Abstract
The concept of using stimulants to treat cocaine and methamphetamine dependence is largely based on the “replacement” therapy model that has shown efficacy for treating nicotine and opiate dependence. Although results have been mixed, some evidence supports using stimulant medication to reduce cocaine use. There are not enough data to date to determine the efficacy of stimulants for methamphetamine dependence. Draw-backs of stimulants as treatments include the potential for abuse of the treatment, which necessitates careful screening and monitoring of patients. Possible reasons for efficacy of stimulants include enhancement of monoamine function dysregulated by chronic cocaine or methamphetamine use. Newer medications that enhance dopamine function but lack the abuse potential of older stimulants are being studied. It is hoped that these medications will provide safe, effective treatment for cocaine and methamphetamine dependence, but more research on this topic is needed.
Introduction
The first step in examining the use of stimulants to treat cocaine and methamphetamine abuse is to define what constitutes a stimulant. This is complicated by the fact that stimulants are classified as such based on behavioral effects rather than chemical structure or biochemical target. For the purpose of this review, we use the classical definition of a stimulant, which is a drug that increases the level of activity, arousal, or alertness through effects on the central nervous system. Based on this definition, several stimulant medications have been studied for the treatment of cocaine and methamphetamine abuse. These medications include dextroamphetamine, mazindol, methylphenidate, and modafinil. In this review, we provide a background for the rationale for using stimulants to treat cocaine and methamphetamine abuse, then systematically review the literature to date supporting and refuting the use of these drugs. Lastly, we discuss the future of stimulant use for the treatment of cocaine and methamphetamine abuse.
Rationale for Use
The rationale often cited for using stimulants to treat cocaine dependence is a “replacement” or “agonist” approach, similar to the use of methadone to treat opiate dependence [1,2••]. However, as stimulants do not bind to a single receptor (eg, the μ opiate receptor), the analogy is not entirely accurate. Stimulants largely do not act as agonists in that they do not mimic the effects of a naturally occurring neurotransmitter but are more likely to increase release or block reuptake of neurotransmitters.
Thus, although the agonist model is not completely in keeping with the neuropharmacology of stimulants, there are some similarities between classical agonists and stimulants to suggest that they may be effective in treating cocaine and methamphetamine dependence. These include generally good patient acceptance of the treatment medication and familiarity with the drug’s side effects, which could potentially enhance compliance [1].
However, there are other reasons related to the neuropharmacology of stimulants to suggest that they may be effective in treating cocaine and methamphetamine dependence. Cocaine is a potent uptake inhibitor of central monoamines [3]. Likewise, methamphetamine is a potent releaser and reuptake inhibitor of central monoamines [4]. Thus, cocaine and methamphetamine acutely increase extracellular levels of serotonin, dopamine, and norepinephrine in the brain. However, studies point to a different effect of chronic use of these drugs in humans. Several studies reported a blunted neuroendocrine response to serotonin-releasing agents or agonists in cocaine-dependent individuals compared with controls, providing evidence of reduced serotonin function after chronic use [5]. Prior studies using positron emission tomography (PET) have also shown evidence of reduced dopamine release or decreased receptor availability in cocaine-dependent individuals compared with controls that persist for days to weeks after cessation of cocaine use [6,7,8•,9]. Similar findings of reduced dopamine function have been demonstrated in methamphetamine users [10,11].
The significance of reduced monoamine function for behavior also has been well documented. In a study that found that cocaine dependence was associated with reduced dopamine release in the striatum as measured by PET, the amount of this reduction correlated with the choice of cocaine over money in a behavioral laboratory procedure. This provided evidence that the amount of reduction in dopamine function in cocaine users is related to the choice to use cocaine [8•]. Other studies on the role of dopamine and behavior in non–drug-using populations have shown that reduced dopamine function is associated with poorer decision-making [12]. This could at least partially explain the impairment in decision-making in cocaine- and methamphetamine-dependent patients [13,14].
Platelet paroxetine binding, a measure of serotonin function, was found to be lower in cocaine users than in controls, and the level of paroxetine binding inversely correlated with measures of impulsivity and aggression [15]. This study is consistent with the well-documented association between lower serotonin function and impulsivity [16].
Preclinical Studies
Given the involvement of the dopamine, serotonin, and norepinephrine systems in the acute and chronic effects of cocaine and methamphetamine use, a great deal of preclinical research has focused on medications that modulate these systems. There is no consensus regarding targeting single versus multiple transmitter systems; however, the dopamine system is an important target given its prominent role in drug reinforcement [17].
Here we focus on a monoamine reuptake blocker (methylphenidate), monoamine releaser (d-amphetamine), and the novel stimulant modafinil.
Methylphenidate, approved by the US Food and Drug Administration for treating attention-deficit/hyperactivity disorder (ADHD), blocks monoamine reuptake transporters with greater selectivity for the dopamine transporter versus the serotonin or norepinephrine transporter. Microdialysis studies indicate that this compound, like cocaine, enhances extracellular levels of dopamine in the nucleus accumbens, a brain region critical for drug reward [18]. Preclinical behavioral studies also support methylphenidate as a substitution medication. In the drug discrimination paradigm—a model of the subjective effects of drugs in humans—methylphenidate substitutes for cocaine, suggesting that both compounds generate a similar internal cue in animals [19]. Another similarity between the medications is found in the self-administration assay, a model of drug reinforcement, as cocaine and methylphenidate are self-administered in animals. Self-administration studies also indicate the potential use of methylphenidate to treat cocaine dependence, as chronic oral methylphenidate treatment attenuated cocaine reinforcement in rodents [20]. Collectively, these data support the utility of methylphenidate and related compounds as substitution medications for stimulant dependence.
Like methylphenidate, d-amphetamine enhances extracellular monoamine levels, albeit via a different mechanism.
Methylphenidate enhances transmitter levels by blocking reuptake transporters; however, the amphetamines reverse monoamine transporters via a carrier-mediated exchange system, resulting in increased release of monoamines. Administration of d-amphetamine to rodents increases extracellular dopamine and norepinephrine, with a much lesser effect on serotonin levels. Like methylphenidate, d-amphetamine substitutes for cocaine in rodents, indicating subjective similarities between these stimulants. Negus and Mello [21] elegantly demonstrated the ability of d-amphetamine to reduce cocaine self-administration. For example, chronic infusion of d-amphetamine blocked cocaine self-administration in primates with minimal alteration in food reinforcement, indicating that this effect of d-amphetamine is selective for drug versus natural reinforcers [21]. These findings are consistent with human studies, as discussed later.
Modafinil is a stimulant approved by the US Food and Drug Administration for the treatment of excessive daytime sleepiness associated with narcolepsy. Modafinil’s mechanism of action is not completely understood, although it does enhance dopaminergic, adrenergic, and glutamatergic transmission [22]. Modafinil partially substitutes for cocaine in the drug discrimination paradigm, suggesting some similarity between the compounds [23]. Modafinil is self-administered by animals but only at high doses [23], suggesting lesser abuse liability than cocaine. Modafinil’s subtle stimulant-like effects make it an attractive medication to treat stimulant dependence, and clinical studies have yielded some positive results.
All the stimulants described previously directly or indirectly increase brain monoamines that were depleted by chronic cocaine or methamphetamine abuse. In addition to these medications’ ability to reduce drug-taking, stimulants may also improve the cognitive deficits associated with abuse of these drugs. Studies of stimulants’ acute effect on behavioral laboratory measures of impulsivity have largely shown that they improve these measures in healthy controls [24], and their efficacy in improving impulsivity in children with ADHD is well documented [25]. However, some evidence indicates that there is no improvement in at least some measures of impulsivity in stimulant abusers after acute amphetamine administration [26]. One reason for the lack of consistency in behavioral effects of stimulants in drug abusers is that there may be subgroups of individuals with stimulant abuse who have higher dopamine function that would not be improved by increased dopamine levels. As reviewed by Elkashef and Vocci [17], data suggest that about two thirds of chronic cocaine users have evidence of reduced brain dopamine function, with the rest having normal or high dopamine function. This makes it less likely that a single medication would be efficacious for all cocaine abusers and points out the importance of identifying subgroups of patients with specific characteristics that would respond to stimulants.
Another rationale for using stimulants to treat cocaine or methamphetamine dependence is a “self-medication” model in which cocaine or methamphetamine users begin using these drugs as a method of self-medicating symptoms of ADHD [1].
Studies indicate that 10% to 20% of cocaine-dependent individuals have ADHD [27]. The neurobiologic underpinnings underlying this comorbidity are not well understood, although both disorders involve alterations in the dopamine system and have a large genetic component. The factors triggering onset of stimulant use in these individuals are not well understood; however, some hypothesize that the stimulant is first used as a form of “self-medication” to reduce ADHD symptoms, with the development of dependence after repeated use [27]. This comorbidity is particularly important, as these individuals exhibit an earlier onset of drug use and more frequent and intense patterns of use. Also, people with stimulant dependence and ADHD have a more complicated pattern of remission/relapse with poorer treatment outcomes [28]. The negative impact of ADHD on treatment outcomes suggests that the optimal treatment strategy for these individuals should address stimulant dependence and ADHD.
The optimal pharmacotherapeutic treatment of comorbid ADHD and stimulant dependence is the subject of ongoing debate. The first-line treatment for single-diagnosis ADHD is often psychostimulants, but they have been infrequently used to treat ADHD in stimulant abusers for various reasons, including concerns that administering psychostimulant ADHD medications to active or recent stimulant abusers may further exacerbate their drug use. As a result, such comorbid individuals are required to be abstinent for 3 to 12 months before initiation of treatment with stimulants.
However, recent research findings have proven this to be incorrect, as administering methylphenidate or the amphetamines does not increase cocaine abuse, produce drug craving, or elicit relapse. In fact, psychostimulant administration decreases cocaine use. In line with these data, children with ADHD who are treated with psychostimulants are less likely to develop substance abuse disorders as adolescents and adults than their untreated counterparts [29].
Recent evidence indicating the utility of amphetamines for treatment of single-diagnosis cocaine dependence in addition to the longstanding track record demonstrating efficacy of psychostimulants for the treatment of single-diagnosis ADHD suggests that psychostimulants may be effective as a treatment for dual-diagnosis ADHD/cocaine dependence. This possibility was supported by a recent open trial by Levin and colleagues [30] indicating that methylphenidate decreased the severity of ADHD symptoms, cocaine use, and drug craving in individuals with dual-diagnosis ADHD/cocaine dependence. A double-blind, placebo-controlled clinical trial subsequently produced mixed results, with no difference in retention or probability of cocaine-positive urines between methyl-phenidate- and placebo-treated individuals. However, a secondary analysis did show significant differences in the slopes of probability of cocaine-positive urine over time, with methylphenidate-treated patients having a decreased probability of cocaine use over time, and the placebo-treated individuals showing an increased probability in the same period [31•].
Having listed the reasons that stimulants may be used to treat cocaine and methamphetamine dependence, there are also some reasons limiting their potential use. The most obvious of these reasons is the abuse potential of stimulants. Data from epidemiologic studies show that prescription stimulants can be diverted to misuse [32], and there is concern among clinicians about substituting one addicting drug for another. Likewise, animal studies suggest that stimulants could increase self-administration of cocaine and methamphetamine via a “priming” effect [33]. However, the effects in humans may differ. In a study using 11C raclopride binding in PET, it was shown that oral methylphenidate increased dopamine in striatum but was not associated with craving unless paired with cocaine cues [34]. Likewise, diversion has not been seen in controlled clinical trials [35,36].
Treatment Studies in Humans
Most treatment studies using stimulants for cocaine and methamphetamine have been conducted in cocaine-dependent individuals. As there are many examples in which open-label trials of medications for cocaine dependence have later not been supported by placebo-controlled studies, we focus on placebo-controlled studies in this review. Table 1 lists the controlled studies that have been published using drugs that could be described as stimulants (using the definition described previously for treatment of cocaine and methamphetamine dependence).
Table 1.
Results of placebo-controlled trials of stimulants for treating cocaine dependence
| Study | Treatment | Participants | Results |
|---|---|---|---|
|
| |||
| Grabowski et al. [35] | d-amphetamine extended release; 2 titration groups (15- to 30-mg/d and 30- to 60-mg/d vs placebo) | Cocaine dependent; randomized; n = 128 | Improved retention in 15- to 30-mg/d treatment group; reduced cocaine-positive urines in 60-mg/d treatment group |
| Grabowski et al. [36] | d-amphetamine extended release; 2 titration groups (15- to 30-mg/d and 30- to 60-mg/d and methadone vs placebo) | Cocaine and opiate dependent; randomized; n = 94 | Reduced cocaine-positive urines in 30- to 60-mg/d treatment group |
| Shearer et al. [37] | d-amphetamine immediate release, 60 mg/d, vs placebo | Cocaine dependent; n = 30 | No significant differences between groups on retention or cocaine-positive urines |
| Margolin et al. [47] | Mazindol, 1 mg/d, vs placebo | Cocaine and opiate dependent; n = 37 | No significant differences between groups on retention or cocaine-positive urines |
| Stine et al. [48] | Mazindol, 2 mg/d, vs placebo | Cocaine dependent; n = 43 | No significant differences between groups on retention or cocaine-positive urines |
| Grabowski et al. [49] | Methylphenidate (immediate and extended release), 45 mg/d, vs placebo | Cocaine dependent; n = 49 | No significant differences between groups on retention or cocaine-positive urines |
| Schubiner et al. [50] | Methylphenidate, up to 90 mg/d, vs placebo | Cocaine dependent and adult ADHD; n = 48 | No significant differences between groups on retention or cocaine-positive urines; improved ADHD symptoms in methylphenidate group |
| Levin et al. [31•] | Methylphenidate, up to 60 mg/d, vs placebo | Cocaine dependent and adult ADHD; n = 106 | No significant difference between groups on retention or proportion of cocaine-positive urines; the slope of cocaine-positive urines over time differed with methylphenidate-treated participants; decreased probability of cocaine-positive urine over time |
| Dackis et al. [51] | Modafinil, 400 mg/d | Cocaine dependent; n = 62 | Reduced cocaine-positive urines in modafinil treatment group |
ADHD—attention-deficit/hyperactivity disorder.
Of the studies published to date, those using d-amphetamine as a treatment have shown some of the more promising results. Three placebo-controlled trials have been published using doses of up to 60 mg of d-amphetamine. Two studies by the same group [35,36] have shown a reduction in cocaine-positive urine drug screens in cocaine-dependent individuals treated with extended-release d-amphetamine compared with placebo-treated individuals. One of these studies used dual-diagnosis cocaine-opiate–dependent individuals also being treated with methadone [36]. A third study with a much smaller number of participants did not find a statistically significant effect of d-amphetamine for retention or reduction in cocaine-positive urine drug screens using immediate-release d-amphetamine [37]. In these studies, the dosage of d-amphetamine was titrated up from a starting dose no higher than 30 mg to minimize the potential cardiovascular complications. Although some cross-tolerance to the effects of d-amphetamine would be expected in cocaine users, acute doses of 40 mg or higher without titration can produce substantial increases in blood pressure (Moeller et al., unpublished data). Patients were also carefully screened to exclude cardiovascular disease. In cocaine-dependent individuals who had been screened this way, the risk of side effects or complications was minimal.
Because the monoamine oxidase inhibitor selegiline is metabolized to L-amphetamine, L-methamphetamine, and phenylethylamine, it has been argued that a potential mechanism of action of selegiline in the treatment of cocaine dependence could be through its metabolites [38]. However, a large, placebo-controlled trial found that transdermal selegiline was not effective in reducing cocaine use [38].
The wakefulness-promoting medication modafinil also has been studied as a potential treatment for cocaine dependence. Although many trials with modafinil are ongoing, only one placebo-controlled trial has been published to date. In that study, Dackis et al. [39] administered modafinil, 400 mg/d, or placebo to 62 cocaine-dependent individuals for 8 weeks. They also were treated using cognitive-behavioral therapy over the 8 weeks. Individuals were excluded if they had any substance dependence other than cocaine or nicotine. Results of this study were significantly fewer cocaine-positive urines and significantly greater likelihood of achieving protracted abstinence in the modafinil-treated individuals. Side effects that prompted reduced dosing of the medication occurred in six of the modafinil-treated individuals. These included insomnia, headache, nausea, tachycardia, and anxiety.
Stimulants for Methamphetamine Dependence
In one retrospective examination of the charts of amphetamine-abusing patients who received d-amphetamine treatment, there appeared to be a decrease in intravenous and oral administration of illicit amphetamine [40].
Another retrospective study of amphetamine-dependent schizophrenia patients prescribed d-amphetamine showed that the patients seemed to exhibit decreased illicit amphetamine use and increased compliance with coadministered neuroleptics [41]. Although these small, retrospective, and pilot studies have shown that d-amphetamine substitution is likely safe and effective for treating amphetamine and/or methamphetamine dependence [40] and efficacious at retaining patients [42], studies looking at pure methamphetamine dependence are more rare, and randomized, controlled trials are needed to elucidate more information about the use of d-amphetamine for agonist therapy of methamphetamine dependence.
As methylphenidate works in a similar mechanism of action as d-amphetamine, blocking presynaptic reuptake, it has also been suggested as a treatment for methamphetamine dependence. In a case series detailed by Laqueille et al. [43], methylphenidate was well tolerated and useful for treating symptoms of depression seen in patients withdrawing from amphetamines. In a study comparing aripiprazole, methylphenidate, and placebo for amphetamine dependence, amphetamine use was significantly reduced in methylphenidate-treated patients compared with placebo-treated individuals [44]. This study allowed for inclusion of methamphetamine and amphetamine users; however, it was also stated that none of the participants were found to be using methamphetamine. Speculation from this study suggested that slow-release methylphenidate may be an appropriate and effective treatment for amphetamine dependence.
Future Directions
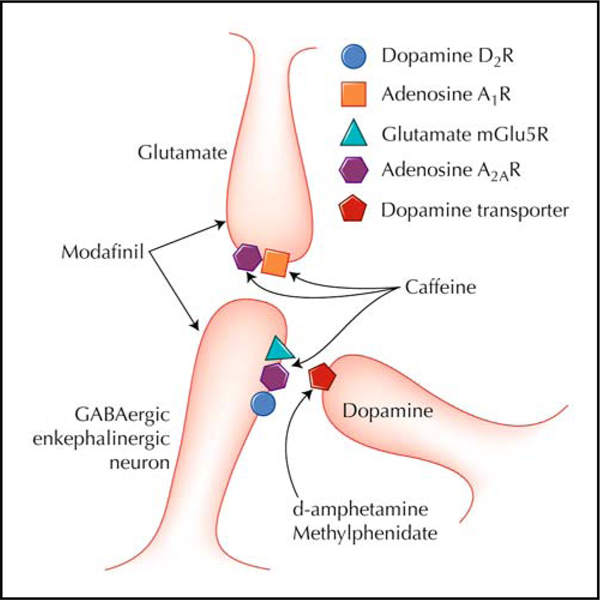
Several clinical trials of stimulants for cocaine and methamphetamine dependence are under way. Several larger trials using modafinil are ongoing, and results should be published within the next year. In addition, other stimulants that have direct or indirect effects on dopamine function are being examined as potential treatments (Fig. 1), including a different class of stimulants, the adenosine receptor antagonists [45•].
Figure 1.
Potential receptor sites of action of stimulants for the treatment of cocaine and methamphetamine dependence. GABA—γ-aminobutyric acid. (Adapted from Ferre et al. [45•].).
Adenosine receptor antagonists also function as stimulant drugs, with caffeine being the prototypical stimulant in this class [46]. Caffeine is a nonselective adenosine receptor antagonist that binds to adenosine A1 receptors and A2A receptors [46]. Based on the clinical observation that cocaine abstinence symptoms were reduced by caffeine use and a renewed interest in adenosine receptor antagonists as potential treatments for drug abuse, clinical trials using caffeine to reduce cocaine withdrawal symptoms and reduce cocaine use are under way. In addition to caffeine, other compounds are more selective adenosine A2A receptor antagonists. Several of these selective A2A antagonists are being studied as treatments for Parkinson’s disease. Their efficacy in Parkinson’s disease appears to be related to the fact that the A2A receptor forms heteroreceptors with dopamine receptors, which reduce these receptors’ function. Thus, an A2A antagonist would enhance dopamine’s effect on dopamine receptors. As dopamine is central in reward and addiction by enhancing the responsiveness of dopamine receptors for dopamine function, A2A antagonists could be efficacious in treating cocaine and methamphetamine dependence.
Conclusions
Several different classes of stimulants have been tried as treatment for cocaine dependence, with mixed results. Some stimulants have reduced cocaine use and/or improved treatment retention, whereas others have not shown superiority over placebo. Studies are also under way using stimulants for methamphetamine dependence, but results of these trials have not been published yet. One disadvantage of stimulants is their potential for abuse. Several clinical trials are under way using classes of stimulants that appear to have a relatively low abuse potential. If these studies are successful, they may provide a safe and effective pharmacologic treatment for cocaine and methamphetamine dependence.
Acknowledgments
Disclosures
Dr. Moeller serves as a consultant for GlaxoSmithKline and Ortho-McNeil and has received contract support from Ortho-McNeil. No other potential conflicts of interest relevant to this article were reported.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Grabowski J, Shearer J, Merrill J, Negus SS: Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 2004, 29:1439–1464. [DOI] [PubMed] [Google Scholar]
- 2. Castells X, Casas M, Vidal X, et al. : Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials. Addiction 2007, 102:1871–1887. •• This is a meta-analysis of placebo-controlled studies published when stimulants were used to treat cocaine dependence. It gives a good summary of most of the studies published to date.
- 3.Ritz MC, Cone EJ, Kuhar MJ: Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci 1990, 46:635– 645. [DOI] [PubMed] [Google Scholar]
- 4.Vocci FJ, Appel NM: Approaches to the development of medications for the treatment of methamphetamine dependence. Addiction 2007, 102(Suppl 1):96–106. [DOI] [PubMed] [Google Scholar]
- 5.Buydens-Branchey L, Branchey M, Hudson J, et al. : Serotonergic function in cocaine addicts: prolactin responses to sequential D,L-fenfluramine challenges. Biol Psychiatry 1999, 45:1300–1306. [DOI] [PubMed] [Google Scholar]
- 6.Volkow ND, Fowler JS, Wolf AP, et al. : Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry 1990, 147:719–724. [DOI] [PubMed] [Google Scholar]
- 7.Martinez D, Broft A, Foltin RW, et al. : Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology 2004, 29:1190–1202. [DOI] [PubMed] [Google Scholar]
- 8. Martinez D, Narendran R, Foltin RW, et al. : Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry 2007, 164:622– 629. • The data in this article clearly show that chronic cocaine-dependent individuals have reduced dopamine function. The article also reviews previous studies that have examined this issue.
- 9.Volkow ND, Fowler JS, Wang GJ, et al. : Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993, 14:169–177. [DOI] [PubMed] [Google Scholar]
- 10.Volkow ND, Chang L, Wang GJ, et al. : Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001, 158:2015–2021. [DOI] [PubMed] [Google Scholar]
- 11.McCann UD, Wong DF, Yokoi F, et al. : Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci 1998, 18:8417–8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sevy S, Hassoun Y, Bechara A, et al. : Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology 2006, 188:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stout JC, Busemeyer JR, Lin A, et al. : Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev 2004, 11:742–747. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez R, Bechara A, Martin EM: Executive functions among individuals with methamphetamine or alcohol as drugs of choice: preliminary observations. J Clin Exp Neuropsychol 2007, 29:155–159. [DOI] [PubMed] [Google Scholar]
- 15.Patkar AA, Gottheil E, Berrettini WH, et al. : Relationship between platelet serotonin uptake sites and measures of impulsivity, aggression, and craving among African-American cocaine abusers. Am J Addict 2003, 12:432– 447. [PubMed] [Google Scholar]
- 16.Moeller FG, Barratt ES, Dougherty DM, et al. : Psychiatric aspects of impulsivity. Am J Psychiatry 2001, 158:1783–1793. [DOI] [PubMed] [Google Scholar]
- 17.Elkashef A, Vocci F: Biological markers of cocaine addiction: implications for medications development. Addict Biol 2003, 8:123–139. [DOI] [PubMed] [Google Scholar]
- 18.Volkow ND, Li TK: Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci 2004, 5:963–970. [DOI] [PubMed] [Google Scholar]
- 19.Schweri MM, Deutsch HM, Massey AT, Holtzman SG: Biochemical and behavioral characterization of novel methylphenidate analogs. J Pharmacol Exp Ther 2002, 301:527–535. [DOI] [PubMed] [Google Scholar]
- 20.Thanos PK, Michaelides M, Benveniste H, et al. : Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav 2007, 87:426– 433. [DOI] [PubMed] [Google Scholar]
- 21.Negus SS, Mello NK: Effects of chronic d-amphetamine treatment on cocaine- and food-maintained responding under a second-order schedule in rhesus monkeys. Drug Alcohol Depend 2003, 70:39–52. [DOI] [PubMed] [Google Scholar]
- 22.Dackis C, O’Brien C: Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci 2003, 1003:328–345. [DOI] [PubMed] [Google Scholar]
- 23.Gold LH, Balster RL: Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology (Berl) 1996, 126:286–292. [DOI] [PubMed] [Google Scholar]
- 24.de Wit H, Enggasser JL, Richards JB: Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 2002, 27:813–825. [DOI] [PubMed] [Google Scholar]
- 25.Faraone SV, Biederman J: Efficacy of Adderall for attention-deficit/hyperactivity disorder: a meta-analysis. J Atten Disord 2002, 6:69–75. [DOI] [PubMed] [Google Scholar]
- 26.Fillmore MT, Rush CR, Marczinski CA: Effects of d-amphetamine on behavioral control in stimulant abusers: the role of prepotent response tendencies. Drug Alcohol Depend 2003, 71:143–152. [DOI] [PubMed] [Google Scholar]
- 27.Levin FR, Evans SM, Kleber HD: Prevalence of adult attention-deficit hyperactivity disorder among cocaine abusers seeking treatment. Drug Alcohol Depend 1998, 52:15–25. [DOI] [PubMed] [Google Scholar]
- 28.Carroll KM, Power ME, Bryant K, Rounsaville BJ: One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J Nerv Ment Dis 1993, 181:71–79. [DOI] [PubMed] [Google Scholar]
- 29.Faraone SV, Wilens TE: Effect of stimulant medications for attention-deficit/hyperactivity disorder on later substance use and the potential for stimulant misuse, abuse, and diversion. J Clin Psychiatry 2007, 68(Suppl 11):15–22. [PubMed] [Google Scholar]
- 30.Levin FR, Evans SM, McDowell DM, Kleber HD: Methylphenidate treatment for cocaine abusers with adult attention-deficit/hyperactivity disorder: a pilot study. J Clin Psychiatry 1998, 59:300–305. [DOI] [PubMed] [Google Scholar]
- 31. Levin FR, Evans SM, Brooks DJ, Garawi F: Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend 2007, 87:20–29. • Placebo-controlled clinical trial using a stimulant to treat dual-diagnosis cocaine-dependent and ADHD patients. It also provides a summary of previous trials in this area.
- 32.Johnston LD, Bachman JG, Schulenberg JE: Monitoring the future national survey results on drug use, 1975–2005. Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2006. [Google Scholar]
- 33.de Wit H, Stewart J: Reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology (Berl) 1981, 75:134–143. [DOI] [PubMed] [Google Scholar]
- 34.Volkow ND, Wang GJ, Telang F, et al. : Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 2008, 39:1266–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabowski J, Rhoades H, Schmitz J, et al. : Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol 2001, 21:522–526. [DOI] [PubMed] [Google Scholar]
- 36.Grabowski J, Rhoades H, Stotts A, et al. : Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology 2004, 29:969–981. [DOI] [PubMed] [Google Scholar]
- 37.Shearer J, Wodak A, van Beek I, et al. : Pilot randomized double blind placebo-controlled study of dexamphetamine for cocaine dependence. Addiction 2003, 98:1137–1141. [DOI] [PubMed] [Google Scholar]
- 38.Elkashef A, Fudala PJ, Gorgon L, et al. : Double-blind, placebo-controlled trial of selegiline transdermal system (STS) for the treatment of cocaine dependence. Drug Alcohol Depend 2006, 85:191–197. [DOI] [PubMed] [Google Scholar]
- 39.Dackis CA, Kampman KM, Lynch KG, et al. : A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005, 30:205–211. [DOI] [PubMed] [Google Scholar]
- 40.White R: Dexamphetamine substitution in the treatment of amphetamine abuse: an initial investigation. Addiction 2000, 95:229–238. [DOI] [PubMed] [Google Scholar]
- 41.Carnwath T, Garvey T, Holland M: The prescription of dexamphetamine to patients with schizophrenia and amphetamine dependence. J Psychopharmacol 2002, 16:373–377. [DOI] [PubMed] [Google Scholar]
- 42.Shearer J, Wodak A, Mattick RP, et al. : Pilot randomized controlled study of dexamphetamine substitution for amphetamine dependence. Addiction 2001, 96:1289–1296. [DOI] [PubMed] [Google Scholar]
- 43.Laqueille X, Dervaux A, El Omari F, et al. : Methylphenidate effective in treating amphetamine abusers with no other psychiatric disorder. Eur Psychiatry 2005, 20:456–457. [DOI] [PubMed] [Google Scholar]
- 44.Tiihonen J, Kuoppasalmi K, Fohr J, et al. : A comparison of aripiprazole, methylphenidate, and placebo for amphetamine dependence. Am J Psychiatry 2007, 164:160–162. [DOI] [PubMed] [Google Scholar]
- 45. Ferre S, Diamond I, Goldberg SR, et al. : Adenosine A2A receptors in ventral striatum, hypothalamus and nociceptive circuitry implications for drug addiction, sleep and pain. Prog Neurobiol 2007, 83:332–347. • This review article describes recent advances in the neurobiology of adenosine receptors. It also discusses potential applications of adenosine receptor antagonists for drug addiction.
- 46.Ferre S: An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem 2008, 105:1067–1079. [DOI] [PubMed] [Google Scholar]
- 47.Margolin A, Avants SK, Kosten TR: Mazindol for relapse prevention to cocaine abuse in methadone-maintained patients. Am J Drug Alcohol Abuse 1995, 21:469–481. [DOI] [PubMed] [Google Scholar]
- 48.Stine SM, Krystal JH, Kosten TR, Charney DS: Mazindol treatment for cocaine dependence. Drug Alcohol Depend 1995, 39:245–252. [DOI] [PubMed] [Google Scholar]
- 49.Grabowski J, Roache JD, Schmitz JM, et al. : Replacement medication for cocaine dependence: methylphenidate. J Clin Psychopharmacol 1997, 17:485– 488. [DOI] [PubMed] [Google Scholar]
- 50.Schubiner H, Saules KK, Arfken CL, et al. : Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp Clin Psychopharmacol 2002, 10:286–294. [DOI] [PubMed] [Google Scholar]
- 51.Dackis CA, Kampman KM, Lynch KG, et al. : A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005, 30:205–211. [DOI] [PubMed] [Google Scholar]