Abstract
Introduction
Owing to the COVID-19 pandemic, there has been significant disruption to all surgical specialties. In the UK, units have cancelled elective surgery and a decrease in aerosol generating procedures (AGPs) was favoured. Centres around the world advocate the use of negative pressure environments for AGPs in reducing the spread of infectious airborne particles. We present an overview of operating theatre ventilation systems and the respective evidence with relation to surgical site infection (SSI) and airborne pathogen transmission in light of COVID-19.
Methods
A literature search was conducted using the PubMed, Cochrane Library and MEDLINE databases. Search terms included “COVID-19”, “theatre ventilation”, “laminar”, “turbulent” and “negative pressure”.
Findings
Evidence for laminar flow ventilation in reducing the rate of SSI in orthopaedic surgery is widely documented. There is little evidence to support its use in general surgery. Following previous viral outbreaks, some centres have introduced negative pressure ventilation in an attempt to decrease exposure of airborne pathogens to staff and surrounding areas. This has again been suggested during the COVID-19 pandemic. A limited number of studies show some positive results for the use of negative pressure ventilation systems and reduction in spread of pathogens; however, cost, accessibility and duration of conversion remain an unexplored issue. Overall, there is insufficient evidence to advocate large scale conversion at this time. Nevertheless, it may be useful for each centre to have its own negative pressure room available for AGPs and high risk patients.
Keywords: Laminar flow, Coronavirus, COVID-19, SARS, General surgery, Operating room
Introduction
Airborne microbial contamination and subsequent infection can occur in the operating theatre.1 Theatre airflow systems and technology have evolved to help reduce this to a minimum. Variations have been in use for over 60 years.2 They can utilise a combination of air filtration, dilution of airborne contaminants, prevention of entry of airborne contaminants into theatre and control of airflow direction.3
Much of the evidence pertaining to airflow in operating theatres is based on studies in orthopaedic surgery owing to the significant implications that surgical site infection (SSI) can have on bone and joint infections, especially in relation to prosthetic implantation.1,4,5 Less emphasis, however, has been placed on theatre airflow in other surgical specialties, including general and colorectal surgery, possibly as a result of so many other factors influencing SSI, particularly in non-elective colorectal surgery.
Over and above the consideration of patients and SSI with respect to theatre airflow and microbial contamination, the recent COVID-19 pandemic has focused minds on the topic of ventilation once more. On this occasion, the issue of staff contamination both in and outside the operating theatre has come to the fore. This international public health emergency, which overwhelmed hospitals and countries alike, caused non-emergency surgery to come to a near standstill in the UK during the initial surge.6 Among the many changes made in theatre practice was the focus on protection of healthcare staff. Much attention has been paid to the provision of appropriate personal protective equipment (PPE) as well as the development of treatment protocols and training initiatives to minimise viral exposure of operating theatre personnel and healthcare providers.7
The importance of aerosol-based transmission was established early during the global pandemic8 with subsequent limitation of aerosol generating procedures (AGPs). A multitude of recommendations emerged including advice regarding use of PPE, consideration of laparoscopic techniques and patient coronavirus testing prior to surgery where available.9
In addition, there have been recommendations made for the use of negative pressure environments, including in operating theatres, particularly for AGPs.6,10 This is relevant because all discussion on measures in theatre to reduce staff exposure must be taken in the context of the ventilation system in question. This paper presents an overview as a primer for surgeons on different ventilation systems in order to help understand and contextualise measures taken to reduce SSI and/or microbial staff exposure in the operating theatre.
Methods
A literature search was conducted using the PubMed, Cochrane Library and MEDLINE® databases. Search terms included “COVID-19”, “theatre ventilation”, “laminar”, “turbulent” and “negative pressure”.
Findings
The majority of operating theatres can be classed as having laminar or conventional/turbulent ventilation. Negative pressure environments were introduced after the severe acute respiratory syndrome (SARS) pandemic in Hong Kong in 2003. This theatre technology is available and may have a role to play following COVID-19.
Laminar airflow
In the UK in the 1960s and 1970s, Sir John Charnley pioneered the use of laminar flow theatre ventilation systems in orthopaedic surgery. He was subsequently able to demonstrate its success in reducing SSI when used in conjunction with other infection reducing strategies.11
Laminar flow design requires installation of either vertical or horizontal air filters supplying clean air via high efficiency particulate air (HEPA) filters.11 The filtered air travels from the operative field to the periphery under positive pressure and is extracted by an exhaust grill.12 Laminar systems can change the air in operating theatres up to 300 times per hour.11
Microbiological sampling methods are used to measure the concentration of microorganisms in an operating theatre. Laminar flow systems supply air containing <10 colony forming units per cubic meter (cfu/m3) whereas conventional ventilation systems are closer to 180cfu/m3.11, 13
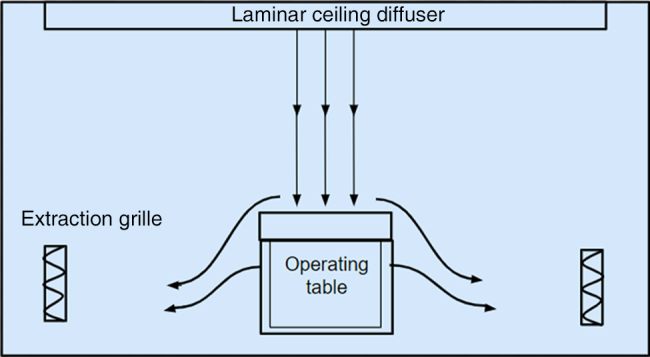
In the vertical design, continuous filtered air is supplied directly on to the operating table,11 resulting in a continuous column of air in a uniform direction and velocity (Figure 1). The air is then dispersed away from the operating table and removed from the theatre under positive pressure. This allows contaminants to be directed away from the clean operating area and prevents contaminants from outside the theatre area entering the room.13
Figure 1 .
Laminar airflow
In horizontal systems, the HEPA filters are installed into one or part of a wall in the operating theatre and with careful planning of staff position, filtered air is supplied in the patient direction.11 Both configurations use filtered air directed towards the patient and subsequently, any airborne contamination from theatre staff is directed away. The general consensus is that vertical laminar flow is superior to horizontal flow.14 In those circumstances where vertical flow may not be an option (economic/architectural issues), horizontal flow is still advocated.
Many other factors can influence wound contamination and SSI; these include sterility of surgical instruments, theatre staff body suits, maintaining normothermia, forced air versus fabric warming, surgical lighting and prophylactic antibiotics.11,13,15 Other considerations are cost and availability, which lie outside the scope of this review.
A large body of evidence exists on the relationship between SSI and use of laminar flow for orthopaedic procedures with prostheses.1,4,5 Despite some controversy regarding its overall effectiveness,4,16 laminar flow is recommended by both the National Institute for Health and Care Excellence,17 and the British Orthopaedic Association.18
Evidence pertaining to general surgery over the use of laminar flow operating theatres is less robust. There are no current recommendations for laminar flow in general surgery from the surgical19 or anaesthetic communities.20 Most studies investigating the use of laminar flow in general surgery have been retrospective, and have failed to demonstrate a benefit in SSI rate in appendicectomy, cholecystectomy, colonic and haemorrhoid surgery.21 Furthermore, a meta-analysis of 63,472 general and vascular surgical procedures also concluded no significant benefit.4
Conventional airflow
Conventional theatre ventilation uses a supply of variably filtered air being introduced to the operating theatre via ceiling diffusers (Figure 2).11 This can be released from multiple ceiling points at different angles depending on installation. The direction is therefore not necessarily uniform or from directly over the operating field, creating turbulent flow.
Figure 2 .
Conventional airflow
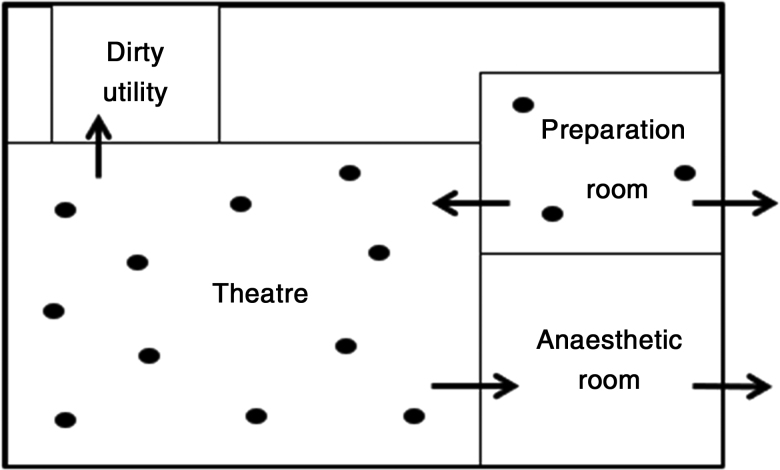
The air is subsequently removed from the room via pressure release dampers (usually at floor level) and any gap in the integrity of the walls (doors/panels).11 The process is dependent on positive pressure to remove air from clean areas (theatre/preparation rooms) to less clean areas (anaesthetic/waste extraction rooms).13
The mechanism of conventional theatre airflow is dilution of airborne contaminants and prevention of introduction of further airborne contaminants from outside the operating theatre.13 The air change rate is generally up to 25 times per hour.11 The US Centres for Disease Control and Prevention recommend at least 15 air changes per hour.22
As described, there is substantial evidence comparing laminar and conventional theatre airflow. However, there is minimal evidence regarding natural ventilation versus conventional ventilation in terms of SSI. In some guidance, natural/window ventilation is acceptable (eg for minor procedures, and with specific window layouts and room sizes).14,23 A study looking at 4 types of ventilation in 277 surgical procedures showed that window ventilation was associated with the highest contamination intraoperatively (13.3cfu/h vs 0.8–6.4cfu/h for other ventilation methods).24
Negative pressure environment
Since the SARS epidemic of 2003, more attention has been focused on adapting operating theatres into negative pressure rooms. This reduces release of infective particles into nearby corridors/wards and is achieved by sealing all doors attached to the theatre as well as installing strong exhaust fans where the original exhausts were located.12 In some areas affected by SARS in 2003, negative pressure theatres were created and kept in use for further infectious outbreaks as well as for patients with influenza and tuberculosis.15 During the COVID-19 pandemic, papers from around the world have once again recommended negative pressure environments in theatre,6,15,25,26 for endoscopy,27 and for intubation28 and extubation of patients.29
There is no literature comparing different operating theatre environments in relation to COVID-19 and transmission to other people. One study of a theatre that converted from a positive to a negative pressure space used simulation to demonstrate a decreased risk of transmissions in nearby areas, although the difference in the dispersion of bacteria when comparing the two environments was minimal.12 A further study by the same authors has shown that negative pressure results in adequate protection of staff members from patient released particles as well as protection of the patient from staff particles.15 The authors also investigated different configurations of medical lamp positioning, which shows a possible effect on bacterial dispersion.15 These studies all took place in a single centre in Hong Kong and the results were based on simulated environments.
Most operating theatres have a positive pressure environment and would therefore require modification to convert to a negative pressure space. This can be a difficult and time consuming process, and access to negative pressure rooms remains an issue.12 In some settings, only anaesthetic and ante-rooms have negative pressure, with the rest remaining in positive pressure.
There is no published direct cost comparison for converting theatre ventilation systems. Cost is dependent on the original ventilation system in place, theatre space and the surrounding ward layout. Consideration must also be given to the time taken for conversion and the delay this would have on patient care. This may result in only hospitals with multiple operating theatres using this as a feasible option.
COVID-19 and surgical procedures
While COVID-19 appears to be largely transmitted by respiratory droplet infection,30 the virus has been detected in other body fluids31,32 although actual infectivity from these has yet to be confirmed. AGPs therefore carry the highest risk of transmission.8 For this reason, limitation of these procedures and full use of PPE is advised9 with the option of having a negative pressure environment in the anaesthetic room.12
Although surgical smoke and the use of diathermy has been of concern, there is no evidence that COVID-19 is transmissible via smoke. Guidance suggests using devices with caution and evacuation/filtration systems attached.9,33,34 There has been no mention of negative pressure consideration or change in theatre ventilation in UK guidance.35
For any surgical procedure, evidence suggests that COVID-19 positive patients have a mortality rate of up to 23% and a 50% pulmonary complication rate.36 This calls for significant consideration to be made regarding the consent and decision making process for both elective and emergency surgery during this time.
Conclusions
The theatre environment in which we operate has evolved over time with a goal of reducing patient exposure to airborne contaminants to improve outcomes. While the theatre environment (and in particular, theatre ventilation) has been investigated in orthopaedic surgery, less focus has been placed on other specialties, with no specific recommendations for general surgery. The recent COVID-19 pandemic has once again directed our attention towards the issue of theatre ventilation, although the emphasis has now shifted from prevention of SSI to prevention of pathogenic exposure to healthcare staff and patients.
A variety of ventilation strategies exist including unventilated treatment rooms, laminar flow, conventional flow and negative pressure environments, each with individual attributes. Recommendations for operating theatre ventilation systems to limit pathogenic or viral exposure of healthcare staff and patients point towards the use of negative pressure theatre spaces. The evidence for this, however, is limited and insufficient to direct the largescale conversion of theatres to negative pressure environments, particularly when considering the cost of conversion, investment of time and loss of theatre activity. It is also difficult to conclude the benefit when no data exist regarding how the use of full PPE alters exposure of healthcare staff in each type of theatre environment.
Nevertheless, operating for many hours in full PPE is exhausting and anecdotally, it is more difficult as communication is poorer than when wearing normal surgical attire. Surgical teams would doubtless be attracted to a theatre ventilation system that permitted them to not have to wear full PPE. A more pragmatic option may be to designate selected negative pressure environments in each hospital trust to be utilised during high risk procedures, AGPs or for high risk patients.
References
- 1.Thomas AM, Simmons MJ. The effectiveness of ultra-clean air operating theatres in the prevention of deep infection in joint arthroplasty surgery. Bone Joint J 2018; 100-B: 1264–1269. 10.1302/0301-620X.100B10.BJJ-2018-0400.R1 [DOI] [PubMed] [Google Scholar]
- 2.Humphreys H, Taylor EW. Operating theatre ventilation standards and the risk of postoperative infection. J Hosp Infect 2002; 50: 85–90. 10.1053/jhin.2001.1126 [DOI] [PubMed] [Google Scholar]
- 3.Hoffman PN, Williams J, Stacey Aet al. Microbiological commissioning and monitoring of operating theatre suites. J Hosp Infect 2002; 52: 1–28. 10.1053/jhin.2002.1237 [DOI] [PubMed] [Google Scholar]
- 4.Bischoff P, Kubilay NZ, Allegranzi Bet al. Effect of laminar airflow ventilation on surgical site infections: a systematic review and meta-analysis. Lancet Infect Dis 2017; 17: 553–561. 10.1016/S1473-3099(17)30059-2 [DOI] [PubMed] [Google Scholar]
- 5.Lidwell OM, Lowbury EJ, Whyte Wet al. Effect of ultraclean air in operating rooms on deep sepsis in the joint after total hip or knee replacement: a randomised study. Br Med J (Clin Res Ed ) 1982; 285: 10–14. 10.1136/bmj.285.6334.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Simone B, Chouillard E, Di Saverio Set al. Emergency surgery during the COVID-19 pandemic: what you need to know for practice. Ann R Coll Surg Engl 2020; 102: 323–332. 10.1308/rcsann.2020.0097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coccolini F, Perrone G, Chiarugi Met al. Surgery in COVID-19 patients: operational directives. World J Emerg Surg 2020; 15: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson EL, Turnham P, Griffin JR, Clarke CC. Consideration of the aerosol transmission for COVID-19 and public health. Risk Anal 2020; 40: 902–907. 10.1111/risa.13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Royal College of Surgeons of England. Updated intercollegiate general surgery guidance on COVID-19. https://www.rcseng.ac.uk/coronavirus/joint-guidance-for-surgeons-v2 (cited December 2020).
- 10.Wexner SD, Cortés-Guiral D, Gilshtein Het al. COVID-19: impact on colorectal surgery. Colorectal Dis 2020; 22: 635–640. 10.1111/codi.15112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James M, Khan WS, Nannaparaju MRet al. Current evidence for the use of laminar flow in reducing infection rates in total joint arthroplasty. Open Orthop J 2015; 9: 495–498. 10.2174/1874325001509010495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chow TT, Kwan A, Lin Z, Bai W. Conversion of operating theatre from positive to negative pressure environment. J Hosp Infect 2006; 64: 371–378. 10.1016/j.jhin.2006.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.International Federation of Infection Control. Laminar flow in orthopaedic operations – essential if you can afford it. https://theific.org/wp-content/uploads/2014/10/016.pdf (cited December 2020).
- 14.GOV.UK. Heating and ventilation of health sector buildings (HTM 03-01). https://www.gov.uk/government/publications/guidance-on-specialised-ventilation-for-healthcare-premises-parts-a-and-b (cited December 2020). [Google Scholar]
- 15.Chow TT, Kwan A, Lin Z, Bai W. A computer evaluation of ventilation performance in a negative-pressure operating theater. Anesth Analg 2006; 103: 913–918. 10.1213/01.ane.0000237404.60614.24 [DOI] [PubMed] [Google Scholar]
- 16.Gastmeier P, Breier AC, Brandt C. Influence of laminar airflow on prosthetic joint infections: a systematic review. J Hosp Infect 2012; 81: 73–78. 10.1016/j.jhin.2012.04.008 [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence. Joint replacement (primary): hip, knee and shoulder. [l] Evidence review for ultra-clean air. https://www.nice.org.uk/guidance/ng157/evidence/i-ultraclean-air-pdf-315756469332 (cited December 2020). [PubMed]
- 18.British Orthopaedic Association. Helping Consultants Get Things Right: The BOA Advisory Book. London: BOA; 2014. [Google Scholar]
- 19.National Institute for Health and Care Excellence. Surgical Site Infections: Prevention and Treatment (NG125). London: NICE; 2019. [PubMed] [Google Scholar]
- 20.Association of Anaesthetists. Infection Prevention and Control 2020. London: AAGBI; 2020. [Google Scholar]
- 21.Brandt C, Hott U, Sohr Det al. Operating room ventilation with laminar airflow shows no protective effect on the surgical site infection rate in orthopedic and abdominal surgery. Ann Surg 2008; 248: 695–700. 10.1097/SLA.0b013e31818b757d [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Environmental infection control guidelines. https://www.cdc.gov/infectioncontrol/guidelines/environmental (cited December 2020).
- 23.Humphreys H, Coia JE, Stacey Aet al. Guidelines on the facilities required for minor surgical procedures and minimal access interventions. J Hosp Infect 2012; 80: 103–109. 10.1016/j.jhin.2011.11.010 [DOI] [PubMed] [Google Scholar]
- 24.Hirsch T, Hubert H, Fischer Set al. Bacterial burden in the operating room: impact of airflow systems. Am J Infect Control 2012; 40: e228–e232. 10.1016/j.ajic.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 25.Ti LK, Ang LS, Foong TW, Ng BS. What we do when a COVID-19 patient needs an operation: operating room preparation and guidance. Can J Anaesth 2020; 67: 756–758. 10.1007/s12630-020-01617-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong J, Goh QY, Tan Zet al. Preparing for a COVID-19 pandemic: a review of operating room outbreak response measures in a large tertiary hospital in Singapore. Can J Anaesth 2020; 67: 732–745. 10.1007/s12630-020-01620-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu PW, Ng SC, Inoue Het al. Practice of endoscopy during COVID-19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE-COVID statements). Gut 2020; 69: 991–996. 10.1136/gutjnl-2020-321185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brown CA, Mosier JM, Carlson JN, Gibbs MA. Pragmatic recommendations for intubating critically ill patients with suspected COVID-19. J Am Coll Emerg Physicians Open 2020; 1: 80–84. 10.1002/emp2.12063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.D’Silva DF, McCulloch TJ, Lim JS et al. Extubation of patients with COVID-19. Br J Anaesth 2020; 125: e192–e195. 10.1016/j.bja.2020.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Coronavirus disease (COVID-19): how is it transmitted? https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted (cited December 2020).
- 31.Coccolini F, Tartaglia D, Puglisi Aet al. SARS-CoV-2 is present in peritoneal fluid in COVID-19 patients. Ann Surg 2020; 272: e240–e242. 10.1097/SLA.0000000000004030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Du RH, Li Bet al. Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect 2020; 9: 386–389. 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karuppal R, Surendran S, Patinharayil Get al. It is time for a more cautious approach to surgical diathermy, especially in COVID-19 outbreak: a schematic review. J Orthop 2020; 20: 297–300. 10.1016/j.jor.2020.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vigneswaran Y, Prachand VN, Posner MCet al. What is the appropriate use of laparoscopy over open procedures in the current COVID-19 climate? J Gastrointest Surg 2020; 24: 1686–1691. 10.1007/s11605-020-04592-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Royal College of Surgeons of England. Recovery of surgical services during and after COVID-19. https://www.rcseng.ac.uk/coronavirus/recovery-of-surgical-services (cited December 2020).
- 36.COVIDSurg Collaborative. Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet 2020; 396: 27–38. 10.1016/S0140-6736(20)31182-X [DOI] [PMC free article] [PubMed] [Google Scholar]