Abstract
Bacterial larvicides Bacillus thuringiensis var. israelensis (Bti) and Bacillus sphaericus (Bs) have been used extensively for mosquito control. However, their efficacy varies greatly mainly due to factors related to target mosquitoes, larval habitat conditions, and inherent larvicide properties. We evaluated the efficacy of Bti (Bactivec®) and Bs (Griselesf®) for control of Anopheles gambiae complex, Culex quinquefasciatus and Aedes aegypti larvae under laboratory and semi-field conditions in northeastern Tanzania. Laboratory bioassays were conducted with five to six different concentrations of Bti and Bs, replicated four times and the experiment repeated on three different days. Larvae mortality was recorded at 24 or 48 h after the application of larvicide and subjected to Probit analysis. Laboratory bioassays were followed by semi-field trials to establish initial and residual activity of Bti and Bs. Semi-field trials were conducted in artificial larval habitats in the open sunlit ground and in “mosquito spheres”. These artificial larval habitats were colonized with mosquito larvae, treated with Bti and Bs, and the impact of treatments on mosquito larvae was monitored daily. Lethal concentration values that caused 50% and 95% mortalities of test larvae (LC50 and LC95) showed that An. gambiae complex and Cx. quinquefasciatus tested were highly susceptible to Bti and Bs under laboratory conditions. Likewise, larvae of Ae. aegypti were highly susceptible to Bti, with LC95 value as low as 0.052 mg/l. However, Ae. aegypti larvae were not susceptible to Bs under practical doses of laboratory settings. In semi-field trials, all treatment dosages for Bti provided 91.0–100% larval mortality within 24 h whereas Bs resulted in 96.8–100% larval mortality within the same time-frame. Bs had a more prolonged residual activity, with pupal reductions range of 55.7–100% for 9 days at all application rates while the corresponding pupal reduction with Bti was 15.4–100% for 5 days. Due to the low residual activity of Bti and Bs tested, weekly application at a maximum label rate would be appropriate to reduce mosquito larvae in natural larval habitats. Based on laboratory findings, Bs product tested would not be recommended for use in the control of Ae. aegypti.
Keywords: Anopheles gambiae complex, Culex quinquefasciatus, Aedes aegypti, Bacillus thuringiensis var. israelensis, Bacillus sphaericus, Northeastern Tanzania
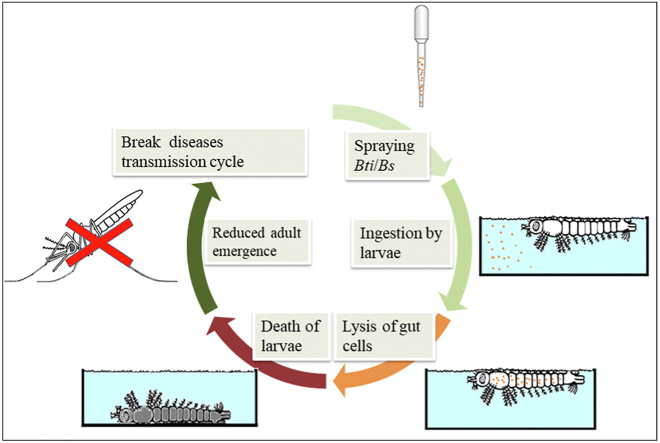
Graphical abstract
Highlights
-
•
Bacillus thuringiensis var. israelensis (Bti) and Bacillus sphaericus (Bs) tested in laboratory and semi-field conditions.
-
•
Low concentrations of Bti and Bs were effective in controlling larvae of An. gambiae complex and Cx. quinquefasciatus.
-
•
In laboratory conditions, larvae of Aedes aegypti were highly susceptible to Bti but not to Bs.
-
•
The Bti and Bs products tested were less persistent and would require weekly application in natural larval habitats.
1. Background
Mosquito-borne diseases pose a major threat to the health of human populations in tropical and subtropical areas of the world (Tolle, 2009). Over the past decades, the burden of more traditional mosquito-borne diseases such as malaria and lymphatic filariasis (LF) has been compounded by emerging and re-emerging mosquito-borne arboviruses like yellow fever, dengue, chikungunya and Zika (Benelli, 2016; Huang et al., 2019). The distribution of these diseases has expanded and caused epidemics in different parts of the world. In recent years, outbreaks of one or more mosquito-borne arboviruses have been reported in the Pacific and America (Musso et al., 2018; Paixão et al., 2018; Espinal et al., 2019), Asia (Gautam et al., 2017), and the African region (Weetman et al., 2018). The impact of mosquito-borne arboviruses has also been felt in other regions beyond endemic areas (Gautam et al., 2017). Unlike malaria and LF which are responsive to chemotherapy, control of mosquito-borne arboviruses relies mainly on the prevention of mosquito bites and good clinical care to the infected individuals. Except for yellow fever, vaccines for other emerging mosquito-borne arboviruses are in different phases of development (Jentes et al., 2011; Gautam et al., 2017).
Prevention of mosquito bites has remained an important strategy to reduce mosquito-borne diseases. Efforts to control mosquitoes, particularly malaria vectors have relied mainly on the use of long-lasting insecticide-treated bednets (LLINs) and/or indoor residual spraying (IRS). However, these insecticide-based mosquito control interventions are threatened by development and widespread insecticide resistance and behavioral adaptations by the vectors (Kleinschmidt et al., 2018; Protopopoff et al., 2018). On the other hand, Culex quinquefasciatus, an important nuisance, and filarial mosquito vector remained largely unaffected by insecticides applied for malaria vector control (Magesa et al., 1991). Moreover, insecticide resistance has emerged in Aedes aegypti mosquito populations worldwide (Vontas et al., 2012).
Integrated mosquito control interventions have a proven record of lowering mosquito-borne disease transmission and even eradication of mosquitoes (see examples in Killeen et al., 2002a). It has been shown that, unlike adult mosquitoes, larvae cannot change their behavior to avoid a control intervention targeted at larval habitats (Killeen et al., 2002b). Moreover, larval control strategies also serve to extend the useful life of insecticides against adult mosquitoes and the strategy is equally effective in controlling both indoor- and outdoor-biting mosquitoes. Integrating larviciding with adult mosquito control interventions like LLINs and/or IRS has been considered to be a highly effective strategy to control malaria (Fillinger et al., 2009). Larviciding with chemical agents was historically an important component of malaria vector control in endemic countries (Shousha, 1948; Killeen et al., 2002a). However, due to significant adverse effects on other non-target species, preference has been shifted to the use of microbial larvicides, Bacillus thuringiensis var. israelensis (Bti) and Bacillus sphaericus (Bs), which selectively kill mosquito larvae with negligible effect on non-target organisms (Mittal, 2003; Walker & Lynch, 2007). However, the efficacy of Bti and Bs has also been reported to vary greatly, mainly due to factors related to target mosquitoes, larval habitat conditions, and inherent larvicide properties (Mulla et al., 1990; Lacey, 2007; Walker & Lynch, 2007). Due to this heterogeneity of their activity, the efficacy of a particular microbial larvicide product needs to be validated against the natural mosquito population in different ecological settings of endemic areas before their widescale application.
Mosquito larval control by applying Bti and Bs has been widely practiced in different ecological settings in Tanzania (Ragoonanansingh et al., 1992; Fillinger et al., 2008; Geissbühler et al., 2009; Magesa et al., 2009; Kramer et al., 2014; Msellemu et al., 2016; Mazigo et al., 2019). In the control areas, bacterial larvicide interventions were found to be effective in controlling malaria vectors, safe to non-target organisms (Magesa et al., 2009), accepted by the general community (Magesa et al., 2009; Mboera et al., 2014; Mazigo et al., 2019), and their cost compared fairly well with those of other malaria vector control measures practiced in sub-Saharan Africa (Worrall & Fillinger, 2011; Maheu-Giroux & Castro, 2014; Rahman et al., 2016). With an ambitious goal to eliminate lymphatic filariasis and the recent outbreaks of mosquito-borne arboviruses in the country, the government has expanded the larviciding programmes to target also Cx. quinquefasciatus and Ae. aegypti. However, before the large-scale application of larvicides, it is important to establish baseline information on the susceptibility status of the target mosquito vectors. This information is useful for monitoring changes in the susceptibility of the target mosquito vectors in the future (Wirth et al., 2001). The present study evaluated the efficacy of locally produced microbial larvicides Bactivec® (Bti) and Griselesf® (Bs) against mosquito larvae under laboratory and semi-field conditions.
2. Materials and methods
2.1. Study area and design
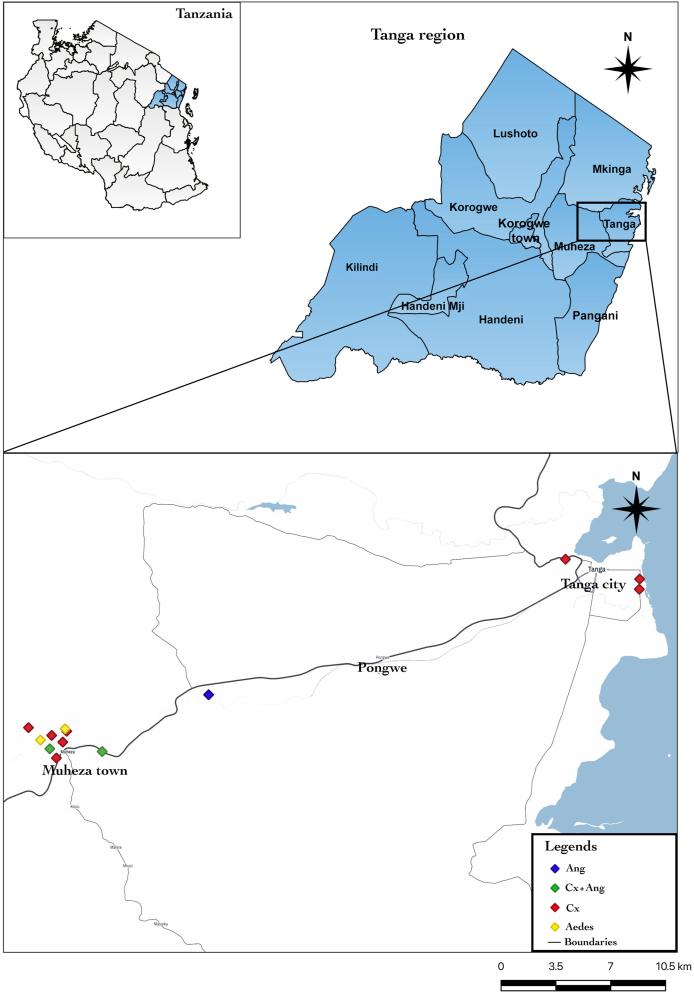
The study was conducted at the compounds of the Amani Medical Research Centre in Muheza, Tanga, north-eastern Tanzania (−5.1638°S, 38.7932°E) from June to August 2019. Laboratory bioassays were conducted at the institute’s insecticide testing facility whereas semi-field trials were conducted at open ground and in the “mosquito spheres” (a simulated field test facility) at the institute’s premises previously described (Kitau et al., 2010). Mosquito larvae for laboratory bioassays and “mosquito spheres” trials were collected from different ecological settings in Muheza and Tanga City, north-eastern Tanzania. Anopheles gambiae complex larvae were collected from a variety of larval habitats including hoof prints, rice fields, ponds, and roadside canals. Larvae of Cx. quinquefasciatus and Ae. aegypti were collected from urban areas of Tanga City and around Muheza town, respectively (Fig. 1). Culex quinquefasciatus larvae were sampled from drainage canals, small pools, and polluted marshes near human habitats, whereas Ae. aegypti larvae were mostly collected from abandoned car tires. Upon collection, larvae were transferred to the “mosquito spheres”, sorted by larval instars, and maintained following recommended standard mosquito rearing techniques (Benedict, 2007). The collected larvae were not subjected to larval bioassay for at least 12 h to allow them to adjust to the change of the environment (acclimatization).
Fig. 1.
Location of study sites in Tanga, northeastern Tanzania. Abbreviations: Ang., Anopheles gambiae complex; Cx + Ang, Culex + An. gambiae complex; Cx, Culex.
2.2. Laboratory trials
Susceptibility of field-collected An. gambiae complex, Cx. quinquefasciatus and Ae. aegypti to Bti and Bs were compared to standard insectary-reared larvae of the respective species, namely An. gambiae (sensu stricto) (Kisumu strain), Cx. quinquefasciatus (Tropical Pesticides Research Institute, TPRI strain) and Ae. aegypti (London School of Hygiene and Tropical Medicine, LSHTM strain). The standard reference colony of An. gambiae (s.s.) (Kisumu strain) had been maintained for several generations at the insectary facility of the Amani Medical Research Centre whereas Cx. quinquefasciatus (TPRI strain) and Ae. aegypti (LSHTM strain) were generously provided by the Pan-African Malaria Vector Control Consortium (PAMVEC) test facility at Kilimanjaro Christian Medical University College in Moshi, Tanzania.
2.3. Test biolarvicides preparation
Tested bacterial larvicides Bactivec® (Bti) and Griselesf® (Bs) contain spores and endotoxin crystals of Bacillus thuringiensis var. israelensis (serotype H-14, strain 266/2; biopotency ≥ 1200 international toxic units (ITU)/mg) and Bacillus sphaericus (strain 2362, potency 268 ITU/mg) as active ingredients, respectively. The two biolarvicide products were supplied by Tanzania Biotech Products Limited located in Kibaha, Tanzania. According to the product label, the concentration of Bti and Bs was 6 g/l and 5 g/l, respectively. Manufacturers recommended dosage (label rate) for field application was 2–5 ml/m2 for Bti and 5–10 ml/m2 for Bs.
To prepare a stock solution, 2 ml aliquots of ready-to-use Bti and Bs were measured and stored in a refrigerator at 2–8 °C until use. On the test day, a 2 ml aliquot stock solution for either Bti or Bs was serially diluted in distilled water as previously recommended (WHO, 2005). For Bti, a 10-fold dilution series was prepared by first transferring 2 ml of stock solution to 18 ml of distilled water to make 0.6 mg/ml concentration, and then by subsequently repeating this procedure by transferring 2 ml of the latest solution to 18 ml of distilled water to make 6 × 10−2, 6 × 10−3 and 6 × 10−4 mg/ml. Following the same procedure, 10-fold serial dilution for Bs gave 5 × 10−1, 5 × 10−2, 5 × 10−3 and 5 × 10−4 mg/ml. The last three dilutions (Bti: 6 × 10−2, 6 × 10−3 and 6 × 10−4 mg/ml; Bs: 5 × 10−2, 5 × 10−3 and 5 × 10−4 mg/ml) were used in the subsequent larvicide bioassays.
2.4. Larval bioassay experiments
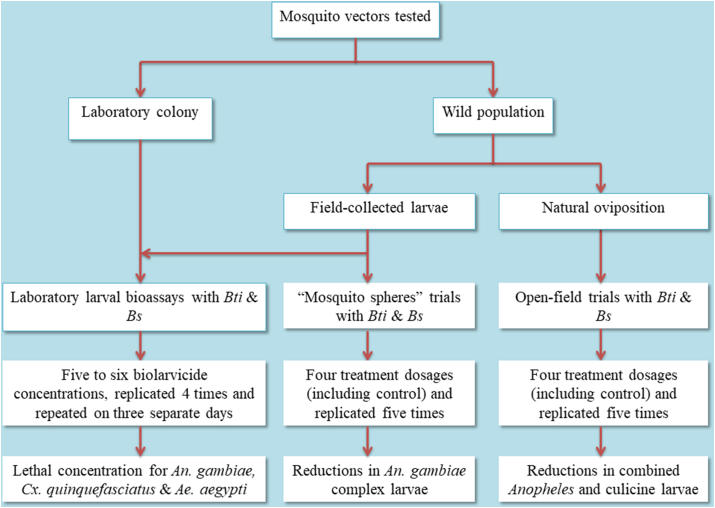
Larval bioassay experiments with An. gambiae complex, Cx. quinquefasciatus and Ae. aegypti were run on separate days. For each species, two separate larval bioassay experiments testing the susceptibility of wild populations to Bti and Bs were conducted. Two additional bioassay experiments, each testing Bti and Bs against a susceptible reference laboratory strain of the respective species were conducted for comparison. In each larval bioassay (with either Bti or Bs), five to six biolarvicide concentrations (including a negative control) were tested in four replicates and repeated on three different days (Fig. 2). At the start of each experiment, 25 third-instar larvae were transferred from the larval rearing pans to the labeled disposable paper cups with 100 ml of non-chlorinated tap water by use of disposable Pasteur pipettes. They were then observed for 1 h to identify and replace any larvae not showing a normal vigor. Using a pipette with disposable tips, and starting with the lowest concentration, appropriate volumes established in range finding bioassays (1.0–0.1 ml) of each of the three last dilutions of Bti/Bs were then added to the experimental cups (with mosquito larvae in 100 ml of tap water). In control test cups, distilled water was used instead. The test cups were held at an average ambient temperature of 28.1 °C and photoperiods of 12 h light followed by 12 h of darkness. For Bs experiments, which were run for 48 h, test larvae were provided with larval food after 24 h from the onset of each experiment. Larval mortality was recorded at 24 and 48 h after the addition of Bti and Bs, respectively, by counting the live larvae remaining. Larvae were considered dead when they sunk at the bottom of the test cups and were incapable of rising to the surface or floating on the surface but could not be induced to move when probed with a pipette tip or when the water is disturbed. Before and during bioassays, larvae of An. gambiae complex were fed Aquafin® fish food (China) while Cx. quinquefasciatus and Ae. aegypti were fed Whiskas® cat food (Mars Africa, South Africa).
Fig. 2.
Study design.
2.5. Semi-field trials
Semi-field trials were conducted with two approaches, namely open-field and “mosquito spheres” (simulated field) trials. The open-field trials were conducted following the method of Fillinger et al. (2003). In brief, twenty plastic tubs (0.4 m in diameter) were buried into the open sunlit ground in 4 lines of 5 tubs (1 m apart) at Amani Research Centre compounds (Fig. 2, Fig. 3). Soil and mud from active An. gambiae complex breeding habitats were added to each tub (one-third of its volume) to provide suitable biotic and abiotic conditions for mosquitoes. Tubs were subsequently filled with water (to a depth of 0.14 m) from known An. gambiae complex breeding habitats and left to allow natural oviposition by malaria vectors. Natural oviposition was recorded about 3 days after setting the tubs and included both Anopheles and culicine larvae. Care was taken not to allow adult mosquitoes to emerge, by removing pupae from all tubs once a day. Mosquito oviposition did not occur in all tubs, and hence larval density in tubs was matched by transferring larvae to the tubs with fewer or no larvae so that control and treatment tubs had relatively similar densities at the start of biolarvicide application.
Fig. 3.
Open-field trials site and set-up of the artificial larval habitats.
Treatment and control tubs were assigned randomly using a web-based randomization tool (http://www.randomization.com). For Bti experiments, five tubs served as controls, whereas five each of the remaining 15 tubs were treated with minimum label rate (2 ml/m2), maximum label rate (5 ml/m2), and twice the maximum label rate (10 ml/m2). Similarly, for Bs experiments, five tubs served as controls, whereas five each of the remaining 15 tubs were treated with minimum label rate (5 ml/m2), maximum label rate (10 ml/m2), and twice the maximum label rate (20 ml/m2). Bti and Bs were applied using a 2-l hand-held sprayer (Shifachem limited, Mombasa, Kenya) with a fixed volume (250 ml) per tub and sprayed evenly over the entire water surface. Thereafter, all tubs were examined daily and the number of immature mosquitoes was estimated using a standard 350 ml capacity mosquito dipper (BioQuip Products, CA, USA) by taking five dips per tub, four from the periphery and one from the center. Immature mosquitoes were classified into three categories: early instars (first and second stage larvae); late instars (third and fourth stage larvae); and pupae. All larvae were counted, classified to the genus and development stage, recorded, and then returned to their respective sites. Pupae were counted, recorded, removed from the tubs and reared to adults for morphological identification.
In addition, “mosquito spheres” trials were conducted with the same set-up as that of the open-field trials to complement the open-field trials that depend entirely on the natural oviposition cycle of wild mosquitoes and had a mixture of both Anopheles and culicine species. In these trials, 25 third-instar larvae of field-collected An. gambiae complex were added to each experimental tub. They were then treated with the two bacterial larvicides and larvae mortality scored as explained for the open-field trials. In this set-up, the residual effect of Bti and Bs was assessed by adding a new batch of 25 An. gambiae complex larvae in each of the biolarvicide-treated and control tubs (Fig. 2, Fig. 4).
Fig. 4.
“Mosquito spheres” trials and set-up of the simulated field experiments in artificial larval habitats: exterior (A) and interior (B) views of “mosquito spheres”.
2.6. Identification of mosquitoes
A sub-population of field-collected larvae of An. gambiae complex, Cx. quinquefasciatus and Ae. aegypti were reared to adults for subsequent morphological identification. Moreover, pupae that emerged from the offspring of mosquitoes that oviposited in the experimental tubs (open-field trials) were also reared to adults for further identification. Morphological identification was conducted using relevant keys to identify An. gambiae complex (Gillies & Coetzee, 1987), Cx. quinquefasciatus (Edwards, 1941) and Ae. aegypti (Huang, 2004). A previous study conducted near the sampling sites identified An. gambiae (s.s.) and An. arabiensis sibling species of the An. gambiae complex to be prevalent, with former species accounting for 96.5% of the population (Kabula et al., 2016).
2.7. Data analysis
Data were entered in Excel and subsequently analyzed separately for the laboratory and semi-field trials. For laboratory trials, an experiment was considered valid if larval mortality in the control was less than 5%. The concentration of Bti and Bs that caused 50% and 95% mortality of test larvae (LC50 and LC95) were calculated by the Probit/Logit analysis programme PoloPlus (Robertson & Preisler, 2003). The percentage reduction in larval densities was calculated using the formula developed by Mulla et al. (1971) and estimated as: Percentage reduction = 100 – (C1/T1 × T2/C2) × 100, where C1 and C2 are the average numbers of larvae in the control tubs pre- and post-treatment, respectively, and T1 and T2 are the average numbers of larvae in the tubs treated with experimental formulations pre- and post-treatment, respectively. The average number of early instars, late instars, and pupae in the control and treatment tubs were compared daily by non-parametric Kruskal-Wallis multiple-comparison Z-value tests using STATA 16.0 (Stata Corp LP, College Station, Texas, USA). P-value < 0.05 was considered statistically significant.
3. Results
3.1. Laboratory trials
Laboratory bioassays with Bactivec® (Bti, ≥ 1200 ITU/mg) against third-instar larvae of field-collected An. gambiae complex showed that after 24 h of exposure, concentrations of 0.029 mg/l and 0.096 mg/l caused 50% and 95% mortality of the test larvae, respectively. The same level of mortality was obtained after 24 h for the laboratory strain (An. gambiae (s.s.) Kisumu strain) at concentrations of 0.023 mg/l and 0.063 mg/l, respectively. Laboratory bioassays with Griselesf® (Bs, 268 ITU/mg) showed that after 48 h of exposure, concentrations of 0.022 mg/l and 0.071 mg/l caused 50% and 95% mortality of third-instar larvae of field-collected An. gambiae complex, respectively. The same level of mortality was obtained after 48 h for the laboratory strain (An. gambiae (s.s.), Kisumu strain) at concentrations of 0.029 mg/l and 0.086 mg/l, respectively. The 95% confidence intervals (95% CI) for LC50 and LC95 of the laboratory strain and field-collected An. gambiae complex showed extensive overlaps, indicating a lack of significant variation in susceptibility of tested mosquitoes for both Bti and Bs (Table 1).
Table 1.
Laboratory bioassay results for Bacillus thuringiensis var. israelensis (Bactivec®) and Bacillus sphaericus (Griselesf®) treatments against larvae of Anopheles gambiae complex, Culex quinquefasciatus and Aedes aegypti
| Mosquito species (strain) | Biolarvicide | No. testeda | LC50b (95% CI) | LC95b (95% CI) | Slope ± SE | χ2 (df) | Heterogeneityc |
|---|---|---|---|---|---|---|---|
| An. gambiae (s.s.) (laboratory) | Bti | 1800 | 0.023 (0.022–0.025) | 0.063 (0.056–0.072) | 3.814 ± 0.186 | 2.789 (3) | 0.930 |
| Bs | 2100 | 0.029 (0.021–0.040) | 0.086 (0.058–0.188) | 3.491 ± 0.159 | 38.974 (4) | 9.743 | |
| An. gambiae complex (wild) | Bti | 1800 | 0.029 (0.020–0.042) | 0.096 (0.061–0.244) | 3.167 ± 0.146 | 23.728 (3) | 7.909 |
| Bs | 2100 | 0.022 (0.013–0.034) | 0.071 (0.042–0.292) | 3.204 ± 0.153 | 67.020 (4) | 16.755 | |
| Cx. quinquefasciatus (laboratory) | Bti | 1800 | 0.026 (0.015–0.047) | 0.106 (0.056–0.633) | 2.709 ± 0.108 | 54.817 (3) | 18.272 |
| Bs | 1800 | 0.017 (0.013–0.022) | 0.040 (0.028–0.099) | 4.386 ± 0.227 | 28.194 (3) | 9.398 | |
| Cx. quinquefasciatus (wild) | Bti | 1800 | 0.028 (0.020–0.038) | 0.123 (0.079–0.267) | 2.548 ± 0.100 | 19.956 (3) | 6.652 |
| Bs | 1800 | 0.021 (0.013–0.036) | 0.054 (0.033–0.486) | 3.980 ± 0.192 | 58.924 (3) | 19.641 | |
| Ae. aegypti (laboratory) | Bti | 1800 | 0.018 (0.012–0.027) | 0.052 (0.032–0.265) | 3.545 ± 0.175 | 41.277 (3) | 13.759 |
| Ae. aegypti (wild) | Bti | 1800 | 0.037 (0.033–0.041) | 0.099 (0.083–0.124) | 3.859 ± 0.166 | 4.519 (3) | 1.506 |
Abbreviations: Bti, Bacillus thuringiensis var. israelensis; Bs, Bacillus sphaericus; CI, confidence interval; SE, standard error; df, degrees of freedom.
1500 subjects and 300 controls in all tests except for Bs experiments with An. gambiae complex where there were 1800 subjects and 300 controls (control mortality did not exceed 4% in any experiment).
mg/litre at 24 and 48 h for Bti and Bs, respectively.
Definition: heterogeneity in the context of bioassay is the value of Chi-square divided by the degrees of freedom, a factor used to measure how well the values predicted by the model compared with the actual value observed in bioassay.
Laboratory trials with Cx. quinquefasciatus showed that after 24 h of exposure, Bti concentrations of 0.028 mg/l and 0.123 mg/l caused 50% and 95% mortality of third-stage larvae (the offspring of the first generation (F1) adults of the field-collected Cx. quinquefasciatus larvae), respectively. LC50 and LC95 for the susceptible reference laboratory strain of Cx. quinquefasciatus were 0.026 mg/l and 0.106 mg/l, respectively. For Bs experiments, after 48 h of exposure, the LC50 and LC95 of third-stage larvae (the offspring of the F1 adults of the field-collected Cx. quinquefasciatus larvae) were 0.021 mg/l and 0.054 mg/l, respectively. LC50 and LC95 for the susceptible reference laboratory strain of Cx. quinquefasciatus were 0.017 mg/l and 0.040 mg/l, respectively. Examination of 95% CI indicated a lack of significant variation in susceptibility between field-collected and susceptible reference laboratory strains (Table 1).
Bioassay experiments with Ae. aegypti revealed that 50% and 95% mortality of test larvae was obtained after 24 h of exposure of third-stage larvae (the offspring of the F1 adults of the field-collected Ae. aegypti larvae) to Bti at 0.037 mg/l and 0.099 mg/l concentrations, respectively. LC50 and LC95 for the susceptible reference laboratory strain of Ae. aegypti were 0.018 mg/l and 0.052 mg/l, respectively (Table 1). The larvae of Ae. aegypti tested were found to be not susceptible to Bs under practical doses of laboratory settings.
3.2. Semi-field trials
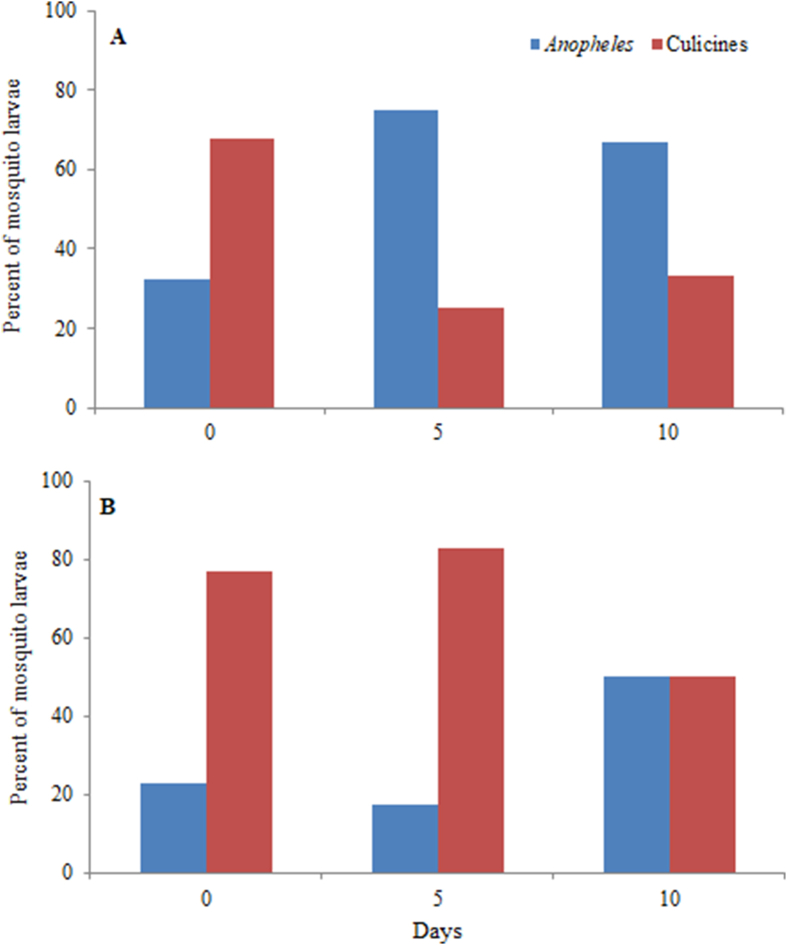
In the open-field settings, both Anopheles and culicines oviposited in the experimental tubs. In both Bti and Bs trials, species of the Culicinae were the majority of larvae seen at the beginning of the experiment; these progressively declined in Bti trials but predominated in Bs trials (Fig. 5). Culicines observed in the experimental tubs were mainly Cx. quinquefasciatus, Cx. tigripes and Ae. aegypti whereas all Anopheles species belonged to the An. gambiae complex. Results for Anopheles and culicines were pooled during analysis.
Fig. 5.
Proportion of Anopheles and culicine mosquitoes in control tubs surveyed at the start (Day 0), middle (Day 5) and the end (Day 10) of monitoring in the open-field trials. ABti trials. BBs trials.
The mean number and percentage reduction of early instars, late instars, and pupae following one round of Bti application in the open-field trial are shown in Table 2. Bti treatment resulted in 91.0–100% larval mortality within 24 h at all treatment dosages. Considering late instars alone, a reduction rate of 78.6–100% was observed up to 4 days post-treatment at all application rates. The residual activity of Bti was found to be low, indicated by a continuing re-colonization of the treated tubs with early instars. All application rates tested were effective up to 5 days post-treatment for reducing late instars and pupae (Table 2). Comparison of larval density between Bti treated and control tubs revealed a progressive decline in immature stages of mosquitoes in treated tubs. A significant reduction of late instars of Anopheles and culicines was recorded for up to 4 days post-treatement at all application dosages (Table 3).
Table 2.
Effects of Bacillus thuringiensis var. israelensis (Bactivec®) on densities of immature stages of mosquitoes (Anopheles and culicines combined) and percent reduction in open-field trials during three subsequent treatments (T) with varying doses
| Day | Average number per dip |
Percentage reduction |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early instars |
Late instars |
Pupae |
Early instars |
Late instars |
Pupae |
||||||||||||||||
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | C | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| 0a | 5.0 | 5.1 | 2.8 | 3.0 | 2.4 | 5.2 | 5.5 | 3.2 | 3.0 | 3.0 | 2.8 | 3.2 | – | – | – | – | – | – | – | – | – |
| 1 | 6.1 | 0.5 | 0 | 0.1 | 4.6 | 0.1 | 0.1 | 0.9 | 2.6 | 1.2 | 1.6 | 2.2 | 92.0 | 100 | 98.4 | 99.0 | 99.0 | 91.0 | 53.8 | 38.5 | 15.4 |
| 2 | 5.3 | 1.5 | 0.7 | 2.1 | 2.8 | 0.2 | 0 | 0.8 | 5.6 | 0.2 | 0 | 0 | 72.3 | 76.4 | 34.0 | 96.7 | 100 | 78.6 | 96.4 | 100 | 100 |
| 3 | 0.9 | 0.5 | 0.4 | 4.6 | 2.9 | 0 | 0.2 | 0.3 | 5.2 | 2.6 | 0.8 | 1.0 | 45.5 | 20.6 | 0 | 100 | 97.0 | 92.2 | 50.0 | 83.5 | 82.0 |
| 4 | 4.4 | 4.7 | 0.8 | 4.5 | 2.0 | 0 | 0 | 0.3 | 5.0 | 0.8 | 0 | 0 | 0 | 67.5 | 0 | 100 | 100 | 88.8 | 84.0 | 100 | 100 |
| 5 | 1.8 | 3.6 | 0.9 | 3.4 | 1.0 | 1.3 | 0.4 | 0.5 | 3.2 | 0 | 0 | 0 | 0 | 10.7 | 0 | 40.0 | 82.5 | 62.5 | 100 | 100 | 100 |
| 6 | 2.4 | 3.6 | 1.2 | 1.7 | 0.3 | 0.4 | 0.5 | 0.6 | 0 | 2.8 | 0 | 0 | 0 | 10.7 | 0 | 38.5 | 27.3 | – | – | – | – |
| 7 | 3.5 | 2.6 | 0.1 | 0.8 | 0.6 | 0 | 0.4 | 0.3 | 1.6 | 3.2 | 0.4 | 0.8 | 27.2 | 94.9 | 61.9 | 100 | 70.9 | 62.5 | 0 | 73.2 | 53.1 |
| 8 | 0.7 | 1.3 | 0.9 | 0.8 | 0.5 | 0.4 | 0.1 | 1.8 | 0.8 | 2.0 | 0.4 | 0.8 | 0 | 0 | 0 | 63.1 | 91.3 | 0 | 0 | 46.4 | 6.3 |
| 9 | 1.1 | 0.5 | 0.1 | 0.7 | 0.6 | 0.5 | 0.5 | 0.3 | 1.6 | 1.0 | 0.6 | 0.4 | 55.4 | 83.8 | 0 | 61.5 | 63.6 | 62.5 | 37.5 | 59.8 | 76.6 |
| 10 | 0.8 | 0.6 | 1.0 | 1.8 | 1.0 | 0.4 | 0.3 | 0.2 | 0.6 | 4.0 | 1.6 | 2.2 | 26.5 | 0 | 0 | 81.5 | 86.9 | 85.0 | 0 | 0 | 0 |
Abbreviations: T1, minimum label rate (2 ml/m2); T2, maximum label rate (5 ml/m2); T3, twice the maximum label rate (10 ml/m2); C, control.
Day of larvicide application.
Table 3.
Comparison of density of immature mosquito stages between control and treated tubs in open-field trials: P-values calculated by non-parametric Kruskal-Wallis multiple-comparison Z-value test
| Larvicide | Daya | Effect of treatment compared to control |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Early instars |
Late instars |
Pupae |
||||||||
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | ||
| Bti | 1 | ns | 0.0013 | 0.0041 | 0.0041 | 0.0041 | 0.0181 | ns | ns | ns |
| 2 | 0.0192 | ns | 0.0457 | 0.0113 | 0.0010 | 0.0175 | ns | 0.0225 | 0.0225 | |
| 3 | ns | 0.0221 | ns | 0.0013 | 0.0066 | 0.0173 | ns | ns | ns | |
| 4 | ns | 0.0382 | ns | 0.0013 | 0.0013 | 0.0173 | ns | 0.0225 | 0.0225 | |
| 5 | ns | ns | ns | ns | 0.0410 | 0.0330 | 0.0225 | 0.0225 | 0.0225 | |
| 6 | ns | ns | ns | 0.0410 | ns | ns | ns | 0.0225 | 0.0225 | |
| 7 | ns | ns | ns | 0.0010 | 0.0260 | 0.0260 | ns | ns | ns | |
| 8 | ns | ns | ns | 0.0100 | 0.0040 | ns | ns | ns | ns | |
| 9 | ns | 0.004 | ns | ns | 0.0120 | 0.0280 | ns | ns | ns | |
| 10 | ns | ns | ns | 0.0220 | 0.0281 | 0.0070 | ns | ns | ns | |
| Bs | 1 | 0.0032 | 0.0032 | 0.0032 | 0.0032 | 0.0032 | 0.0106 | ns | ns | 0.0144 |
| 2 | 0.0342 | 0.0318 | 0.0032 | 0.0032 | 0.0032 | 0.0032 | 0.0363 | 0.0363 | 0.0363 | |
| 3 | ns | ns | ns | 0.0318 | ns | 0.0106 | ns | 0.0363 | 0.0363 | |
| 4 | ns | ns | ns | 0.0382 | 0.0032 | 0.0032 | 0.0363 | 0.0363 | ns | |
| 5 | ns | ns | ns | ns | ns | ns | 0.0363 | 0.0363 | 0.0363 | |
| 6 | ns | ns | 0.0267 | 0.0224 | ns | ns | 0.0363 | 0.0363 | 0.0363 | |
| 7 | ns | ns | ns | 0.0295 | ns | ns | ns | 0.0363 | 0.0363 | |
| 8 | ns | ns | ns | 0.0106 | 0.0032 | 0.0451 | ns | 0.0363 | ns | |
| 9 | ns | ns | ns | ns | 0.0106 | ns | ns | ns | ns | |
| 10 | ns | ns | ns | ns | ns | ns | 0.0091 | ns | ns | |
Abbreviations: T1, minimum label rate; T2, maximum label rate; T3, twice the maximum label rate for the respective Bti and Bs trials; ns, not significant (P > 0.05).
Days post-treatment.
The effect of a single application of Bs on larval density and the corresponding percentage reductions are shown in Table 4. Bs application resulted in 96.8–100% larval mortality within 24 h at all application rates. Bs had a more prolonged residual activity, with pupal reductions ranging from 55.7 to 100% for 9 days at all application rates (Table 4). By using a manufacturer’s recommended dosage of 10 ml/m2, Bs caused a significant reduction of pupae of combined Anopheles and culicines for up to 8 days post-treatment when compared to untreated tubs (Table 3).
Table 4.
Effects of Bacillus sphaericus (Griselesf®) on densities of immature stages of mosquitoes (Anopheles and culicines combined) and percent reduction in open-field settings during three subsequent treatments (T) with varying doses
| Day | Average number per dip |
Percentage reduction |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Early instars |
Late instars |
Pupae |
Early instars |
Late instars |
Pupae |
||||||||||||||||
| C | T1 | T2 | T3 | C | T1 | T2 | T3 | C | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |
| 0a | 1.8 | 1.5 | 3.2 | 1.8 | 2.1 | 3.5 | 4.1 | 4.1 | 1.2 | 2.2 | 2.4 | 3.8 | – | – | – | – | – | – | – | – | – |
| 1 | 1.5 | 0.0 | 0 | 0 | 1.6 | 0 | 0 | 0.1 | 3.2 | 2.6 | 1.6 | 3.8 | 100 | 100 | 100 | 100 | 100 | 96.8 | 55.7 | 75.0 | 62.5 |
| 2 | 4.1 | 0.7 | 0.6 | 0 | 0.8 | 0 | 0 | 0 | 2.8 | 0 | 0 | 0 | 79.5 | 91.8 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 3 | 2.0 | 0.6 | 1.5 | 1.5 | 0.5 | 0.6 | 0.8 | 0.1 | 2.0 | 0.4 | 0 | 0 | 64.0 | 57.8 | 25.0 | 28.0 | 18.0 | 89.8 | 89.1 | 100 | 100 |
| 4 | 4.6 | 1.0 | 1.4 | 3.1 | 0.6 | 1.5 | 0 | 0 | 3.4 | 0 | 0 | 0.2 | 73.9 | 82.9 | 32.6 | 0 | 100 | 100 | 100 | 100 | 98.1 |
| 5 | 3.2 | 1.6 | 1.3 | 1.6 | 1.4 | 0.5 | 1.2 | 0.9 | 0.4 | 0 | 0 | 0 | 40.0 | 51.3 | 50.0 | 78.6 | 48.6 | 67.1 | 100 | 100 | 100 |
| 6 | 2.4 | 1.2 | 0.5 | 0.4 | 2.3 | 0.3 | 1.2 | 1.0 | 1.0 | 0 | 0 | 1.6 | 40.0 | 88.3 | 83.3 | 92.2 | 73.3 | 77.7 | 100 | 100 | 100 |
| 7 | 0.5 | 0.4 | 0.5 | 0.9 | 0.2 | 0.5 | 0.6 | 0.8 | 1.6 | 0.2 | 0 | 0 | 4.0 | 43.8 | 0 | 0 | 0 | 0 | 93.2 | 100 | 100 |
| 8 | 1.3 | 1.7 | 0.3 | 0.8 | 0.2 | 0.1 | 0 | 0.3 | 0.8 | 0.2 | 0 | 0.4 | 0 | 87.0 | 38.5 | 70.0 | 100 | 23.2 | 86.4 | 100 | 84.2 |
| 9 | 0.3 | 0.3 | 0.4 | 0.7 | 0.4 | 0.4 | 0.1 | 0.5 | 2.8 | 2.2 | 1.6 | 2.0 | 0 | 25.0 | 0 | 40.0 | 87.2 | 36.0 | 57.2 | 71.4 | 77.4 |
| 10 | 0.3 | 0.5 | 0.2 | 1.1 | 0.1 | 0.3 | 0.8 | 0.4 | 2.2 | 3.4 | 1.2 | 1.4 | 0 | 62.5 | 0 | 0 | 0 | 0 | 15.7 | 72.7 | 79.9 |
Abbreviations: T1, minimum label rate (5 ml/m2); T2, maximum label rate (10 ml/m2); T3, twice the maximum label rate (20 ml/m2); C, control.
Day of application.
The effect of a single application of Bti on mortality of third-instar field-collected larvae of An. gambiae complex in “mosquito spheres” trials is shown in Table 5. In the initial batch of larvae introduced, treatment with Bti resulted in 96.1–100% larval mortality within 24 h at all application rates. Irrespective of the application rate, a relatively low residual effect of Bti was detected with the subsequent introduction of a new batch of larvae in the treated tubs (Table 5).
Table 5.
Effects of Bacillus thuringiensis var. israelensis (Bactivec®) on densities of late instar of An. gambiae complex and percent reduction in “mosquito spheres” trials during three subsequent treatments (T) with varying doses
| Day | Average number per dip |
Percentage reduction |
|||||
|---|---|---|---|---|---|---|---|
| Late instars |
Late instars |
||||||
| Control | T1 | T2 | T3 | T1 | T2 | T3 | |
| 0a | 25.0 | 25.0 | 25.0 | 25.0 | – | – | – |
| 1 | 15.4A | 0.6B | 0B | 0B | 96.1 | 100 | 100 |
| 3 | 5.8A | 4.6A | 3.6A | 5.0A | 20.7 | 37.9 | 13.8 |
| 5 | 20.2A | 10.0A | 15.6A | 12.0A | 50.5 | 22.8 | 40.6 |
| 7 | 18.4A | 14.4A | 12.6A | 15.4A | 21.7 | 31.5 | 16.3 |
Note: In each row, larval density figures sharing the same superscript letter do not differ significantly.
Abbreviations: T1, minimum label rate (2 ml/m2); T2, maximum label rate (5 ml/m2); T3, twice the maximum label rate (10 ml/m2).
The first batch of 25 An. gambiae complex larvae were introduced in the treatment tubs and application of Bti; a new batch of 25 larvae were introduced on days 2, 4 and 6 (not shown).
For Bs, the effect of a single application on mortality of third-instar larvae of field-collected An. gambiae complex is shown in Table 6. At all application rates, treatment with Bs resulted in 98.4–100% larval mortality within 24 h. Following three subsequent introductions of a new batch of larvae in the treated tubs, Bs showed a relatively higher residual effect producing larval mortality ranging from 60.6 to 100% for up to 7 days at all application rates (Table 6).
Table 6.
Effects of Bacillus sphaericus (Griselesf®) on densities of late instars of An. gambiae complex and percent reduction in “mosquito spheres” trials during three subsequent treatments (T) with varying doses
| Day | Average number per dip |
Percentage reduction |
|||||
|---|---|---|---|---|---|---|---|
| Late instars |
Late instars |
||||||
| Control | T1 | T2 | T3 | T1 | T2 | T3 | |
| 0a | 25.0 | 25.0 | 25.0 | 25.0 | – | – | – |
| 1 | 12.6A | 0.2B | 0B | 0B | 98.4 | 100 | 100 |
| 3 | 11.6A | 3.2B | 0B | 0.4B | 72.4 | 100 | 96.6 |
| 5 | 22.6A | 3.2B | 2.0B | 0B | 85.8 | 91.2 | 100 |
| 7 | 18.8A | 4.6A | 7.4A | 3.0A | 75.5 | 60.6 | 84.0 |
Notes: In each row, larval density figures sharing the same superscript letter do not differ significantly.
Abbreviations: T1: minimum label rate (5 ml/m2); T2: maximum label rate (10 ml/m2); T3: twice the maximum label rate (20 ml/m2).
The first batch of 25 An. gambiae complex larvae were introduced in the treatment tubs and application of Bs; a new batch of 25 larvae were introduced on days 2, 4 and 6 (not shown).
4. Discussion
Larvae source management (LSM) by targeting immature stages of mosquitoes in their natural breeding habitats has the potential to effectively control mosquito-borne diseases and is the only proven method for control of arboviruses transmitted by Ae. aegypti and Ae. albopictus (WHO, 2009). When integrated with adult mosquito control interventions, LSM has been shown to provide an important supplementary role in mosquito-borne disease control (Fillinger et al., 2009). Despite the potential role of LSM in mosquito vector control, the intervention has not been widely deployed, particularly in sub-Saharan Africa where malaria and other mosquito-borne diseases are more prevalent (Fillinger & Lindsay, 2011). However, the emergence and widespread insecticide resistance and behavioral adaptation by mosquito vectors threaten the efficacy of adult mosquito control interventions. This calls for integrated mosquito control interventions targeting all stages of the mosquito life-cycle to control and eventually eliminate mosquito-borne diseases.
The findings of the laboratory trials showed that at low dosage rates, field-collected larvae of An. gambiae complex and Cx. quinquefasciatus were fully susceptible to Bti and Bs when compared to their respective standard susceptible reference laboratory strains. However, larvae of Ae. aegypti were found to be susceptible to Bti but not to Bs. When considering LC95 values (which represent the minimum effective dosages for field application), the tested An. gambiae complex and Cx. quinquefasciatus were found to be equally susceptible to Bti and Bs. Laboratory studies previously conducted across sub-Saharan Africa have shown high levels of efficacy of different formulations of Bti and Bs against malaria mosquito vectors (Derua et al., 2019). Findings of reduced susceptibility of Ae. aegypti to Bs recorded in the present study corroborates previous studies conducted elsewhere (Lacey et al., 1988; Davidson, 1995; Monnerat et al., 2004).
Results from the open-field trials with Bti indicated that a maximum label rate of 5 ml/m2 (equivalent to the surface application of 5 litres/ha) was sufficient to suppress late instars and the resulting pupae of Anopheles and culicines for up to 5 days. The low residual effect of Bti recorded in open-field trials was also observed in the “mosquito spheres” trials where field-collected larvae were added to the larvicide-treated tubs at regular intervals. The relatively low residual activity of the Bti formulation tested corroborates other evaluations of Bti-based products conducted elsewhere in sub-Saharan Africa (Karch et al., 1991; Fillinger et al., 2003; Majambere et al., 2007; Nartey et al., 2013). On the other hand, open-field trials with Bs at the maximum label rate of 10 ml/m2 (equivalent to the surface application of 10 liters/ha) provided a significant reduction of Anopheles and culicine pupae for up to 8 days. The residual larvicidal activity of Bs achieved compares fairly well with Bs water-dispersible granules (WDG) formulation evaluated in different ecological settings in sub-Saharan Africa (Fillinger et al., 2003; Majambere et al., 2007; Baffour-Awuah et al., 2014). When compared to Bti, Bs had a relatively greater residual activity observed in open-field trials and this was also confirmed in the “mosquito spheres” trials against larvae of the An. gambiae complex. It has been reported that Bs-based products provide greater residual larvicidal activity because of the longer persistence of the spores in the environment and their recycling potential in the gut of exposed larvae after dying (Becker et al., 1995).
The findings of the present study show that both Bti and Bs formulations tested were effective against larvae of mosquito vectors in the laboratory and semi-field settings. However, the formulations tested were found to exhibit low residual activity in the open-field and “mosquito spheres” trials. Based on these findings, and the results of bacterial larvicide evaluations undertaken in different ecological settings in sub-Saharan Africa (Derua et al., 2019), weekly application cycles for either Bti or Bs formulations at the maximum label rate are appropriate for the control of mosquito vectors. Furthermore, due to the relatively low residual activity of the Bti formulation, this product would be more suitable for application during the heavy rainy season where the residual effect cannot be achieved even with larvicide with higher residual activity due to continuous dilution and washing of the larval habitats away by rain (Fillinger & Lindsay, 2006). Moreover, the application of Bti at regular intervals will delay the risk of resistance development in larval populations as resistance has been recorded in Bs interventions (Rao et al., 1995; Nielsen-Leroux et al., 2002; Mulla et al., 2003). Since the residuality of Bti and Bs is believed to be enhanced by repeated application as previously reported (Karch et al., 1990, 1991; Fillinger & Lindsay, 2006), monitoring of persistence of the larvicide product will help inform control programmes on appropriate re-treatment regimens as the larvicide intervention matures.
The empirical results reported in this study should be considered in the light of some limitations. In laboratory bioassays, due to insufficient number of field-collected larvae, third-stage larvae of the laboratory strains of Cx. quinquefasciatus and Ae. aegypti were compared with the offspring of the first generation (F1) adults of the field-collected larvae. On the other hand, in the open-field settings, a mixture of Anopheles and culicine mosquitoes oviposited in the experimental tubs and a relatively low density of larvae in the tubs did not permit meaningful analysis of larval reduction by species. In this regard, Anopheles and culicine mosquito larvae were pooled during the analysis of the biolarvicide treatment effects as supported by other studies (Fillinger et al., 2003; Majambere et al., 2007). Furthermore, the findings revealed that the efficacy and persistence of Bti and Bs in pooled data were fairly comparable to those of simulated field studies in the “mosquito spheres” trials where only An. gambiae complex was tested. Since coexistence between Anopheles and culicine mosquitoes is common in the natural larval habitats (Mwangangi et al., 2008; Kweka et al., 2011), the findings of this study suggest that Bti and Bs work fairly well in controlling co-existing larvae of mosquito vectors. Despite the limitations, the efficacy of Bti (Bactivec®) and Bs (Griselesf®) recorded in this study agrees with those conducted elsewhere in sub-Saharan Africa (Derua et al., 2019) and hence provide guidance in their application in similar settings in Tanzania and possibly beyond.
5. Conclusions
The present study showed that at low concentrations, the Bti (Bactivec®) formulation tested caused significant mortality of An. gambiae complex, Cx. quinquefasciatus and Ae. aegypti larvae. Moreover, the tested mosquito larvae were highly susceptible to Bs (Griselesf®) except those of Ae. aegypti. However, due to the low residual activity of Bti and Bs observed in the semi-field trials, weekly application cycles at the maximum label rate will be required for the effective control of tested mosquitoes in natural larval habitats. The findings of the laboratory trials indicated that the Bs product tested is not effective against Ae. aegypti and would not be recommended for use in its control.
Funding
Not applicable.
Ethical approval
Not applicable.
CRediT author statement
Conceived and designed the study: Yahya Derua, Robert Malima, William Kisinza and Yunus Mgaya. Laboratory and semi-field experiments: Edward Sambu, Aza Kimambo, Bernard Batengana, Victor Mwingira and Yahya Derua. Data analysis: Yahya Derua, Patrick Tungu and Pendael Machafuko. Writing - original draft: Yahya Derua. Writing - review and editing: Yahya Derua, Robert Malima, William Kisinza, Edward Sambu, Aza Kimambo, Patrick Tungu, Pendael Machafuko, Bernard Batengana, Victor Mwingira and Yunus Mgaya. All authors read and approved the final manuscript.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the laboratory and insectary staff of the Amani Medical Research Centre, namely Wema Sudi, Josephine Nyongole, Amina Ibrahim, Stellah Mhando, Mwanaisha Amiri, Isaya Kibwana, Justine Mnkeni, Charles Kayamba, Mary Rabson, Gladness Kiyaya, Grace Magogo and Kisesa Kasimu, for their technical support during the larvae collection in the field, mosquito larval rearing, and larval bioassays. We thank Frank Mbua, Steven Kassim, Zuberi Hatibu and Abdallah Juma for preparing a layout for semi-field trials including clearing the ground and burying plastic tubs.
References
- Baffour-Awuah S., Owusu-Dabo E., Kruppa T., Annan A., Nartey R., Dogbe J., et al. Lysinibacillus sphaericus biolarvicide, an efficacious tool in the control of Anopheles gambiae in Kumasi, Ghana. East Afr. J. Public Health. 2014;11:851–861. [Google Scholar]
- Becker N., Zgomba M., Petric D., Beck M., Ludwig M. Role of larval cadavers in recycling processes of Bacillus sphaericus. J. Am. Mosq. Control Assoc. 1995;11:329–334. [PubMed] [Google Scholar]
- Benedict M.Q. CDC; Atlanta: 2007. Methods in Anopheles research.https://www.beiresources.org/portals/2/MR4/MR4_Publications/Methods%20in%20Anopheles%20Research%202014/2014MethodsinAnophelesResearchManualFullVersionv2tso.pdf [Google Scholar]
- Benelli G. Spread of Zika virus: The key role of mosquito vector control. Asian Pac. J. Trop. Biomed. 2016;6:468–471. [Google Scholar]
- Davidson E.W. Biochemistry and mode of action of the Bacillus sphaericus toxins. Mem. Inst. Oswaldo Cruz. 1995;90:81–86. doi: 10.1590/s0074-02761995000100018. [DOI] [PubMed] [Google Scholar]
- Derua Y.A., Kweka E.J., Kisinza W.N., Githeko A.K., Mosha F.W. Bacterial larvicides used for malaria vector control in sub-Saharan Africa: Review of their effectiveness and operational feasibility. Parasit. Vectors. 2019;12 doi: 10.1186/s13071-019-3683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards F.W. British Museum (Natural History); London: 1941. Mosquitoes of the Ethiopian Region. III. Culicine adults and pupae. [Google Scholar]
- Espinal M.A., Andrus J.K., Jauregui B., Waterman S.H., Morens D.M., Santos J.I., et al. Emerging and reemerging Aedes-transmitted arbovirus infections in the region of the Americas: Implications for health policy. Am. J. Public Health. 2019;109:387–392. doi: 10.2105/AJPH.2018.304849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U., Kannady K., William G., Vanek M.J., Dongus S., Nyika D., et al. A tool box for operational mosquito larval control: Preliminary results and early lessons from the urban malaria control programme in Dar es Salaam, Tanzania. Malar. J. 2008;7 doi: 10.1186/1475-2875-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U., Knols B.G.J., Becker N. Efficacy and efficiency of new Bacillus thuringiensis var. israelensis and Bacillus sphaericus formulations against Afrotropical anophelines in Western Kenya. Trop. Med. Int. Health. 2003;8:37–47. doi: 10.1046/j.1365-3156.2003.00979.x. [DOI] [PubMed] [Google Scholar]
- Fillinger U., Lindsay S.W. Suppression of exposure to malaria vectors by an order of magnitude using microbial larvicides in rural Kenya. Trop. Med. Int. Health. 2006;11:1629–1642. doi: 10.1111/j.1365-3156.2006.01733.x. [DOI] [PubMed] [Google Scholar]
- Fillinger U., Lindsay S.W. Larval source management for malaria control in Africa: Myths and reality. Malar. J. 2011;10:353. doi: 10.1186/1475-2875-10-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillinger U., Ndenga B., Githeko A., Lindsay S.W. Integrated malaria vector control with microbial larvicides and insecticide-treated nets in western Kenya: A controlled trial. Bull. World Health Organ. 2009;87:655–665. doi: 10.2471/BLT.08.055632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam R., Mishra S., Milhotra A., Nagpal R., Mohan M., Singhal A., et al. Challenges with mosquito-borne viral diseases: Outbreak of the monsters. Curr. Top. Med. Chem. 2017;17:2199–2214. doi: 10.2174/1568026617666170130122921. [DOI] [PubMed] [Google Scholar]
- Geissbühler Y., Kannady K., Chaki P.P., Emidi B., Govella N.J., Mayagaya V., et al. Microbial larvicide application by a large-scale, community-based program reduces malaria infection prevalence in urban Dar es Salaam, Tanzania. PLoS One. 2009;4 doi: 10.1371/journal.pone.0005107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies M.T., Coetzee M.A. South Africa Institute for Medical Research; Johannesburg: 1987. Supplement to the anophelinae of Africa south of the sahara (Afrotropical Region) [Google Scholar]
- Huang Y. The subgenus stegomyia of Aedes in the afrotropical region with keys to the species (Diptera: Culicidae) Zootaxa. 2004;700:1. [Google Scholar]
- Huang Y.J.S., Higgs S., Vanlandingham D.L. Emergence and re-emergence of mosquito-borne arboviruses. Curr. Opin. Virol. 2019;34:104–109. doi: 10.1016/j.coviro.2019.01.001. [DOI] [PubMed] [Google Scholar]
- Jentes E.S., Poumerol G., Gershman M.D., Hill D.R., Lemarchand J., Lewis R.F., et al. The revised global yellow fever risk map and recommendations for vaccination, 2010: Consensus of the Informal WHO Working Group on geographic risk for yellow fever. Lancet Infect. Dis. 2011;11:622–632. doi: 10.1016/S1473-3099(11)70147-5. [DOI] [PubMed] [Google Scholar]
- Kabula B., Tungu P., Rippon E.J., Steen K., Kisinza W., Magesa S., et al. A significant association between deltamethrin resistance, Plasmodium falciparum infection and the Vgsc-1014S resistance mutation in Anopheles gambiae highlights the epidemiological importance of resistance markers. Malar. J. 2016;15 doi: 10.1186/s12936-016-1331-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch S., Manzambi Z.A., Salaun J.J. Field trials with Vectolex (Bacillus sphaericus) and Vectobac (Bacillus thuringiensis (H-14)) against Anopheles gambiae and Culex quinquefasciatus breeding in Zaire. J. Am. Mosq. Control Assoc. 1991;7:176–179. [PubMed] [Google Scholar]
- Karch S., Monteny N., Jullien J.L., Sinegre G., Coz J. Control of Culex pipiens by Bacillus sphaericus and the role of nontarget arthropods in its recycling. J. Am. Mosq. Control Assoc. 1990;6:47–54. [PubMed] [Google Scholar]
- Killeen G.F., Fillinger U., Kiche I., Gouagna L.C., Knols B.G.J. Eradication of Anopheles gambiae from Brazil: Lessons for malaria control in Africa? Lancet Inf. Dis. 2002;2:618–627. doi: 10.1016/s1473-3099(02)00397-3. [DOI] [PubMed] [Google Scholar]
- Killeen G.F., Fillinger U., Knols B.G.J. Advantages of larval control for African malaria vectors: Low mobility and behavioural responsiveness of immature mosquito stages allow high effective coverage. Malar. J. 2002;1:8. doi: 10.1186/1475-2875-1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitau J., Pates H., Rwegoshora T.R., Rwegoshora D., Matowo J., Kweka E.J., et al. The effect of Mosquito Magnet® Liberty Plus trap on the human mosquito biting rate under semi-field conditions. J. Am. Mosq. Control Assoc. 2010;26:287–294. doi: 10.2987/09-5979.1. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt I., Bradley J., Knox T.B., Mnzava A.P., Kafy H.T., Mbogo C., et al. Implications of insecticide resistance for malaria vector control with long-lasting insecticidal nets: A WHO-coordinated, prospective, international, observational cohort study. Lancet Infect. Dis. 2018;18:640–649. doi: 10.1016/S1473-3099(18)30172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R.A., Mboera L.E.G., Senkoro K., Lesser A., Shayo E.H., Paul C.J., Miranda M.L. A randomized longitudinal factorial design to assess malaria vector control and disease management interventions in rural Tanzania. Int. J. Environ. Res. Public Health. 2014;11:5317–5332. doi: 10.3390/ijerph110505317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kweka E.J., Zhou G., Lee M., Gilbreath T.M., Mosha F., Munga S., et al. Evaluation of two methods of estimating larval habitat productivity in western Kenya highlands. Parasit. Vectors. 2011;4:110. doi: 10.1186/1756-3305-4-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey L.A. Bacillus thuringiensis var. israelensis and Bacillus sphaericus for mosquito control. J. Am. Mosq. Control Assoc. 2007;23:133–163. doi: 10.2987/8756-971X(2007)23[133:BTSIAB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Lacey L.A., Lacey C.M., Peacock B., Thiery I. Mosquito host range and field activity of Bacillus sphaericus isolate 2297 (serotype 25) J. Am. Mosq. Control Assoc. 1988;4:51–56. [PubMed] [Google Scholar]
- Magesa S.M., Athumani Y., Barongo V., Sambu E.Z., Senkoro K.P., Mboera L.E.G., et al. National Institute for Medical Research; Dar es Salaam, Tanzania: 2009. Efficacy of Bacillus thuringiensis var. israelensis (Bactivec®) and Bacillus sphaericus (Griselesf®) for control of mosquito larvae: A field trial in Mvomero and Bagamoyo districts. [Google Scholar]
- Magesa S.M., Wilkes T.J., Mnzava A.E.P., Njunwa K.J., Myamba J., Kivuyo M.D.P., et al. Trial of pyrethroid impregnated bednets in an area of Tanzania holoendemic for malaria Part 2. Effects on the malaria vector population. Acta Trop. 1991;49:97–108. doi: 10.1016/0001-706x(91)90057-q. [DOI] [PubMed] [Google Scholar]
- Maheu-Giroux M., Castro M.C. Cost-effectiveness of larviciding for urban malaria control in Tanzania. Malar. J. 2014;3 doi: 10.1186/1475-2875-13-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majambere S., Lindsay S.W., Green C., Kandeh B., Fillinger U. Microbial larvicides for malaria control in the Gambia. Malar. J. 2007;6 doi: 10.1186/1475-2875-6-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazigo H.D., Massawe I.S., Rumisha S.F., Kweka E.J., Mboera L.E.G. Rice farmers’ perceptions and acceptability in the use of a combination of biolarvicide (Bacillus thuringiensis var. israeliensis) and fertilizers application for malaria control and increase rice productivity in a rural district of central Tanzania. Malar. J. 2019;18 doi: 10.1186/s12936-019-2697-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mboera L.E.G., Kramer R.A., Miranda M.L., Kilima S.P., Shayo E.H., Lesser A. Community knowledge and acceptance of larviciding for malaria control in a rural district of east-central Tanzania. Int. J. Environ. Res. Public Health. 2014;11:5137–5154. doi: 10.3390/ijerph110505137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal P.K. Biolarvicides in vector control: Challenges and prospects. J. Vector Borne Dis. 2003;40:20–32. [PubMed] [Google Scholar]
- Monnerat R., Da Silva S.F., Dias D.S., Martins É.S., Praça L.B., Jones G.W., et al. Screening of Brazilian Bacillus sphaericus strains for high toxicity against Culex quinquefasciatus and Aedes aegypti. J. Appl. Entomol. 2004;128:469–473. [Google Scholar]
- Msellemu D., Namango H.I., Mwakalinga V.M., Ntamatungiro A.J., Mlacha Y., Mtema Z.J., et al. The epidemiology of residual Plasmodium falciparum malaria transmission and infection burden in an African city with high coverage of multiple vector control measures. Malar. J. 2016;15 doi: 10.1186/s12936-016-1340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulla M.S., Darwazeh H.A., Zgomba M. Effect of some environmental factors on the efficacy of Bacillus sphaericus 2362 and Bacillus thuringiensis (H-14) against mosquitoes. Bull. Soc. Vector Ecol. 1990;15:166–175. [Google Scholar]
- Mulla M.S., Norland R.L., Fanara D.M., Darwazeh H.A., McKean D.W. Control of chironomid midges in recreational lakes. J. Econ. Entomol. 1971;64:300–308. [Google Scholar]
- Mulla M.S., Thavara U., Tawatsin A., Chomposri J., Su T. Emergence of resistance and resistance management in field populations of tropical Culex quinquefasciatus to the microbial control agent Bacillus sphaericus. J. Am. Mosq. Control Assoc. 2003;19:39–46. [PubMed] [Google Scholar]
- Musso D., Rodriguez-Morales A.J., Levi J.E., Cao-Lormeau V.M., Gubler D.J. Unexpected outbreaks of arbovirus infections: Lessons learned from the Pacific and tropical America. Lancet Infect. Dis. 2018;18:e355–e361. doi: 10.1016/S1473-3099(18)30269-X. [DOI] [PubMed] [Google Scholar]
- Mwangangi J.M., Muturi E.J., Shililu J.I., Jacob B., Kabiru E.W., Mbogo C.M., et al. Distribution of mosquito larvae within the paddy and its implication in larvicidal application in Mwea rice irrigation scheme, central Kenya. J. Am. Mosq. Control Assoc. 2008;24:36–41. doi: 10.2987/5586.1. [DOI] [PubMed] [Google Scholar]
- Nartey R., Owusu-Dabo E., Kruppa T., Baffour-Awuah S., Annan A., Oppong S., et al. Use of Bacillus thuringiensis var. israelensis as a viable option in an integrated malaria vector control programme in the Kumasi metropolis, Ghana. Parasit. Vectors. 2013;6 doi: 10.1186/1756-3305-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen-Leroux C., Pasteur N., Prètre J., Charles J., Sheikh H.B., Chevillon C. High resistance to Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae): The complex situation of west Mediterranean countries. J. Med. Entomol. 2002;39:729–735. doi: 10.1603/0022-2585-39.5.729. [DOI] [PubMed] [Google Scholar]
- Paixão E.S., Teixeira M.G., Rodrigues L.C. Zika, chikungunya and dengue: The causes and threats of new and reemerging arboviral diseases. BMJ Glob. Health. 2018;3 doi: 10.1136/bmjgh-2017-000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protopopoff N., Mosha J.F., Lukole E., Charlwood J.D., Wright A., Mwalimu C.D., et al. Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: A cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391:1577–1588. doi: 10.1016/S0140-6736(18)30427-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragoonanansingh R.N., Njunwa K.J., Curtis C.F., Becker N. A field study of Bacillus sphaericus for the control of culicine and anopheline mosquito larvae in Tanzania. Bull. Soc. Vector Ecol. 1992;17:45–50. [Google Scholar]
- Rahman R., Lesser A., Mboera L., Kramer R. Cost of microbial larviciding for malaria control in rural Tanzania. Trop. Med. Int. Health. 2016;21:1468–1475. doi: 10.1111/tmi.12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D.R., Mani T.R., Rajendran R., Joseph A.S., Gajanana A., Reuben R. Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi, India. J. Am. Mosq. Control Assoc. 1995;11:1–5. [PubMed] [Google Scholar]
- Robertson J.L., Preisler H.,R.R. LeOra Software; Petaluma, CA, USA: 2003. PoloPlus: Probit and logit analysis user's guide. [Google Scholar]
- Shousha A.T. Species-eradication. The eradication of Anopheles gambiae from upper Egypt, 1942–1945. Bull. World Health Organ. 1948;1:309. [PMC free article] [PubMed] [Google Scholar]
- Tolle M.A. Mosquito-borne diseases. Curr. Probl. Pediatr. Adolesc. Health Care. 2009;39:97–140. doi: 10.1016/j.cppeds.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Vontas J., Kioulos E., Pavlidi N., Morou E., della Torre A., Ranson H. Insecticide resistance in the major dengue vectors Aedes albopictus and Aedes aegypti. Pestic. Biochem. Physiol. 2012;104:126–131. [Google Scholar]
- Walker K., Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: Review of achievements and potential. Med. Vet. Entomol. 2007;21:2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- Weetman D., Kamgang B., Badolo A., Moyes C.L., Shearer F.M., Coulibaly M., et al. Aedes mosquitoes and Aedes-borne arboviruses in Africa: Current and future threats. Int. J. Environ. Res. Public Health. 2018;15:220. doi: 10.3390/ijerph15020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . World Health Organization; Geneva: 2005. Guidelines for laboratory and field testing of mosquito larvicides.http://whqlibdoc.who.int/hq/2005/WHO_CDS_WHOPES_GCDPP_2005.13.pdf [Google Scholar]
- WHO . World Health Organization; Geneva: 2009. Dengue guidelines for diagnosis, treatment, prevention and control.https://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf [PubMed] [Google Scholar]
- Wirth M.C., Ferrari J.A., Georghiou G.P. Baseline susceptibility to bacterial insecticides in populations of Culex pipiens complex (Diptera: Culicidae) from California and from the Mediterranean Island of Cyprus. J. Econ. Entomol. 2001;94:920–928. doi: 10.1603/0022-0493-94.4.920. [DOI] [PubMed] [Google Scholar]
- Worrall E., Fillinger U. Large-scale use of mosquito larval source management for malaria control in Africa: A cost analysis. Malar. J. 2011;10:338. doi: 10.1186/1475-2875-10-338. [DOI] [PMC free article] [PubMed] [Google Scholar]