Visual Abstract
Keywords: progressive supranuclear palsy, corticobasal degeneration, PET, pathology, AV-1451
Abstract
Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) are 4-repeat (4R) tauopathies with overlapping, but also morphologically distinct, tau immunoreactive lesions that vary in count by brain region. 18F-flortaucipir PET uptake has been reported to correlate with overall tau burden, and—in 1 CBD case—to have greater affinity to threads than tangles. We determined whether 18F-flortaucipir uptake is associated with histologic lesion type in 4R tauopathies. Methods: We performed semiquantitative regional lesion counts on pretangles/neurofibrillary tangles, threads, oligodendroglial coiled bodies, tufted astrocytes, and astrocytic plaques in 29 cases of autopsied 4R tauopathy (PSP, 16; CBD, 13). Regression models were used for statistical analyses. Results: 18F-flortaucipir uptake marginally correlated with threads in the precentral cortex (P = 0.04) and with astrocytic lesions in the red nucleus (P = 0.05). Conclusion: The findings do not support 18F-flortaucipir’s having differential affinity to any 4R tau lesion type.
Progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD) are 4-repeat (4R) tauopathies that are pathologically characterized by overlapping lesion distributions and morphologies, as well as distinct tau immunoreactive lesions that vary in count (amount), depending on which brain region is assessed (1,2). Both PSP and CBD are characterized by the presence of pretangles/neurofibrillary tangles (neuronal), oligodendroglial coiled bodies (glial), and threads (neuronal > glial), whereas PSP has only tufted astrocytes (glial) and CBD has only astrocytic plaques (glial) (1,2). CBD has a predominantly corticostriatal distribution of lesion burden with less involvement of brain stem regions, whereas PSP has a predominantly brain stem distribution of lesion burden.
18F-flortaucipir (also known as 18F-Av-1451 and 18F-T807) is a small indole ligand that is made with a radioactive fluorine isotope that robustly binds to paired helical filament (3R + 4R) tau that occurs in Alzheimer disease brains (3,4). Therefore, 18F-flortaucipir is considered an antemortem biomarker for visualizing underlying paired helical filament tau in Alzheimer disease. Regional 18F-flortaucipir PET uptake has been described in clinical syndromes suspected to have underlying 4R tau (5) and in a few autopsied cases of 4R tauopathies where uptake was reported to correlate with histopathologic tau lesion burden (6,7). In these individual cases, a correlation was identified by assessing the relationship between 18F-flortaucipir uptake across multiple brain regions and histopathologic tau lesion count, whereby count was treated as a single variable combining all lesion types. 18F-flortaucipir uptake has been shown to have greater affinity for threads than for tangles, again across multiple brain regions, in a single CBD case (7). More recently, in an autopsied case series we found correlations between 18F-flortaucipir and histologic tau to be selective and limited to only a few brain regions, particularly the red nucleus of the midbrain (8). We did not assess lesion type, however.
Given these findings, we aimed to investigate in a relatively large autopsy-confirmed cohort of PSP and CBD cases with antemortem 18F-flortaucipir whether ligand uptake does indeed show evidence of differential affinity for threads and, if so, whether such a relationship would be region-specific or whether different regions would show different lesion affinities. We hypothesize that 18F-flortaucipir uptake would correlate to overall tau burden in the red nucleus but not to any single specific lesion since all lesion types are represented in the red nucleus in CBD or PSP.
MATERIALS AND METHODS
Participant Cohort
All participants were prospectively recruited by the Neurodegenerative Research Group at Mayo Clinic, Minnesota, as part of a National Institutes of Health–funded grant and met 2 inclusion criteria: completion of antemortem volumetric head MRI, 18F-flortaucipir PET, and Pittsburgh compound B PET; and a brain autopsy with a pathologic diagnosis of PSP or CBD between April 7, 2016, and April 6, 2021.
The study was approved by the Mayo Clinic Institutional Review Board, and all patients gave written informed consent to participate in the study.
Neuropathologic Evaluation and Diagnosis
All patients had a standardized neuropathologic evaluation in accordance with accepted published methodologies (9), including immunohistochemistry to detect 4R tau immunoreactive lesions using an antibody to phospho-tau (CP13; 1:1,000; IgG1 to phospho-serine 202, gift from the late Peter Davies, Feinstein Institute). PSP and CBD pathologic diagnoses were rendered according to published criteria (1,2). PSP was diagnosed if there were globose neurofibrillary tangles, tufted astrocytes, and oligodendroglial coiled bodies in cardinal nuclei (2), whereas CBD was diagnosed if there were balloon neurons, as well as tau-specific lesions of pretangles, astrocytic plaques, and threads in cardinal nuclei (1). Additional pathologic findings were documented as previously described (8).
Semiquantitative 4R Tau Lesion Count
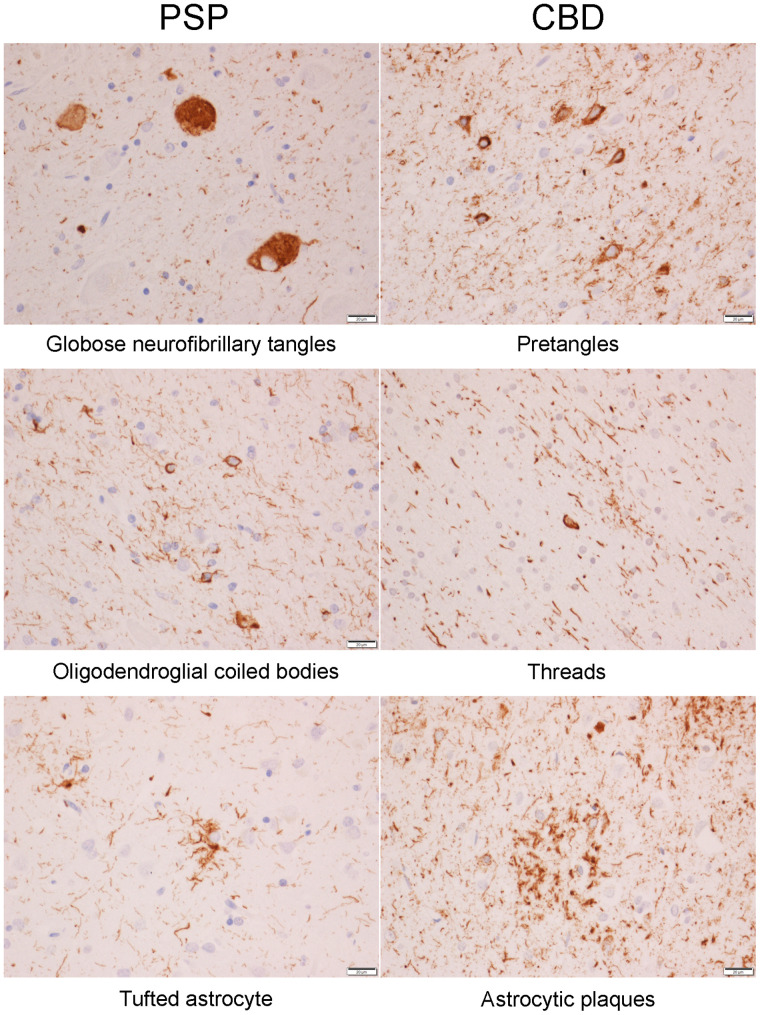
We assessed the burden (lesion count) of tau-specific lesions with anti-CP13 antibodies and immunohistochemistry. Lesions were counted by a single neuropathologist in the cerebellar dentate, midbrain tegmentum, substantia nigra, red nucleus, subthalamic nucleus, ventral thalamus, globus pallidus, striatum, superior frontal cortex, and precentral (motor) cortex. For each of these 10 regions, we determined lesion count for pretangles/neurofibrillary tangles, coiled bodies, tufted astrocytes (PSP only), and astrocytic plaques (CBD only) (Fig. 1) scored using a semiquantitative 4-point scale as follows: 0, absent lesions; 1+, few lesions (1–3 per ×200 field); 2+, moderate number of lesions (4–6 per ×200 field); and 3+, frequent number of lesions (7 or more per ×200 field). The density of neuropil threads was also graded on the same 0–3 scale (Fig. 1). This semiquantitative scale was published in 2006, in an article that displays specific exemplars or representative images for each score, for 3 of the lesions analyzed in this study (10).
FIGURE 1.
Different types of 4R tau lesions in PSP and CBD. Bar at lower right of each image indicates magnification level.
PET Analysis
All participants underwent a standardized MRI protocol at 3 T that included a magnetization-prepared rapid gradient-echo (MPRAGE) sequence. 18F-flortaucipir and Pittsburgh compound B PET scans were acquired using a PET/CT scanner (Discovery MR750; GE Healthcare) (11). All MPRAGE scans were normalized to the Mayo Clinic Adult Lifespan Template and segmented via unified segmentation with Mayo Clinic Adult Lifespan Template priors or settings. 18F-flortaucipir images were coregistered to the MPRAGE using 6°-of-freedom registration in SPM12. Region-level data were calculated for the MPRAGE-space 18F-flortaucipir PET to allow for correlations with histopathology. Our analysis was performed on the hemisphere to match the hemisphere sampled at autopsy. To assess for correlations between 18F-flortaucipir uptake and histology lesion counts, we selected regions of interest that matched the regions assessed at autopsy. The Mayo Clinic Adult Lifespan Template atlas was used to assess uptake in the superior frontal, motor cortex, thalamus, globus pallidus, and striatum; the Deep Brain Stimulation Intrinsic Template (12) atlas for uptake in the substantia nigra, subthalamic nucleus, and red nucleus; and an in-house–developed atlas for the midbrain and cerebellar dentate, as previously described (5). All regional uptake values were divided by median uptake in the cerebellar crus gray matter to calculate SUV ratio (SUVR). A 2-compartment partial-volume correction was performed (13). The analyses were also repeated using PET data without partial-volume correction. Regional volumes were also calculated from the MPRAGE for the same 10 regions to allow for the assessment of potential confounding effects due to atrophy. Global Pittsburgh compound B SUVRs were calculated as previously described (14) and converted to centiloid units as follows: centiloid = 100 × [(−0.1620 + 0.9467 × global Pittsburgh compound B SUVR) − 1.009]/1.067 (15).
Statistical Analysis
For each region, we fit a linear regression model with log-transformed tau PET SUVR as the response and semiquantitative tau lesion burden scores as the predictors of interest. We incorporated the lesion count scores in several ways to have a more complete understanding of their effects. In our first approach, we included the scores for the 4 lesion types as 4 separate predictors in the model. In the second approach, we included the sum of the scores of the preneurofibrillary or neurofibrillary tangles plus tau threads as a first predictor and the sum of the scores of coiled bodies and astrocytic lesions as a second predictor. In the last approach, we summed all 4 semiquantitative scores to form a single numeric composite. We used the natural logarithm as the response in these models to reduce skew, maintain the regression assumption of constant variance, and allow an interpretation of the effect of tau burden in terms of percentage difference in PET uptake. All models included time from PET scan to death, age at death, and total intracranial volume as covariates.
RESULTS
We identified 29 cases that met our inclusion criteria of having had an antemortem 18F-florataucipir PET scan, died with autopsy, and had a pathologic diagnosis of PSP (n = 16) or CBD (n = 13). Table 1 shows the demographic characteristics of all 29, as well as differences between those with PSP and those with CBD. Those with CBD were younger by an average of 3 y at the time of the scan and were more likely to have aging-related tau astrogliopathy and argyrophilic grains disease at death as secondary pathologies.
TABLE 1.
Participant Characteristics
| Characteristic | All (n = 29) | CBD (n = 13) | PSP (n = 16) | P |
|---|---|---|---|---|
| Female | 12 (41%) | 6 (46%) | 6 (38%) | 0.72 |
| Education (y) | 16 (12, 16) | 14 (12, 16) | 16 (14, 16) | 0.29 |
| Age at death (y) | 74 (68, 78) | 68 (60, 74) | 78 (69, 80) | 0.009 |
| Age at tau PET (y) | 70 (67, 77) | 67 (58, 73) | 76 (68, 78) | 0.02 |
| Tau PET to death (y) | 1.3 (0.93, 2.43) | 1.2 (0.93, 1.50) | 1.4 (1.0, 2.5) | 0.71 |
| APOE carrier | 3 (12%) | 1 (9%) | 2 (14%) | >0.99 |
| 22 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 23 | 5 (20%) | 2 (18%) | 3 (21%) | |
| 33 | 17 (68%) | 8 (73%) | 9 (64%) | |
| 34 | 2 (8%) | 0 (0%) | 2 (14%) | |
| 44 | 1 (4%) | 1 (9%) | 0 (0%) | |
| NFT-positive | 27 (93%) | 12 (92%) | 15 (94%) | >0.99 |
| Braak NFT stage | 0.18 | |||
| 1 | 4 (15%) | 0 (0%) | 4 (29%) | |
| 2 | 5 (19%) | 4 (33%) | 1 (7%) | |
| 3 | 9 (35%) | 5 (42%) | 4 (29%) | |
| 4 | 8 (31%) | 3 (25%) | 5 (36%) | |
| 5 | 0 (0%) | 0 (0%) | 0 (0%) | |
| 6 | 0 (0%) | 0 (0%) | 0 (0%) | |
| ARTAG-positive | 18 (64%) | 11 (85%) | 7 (47%) | 0.05 |
| Grain disease–positive | 13 (46%) | 7 (54%) | 6 (40%) | 0.71 |
| MoCA | 18 (10, 22) | 12 (7, 20) | 18 (16, 23) | 0.18 |
| UPDRS III | 56 (44, 72) | 53 (44, 72) | 59 (47, 72) | 0.61 |
| PiB PET SUVRs | 1.43 (1.37, 1.75) | 1.41 (1.37, 1.44) | 1.73 (1.37, 1.93) | 0.30 |
| aβ centiloid | 17 (12, 46) | 16 (12, 18) | 44 (12, 61) | 0.30 |
APOE = apolipoprotein E; NFT = neurofibrillary tangle; ARTAG = aging-related tau astrogliopathy; MoCA = Montreal Cognitive Assessment; UPDRS III = Movement Disorders Society sponsored revision of Unified Parkinson Disease Rating Scale; PiB = Pittsburgh compound B; Aβ = β-amyloid.
Data are median and interquartile range or number and percentage. P values are from Fisher exact test or Wilcoxon rank sum test when appropriate.
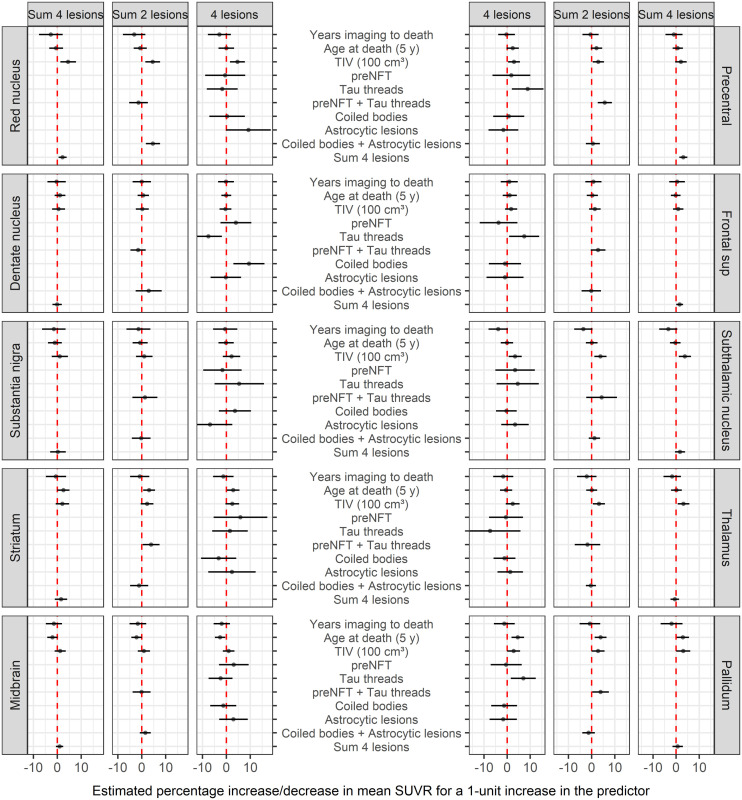
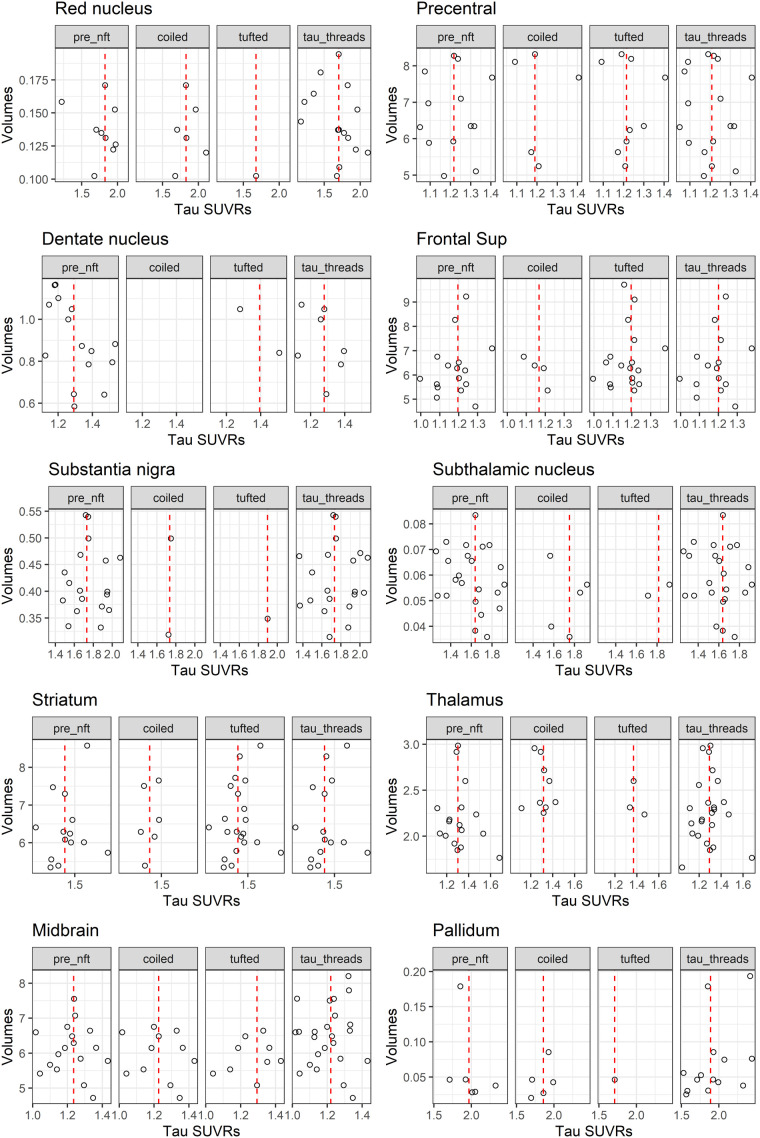
The distribution of pathology across regions is shown in Figure 2. The only associations between tau pathology and 18F-flortaucipir uptake that reached marginal significance were between 18F-flortaucipir uptake and total lesion burden in the red nucleus (P = 0.05) and the precentral gyrus (P = 0.04) (Fig. 3). The association in the red nucleus was driven mainly by glial lesions, that is, astrocytic lesions, whereas the association in the precentral gyrus was driven by neuronal lesions, that is, threads. We also observed a trend for the subthalamic nucleus to show relationships like those of the red nucleus. The results remained the same using PET data without partial-volume correction (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org). There was no evidence that volumes differed by 18F-flortaucipir uptake or that volumes were smaller in those with low uptake (Fig. 4).
FIGURE 2.
Histograms showing distribution of lesion count scores across cases for each region and each tau lesion type.
FIGURE 3.
Relationships between 18F-flortaucipir uptake (SUVR) and lesion type, adjusting for time from imaging to death, age at death, and total intracranial volume. NFT = neurofibrillary tangle; TIV = total intracranial volume.
FIGURE 4.
Scatterplots showing relationship between tau SUVR and volume across regions in cases that had frequent lesion counts (score = 3). Red dashed line represents median tau SUVR. nft = neurofibrillary tangles.
DISCUSSION
In this study of 29 cases of autopsied PSP and CBD (4R tauopathies) with antemortem 18F-flortaucipir imaging, we found marginal associations between ligand uptake and histopathologic lesion count when combining all lesion types in 2 regions only, the red nucleus and the precentral gyrus. The association in the precentral gyrus appeared to be more related to threads, whereas in the red nucleus it appeared to be related to astrocytic lesions.
It is well known that 18F-flortaucipir binds strongly to tau in Alzheimer disease but not to tau in PSP and CBD. Two hypotheses for this differential affinity include binding affinity’s being related to the tau isoform or to the structure of the tau filaments. That is, differential binding of 18F-flortaucipir is related to the ligand’s having stronger affinity for tau enriched with mixed 3R + 4R tau isoforms than for tau enriched with predominantly 4R tau isoforms (3) or to 18F-flortaucipir’s binding better to paired helical filamentous tau that characterizes the tau filaments in Alzheimer disease (3). It is also possible, however, that differential binding is related to ultrastructural differences between the tau in Alzheimer disease and the tau in 4R tauopathies. A recent cryoelectron microscopic study and another study showed that 4R tauopathies have unique tau aggregated ultrastructural confirmations that differ from that of Alzheimer disease (16,17).
Neither threads nor astrocytic lesions in PSP and CBD are enriched with mixed 3R + 4R tau or are characterized by paired helical filaments. The marginal correlations identified in the 2 regions in this study confirm our previous findings (8). However, they do not support 18F-flortaucipir’s having differential affinity for any one lesion type. We are not able to biologically elucidate why there were correlations of significance between ligand uptake and those 2 specific lesion types in red nucleus and precentral gyrus. One explanation could be that there is some Alzheimer (3R + 4R paired helical filament) tau that is codeposited with 4R tau in these 2 regions. An argument against this explanation is that the highest Braak neurofibrillary tangle stage in any of the 29 patients in this study was IV (most were Braak neurofibrillary tangle stages 0–III), and hence there was no Alzheimer-type tau in any of the 10 regions analyzed in this study. Furthermore, we have previously shown that 18F-flortaucipir PET is not sensitive to low levels of tau pathology (8). Another is that we are observing correlations that are influenced by regional off-target binding. This latter explanation is supported by previous studies that have found evidence that 18F-flortaucipir binds to neuromelanin and melanin-containing cells (3,18), iron (19), monoamine oxidase B inhibitors (20), and vascular structures (21), to name a few. It is also a possibility that binding could be related to a yet-to-be-identified pathologic lesion or process that may or may not vary in burden by regions in PSP and CBD.
The comprehensive analyses, including modeling the relationships with and without partial-volume correction, excluded the likelihood that our results were influenced by volume loss. Other strengths to our study include the large number of autopsy-confirmed cases with a 4R tauopathy that had antemortem 18F-flortaucipir and that all underwent semiquantitative scoring by a single neuropathologist with over 30 y of experience with PSP and CBD pathology. Limitations include the fact that a quantitative histologic measure was not used, although semiquantitative scales were also used in the recent international study of the distribution patterns of tau pathology in PSP, which was performed with the intent to inform tau-neuroimaging distribution patterns (22). Other limitations are that the distribution of lesion severity within regions of interest—and the fact that the histologic region of interest represents a small sampled area within the larger 18F-flortaucipir region of interest—could have impacted the ability to detect correlations. Furthermore, PET SUVRs can be influenced by different biologic and technical factors (23).
CONCLUSION
The results of this study do not support differential affinity of 18F-flortaucipir for any histologic lesion, including threads, in 4R tauopathies.
ACKNOWLEDGMENT
We acknowledge the Mayo ADIR Laboratory for PET processing pipelines.
DISCLOSURE
This study was funded by NIH grants RF1-NS112153, R01-DC12519, R01-NS89757, and R01-DC14942. Val Lowe serves on the scientific advisory board of AVID Radiopharmaceuticals. No other potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Is 18F-flortaucipir uptake in 4R tauopathies related to histologic lesion type?
PERTINENT FINDINGS: In this PET pathologic study of 29 autopsied patients who underwent 18F-flortaucipir PET while alive and later died with autopsy-confirmed PSP or CBD, we semiquantified histologic lesion types in multiple brain regions and looked for associations between ligand uptake and lesion type using linear regression. We found only 2 weak associations.
IMPLICATIONS FOR PATIENT CARE. There is little evidence linking 18F-flortaucipir uptake to any one lesion type in 4R tauopathies. Hence, other factors must be considered when designing second and third generations of 4R tau PET ligands.
REFERENCES
- 1. Dickson DW, Bergeron C, Chin SS, et al. Office of Rare Diseases neuropathologic criteria for corticobasal degeneration. J Neuropathol Exp Neurol. 2002;61:935–946. [DOI] [PubMed] [Google Scholar]
- 2. Hauw JJ, Daniel SE, Dickson D, et al. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–2019. [DOI] [PubMed] [Google Scholar]
- 3. Lowe VJ, Curran G, Fang P, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xia CF, Arteaga J, Chen G, et al. [18F]T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimers Dement. 2013;9:666–676. [DOI] [PubMed] [Google Scholar]
- 5. Whitwell JL, Lowe VJ, Tosakulwong N, et al. [18F]AV-1451 tau positron emission tomography in progressive supranuclear palsy. Mov Disord. 2017;32:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Josephs KA, Whitwell JL, Tacik P, et al. [18F]AV-1451 tau-PET uptake does correlate with quantitatively measured 4R-tau burden in autopsy-confirmed corticobasal degeneration. Acta Neuropathol (Berl). 2016;132:931–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McMillan CT, Irwin DJ, Nasrallah I, et al. Multimodal evaluation demonstrates in vivo 18F-AV-1451 uptake in autopsy-confirmed corticobasal degeneration. Acta Neuropathol (Berl). 2016;132:935–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghirelli A, Tosakulwong N, Weigand SD, et al. Sensitivity-specificity of tau and amyloid beta positron emission tomography in frontotemporal lobar degeneration. Ann Neurol. 2020;88:1009–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol (Berl). 2012;123:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Josephs KA, Mandrekar JN, Dickson DW. The relationship between histopathological features of progressive supranuclear palsy and disease duration. Parkinsonism Relat Disord. 2006;12:109–112. [DOI] [PubMed] [Google Scholar]
- 11. Whitwell JL, Ahlskog JE, Tosakulwong N, et al. Pittsburgh compound B and AV-1451 positron emission tomography assessment of molecular pathologies of Alzheimer’s disease in progressive supranuclear palsy. Parkinsonism Relat Disord. 2018;48:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ewert S, Plettig P, Li N, et al. Toward defining deep brain stimulation targets in MNI space: a subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage. 2018;170:271–282. [DOI] [PubMed] [Google Scholar]
- 13. Schwarz CG, Therneau TM, Weigand SD, et al. Selecting software pipelines for change in flortaucipir SUVR: balancing repeatability and group separation. Neuroimage. 2021;238:118259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jack CR, Jr, Wiste HJ, Weigand SD, et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017;13:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schwarz CG, Tosakulwong N, Senjem ML, et al. Considerations for performing level-2 centiloid transformations for amyloid PET SUVR values. Sci Rep. 2018;8:7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang W, Tarutani A, Newell KL, et al. Novel tau filament fold in corticobasal degeneration. Nature. 2020;580:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarutani A, Miyata H, Nonaka T, et al. Human tauopathy-derived tau strains determine the substrates recruited for templated amplification. Brain. 2021;144:2333–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marquié M, Normandin MD, Vanderburg CR, et al. Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann Neurol. 2015;78:787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi JY, Cho H, Ahn SJ, et al. Off-target 18F-AV-1451 binding in the basal ganglia correlates with age-related iron accumulation. J Nucl Med. 2018;59:117–120. [DOI] [PubMed] [Google Scholar]
- 20. Vermeiren C, Motte P, Viot D, et al. The tau positron-emission tomography tracer AV-1451 binds with similar affinities to tau fibrils and monoamine oxidases. Mov Disord. 2018;33:273–281. [DOI] [PubMed] [Google Scholar]
- 21. Saint-Aubert L, Lemoine L, Chiotis K, Leuzy A, Rodriguez-Vieitez E, Nordberg A. Tau PET imaging: present and future directions. Mol Neurodegener. 2017;12:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kovacs GG, Lukic MJ, Irwin DJ, et al. Distribution patterns of tau pathology in progressive supranuclear palsy. Acta Neuropathol (Berl). 2020;140:99–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Adams MC, Turkington TG, Wilson JM, Wong TZ. A systematic review of the factors affecting accuracy of SUV measurements. AJR. 2010;195:310–320. [DOI] [PubMed] [Google Scholar]