Abstract
Objective:
To investigate the preliminary efficacy of a high n-3 plus low n-6 (H3-L6) dietary intervention in improving mood stability in Bipolar Disorder (BD) when compared to dietary intervention with usual U.S. levels of n-6 and n-3 polyunsaturated fatty acid (PUFA) intakes (control diet, CD).
Methods:
This 2-arm, parallel-group, randomized, modified double-blind, controlled 48-week study of 12-week intensive diet intervention in subjects with BD was conducted at a single suburban-rural site in the mid-Atlantic region. Participants with DSM-IV TR BD I or II with hypomanic or depressive symptoms were randomized, stratified on gender (N = 82). The intervention included the provision of group-specific study foods and dietary counseling. Variability of mood symptoms was measured by a twice-daily, 12-week ecological momentary analysis (EMA) paradigm, and group differences were analyzed using multilevel models. Circulating n-3 and n-6 fatty acids were measured at baseline and after 4, 8, and 12 weeks of diet exposure.
Results:
All 82 randomized participants were included in biochemical analyses. Seventy participants completed at least 2 EMA surveys and were included in primary EMA analyses. Variability in mood, energy, irritability, and pain as measured using EMA was reduced in the H3-L6 group compared to the CD group. No significant differences in mean ratings of mood symptoms, or any other symptom measures, were detected. The dietary intervention effect on target PUFAs significantly differed by the group over time.
Conclusions:
A dietary intervention adjunctive to usual care showed preliminary efficacy in improving variability in mood symptoms in participants with BD.
Trial Registration:
Keywords: bipolar disorder, diet, depression, fatty acids, omega-3, omega-6, food, unsaturated
1 |. BACKGROUND
Bipolar disorders (BDs) are chronic relapsing, remitting illnesses in which individuals experience cyclic and abnormally elevated and/or depressed mood states.1 BDs affect about 1–2.4% of the population,2 but are 17th in the causes of years of life lived with disability.3 As shown in the BP CHOICE study, the BALANCE study, and others, even with pharmacotherapy delivered in optimal conditions, many individuals with BDs remain symptomatic and have significant mood variability.4,5 Instability of mood in BDs predicts relapse, leads to functional impairment and is a marker of illness severity even between major mood episodes.4–8 Because many individuals treated for BDs do not achieve adequate control of mood symptoms and experience substantial adverse effects from BD treatments, there is a need for the augmentation of pharmacological maintenance treatments for this disorder. Dietary modification of food-based essential polyunsaturated fatty acids (PUFAs) may offer one avenue for a safe, tolerable, targeted augmentation of therapy in BD.
Polyunsaturated fatty acids (PUFAs) make up approximately 35% of lipids in the brain, and n-3 and n-6 PUFAs have been implicated in mood disorders.9 PUFAs are nutritionally essential, and serve a critical function to increase fluidity in the cell membranes, serve as precursors for autacoids (i.e., oxylipins, endocannabinoids) that modulate synaptic neurotransmission, oxidative stress, excitotoxicity, and inflammation, in addition to a critical role in generating pain-inducing compounds such as prostaglandin E2 (PGE2).9 While epidemiological and ecological studies have reported that higher consumption of seafood rich in the n-3 PUFAs eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) was associated with lower prevalence rates of BD,10,11 results of n-3 supplementation trials in BD are conflicting.12–18 Because pathways involved in n-3 and n-6 PUFA share enzymes, supplementation of n-3 EPA and DHA alone may not be sufficient to affect BD symptoms. Lowering the intake of the n-6 PUFA linoleic acid (LA) decreases THE metabolic turnover of the n-6 PUFA arachidonic acid (AA) in the brain in animal models,19 and may augment biochemical changes produced by increases in dietary n-3 PUFA.20 Post-mortem and clinical studies have suggested altered metabolism of PUFA in BD,21 and pharmacological studies in rodent models have demonstrated that several effective medications for BD decrease metabolism of the n-6 AA.22 Based on these lines of evidence, we sought to investigate whether a combined intervention lowering dietary n-6 LA while increasing dietary n-3 EPA and DHA may be an effective adjunctive treatment for BD.
To measure mood instability on a daily basis, ecological momentary assessment (EMA) paradigms are ideal. EMA is designed to subjectively record mood ratings on a more frequent basis and in real time when individuals are going about daily activities, allowing for a specific assessment of the variability of mood over time.23 This eliminates the need for the participant to recall and integrate mood shifts over time when reporting and provides a window into the daily experience of individuals with BD. EMA has been used successfully to measure the variability of mood in BD over time and has demonstrated that individuals with BD have different daily mood patterns than individuals without BD.24–27 EMA thus provides a tool to specifically measure mood instability experienced by participants in daily life during an intervention.
We hypothesized that a dietary intervention designed to raise n-3 EPA and DHA and lower n-6 LA would reduce day-to-day instability in mood symptoms and reduce general psychological distress in individuals with BD. This trial was therefore designed to test the effect of a high n-3 EPA +DHA plus low n-6 LA (H3-L6) targeted dietary manipulation on the variability of mood in individuals with BD, with mood and related symptoms measured in an EMA paradigm. The primary clinical outcome of the trial was variability of mood during the time of intensive intervention and general psychosocial distress and functioning. The main biochemical endpoints of the trial were to evaluate whether the experimental dietary intervention altered n-6 AA, n-3 DHA, and their bioactive metabolites in patients with BD. We hypothesized that compared to the control diet, the experimental intervention would significantly decrease circulating n-6 AA and increase n-3 DHA, and increase 17-hydroxy DHA (17-HDHA) and decrease PGE2. Herein, results of EMA-measured BD symptoms and blood levels of n-3 and n-6 PUFAs, 17-HDHA, and PGE2 are described throughout an intensive 12-week randomized, modified double-blinded H3-L6 dietary intervention augmenting usual care.
2 |. METHODS
2.1 |. Study design
The study was designed to evaluate the therapeutic efficacy of augmentation of usual care with either a high n-3 EPA + DHA (1,500 mg per day) plus low n-6 LA (2% energy or en%) diet or a control diet standardized to the usual American distribution of n-3 EPA + DHA (150 mg per day) and n-6 LA (7% energy or en%).28
2.2 |. Recruitment, screening, eligibility, consent, and enrollment procedures
The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was IRB approved by the Penn State College of Medicine IRB #00268. Participants were identified from a registry, clinics, and advertisement. Eligibility was evaluated during a screening phone call and confirmed at the screening visit. Subjects over age 18, with a history of BD, hypomanic (Clinician-Administered Rating Scale for Mania (CARS-M)29 ≥7), or depressive symptoms (Montgomery-Asberg Depression Rating Scale (MADRS)30 ≥6) in the past month, and current psychiatric treatment were included. Subjects were excluded if they reported hospitalization for BD at the time of the phone call, suicidal or homicidal ideation, active substance dependence or eating disorder, pregnancy, active treatment for major medical illness, history of specific food allergies such as, but not limited to, fish, gluten, or dairy products, and strong aversion to consuming fish. Written informed consent was documented at the first screening visit prior to the conduct of the research and enrollment.
2.3 |. Study visit procedures and randomization
At the screening visit, participants’ demographic and basic health information was collected, and a basic physical examination was conducted. During the scheduled in-person screening appointment, a clinician confirmed DSM-IV BD based on the Mini International Neuropsychiatric Interview (MINI).31 Smartphone devices were provided to participants for the collection of daily self-report surveys. Participants were instructed to avoid changes in over-the-counter medication and nutritional supplements during the course of this study.
After completion of the 2-week baseline period, participants attended a week 0 randomization visit. At this visit, mood and related symptoms were rated by clinicians and self-report. Subjects were randomized 1:1 to H3-L6 or CD, stratified on self-reported gender to minimize any confounding of the results due to potential gender-based difference in mood variability. Due to a technical design issue, nine participants utilized slots in the randomization allocation sequence that were not intended to be randomized, and thus were never distributed a diet allocation. After the randomization procedure was completed, the participant met with the dietitian to review the 3-day food record, receive diet education and study foods specific to their assigned diet arm, report any adverse events, and complete the Credibility and Expectation of Treatment Questionnaire.
At week 2, 4, 6, 8, 10, and 12 visits, the participant met with the dietitian to review diet adherence and food records, receive dietary counseling, pick up food supplies, and report any adverse events. Additionally, at weeks 4, 8, and 12, participants were given a physical exam, repeated clinician-rated and self-report questionnaires. Participants were permitted to skip one visit and remain in the study. In one instance, a participant missed two consecutive study visits and was allowed to remain in the trial because it was confirmed that the participant had enough food to remain compliant with the study diet.
2.4 |. Intervention
2.4.1 |. Diet education, counseling, and website
The H3-L6 and control diets were adapted from a previously published method shown to achieve n-3 and n-6 targets as shown by diet recall and plasma outcome measures.32 Participants randomized to both diet groups received equal intensity of interaction with the study team, dietary counseling and education materials, and amounts of study food provision.32 Individual diet education and counseling sessions were conducted by a registered dietitian, and participants received access to a website containing educational material for their assigned diet. Participants received food strategically selected and/or formulated to meet target nutrient ranges for the assigned diet arm. Specially formulated oils and recipes were prepared by the University of North Carolina Health Care, Metabolic and Nutrition Research Core (MNRC), and select foods devoid of specific oils were purchased from local grocery stores. The diet was designed to be calorically neutral for each participant and to not induce weight loss. Further description of the dietary methods and modified blinding procedures are included in supplemental materials.
2.5 |. Study instruments and data collection
2.5.1 |. Ecological Momentary Assessment
Participants were provided with a research-programmed Android smartphone at the screening visit and were expected to respond to daily notifications programmed on that phone for 14 weeks (2 weeks between the baseline visit and week 0 visit plus the 12 weeks during Phase 1). Smartphones were rented and programmed prior to the start of the study in 2014 through the Survey Research Center at Penn State University Park, Dynamic Real Time Ecological Ambulatory Methodologies group (DREAM), an established center with expertise in the use of real time methods for capturing data. A Mood and Stress survey was delivered randomly two times per day in an EMA paradigm. Participants were asked to rate their levels of mood, energy, speed of thoughts, impulsive thoughts and actions, anxiety, irritability, and pain on a visual analog scale (0– 100) (see Supplemental Methods).
2.5.2 |. Clinician and self-reported questionnaires
Participants completed a Credibility and Expectations of Treatment form during week 0 visit after randomization and in absence of the research staff. Additional clinician and self-reported questionnaires were gathered and results will be reported separately (see supplemental methods).
2.6 |. Biochemical samples and processing
Blood samples were collected at week 0, week 4, week 8, week 12, and week 48. Participants fasted for at least six hours immediately preceding the visits, and blood was drawn into vacutainers containing EDTA and serum separator tubes. After centrifugation for 10 minutes, the plasma supernatant was transferred to plastic tubes and maintained on dry ice or at −80 degrees Celsius until processing. Plasma was added to butylated hydroxytoluene (BHT)/methanol and after addition of internal standard fatty acids were extracted according to the method of Folch et al33 and as previously described.34 Lipid extracts were then transmethylated in 14% BF3/methanol and methyl esters were extracted with hexane and analyzed by gas chromatography-flame ionization detection as previously described.34 Internal standards were used to calculate tissue fatty acid concentrations. Serum samples were collected at weeks 0 and 12. Oxylipins including 17-HDHA and PGE2 were extracted from serum following the methods of Yuan et al. as modified by Domenichiello et al.35,36
2.7 |. Outcome measures
The primary outcome of the study was mood variability as assessed using EMA after 12 weeks of intensive dietary intervention. Analyses pertaining to general psychological distress outcomes will be reported separately. The primary biochemical endpoints of the trial were plasma n-6 AA and n-3 DHA, and their oxylipin derivatives. We hypothesized that the experimental intervention would significantly alter (1a) n-6 AA and n-3 DHA; and (1b) 17-hydroxy DHA and reduce PGE2, compared to the control diet. Plasma PUFAs were assayed from blood collected at week 0 visit and weeks 4, 8, and 12 to determine if the effect of the dietary intervention on circulating levels of PUFA differed throughout the intensive intervention phase of the trial.
2.8 |. Sample size calculation
The sample size calculation was based on a Monte Carlo simulation-based technique, estimating the average reduction in standard deviation (SD) from the baseline 2 week period to week 12 (end of Phase I Intensive Intervention) between groups. A 15% drop-out rate was assumed prior to week 12. A total of N = 40 participants in each group (80 total) yielded 80% power to detect an average 10% reduction in SD in the intervention group compared to an average 0% reduction in the control group using a two-sided Wilcoxon rank-sum test with the significance level of 0.05.
2.9 |. Statistical analysis
Descriptive summary statistics for continuous and categorical measures collected from the screening and baseline visits are reported to characterize the study sample. Mean and SD or (25%, 50%, 75%) quartiles were provided for continuous measures and frequencies with percentages (%) for categorical measures are presented by treatment group. Prior to analysis, the daily data collected via smartphone were pre-processed and filtered to create a comprehensive and cohesive analytical database of usable daily logs. Details pertaining to the Mood and Stress phone survey and the Sleep and Diet phone survey (used to summarize self-reported dietary intervention compliance) are outlined in more detail within the supplemental material. Mood symptoms were assessed under an ecological momentary assessment paradigm to assess within-day, within-person variability during the duration of the intervention. Group comparisons between the diet groups were performed using two-sample t-tests or Wilcoxon rank-sum tests, as appropriate. Within each diet group, the pre-versus post-intervention comparisons were conducted using paired t-tests or Wilcoxon signed-rank tests, as appropriate. To analyze the variability in the symptom responses recorded via EMA, the intraclass correlation coefficient (ICC) for each group was calculated to examine the relative magnitude of between-person versus within-person variation. Multilevel mixed-effects models were applied to analyze the variation in BD symptoms between intervention groups by incorporating potential individual differences across groups in within-person variation and a random intercept accounting for between-subject heterogeneity in each symptom. The estimates for fixed effects were based on maximum likelihood, and for the parameters in residuals and random effects, restricted maximum likelihood was applied. p-values corrected for multiple comparisons are provided if the adjustment changed the determination of significance; otherwise, only uncorrected p-values are reported.
All randomized participants were included in linear mixed-effects models analyzing the continuous fatty acid concentration outcomes of interest. Fatty acid concentrations were analyzed in ug/ml and reported as a percentage of total fatty acid plasma levels. Of the 82 randomized participants, 11 had fatty acid concentration data available at week 0 visit only, with no follow-up plasma sample data available. The linear mixed-effects models assume a first-order autoregressive covariance structure, and include a categorical time (visit week number) effect, the dietary intervention group effect, and a term for the group-by-time interaction while controlling for gender as the stratified randomization covariate. A random intercept term for each participant was used to further account for within-person correlations of repeated measures over time. To better meet the normality assumption of the model, a natural log transformation was applied to all fatty acid outcome data, therefore geometric means and ratios of geometric means are reported as the result of back-transformation (via exponentiation) of the resulting estimates.
Analysis of prostaglandin E2 (PGE2) and 17-hydroxytetranenoic (17-HDHA) from weeks 0 and 12 in units of ng/ml of serum is described in supplemental materials. A generalized linear mixed model was used to analyze PGE2 using a binary outcome with correlated errors assuming an unstructured covariance structure to account for repeated measurements across time (two visits: week 0 and week 12), modeling the probability of a measurable value being obtained (above LOQ). The marginal binary correlated errors model included fixed effects terms for the dietary intervention group effect, the categorical time (visit week number) effect, and a term for the group-by-time interaction while controlling for gender as the stratified randomization covariate. All hypothesis tests were two-sided with a significance level of 0.05.
Data were analyzed using SAS 9.4 Software with the MIXED Procedure.
2.9.1 |. Intent-to-treat and missing data
The primary approach for the analyses was intent-to-treat; patients were analyzed within their randomized group, regardless of protocol violations, dietary intervention compliance, or drop-out. Likelihood-based approaches (i.e., mixed-effect models) were employed, assuming missing data were missing-at-random to obtain valid inferences. For the primary interest of comparing pre-intervention versus post-intervention variability between the two diet groups in the EMA analysis, subjects with at least 2 usable EMA surveys logged during both the baseline period and the follow-up period were included. Demographic variables (age, gender, race, marital status, employment) and baseline EMA variability were compared for the included and excluded participants to assess for potential bias in the sampling.
3 |. RESULTS
Eighty-two participants were randomized in a gender-stratified protocol to an H3-L6 or CD diet. The sample included participants aged 20–75 years (mean 43.5 ± 13.9 years) and was 83% female. Sociodemographic and baseline clinical descriptors were similar between groups and are shown in Table 1. Biometrics were monitored for safety throughout the trial (Supplemental Table 1). Participant flow is described in the CONSORT diagram (Figure 1). The Expectation and Credibility Questionnaire scores were similar between groups, indicating both groups had a similar expectation of improvement. Additional description of the clinical features of the sample are presented in Table S2.
TABLE 1.
Demographics and clinical description of N = 82 randomized participants with BD
| H3-L6 Diet (N = 41) | Control Diet (N = 41) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 42.7 | 13.7 | 44.3 | 14.2 |
| BMIa | 30.5 | 7.9 | 32.8 | 7.5 |
| Systolic blood pressure (mm/Hg)b | 125.6 | 16.9 | 125.3 | 14.5 |
| Diastolic blood pressure (mm/Hg)b | 78.4 | 9.5 | 78.6 | 8.5 |
| Age at onset depressionc | 16.9 | 7.9 | 18.2 | 8.6 |
| Age at onset maniad | 21.2 | 8.6 | 22.1 | 9.0 |
| Expectation and credibility questionnairee | 35.3 | 5.6 | 34.4 | 9.0 |
| Median | (P25, P75) | Median | (P25, P75) | |
| No. of lifetime depressive episodesf | 30.0 | (10.0, 66.0) | 11.0 | (6.0, 56.5) |
| No. of lifetime manic episodesg | 5.0 | (8.0, 76.0) | 10.0 | (5.0, 56.0) |
| N | Col. % | N | Col. % | |
| Sex | ||||
| Female | 34 | 82.9 | 34 | 82.9 |
| Race | ||||
| White | 38 | 92.7 | 34 | 82.9 |
| Black/African-American | 2 | 4.9 | 3 | 7.3 |
| Asian | 0 | 0.0 | 1 | 2.4 |
| More than one race | 1 | 2.4 | 2 | 4.9 |
| Unknown or not reported | 0 | 0.0 | 1 | 2.4 |
| Ethnicity | ||||
| Not hispanic | 40 | 97.6 | 38 | 92.7 |
| Hispanic | 1 | 2.4 | 2 | 4.9 |
| Unknown | 0 | 0.0 | 1 | 2.4 |
| Marital status | ||||
| Married | 11 | 26.8 | 20 | 48.8 |
| Separated | 3 | 7.3 | 1 | 2.4 |
| Divorced | 13 | 31.7 | 8 | 19.5 |
| Widowed | 1 | 2.4 | 0 | 0.00 |
| Never married | 13 | 31.7 | 12 | 29.3 |
| Employment | ||||
| Unemployed | 6 | 14.6 | 5 | 12.2 |
| Disabled | 7 | 17.1 | 6 | 14.6 |
| Employed | 20 | 48.8 | 17 | 41.5 |
| Student | 4 | 9.8 | 4 | 9.8 |
| Retired | 2 | 4.9 | 8 | 19.5 |
| Employed/student | 2 | 4.9 | 1 | 2.4 |
| BD diagnosis | ||||
| BDI | 37 | 90.2 | 39 | 95.1 |
| BDII | 3 | 7.3 | 1 | 2.4 |
| BD NOS | 1 | 2.4 | 1 | 2.4 |
Note: There were no statistically significant associations observed between the randomized diet groups.
Abbreviations: BD, bipolar disorder, BMI is calculated as (weight lbs./(baseline height in.)2) × 703; BMI, body mass index; Col. %, column percent; NOS, not otherwise specified; P25, 25th percentile; P75, 75th percentile; SD, standard deviation.
n = 1 missing value in the H3-L6 diet group; height measured at week 4 was used for one instance where baseline height was missing.
n = 1 missing value in each group.
n = 2 missing values in each group.
n = 5 missing values in the H3-L6 diet group and n = 1 missing value in control diet group.
n = 5 missing values in the H3-L6 diet group and n = 2 missing values in control diet group. Score represents a 5-item sum (with possible scores ranging from 0–45). Each item was asked on a 10-point scale (0–9), where higher scores represent greater expectations and credibility of treatment.
n = 4 missing values in H3-L6 diet group and n = 1 missing value in control diet group.
n = 5 missing values in H3-L6 diet group and n = 6 missing values in control diet group.
FIGURE 1.
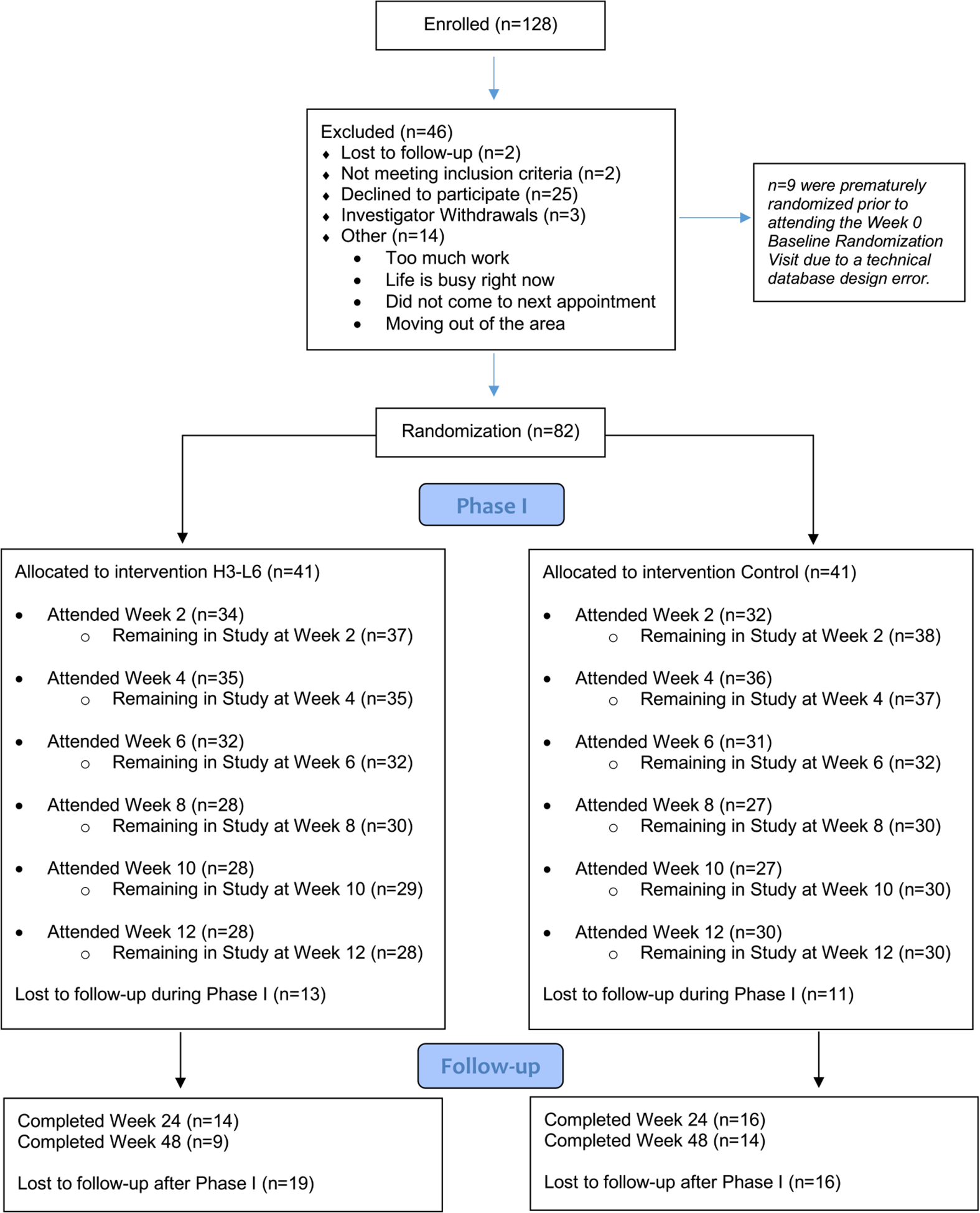
CONSORT study diagram
For all three descriptive summary methods explored to evaluate self-reported compliance to the diet (refer to supplemental material), the H3-L6 diet group reported a compliance rate of 86.4% and the CD group reported a compliance rate of 84.4% during the intensive intervention phase of the study. The mean and standard deviation of the visual analog scale (VAS) for each mood symptom during the pre-intervention baseline and the post-intervention period are shown in Table 2, where mean levels of mood, energy, speed of thoughts, impulsive thoughts, impulsive actions, irritability, and pain did not differ within groups pre/post-intervention. Anxiety was reduced in the H3-L6 group (p = 0.02), but the mean levels of symptoms did not differ between dietary groups for any of the items aggregated post-intervention (Table 2). 70 participants were included for the analysis of EMA data. Baseline demographic characteristics and EMA variability did not significantly differ between participants that were included in the EMA analysis and those excluded due to missing data. Further sensitivity analyses were not explored.
TABLE 2.
Means and standard deviations (SD) of mood symptoms and pain stratified by intervention group pre- and post-intervention
| Outcomec | H3-L6 Diet (N = 33)a | Control Diet (N = 37)a | Between Groups (Postb)s P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Postb | Within Group P-valued | Pre | Postb | Within Group P-value | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Mood | 50.41 | 20.15 | 50.72 | 18.38 | 0.79 | 52.28 | 21.11 | 53.32 | 18.92 | 0.84 | 0.65 |
| Energy | 44.97 | 22.30 | 44.44 | 19.38 | 0.96 | 42.88 | 23.26 | 43.97 | 20.36 | 0.99 | 0.43 |
| Speed of thoughts | 46.13 | 22.70 | 42.86 | 21.76 | 0.30 | 44.28 | 23.38 | 44.43 | 24.79 | 0.84 | 0.87 |
| Impulsivity (thoughts) | 28.02 | 27.08 | 24.76 | 25.57 | 0.39 | 25.20 | 24.08 | 22.06 | 25.65 | 0.85 | 0.93 |
| Impulsivity (actions) | 20.97 | 22.82 | 18.47 | 21.21 | 0.08 | 19.62 | 20.69 | 17.44 | 22.24 | 0.83 | 0.79 |
| Anxiety | 44.68 | 29.90 | 38.94 | 27.53 | 0.02* | 39.95 | 28.48 | 36.99 | 31.07 | 0.29 | 0.74 |
| Irritability | 36.50 | 30.38 | 31.51 | 26.64 | 0.18 | 37.70 | 29.08 | 35.43 | 30.31 | 0.73 | 0.25 |
| Pain | 29.44 | 28.93 | 31.08 | 29.56 | 0.06 | 32.33 | 27.95 | 37.83 | 28.72 | 0.25 | 0.40 |
Phone data for the Mood and Stress survey from 12 of the 82 randomized participants could not be used in this analysis. N = 2 participants did not log any usable phone date during the Baseline period (prior to week 0 randomization visit). N = 9 participants did not log any usable phone data during the follow-up period (after week 0 randomization visit), and N = 1 participant had no usable phone data for the study due to the malfunctioning date-time mechanism on the phone that they were provided. Therefore, N = 70 participants were included in the EMA analysis utilizing Mood and Stress surveys.
Note that ‘Pre’ data refers to all usable phone surveys logged between the participant’s date of enrollment and their date of randomization at week 0 visit (total of 1711 phone surveys included here).
Note that ‘Post’ data in this table references all aggregated phone survey data that was logged after week 0 randomization visit until each participant’s last attended Phase 1 study visit (total of 5512 phone surveys included here). This table utilizes a total of 7223 phone surveys from the N = 70 participants included in this analysis. Group comparisons between the diet groups were performed using two-sample t-tests or Wilcoxon rank-sum tests, as appropriate. Within each diet group, the pre-versus post-intervention comparisons were conducted using paired t-tests or Wilcoxon signed-rank tests, as appropriate.
Outcomes were measured twice per day on a visual analog scale (Range 0–100) using an Ecological Momentary Assessment paradigm. Exact question text provided in supplement.
P-values after multiple comparison adjustment based on the Bonferroni correction: Mood, 1.00; Energy,1.00; Speed of Thoughts, 1.00; Impulsivity (thoughts), 1.00; Impulsivity (actions), 0.16; Anxiety, 0.14; Irritability, 1.00; Pain, 0.48.
p < 0.05.
The primary clinical outcome of the trial was the variability of mood during the intensive intervention phase. The mean and standard deviation of within-person variability (measured using standard deviation) of the VAS score of each item is shown in Table 3, comparing the baseline period to the period preceding the last attended Phase 1 study visit. In the H3-L6 intervention group, reductions are seen in variability of mood (p < 0.01), energy (p = 0.026), speed of thoughts (p = 0.006), anxiety (p = 0.04), and pain (p = 0.002), but not for impulsivity of thoughts, impulsivity of actions, or irritability. In the CD group, variability of energy (p = 0.022) and impulsivity of actions (p = 0.03) were reduced post-intervention. In this analysis comparing only the first and last visit and without accounting for baseline symptoms, within-person standard deviation did not significantly differ between groups post-intervention.
TABLE 3.
Within-person variability as measured by standard deviation of mood symptoms and pain stratified by intervention group pre- and post-intervention
| Outcomea Within-Person Variability in… | Diet H3-L6 (N=33)b Control (N=37)b | Pre | Postc | Within Group P-value | Between Groups (Postc) P-value | |
|---|---|---|---|---|---|---|
| Mood | H3-L6 | Mean (SD) | 17.27 (5.40) | 13.49 (8.47) | <0.01* | 0.17 |
| Range Min-Max | 4.66–29.96 | 2.12–44.55 | ||||
| Control | Mean (SD) | 17.24 (8.14) | 17.19 (13.14) | 0.99 | ||
| Range Min-Max | 2.12–36.79 | 1.88–70.71 | ||||
| Energy | H3-L6 | Mean (SD) | 18.90 (5.94) | 15.28 (9.96) | 0.026* | 0.44 |
| Range Min-Max | 8.31–32.01 | 3.06–55.15 | ||||
| Control | Mean (SD) | 19.84 (5.97) | 17.03 (8.35) | 0.022* | ||
| Range Min-Max | 8.40–29.27 | 0–35.56 | ||||
| Speed of Thoughts | H3-L6 | Mean (SD) | 18.29 (7.40) | 13.43 (9.82) | 0.006* | 0.22 |
| Range Min-Max | 2.57–35.27 | 0–45.25 | ||||
| Control | Mean (SD) | 18.41 (8.78) | 17.02 (13.95) | 0.43 | ||
| Range Min-Max | 0.71–32.41 | 1.56–62.93 | ||||
| Impulsivity (thoughts) | H3-L6 | Mean (SD) | 19.09 (10.03) | 15.40 (12.68) | 0.17 | 0.97 |
| Range Min-Max | 0.2–37.85 | 0–58.69 | ||||
| Control | Mean (SD) | 18.32 (8.38) | 15.49 (10.74) | 0.11 | ||
| Range Min-Max | 0–35.68 | 0–41.79 | ||||
| Impulsivity (actions) | H3-L6 | Mean (SD) | 16.30 (7.82) | 12.84 (10.29) | 0.13 | 0.56 |
| Range Min-Max | 0.4–29.73 | 0–42.43 | ||||
| Control | Mean (SD) | 15.56 (7.98) | 11.35 (10.40) | 0.03* | ||
| Range Min-Max | 0.71–38.58 | 0–45.90 | ||||
| Anxiety | H3-L6 | Mean (SD) | 20.73 (7.89) | 17.01 (9.69) | 0.04* | 0.29 |
| Range Min-Max | 4.48–38.36 | 4.95–52.33 | ||||
| Control | Mean (SD) | 22.83 (9.59) | 19.97 (12.91) | 0.19 | ||
| Range Min-Max | 3.92–48.08 | 3.49–64.35 | ||||
| Irritability | H3-L6 | Mean (SD) | 22.06 (8.53) | 19.06 (12.62) | 0.16 | 0.64 |
| Range Min-Max | 5.13–39.45 | 1.41–60.81 | ||||
| Control | Mean (SD) | 24.20 (9.97) | 20.61 (14.36) | 0.10 | ||
| Range Min-Max | 7.42–41.74 | 1.94–70.71 | ||||
| Pain | H3-L6 | Mean (SD) | 13.78 (6.52) | 9.43 (6.24) | 0.002* | 0.21 |
| Range Min-Max | 2.97–28.18 | 0–24.35 | ||||
| Control | Mean (SD) | 14.67 (9.23) | 11.72 (8.46) | 0.11 | ||
| Range Min-Max | 0–35.33 | 0–33.45 |
Outcomes were measured twice per day on a visual analog scale (Range 0–100) using an Ecological Momentary Assessment paradigm. Exact question text provided in supplement.
Phone data for the Mood and Stress survey from 12 of the 82 randomized participants could not be used in this analysis. N = 2 participants did not log any usable phone date during the Baseline period (prior to week 0 randomization visit). N = 9 participants did not log any usable phone data during the follow-up period (after week 0 randomization visit), and N = 1 participant had no usable phone data for the study due to the malfunctioning date-time mechanism on the phone that they were provided. Therefore, N = 70 participants were included in the EMA analysis utilizing Mood and Stress surveys.
Note that ‘Pre’ data refers to all usable phone surveys logged between the participant’s date of enrollment and their date of randomization at week 0 visit (total of 1711 phone surveys included here). Note that ‘Post’ data in this table references only phone survey data logged between each participant’s last two attended study visits during Phase 1 of the trial (total of 938 phone surveys included here). For example, if the participant completed Phase 1, week 12 visit would be considered their last attended study visit. Therefore, the data used for this table would have included all phone surveys logged between their week 10 visit and their week 12 visit. There was one participant who skipped two consecutive study visits (week 8 and week 10). Any usable phone data logged between their week 6 visit and their week 12 visit was assigned to their last visit (week 12). There were two participants who skipped their week 10 visit, therefore any usable phone data logged between their week 8 visit and their week 12 visit was assigned to their last visit (week 12). There was one participant who skipped their week 2 visit, therefore any usable phone data logged between their week 0 visit and their week 4 visit was assigned to their last visit (week 4). The smallest and largest number of surveys attributed to week 0 visit for a participant in either group were 2 and 55, respectively. The smallest and largest number of surveys attributed to any last attended visit for a participant in either group during Phase 1 were 1 and 36, respectively. This table utilizes a total of 2649 phone surveys. Group comparisons between the diet groups were performed using two-sample t-tests or Wilcoxon rank-sum tests, as appropriate. Within each diet group, the pre-versus post-intervention comparisons were conducted using paired t-tests or Wilcoxon signed-rank tests, as appropriate.
p < 0.05.
A multilevel analysis was performed to identify differences in within-person variability between groups throughout the 12-week intensive intervention while controlling for baseline symptoms and found the H3-L6 intervention group had a significantly lower variability in mood, energy, irritability, and pain (all p < 0.001, Table 4). In this analysis, baseline symptoms for all items had significant effects on the mean level of post-intervention symptoms (p < 0.05). Using multilevel analysis, groups did not differ in mean levels of symptoms throughout the 12-week intensive intervention (p > 0.05) (Table 4). The effect size as depicted as percent change in within-person variability of the H3-L6 group, compared to the CD group, is shown in Figure 2.
TABLE 4.
Multilevel analysis of variability in visual analog scale scores of mood symptoms and pain during the 12-week intensive diet intervention, adjusted for variability during the baseline period
| Outcomea | H3-L6 ICC | Control ICC | Diet intervention effect on within-person variability | Between diet intervention group difference effect on mean level of symptoms | ||
|---|---|---|---|---|---|---|
| Estimateb | P-value | Estimateb | P-value | |||
| Mood | 0.30 | 0.29 | 0.20 | <0.001 | 0.44 | 0.85 |
| Energy | 0.25 | 0.23 | 0.17 | <0.001* | −0.30 | 0.88 |
| Speed of Thoughts | 0.42 | 0.50 | 0.08 | 0.052 | 2.13 | 0.45 |
| Impulsivity (thoughts) | 0.58 | 0.61 | −0.07 | 0.09 | 2.13 | 0.53 |
| Impulsivity (actions) | 0.52 | 0.55 | 0.05 | 0.20 | 2.26 | 0.40 |
| Anxiety | 0.49 | 0.57 | 0.05 | 0.18 | 2.05 | 0.50 |
| Irritability | 0.46 | 0.50 | 0.24 | <0.001* | 4.25 | 0.23 |
| Pain | 0.77 | 0.64 | 0.46 | <0.001* | 0.64 | 0.84 |
Note: Phone data for the Mood and Stress survey from 12 of the 82 randomized participants could not be used in this analysis. N = 2 participants did not log any usable phone date during the Baseline period (prior to week 0 randomization visit). N = 9 participants did not log any usable phone data during the follow-up period (after week 0 randomization visit), and N = 1 participant had no usable phone data for the study due to the malfunctioning date-time mechanism on the phone that they were provided. Therefore, N = 70 participants were included in the EMA analysis utilizing Mood and Stress surveys. Note that the baseline period refers to all usable phone surveys logged between the participant’s date of enrollment and their date of randomization at week 0 visit. This table utilizes a total of 7223 phone surveys from the N = 70 participants included in this analysis.
Abbreviation: ICC, intraclass correlation coefficient.
Exact question text provided in supplement.
Reference level is H3-L6 diet group. Positive estimates indicate higher variability and higher mean level of symptoms in the Control diet group than in H3-L6 diet group, respectively.
p<0.05.
FIGURE 2.
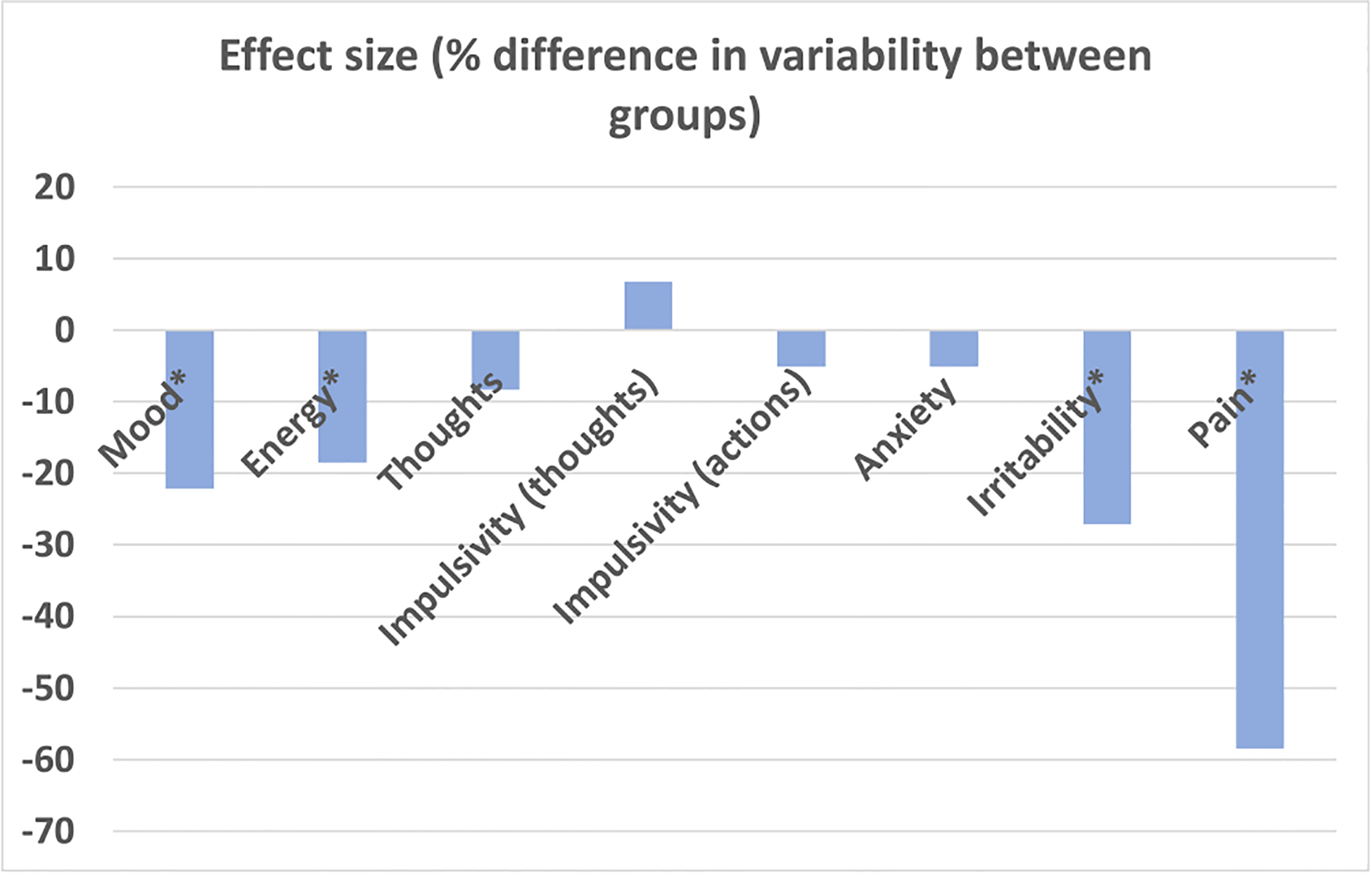
Percent change in outcome variability in H3-L6 group, compared to control diet group. *p<0.004. Calculated as 1-eβ, where β is reported in Table 4 as the estimate of the diet intervention effect on within-person variability for each outcome measure
The H3-L6 dietary intervention was designed to lower n-6 LA intake and raise n-3 EPA and DHA intake. The effect of the dietary intervention on plasma n-6 LA and n-3 EPA and DHA during the intensive intervention phase significantly differed over time (diet group by time interaction p < 0.01; Table S3). The n-6 LA geometric mean estimates slightly reduce over time in the H3-L6 group compared to the control group, and the n-3 EPA and DHA seem to increase more over time in the H3-L6 group compared to the control group (Figure 3). Changes in AA, ALA, palmitic, and oleic acid did not differ by group over the intensive intervention period (Table S3). 17-hydroxy DHA in serum was below the limit of quantification in most samples (see supplemental material and Table S4). The odds of a PGE2 value being measurable (above LOQ) did not significantly differ between groups at either time point, or across time points (diet group by time interaction p = 0.99; see supplemental material and Table S5).
FIGURE 3.
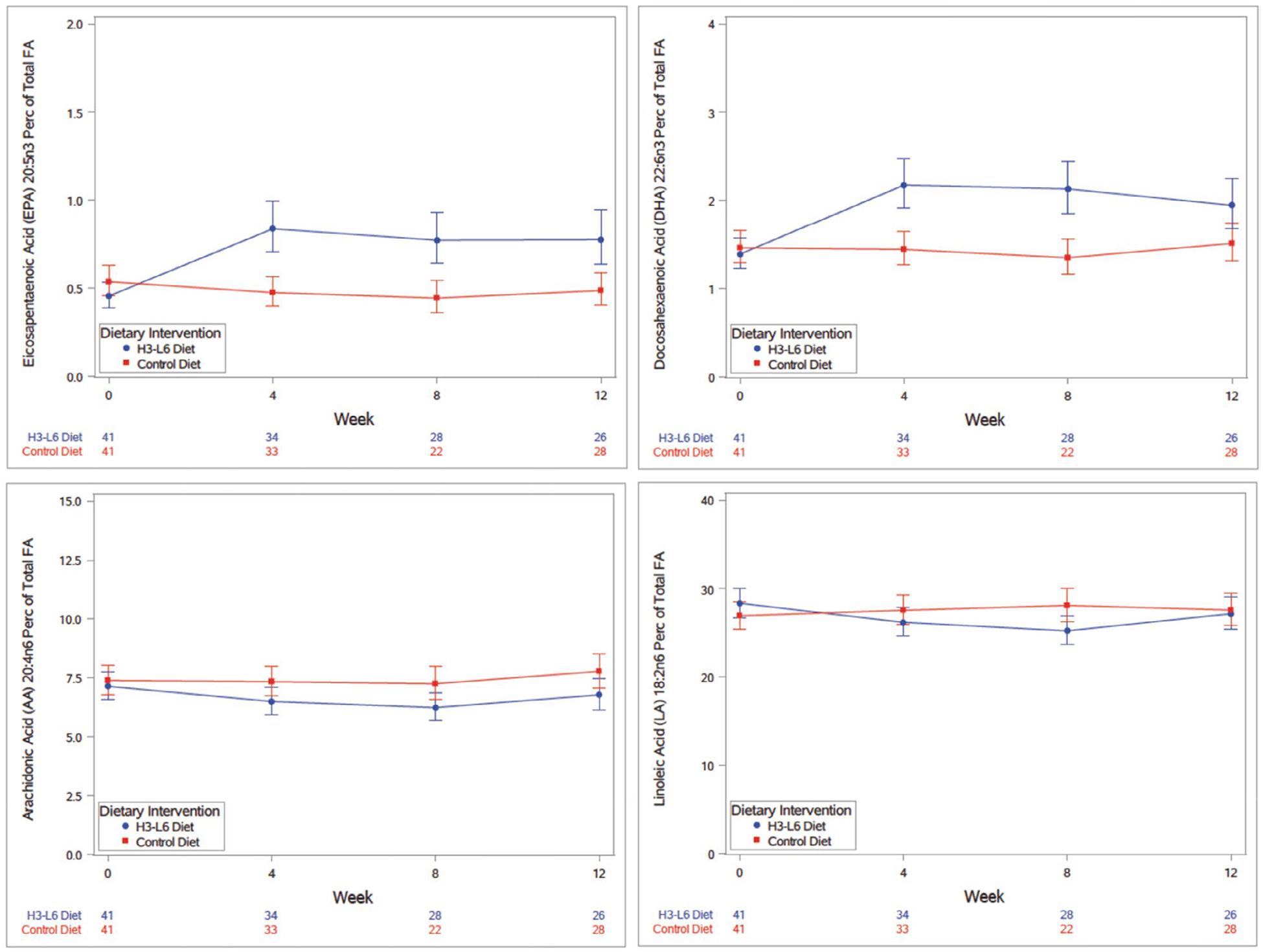
Plotted mixed model geometric mean estimates for eicosapentaenoic acid, docasahexaenoic acid, arachidonic acid, and linoleic acid concentrations as percent of total fatty acid concentration over the duration of Phase 1 intensive dietary intervention with corresponding 95% confidence intervals, adjusted for gender. Perc, percent; FA, fatty acid. X-axis table delineates the number of samples analyzed at each time point by diet group (study week visit number), where week 0 represents the Baseline visit (participants were randomized to their dietary intervention group at week 0) and week 12 was the last Phase 1 study visit for the intensive dietary intervention phase
During the trial, four instances were investigated as adverse events. Two were determined to not be related to the intervention, and one reported nausea from study bread, and one reported reaction to sucralose in study food.
4 |. DISCUSSION
In this randomized, controlled trial, a dietary intervention designed to lower n-6 LA and increase n-3 EPA and DHA PUFA levels were associated with significant reductions in variability of mood, energy, irritability, and pain in patients with symptomatic BD during a 12-week period. The H3-L6 intervention was successful in reducing circulating LA and raising DHA and EPA, though the AA concentration did not differ between groups. In this trial, both arms received equally intensive interventions, including nutritional education to make alterations in dietary intake, and both interventions were perceived to be equally credible.
Dietary trials are difficult to conduct due to challenges in affecting dietary intake through behavior change,37 and can be designed using a change in whole food intake or nutritional supplementation through other means. The recent SMILES trial, which was designed as a single-blind RCT of a Mediterranean diet intervention delivered through dietary counseling compared to a social support condition for moderate to severe depression,38 reported improvement in depression in the dietary intervention group. Prior trials in BD targeting n-3 PUFA have used supplements to make specific changes in nutrients. An initial clinical trial for use of n-3 fish oil (EPA + DHA) supplementation in the depressive episode of BD for adult patients showed promising results14; however, subsequent studies failed to replicate these findings,15–17,39–41 Studies of youth with BD spectrum have shown more mixed results with some studies finding promising improvements.41–45
The trial described here altered n-3 EPA and DHA through dietary intervention rather than nutritional supplementation and added dietary reduction of n-6 LA to the intervention. We hypothesized that diet-induced reduction of the abundance and/or metabolism of n-6 AA is a key mechanism underlying the effects of the H3-L6 intervention on mood variability. Anti-manic drugs are reported to reduce the brain turnover of n-6 AA to downstream metabolites, some of which stimulate inflammatory cytokine release,46 without increasing turnover of n-3 docosahexaenoic acid (DHA),46–51 suggesting that reduction of the abundance or metabolism of AA may play a key role in BD treatment. Putative mechanisms of mood stabilization may include mediation of neuroinflammatory processes by the PUFAs or their derivatives that may lead to neurotoxicity and chronicity of illness in BD.22,52–54 AA and DHA serve key physiological functions in the nervous system by increasing membrane fluidity in the cell membranes and serving as precursors for autacoids (i.e., oxylipins, endocannabinoids)55,56 that regulate synaptic neurotransmission, oxidative stress, excitotoxicity, and neuroinflammation, but concentrations may not be elevated in the post-mortem brains of people with BD,57–59 indicating that potential effects of these fatty acids in BD are due to changes in their metabolism.21 AA is metabolized by the cyclooxegnase (COX) enzymes into prostanoids, including thromboxane and prostaglandins. Efficacy of reducing brain n-6 AA metabolism and increasing n-3 EPA and DHA metabolism in BD also would be consistent with reported adjunctive effects of COX inhibitors, including aspirin (COX-1 and COX-2 inhibitor and acetylator) and celexcoxib (COX-2 inhibitor) in BD.60,61 Several clinical studies have reported differences in levels of AA and plasma ratios of n-6 to n-3 fatty acids in BD patients compared to healthy controls, and, in some cases, PUFA markers related to symptom severity.62–68 Differences in the ratio of unesterified to esterified circulating EPA have been found between BD and control groups, while several groups have found reduced levels of DHA.62,64,68,69 While the H3-L6 group did not show a difference in concentration of circulating AA during the intervention, there was a group-by-time interaction observed for LA.
Lowering dietary n-6 LA has been proposed as a key component to efficacy of a similar dietary H3-L6 intervention in migraine headache.22,32,70,71 The prevalence of migraine headache in BD is 30%, twice as high as in the general population, and both conditions can respond to similar medications, such as valproic acid.4,72,73 In this study, we included a measurement of pain to understand if the pain was affected by the H3-L6 intervention in those with BD and pain. The variability of pain was reduced, and further investigation is needed to determine if this improved pain overall and improved mood in those with pain.
Based on the putative clinical and neuroinflammatory links between BD and migraines, lowering dietary n-6 LA in addition to increasing dietary n-3 EPA + DHA is a promising adjunctive treatment for BD.
The present trial differs from previous trials in the design of measurement of mood outcome. While prior trials were designed to improve an acute mood episode, this trial was designed to measure the change in the daily variability in mood using an ecological momentary assessment paradigm. This design was selected for two reasons. Since mood instability has been shown to have long-term effects in increasing risk for relapse of a major mood episode and impact functioning, improvement in mood variability is an important treatment target.74–76 Including participants with milder mood symptoms but not in a major mood episode improved feasibility of completing the dietary intervention with fidelity and this design simulates the setting and stage of illness in which a dietary intervention would most likely be included in treatment.
The results of this trial suggest that a combined dietary intervention of increasing n-3 EPA + DHA while lowering n-6 LA can decrease instability of mood, energy, irritability, and pain compared to usual U.S. levels of n-6 LA and n-3 EPA + DHA. Additional questions to explore include the determination of the key components of the diet that drove the changes in circulating n-3 EPA and DHA and n-6 LA, and further exploration of whether changes in biological pathways downstream of these PUFAs were associated with the observed effect on mood.
Strengths and Limitations: A strength of this trial is in the use of two dietary-driven interventions delivered by dietitians with equal interaction in both groups including dietary counseling and provision of whole foods. Many dietary trials approach control conditions without using an intervention by advising participants to continue on habitual diet. Both interventions were considered credible, which is important in the interpretation of results in such an intervention. Additionally, the use of EMA is a strength of the trial’s data collection mechanisms, allowing for the collection of rich and more frequently assessed data points to more accurately detect and analyze the sensitive variability of mood over a time course that is not frequently studied in treatment trials. While it has been hypothesized that electronic monitoring alone may improve mood, recent trials have not shown that to be the case.77 In this study, both groups received electronic monitoring equally, and improvement in mean mood symptoms was not observed in either intervention group.
The sample size of this trial is a limitation of our findings, and results will need to be replicated in a larger trial. The sample was predominantly female, and the study was not powered to stratify analyses by gender. Due to the complexity of the biological cascades, study results do not prove that changes in the n-3 or n-6 intake are responsible for the improvement in mood through direct or indirect impacts. Generalizability may be limited outside of an ambulatory clinical population of patients willing to enter a clinical trial with a diet intervention. While we have shown that there was no expectation bias in the H3-L6 intervention group, an important caveat is that every participant was willing to change their dietary practices upon enrollment into the trial. Further study of the key changes that drove the results will determine if an intervention can be tailored to improve ease of use.
Increasing N-3 EPA + DHA while decreasing n-6 LA through a food-based intervention decreased day-to-day variability in mood, energy, and irritability. Larger future trials are needed to explore whether the preliminary therapeutic efficacy observed is reproducible, to optimize the intervention, and to investigate underlying mechanisms.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in NIMH Data Archive at https://nda.nih.gov/edit_collection.html?id=2219, reference number 2219.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the study participants and acknowledge the following individuals for research assistance: Caitlin Millett, Aubrey Reider, Elise Ball, Katerina Martin, Daniel Conroy, Jim Loewke for assistance in data collection. We acknowledge Dr. Stanley Rappaport for guidance in formulating study objectives.
Funding information
This project was funded by grant # 13T-013 from the Stanley Medical Research Institute (main funding source). Additional support was provided by the Penn State Clinical & Translational Research Institute, Pennsylvania State University Clinical Translational Science Award, National Institutes of Health/National Center for Advancing Translational Sciences Grant Number UL1 TR000127 and UL1 TR002014 (REDCap project for providing infrastructure support), and the intramural programs of the National Institute on Aging and the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
CONFLICT OF INTEREST
ES reports having received a stipend for work as an associate editor from the Journal of Clinical Psychiatry and past participation in an advisory board for Myriad Neuroscience. The other authors report no financial relationships with commercial interests.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2007;64(5):543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geddes JR, Goodwin GM, Rendell J, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet. 2010;375(9712):385–395. [DOI] [PubMed] [Google Scholar]
- 5.Nierenberg AA, McElroy SL, Friedman ES, et al. Bipolar CHOICE (Clinical Health Outcomes Initiative in Comparative Effectiveness): a pragmatic 6-month trial of lithium versus quetiapine for bipolar disorder. J Clin Psychiatry. 2016;77(1):90–99. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Glenn T, Alda M, et al. Comparison of pre-episode and pre-remission states using mood ratings from patients with bipolar disorder. Pharmacopsychiatry. 2011;44(Suppl 1):S49–53. [DOI] [PubMed] [Google Scholar]
- 7.Bauer M, Glenn T, Keil M, et al. Brief depressive symptoms in patients with bipolar disorder: analysis of long-term self-reported data. Aust N Z J Psychiatry. 2012;46(11):1068–1078. [DOI] [PubMed] [Google Scholar]
- 8.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. [DOI] [PubMed] [Google Scholar]
- 9.Liu JJ, Green P, John Mann J, Rapoport SI, Sublette ME. Pathways of polyunsaturated fatty acid utilization: implications for brain function in neuropsychiatric health and disease. Brain Res. 2015;1597:220–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Noaghiul S, Hibbeln JR. Cross-national comparisons of seafood consumption and rates of bipolar disorders. Am J Psychiatry. 2003;160(12):2222–2227. [DOI] [PubMed] [Google Scholar]
- 11.Hibbeln JR, Nieminen LR, Blasbalg TL, Riggs JA, Lands WE. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am J Clin Nutr. 2006;83(6 Suppl):1483S–1493S. [DOI] [PubMed] [Google Scholar]
- 12.Freeman MP, Rapaport MH. Omega-3 fatty acids and depression: from cellular mechanisms to clinical care. J Clin Psychiatry. 2011;72(2):258–259. [DOI] [PubMed] [Google Scholar]
- 13.Stoll AL, Locke CA, Marangell LB, Severus WE. Omega-3 fatty acids and bipolar disorder: a review. Prostaglandins Leukot Essent Fatty Acids. 1999;60(5– 6):329–337. [DOI] [PubMed] [Google Scholar]
- 14.Stoll AL, Severus WE, Freeman MP, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry. 1999;56(5):407–412. [DOI] [PubMed] [Google Scholar]
- 15.Keck PE, Mintz J, McElroy SL, et al. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry. 2006;60(9):1020–1022. [DOI] [PubMed] [Google Scholar]
- 16.Murphy BL, Stoll AL, Harris PQ, et al. Omega-3 fatty acid treatment, with or without cytidine, fails to show therapeutic properties in bipolar disorder: a double-blind, randomized add-on clinical trial. J Clin Psychopharmacol. 2012;32(5):699–703. [DOI] [PubMed] [Google Scholar]
- 17.Frangou S, Lewis M, McCrone P. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry. 2006;188:46–50. [DOI] [PubMed] [Google Scholar]
- 18.Kraguljac NV, Montori VM, Pavuluri M, Chai HS, Wilson BS, Unal SS. Efficacy of omega-3 fatty acids in mood disorders -a systematic review and metaanalysis. Psychopharmacol Bull. 2009;42(3):39–54. [PubMed] [Google Scholar]
- 19.Taha AY, Blanchard HC, Cheon Y, et al. Dietary linoleic acid lowering reduces lipopolysaccharide-induced increase in brain arachidonic acid metabolism. Mol Neurobiol. 2017;54(6):4303–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taha AY, Cheon Y, Faurot KF, et al. Dietary omega-6 fatty acid lowering increases bioavailability of omega-3 polyunsaturated fatty acids in human plasma lipid pools. Prostaglandins Leukot Essent Fatty Acids. 2014;90(5):151–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rapoport SI. Lithium and the other mood stabilizers effective in bipolar disorder target the rat brain arachidonic acid cascade. ACS Chem Neurosci. 2014;5(6):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders EF, Ramsden CE, Sherazy MS, Gelenberg AJ, Davis JM, Rapoport SI. Reconsidering dietary polyunsaturated fatty acids in bipolar disorder: a translational picture. J Clin Psychiatry. 2016;77(10):e1342–e1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Myin-Germeys I, Peeters F, Havermans R, et al. Emotional reactivity to daily life stress in psychosis and affective disorder: an experience sampling study. Acta Psychiatr Scand. 2003;107(2):124–131. [DOI] [PubMed] [Google Scholar]
- 24.Depp CA, Kim DH, de Dios LV, Wang V, Ceglowski J. A pilot study of mood ratings captured by mobile phone versus paper-and-pencil mood charts in bipolar disorder. J Dual Diagn. 2012;8(4):326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwartz S, Schultz S, Reider A, Saunders EF. Daily mood monitoring of symptoms using smartphones in bipolar disorder: a pilot study assessing the feasibility of ecological momentary assessment. J Affect Disord. 2016;191:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Mukherjee D, Krishnamurthy VB, et al. Use of ecological momentary assessment to detect variability in mood, sleep and stress in bipolar disorder. BMC Res Notes. 2019;12(1):791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faurholt-Jepsen M, Munkholm K, Frost M, Bardram JE, Kessing LV. Electronic self-monitoring of mood using IT platforms in adult patients with bipolar disorder: a systematic review of the validity and evidence. BMC Psychiatry. 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mann JD, Faurot KR, MacIntosh B, et al. A sixteen-week three-armed, randomized, controlled trial investigating clinical and biochemical effects of targeted alterations in dietary linoleic acid and n-3 EPA+DHA in adults with episodic migraine: study protocol. Prostaglandins Leukot Essent Fatty Acids. 2018;128:41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Altman EG, Hedeker DR, Janicak PG, Peterson JL, Davis JM. The Clinician-Administered Rating Scale for Mania (CARS-M): development, reliability, and validity. Biol Psychiatry. 1994;36(2):124–134. [DOI] [PubMed] [Google Scholar]
- 30.Davidson J, Turnbull CD, Strickland R, Miller R, Graves K. The Montgomery-Asberg Depression Scale: reliability and validity. Acta Psychiatr Scand. 1986;73(5):544–548. [DOI] [PubMed] [Google Scholar]
- 31.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 32.MacIntosh BA, Ramsden CE, Faurot KR, et al. Low-n-6 and low-n-6 plus high-n-3 diets for use in clinical research. Br J Nutr. 2013;110(3):559–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Folch J, Lees M, Sloan Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 34.Ramsden CE, Ringel A, Majchrzak-Hong SF, et al. Dietary linoleic acid-induced alterations in pro- and anti-nociceptive lipid autacoids: Implications for idiopathic pain syndromes? Mol Pain. 2016;12:174480691663638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domenichiello AF, Jensen JR, Zamora D, et al. Identifying oxidized lipid mediators as prognostic biomarkers of chronic post-traumatic headache. Pain. 2020;161(12):2775–2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan ZX, Majchrzak-Hong S, Keyes GS, Iadarola MJ, Mannes AJ, Ramsden CE. Lipidomic profiling of targeted oxylipins with ultra-performance liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2018;410(23):6009–6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berk M, Jacka FN. Diet and depression-from confirmation to implementation. JAMA. 2019;321(9):842–843. [DOI] [PubMed] [Google Scholar]
- 38.Jacka FN, O’Neil A, Opie R, et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiu CC, Huang SY, Chen CC, Su KP. Omega-3 fatty acids are more beneficial in the depressive phase than in the manic phase in patients with bipolar I disorder. J Clin Psychiatry. 2005;66(12):1613–1614. [DOI] [PubMed] [Google Scholar]
- 40.Frangou S, Lewis M, Wollard J, Simmons A. Preliminary in vivo evidence of increased N-acetyl-aspartate following eicosapentanoic acid treatment in patients with bipolar disorder. J Psychopharmacol. 2007;21(4):435–439. [DOI] [PubMed] [Google Scholar]
- 41.Gracious BL, Chirieac MC, Costescu S, Finucane TL, Youngstrom EA, Hibbeln JR. Randomized, placebo-controlled trial of flax oil in pediatric bipolar disorder. Bipolar Disord. 2010;12(2):142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73(1):81–86. [DOI] [PubMed] [Google Scholar]
- 43.Wozniak J, Biederman J, Mick E, et al. Omega-3 fatty acid monotherapy for pediatric bipolar disorder: a prospective open-label trial. Eur Neuropsychopharmacol. 2007;17(6–7):440–447. [DOI] [PubMed] [Google Scholar]
- 44.Clayton EH, Hanstock TL, Hirneth SJ, Kable CJ, Garg ML, Hazell PL. Reduced mania and depression in juvenile bipolar disorder associated with long-chain omega-3 polyunsaturated fatty acid supplementation. Eur J Clin Nutr. 2009;63(8):1037–1040. [DOI] [PubMed] [Google Scholar]
- 45.Fristad MA, Young AS, Vesco AT, et al. A Randomized controlled trial of individual family psychoeducational psychotherapy and omega-3 fatty acids in youth with subsyndromal bipolar disorder. J Child Adolesc Psychopharmacol. 2015;25(10):764–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rao JS, Rapoport SI. Mood-stabilizers target the brain arachidonic acid cascade. Curr Mol Pharmacol. 2009;2(2):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reese EA, Cheon Y, Ramadan E, et al. Gabapentin’s minimal action on markers of rat brain arachidonic acid metabolism agrees with its inefficacy against bipolar disorder. Prostaglandins Leukot Essent Fatty Acids. 2012;87(2–3):71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheon Y, Park JY, Modi HR, et al. Chronic olanzapine treatment decreases arachidonic acid turnover and prostaglandin E₂ concentration in rat brain. J Neurochem. 2011;119(2):364–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Modi HR, Taha AY, Kim HW, Chang L, Rapoport SI, Cheon Y. Chronic clozapine reduces rat brain arachidonic acid metabolism by reducing plasma arachidonic acid availability. J Neurochem. 2013;124(3):376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shimshoni JA, Basselin M, Li LO, Coleman RA, Rapoport SI, Modi HR. Valproate uncompetitively inhibits arachidonic acid acylation by rat acyl-CoA synthetase 4: relevance to valproate’s efficacy against bipolar disorder. Biochim Biophys Acta. 2011;1811(3):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HJ, Rao JS, Chang L, Rapoport SI, Kim HW. Chronic imipramine but not bupropion increases arachidonic acid signaling in rat brain: is this related to ‘switching’ in bipolar disorder? Mol Psychiatry. 2010;15(6):602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Mol Psychiatry. 2011;16(4):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapoport SI, Basselin M, Kim HW, Rao JS. Bipolar disorder and mechanisms of action of mood stabilizers. Brain Res Rev. 2009;61(2):185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804–817. [DOI] [PubMed] [Google Scholar]
- 55.Ramsden CE, Zamora D, Makriyannis A, et al. Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain. 2015;16(8):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Di Miceli M, Bosch-Bouju C, Layé S. PUFA and their derivatives in neurotransmission and synapses: a new hallmark of synaptopathies. Proc Nutr Soc. 2020;79(4):388–403. [DOI] [PubMed] [Google Scholar]
- 57.Igarashi M, Ma K, Gao F, et al. Brain lipid concentrations in bipolar disorder. J Psychiatr Res. 2010;44(3):177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamazaki K, Maekawa M, Toyota T, Dean B, Hamazaki T, Yoshikawa T. Fatty acid composition of the postmortem prefrontal cortex of patients with schizophrenia, bipolar disorder, and major depressive disorder. Psychiatry Res. 2015;227(2–3):353–359. [DOI] [PubMed] [Google Scholar]
- 59.Hamazaki K, Choi KH, Kim HY. Phospholipid profile in the postmortem hippocampus of patients with schizophrenia and bipolar disorder: no changes in docosahexaenoic acid species. J Psychiatr Res. 2010;44(11):688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bavaresco DV, Colonetti T, Grande AJ, et al. Efficacy of celecoxib adjunct treatment on bipolar disorder: systematic review and metaanalysis. CNS Neurol Disord Drug Targets. 2019;18(1):19–28. [DOI] [PubMed] [Google Scholar]
- 61.Stolk P, Souverein PC, Wilting I, et al. Is aspirin useful in patients on lithium? A pharmacoepidemiological study related to bipolar disorder. Prostaglandins Leukot Essent Fatty Acids. 2010;82(1):9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chiu C-C, Huang S-Y, Su K-P, et al. Polyunsaturated fatty acid deficit in patients with bipolar mania. Eur Neuropsychopharmacol. 2003;13(2):99–103. [DOI] [PubMed] [Google Scholar]
- 63.Sublette ME, Bosetti F, DeMar JC, et al. Plasma free polyunsaturated fatty acid levels are associated with symptom severity in acute mania. Bipolar Disord. 2007;9(7):759–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Selective deficits in erythrocyte docosahexaenoic acid composition in adult patients with bipolar disorder and major depressive disorder. J Affect Disord. 2010;126(1–2):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans SJ, Kamali M, Prossin AR, et al. Association of plasma omega-3 and omega-6 lipids with burden of disease measures in bipolar subjects. J Psychiatr Res. 2012;46(11):1435–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans SJ, Prossin AR, Harrington GJ, et al. Fats and factors: lipid profiles associate with personality factors and suicidal history in bipolar subjects. PLoS One. 2012;7(1):e29297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evans SJ, Ringrose RN, Harrington GJ, Mancuso P, Burant CF, McInnis MG. Dietary intake and plasma metabolomic analysis of polyunsaturated fatty acids in bipolar subjects reveal dysregulation of linoleic acid metabolism. J Psychiatr Res. 2014;57:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saunders EF, Reider A, Singh G, Gelenberg AJ, Rapoport SI. Low unesterified:esterified eicosapentaenoic acid (EPA) plasma concentration ratio is associated with bipolar disorder episodes, and omega-3 plasma concentrations are altered by treatment. Bipolar Disord. 2015;17(7):729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pomponi M, Janiri L, La Torre G, et al. Plasma levels of n-3 fatty acids in bipolar patients: deficit restricted to DHA. J Psychiatr Res. 2013;47(3):337–342. [DOI] [PubMed] [Google Scholar]
- 70.Ramsden CE, Faurot KR, Zamora D, et al. Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. 2013;154(11):2441–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramsden CE, Faurot KR, Zamora D, et al. Targeted alterations in dietary n-3 and n-6 fatty acids improve life functioning and reduce psychological distress among patients with chronic headache: a secondary analysis of a randomized trial. Pain. 2015;156(4):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saunders EFH, Nazir R, Kamali M, et al. Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with co-morbid migraine. J Clin Psychiatry. 2014;75(5):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linde M, Mulleners WM, Chronicle EP, McCrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;(6):CD010611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simon GE, Bauer MS, Ludman EJ, Operskalski BH, Unutzer J. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237–1245. [DOI] [PubMed] [Google Scholar]
- 75.Bauer M, Glenn T, Grof P, et al. Frequency of subsyndromal symptoms and employment status in patients with bipolar disorder. Soc Psychiatry Psychiatr Epidemiol. 2009;44(7):515–522. [DOI] [PubMed] [Google Scholar]
- 76.Bauer M, Glenn T, Grof P, Schmid R, Pfennig A, Whybrow PC. Subsyndromal mood symptoms: a useful concept for maintenance studies of bipolar disorder? Psychopathology. 2010;43(1):1–7. [DOI] [PubMed] [Google Scholar]
- 77.Faurholt-Jepsen M, Vinberg M, Christensen EM, et al. Differences in psychomotor activity during remission in unipolar disorder and bipolar disorder. Bipolar Disord. 2011;13:43. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in NIMH Data Archive at https://nda.nih.gov/edit_collection.html?id=2219, reference number 2219.


