278 (97.5) 0 0 7 (2.5) |
755 (90.9) 43 (5.2) 4 (0.5) 29 (3.5) |
<0.001 | <0.001 | <0.001 |
| Main place of care, n (%) | ||||||
| CCU/ICU ward§ |
473 (93.1) 35 (6.9) |
171 (56.6) 131 (43.4) |
504 (60.6) 327 (39.4) |
<0.001 | <0.001 | 0.222 |
| Specialist, input n (%) | ||||||
| Cardiologist Specialist HF nurse Non-cardiologist |
448 (88.2) 0 (0) 60 (11.8) |
297 (99.3) 0 (0) 2 (0.7) |
493 (62.2) 125 (15.8) 175 (22.1) |
<0.001 | <0.001 | <0.001 |
| Patient outcomes | ||||||
| Death in hospital, n (%) | 38 (7.5) | 36 (11.9) | 67 (8.0) | 0.034 | 0.75 | 0.039 |
| Length of hospital stay - median (IQR) | 6 (4–10) | 8 (7–11) | 8.5 (4–15) | <0.001 | <0.001 | 0.947 |
*This refers to the comorbidities included in the registry only.
†Medications on discharge (this does not include other drug combinations such as mineralocortoid antagonist with beta-blockers or ACEi).
‡Device therapy on or prior to admission.
§This refers to both medical (including cardiology) and non-medical wards.
ACEi, ACE inhibitor; AGH, Apollo Gleneagles Hospital; ARB, angiotensin receptor blockade; CABG, coronary artery bypass grafting; CCU, coronary care unit; CKD, chronic kidney disease with estimated glomerular filtration rate below 60mL/min/1.73m2; COPD, chronic obstructive pulmonary disease; HF, heart failure; ICU, intensive care unit; IHD, ischaemic heart disease; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NRS, Nil Ratan Sircar Hospital; PCI, percutaneous coronary intervention; UHCW, University Hospitals Coventry and Warwickshire.
Baseline characteristics
Demographics and clinical characteristics are summarised in table 1. Overall mean age was 69.9±14.5 years with 61% male patients. When assessed individually, each hospital had a distinct phenotype (figure 1). The private sector of AGH treated a relatively young HF population with significantly more frequent hypertension, diabetes, IHD and a preponderance of HFrEF (76.4%) similar to NRS (71.5%). Although NRS served the youngest population, it had the highest prevalence of rheumatic fever and patients with ≥2 comorbidities. UK population was significantly older (77±12.9 years)—over a decade older than Indian counterparts. UHCW largely stood as an intermediate phenotype between AGH and NRS in terms of comorbidities, although with less HFrEF (57%) as it was counterbalanced by a prevailing proportion of HF with preserved ejection fraction (HFpEF) (32.7%, vs AGH 22.6%, NRS 19.2%, p<0.001).
Figure 1.
Infographic illustration of different heart failure phenotypes and clinical outcomes between countries, regions and healthcare sectors in Kolkata, India and Coventry, UK. AGH, Apollo Gleneagles Hospital; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NRS, Nil Ratan Sircar Hospital; NYHA, New York Heart Association; UHCW, University Hospitals Coventry and Warwickshire.
On presentation, public users of NRS tended to be more symptomatic with a higher risk profile. New York Heart Association (NYHA) III-IV breathlessness with significantly lower average systolic blood pressure (SBP) and higher heart rates were experienced by 95.3% of its patients. Both Indian groups were more prone to NYHA IV breathlessness than peripheral oedema (suggesting acute left ventricular failure), whereas UHCW had significantly more cases of severe peripheral oedema. This correlated with a much higher admission weight at UHCW—by a difference of 16 kg greater than the lean population of NRS.
Clinical care
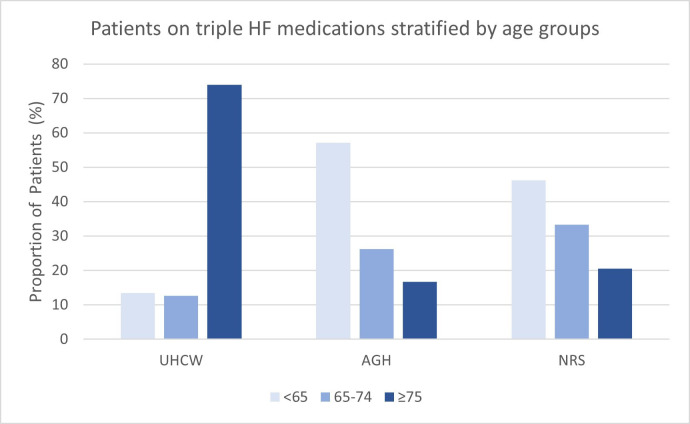
HF medications prescribed on discharge were grouped into monotherapy, dual therapy and triple therapy. Expectedly, diuretics were most extensively prescribed in all sites. Unexpectedly, AGH had the least number of patients discharged on triple GDMT (8.3%, p<0.001) compared with patients in the public sector (UHCW 15.1% vs NRS 12.9%, respectively, p=0.36). This pattern remained the case across HFrEF subgroups (online supplemental appendix S1). Another interesting finding is that when stratified to age, most patients in UHCW receiving GDMT were ≥75 years old whereas the reverse was observed in the Indian hospitals (figure 2). Compared with UHCW, RAASi was markedly underused in both Indian hospitals, partly contributed by the higher percentage of contraindications to ACE-inhibitors.
Figure 2.
Age distribution of patients with HF on ACE inhibitors/angiotensin receptor blockers, beta-blockers and mineralocorticoid receptor antagonists across the three hospitals. For UHCW, patients on triple therapy were primarily ≥75 years old, while this was the reverse in the Indian hospitals. AGH, Apollo Gleneagles Hospital; HF, heart failure; NRS, Nil Ratan Sircar Hospital; UHCW, University Hospitals Coventry and Warwickshire.
openhrt-2022-001964supp001.pdf (51.4KB, pdf)
Cardiac resynchronisation therapy (CRT) or implantable cardioverter-defibrillators (ICD) was low prior to or during admission with similar rates in UHCW and AGH (5.1% vs 3.1%, p=0.09). For NRS, no cases of CRT or ICD were identified. On further analysis, 42 patients in NRS met criteria for CRT implantation (ie, NYHA III-IV, LVEF ≤35% with left bundle branch block) who would have been potential candidates.
Organisational care
A striking difference was seen with place of care. While all hospitals admitted the majority of their patients to high-dependency units (CCU/ICU), this was most noticeable in AGH (93.1%, NRS 56.5%, UHCW 60.6%, p<0.001). Given the pro forma design, it was not possible to distinguish between care on CCU versus ICU. That said, cardiologist input for HF was greatest for NRS than AGH (99.3% vs AGH 88.2%, p<0.001) suggesting that a reasonable quantity of AGH patients were cared for by intensivists or general physicians instead. A unique feature of UHCW (not available in AGH and NRS) was the HFNS, who ensured that patients with AHF still received specialist input regardless of place of care.
Predictors of all-cause in-hospital mortality
As presented in table 2, independent prognostic factors were admission haemodynamics, electrolyte derangements, clinical and organisational care. SBP was a significant predictor of in-hospital mortality across all hospitals with the greatest effect seen in NRS. Statistically, every 5 mm Hg SBP increase was associated with a 22% reduction in OR of death. In clinical terms, it is the lower blood pressure at the time of admission that increases the risk of death from acute HF. For clinical care, patients not on any HF medications had markedly higher odds of death in the Indian cohort (AGH OR 7.8, 95% CI 2.25 to 26.9, p<0.001; NRS 10.2, 95% CI 2.02 to 51.1, p<0.005). This effect was absent in UHCW. Yet, being on GDMT was not a significant protective factor.
Table 2.
Predictors of all-cause in-hospital mortality (multivariable logistic analysis)
| Potential predictors | OR (95% CI), p value | ||
| AGH (n=508) | NRS (n=302) | UHCW (n=842) | |
| Demographic variables | |||
| Age (years)/5* | 1.04 (0.89 to 1.21), 0.646 | 1.13 (0.95 to 1.36), 0.167 | 1.13 (1.00 to 1.27), 0.057 |
| Female gender | 0.57 (0.25 to 1.30), 0.180 | 0.71 (0.28 to 1.84), 0.488 | 1.07 (0.63 to 1.83), 0.799 |
| Medical history | |||
| Number of comorbidities | |||
| 2–8 ≤1† |
0.43 (0.19 to 0.99), 0.047 | 2.08 (0.81 to 5.34), 0.130 | 0.92 (0.54 to 1.57), 0.760 |
| Presenting patient characteristics | |||
| Breathlessness: | |||
| At rest or minimal activity No marked limitation† |
1.25 (0.51 to 3.05), 0.631 | 0.76 (0.24 to 2.42), 0.646 | 1.46 (0.78 to 2.73), 0.232 |
| Peripheral oedema: | |||
| Severe Moderate Mild No† |
6.26 (0.98 to 40.1), 0.053 1.56 (0.64 to 3.84), 0.330 4.74 (1.67 to 13.46), 0.004 |
2.08 (0.44 to 9.90), 0.357 0.49 (0.15 to 1.62), 0.244 0.55 (0.18 to 1.71), 0.300 |
2.34 (0.92 to 5.94), 0.075 1.60 (0.66 to 3.87), 0.294 0.80 (0.29 to 2.20), 0.665 |
| Heart rate/5* | 0.99 (0.91 to 1.07), 0.749 | 1.01 (0.94 to 1.09), 0.704 | 1.04 (0.97 to 1.11), 0.235 |
| Systolic blood pressure/5* | 0.89 (0.84 to 0.95),<0.001 | 0.78 (0.70 to 0.87),<0.001 | 0.92 (0.88 to 0.97), 0.003 |
| Clinical and organisational care | |||
| Device therapy | 0.22 (0.05 to 1.00), 0.051 | 0.19 (0.01 to 2.78), 0.224 | 0.58 (0.20 to 1.68), 0.314 |
| Main place of care: | |||
| CCU/ICU ward† |
4.34 (0.52 to 36.24), 0.176 | 5.07 (1.42 to 18.08), 0.013 | 0.43 (0.16 to 1.16), 0.096 |
| Specialist: | |||
| Cardiologist Specialist HF nurse Non-cardiologist† |
1.04 (0.35 to 3.04), 0.948 N/A |
Not included in model since >99% patients cared by a cardiologist | 0.66 (0.22 to 1.93), 0.442 0.99 (0.49 to 1.97), 0.967 |
*OR is per increase of five units.
†Reference category.
AGH, Apollo Gleneagles Hospital; CCU, coronary care unit; HF, heart failure; ICU, intensive care unit; N/A, not available; NRS, Nil Ratan Sircar hospital; UHCW, University Hospitals Coventry and Warwickshire.
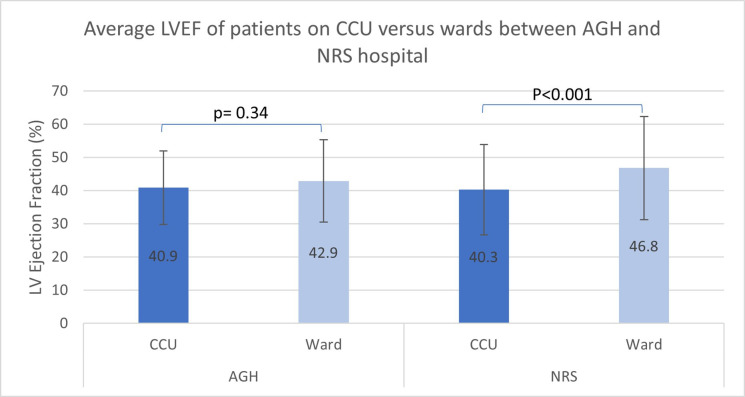
Organisational care was interesting. In NRS, patients admitted to CCU/ICU had a higher risk of in-hospital death (OR 5.07, 95% CI 1.42 to 18.08, p=0.013), while a similar but insignificant trend was seen for AGH. For NRS, this is somewhat explained by the significantly lower LVEF in patients on CCU than on the wards (LVEF 40.3%±13.6% vs 46.8±15.5%, p<0.001) (figure 3). Although not statistically significant, input from a cardiologist or HFNS emerged as a potential protective factor in UHCW.
Figure 3.
Average LVEF of patients between CCU and the wards across the two Indian hospitals. This was significantly different in NRS only which may be one possible reason for the higher mortality rate of patients cared for on CCU, compared with those on the wards. AGH, Apollo Gleneagles Hospital; CCU, coronary care unit; LVEF, left ventricular ejection fraction; NRS, Nil Ratan Sircar Hospital.
Predictors of length of stay
As presented in table 3, independent factors that prolonged hospitalisation were variable between hospitals. For UHCW, increased age and female gender significantly increased LOHS. Multiple comorbidities increased LOHS for the Indian cohort only. Exclusive to UHCW and NRS public sectors, increased breathlessness and peripheral oedema significantly lengthened LOHS. A noteworthy difference is that being under a private cardiologist in AGH was associated with reduced LOHS by 21% (p=0.002) compared with non-cardiologist care. However for UHCW, patients under a cardiologist (rate ratio (RR) 1.35, p=0.035) or receiving HFNS input (RR 2.15, p<0.001) were associated with prolonged hospitalisation. That said, the lower mortality and reduced LOHS in AGH are most probably explained by the fact that patients were less sick compared with the other hospitals.
Table 3.
Predictors of hospital length of stay (negative binomial analysis)
| Potential predictor | Rate ratio (95% CI), p value | ||
| AGH (n=470) | NRS (n=266) | UHCW (n=775) | |
| Demographic variables | |||
| Age (years)/5* | 1.01 (0.99 to 1.04), 0.293 | 0.99 (0.98 to 1.01), 0.425 | 1.06 (1.03 to 1.09),<0.001 |
| Gender: | |||
| Female Male† |
0.96 (0.86 to 1.07), 0.472 | 0.97 (0.89 to 1.06), 0.533 | 1.15 (1.01 to 1.30), 0.029 |
| Medical history | |||
| Number of comorbidities | |||
| 2–8 ≤1† |
1.16 (1.00 to 1.33), 0.048 | 1.13 (1.03 to 1.23), 0.007 | 0.97 (0.85 to 1.10), 0.617 |
| Presenting patient characteristics | |||
| Breathlessness: | |||
| At rest or minimal activity No to marked limitation† |
0.98 (0.87 to 1.11), 0.782 | 1.19 (1.07 to 1.33), 0.002 | 1.29 (1.09 to 1.52), 0.003 |
| Peripheral oedema: | |||
| Severe Moderate Mild No† |
0.81 (0.54 to 1.24), 0.333 1.05 (0.92 to 1.19), 0.495 0.96 (0.76 to 1.20), 0.716 |
1.13 (0.95 to 1.34), 0.157 0.89 (0.79 to 1.00), 0.047 0.88 (0.79 to 0.98), 0.019 |
1.79 (1.39 to 2.29), <0.001 1.20 (0.96 to 1.50), 0.101 1.02 (0.78 to 1.34), 0.874 |
| Heart rate/5* | 1.02 (1.00 to 1.03), 0.009 | 1.00 (0.99 to 1.01), 0.893 | 1.00 (0.98 to 1.02), 0.985 |
| Systolic blood pressure/5* | 0.99 (0.99 to 1.00), 0.197 | 0.99 (0.99 to 1.00), 0.097 | 0.98 (0.96 to 0.99), <0.001 |
| Clinical and organisational care | |||
| Device therapy | 1.07 (0.92 to 1.25), 0.357 | 0.98 (0.75 to 1.27), 0.867 | 1.28 (1.04 to 1.57), 0.021 |
| Main place of care: | |||
| CCU/ICU ward† |
1.27 (1.02 to 1.58), 0.035 | 0.99 (0.90 to 1.09), 0.873 | 1.15 (0.90 to 1.47), 0.275 |
| Specialist: | |||
| Cardiologist Specialist HF nurse Non-cardiologist† |
0.79 (0.67 to 0.92), 0.002 N/A |
1.03 (0.53 to 2.02), 0.924 N/A |
1.35 (1.02 to 1.78), 0.035 2.15 (1.75 to 2.65), <0.001 |
*OR is per increase of five units.
†Reference category.
CCU, coronary care unit; HF, heart failure; ICU, intensive care unit; N/A, not available; UHCW, University Hospitals Coventry and Warwickshire.
Discussion
Shining a novel light on HF registries, KOLCOV HF study has demonstrated variations in practices and outcomes of AHF not only between countries, but also intra-regionally between private (AGH) and public (NRS) health systems of Kolkata. Even with universal health coverage, Coventry also displayed similarities to Kolkata in socioeconomic status (indicated by its index of deprivation) and mutual HF characteristics with AGH and NRS. This raises an important caveat when interpreting international registries because the picture of AHF drawn of a particular country may be incomplete and not entirely generalisable. One may argue that random effects balance out, but unequal balances of private and public hospitals within cities, for example, in India,5 may still skew the data. Apart from socioeconomic disparities, KOLCOV has shown that organisational factors also play a role in health inequalities. To develop individualised strategies to tackle this, we evaluate our findings against multinational registries to unearth any meaningful lessons.
Differences in all-cause in-hospital mortality
The large survival gap in AHF between Western countries and South Asia is well-recognised. US registries report in-hospital HF mortality as low as 3%–4%,19–21 European registries between 5%–7%22 23 and South Asians at least 8%.3 7 24 This latter figure echoes that of UHCW and AGH. However, only 3 miles away from AGH, the publicly run NRS had the highest mortality (11.9%). Averaging mortality rates for a city, let alone a country, would have overlooked this marked contrast. The elevated deaths may be driven by non-cardiovascular causes, for example, infections, especially since rheumatic fever was most prevalent in NRS. In fact, improvements in global cardiovascular mortality have been offset by rising non-cardiovascular deaths, namely from infections and respiratory diseases.25 Hence, to reduce all-cause mortality, cardiologists and policymakers should prioritise on improving the management of comorbidities regardless of age.26
Distinct clinical phenotypes
Consistent with other registries, patients with AHF from South Asia were generally 10 years younger than Western counterparts.3 7 19 23 24 Our findings support this, along with a similar 70:30 male to female ratio. It is concerning that the youngest patients with HF of NRS were associated with the highest mortality—a similar observation for an Indian cohort from a subgroup analysis of PARADIGM-HF.27 This may reflect more advanced disease, as judged by the higher proportion of Indian patients with NYHA III-IV breathlessness, in agreement with ASIAN-HF and INTER-CHF registries.3 7 Patients admitted to AGH were evidently less sick with a higher admission BP and lower comorbidity burden than NRS, which would account for it having the lowest mortality and shortest length of stay. In contrast, NRS patients had significantly lower SBP with tachycardia on admission, suggesting a wet-and-cold profile (ie, congested with hypoperfusion) which has a higher risk of death than wet-and-warm.16 22 28 One reason for this sicker profile could be late presentation of a relatively poorer, less educated and predominantly rural population accessing services at NRS. From India’s Consensus, 72% of its population resides in rural areas, far away from metropolitan hospitals and hence, most would require transport by train to reach NRS.5 Educational awareness and accessibility are therefore fundamental to restore equity in HF care.
Distribution of HFrEF and HFpEF also differed between UK and India, but this may change. According to UK NHFA, HFpEF is emerging as the predominant form in parallel with UHCW’s cohort.13 HFrEF overshadows the Indian HF population, for now. With rising prevalence of diabetes, hypertension and IHD, it is a matter of time before HFpEF overtakes. This may eventually translate to even higher mortality rates unless prompt action is taken. Clearly, the success of future HF management requires modification of the natural history of HF through aggressive comorbidity control to halt the progression of patients to HFpEF, a syndrome with dismal prognosis and treatment options.29
Disparities in medical and device therapies
One of NICOR’s key performance indicators (KPI) is for >85% HFrEF to receive GDMT on discharge. None of the hospitals reached this level, irrespective of their financial infrastructure. Significant underuse of GDMT, an independent predictor of mortality, was also noted in the Trivandrum Indian registry.4 24 Contraindications to RAASi were high in Kolkata but did not fully account for the low prescription levels. Side effects may contribute since a higher incidence of ACEi-related cough has been reported among Indians than Western cohorts.30 Hence, it is acknowledged that some patients may be on optimal therapy but not necessarily triple therapy due to such contraindication or drug intolerance. Some suggest that frailty in the elderly may be another prohibiting factor to optimal therapy.23 31 Our registry does not support this since Kolkata patients were mainly young and for UHCW, the main age group to receive GDMT were ≥75 years (figure 3). Importantly, physicians and patients should acknowledge this ‘risk-treatment paradox’ where those at greatest risk (eg, relatively older with CKD, lower SBP and higher NYHA class) are more likely to benefit but least likely to receive GDMT for fear of side effects.20 32 33 Education is pivotal to addressing this gap. The advent of sacubitril-valsartan and sodium glucose-transporter 2 inhibitors in the management of HFrEF since the completion of this HF registry may further increase the inequality gap, due to increased cost and potential compliance issues from increased medication burden.
Implantation of CRT/ICD devices were also sparse. UHCW had the highest implantation rate (although 5.1%), AGH similar while NRS had no cases even though 42 patients were retrospectively identified as potential CRT candidates. This was mirrored by previous registries and surveys.3 4 34 Reasons may include cost, limited expertise availability or cross-cultural differences. A survey investigating patients’ willingness to adopt unfamiliar therapies or new technologies (termed ‘innovativeness’) found that Caucasians had higher innovativeness than ethnic minorities.35 The public Indian hospital had no patients undergoing CRT or ICD; this would have impacted on mortality in the long-term. In any case, no single drug or device is yet effective in swaying the clinical course of AHF. The cornerstone of successful care involves a multidisciplinary approach to address precipitants of HF, reduce congestion and support organs, through which an optimal organisational framework is key.
Variations in organisational care between health systems
NICOR recommends that >85% of AHF cases should receive cardiologist input and >60% to be admitted to cardiology, both of which are associated with improved mortality for HFrEF and HFpEF.13 36 AGH exceeded these KPIs, followed by NRS and least with UHCW. Admission to CCU/ICU in AGH may not necessarily be due to clinical but financial reasons (eg, expenses for oxygen use). Conversely, with limited CCU/ICU capacity in the public sector, it is essential to identify clinical features of high-risk patients who require intensive monitoring. Based on other HF registries,3 24 37 this would include patients with multiple comorbidities presenting with marked breathlessness at rest, severe peripheral oedema and a low SBP, of which the latter was found to be a significant predictor in KOLCOV. Furthermore, due to limited bed capacity in public hospitals, some patients with AHF end up on non-cardiology wards, with NRS having a higher proportion of this group than UHCW. The aforementioned HFNS team can help offer specialist input to these patients regardless of place of care, potentially mitigating the high mortality for NRS. This does not currently exist in Kolkata but could be developed.
Admission to CCU or a cardiology ward may not always be beneficial to all patients. Some with overarching medical needs would be best provided by another specialty. For example, patients with HF with complex airways disease on non-invasive ventilation would appropriately be cared for on a respiratory ward and cases of advanced dementia better managed by gerontology.12 Additionally, LOHS varied between settings, which in turn may influence the prescription of GDMT on discharge. AGH had the shortest LOHS (6 days) and lowest proportion of patients on GDMT. Private physicians may favour discharging patients earlier once the acute phase of HF has resolved, with less impetus to initiate all GDMT pre-discharge. One reason is to reduce the financial burden on patients without health insurance, paying out of pocket. European registries reveal a mean LOHS of 9 days23 37 similar to UHCW and NRS (8–8.5 days). Indeed, very short or long LOHS has been associated with worser mortality in AHF.38 Ideally, physicians should ensure that evidence-based measures are completed before discharge.
Strengths and limitations
While many observational registries rely on voluntary participation open to selection bias, KOLCOV employs the NICOR database to capture a representative UK sample. Another strength is that HF diagnoses were verified by the cardiology team rather than dependent on ICD-9 codes, usually entered by non-clinical coders on billing or discharge records, which can be prone to errors.12 Given the intrinsic weakness of observational registries, the data do not define cause-and-effect but associations between variables and outcomes. Some variables have relatively wide CIs, reflecting a fairly small number of deaths but as previously shown, our mortality data correspond with larger registries. Further data on all-cause versus cardiovascular mortality, modality of referral and de novo versus acute-on-chronic HF decompensation would have strengthened this registry, but we believe that this limitation does not alter the overall message and insight offered by KOLKOV. Finally, outcomes beyond discharge, for example, 1 year mortality were not included, but this can introduce attrition bias since many Indian patients live in rural areas without reliable means of follow-up.
Conclusion
Further enriching current data on global HF trends, we have shown that there can be both similarities and marked differences in HF characteristics, treatment patterns and outcomes intra-regionally and between low–middle versus high-income countries across private, public and universal healthcare systems. The type of healthcare model can impact on the access to emergency care, time of presentation from symptom onset and medication adherence on discharge, all of which can ultimately influence HF prognosis. This alerts physicians and policymakers to take caution when applying country-level data locally especially when developing strategies to address local evidence-practice gaps in HF. Such strategies should be individualised to their distinctive HF population, organisational structure and healthcare model, implementing strengths of the NICOR initiatives to tackle health inequalities.
Footnotes
Twitter: @banerjeep
Contributors: PB, SB and SKH conceptualised and designed the study. AD, MR, NW and MD contributed to conduction of study including patient recruitment, consent and data collection. PK was responsible for statistical analysis of data. PB, SKH, PK, PT and PB contributed to data interpretation. PT contributed to statistical analysis and prepared the draft manuscript with important input and further revisions from PK, PB and SB. All authors approved final version of the manuscript to be published. PB is the guarantor of this publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 2014;63:1123–33. 10.1016/j.jacc.2013.11.053 [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. The war against heart failure: the Lancet lecture. Lancet 2015;385:812–24. 10.1016/S0140-6736(14)61889-4 [DOI] [PubMed] [Google Scholar]
- 3.MacDonald MR, Tay WT, Teng T-HK, et al. Regional variation of mortality in heart failure with reduced and preserved ejection fraction across Asia: outcomes in the ASIAN-HF registry. J Am Heart Assoc 2020;9:e012199. 10.1161/JAHA.119.012199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harikrishnan S, Jeemon P, Ganapathi S, et al. Five-year mortality and readmission rates in patients with heart failure in India: results from the Trivandrum heart failure registry. Int J Cardiol 2021;326:139–43. 10.1016/j.ijcard.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guha S, Harikrishnan S, Ray S, et al. CSI position statement on management of heart failure in India. Indian Heart J 2018;70 Suppl 1:S1–72. 10.1016/j.ihj.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tromp J, Bamadhaj S, Cleland JGF, et al. Post-discharge prognosis of patients admitted to hospital for heart failure by world region, and national level of income and income disparity (REPORT-HF): a cohort study. Lancet Glob Health 2020;8:e411–22. 10.1016/S2214-109X(20)30004-8 [DOI] [PubMed] [Google Scholar]
- 7.Dokainish H, Teo K, Zhu J, et al. Global mortality variations in patients with heart failure: results from the International Congestive Heart Failure (INTER-CHF) prospective cohort study. Lancet Glob Health 2017;5:e665–72. 10.1016/S2214-109X(17)30196-1 [DOI] [PubMed] [Google Scholar]
- 8.Benedetto U, Dimagli A, Gibbison B, et al. Disparity in clinical outcomes after cardiac surgery between private and public (NHS) payers in England. Lancet Reg Health Eur 2021;1:100003. 10.1016/j.lanepe.2020.100003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Group CCC . Insight- the English indices of deprivation 2019. summary for coventry. 30/03/2021 ED. (online). Available: English_Indices_of_Deprivation_2019___Coventry_summary.pdf [Accessed 30 Mar 2021].
- 10.Council CC . Population and demographics: ethnicity and Religion.2011 (census). Available: https://www.coventry.gov.uk/info/195/facts_about_coventry/2435/population_and_demographics/3 [Accessed 30 Mar 2021].
- 11.Foundation BH . Local heart and circulatory disease statistics from the British heart Foundation. coventry and Warwickshire (STP), 2021. Available: coventry-and-warwickshire-bhf-statistics.pdf [Accessed 30 Mar 2021].
- 12.Tran P, McDonald M, Kunaselan L, et al. A hundred heart failure deaths: lessons learnt from the Dr Foster heart failure hospital mortality alert. Open Heart 2019;6:e000970. 10.1136/openhrt-2018-000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.13. National Heart Failure Audit (NHFA) . 2020 summary report (2018/19 data) National Institute for cardiovascular outcomes research (NICOR. Institute of cardiovascular science, University College London, 2020. https://www.nicor.org.uk/wp-content/uploads/2020/12/National-Heart-Failure-Audit-2020-FINAL.pdf [Google Scholar]
- 14.Das M, Pal S, Ghosh A. Prevalence of cardiovascular disease risk factors by habitat: a study on adult Asian Indians in West Bengal, India. Anthropol Anz 2011;68:253–64. 10.1127/0003-5548/2011/0099 [DOI] [PubMed] [Google Scholar]
- 15.Authority NHR . KOLCOV HF study: the Kolkata-Coventry heart failure comparative registry study of acute heart failure. IRAS ID 158727 NHS England. KOLCOV HF Study - Health Research Authority
- 16.Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. 10.1093/eurheartj/ehw128 [DOI] [PubMed] [Google Scholar]
- 17.Cattle BA, Baxter PD, Greenwood DC, et al. Multiple imputation for completion of a national clinical audit dataset. Stat Med 2011;30:2736–53. 10.1002/sim.4314 [DOI] [PubMed] [Google Scholar]
- 18.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and COX regression. Am J Epidemiol 2007;165:710–8. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 19.Kociol RD, Hammill BG, Fonarow GC, et al. Generalizability and longitudinal outcomes of a national heart failure clinical registry: comparison of acute decompensated heart failure national registry (ADHERE) and non-ADHERE Medicare beneficiaries. Am Heart J 2010;160:885–92. 10.1016/j.ahj.2010.07.020 [DOI] [PubMed] [Google Scholar]
- 20.Peterson PN, Rumsfeld JS, Liang L, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American heart association get with the guidelines program. Circ Cardiovasc Qual Outcomes 2010;3:25–32. 10.1161/CIRCOUTCOMES.109.854877 [DOI] [PubMed] [Google Scholar]
- 21.Abraham WT, Fonarow GC, Albert NM, et al. Predictors of in-hospital mortality in patients hospitalized for heart failure: insights from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J Am Coll Cardiol 2008;52:347–56. 10.1016/j.jacc.2008.04.028 [DOI] [PubMed] [Google Scholar]
- 22.Chioncel O, Mebazaa A, Maggioni AP, et al. Acute heart failure congestion and perfusion status - impact of the clinical classification on in-hospital and long-term outcomes; insights from the ESC-EORP-HFA Heart Failure Long-Term Registry. Eur J Heart Fail 2019;21:1338–52. 10.1002/ejhf.1492 [DOI] [PubMed] [Google Scholar]
- 23.Nieminen MS, Brutsaert D, Dickstein K, et al. EuroHeart failure survey II (EHFS II): a survey on hospitalized acute heart failure patients: description of population. Eur Heart J 2006;27:2725–36. 10.1093/eurheartj/ehl193 [DOI] [PubMed] [Google Scholar]
- 24.Sanjay G, Jeemon P, Agarwal A, et al. In-hospital and three-year outcomes of heart failure patients in South India: the Trivandrum heart failure registry. J Card Fail 2018;24:842–8. 10.1016/j.cardfail.2018.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conrad N, Judge A, Canoy D, et al. Diagnostic tests, drug prescriptions, and follow-up patterns after incident heart failure: a cohort study of 93,000 UK patients. PLoS Med 2019;16:e1002805. 10.1371/journal.pmed.1002805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Conrad N, Judge A, Canoy D, et al. Temporal Trends and Patterns in Mortality After Incident Heart Failure: A Longitudinal Analysis of 86 000 Individuals. JAMA Cardiol 2019;4:1102–11. 10.1001/jamacardio.2019.3593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kristensen SL, Martinez F, Jhund PS, et al. Geographic variations in the PARADIGM-HF heart failure trial. Eur Heart J 2016;37:3167–74. 10.1093/eurheartj/ehw226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol 2003;41:1797–804. 10.1016/S0735-1097(03)00309-7 [DOI] [PubMed] [Google Scholar]
- 29.Banerjee P, Motiwala A, Mustafa HM, et al. Does left ventricular diastolic dysfunction progress through stages? Insights from a community heart failure study. Int J Cardiol 2016;221:850–4. 10.1016/j.ijcard.2016.07.091 [DOI] [PubMed] [Google Scholar]
- 30.Singh NP, Uppal M, Anuradha S, et al. Angiotensin converting enzyme inhibitors and cough--a north Indian study. J Assoc Physicians India 1998;46:448–51. [PubMed] [Google Scholar]
- 31.Parmar KR, Xiu PY, Chowdhury MR, et al. In-hospital treatment and outcomes of heart failure in specialist and non-specialist services: a retrospective cohort study in the elderly. Open Heart 2015;2:e000095. 10.1136/openhrt-2014-000095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterson PN, Rumsfeld JS, Liang L, et al. Treatment and risk in heart failure: gaps in evidence or quality? Circ Cardiovasc Qual Outcomes 2010;3:309–15. 10.1161/CIRCOUTCOMES.109.879478 [DOI] [PubMed] [Google Scholar]
- 33.Greene SJ, Butler J, Albert NM, et al. Medical Therapy for Heart Failure With Reduced Ejection Fraction: The CHAMP-HF Registry. J Am Coll Cardiol 2018;72:351–66. 10.1016/j.jacc.2018.04.070 [DOI] [PubMed] [Google Scholar]
- 34.Shenthar J, Bohra S, Jetley V, et al. A survey of cardiac implantable electronic device implantation in India: by Indian Society of Electrocardiology and Indian heart rhythm Society. Indian Heart J 2016;68:68–71. 10.1016/j.ihj.2015.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groeneveld PW, Sonnad SS, Lee AK, et al. Racial differences in attitudes toward innovative medical technology. J Gen Intern Med 2006;21:559–63. 10.1111/j.1525-1497.2006.00453.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawley A, He J, Crabtree A, et al. The impact of an integrated heart failure service in a medium-sized district general hospital. Open Heart 2020;7:e001218. 10.1136/openhrt-2019-001218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavazzi L, Maggioni AP, Lucci D, et al. Nationwide survey on acute heart failure in cardiology ward services in Italy. Eur Heart J 2006;27:1207–15. 10.1093/eurheartj/ehi845 [DOI] [PubMed] [Google Scholar]
- 38.Sud M, Yu B, Wijeysundera HC, et al. Associations Between Short or Long Length of Stay and 30-Day Readmission and Mortality in Hospitalized Patients With Heart Failure. JACC Heart Fail 2017;5:578–88. 10.1016/j.jchf.2017.03.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-001964supp001.pdf (51.4KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.