Abstract
Introduction:
Pigs deficient in three glycosyltransferase enzymes (triple-knockout [TKO] pigs, that is, not expressing the three known carbohydrate xenoantigens) and expressing ‘protective’ human transgenes are considered a likely source of organs for transplantation into human recipients. Some human sera have no or minimal natural antibody binding to red blood cells (RBCs) and peripheral blood mononuclear cells (PBMCs) from TKO pigs. However, all Old World monkeys exhibit natural antibody binding to TKO pig cells. The xenoantigen targets of Old World monkey natural antibodies are postulated to be carbohydrate moieties exposed when the expression of the carbohydrate N-glycolylneuraminic acid (Neu5Gc) is deleted. The aim of this study was to compare the survival in baboons and histopathology of renal grafts from pigs that either (a) expressed Neu5Gc (GTKO pigs; Group 1) or (b) did not express Neu5Gc (GTKO/CMAHKO [DKO] or TKO pigs; Group 2).
Methods:
Life-supporting renal transplants were carried out using GTKO (n = 5) or DKO/TKO (n = 5) pig kidneys under an anti-CD40mAb-based immunosuppressive regimen.
Results:
Group 1 baboons survived longer than Group 2 baboons (median 237 vs. 35 days; mean 196 vs. 57 days; p < 0.07) and exhibited histopathological features of antibody-mediated rejection in only two kidneys. Group 2 exhibited histopathological features of antibody-mediated rejection in all five grafts, with IgM and IgG binding to renal interstitial arteries and peritubular capillaries. Rejection-free survival was significantly longer in Group 1 (p < 0.05).
Conclusions:
The absence of expression of Neu5Gc on pig kidney grafts is associated with increased binding of baboon antibodies to pig endothelium and reduced graft survival.
Keywords: baboon, genetically-engineered, histopathology, kidney, Neu5Gc, N-glycolylneuraminic acid, pig, xenotransplantation
1 |. INTRODUCTION
Over the last several years, considerable advances in genetic engineering of pigs have been achieved, resulting in improved survival of lifesupporting genetically-engineered pig organ (kidney and heart) transplants in nonhuman primates (NHPs). In particular, deletion of expression of pig glycoantigens against which humans exhibit natural antibodies has contributed to the longer pig graft survival that is now measured in months or even years.
Sera from many humans contain no or minimal IgM or IgG antibodies that bind to cells from pigs in which all three known pig glycoxenoantigens have been deleted (triple-knockout [TKO] pigs)1–2 (Table S1), suggesting that organs from these pigs would not be rejected by (naturally occurring) antibodies to these epitopes in clinical xenotransplantation. TKO pigs with transgenic expression of human proteins, including (i) regulators of hemostasis (e.g., thrombomodulin, endothelial protein C receptor), and of (ii) complement pathway activators (e.g., CD46, CD55), and (iii) anti-inflammatory (anti-apoptotic) proteins (CD39, CD47, HO-1) have resulted in a considerable improvement in the results of pig-to-NHP organ xenotransplantation.3–4
Despite these genetic modifications, the adaptive immune response may still induce antibody- or cell-mediated rejection, which necessitates immunosuppressive therapy targeting humoral (B cells) and/or cellular (T cells) immunity. The administration of agents that target the CD40:CD154 costimulation pathway has had a significant beneficial impact on pig renal5–8 and cardiac9–10 graft survival in NHPs.
However, there is a discordance between humans and Old World NHPs in their response to TKO pig cells and organs. Baboons and other Old World NHPs have natural IgM antibodies that bind to TKO and GTKO/CMAHKO (double-knockout, DKO) pig cells,1 and this binding may be associated with potent complement-dependent cytotoxicity.1–2,11–12 These antibodies bind to currently unknown xenoantigen(s) that likely are carbohydrate moieties exposed after the loss of expression of the carbohydrate, N-glycolylneuraminic acid (Neu5Gc).13
We here report our initial observations on graft survival and provide a detailed evaluation of the histopathological features of pig kidneys and ureters after transplantation into immunosuppressed baboons, where the grafts were from pigs that either expressed Neu5Gc (i.e., GTKO pigs, Group 1) or did not express Neu5Gc (i.e., TKO or DKO pigs, Group 2). Some data from Group 1 have been presented previously.6,8
2 |. MATERIALS AND METHODS
2.1 |. Pigs
Genetically-engineered pigs (Revivicor, Blacksburg, VA, USA), weighing 15–30 kg, aged 2–3 months, and of blood group O (nonA), served as sources of kidneys. All pigs were α1,3-galactosyltransferase gene-knockout (GTKO), and some had additional knockout of cytidine monophosphate-N-acetylneuraminic acid hydroxylase (CMAH) (i.e., were DKO) or knockout of CMAH and β-1,4N-acetylgalactosaminyltransferase (i.e., TKO) (Table S1). All had variable other genetic manipulations aimed at protecting the graft from the primate immune, coagulation, and/or inflammatory responses (Table 1).6–7,14 Transgenic protein expression was confirmed in all cases by flow cytometry of pig aortic endothelial cells (data not shown). All pigs were cytomegalovirus-negative.15
TABLE 1.
Baboon number, donor pig phenotype, cause for termination, and evidence of immune-mediated rejection
| Baboon | Donor pig phenotype | Survival (days) | Cause of death | Immune-mediated rejection |
|---|---|---|---|---|
| Group 1: Kidney is not from a CMAHKO pig | ||||
| B9315 | GTKO/CD46/CD55/TBM/EPCR/CD39 | 136 | Infection | None |
| B17315 | GTKO/CD46/CD55/EPCR/TFPI/CD47 | 237 | Infection | None |
| B17615 | GTKO/CD46/CD55/EPCR/TFPI/CD47 | 260 | Infection | None |
| B10815 | GTKO/CD46/TBM/EPCR/CD47/HO-1 | 90 | Edema | AMR |
| B5916 | GTKO/CD46/TBM | 255 | Rejection | AMR |
| Group 2: Kidney is from a CMAHKO pig | ||||
| B8216 | GTKO/CMAHKO/CD46/CD55/TBM/EPCR | 7 | Edema | AMR |
| B1417 | TKO/CD46/CD55/TBM/EPCR/CD47/HO-1 | 61 | Rejection | AMR/CCXR |
| B3917 | TKO/CD46/CD55/TBM/EPCR/CD47/HO-1 | 0 | Rejection | Suspected HAR |
| B15816 | GTKO/CMAHKO/CD46/CD55/TBM/EPCR/CD47/HO-1 | 35 | Rejection | Infection/AMR |
| B517 | GTKO/CMAHKO/CD46/CD55/TBM/EPCR/CD47/HO-1 | 183 | Trauma | AMR |
Abbreviations: AMR, antibody-mediated rejection and thrombosis; CCXR, chronic cellular xenograft rejection; CD46, membrane cofactor protein; CD55, decay accelerating factor; CD47, integrin associated protein; CMAHKO, cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene-knockout; EPCR, endothelial protein C receptor; GTKO, α1,3-galactosyltransferase gene-knockout; HAR, hyperacute rejection; HO-1, human heme oxygenase-1; TBM, thrombomodulin; TFPI, tissue factor pathway inhibitor; TKO, triple-knockout.
2.2 |. Baboons
Baboons (Papio spp) from the Division of Animal Resources of the University of Oklahoma Health Sciences Center, Oklahoma City, OK, or from the Michale E. Keeling Center, MDAnderson Cancer Center, Bastrop, TX, 3–4 year old, weighing 7–10 kg, were recipients. The baboons were divided into two groups based on whether they received a graft from a GTKO pig (Group 1) or from a pig that did not express either Gal of Neu5Gc (DKO or TKO, Group 2) (Table 1). All recipients were selected on the basis of low anti-pig antibody levels either to GTKO pig cells (Group 1) or to DKO/TKO pig cells (Group 2), as previously described.16
Animal care was in accordance with the Guide for the Care and Use of Laboratory Animals issued by the Institute of Laboratory Animal Research (8th edition, 2010). Protocols were approved by the Institutional Animal Care and Use Committees of the University of Pittsburgh, PA, USA or the University of Alabama at Birmingham, AL, USA.
2.3 |. Surgical procedures
Intravascular catheter placement in baboons, donor pig nephrectomy, and life-supporting pig kidney transplantation in baboons have been described previously.17–18
2.4 |. Immunosuppressive, anti-inflammatory, and supportive therapy
Details of therapy have been reported previously (Table S2).7–8
2.5 |. Monitoring of recipient baboons
Monitoring was conducted as described in detail previously.7–8,19–20
2.6 |. Histopathologic assessment of pig kidney xenografts
Formalin-fixed kidney tissue was embedded in paraffin. Sections (approximately 3 μM) were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), and Masson’s trichrome. In the scoring of histologic abnormalities, we experienced that earlier classifications for solid organ xenograft rejection21–22 did not suffice, which may be related to the more extensive genetic modification of pigs after these classifications were published. Also, the features in the Banff classification of acute or chronic allograft rejection23 did not consistently apply to the xenografts as evaluated by a board certified MD pathologist with expertise in renal allotransplant pathology (HF). We therefore followed the approaches published by Shimizu et al.,24–29 with a detailed assessment of a series of histologic features using previously established criteria8 performed by a board certified veterinary anatomic pathologist (JBF) and PhD scientist (HJS) with extensive expertise in xenotransplant pathology. Lesions were scored in a semi-quantitative fashion, as follows: 0 = no abnormailites, and 1 = mild, 2 = moderate, and 3 = severe histopathologic changes. Ureter pathology was evaluated using the same scoring schema. The scoring schemes are detailed in Tables S3 and S4 for (i) glomeruli and (ii) renal interstitium and ureter.
For each sample from an individual organ, the average score was calculated from 40 (kidney) or 30 (ureter) random 400x images; data presented are the average values (10 ureter images were not always available). The scoring was conducted in a blinded fashion by two independent pathologists, who were not informed about details of pig genetics, clinical courses, or medical management.
The scoring schema also included immunohistochemistry for C3c, IgG, and IgM, as described previously.8 Additionally, we performed immunohistochemistry for C4d (clone 12D11, Mouse IgG1, Abcam, Branford, CT) using a standard citrate buffer-based antigen retrieval procedure and similar methodology as described previously.8
2.7 |. Statistical analysis
Continuous variables were expressed as mean and standard deviation. Discontinuous variables, such as scores in histopathology, were expressed as median and range. Statistical testing between groups was performed using unpaired Welch’s t-test (parametric variables) or Mann–Whitney U-test for discontinuous (non-parametric) variables. A p value of <0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism 9 software (San Diego, CA, USA).
3 |. RESULTS
One baboon in each group required early euthanasia for acute gastric dilatation, which we have seen previously in baboons in the early posttransplant period.30 On microscopic examination, neither kidney graft showed histopathological changes indicative of immunologic rejection (either antibody-mediated or acute cellular rejection). These two cases were excluded from further evaluation, leaving group sizes of n = 5 informative cases (Table 1). Some data on the Group 1 animals have been reported previously.6,8
Survival of Group 1 baboons was longer than of those in Group 2 (Table 1; median 237 vs. 35 days; mean 196 vs. 57 days; p < 0.07). Three of five baboons in Group 1 were ultimately euthanized due to infectious complications unrelated to clinical parameters of xenograft function. In contrast, all Group 2 baboons displayed clinical evidence of kidney rejection (elevations in serum creatinine, urine protein, reduced urine output, and decreased vascular blood flow). Rejection-free survival was significantly longer in Group 1 (p < 0.05).
All grafts in Group 2 showed antibody binding to renal vasculature, specifically, evidence of IgM and IgG deposited on renal interstitial arteries, peritubular capillaries, varying degrees of microvascular inflammation, and vascular thrombosis (Tables 2–5). None of the baboons in either group exhibited evidence of consumptive coagulopathy, indicated by thrombocytopenia, reduced plasma fibrinogen levels, or elevated fibrinogen D-dimers, at the onset of clinical features of rejection.
TABLE 2.
Major histopathological findings in the kidney graft
| Baboon | Histopathological findings | IMR | IMR-D/S |
|---|---|---|---|
| Group 1: Kidney is not from a CMAHKO pig | |||
| B9315 | Interstitial hemorrhage, edema, glomerular/tubular necrosis, and tubular atrophy | None | N/A |
| B17315 | Interstitial edema, fibrosis, and tubular atrophy | None | N/A |
| B17615 | Interstitial fibrosis and tubular atrophy | None | N/A |
| B10815 | Interstitial fibrin thrombi, dilation of Bowman’s space, and interstitial edema | AMR | Renal interstitium, multifocal/mild |
| B5916 | Thrombotic microangiopathy, interstitial fibrin thrombi, fibrosis and edema | AMR | Glomerular: global/severe Renal interstitium: multifocal/ moderate |
| Group 2: Kidney is from a CMAHKO pig | |||
| B8216 | Acute tubular injury, multifocal foci of interstitial vasculitis fibrin thrombi, and interstitial neutrophilic infiltrate | IRI/AMR | Renal interstitium: multifocal/mild |
| B1417 | Hemorrhage, fibrin thrombi, tubular necrosis, fibrosis, neutrophil and mononuclear cell infiltration | AMR/CCXR | Renal interstitium: diffuse/ severe |
| B3917 | Hemorrhage, congestion, fibrin thrombi, tubular necrosis, and neutrophilic infiltrate | HAR | Renal interstitium: diffuse/ severe |
| B15816 | Segmental edema associated with ascending pyelonephritis, gram-negative bacteria, multifocal foci of fibrin thrombi | AMR | Renal interstitium: multifocal/mild |
| B517 | Glomerular necrosis and dilation of Bowman’s space, interstitial edema, and fibrosis | AMR | N/A |
Abbreviations: AMR, antibody-mediated thromboses and rejection; CCXR, chronic cellular xenograft rejection; HAR, hyperacute rejection; IRI, ischemia/reperfusion injury; IMR, Immune mediated rejection; D/S, distribution and severity; N/A, no evidence of immune-mediated rejection.
TABLE 5.
Summary of renal interstitial immunoglobulin depositions
| Baboon | V-C3c | V-C4d | V-IgM | V-IgG | PTC-C3c | PTC-C4d | PTC-IgM | PTC-IgG |
|---|---|---|---|---|---|---|---|---|
| Group 1: Kidney is not from a CMAHKO pig | ||||||||
| B9315 | 0.5 | 0.5 | 0.5 | 0 | 0.9 | 0.5 | 0.1 | 0 |
| B17315 | 0.25 | n/a | 0 | 0 | 2.0 | n/a | 0.1 | 0.08 |
| B17615 | 0.38 | n/a | 0 | 0.42 | 0.9 | n/a | 0 | 0.03 |
| B10815 | 0.15 | 1.44 | 0.16 | 0.43 | 0.1 | 1.1 | 0.1 | 1.5 |
| B5916 | 0.5 | 1.25 | 1.28 | 0.08 | 1.0 | 0.9 | 1.7 | 0.26 |
| Median | 0.38 | 1.25 | 0.16 | 0.08 | 0.9 | 0.83 | 0.1 | 0.08 |
| Group 2: S is from a CMAHKO pig | ||||||||
| B8216 | 0.5 | 1.3 | 0.7 | 0.50 | 2.5 | 1.35 | 2.3 | 1.2 |
| B1417 | 0.76 | 1.11 | 0.87 | 0.77 | 2.0 | 1.19 | 1.4 | 0.88 |
| B3917 | 0.67 | 0.7 | 0.89 | 0.5 | 0.2 | 0.03 | 0.9 | 1.0 |
| B15816 | 0.56 | 1.13 | 0.83 | 0.35 | 0.8 | 0.89 | 0.8 | 0.31 |
| B517 | 0.58 | 1.48 | 0.47 | 0.64 | 1.83 | 1.61 | 1.4 | 1.0 |
| Median | 0.58 | 1.13 | 0.83 | 0.5 | 1.83 | 1.19 | 1.4 | 1.0 |
| p value | 0.017 | >0.99 | 0.21 | 0.019 | 0.36 | 0.31 | 0.046 | 0.18 |
Note: Data presented are the average score for each individual animal.
Abbreviations: PTC-C3c, peritubular capillary C3c deposition; PTC-C4d, peritubular capillary C4d deposition; PTC-IgG, peritubular capillary IgG deposition; PTC-IgM, peritubular capillary IgM deposition; V, renal arterial C3c deposition; V-IgG, renal arterial IgG deposition; V-IgM, renal arterial IgM deposition. Median values, range and non-parametric statistical significance (Mann–Whitney U test).
3.1 |. Clinical courses
Since there was considerable variation in survival and histologic features, we present brief narratives of the clinical courses of individual baboons to demonstrate this variation (Table 1).
3.2 |. Group 1: GTKO pigs expressing Neu5Gc
3.2.1 |. B5916
Open kidney needle biopsies at 2 and 6 months showed evidence of glomerular fibrin thrombi, interstitial edema, and acute tubular injury (Figure 1A) with minimal progression between these time-points, suggesting no increase in the severity of antibody-mediated rejection. On ultrasound, the ureter was not dilated, but the wall was thickened. After 247 days, the baboon developed clinical manifestations of nephrotic syndrome, and the baboon was euthanized on day 255.
FIGURE 1.
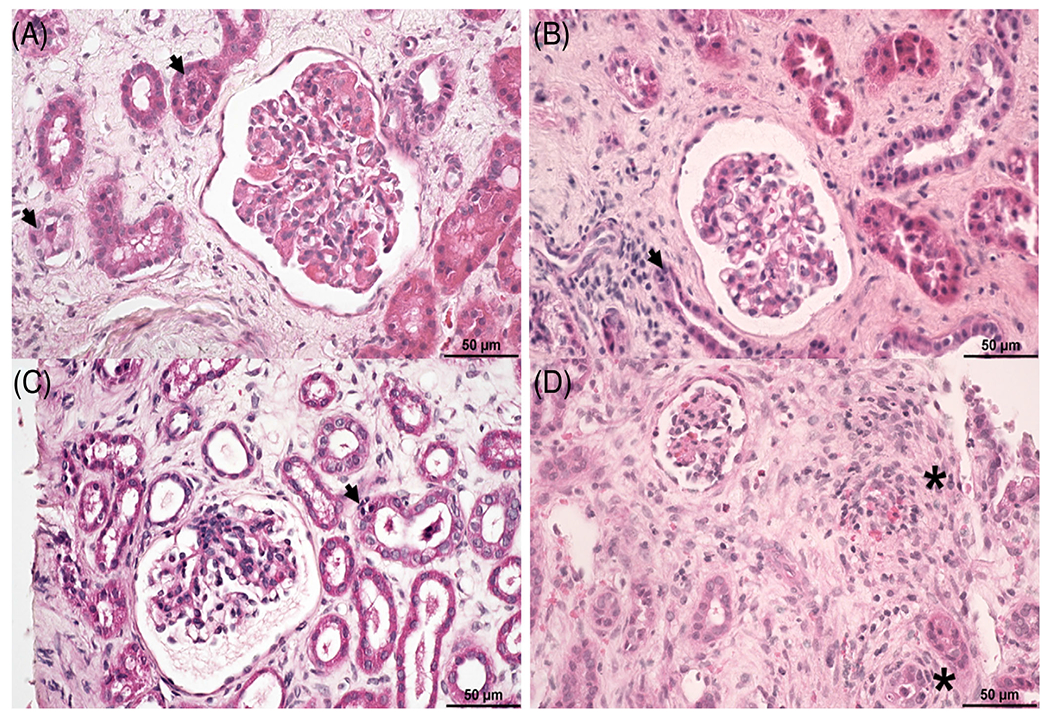
Graft biopsies from baboons in both groups displayed evidence of interstitial edema and fibrosis. (A) Biopsy from B5916 (Group 1) displayed (i) mild segmental thrombotic microangiopathy, (ii) diffuse moderate interstitial edema, and (iii) acute tubular injury. (B) Biopsy from B17315 (Group 1) displayed (i) diffuse moderate interstitial edema, (ii) diffuse mild interstitial fibrosis, and (iii) acute tubular injury. (C) Biopsy from B517 (Group 2) displayed diffuse mild interstitial edema and acute tubular injury. (D) Biopsy from B1417 (Group 2) displayed (i) diffuse moderate interstitial fibrosis, (ii) diffuse mild interstitial edema, and (iii) scattered mononuclear cell infiltrates, some located within peritubular capillaries adjacent to renal tubules or located in scarred interstitium (asterisk). Hematoxylin and eosin (Size bar 50 μm)
3.2.2 |. B9315
After 100 days, serum creatinine slowly increased. On day 103, the ureter was dilated, and an inflammatory mid-ureteric stricture (not associated with the anastomosis to the bladder) was bypassed by anastomosing the proximal ureter (proximal to the stricture) to the bladder. A needle biopsy of the kidney graft showed interstitial edema, but no evidence of antibody-mediated or cellular rejection. The animal was terminated on day 136 because of septic shock due to Myroides spp.6,8,31
3.2.3 |. B17315
Open needle biopsies were performed at 4 and 7 months after transplantation. Both showed evidence of mild interstitial edema, fibrosis, and acute tubular injury, but no features of antibody-mediated or cellular rejection (Figure 1B). On ultrasound, the ureter was grossly dilated, and the kidney manifested hydronephrosis. On day 223, the proximal dilated ureter was re-anastomosed to the bladder (bypass procedure), which effectively reversed the hydronephrosis. The baboon was found dead on day 237 from an infectious peritonitis (based on the culture of methicillin-resistant Staphylococcus aureus and Enterococcus faecium from the peritoneal cavity7).
3.2.4 |. B17615
The ureter became dilated, and the kidney developed hydronephrosis. An inflammatory ureteric stricture was bypassed by anastomosing the proximal ureter to the bladder on day 187, reversing the hydronephrosis. A needle biopsy of the kidney showed interstitial edema, but no features of rejection (similar to baboon 17315, Figure 1B).7 The baboon was euthanized on day 260 because of Pneumocystis pneumonia-induced decline in pulmonary function, while renal function was normal.7
3.2.5 |. B10815
At 2 months, after omitting two doses of anti-CD40mAb because of neutropenia, serum creatinine increased to 5.3 mg/dl. Rescue therapy was only partially successful in restoring renal function. The baboon developed ascites and was euthanized on day 90. Serum albumin levels were within a normal range at the time of necropsy, and proteinuria was not noted. Therefore, the cause of ascites is uncertain. At necropsy, the distal ureter wall was thickened, demonstrating extensive granulation tissue formation (Figure S1A–D), with thromboses within small caliber vessels in the ureteric submucosa and serosa; however, no evidence of urine leakage was noted.
3.3 |. Group 2: DKO/TKO pigs not expressing Neu5Gc
3.3.1 |. B517
Graft needle biopsies on days 45 and 98 revealed no histologic evidence of antibody-mediated or cellular rejection, although there was diffuse interstitial edema and evidence of acute tubular injury (Figure 1C). The baboon remained clinically well until day 176, when it suffered trauma to the lower left leg, which became ischemic, requiring euthanasia on day 183. At necropsy, the kidney showed features of antibody-mediated rejection.
3.3.2 |. B8216
Serum creatinine increased to 2.5 mg/dl on day 2 after transplantation and to 6.1 mg/dl on day 7, necessitating euthanasia. The baboon had severe peripheral edema, but no features of a consumptive coagulopathy. Urine protein at euthanasia was 163 mg/dl. On ultrasound, the ureter, but not kidney, exhibited evidence of gross dilation.
3.3.3 |. B1417
At 27 days, the serum creatinine increased suddenly to 5.0 mg/dl, and proteinuria increased. On day 35, a kidney biopsy showed multifocal hemorrhage, evidence of acute tubular injury, neutrophilic infiltration, segmental moderate glomerular thrombosis, some interstitial edema,fibrosis, and multiple foci of mononuclear cell infiltration within the renal interstitium (Figure 1D). Antibody-mediated rejection was suspected, and rescue therapy with high-dose steroids and the TNF-α antagonist etanercept (0.5 mg/kg) was instituted, which resulted in an immediate reduction in serum creatinine to 2.0 mg/dl. Survival was extended by almost 1 month, when an increase in creatinine to 8.9 mg/dl necessitated euthanasia on day 61. On ultrasound, the ureter was not dilated, nor did the kidney exhibit features of hydronephrosis
3.3.4 |. B3917
Immediately on reperfusion, the pig kidney became pink and remained so until the abdomen was closed. However, the baboon passed no urine, and therefore, after 6 h, the abdomen was explored. The kidney and ureter were enlarged, and showed a diffuse, dark livid discoloration, typical macroscopic features of hyperacute rejection. There was no reason to consider ischemia-reperfusion injury as the cause of graft failure as the ischemia time was significantly lower than other transplants (data not shown). Furthermore, this baboon exhibited higher baseline levels of anti-pig IgG antibody binding to peripheral blood mononuclear cells (PBMCs) and red blood cells (RBCs) than the other baboons and increased CDC 1.5 fold greater than averages of CDC values for Group 1 and Group 2 to GTKO and TKO PBMCs (92% vs. 59% and 72% vs. 47%, respectively) than other animals in both groups. For these reasons, we decided upon a presumptive diagnosis of hyperacute rejection, which is somewhat perplexing, given the confirmed expression of human CD46 and CD55 and preoperative use of CVF.
3.3.5 |. B15816
The kidney functioned well until day 30, when serum creatinine rose to 2.0 mg/dl, and edema of the thighs developed. On ultrasound, the ureter was dilated. Rejection was suspected, and a course of high-dose corticosteroids and etanercept was initiated. There was no clinical improvement, and the baboon was euthanized on day 35. On microscopy, there was evidence of ascending tubular injury with histology showing numerous intra-tubular neutrophils and signs of bacterial infection, suggesting the development of an ascending pyelonephritis.
3.4 |. Histopathology of graft biopsies and grafts at euthanasia
The most common histopathologic glomerular lesion noted in both groups at necropsy was the presence of varying degrees of dilation of Bowman’s space (Table 3 and Figure 2A,B), which was noted along with dilation of cortical collecting ducts (data not shown). We feel that these findings are indicative of edema pertaining to urinary tract outflow obstruction, which was consistent with ureteric dilation seen by ultrasound. With the exception of severe global thrombotic microangiopathy and deposition of IgM, C3c, and C4d within the glomeruli in B5916 (Figures 2C,D, and data not shown), no kidney in either group displayed clear evidence of thrombotic microangiopathy, nor were there any significant differences in scores for glomerular C3c, C4d, IgM, or IgG deposition (Table 3, Figure 2).
TABLE 3.
Summary of renal glomerular histopathologic changes
| Baboon | TMA | Mm | Necrosis | Dilation | C3c | C4d | IgM | IgG |
|---|---|---|---|---|---|---|---|---|
| Group 1: Kidney is not from a CMAHKO pig | ||||||||
| B9315 | 0.4 | 1.7 | 1.6 | 0.4 | 1.0 | 1.3 | 0.5 | 0.2 |
| B17315 | 0.02 | 1.0 | 0 | 2.0 | 2.0 | n/a | 1.4 | 0.2 |
| B17615 | 0.1 | 1.4 | 0 | 0.9 | 1.2 | n/a | 0.05 | 0.3 |
| B10815 | 0.02 | 0.3 | 0 | 2.0 | 0.4 | 0.95 | 0.8 | 1.1 |
| B5916 | 2.3 | 1.0 | 0.6 | 1.1 | 1.2 | 2.1 | 2.7 | 0.8 |
| Median | 0.4 | 1.0 | 0.6 | 1.1 | 1.2 | 1.3 | 0.8 | 0.3 |
| Group 2: Kidney is from a CMAHKO pig | ||||||||
| B8216 | 0.3 | 0.7 | 0.2 | 1.6 | 0.8 | 0.89 | 1.0 | 0.7 |
| B1417 | 0.4 | 0.6 | 0.2 | 1.5 | 0.6 | 1.05 | 0.7 | 0.5 |
| B3917 | 0.2 | 0 | 0.6 | 0.1 | 0.06 | 0.03 | 0.4 | 0.3 |
| B15816 | 0.02 | 1.1 | 0.02 | 2.0 | 0.8 | 1.61 | 1.0 | 0.7 |
| B517 | 0.6 | 2.0 | 1.1 | 2.0 | 0.2 | 1.35 | 0.4 | 0.4 |
| Median | 0.3 | 0.7 | 0.2 | 1.5 | 0.6 | 1.35 | 0.4 | 0.5 |
| p value | 0.554 | 0.764 | 0.937 | 0.745 | 0.068 | 0.34 | 0.414 | 0.839 |
Note: Median values and non-parametric statistical significance (Mann–Whitney U test) analyses are reported.
Abbreviations: C3c, glomerular complement 3c staining; dilation, expansion of Bowman’s space; IgG, glomerular IgG staining; IgM, glomerular IgM staining; Mm, mesangial matrix expansion; Necrosis, glomerular necrosis; TMA, thrombotic microangiopathy.
FIGURE 2.
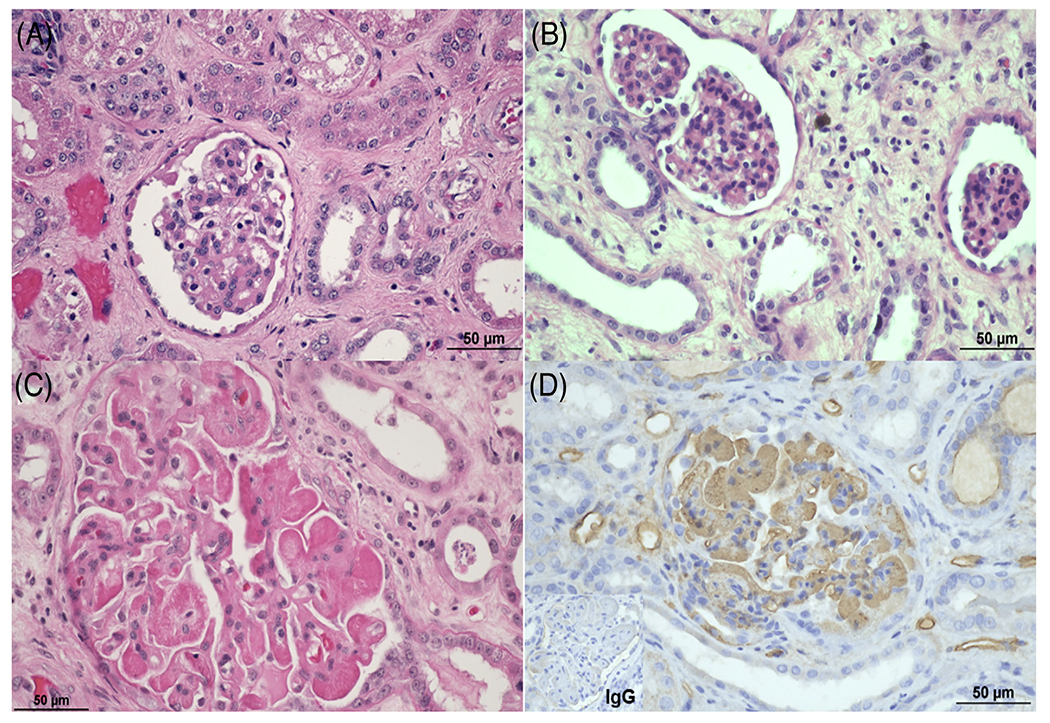
Glomerular histopathologic lesions seen in CMAHKO donor kidneys. Expansion of Bowman’s space (edema) was commonly seen in baboons in both groups. (A) B17315 (Group 1) and (B) B517 (Group 2) both displayed moderate amounts of edema in Bowman’s space. (C) B5916 (Group1) displayed global severe thrombotic microangiopathy with (D) global, intense IgM deposition. (A-C) Hematoxylin and eosin, (D) immunohistochemistry for IgM (brown staining), and IgG (see insert in lower left corner). (Size bar 50 μm)
Evaluation of the renal interstitium revealed differences in pathology of the interstitial arteries and peritubular capillaries. Group 2 kidneys exhibited increased fibrin thrombi deposition, vasculitis, and peritubular capillary infiltrates (Table 4 and Figure 3). Furthermore, there were trends for increased deposition of C3c, IgM, and IgG in renal interstitial vessels (Table 5), but not of C4d. Lastly, there were T and B cell infiltrates of varying size in both groups as determined by staining for CD3, IgM, and IgG. Compared to Group 1, B1417 and B517 in Group 2 exhibited a trend increased numbers of CD3-positive T cells and to a much lesser extent IgM and IgG-positive B cells (Figure 4A–D). Overall, these findings suggest a greater incidence of antibody-mediated thromboses centered around the renal interstitial arteries and peritubular capillaries in baboons receiving CMAHKO pig kidneys (Group 2).
TABLE 4.
Summary of renal interstitial histopathologic changes
| Baboon | Hem | Edema | TIF | PTC | Tublitis | TubA | TubN | Thrombi | v | ci |
|---|---|---|---|---|---|---|---|---|---|---|
| Group 1: Kidney is not from a CMAHKO pig | ||||||||||
| B9315 | 1.6 | 2.7 | 0.6 | 0. | 0.5 | 2.8 | 1.2 | 0.5 | 0.5 | 1.3 |
| B17315 | 0.1 | 1.5 | 0.8 | 0.4 | 0.3 | 1.3 | 0.7 | 0.1 | 0 | 1.4 |
| B17615 | 0.0 | 0.3 | 0.9 | 0.5 | 0.3 | 1.1 | 0.5 | 0 | 0 | 1.1 |
| B10815 | 0 | 1.8 | 0.4 | 1.0 | 0.4 | 2.7 | 0 | 0.2 | 0.1 | 1.9 |
| B5916 | 0.1 | 1.5 | 0.3 | 1.0 | 0.2 | 0.8 | 0.4 | 0.6 | 0.5 | 0.5 |
| Median | 0.1 | 1.5 | 0.6 | 0.6 | 0.3 | 1.3 | 0.5 | 0.2 | 0.1 | 1.3 |
| Group 2: Kidney is from a CMAHKO pig | ||||||||||
| B8216 | 0.5 | 1.2 | 0.2 | 1.2 | 0.6 | 0.6 | 0.6 | 0.8 | 0.6 | 0.3 |
| B1417 | 1.8 | 1.5 | 1.6 | 1.2 | 1.1 | 2.5 | 1.3 | 1.3 | 1.2 | 2.5 |
| B3917 | 2.0 | 0.2 | 0 | 1.8 | 0.2 | 0 | 2.0 | 0.5 | 0.7 | 0 |
| B15816 | 0.1 | 1.8 | 1.0 | 1.0 | 0.7 | 2.6 | 0.5 | 0.4 | 0.7 | 0.9 |
| B517 | 0.1 | 2.2 | 0.6 | 0.8 | 0.5 | 1.0 | 1.3 | 1.1 | 0.9 | 1.6 |
| Median | 0.5 | 1.5 | 0.6 | 1.2 | 0.6 | 1.0 | 1.3 | 0.5 | 0.92 | 0.9 |
| p value | 0.35 | 0.73 | 0.81 | 0.048 | 0.12 | 0.59 | 0.12 | 0.095 | 0.008 | 0.75 |
Note: Data presented are the average score for each individual animal.
Abbreviations: ci, interstitial fibrosis; edema, interstitial edema; Hem, interstitial hemorrhage; PTC, peritubular capillary inflammation; TIF, total interstitial inflammation; TubA, tubular atrophy; TubN, tubular necrosis; thrombi, renal interstitial arterial thrombi; v, vasculitis.
Median values, range and non-parametric statistical significance (Mann–Whitney U test).
FIGURE 3.
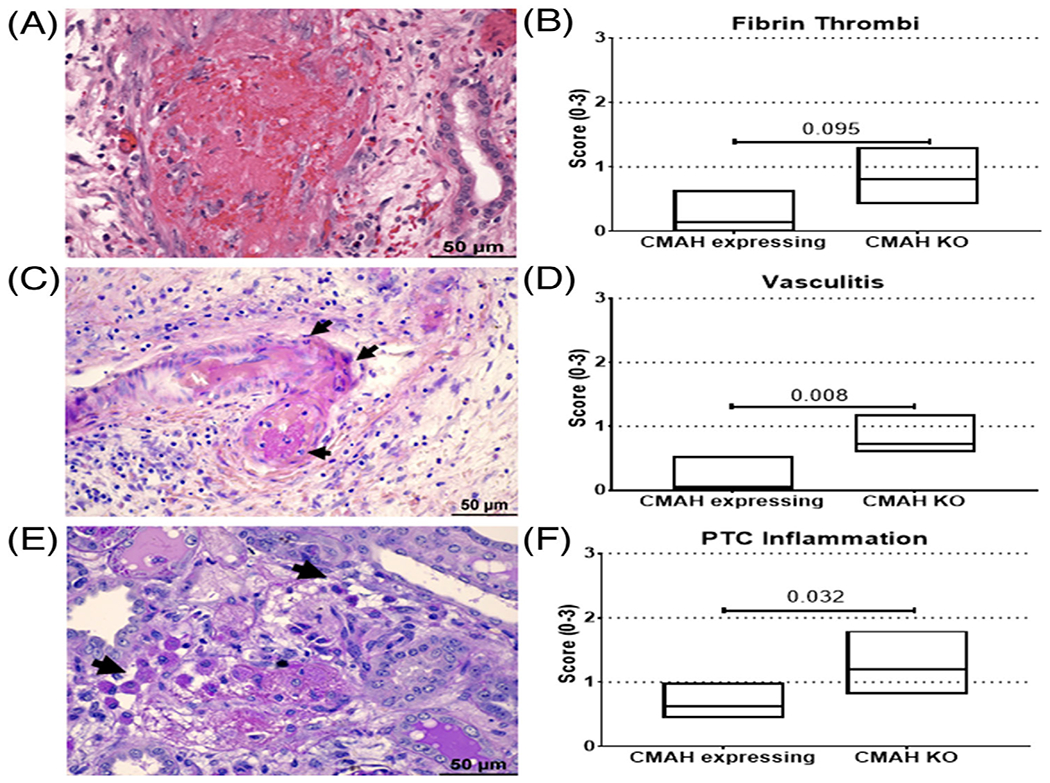
CMAHKO kidneys displayed an increased incidence of intimal arteritis, fibrin thrombi, and peritubular capillary inflammation. Kidneys from CMAHKO donors manifested an increased incidence of (A and B) fibrin thrombi, (C and D) vasculitis, and (E and F) peritubular capillary (PTC) inflammation. (A) Diffuse fibrin thrombi with transmural inflammation and fibrinoid necrosis is present in renal arteries. Representative image is from baboon 1417 (Group 2) H+E stain. (C) Renal artery exhibits transmural necrosis with neutrophilic infiltrate (arrows) and intraluminal periodic acid-Schiff (PAS) positive fibrin thrombus. Representative image from baboon 1417 (Group 2) PASH stain. (E) Highlighted peritubular capillaries (arrows) contain significant numbers of mononuclear cells (most likely macrophage) containing PAS-positive cytoplasmic material and adjacent immature fibrin thrombus (fibrillary, violet, PAS positive material). Representative image from baboon 8216 (Group 2) PASH stain. Non-parametric statistics was assessed by Mann–Whitney U-test; p values are presented with horizontal lines. (Size bar 50 μm)
FIGURE 4.
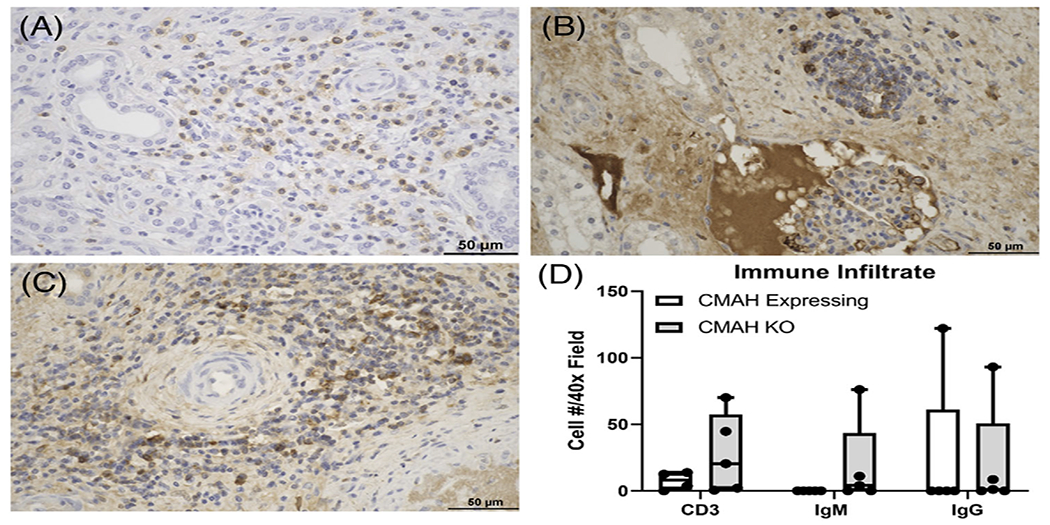
Increased T and B cell infiltrates in the renal interstitium of CMAHKO kidney transplants. (A-C) Representative images of (A) CD3, (B) IgM, and (C) IgG from B1417 (Group 2). The majority of cells reside around medium caliber arteries and within the renal interstitium. (D) Numbers of CD3+ T cells, IgM+ B cells, and IgG+ B cells in CMAHKO pig kidney transplants. No statistically significant differences were noted despite trends for increased T and B cell infiltrates in some of the CMAHKO kidneys. Immunohistologic staining (brown color). Variance in each group is expressed as standard deviation (n = 4 data points/group). (Size bar 50 μm)
Ureters in both groups commonly revealed mononuclear cell infiltrates, edema, and fibrosis in the submucosa and serosa (Figure 5). All baboons displayed some gross evidence of ureteric dilation with stricture formation at or proximal to the bladder anastomoses. This aside, there were no significant differences in histologic scores of ureter histopathology between the two groups with the exception of increased urothelial necrosis in Group2 (Tables 6 and 7). Evaluation of ureter thickness with graft survival did not reveal any significant correlation (Figure S2A,B). However, the extent of fibrosis and graft survival revealed a significant correlation (Figure S2C,D), suggesting that fibrotic changes in the ureter accumulate over a prolonged period of graft survival.
FIGURE 5.
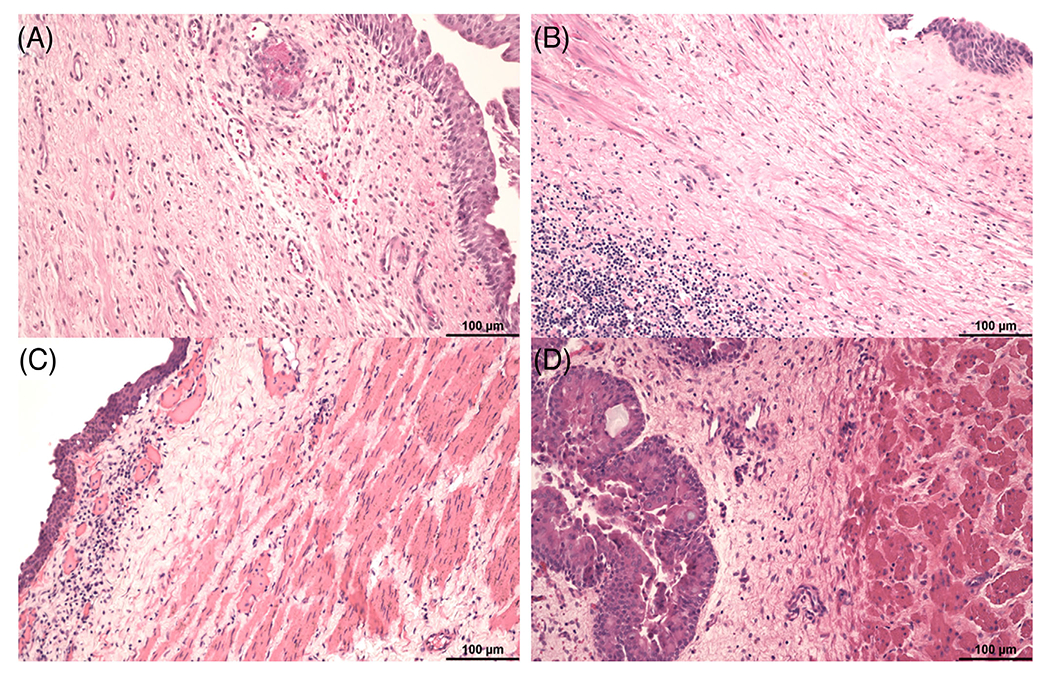
Submucosal edema and fibrosis was commonly noted in ureters from both groups. The submucosa was expanded by edema, fibrosis, and scattered mononuclear cell infiltrates in (A) B5916 (Group 1), (B) B1417 (Group 2), (C) B9315 (Group 1), and (D) B15816 (Group 2). Hematoxylin and eosin (Size bar 100 μm)
TABLE 6.
Summary of major gross and histopathological findings in the ureter
| Baboon | Histopathological findings | Dilation | Stricture formation | Surgical correction |
|---|---|---|---|---|
| Group 1: Kidney is not from a CMAHKO pig | ||||
| B9315 | Submucosal edema, fibrosis, and mononuclear cell infiltrates | Yes | Yes | Yes |
| B17315 | Submucosal edema, fibrosis, and mononuclear cell infiltrates | Yes | No | No |
| B17615 | Transmural fibrosis, edema, segmental necrosis, and intimal arteritis | Yes | Yes | Yes |
| B10815 | Transmural fibrosis, edema, fibrin thrombi (muscularis and serosal layers), and intimal arteritis | Yes | Yes | No |
| B5916 | Submucosal edema, fibrosis, fibrin thrombi, and intimal arteritis | No* | No | No |
| Group 2: Kidney is from a CMAHKO pig | ||||
| B8216 | Submucosal and serosal edema, serosal fat necrosis, fibrin thrombi and multifocal foci of hemorrhage | No | No | No |
| B1417 | Submucosal edema, fibrosis, and mononuclear cell infiltrates | No | No | No |
| B3917 | Submucosal edema, congestion, and fibrin thrombi | Yes | No | No |
| B15816 | Submucosal edema | Yes | No | No |
| B517 | Submucosal and serosal edema and mononuclear cell infiltrates | Yes | No | No |
Baboon 5916, ureter mucosa appeared thickened, but not dilated on ultrasound examination.
TABLE 7.
Summary of ureter histopathologic changes
| Baboon | Hemorrhage | Edema | Necrosis | i | v | Thrombi | ci | Thickness (mm)* |
|---|---|---|---|---|---|---|---|---|
| Group 1: Kidney is from a CMAH expressing pig | ||||||||
| B9315 | 0.27 | 1.05 | 0.05 | 0.1 | 0 | 0 | 1.2 | 1.06 |
| B17315 | 0 | 1.1 | 0.7 | 0.35 | 0.1 | 0 | 1.8 | 1.84 |
| B17615 | 0.35 | 1.35 | 0 | 0.6 | 0.3 | .13 | 1.3 | 8.97 |
| B10815 | 0 | 1.0 | 0 | 0.5 | 0.35 | 0.3 | 1.25 | 5.67 |
| B5916 | 0.1 | 1.1 | 0 | 0.3 | 0.1 | 0.48 | 1.5 | 8.68 |
| Median | 0.1 | 1.1 | 0 | 0.35 | 0.1 | 0.13 | 1.3 | 4.64 |
| Group 2: Kidney is from a CMAHKO pig | ||||||||
| B8216 | 1.5 | 2.0 | 1 | 2.0 | 0 | 0.3 | 0 | 2.26 |
| B1417 | 0.18 | 0.36 | 1.3 | 1.3 | 0.18 | 0 | 1.8 | 2.78 |
| B3917 | 3.0 | 0 | 0.73 | 0.6 | 0.3 | 0.6 | 0 | 1.83 |
| B15816 | 0.05 | 1.5 | 0.4 | 0.3 | 0.3 | 0 | 2.0 | 4.71 |
| B517 | 0 | 1.1 | 0.13 | 0.43 | 0.18 | 0.13 | 1.4 | 2.95 |
| Median | 0.18 | 1.1 | 0.4 | 0.6 | 0.18 | 1.0 | 2.0 | 2.91 |
| p value | 0.54 | >0.99 | 0.0476 | 0.21 | 0.99 | 0.56 | 0.33 | 0.24 |
Hemorrhage, edema, necrosis, inflammation (i), vasculitis (v), and fibrosis (ci) were scored as described in Table 3.
Ureter thickness was assessed on low magnification (2x) by measuring the distance (μM) from mucosal apical surface to serosal apical surface. Mean thickness is reported for each group, and an unparied Student’s t test was used to evaluate for statistical for significance (p < 0.05). For all other semi-quantitative parameters, data are reported as median values and non-parametric statistical significance (Mann–Whitney U test) analyses are reported.
Summarizing the histopathological features, antibody-mediated rejection of varying severity was seen in all grafts in Group 2, manifesting primarily as fibrin thrombi in medium-to-large caliber arteries and peritubular capillaries, with increased IgM and IgG antibody binding. In Group 1, only two kidneys (B5916, B10815) demonstrated some features consistent with antibody-mediated rejection and thromboses, and with graft rejection. Ureters displayed similar features with the exception of increased urothelial necrosis and incidence of transmural fibrosis in grafts surviving for longer periods of time.
4 |. DISCUSSION
Recipient survival and rejection-free survival were both longer in Group 1 than in Group 2 (Table 1), suggesting that deletion of expression of Neu5Gc in the pigs used in Group 2 was detrimental to the outcome of the kidney transplant in baboons. In addition to clinical evidence of kidney graft rejection, the histopathology confirmed features indicative of antibody-mediated rejection and vascular thromboses. Specifically, with respect to histopathologic features associated with AMR and thromboses we see increased incidence of vascular inflammation, fibrin thrombi, and immunoglobulin deposition in affected renal interstitial arteries and peritubular capillaries.
In contrast to our previous studies, in which features of thrombotic microangiopathy associated with a consumptive coagulopathy were almost ubiquitous when graft loss was imminent,32 the present study did not manifest these features in either group (with exception of B5916 in Group 1; Figures 2C,D). This is presumably associated with the greater protection offered by the increased extent of genetic engineering of the pigs (It cannot be ascribed to the immunosuppressive regimen, which was largely unchanged.). Although this is an encouraging observation, it indicates that a current diagnosis of antibody-mediated rejection can no longer rest on the development of a consumptive coagulopathy, and that other parameters are needed to define antibody-mediated rejection. The diagnosis should be associated with the histopathology, that is, fibrin thrombi in medium-to-large arteries and peritubular capillaries, with increased IgM and IgG antibody binding.
Edema was a consistent finding in pig kidney xenografts in both groups, irrespective of antibody-mediated rejection and vascular thrombosis. Interstitial edema is seen in kidney allotransplant rejection and occurs as a result of obstruction of urine outflow.33 Dilation of renal tubules and Bowman’s space was also prominent in transplanted kidneys, and ureters demonstrated evidence of hydronephrosis and hydroureter. In renal xenotransplantation, the etiology is likely multifactorial, including antibody-mediated damage to the renal vasculature, ureter-related obstruction, and possibly uncharacterized physiologic incompatibilities.34–36
In relation to obstruction of urine outflow, both groups displayed evidence of thickening of the walls of the pig ureters, which we suggest was associated with the systemic inflammatory response documented in all baboons.36–38 Histopathological changes to the ureters revealed minimal differences between the groups with the exception of increased urothelial necrosis in Group 2 (Table 7). Three baboons in Group 1 developed partial strictures that required bypass surgery and demonstrated additional pathologic lesions, including vascular thrombosis and moderate-to-severe periarteritis. None of the pig ureters in Group 2 baboons developed partial strictures or required surgical bypass, although ureteric dilation or thickening was similar to that in baboons in Group 1. We suggest that these differences may, in part, be associated with the significantly shorter graft survival in Group 2, this being insufficient for the development of partial strictures. There was a significant correlation between the extent of fibrosis and graft survival (Figure S2), further supporting this hypothesis.
In a life-supporting CD55-transgenic pig-to-cynomolgus monkey kidney xenotransplantation model, Baldan et al.39 reported that (i) ureteral rejection was observed in 85% of cases, at least partially contributing to ureteral stenosis, and (ii) there was a close relationship between ureter and kidney in terms of type and severity of rejection. Antibody-mediated disruption of blood flow to the ureters over time likely contributes to pathologic changes and alterations in urine flow. Understanding the pathogenesis of ureteric changes and their contribution to partial urine outflow obstruction is critical in selecting appropriate therapeutic interventions to maintain collateral circulation to the ureter and maximize ureter viability in future transplants.
Overall, the present findings suggest that transplantation of kidneys from pigs in which expression of Neu5Gc has been deleted (Group 2) is associated with a higher occurrence of antibody binding to the vascular endothelium and reduced graft survival in baboons. This antibody-mediated injury is presumably related to expression of novel xenoantigens in pigs in which Neu5Gc has been deleted. In view of the potent complement-dependent cytotoxicity that is associated with binding of baboon antibodies to CMAHKO pig cells,2,11–12 it is perhaps surprising that there was not even more rapid injury to these kidneys. One CMAHKO pig kidney in Group 2 functioned well for 183 days (despite some histopathological evidence of antibody-mediated injury) at which time the baboon required euthanasia for unrelated lower limb trauma. This is encouraging, because it indicates that a combination of human “protective” transgenes may overcome to a certain extent the barrier presented by CMAHKO in pig organs transplanted into NHPs. Nevertheless, to avoid this complication, the transplantation of a kidney from a pig with multiple genetic modifications but expressing Neu5Gc into baboon recipients would be preferable to validate the efficacy of immunosuppressive regimens and other genetic modifications in pig “donors” with respect to translation into the clinic11,12; however, in the clinical setting deletion of Neu5Gc would be preferrable based on in vitro studies with human sera.
Supplementary Material
ACKNOWLEDGMENTS
Work on xenotransplantation at the University of Alabama at Birmingham is supported in part by NIH NIAID U19 grant AI090959 and in part by a grant to UAB from United Therapeutics, Silver Spring, MD. The anti-CD40 2C10R4 monoclonal antibody used in these studies was provided by the NIH NHP Reagent Resource funded by NIH grants AI126683 and OD010976. Some of the baboons used in these studies were from the Michale E. Keeling Center, MD Anderson Cancer Center, Bastrop, Tx, which is supported in part by a NIH grant NIHOD024628-02.
Abbreviations:
- CMAH-KO
cytidine monophosphate-N-acetylneuraminic acid hydroxylase gene-knockout (deletion of expression of Neu5Gc)
- DKO
double-knockout (deletion of expression of Gal and Neu5Gc)
- Gal
galactose-α1,3-galactose
- GTKO
1,3-galactosyltransferase gene-knockout (deletion of expression of Gal)
- Neu5Gc
N-glycolylneuraminic acid
- NHP
nonhuman primate
- TKO
triple-knockout (deletion of expression of all three known pig carbohydrate xenoantigens)
Footnotes
CONFLICT OF INTEREST
David Ayares is an employee of Revivicor, Inc. Hendrik-Jan Schuurman is director of SchuBiomed Consultancy and provides consultancy in the biomedical sector worldwide. No other author reports a conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found in the online version of the article at the publisher’s website.
REFERENCES
- 1.Estrada JL, Martens G, Li P, et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation. 2015;22:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamamoto T, Hara H, Iwase H, et al. The final obstacle to successful preclinical xenotransplantation. Xenotransplantation. 2020;27:e12596. [DOI] [PubMed] [Google Scholar]
- 3.Cooper DKC, Ezzelarab M, Iwase H, Hara H. Perspectives on the optimal genetically-engineered pig in 2018 for initial clinical trials of kidney or heart xenotransplantation. Transplantation. 2018;102(12):1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper DKC, Hara H, Iwase H, et al. Justification of specific genetic modifications in pigs for clinical kidney or heart xenotransplantation. Xenotransplantation. 2019;26(4):e12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamada K,Yazawa K, Shimizu A, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3- galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005;11(1):32–34. [DOI] [PubMed] [Google Scholar]
- 6.Iwase H, Liu H, Wijkstrom M, et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation. 2015;22:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwase H, Hara H, Ezzelarab M, et al. Immunological and physiological observations in baboons with life-supporting genetically engineered pig kidney grafts. Xenotransplantation. 2017;24(2). 10.1111/xen.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto T, Hara H, Foote J, et al. Life-supporting kidney xenotransplantation from genetically-engineered pigs in baboons: a comparison of two immunosuppressive regimens. Transplantation. 2019;103(10):2090–2104. [DOI] [PubMed] [Google Scholar]
- 9.Mohiuddin MM, Singh AK, Corcoran PC, et al. Role of anti-CD40 antibody-mediated costimulation blockade on non-Gal antibody production and heterotopic cardiac xenograft survival in a GTKO.hCD46Tg pig-to-baboon model. Xenotransplantation. 2014;21(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Längin M, Mayr T, Reichart B, et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature. 2018;564:430–433. [DOI] [PubMed] [Google Scholar]
- 11.Yamamoto T, Iwase H, Patel D, et al. Old world monkeys are less than ideal transplantation models for testing organs lacking three carbohydrate antigens (triple-knockout). Sci Rep. 2020;10:9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui Y, Yamamoto T, Raza SS, et al. Evidence for GTKO/β4GalNT2KO pigs as the preferred organ-source for Old World nonhuman primates as a preclinical model of xenotransplantation. Transplant Direct. 2020;6(8):e590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang J, Xie C, Lu Y, et al. Potential antigens involved in delayed xeongraft rejection in a GGTA1/CMAH DKO pig-to-monkey model. Sci Rep. 2017;7:10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwase H, Ekser B, Satyananda V, et al. Pig-to-baboon heterotopic heart transplantation-exploratory preliminary experience with pigs transgenic for human thrombomodulin and comparison of three costimulation blockade-based regimens. Xenotransplantation. 2015;22(3):211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller NJ, Kuwaki K, Dor FJ, et al. Reduction of consumptive coagulopathy using porcine cytomegalovirus-free cardiac porcine grafts in pig-to-primate xenotransplantation. Transplantation. 2004;78(10):1449–1453. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Iwase H,Wolf RF, et al. Are there advantages in the use of specific pathogen-free baboons in pig organ xenotransplantation models?. Xenotransplantation. 2014;21(3):287–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ezzelarab M, Garcia B, Azimzadeh A, et al. The innate immune response and activation of coagulation in alpha1,3-galactosyltransferase gene-knockout xenograft recipients. Transplantation. 2009;87(6):805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin CC, Ezzelarab M, Shapiro R, et al. Recipient tissue factor expression is associated with consumptive coagulopathy in pig-to-primate kidney xenotransplantation. Am J Transplant. 2010;10(7):1556–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwase H, Ekser B, Satyananda V, et al. Initial in vivo experience of pig artery patch transplantation in baboons using mutant MHC (CIITA-DN) pigs. Transpl Immunol. 2015;32(2):99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao B, Long C, Lee W, et al. Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLoS One. 2017;12(7):e0180768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schuurman HJ, Cheng J, Lam T. Pathology of xenograft rejection: a commentary (review article). Xenotransplantation. 2003;10:293–299. [DOI] [PubMed] [Google Scholar]
- 22.Rose AG, Cooper DKC. A histopathologic grading system of hyperacute (humoral, antibody-mediated) cardiac xenograft and allograft rejection. J Heart Lung Transplant. 1996;15:804–817. [PubMed] [Google Scholar]
- 23.Roufosse C, Simmonds N, Clahsen-van Groningen M, et al. A 2018 reference guide to the Banff classification of renal allograft pathology. Transplantation. 2018;102(11):1795–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimizu A, Yamada K, Sachs DH, et al. Persistent rejection of peritubular capillaries and tubules is associated with progressive interstitial fibrosis. Kidney Int. 2002;61(5):1867–1879. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu A, Meehan SM, Kozlowski T, et al. Acute humoral xenograft rejection: destruction of the microvascular capillary endothelium in pig-to-nonhuman primate renal grafts. Lab Invest. 2000;80(6):815–830. [DOI] [PubMed] [Google Scholar]
- 26.Shimizu A, Yamada K, Sachs DH, et al. Mechanisms of chronic renal allograft rejection. II. Progressive allograft glomerulopathy in miniature swine. Lab Invest. 2002;82(6):673–686. [DOI] [PubMed] [Google Scholar]
- 27.Shimizu A, Yamada K, Yamamoto S, et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J Am Soc Nephrol. 2005;16(9):2732–2745. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu A, Yamada K. Histopathology of xenografts in pig to non-human primate discordant xenotransplantation. Clin Transplant. 2010;24(22):11–15. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu A, Yamada K, Robson SC, et al. Pathologic characteristics of transplanted kidney xenografts. J Am Soc Nephrol. 2012;23:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campanile N, Rood PPM, Yeh P, Casu A, Bottino R, Cooper DKC. Acute gastric dilatation after porcine islet transplantation in a cynomolgus monkey – case history and review of the literature. Xenotransplantation. 2007;14(3):265–270. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Iwase H, Wijkstrom M, et al. Myroides infection in a baboon after prolonged pig kidney graft survival. Transplantation Direct. 2015;1(4):e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knosalla C, Gollackner B, Bühler L, et al. Correlation of biochemical and hematological changes with graft failure following pig heart and kidney transplantation in baboons. Am J Transplant. 2003;3(12):1510–1519. [DOI] [PubMed] [Google Scholar]
- 33.Kumar S, Ameli-Renani S, Hakin A, et al. Urethral obstruction following renal transplantation: causes, diagnosis, and management. Br J Radiol. 2014;87(1044):20140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iwase H, Klein E, Cooper DKC. Physiological aspects of pig kidney transplantation in primates. Comp Med. 2018;68(5):332–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwase H, Yamamoto T, Cooper DKC. Episodes of hypovolemia/dehydration in baboons with pig kidney transplants: a new syndrome of clinical importance? Xenotransplantation. 2019;26(2):e12472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ezzelarab MB, Cooper DKC. Systemic inflammation in xenograft recipients (SIXR): a new paradigm in pig-to-primate xenotransplantation? Int J Surg. 2015;23(Pt B):301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iwase H, Ekser B, Zhou H, et al. Further evidence for a sustained systemic inflammatory response in xenograft recipients (SIXR).Xenotransplantation. 2015;22(5):399–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Hara H, Wang Y, Esmon C, Cooper DKC, Iwase H. Evidence for the important role of inflammation in xenotransplantation. J Inflamm (Lond). 2019;16:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baldan N, Rigotti P, Calabrese F, et al. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004;4(4):475–481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


