Several biomarkers have emerged in the past few decades to quantify pathologic brain changes related to Alzheimer disease (AD). In particular, PET radiotracers that bind selectively to amyloid-β plaques and tau neurofibrillary tangles have advanced AD research and drug development by enabling the detection and quantification of the neuropathologic lesions that define AD in living people.
Among amyloid-β PET tracers, 11C-Pittsburgh compound B was the first to be developed, followed by 18F-labeled tracers approved for clinical use (i.e., 18F-florbetapir, 18F-florbetaben, and 18F-flutametamol). Cumulative evidence has demonstrated the diagnostic utility of amyloid PET in differentiating AD from nonamyloid neurodegenerative diseases (1,2). However, amyloid PET retention begins 2 decades before the onset of clinical symptoms and reaches a relative plateau throughout most of the neocortex early in the evolution of AD (before, or coincident with, early clinical symptoms). As a result, the distribution and burden of amyloid correlate poorly with disease stage or clinical measures in symptomatic patients and do not colocalize with markers of regional neurodegeneration (3,4).
18F-flortaucipir was the first PET tracer to show high affinity and selectivity for AD neurofibrillary tangles, followed by next-generation tau tracers such as 18F-MK6240, 18F-RO948, 18F-PI2620, 18F-GTP1, and 18F-PM-PBB3. 18F-flortaucipir is, to date, the only tau PET tracer to receive Food and Drug Administration approval for clinical use in the United States. 18F-flortaucipir PET distinguishes AD from other underlying neuropathologies, including non-AD tauopathies to which the tracer shows a low binding affinity (5–8). In contrast to the early widespread distribution of amyloid PET binding, tau PET signal originates in the entorhinal cortex and other medial temporal regions. In the presence of amyloid, signal progressively spreads into the inferior temporal gyrus, followed by the lateral occipital cortex, posterior cingulate/precuneus, lateral temporoparietal regions, and, finally, prefrontal cortex. This evolution is similar (though not identical) to Braak neuropathologic staging of tau neurofibrillary tangles and closely colocalizes with brain atrophy and hypometabolism patterns as measured by MRI or 18F-FDG PET (3,9). Increasing spread of tau is associated with clinical impairment (10–12), with the tau PET binding topography associated with domain-specific cognitive deficits (13,14) and distinct AD clinical variants (9,15,16),
The relationships between amyloid and tau PET and clinical measures largely replicate clinicopathologic studies showing that the stage and extent of neurofibrillary tau tangles are strongly associated with antemortem clinical status and cognitive deficits (17,18), whereas amyloid neuropathology correlates weakly with antemortem clinical impairment. Moreover, the regional distribution of neurofibrillary tau tangles after death also relates to distinct clinical presentations and syndromes (19), whereas amyloid-β plaque distribution generally does not. Postmortem pathology and in vivo imaging evidence together suggest a spatial and temporal decoupling between amyloid-β plaque accumulation and neurodegeneration, whereas neurofibrillary tangles are more closely associated with regional neurodegeneration and clinical impairment. These observations support the hypothesis that amyloid-β may influence neuronal integrity only indirectly by facilitating tau spreading (20), leading to synaptic and cell loss and ultimately translating into cognitive and functional decline.
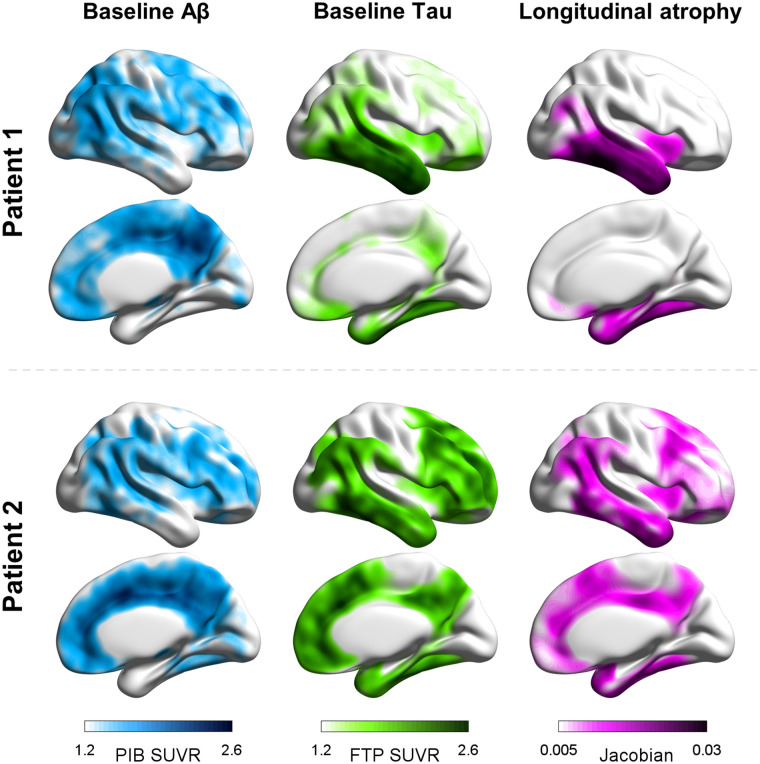
In cross-sectional multimodal imaging studies along the clinical AD spectrum, tau accumulation has also been observed in areas without overt neurodegeneration (3,9,15,21). These findings support the hypothesis that tau elevation may locally precede neurodegeneration, which would be a downstream event in the cascade associated with AD. Such hypotheses are reinforced by significant associations between baseline tau PET patterns and prospective MRI atrophy (22,23). A recent study by La Joie et al. (22) showed that in patients with clinically mild AD, the burden and regional distribution of tau pathology at baseline, as measured with 18F-flortaucipir PET, can forecast the severity and topography of prospective brain atrophy over the following 15 mo. In contrast, neither the severity nor the topography of amyloid PET was found to be informative of atrophy progression. At a group level, regional tau PET uptake at baseline explained more than 40% of unique variance in atrophy at follow-up, even when corrected for baseline cortical thickness, versus 3% of variance explained by regional amyloid PET. Importantly, the close association between baseline tau PET and subsequent atrophy was found not only at the group level but also in each individual patient (Fig. 1). In line with this finding, tau PET also correlates strongly with retrospective longitudinal atrophy (years preceding PET) in both cognitively unimpaired individuals and patients with clinical AD (24,25).
FIGURE 1.
Single-subject amyloid and tau PET patterns at baseline and cortical atrophy over time. (Left and middle) 11C-Pittsburgh compound B (PIB) and 18F-flortaucipir (FTP) PET SUV ratio maps, respectively, with higher values indicate more severe pathology. (Right) Patterns derived from prospective longitudinal structural MRI scans after PET, with positive Jacobians indicating shrinkage over time. Patient 1 is 71-y-old with mild AD dementia; patient 2 is 61-y-old with mild AD dementia.
Tau PET is a sensitive predictor not only of structural brain changes in AD but also of prospective cognitive decline. Several studies have described strong associations between baseline tau PET and cognitive changes over time across the AD clinical spectrum. In head-to-head comparisons, tau PET binding in temporoparietal regions outperformed amyloid PET and structural MRI measures in predicting cognitive decline (26–28), especially in patients at early AD stages (26). This result was replicated by local and multicenter studies using different tau PET tracers (i.e., 18F-flortaucipir and 18F-RO948), retrospective and prospective longitudinal cognitive assessments, and participants with different severities of impairment. These converging findings suggest that tau PET is a promising prognostic tool for predicting cognitive decline and that tau pathology may be the main driver of neurodegeneration and cognitive symptoms.
Tau PET may play an important role in future precision medicine approaches to AD care by enabling prediction of specific neurodegeneration and cognitive trajectories in individual patients. A recent study that evaluated tau PET patterns in a large, multisite dataset (n = 1,612) revealed substantial variability across patients, highlighting 4 distinct spatiotemporal patterns, each associated with specific demographic and clinical features (29). This heterogeneity highlights the limitations of a one-size-fits-all approach to predicting tau, neurodegeneration, or clinical change. Furthermore, individual factors modify the relationship between tau accumulation and cognition, with younger age, female sex, higher educational attainment, and higher baseline cortical thickness all associated with increased resistance against the deleterious effect of pathology on cognitive performance (30). Some studies suggest that the relationship between tau, neurodegeneration, and cognition may also vary with race and ethnicity as proxies for social determinants of health (31), though much more work on diverse cohorts is needed to better understand these relationships. Finally, the development of biomarkers that measure common non-AD pathologies (e.g., vascular lesions, TDP43, and α-synuclein aggregates) will be critical for achieving a more adequate prognosis, as these processes are highly prevalent in patients with AD (32) and contribute significantly to neurodegeneration and cognitive decline.
Plasma biomarkers have recently emerged as promising and accessible biomarkers for tau pathology in AD (33). Plasma p-tau 181, p-tau 217, and p-tau 231 increase in early stages of AD, discriminate patients with AD from those with non-AD conditions, and show moderate correlations with tau PET uptake (34,35). Although plasma p-tau measures Aβ-induced changes in tau phosphorylation and secretion, tau PET measures the overall burden and topographic distribution of neurofibrillary tangles. Therefore, plasma p-tau measurements and tau PET provide additive and complementary information on tau pathology and prognosis. In a recent head-to-head study, baseline plasma p-tau 217 best predicted longitudinal increases in tau PET in preclinical AD, whereas baseline tau PET was the better predictor in symptomatic patients (36). Future work will determine whether baseline patterns of tau PET can also predict domain-specific changes in cognition (e.g., medial temporal tau predicting changes in episodic memory, or occipital tau predicting changes in visuospatial function).
The close relationship between tau burden, prospective neurodegeneration, and consequent clinical decline is particularly important in the development of novel AD therapies. Effective treatments for AD may ultimately require combination therapies targeting both amyloid-β and tau pathology as well as other elements of AD pathophysiology. Antitau therapies could be effective in preventing synaptic loss and atrophy, thus slowing clinical decline, whereas antiamyloid therapies in early stages could prevent tau spreading. Thus, tau PET may be a good tool to stratify patients in clinical trials of disease-modifying therapies, with personalized estimations of neurodegeneration and cognitive trajectories, enhancing the chance to identify the best time window and the cohort in which a given therapy can be most effective. In a recent phase 2 trial of donanemab (37), a monoclonal antibody targeting the pyroglutamate epitope on Aβ plaques, 18F-flortaucipir PET was used to limit trial inclusion to patients with intermediate tau deposition. This innovative approach to patient stratification shifts the focus from amyloid to tau pathology, enabling more accurate prediction of clinical progression. Including patients with an intermediate amount of tau pathology addresses the concern that antiamyloid therapies may not be beneficial at advanced disease stages (high tau) but also reduces the risk of including patients who may not progress clinically during the course of the trial (low tau). In the trial, significant amyloid PET lowering by donanemab was associated with slower tau PET progression in the frontal and temporal cortices and with modestly slower cognitive and functional decline compared with placebo. This successful trial foreshadows a future in which tau PET may have an important role in establishing eligibility and evaluating response to novel disease-modifying therapies. However, future clinical trials also need to evaluate alternative tau PET tracers, which may be more sensitive than 18F-flortaucipir PET for early Braak tau stages in order to ensure the inclusion of patients with early AD-tau pathology, as these patients may benefit most from antiamyloid and other therapeutic approaches.
In conclusion, tau PET is a highly promising tool that will likely play an important role in future precision medicine approaches to AD care. Tau PET is both highly specific for AD neuropathology and (in contrast to amyloid PET) strongly associated with neurodegeneration and clinical outcomes. Further work is needed to fully leverage the potential of tau PET to predict individual patient trajectories, understand the complex pathophysiology of the disease, and ultimately accelerate the development of effective therapies.
DISCLOSURE
Gil Rabinovici receives research support from Avid Radiopharmaceuticals, GE Healthcare, Genentech, and Life Molecular Imaging. He has received consultation fees from Eli Lilly, Roche, and Genentech. He serves on a data safety monitoring board for Johnson & Johnson and as an associate editor for JAMA Neurology. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ossenkoppele R, Jansen WJ, Rabinovici GD, et al. Prevalence of amyloid PET positivity in dementia syndromes: a meta-analysis. JAMA. 2015;313:1939–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iaccarino L, La Joie R, Edwards L, et al. Spatial relationships between molecular pathology and neurodegeneration in the Alzheimer’s disease continuum. Cereb Cortex. 2021;31:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. La Joie R, Perrotin A, Barré L, et al. Region-specific hierarchy between atrophy, hypometabolism, and 2-amyloid (Aβ) load in Alzheimer’s disease dementia. J Neurosci. 2012;32:16265–16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ossenkoppele R, Rabinovici GD, Smith R, et al. Discriminative accuracy of [18F]flortaucipir positron emission tomography for Alzheimer disease vs other neurodegenerative disorders. JAMA. 2018;320:1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jack CR, Wiste HJ, Botha H, et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142:3230–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fleisher AS, Pontecorvo MJ, Devous MD, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020;77:829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soleimani-Meigooni DN, Iaccarino L, La Joie R, et al. 18F-flortaucipir PET to autopsy comparisons in Alzheimer’s disease and other neurodegenerative diseases. Brain. 2020;143:3477–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ossenkoppele R, Schonhaut DR, Schöll M, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schöll M, Lockhart SN, Schonhaut DR, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Maass A, Landau S, Baker S, et al. Comparison of multiple tau PET measures as biomarkers in aging and Alzheimer’s disease. Neuroimage. 2017;157:448–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lowe VJ, Wiste HJ, Senjem ML, et al. Widespread brain tau and its association with ageing, Braak stage and Alzheimer’s dementia. Brain. 2018;141:271–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bejanin A, Schonhaut DR, La Joie R, et al. Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain. 2017;140:3286–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ossenkoppele R, Smith R, Ohlsson T, et al. Associations between tau, Aβ, and cortical thickness with cognition in Alzheimer disease. Neurology. 2019;92:e601–e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Whitwell JL, Graff‐Radford J, Tosakulwong N, et al. Imaging correlations of tau, amyloid, metabolism, and atrophy in typical and atypical Alzheimer’s disease. Alzheimers Dement. 2018;14:1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Josephs KA, Tosakulwong N, Graff‐Radford J, et al. MRI and flortaucipir relationships in Alzheimer’s phenotypes are heterogeneous. Ann Clin Transl Neurol. 2020;7:707–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nelson PT, Alafuzoff I, Bigio EH, et al. Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol. 2012;71:362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serrano-Pozo A, Qian J, Muzikansky A, et al. Thal amyloid stages do not significantly impact the correlation between neuropathological change and cognition in the Alzheimer disease continuum. J Neuropathol Exp Neurol. 2016;75:516–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen C, Nolan AL, de Paula França Resende E, et al. Alzheimer’s disease clinical variants show distinct regional patterns of neurofibrillary tangle accumulation. Acta Neuropathol (Berl). 2019;138:597–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Busche MA, Hyman BT. Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci. 2020;23:1183–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harrison TM, La Joie R, Maass A, et al. Longitudinal tau accumulation and atrophy in aging and Alzheimer disease. Ann Neurol. 2019;85:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau PET. Sci Transl Med. 2020;12:1–13. [Google Scholar]
- 23. Scott MR, Hampton OL, Buckley RF, et al. Inferior temporal tau is associated with accelerated prospective cortical thinning in clinically normal older adults. Neuroimage. 2020;220:116991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. LaPoint MR, Chhatwal JP, Sepulcre J, Johnson KA, Sperling RA, Schultz AP. The association between tau PET and retrospective cortical thinning in clinically normal elderly. Neuroimage. 2017;157:612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Das SR, Xie L, Wisse LEM, et al. Longitudinal and cross-sectional structural magnetic resonance imaging correlates of AV-1451 uptake. Neurobiol Aging. 2018;66:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ossenkoppele R, Smith R, Mattsson-Carlgren N, et al. Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: a head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol. 2021;78:961–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hanseeuw BJ, Betensky RA, Jacobs HIL, et al. Association of amyloid and tau with cognition in preclinical Alzheimer disease: a longitudinal study. 2019;76:915–924. [Google Scholar]
- 28. Malpetti M, Kievit RA, Passamonti L, et al. Microglial activation and tau burden predict cognitive decline in Alzheimer’s disease. Brain. 2020;143:1588–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vogel JW, Young AL, Oxtoby NP, et al. Four distinct trajectories of tau deposition identified in Alzheimer’s disease. Nat Med. 2021;27:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ossenkoppele R, Lyoo CH, Jester-Broms J, et al. Assessment of demographic, genetic, and imaging variables associated with brain resilience and cognitive resilience to pathological tau in patients with Alzheimer disease. JAMA Neurol. 2020;77:632–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gleason CE, Zuelsdorff M, Gooding DC, et al. Alzheimer’s disease biomarkers in Black and non‐Hispanic White cohorts: a contextualized review of the evidence. Alzheimers Dement. December 6, 2021. [Epub ahead of print]. [Google Scholar]
- 32. Spina S, La Joie R, Petersen C, et al. Comorbid neuropathological diagnoses in early versus late-onset Alzheimer’s disease. Brain. 2021;144:2186–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hampel H, O’Bryant SE, Molinuevo JL, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol. 2018;14:639–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thijssen EH, La Joie R, Strom A, et al. Plasma phosphorylated tau 217 and phosphorylated tau 181 as biomarkers in Alzheimer’s disease and frontotemporal lobar degeneration: a retrospective diagnostic performance study. Lancet Neurol. 2021;20:739–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ashton NJ, Pascoal TA, Karikari TK, et al. Plasma p-tau231: a new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol (Berl). 2021;141:709–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leuzy A, Smith R, Cullen NC, et al. Biomarker-based prediction of longitudinal tau positron emission tomography in Alzheimer disease. JAMA Neurol. 2022;79:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mintun MA, Lo AC, Duggan Evans C, et al. Donanemab in early Alzheimer’s disease. N Engl J Med. 2021;384:1691–1704. [DOI] [PubMed] [Google Scholar]