Abstract
Background
The triglyceride glucose (TyG) index has been proposed as a reliable marker of insulin resistance (IR) and an independent predictor of cardiovascular disease risk. However, its prognostic value in patients with acute decompensated heart failure (ADHF) remains unclear.
Methods
A total of 932 hospitalized patients with ADHF from January 1st, 2018 to February 1st, 2021 were included in this retrospective study. The TyG index was calculated as ln [fasting triglyceride level (mg/dL) × fasting plasma glucose level (mg/dL)/2]. Patients were divided into tertiles according to TyG index values. The primary endpoints were all-cause death, cardiovascular (CV) death and major adverse cardiac and cerebral events (MACCEs) during follow-up. We used multivariate adjusted Cox proportional hazard models and restricted cubic spline analysis to investigate the associations of the TyG index with primary endpoints.
Results
During a median follow-up time of 478 days, all-cause death, CV death and MACCEs occurred in 140 (15.0%), 103 (11.1%) and 443 (47.9%) cases, respectively. In multivariate Cox proportional hazard models, the risk of incident primary endpoints was associated with the highest TyG tertile. After adjustment for confounding factors, hazard ratios (HRs) for the highest tertile (TyG index ≥ 9.32) versus the lowest tertile (TyG index < 8.83) were 2.09 (95% confidence interval [CI], 1.23–3.55; p = 0.006) for all-cause death, 2.31 (95% CI, 1.26–4.24; p = 0.007) for CV death and 1.83 (95% CI, 1.18–3.01; p = 0.006) for MACCEs. Restricted cubic spline analysis also showed that the cumulative risk of primary endpoints increased as TyG index increased. When the TyG index was used as a continuous variable, the hazard ratios of the three primary endpoints rapidly increased within the higher range of the TyG index (all cause death, TyG > 9.08; CV death, TyG > 9.46; MACCEs, TyG > 9.87).
Conclusions
The elevated TyG index was independently associated with poor prognosis, and thus would be useful in the risk stratification in patients with ADHF.
Keywords: Triglyceride glucose index, Acute decompensated heart failure, All-cause death, Cardiovascular death, Major adverse cardiac and cerebral events
Introduction
Despite similar clinical presentations, acute decompensated heart failure (ADHF) is a highly heterogeneous syndrome with incompletely understood pathophysiology [1, 2]. In contrast to increasing therapeutic options of chronic heart failure (CHF), no new drug for ADHF has been approved in decades [2, 3]. Considering higher rates of morbidity and mortality in ADHF patients than in those with recently diagnosed HF, it is critical to identify high-risk groups before discharge to improve their prognosis [4, 5]. Previous studies have showed some clinical biomarkers, such as elevated BNP, hyponatremia, anemia, and worsening renal function, are associated with poor outcomes in ADHF [6–9]. However, there are few well-validated biomarkers and effective clinical tools in patients with ADHF [2]. Therefore, appropriate risk stratification of ADHF and further individualization of care needs to be further explored [2, 10].
Insulin resistance (IR), a marker of metabolic disorders and systemic inflammation, is an independent and significant risk factor for HF and cardiovascular (CV) death [11–13]. The homeostasis model assessment (HOMA) has been used as a relatively simple and reliable method for assessing IR in previous studies requiring fasting insulin and glucose [14, 15]. The triglyceride-glucose (TyG) index, combined fasting plasma glucose (FPG) and triglyceride levels (TGs), was firstly proposed by Unger G et al. in 2013 as an alternative indicator of IR [16]. Many studies have demonstrated that the TyG index outperformed the HOMA in assessing IR [17–19]. A large number of studies have indicated that the TyG index is positively correlated with the incidence rates of carotid artery atherosclerosis, coronary artery disease, hypertension, myocardial infarction and other cardiovascular diseases [20–24]. Meanwhile, the TyG index is also a reliable and convenient predictor of adverse prognosis in patients with cardiovascular disease [25–27].
However, there are limited clinical studies assessing the TyG index in ADHF. Therefore, we conducted a retrospective cohort study to investigate the relationship between the TyG index and the prognosis in ADHF patients.
Materials and methods
Study population
This is a single-center retrospective analysis of 1376 consecutive ADHF patients admitted to our hospital from January 1st, 2018 to February 1st, 2021. ADHF was defined according to the definitions established in the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure [28]. Of the 1376 patients, 444 were excluded for meeting the exclusion criteria, i.e., (1) lack of data at admission, (2) acute coronary syndrome, (3) advanced cancer, (4) lost to follow up. Finally, 932 patients were included in this study (Fig. 1). This retrospective study was performed in line with the Declaration of Helsinki, with the approval from the ethics committee of Nanjing Drum Tower Hospital.
Fig. 1.
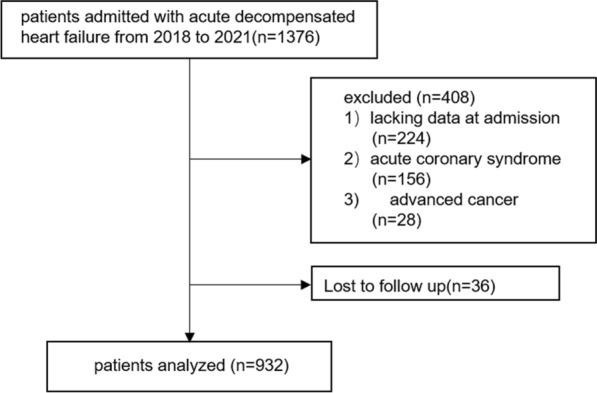
Flow diagram of patient selection
Data collection and definitions
Patient demographics, medical history, laboratory test results, echocardiographic data and medications at admission were collected from the electronic medical recording system by trained physicians. The first group of peripheral venous blood samples was collected after overnight fasting (> 8 h) and was measured in the laboratory department. Body mass index (BMI) was defined as weight (kg)/ (height [m]2). Hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg, any use of the antihypertensive drugs, or a having a history of hypertension. Diabetes mellitus (DM) was defined as fasting plasma glucose (FPG) ≥ 126 mg/dL or hemoglobin A1c (HbA1c) ≥ 6.5%, or a self-reported history of diabetes [29]. Hyperlipidemia was defined as fasting total cholesterol (TC) ≥ 240 mg/dL, low-density lipoprotein cholesterol (LDL-C) > 160 mg/dL, TGs ≥ 150 mg/dL, high-density lipoprotein cholesterol (HDL-C) < 40 mg/dL, or previous use of lipid-lowering drugs.
Hemoglobin, hematocrit, uric acid, red blood cell distribution width (RDW), FPG, HbA1c, total cholesterol, triglycerides, LDL-C, HDL-C, creatinine, C-reactive protein (CRP), B-type natriuretic peptide (BNP), white blood cell count (WBC), serum sodium, serum potassium, albumin (ALB), alanine aminotransferase (ALT) and aspartate transaminase (AST) were measured.
TyG calculation
Endpoints and follow-up
Three predefined primary endpoints were examined, including (1) all-cause death, defined as CV death or non-CV death; (2) CV death, defined as fatal stroke and myocardial infarction (MI), congestive heart failure, malignant arrhythmia, or other structural or functional cardiac diseases; (3) major adverse cardiac and cerebral events (MACCEs), defined as non-fatal MI, non-fatal stroke, or worsening of heart failure. Non-fatal stroke included ischemic and hemorrhagic strokes.
Patients were followed up by telephone and/or clinic visits every six months by well-trained doctors.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and median [interquartile range (IQR)] for those with normal and skewed distributions, respectively. Categorical variables were expressed as number (percentage).
The patients were divided into tertiles according to their TyG index levels: tertile 1 (T1), TyG index < 8.83; tetile 2 (T2), TyG index ≥ 8.83 and < 9.32; tertile 3 (T3), TyG index ≥ 9.32. Continuous variables were compared by analysis of variance (ANOVA) or the Kruskal–Wallis test among the three groups. Categorical variables were compared by the χ2 test among groups.
The cumulative event-free survival rates of the three endpoints were analyzed using the Kaplan–Meier plots and the log-rank test. Additionally, to rule out the effects of confounding factors, adjusted Kaplan–Meier survival curves were generated. Adjusted survival curves were obtained using non-parametric estimated weights [30–32]. Multivariate Cox proportional hazards models were applied to test the associations of the TyG index with the incidence rates of the three primary outcomes. Visualization of Schoenfeld residuals were used to validate the assumptions of proportional hazards before these analyses. The baseline variables with p < 0.1 or clinically significant were selected and included in the Cox proportional hazards models. Multicollinearity was tested in the multivariate models with a threshold of variance inflation factor < 4. Finally, four multivariable regression models were remained: Model 1, adjustment for age, sex, BMI, systolic blood pressure, diastolic blood pressure and heart rate; Model 2, adjustment for variables included in Model 1 plus HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C and LVEF; Model 3, adjustment for variables included in Model 2 plus history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation and hyperlipidemia; Model 4, adjustment for variables included in Model 3 plus statin, aldosterone antagonist, digoxin, diuretic, β-lockers, antiplatelet agent and/or angiotensin-converting enzyme inhibitor (ACEI)/angiotensin receptor inhibitors (ARB)/angiotensin receptor neprilysin inhibitor (ARNI), insulin, sodium-glucose contrasporter-2 inhibitor (SGLT2i), and metformin uses. Hazard ratios (HRs) were calculated, and the results were reported as HRs and 95% confidence intervals (CIs). The lowest tertile of the TyG index was used as a reference in the four models. A restricted cubic spline analysis was performed to reflect the dose–response relationship between the TyG index and the risk of the three primary outcomes. We also conducted subgroup analyses, including age (< 70 versus ≥ 70 years), sex (male versus female), BMI (< 24 versus ≥ 24 kg/m2), and history of diabetes mellitus (no versus yes). Interactions between subgroups were tested by the likelihood ratio test. All data were analyzed with R version 4.1.0 and SPSS for windows version 26, and p < 0.05 was considered to indicate statistical significance.
Results
General patient characteristics
The baseline clinical characteristics of the included patients by TyG tertile are presented in Table 1. The median follow-up time was 478 days. The mean age of the participants was 69 years, and 37.9% participants were female. The proportions of individuals with previous hypertension, ischemic cardiomyopathy (IMD), DM and hyperlipidemia were significantly higher in the highest TyG index tertile, as well as BMI, FPG, HbA1C, CRP, UA, hematocrit, WBC, ALB, total cholesterol, triglyceride, LDL-C and hemoglobin levels; antiplatelet agent, statin, insulin, metformin, sodium-glucose cotransporter 2 inhibitor (SGLT2i) and other hypoglycemic drugs uses were also enhanced (all p < 0.05). Meanwhile, the highest TyG index tertile had younger age; lower HDL-C, RDW, sodium, left atrial diameter (LAD) and LVEF, and lower proportions of valvular heart disease, atrial fibrillation and anemia (all p < 0.05).
Table 1.
Baseline Clinical Characteristics by TyG Group
| Characteristic | Total | Tertile I (Lowest) (n = 310) TyG < 8.83 |
Tertile II (Median) (n = 309) 8.83 ≤ TyG < 9.32 |
Tertile III (Highest) (n = 313) TyG ≥ 9.32 |
p Value | |
|---|---|---|---|---|---|---|
| Female, n (%) | 353(37.9) | 95(26.9) | 133(37.7) | 125(35.4) | < 0.001 | ## |
| Age (years) | 70(61,80) | 73(64,82) | 71(63,79) | 67(56,76) | < 0.001 | #,***,&& |
| BMI (kg/m2) | 24.2(21.6,27.1) | 23.3(20.5,26.1) | 24.1(21.5,26.5) | 25.2(22.7,28.1) | < 0.001 | #,***,&& |
| Heart Rate (bpm) | 81(70,97) | 80(68,95) | 81(70,97) | 84(71,98) | 0.261 | |
| SBP (mmHg) | 129(114,145) | 127(114,147) | 128(113,142) | 130(116,144) | 0.456 | |
| DBP (mmHg) | 75(66,87) | 75(65,85) | 75(66,87) | 76(67,87) | 0.507 | |
| Medical history, n (%) | ||||||
| Ischemic cardiomyopathy | 347(37.2) | 105(33.9) | 99(32) | 143(45.7) | 0.001 | *,&& |
| None-ischemic cardiomyopathy | 197(21.1) | 53(17.1) | 74(23.9) | 70(22.4) | 0.091 | |
| Valvular heart disease | 174(18.7) | 70(22.6) | 60(19.4) | 44(14.1) | 0.022 | * |
| Atrial fibrillation | 404(43.3) | 169(54.5) | 139(45.0) | 96(30.7) | < 0.001 | ***,&&& |
| Hypertension | 563(60.4) | 172(55.5) | 186(60.2) | 205(65.5) | 0.038 | * |
| Hyperlipidemia | 72(43.3) | 5(1.6) | 20(6.5) | 47(15.0) | < 0.001 | #,***,&& |
| Diabetes mellitus | 306(32.8) | 64(20.6) | 83(26.9) | 159(50.8) | < 0.001 | ***,&&& |
| Anemia | 429(46.0) | 177(57.3) | 134(44.1) | 118(37.9) | < 0.001 | ##,* |
| Hemoglobin(g/L) | 128.0(110.1,142.4) | 125.0(107.6,138.1) | 129.5(111.3,141.8) | 132.0(114.0,145.0) | < 0.001 | *** |
| FPG (mg/dL) | 91.8(81.9,112.0) | 82.6(75.6,91.1) | 92.2(83.9,104.9) | 118.44(92.2,151.7) | < 0.001 | ###,***,&&& |
| HbA1c (%) | 6.1(5.6,7.0) | 6.0(5.6,6.4) | 6.1(5.6,6.8) | 6.8(5.8,7.9) | < 0.001 | ***,&&& |
| BNP (pg/mL) | 589.5(307.8,1230.0) | 591.5(339.3,1167.5) | 606(123.5,1088.5) | 552.0(177.3,926.8) | 0.476 | |
| CRP (mg/L) | 4.5(2.9,12.4)) | 4.4(2.9,9.3) | 4.6(3.0,14.7) | 4.4(3.0,14.5) | 0.330 | |
| Hematocrit (%) | 38.4(33.8,42.2) | 37.3(33.1,41.1) | 38.7(34.1,42.1) | 39.10(35.1,43.2) | 0.001 | *** |
| ALB (g/L) | 38.2(35.4,40.5) | 37.4(35.1,39.2) | 38.4(36.0,40.8) | 39.1(35.5,41.4) | < 0.001 | #,*** |
| UA (μmol/L) | 439.0(343.25,550.75) | 419.5(328.3,528.3) | 430.0(325.0,558.5) | 448(372.5,555.0) | 0.033 | * |
| Creatinine (μmol/L) | 84.3(67.0,113.0) | 84.4(69.1,107.1) | 87.0(65.3,113.0) | 85.10(65.7,119.8) | 0.971 | |
| Sodium (mmol/L) | 138.3(135.5,141.5) | 138.3(135.4,141.4) | 139.0(135.8,141.8) | 138.2(135.2,141.2) | 0.458 | |
| Potassium (mmol/L) | 3.9(3.6,4.2) | 3.9(3.5,4.2) | 3.9(3.6,4.2) | 3.90(3.6,4.3) | 0.707 | |
| WBC (10^9/L) | 6.2(5.0,7.8) | 5.5(4.5,6.9) | 6.1(5.1,7.7) | 6.95(5.7,8.7) | < 0.001 | ###,***,&&& |
| RDW (%) | 13.7(12.9,13.7) | 14.1(13.2,15.1) | 13.6(12.9,14.7) | 13.4(12.8,14.3) | < 0.001 | ***,& |
| Total cholesterol (mg/dL) | 138.16(114.07,170.15) | 120.36(103.93,145.11) | 141.26(118.71,168.60) | 156.74(129.93,188.90) | < 0.001 | ###,***,&&& |
| Triglyceride (mg/dL) | 90.27(29.00,54.91) | 61.07(22.43,30.55) | 92.04(35.19,46.40) | 149.57(50.08,83.91) | < 0.001 | ###,***,&&& |
| HDL-C (mg/dL) | 37.15(29.78,46.79) | 40.25(33.06,50.46) | 38.70(30.55,49.50) | 33.28(27.07,40.60) | < 0.001 | ###,*** |
| LDL-C (mg/dL) | 77.79(58.05,101.32) | 65.79(50.46,82.75) | 79.72(60.71,103.25) | 89.78(65.74,116.01) | < 0.001 | ###,***,&& |
| AST (U/L) | 23.0(17.3,33.6) | 23.1(17.5,32.8) | 24.0(17.5,33.0) | 21.60(17.1,35.4) | 0.568 | |
| ALT (U/L) | 19.4(12.9,32.5) | 18.6(11.9,28.2) | 19.5(13.1,31.9) | 20.60(14.1,38.9) | 0.027 | |
| IVSTD (cm) | 0.9(0.8,1.0) | 0.9(0.8,1.0) | 0.90(0.8,1.0) | 0.90(0.8,1.1) | 0.357 | |
| LVPWTD (cm) | 0.9(0.8,1.0) | 0.90(0.8,1.0) | 0.90(0.8,1.0) | 0.90(0.8,1.0) | 0.173 | |
| LVDD (cm) | 5.9(5.2,6.5) | 5.8(5.1,6.5) | 5.9(5.2,6.5) | 5.9(5.2,6.7) | 0.270 | |
| LAD (cm) | 4.8(4.4,5.3) | 5.0(4.5,5.5) | 4.8(4.5,5.3) | 4.7(4.2,5.2) | < 0.001 | #,***,& |
| LVEF, n (%) | 0.048 | * | ||||
| ≤ 40% | 493(52.9) | 144(46.5) | 168(54.4) | 181(57.8) | - | |
| 41% ~ 49% | 154(16.5) | 63(20.3) | 46(14.9) | 45(14.4) | - | |
| ≤ 50% | 285(30.6) | 103(33.2) | 95(30.7) | 87(27.8) | - | |
|
Medications at admission, n (%) |
||||||
| Antiplatelet agent | 412(44.2) | 116(37.4) | 135(43.7) | 161(51.4) | 0.002 | ** |
| ACEI/ARB/ARNI | 150(16.1) | 52(16.8) | 43(13.9) | 55(17.6) | 0.428 | |
| Beta-blocker | 726(77.9) | 236(76.1) | 242(78.3) | 248(79.2) | 0.633 | |
| Statins | 509(54.6) | 154(49.7) | 164(53.1) | 191(61.0) | 0.014 | * |
| Diuretics | 819(87.9) | 270(87.1) | 281(90.9) | 268(53.7) | 0.103 | |
| Loop | 810(86.9) | 268(86.5) | 279(90.0) | 263(84.0) | 0.072 | |
| Thiazide | 51(5.5) | 19(6.1) | 15(4.9) | 17(5.4) | 0.784 | |
| Digoxin | 102(10.9) | 38(12.3) | 24(7.8) | 40(12.8) | 0.079 | |
| Aldosterone antagonist | 375(40.2) | 128(41.3) | 128(41.4) | 119(38.0) | 0.616 | |
| Insulin | 211(22.6) | 41(13.2) | 58(18.8) | 112(35.8) | < 0.001 | **&& |
| Metformin | 106(11.4) | 15(4.8) | 32(10.4) | 59(18.8) | < 0.001 | ##,***&& |
| Sulfonylureas | 4(0.4) | 1(0.1) | 1(0.1) | 2(0.2) | 0.785 | |
| SGLT2i | 27(2.9) | 5(1.6) | 5(1.6) | 17(1.8) | 0.005 | **,&& |
| Other hypoglycemic drugs | 172(18.5) | 34(3.6) | 43(4.6) | 95(10.2) | < 0.001 | ***,&& |
BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, BNP B-type natriuretic peptide, CRP C-creative protein, ALB albumin, UA uric acid, Cr creatinine; Na sodium; K potassium, WBC white blood cell, RDW red blood cell distribution width, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein-C, AST alanine aminotransferase, ALT aspartate transaminase; IVSTD interventricular septal thickness at diastole, LVPWTD left ventricular posterior wall end-diastolic thickness, LVDD left ventricular end-diastolic diameter, LAD left atrial diameter, LVEF left ventricular ejection fraction, ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin receptor inhibitor, ARNI angiotensin receptor neprilysin inhibitor, SGLT2i Sodium-glucose cotransporter 2 inhibitor
#P < 0.05, ##P < 0.01, ### P < 0.001: T1 group vs T2 group
*P < 0.05, **P < 0.01, *** P < 0.001: T1 group vs T3 group
&P < 0.05, &&P < 0.01, &&& P < 0.001: T2 group vs T3 group
Predictive ability of the TyG index for the primary outcomes
During the follow-up period, all-cause death was found in 140 (15.0%) cases, CV death occurred in 103 (11.1%) cases and MACCEs were detected in 443 (47.9%) cases. The patients who died included 45 (14.5%) in the lowest tertile, 42 (13.6%) in the median tertile and 53 (16.9%) in the highest tertile. The patients who died from cardiovascular events included 34 (11%) in the lowest tertile, 28 (9.1%) in the median tertile and 41 (13.1%) in the highest tertile. Individuals with major adverse cardiac and cerebral events included 156 (51.1%) in the lowest tertile, 130 (42.3%) in the median tertile and 157 (50.2%) in the highest tertile.
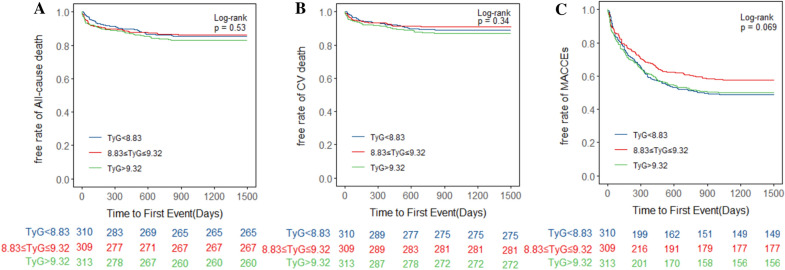
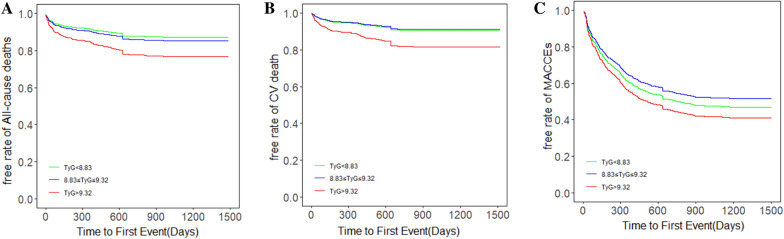
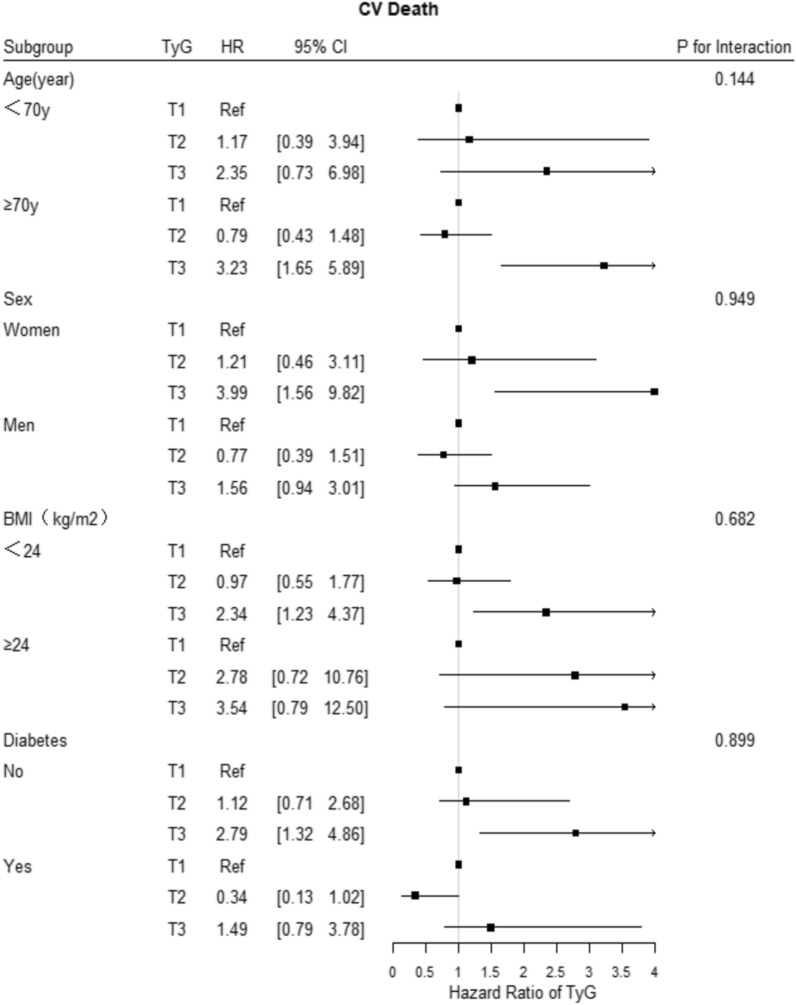
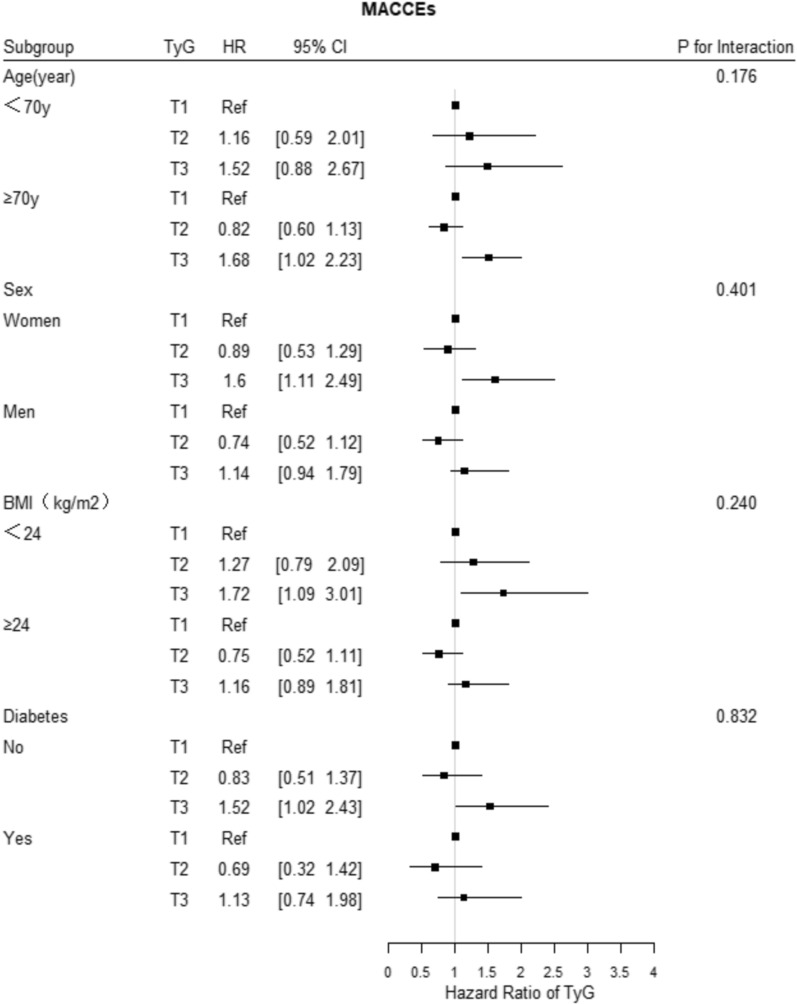
The unadjusted Kaplan–Meier curves demonstrated no significant differences among tertiles in the three predefined primary endpoints (Fig. 2). However, after adjusting for variables in Model 4, we draw the adjusted survival curves, which showed marked differences among the three TyG groups and three endpoints (Fig. 3). Table 2 showed the four multivariate Cox proportional hazards models utilized to test the correlations between the TyG index and primary outcomes. Four Cox proportional hazards models were established for sensitivity analysis. Consequently, the highest tertile of the TyG index was associated with higher incidence of all-cause death and cardiovascular death (all-cause death, adjusted HR = 2.09, 95% CI 1.23–3.55, p = 0.006; cardiovascular death, adjusted HR = 2.31, 95% CI 1.26–4.24, p = 0.007). Meanwhile, there were no significant differences between the lowest and median tertile groups. The incidence of MACCEs was significantly different among tertiles in Model 2, Model 3 and Model 4 (Model 2, T2 adjusted HR = 1.65, 95% CI 1.18–2.30, p = 0.003; T3 adjusted HR = 1.71, 95% CI 1.15–2.58, p = 0.008; Model 3, T2 adjusted HR = 1.64, 95% CI 1.17–2.31, p = 0.004; T3 adjusted HR = 1.72, 95% CI 1.13–2.62, p = 0.012; Model 4, T2 adjusted HR = 1.57, 95% CI 1.11–2.78, p = 0.023; T3 adjusted HR = 1.83, 95% CI 1.18–3.01, p = 0.006), while no significant differences were observed in Model 1.
Fig. 2.
Kaplan–Meier analysis of A All-cause death, B CV death and C MACCEs in various TyG groups. CV, cardiovascular; MACCEs, major adverse cardiac and cerebral events; TyG, triglyceride glucose index
Fig. 3.
Adjusted survival curves for A All-cause death, B CV death and C MACCEs in different TyG groups after adjustment for age, sex, TyG index, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C, LVEF, history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation and hyperlipidemia, and statin, aldosterone antagonist, digoxin, diuretic, β-blocker, antiplatelet agent, ACEI/ARB/ARNI, insulin, sodium-glucose contrasporter-2 inhibitor, and metformin uses. CV, cardiovascular; MACCEs, major adverse cardiac and cerebral events; TyG, triglyceride glucose index
Table 2.
HR (95% CI) of primary outcomes according to TyG index in the four Models
| Model1 | Model2 | Model3 | Model4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcomes | TyG groups | Events, n(%) | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value |
| All-cause death | T1 | 45(14.5) | Ref. | Ref. | Ref. | Ref. | ||||
| T2 | 42(13.6) | 1.11(0.73–1.69) | 0.628 | 1.04(0.67–1.63) | 0.848 | 1.07(0.68–1.67) | 0.765 | 1.07(0.67–1.1.74) | 0.761 | |
| T3 | 53(16.9) | 1.70(1.13–2.57) | 0.011 | 1.75(1.08–2.83) | 0.023 | 1.83(1.12–3.01) | 0.015 | 2.09(1.23–3.55) | 0.006 | |
| CV death | T1 | 34(11.0) | Ref. | Ref. | Ref. | Ref. | ||||
| T2 | 28(9.1) | 0.97(0.59–1.60) | 0.918 | 0.93(0.55–1.58) | 0.801 | 0.91(0.53–1.56) | 0.736 | 0.93(0.52–1.65) | 0.802 | |
| T3 | 41(13.1) | 1.61(1.01–2.58) | 0.046 | 1.89(1.08–3.28) | 0.025 | 1.91(1.08–3.37) | 0.026 | 2.31(1.26–4.24) | 0.007 | |
| MACCEs | T1 | 156(51.1) | Ref. | Ref. | Ref. | Ref. | ||||
| T2 | 130(42.3) | 1.21(0.91–1.60) | 0.186 | 1.65(1.18–2.30) | 0.003 | 1.64(1.17–2.31) | 0.004 | 1.57(1.11–2.78) | 0.023 | |
| T3 | 157(50.2) | 1.32(0.96–1.83) | 0.083 | 1.71(1.15–2.58) | 0.008 | 1.72(1.13–2.62) | 0.012 | 1.83(1.18–3.01) | 0.006 | |
CI confidence interval, TyG triglyceride glucose index, HR hazard ratio
Model 1: adjusted for gender, age, body mass index, systolic blood pressure, diastolic blood pressure and heart rate;
Model 2: adjusted for Model 1 + HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C and LVEF;
Model 3: adjusted for Model 2 + history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation and hyperlipidemia;
Model 4: adjusted for Model 3 + use of statins, aldosterone antagonist, digoxin, diuretics, β-Blockers, antiplatelet agent, ACEI/ARB/ARNI, insulin, sodium-glucose contrasporter-2 inhibitor, and metformin
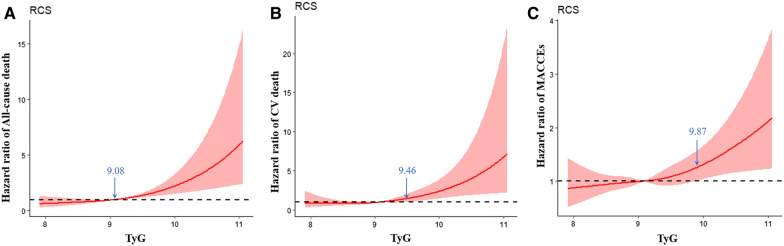
The TyG index as a continuous variable
In Fig. 4, the restricted cubic spline models showed that the risk of the three endpoints was initially flat and then rapidly increased. The risk of all-cause mortality increased rapidly for TyG index above 9.08 (HR per SD increase = 1.48, 95% CI 1.24–1.76, p for non-linearity = 0.001). For TyG index above 9.46, the hazard ratio of CV death per standard deviation of the TyG index was 2.06, (95%CI 1.31–3.21, p for non-linearity = 0.003). The turning point of the TyG index was 9.87 for the risk of MACCEs (HR per SD increase = 1.56, 95% CI 1.14–2.12, p for non-linearity = 0.034).
Fig. 4.
Multivariable-adjusted hazard ratios for A All-cause death, B CV death and C MACCEs based on restricted cubic spines for the TyG index. Red lines represented references for hazard ratios, and red areas represent 95% confidence intervals. The model was adjusted for age, sex, TyG index, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C, LVEF, history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation, hyperlipidemia, and statin, aldosterone antagonist, digoxin, diuretic, β-blocker, antiplatelet agent, ACEI/ARB/ARNI, insulin, sodium-glucose contrasporter-2 inhibitor, and metformin uses
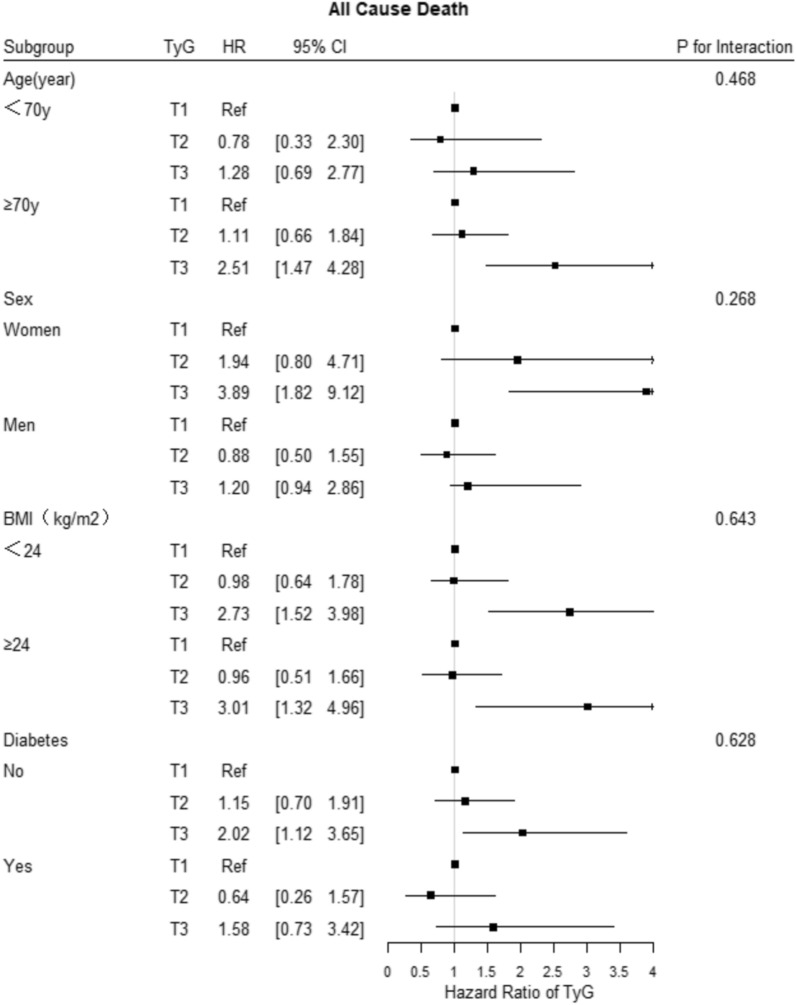
Subgroup analysis
The subgroup analysis showed that the associations of the TyG index tertile with the risk of the three primary outcomes were consistent across the subgroups, including age, sex, BMI, LVEF, and history of diabetes mellitus (Figs. 5, 6, 7). In addition, the more significant trends of the TyG-index were observed among females for the incidence rates of all-cause death and cardiovascular death. Meanwhile, there were no interactions between the TyG index and the variables in subgroup analyses (all p values for interaction > 0.05).
Fig. 5.
Forest plot of all-cause death according to different subgroups. Adjusted model included age, sex, TyG index, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C, LVEF, history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation, hyperlipidemia, and statin, aldosterone antagonist, digoxin, diuretic, β-blocker, antiplatelet agent, ACEI/ARB/ARNI, insulin, sodium-glucose contrasporter-2 inhibitor, and metformin uses
Fig. 6.
Forest plot of CV death according to different subgroups. Adjusted model included age, sex, TyG index, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C, LVEF, history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation, hyperlipidemia, and statin, aldosterone antagonist, digoxin, diuretic, β-blocker, antiplatelet agent, ACEI/ARB/ARNI, insulin, sodium-glucose contrasporter-2 inhibitor, and metformin uses
Fig. 7.
Forest plot of MACCEs according to different subgroups. Adjusted model included age, sex, TyG index, body mass index, systolic blood pressure, diastolic blood pressure, heart rate, HbA1C, C-creative protein, hematocrit, red blood cell distribution width, BNP, sodium, albumin, creatinine, uric acid, ALB, HDL-C, LVEF, history of hypertension, ischemic cardiomyopathy, diabetes mellitus, valvular heart disease, atrial fibrillation, hyperlipidemia, and statin, aldosterone antagonist, digoxin, diuretic, β-blocker, antiplatelet agent, ACEI/ARB/ARNI, insulin, sodium-glucose contrasporter-2 inhibitor, and metformin uses
Discussion
In this retrospective research, we first demonstrated the associations of the TyG index with the risk of all-cause death, CV death, and MACCEs (including non-fatal MI, non-fatal stroke, and rehospitalization due to HF) in patients with ADHF. We found that patients with elevated TyG index were at greater risk of adverse endpoints, regardless of their diabetic status. The results indicated that the TyG index was an independent predictor of adverse events in ADHF patients. Most significantly, this research proposed a simple and efficient method for evaluating IR to optimize risk stratification of mortality and cardiovascular disease recurrence in ADHF patients.
Insulin resistance, TyG index, and CVD risk
Epidemiological studies have shown that ADHF is a prevalent and serious condition with significant morbidity and mortality, imposing a growing global public health burden [33]. We urgently need to explore novel biomarkers, mechanisms, and targeted measures in ADHF. Previous studies have shown that IR is prevalent in patients with HF, and precedes the development of heart failure [34]. IR is also an indicator of heart failure and heart function deterioration [35, 36].
IR is defined as a clinical or experimental condition in which glucose uptake and utilization are impaired [37]. According to previous studies, IR is associated with cardiovascular risk in different populations by inducing imbalances in glucose and lipid metabolism, and triggering oxidative stress and inflammatory response, endothelial dysfunction, and ectopic lipid accumulation [15, 37–39]. HOMA-IR is a comparatively extensive method for IR assessment [14]. The TyG index is strongly correlated with HOMA-IR and the hyperinsulinemic-euglycemic clamp (HIEC), even outperforming the HOMA-IR [18, 19].
In recent years, numerous clinical studies have shown that the TyG index is correlated with the risk of incident cardiovascular disease. Two retrospective cohort studies by Sánchez Iñigo et al. and Li et al. showed that healthy participants with elevated TyG index are at higher risk of cardiovascular events [21, 24]. Another cross-sectional observational study by Thai et al. reported that elevated TyG index is correlated with the incidence and severity of coronary stenosis in patients with type 2 DM [23]. Park et al. reported that the TyG index is an independent predictor of the progression of coronary artery calcification [40]. Liu et al. also reported a high TyG index increase the risk of subclinical myocardial injury [41]. Two studies showed that patients with elevated TyG index are more likely to develop hypertension [22, 42], which was also confirmed in the present research. Moreover, Lu et al. found that in a non-diabetic population, higher TyG index was significantly associated with subclinical atherosclerosis in women compared to men [43]. Further researches have shown that the TyG index is more substantially associated with arterial stiffness, coronary artery calcification, and carotid atherosclerosis than HOMA-IR [17, 20, 44]. In addition, several studies proposed that the TyG index can also predict the risk of major adverse cardiac events in patients with acute coronary syndrome (ACS) and DM [25, 26]. Hu et al. indicated that the TyG index was a better predictor of cardiovascular risk than FPG or HbA1C in ACS patients undergoing percutaneous coronary intervention (PCI) [27].
Association and potential mechanisms between TyG index and ADHF
A retrospective cohort study reported the TyG index is correlated with the prognosis of patients with CHF and type 2 DM [45]. Another study of 132 hospitalized HF patients revealed the TyG index was a novel biomarker of myocardial fibrosis and a valuable risk stratification metric in HF [46]. Our results in the present study are consistent with these observations. Nevertheless, the predictive value of the TyG index in ADHF is not fully recognized. In this study, we investigated the associations of the TyG index with clinical outcomes in patients with ADHF. The mechanisms underlying the association of the TyG index with adverse prognosis of heart failure may include the following. First, IR can induce changes in substrate metabolism and inefficient energy metabolism, thereby hampering the normal myocardial response to injury [11, 34]. In addition, the metabolic efficiency of the myocardium is further dampened in ADHF patients due to the downregulation of genes regulating the beta-oxidation of fatty acids [47, 48]. Secondly, IR contributes to subcellular component abnormalities, including lipotoxicity, oxidative stress, mitochondrial dysfunction, endoplasmic reticulum (ER) stress and impaired calcium signaling [13, 34, 47, 48]. Thirdly, IR promotes inappropriate activation of the sympathetic nervous system and renin–angiotensin–aldosterone system [13, 49]. Fourthly, IR is correlated with hyperglycemia and free fatty acid elevation, inducing immune cells infiltration in adipose tissue and macrophage activation [13]. The secretion of pro-inflammatory mediators by immune cells and macrophage leads to local and systemic low-grade inflammation [13, 47, 50]. As the disease progresses, the vicious cycle of the above conditions contributes to cardiomyocyte injury and death, cardiac hypertrophy and cardiac fibrosis [13, 51]. At the same time, heart failure also contributes to insulin resistance, which in turn leads to further deterioration of heart function [52, 53].
Therefore, for optimizing the risk stratification in patients with heart failure and exploring more potential therapeutic options, it is necessary to examine the link between HF and IR. The TyG index is more convenient for routine screening in clinical practice than HOMA-IR. The TyG index could be monitored in patients with HF, particularly ADHF cases because of difficulty in treatment and poor prognosis.
Study limitations
There were several limitations in this study. Firstly, due to the limitations of a retrospective design, we were unable to dynamically measure TyG in patients over the follow-up period. Secondly, as this was a single-center study with a limited sample size, data bias could not be avoided despite correction for multiple confounding factors. Thirdly, because of the limited clinical information available, the differences between TyG and other IR indicators, including HOMA-IR and HIEC, in the prognosis of ADHF were not studied. Furthermore, prospective cohort studies are required to validate our findings.
Conclusion
In summary, this study demonstrated that the TyG index was directly correlated with poor prognosis in patients with ADHF regardless of DM status. Elevated TyG index could be a predictor and risk stratification tool for all-cause death, cardiovascular death, and MACCEs in patients with ADHF.
Acknowledgements
Not applicable.
Abbreviations
- ADHF
Acute decompensated heart failure
- CHF
Chronic heart failure
- IR
Insulin resistance
- CV
Cardiovascular
- HOMA
The homeostasis model assessment
- TyG
Triglyceride‑glucose
- FPG
Fasting plasma glucose
- TGs
Triglyceride levels
- BMI
Body mass index
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- TC
Total cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- HDL‑C
High‑density lipoprotein cholesterol
- RDW
Red blood cell distribution width
- CRP
C-creative protein
- BNP
B-type natriuretic peptide
- ALB
Albumin
- WBC
White blood cell
- ALT
Aspartate transaminase
- AST
Alanine aminotransferase
- MI
Myocardial infarction
- MACCEs
Major adverse cardiac and cerebral events
- SD
Standard deviation
- IQR
Interquartile range
- ANOVA
Analysis of variance
- IVSTD
Interventricular septal thickness at diastole
- LVPWTD
Left ventricular posterior wall end-diastolic thickness
- LVDD
Left ventricular end-diastolic diameter
- LAD
Left atrial diameter
- LVEF
Left ventricular ejection fraction
- ACEI
Angiotensin-converting enzyme inhibitor
- ARB
Angiotensin receptor inhibitor
- ARNI
Angiotensin receptor neprilysin inhibitor
- SGLT2i
Sodium-glucose contrasporter-2 inhibitor
- HR
Hazard ratio
- CIs
95% Confidence intervals
- IMD
Ischemic cardiomyopathy
- HIEC
Hyperinsulinemic-euglycemic clamp
- PCI
Percutaneous coronary intervention
- ACS
Acute coronary syndrome
- ER
Endoplasmic reticulum
Author contributions
LW and ZHW contributed to the conception and design of the study; RH contributed to manuscript writing; RH, ZYW and JZC contributed to data collection and management; RH, ZHW and XB contributed to the statistics analysis; NJX, GSM and RG participated in the patient follow-up; LW and WMW contributed to manuscript revision and data review. All authors have read and approved the manuscript.
Funding
This work was supported by the following funding: The Natural Science Foundation of China (81870204). Medical Science and technology development Foundation, Nanjing Department of Health (ZKX19017). Medical Science and technology development Foundation, Nanjing Department of Health (YKK19063).
Availability of data and materials
The information and data of the study population were extracted from Hospital Information System. The datasets are not publicly available because the individual privacy of the participants should be protected. Data are however available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study data collections were approved by the Medical Ethics Committee of Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School (2021-532-02). All participants were consent to participate with the verbal consent. Our database is not open to public, the individual privacy of the participants could be well protected.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Weimin Wang, Email: wwmlyg@189.cn.
Zhonghai Wei, Email: weizhonghai@njglyy.com.
Lian Wang, Email: wanglian@njglyy.com.
References
- 1.Essay P, Balkan B, Subbian V. Decompensation in Critical care: early prediction of acute heart failure onset. JMIR Med Inform. 2020;8(8):e19892. doi: 10.2196/19892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Njoroge JN, Teerlink JR. Pathophysiology and therapeutic approaches to acute decompensated heart failure. Circ Res. 2021;128(10):1468–1486. doi: 10.1161/CIRCRESAHA.121.318186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocha BML, Menezes Falcao L. Acute decompensated heart failure (ADHF): a comprehensive contemporary review on preventing early readmissions and postdischarge death. Int J Cardiol. 2016;223:1035–1044. doi: 10.1016/j.ijcard.2016.07.259. [DOI] [PubMed] [Google Scholar]
- 4.Greene SJ, Hernandez AF, Dunning A, Ambrosy AP, Armstrong PW, Butler J, Cerbin LP, Coles A, Ezekowitz JA, Metra M, et al. Hospitalization for recently diagnosed versus worsening chronic heart failure: from the ASCEND-HF trial. J Am Coll Cardiol. 2017;69(25):3029–3039. doi: 10.1016/j.jacc.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 5.Njoroge JN, Cheema B, Ambrosy AP, Greene SJ, Collins SP, Vaduganathan M, Mebazaa A, Chioncel O, Butler J, Gheorghiade M. Expanded algorithm for managing patients with acute decompensated heart failure. Heart Fail Rev. 2018;23(4):597–607. doi: 10.1007/s10741-018-9697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 7.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 8.Vaduganathan M, Claggett B, Packer M, McMurray JJV, Rouleau JL, Zile MR, Swedberg K, Solomon SD. Natriuretic peptides as biomarkers of treatment response in clinical trials of heart failure. JACC Heart Fail. 2018;6(7):564–569. doi: 10.1016/j.jchf.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Sliwa K, Mebazaa A. Acute heart failure. Nat Rev Dis Primers. 2020;6(1):16. doi: 10.1038/s41572-020-0151-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demissei BG, Cotter G, Prescott MF, Felker GM, Filippatos G, Greenberg BH, Pang PS, Ponikowski P, Severin TM, Wang Y, et al. A multimarker multi-time point-based risk stratification strategy in acute heart failure: results from the RELAX-AHF trial. Eur J Heart Fail. 2017;19(8):1001–1010. doi: 10.1002/ejhf.749. [DOI] [PubMed] [Google Scholar]
- 11.Zheng L, Li B, Lin S, Chen L, Li H. Role and mechanism of cardiac insulin resistance in occurrence of heart failure caused by myocardial hypertrophy. Aging (Albany NY) 2019;11(16):6584–6590. doi: 10.18632/aging.102212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wamil M, Coleman RL, Adler AI, McMurray JJV, Holman RR. Increased risk of incident heart failure and death is associated with insulin resistance in people with newly diagnosed type 2 diabetes: UKPDS 89. Diabetes Care. 2021;44(8):1877–1884. doi: 10.2337/dc21-0429. [DOI] [PubMed] [Google Scholar]
- 13.Nishida K, Otsu K. Inflammation and metabolic cardiomyopathy. Cardiovasc Res. 2017;113(4):389–398. doi: 10.1093/cvr/cvx012. [DOI] [PubMed] [Google Scholar]
- 14.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 15.Hanley AJ, Williams K, Stern MP, Haffner SM. Homeostasis model assessment of insulin resistance in relation to the incidence of cardiovascular disease: the San Antonio Heart Study. Diabetes Care. 2002;25(7):1177–1184. doi: 10.2337/diacare.25.7.1177. [DOI] [PubMed] [Google Scholar]
- 16.Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61(10):533–540. doi: 10.1016/j.endonu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, Gnasso A. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. 2013;67(7):665–672. doi: 10.1111/ijcp.12124. [DOI] [PubMed] [Google Scholar]
- 18.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, Jacques-Camarena O, Rodriguez-Moran M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 19.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 20.Kim MK, Ahn CW, Kang S, Nam JS, Kim KR, Park JS. Relationship between the triglyceride glucose index and coronary artery calcification in Korean adults. Cardiovasc Diabetol. 2017;16(1):108. doi: 10.1186/s12933-017-0589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li S, Guo B, Chen H, Shi Z, Li Y, Tian Q, Shi S. The role of the triglyceride (triacylglycerol) glucose index in the development of cardiovascular events: a retrospective cohort analysis. Sci Rep. 2019;9(1):7320. doi: 10.1038/s41598-019-43776-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16(1):175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride glucose index for the detection of asymptomatic coronary artery stenosis in patients with type 2 diabetes. Cardiovasc Diabetol. 2020;19(1):137. doi: 10.1186/s12933-020-01108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46(2):189–197. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 25.Ma X, Dong L, Shao Q, Cheng Y, Lv S, Sun Y, Shen H, Wang Z, Zhou Y, Liu X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):31. doi: 10.1186/s12933-020-01006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, Yang H, Ren LB, Qi W, Li WY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetol. 2020;19(1):80. doi: 10.1186/s12933-020-01054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu C, Zhang J, Liu J, Liu Y, Gao A, Zhu Y, Zhao Y. Discordance between the triglyceride glucose index and fasting plasma glucose or HbA1C in patients with acute coronary syndrome undergoing percutaneous coronary intervention predicts cardiovascular events: a cohort study from China. Cardiovasc Diabetol. 2020;19(1):116. doi: 10.1186/s12933-020-01091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 29.American Diabetes A. 2. Classification and diagnosis of diabetes. Diabetes Care. 2017;40(Suppl 1):S11–S24. doi: 10.2337/dc17-S005. [DOI] [PubMed] [Google Scholar]
- 30.Janak JC, Clemens MS, Howard JT, Le TD, Cancio LC, Chung KK, Gurney JM, Sosnov JA, Stewart IJ. Using the injury severity score to adjust for comorbid trauma may be double counting burns: implications for burn research. Burns. 2018;44(8):1920–1929. doi: 10.1016/j.burns.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Lundgreen CS, Larson DR, Atkinson EJ, Devick KL, Lewallen DG, Berry DJ, Maradit Kremers H, Crowson CS. Adjusted survival curves improve understanding of multivariable Cox model results. J Arthroplasty. 2021;36(10):3367–3371. doi: 10.1016/j.arth.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieto FJ, Coresh J. Adjusting survival curves for confounders: a review and a new method. Am J Epidemiol. 1996;143(10):1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 33.Rider I, Sorensen M, Brady WJ, Gottlieb M, Benson S, Koyfman A, Long B. Disposition of acute decompensated heart failure from the emergency department: an evidence-based review. Am J Emerg Med. 2021;50:459–465. doi: 10.1016/j.ajem.2021.08.070. [DOI] [PubMed] [Google Scholar]
- 34.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J Am Coll Cardiol. 2008;51(2):93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 35.Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, Huang Y. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. 2021;23(8):1746–1753. doi: 10.1111/dom.14388. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee D, Biggs ML, Mercer L, Mukamal K, Kaplan R, Barzilay J, Kuller L, Kizer JR, Djousse L, Tracy R, et al. Insulin resistance and risk of incident heart failure: cardiovascular Health Study. Circ Heart Fail. 2013;6(3):364–370. doi: 10.1161/CIRCHEARTFAILURE.112.000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ormazabal V, Nair S, Elfeky O, Aguayo C, Salomon C, Zuniga FA. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc Diabetol. 2018;17(1):122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. doi: 10.1038/nrendo.2014.29. [DOI] [PubMed] [Google Scholar]
- 39.Vlachopoulos C, Dima I, Aznaouridis K, Vasiliadou C, Ioakeimidis N, Aggeli C, Toutouza M, Stefanadis C. Acute systemic inflammation increases arterial stiffness and decreases wave reflections in healthy individuals. Circulation. 2005;112(14):2193–2200. doi: 10.1161/CIRCULATIONAHA.105.535435. [DOI] [PubMed] [Google Scholar]
- 40.Park K, Ahn CW, Lee SB, Kang S, Nam JS, Lee BK, Kim JH, Park JS. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42(8):1569–1573. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Wu M, Xu J, Sha D, Xu B, Kang L. Association between Triglyceride and glycose (TyG) index and subclinical myocardial injury. Nutr Metab Cardiovasc Dis. 2020;30(11):2072–2076. doi: 10.1016/j.numecd.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 42.Sanchez-Inigo L, Navarro-Gonzalez D, Pastrana-Delgado J, Fernandez-Montero A, Martinez JA. Association of triglycerides and new lipid markers with the incidence of hypertension in a Spanish cohort. J Hypertens. 2016;34(7):1257–1265. doi: 10.1097/HJH.0000000000000941. [DOI] [PubMed] [Google Scholar]
- 43.Lu YW, Chang CC, Chou RH, Tsai YL, Liu LK, Chen LK, Huang PH, Lin SJ. Gender difference in the association between TyG index and subclinical atherosclerosis: results from the I-Lan Longitudinal Aging Study. Cardiovasc Diabetol. 2021;20(1):206. doi: 10.1186/s12933-021-01391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, Kim MJ, Kim MK, Park JS. Association between triglyceride glucose index and arterial stiffness in Korean adults. Cardiovasc Diabetol. 2018;17(1):41. doi: 10.1186/s12933-018-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo W, Zhao L, Mo F, Peng C, Li L, Xu Y, Guo W, Sun A, Yan H, Wang L. The prognostic value of the triglyceride glucose index in patients with chronic heart failure and type 2 diabetes: a retrospective cohort study. Diabetes Res Clin Pract. 2021;177:108786. doi: 10.1016/j.diabres.2021.108786. [DOI] [PubMed] [Google Scholar]
- 46.Yang S, Du Y, Liu Z, Zhang R, Lin X, Ouyang Y, Chen H. Triglyceride-glucose index and extracellular volume fraction in patients with heart failure. Front Cardiovasc Med. 2021;8:704462. doi: 10.3389/fcvm.2021.704462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail Clin. 2012;8(4):609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van de Weijer T, Schrauwen-Hinderling VB, Schrauwen P. Lipotoxicity in type 2 diabetic cardiomyopathy. Cardiovasc Res. 2011;92(1):10–18. doi: 10.1093/cvr/cvr212. [DOI] [PubMed] [Google Scholar]
- 49.Zhou MS, Schulman IH, Zeng Q. Link between the renin-angiotensin system and insulin resistance: implications for cardiovascular disease. Vasc Med. 2012;17(5):330–341. doi: 10.1177/1358863X12450094. [DOI] [PubMed] [Google Scholar]
- 50.Bloomgarden ZT. Inflammation and insulin resistance. Diabetes Care. 2003;26(5):1619–1623. doi: 10.2337/diacare.26.5.1619. [DOI] [PubMed] [Google Scholar]
- 51.Shah RV, Abbasi SA, Heydari B, Rickers C, Jacobs DR, Jr, Wang L, Kwong RY, Bluemke DA, Lima JA, Jerosch-Herold M. Insulin resistance, subclinical left ventricular remodeling, and the obesity paradox: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2013;61(16):1698–1706. doi: 10.1016/j.jacc.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30(2):527–532. doi: 10.1016/S0735-1097(97)00185-X. [DOI] [PubMed] [Google Scholar]
- 53.Amato L, Paolisso G, Cacciatore F, Ferrara N, Ferrara P, Canonico S, Varricchio M, Rengo F. Congestive heart failure predicts the development of non-insulin-dependent diabetes mellitus in the elderly. The Osservatorio Geriatrico Regione Campania Group. Diabetes Metab. 1997;23(3):213–218. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The information and data of the study population were extracted from Hospital Information System. The datasets are not publicly available because the individual privacy of the participants should be protected. Data are however available from the corresponding author on reasonable request.