Abstract
Metastatic castration resistant prostate cancer (mCRPC) is incurable. The expression of the transmembrane protein prostate-specific membrane antigen (PSMA) is markedly increased in most mCRPC lesions. PSMA has been recognized as a viable biologic target for imaging and radionuclide therapy (theranostics) in mCRPC. The PET agents 68Ga-PSMA-11 and 18F-DCFPyL have recently been approved for imaging evaluation of patients with suspected metastasis who are candidates for initial definitive therapy and patients with suspected recurrence based on elevated serum prostate-specific antigen level. Radioligand therapy (RLT) with 177Lu-PSMA-617 (177Lu-vipivotide tetraxetan, Pluvicto, Novartis/AAA) was approved on March 23, 2022, based on the favorable results of the VISION trial. It has been recognized that PET imaging of PSMA expression and glucose metabolism (with 18F-FDG) provides a more comprehensive assessment of the tumor burden and heterogeneity. However, there are many unresolved issues that surround whether or not imaging with 18F-FDG PET is advantageous in the clinical setting of PSMA RLT in mCRPR.
Keywords: prostate, cancer, PSMA, FDG, VISION
The recently published VISION trial grounded on targeting the prostate-specific membrane antigen (PSMA) and the subsequent approval of 177Lu-PSMA-617 by the Food and Drug Administration (FDA) is a momentous milestone for nuclear medicine, adding to the drive that has been generated over the past decade in the growth of theranostics and radiopharmaceutical therapy in cancer management (1). While metastatic castration-resistant prostate cancer (mCRPC) remains incurable despite significant strides in the development of various drug regimens, PSMA-based radioligand therapy (RLT) provides an additional viable option for prolonging life. According to the definition and spirit of theranostics, it is self-evident that the imaging component is an essential partner for assessing the presence, extent, and intensity of the target expression before commencing the therapy companion in the anticipation of favorable response and acceptable biologic and financial toxicities. It is therefore curious to note that the essential step of PSMA imaging in the theranostics process has been a topic of debate (2,3). However, in this discussion, my focus is on whether or not imaging with 18F-FDG is needed or desired in the clinical setting of PSMA RLT.
PIVOTAL RELEVANCE OF TUMOR HETEROGENEITY
It is recognized that there is remarkable molecular heterogeneity between neoplastic cells in an individual tumor mass, between primary tumor and its metastases, and among the metastases, although it appears that intraindividual genomic diversity is more limited than interindividual genomic diversity (4). The multifeature heterogeneity of mCRPC renders its potential cure exceptionally challenging. It is posited that only when the reality of biologic heterogeneity is taken into full consideration, then there may be opportunities for early suitable therapeutic maneuverers to prolong life substantially, preferably with the least compromise on life quality. We have already encountered the heterogeneity concept in nuclear medicine. An example clinical setting includes patients with metastatic thyroid cancer and a negative radioiodine scan but positive 18F-FDG PET/CT scan. Another similar setting involves patients with neuroendocrine tumors who harbor metastases with discordant somatostatin expression and glucose metabolism. Accordingly, discordance of PSMA expression and 18F-FDG uptake is not unanticipated in mCRPC.
In a recent prospective investigation of a cohort of men with metastatic prostate cancer, there was only 22% concordance between 18F-DCFPyL and 18F-FDG, revealing substantial tumor heterogeneity (5). In another study of men with mCRPC undergoing 177Lu-PSMA-617 RLT, at least 1 mismatch PSMA-negative/18F-FDG–positive (PSMA−/18F-FDG+) metastasis was noted in 59% of patients and this mismatch was associated with a significantly shorter overall survival compared with those patients without mismatch lesions (3.3 vs. 6 mo, P = 0.008) (6). A similar finding was reported in an investigation of 54 men with mCRPC who underwent PSMA PET/CT and 18F-FDG PET/CT at baseline before 177Lu-PSMA-617 RLT. Patients with at least 1 PSMA−/18F-FDG+ metastasis at baseline had significantly lower median overall survival than those without mismatch lesions (6.0 vs. 16.0 mo, P < 0.001) (7). The Australian investigators noted that in patients who were excluded from the 177Lu-PSMA-617 RLT clinical trial based on metastases with low PSMA expression and high 18F-FDG uptake, the outcome was poor, with a short median survival of only 2.5 mo, even if the patients received additional systemic treatments (8). New discordant PSMA−/18F-FDG+ lesions can also develop during 177Lu-PSMA-617. Hartrampf et al. noted that after only 2 cycles of PSMA RLT, new PSMA−/18F-FDG+ metastases developed in 13% of their patients (9). The authors paid particular attention to the newly appearing liver metastases. Liver metastases from prostate cancer are not uncommon, being the second most common site (along with lung) after bone, with a clinically evident macro-metastatic incidence of up to 25% and association with worst prognosis despite therapy (10,11). Most liver metastases (∼80%) are PSMA-avid and amenable to PSMA RLT (12). The lack of sufficient PSMA uptake may be due to either low PSMA expression (e.g., genomic dedifferentiation) or reduced target-to-background ratio in relation to high physiologic hepatic uptake of the radiotracer (e.g., 18F-PSMA-1007). In the investigation by Hartrampf et al., the few PSMA− liver metastases were all 18F-FDG+. Except in 1 case, these lesions were also identified on contrast-enhanced CT. These observations imply that aside from effects of the type of PSMA radiotracer that is used and the available ancillary anatomic imaging information in identifying metastatic lesions, the change in tumor biology early in the PSMA RLT, probably through clonal selection with transdifferentiation from an epithelial phenotype to the more aggressive neuroendocrine phenotype, may affect the efficacy of subsequent RLT cycles and the overall impact on patient outcome (13).
WHAT PREDICTS DISCORDANT PSMA−/18F-FDG+ METASTATIC DISEASE?
Chen et al. noted at least 1 PSMA−/18F-FDG+ lesion in 23.2% of their patients with mCRPC who underwent both 68Ga-PSMA-11 PET/CT and 18F-FDG PET/CT. Multivariate regression analysis revealed that dichotomized thresholding of Gleason score (GS) at 8 and serum prostate-specific antigen (PSA) level at 7.9 ng/mL could predict PSMA−/18F-FDG+ mismatch lesions with no mismatch lesions at GS and PSA levels below the threshold levels, 21.7% mismatch with GS < 8 but PSA ≥ 7.9 ng/mL, and as high as 61.5% mismatch metastases when both GS and serum PSA level were above the threshold values (14). Interestingly, in the M0 CRPC clinical setting, Wang et al. reported that a high Gleason grade group was associated significantly with PSMA−/18F-FDG+ disease. Moreover, they noted that castrate-sensitive metastatic disease was rarely associated with PSMA−/18F-FDG+ lesions (15).
Blood parameters (liquid biopsy) may also be helpful as simple predictors of mismatch lesions. Rosar et al. observed that serum neuron-specific enolase (a cytoplasmic enzyme and a marker for tumors of neuroendocrine origin) concentration was significantly and positively associated with 18F-FDG–avid and low PSMA-expressing metastases in patients with mCRPC (16). A recent systematic review reported that serum neuron-specific enolase correlates with prognosis in patients with progressive mCRPC (17). The LuPSMA trial investigators assessed for prognostic biomarkers that included blood parameters (alkaline phosphatase [ALP], lactate dehydrogenase [LDH]), and imaging (whole-body segmented and quantified tumor volume on PET and EXINI index for bone scan). For 18F-FDG PET/CT, lesions were considered if they displayed SUVs greater than mean hepatic parenchyma uptake plus 2 SDs. For PSMA PET/CT, any lesion with an SUV above 3 was considered. The hazard ratios of prognostic biomarkers for overall survival were 2.6, 2.3, 1.2, 1.1, and 0.89 for 18F-FDG+ tumor volume, bone scan index, LDH, ALP, and mean intensity of PSMA-avid tumor uptake, respectively (18). The 18F-FDG+ tumor volume was the most informative prognostic biomarker.
HOW IS A LESION CHARACTERIZED AS PSMA− AND 18F-FDG+?
The definition of PSMA positivity and 18F-FDG negativity is not standardized. The phase 2 LuPSMA trial defined PSMA positivity when the lesion uptake level as measured by maximum SUV (SUVmax) was at least 1.5 times greater than liver SUV. Patients with any 18F-FDG+ disease and corresponding PSMA uptake lower than the selected positivity definition were excluded (19). With these dual imaging criteria, 16% of the patients were excluded. In the phase 2 TheraP trial, PSMA positivity was defined as an SUVmax of at least 20 at a disease site and greater than 10 at all other measurable sites of metastatic disease. Patients were excluded if there were any PSMA−/18F-FDG+ metastases (10% for PSMA− metastases, 18% for 18F-FDG+ metastases) (20). Despite differing PSMA positivity definitions in the 2 trials, these maneuvers preselected patients with relatively high PSMA-expressing metastases, which enriched the potential for favorable outcome in patients undergoing 177Lu-PSMA-617 RLT in these 2 clinical trials (PSA reduction of 50% or more from baseline or PSA50 in 57% and 66% of patients for LuPSMA and TheraP, respectively). The strategy was successful and supported additional clinical trials including the recently published pivotal randomized open-label phase 3 VISION trial comparing standard care plus 177Lu-PSMA-617 with standard care alone (21).
In the VISION trial, only 68Ga-PSMA-11 PET/CT was performed with the eligibility criteria that the patients harbor at least 1 PSMA+ metastatic lesion (defined as uptake greater than that of liver parenchyma in lesions of any size in any organ system) and no PSMA− lesions (defined as uptake equal to or lower than that of liver parenchyma in any lymph node with a short axis of at least 2.5 cm, in any solid organ lesion with a short axis of at least 1.0 cm, or in any bone lesion with a soft-tissue component of at least 1.0 cm in the short axis). With these imaging selection criteria, 12.6% of patients were excluded after PSMA PET/CT imaging. 18F-FDG PET/CT was not performed. Outcome of PSA50 was noted in 46% of patients. The lower PSA50 in the VISION trial in comparison to those reported in the LuPSMA and TheraP trials may be in part due to the differing imaging-based patient eligibility criteria among the trials. It is probable that at least some patients who were eligible for VISION trial would have been excluded from LuPSMA and TheraP trials. It is interesting to concoct how the results of the VISION trial would have been impacted if the patient eligibility criteria included 18F-FDG PET/CT similar to the LuPSMA and TheraP trials. However, in broader term, it remains to be determined if patients with low PSMA expression and discordant 18F-FDG+ lesions should be excluded from PSMA RLT (7).
PROS AND CONS OF 18F-FDG PET/CT INCLUSION IN PSMA RLT
Imaging evaluation of mCRPC with both 18F-FDG and a PSMA radiotracer will provide a more comprehensive assessment of the tumor burden. However, how the levels of PSMA expression and 18F-FDG discordance should impact PSMA RLT management decisions remain an open debate and will need further investigation. It is reasonable to anticipate that patients with tumors that display moderate PSMA expression but with 18F-FDG discordance may be candidates for combination therapy (PSMA RLT plus chemotherapy, immunotherapy, or androgen deprivation therapy (ADT) in patients with polymetastatic disease or PSMA RLT plus stereotactic body radiation therapy with or without ADT in patients with oligometastatic disease). Interim 18F-FDG PET/CT scanning during a course of PSMA RLT may also provide important information on any evolutionary biologic changes of the tumor sites, which may facilitate the tailoring of the subsequent RLT cycles (in terms of timing and dosage) with or without inclusion of other therapies. Clinical trials may be envisioned to address these matters. In this regard, a standardized method to quantify PSMA PET/CT and 18F-FDG PET/CT scans would be helpful to simplify image analysis and interpretation. A 6-tier image scoring system referred to as Pro-PET score has been proposed, although there has been no external validation (22). There are also proposed semiautomated algorithms that can facilitate quantification of total tumor burden on either PSMA PET/CT or 18F-FDG PET/CT (23–25).
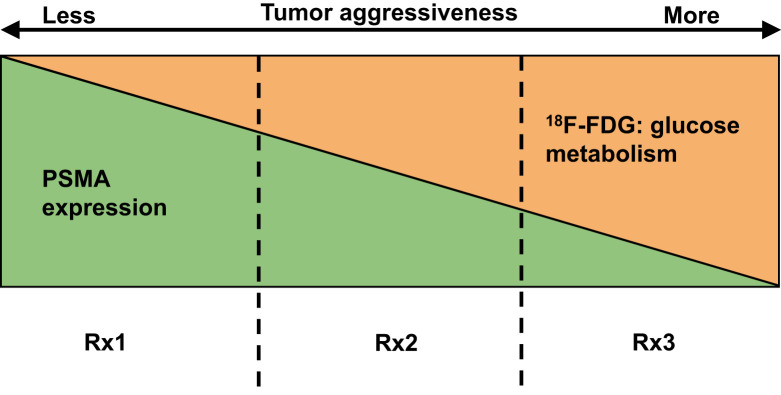
While there are rational motives to include 18F-FDG PET/CT in PSMA RLT, it renders the entire process more complex from multiple points of view. The scans will likely be performed on 2 separate days, which may be inconvenient to patients. The imaging components of the theranostics will need to be interpreted in combination and results provided in a simple standardized format that can inform clinical decision making. While 18F-FDG PET/CT is covered by the Center for Medicare and Medicaid Services (CMS) under the “subsequent treatment strategy” category for prostate cancer, the coverage for PSMA PET/CT has yet to be instituted. It is also unclear if CMS or insurance agencies would be amenable to pay for 2 PET/CT scans in close temporal proximity for the same indication if the outcome benefit for such diagnostic imaging strategy is unestablished. Notwithstanding, the overall cost of imaging will increase, and cost-utility studies will be needed to decipher whether higher cost and incorporation of combined 18F-FDG PET/CT and PSMA PET/CT results improve patient management and outcome. Aside from the important issues of cost and logistics, and as alluded to above, many questions arise that remain unanswered at this time. It is unclear what treatment strategy may be best to treat patients with PSMA−/18F-FDG+ disease (however, this condition ends up being defined) and if these patients should be excluded from PSMA RLT or if some patients may be included as potential candidates for the therapy if PSMA expression can be primed with intervention (e.g., properly timed and dosed ADT) (Fig. 1).
FIGURE 1.
Simplified schematic of spectrum of PSMA and 18F-FDG uptake in mCRPC lesions. Tumor aggressiveness generally increases from left to right, although there can be aggressive tumors without marked hypermetabolism (e.g., neuroendocrine phenotype). Prognosis is also poorer as tumor aggressiveness increases. Vertical dashed lines designate yet-to-be-defined borders of PSMA and 18F-FDG avidity of total tumor burden, which may lead to different therapy strategies. RX1 = in tumors with mainly PSMA+ disease, PSMA RLT may be primary choice of therapy; RX2 = in tumors with mixed PSMA and 18F-FDG avidity, combination therapy (PSMA RLT, chemotherapy, immunotherapy, ADT) may be considered; RX3 = in tumors with low or no PSMA expression and discordant 18F-FDG+ disease, non-RLT may be mainstay strategy, although interventions may be instituted to shift tumor phenotype to left for enabling additional therapeutic approaches that may include PSMA RLT.
POTENTIAL STRATEGY FOR FUTURE
The current evidence suggests that at least in the clinical trial settings, incorporation of both PSMA PET/CT and 18F-FDG PET/CT can be informative and potentially impactful. Post hoc analysis of the pertinent data collected from completed clinical trials can also be contributory. Inclusion of various clinical features such as GS, PSA and its kinetics, prior therapies, and relevant blood indicators may also provide the important stratification parameters for justifying the inclusion or exclusion of 18F-FDG PET/CT imaging in the clinical setting of PSMA RLT.
DISCLOSURE
This study was supported in part by grants R21-EB017568 and P30-CA014089 from the U.S. National Institutes of Health. Hossein Jadvar is on the advisory board of Radiomedix, Inc., is a consultant to Bayer and Blue Earth Diagnostics, and is on the speaker's bureau for Lantheus, all unrelated to this submission. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.U.S. Food and Drug Administration (FDA). FDA approves Pluvicto for metastatic castration-resistant prostate cancer. U.S. FDA website. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castrationresistant-prostate-cancer. Accessed May 16, 2022.. [Google Scholar]
- 2. Srinivas S, Iagaru A. To scan or not to scan: an unnecessary dilemma for PSMA radioligand therapy. J Nucl Med. 2021;62:1487–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calais J, Czernin J. PSMA expression assessed by PET imaging is a required biomarker for selecting patients for PSMA-targeted therapy. J Nucl Med. 2021;62:1489–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kumar A, Coleman I, Morrissey C, et al. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22:369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fourquet A, Rosenberg A, Mena E, et al. A comparison of 18F-DCFPyL, 18F-NaF and 18F-FDG PET/CT in a prospective cohort of men with metastatic prostate cancer. J Nucl Med. 2021;63: 735–741. [DOI] [PubMed] [Google Scholar]
- 6. Khreish F, Ribbat K, Bartholomä M, et al. Value of combined PET imaging with [18F]FDG and [68Ga]Ga-PSMA-11 in mCRPC patients with worsening disease in [177Lu]Lu-PSMA-617 RLT. Cancers (Basel). 2021;13:4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michalski K, Ruf J, Goetz C, et al. Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy. Eur J Nucl Med Mol Imaging. 2021;48:2024–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thang SP, Violet J, Sandhu S, et al. Poor outcomes for patients with metastatic castration-resistant prostate cancer with low prostate-specific membrane antigen (PSMA) expression deemed ineligible for 177Lu-labeled PSMA radioligand therapy. Eur Urol Oncol. 2019;2:670–676. [DOI] [PubMed] [Google Scholar]
- 9. Hartrampf PE, Lapa C, Serfling SE, et al. Development of discordant hypermetabolic prostate cancer lesions in the course of [177Lu]PSMA radioligand therapy and their possible influence on patient outcome. Cancers (Basel). 2021;13:4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ma B, Wells A, Wei L, Zheng J. Prostate cancer liver metastasis: dormancy and resistance to therapy. Semin Cancer Biol. 2021;71:2–9. [DOI] [PubMed] [Google Scholar]
- 11. Singh A, Cheedella NKS, Shakil SA, Gulmi F, Kim DS, Wang JC. Liver metastases in prostate carcinoma represent a relatively aggressive subtype refractory to hormonal therapy and short-term duration response to docetaxel monotherapy. World J Oncol. 2015;6:265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Damjanovic J, Janssen J-C, Prasad V, et al. 68Ga-PSMA-PET/CT for the evaluation of liver metastases in patients with prostate cancer. Cancer Imaging. 2019;19:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bakht MK, Lovnicki JM, Tubman J, et al. Differential expression of glucose transporters and hexokinase in prostate cancer with a neuroendocrine gene signature: a mechanistic perspective for 18F-FDG imaging of PSMA-suppressed tumors. J Nucl Med. 2020;61:904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen R, Wang Y, Zhu Y, et al. The added value of 18F-FDG PET/CT compared to 68Ga-PSMA PET/CT in patients with castration-resistant prostate cancer [abstract]. J Nucl Med. April 23, 2021. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Wang B, Liu C, Wei Y, et al. A prospective trial of 68Ga-PSMA and 18F-FDG PET/CT in nonmetastatic prostate cancer patients with an early PSA progression during castration. Clin Cancer Res. 2020;26:4551–4558. [DOI] [PubMed] [Google Scholar]
- 16. Rosar F, Ribbat K, Ries M, et al. Neuron-specific enolase has potential value as a biomarker for [18F]FDG/[68Ga]Ga-PSMA-11 PET mismatch findings in advanced mCRPC patients. EJNMMI Res. 2020;10:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Muoio B, Pascale M, Roggero E. The role of serum neuron-specific enolase in patients with prostate cancer: a systematic review of the recent literature. Int J Biol Markers. 2018;33:10–21. [DOI] [PubMed] [Google Scholar]
- 18. Ferdinandus J, Violet J, Sandhu S, et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur J Nucl Med Mol Imaging. 2020;47:2322–2327. [DOI] [PubMed] [Google Scholar]
- 19. Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–833. [DOI] [PubMed] [Google Scholar]
- 20. Hofman MS, Emmett L, Sandhu S, et al. ; TheraP Trial Investigators and the Australian and New Zealand Urogenital and Prostate Cancer Trials Group. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. [DOI] [PubMed] [Google Scholar]
- 21. Sartor O, de Bono J, Chi KN, et al. ; VISION Investigators. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med. 2021;385:1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adnan A, Basu S. Concept proposal for a six-tier integrated dual tracer PET-CT (68Ga-PSMA and FDG) image scoring system (‘Pro-PET’ score) and examining its potential implications in metastatic castration-resistant prostate carcinoma theranostics and prognosis. Nucl Med Commun. 2021;42:566–574. [DOI] [PubMed] [Google Scholar]
- 23. O JH, Lim SJ, Wang H, Leal JP, Shu HG, Wahl RL; QIN PET Readers. Quantitation of cancer treatment response by 2-[18F]FDG PET/CT: multi-center assessment of measurement variability using AUTO-PERCIST™. EJNMMI Res. 2021;11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Seifert R, Sandach P, Kersting D, et al. Repeatability of 68Ga-PSMA-HBED-CC PET/CT-derived total molecular tumor volume. J Nucl Med. 2022;63:746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hammes J, Täger P, Drzezga A. EBONI: a tool for automated quantification of bone metastasis load in PSMA PET/CT. J Nucl Med. 2018;59:1070–1075. [DOI] [PubMed] [Google Scholar]