Abstract
Background
Immunoglobulin G (IgG) deficiency increases the risk of acute exacerbations and mortality in chronic obstructive pulmonary disease (COPD). However, the impact of IgG subclass deficiency on mortality in COPD is unknown. Here, we determined which IgG subclass, if any, is associated with increased risk of mortality in COPD.
Methods
We measured serum IgG subclass concentrations of 489 hospitalized patients with COPD who were enrolled in the Rapid Transition Program (clinicaltrials.gov identifier NCT02050022). To evaluate the impact of IgG subclass deficiency on 1-year mortality, Cox proportional hazards regression analyses were performed with adjustments for potential confounders.
Results
Deficiencies in IgG1, IgG2, IgG3, and IgG4 were present in 1.8%, 12.1%, 4.3%, and 11.2% of patients, respectively. One-year mortality was 56% in patients with IgG1 deficiency, 27% in IgG2 deficiency, 24% in IgG3 deficiency, and 31% in IgG4 deficiency. Cox proportional modeling showed that IgG1 and IgG4 deficiencies increased the 1-year mortality risk with an adjusted hazard ratio of 3.92 (95% confidence interval [CI] = 1.55–9.87) and 1.74 (95% CI = 1.02–2.98), respectively. Neither IgG2 nor IgG3 deficiency significantly increased 1-year mortality. Two or more IgG subclass deficiencies were observed in 5.3%. Patients with 2 or more IgG subclass deficiencies had a higher 1-year mortality than those without any deficiencies (46.2% vs. 19.7%, p < 0.001), with an adjusted hazard ratio of 2.22 (95% CI = 1.18–4.17).
Conclusions
IgG1 and IgG4 deficiency was observed in 1.8% and 11.2% of hospitalized patients with COPD, respectively, and these deficiencies were associated with a significantly increased risk of 1-year mortality.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-02052-3.
Keywords: IgG, IgG subclass deficiency, COPD, Mortality
Background
Despite recent advances in the treatment of chronic obstructive pulmonary disease (COPD), it is the 3rd leading cause of death, responsible for over 3 million deaths per year worldwide [1]. Most deaths occur during periods of acute exacerbations (AECOPDs) [2], which are largely triggered by viral or bacterial respiratory tract infections [3, 4]. One important risk factor for recurrent respiratory tract infections is humoral immune deficiency caused by hypogammaglobulinemia [5].
In blood, immunoglobulin G (IgG) is the predominant circulating antibody that plays a crucial role in preventing severe respiratory tract infections [5–8]. Approximately 10–28% of patients with moderate to severe COPD demonstrate hypogammaglobulinemia, which in turn, has been associated with an increased risk for exacerbations, hospitalizations, and mortality [5, 7, 9–11]. There are four distinct subclasses of IgG (IgG1, IgG2, IgG3, and IgG4), and each has a slightly different structure and function in the immune system [12, 13]. While the relationship between total IgG levels and mortality has been established in COPD [5, 11], the impact of IgG subclass deficiencies on COPD remains obscure, especially in hospitalized patients with COPD who are at increased risk of mortality. To date, there has been a paucity of studies with sufficient size and scope that have evaluated IgG subclass deficiencies in hospitalized patients with COPD. Studies have moreover used cross-sectional methodologies, limiting causal inferences [14, 15]. Here, we describe the prevalence of IgG subclass deficiencies and their relationship with 1-year mortality in hospitalized patients with COPD.
Materials and methods
Study participants
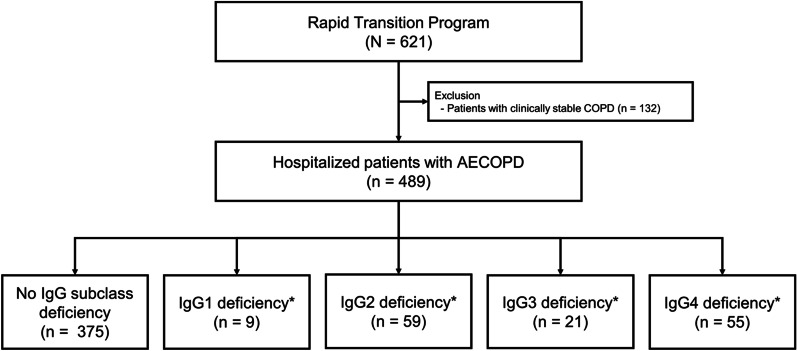
The Rapid Transition Program (ClinicalTrials.gov number: NCT02050022) included hospitalized patients with AECOPD (n = 489) and clinically stable patients with COPD (n = 132) who were seen and managed at St. Paul’s Hospital or Vancouver General Hospital, both in Vancouver, Canada between April 2013 and December 2017 (Fig. 1). The blood samples for hospitalized patients were collected on the first three days of their hospitalization.
Fig. 1.
Study flow chart. *Some patients had two or more IgG subclass deficiencies. IgG immunoglobulin G
Ethical statement
This study was approved by the research ethics board of each institution (certificate number H11-00786 for St. Paul’s Hospital and certificate number H13-00790 for Vancouver General Hospital). Informed consent to participate in this study was received from each patient. None of the patients had bronchiectasis by history or chest imaging. Patients were followed for 1 year after enrollment. Mortality was ascertained through medical records and validated by death certificates. The detailed information about this study was previously published [16].
Study outcome
The primary aim was to evaluate the impact of IgG subclass deficiencies on 1-year mortality in hospitalized patients with COPD.
Measurements of IgG subclasses
Serum samples were processed using standardized protocols and stored in a -80ºC freezer and thawed once for the assay. We measured IgG subclass concentrations in serum using liquid chromatography-tandem mass spectrometry (LC–MS/MS) as previously described [17]. The processing and measurement of all samples were performed in the clinical laboratory at St. Paul’s Hospital, Vancouver, British Columbia, Canada. IgG subclass deficiency was defined when serum IgG subclass levels were below their respective normal ranges (i.e., fell below 2 standard deviations [SDs] from the mean concentrations in a healthy population): IgG1 < 2.80 g/L, IgG2 < 1.15 g/L, IgG3 < 0.24 g/L, and IgG4 < 0.052 g/L [18].
Statistical analysis
Continuous variables are presented as mean ± SD or median (interquartile range). Continuous variables were compared using a t-test or a Mann-Whitney U test, as appropriate. Categorical variables are presented as numbers (%) and were compared using a Chi-squared test or a Fisher’s exact test, as appropriate. To evaluate the survival between subjects with an IgG subclass deficiency and those with no IgG subclass deficiency, we performed a log-rank test. For the analyses of survival according to the number of IgG subclass deficiencies (0, 1, and 2 or more), Bonferroni adjustment was made to account for multiple comparisons. To evaluate the impact of IgG subclass deficiency vs. no IgG subclass deficiency on 1-year mortality, Cox proportional hazards regression analysis was performed with adjustments for age, sex, ethnicity (white vs. other ethnicities), smoking status (current vs. non-current), presence of asthma, and cardiac comorbidity status (i.e., a history of heart failure, myocardial infarction, stable coronary disease, or coronary artery bypass graft surgery). For the Cox analyses, we tested the proportional hazard assumption visually and by using Schoenfeld residuals and the assumption was met except for the IgG2 analysis. All tests were two-sided and p values < 0.05 were considered statistically significant. Data analysis was performed using R software version 4.1.0 (The R Foundation for Statistical Computing Platform, Vienna, Austria), RStudio software version 1.4.1717 (RStudio, Boston, MA), and STATA 15.1 version (StataCorp LP, College Station, TX, USA).
Results
Study participants
The baseline characteristics of 489 patients are summarized in Table 1. The mean (± SD) age of the study population was 67.3 ± 11.6 years and 63.2% were males. Deficiencies in IgG1, IgG2, IgG3, and IgG4 were present in 1.8% (n = 9), 12.1% (n = 59), 4.3% (n = 21), and 11.2% (n = 55) of the cohort, respectively. Approximately 5% of the patients (n = 26) demonstrated two or more IgG subclass deficiencies. The baseline characteristics of patients with each IgG subclass deficiency are summarized in Additional file 1: Table S1.
Table 1.
Baseline characteristics of study participants
| Total (N = 489) | |
|---|---|
| Age, years | 67.3 ± 11.6 |
| Male | 309 (63.2) |
| Ethnicity, white | 391 (81.0) |
| Smoking status | |
| Current smoker | 287 (58.7) |
| Ex-smoker | 173 (35.4) |
| Never smoker | 29 (5.9) |
| Asthma | 119 (24.4) |
| Cardiac comorbidities* | 182 (37.3) |
| Lung function | |
| Post-bronchodilator FVC, L | 2.8 ± 1.1 |
| Post-bronchodilator FVC, %predicted | 77.6 ± 23.1 |
| Post-bronchodilator FEV1, L | 1.5 ± 0.8 |
| Post-bronchodilator FEV1, %predicted | 53.7 ± 23.8 |
| Post-bronchodilator FEV1/FVC | 45.2 ± 24.8 |
| IgG subclass deficiency | |
| IgG1 deficiency | 9 (1.8) |
| IgG2 deficiency | 59 (12.1) |
| IgG3 deficiency | 21 (4.3) |
| IgG4 deficiency | 55 (11.3) |
| 1-year mortality | 101 (20.7) |
Data are presented as numbers (%) or mean ± SD
FVC forced vital capacity, FEV1 forced expiratory volume in 1 s, IgG immunoglobulin G
*Cardiac comorbidities included a history of heart failure, myocardial infarction, stable coronary disease, or coronary artery bypass graft surgery
Impact of IgG subclass deficiency on 1-year mortality
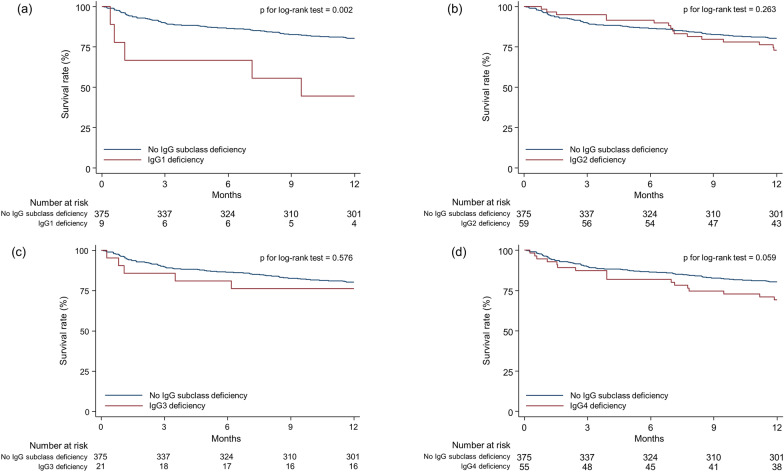
The overall 1-year mortality rate of the study population was 20.7% (101/489): 55.6% (5/9) in patients with IgG1 deficiency, 27.1% (16/59) in IgG2 deficiency, 23.8% (5/21) in IgG3 deficiency, and 30.9% (17/55) in IgG4 deficiency, respectively. Multivariable Cox regression analyses revealed significant relationships between IgG1 and IgG4 deficiencies and increased 1-year mortality (Table 2). Patients with IgG1 deficiency were 3.92 (95% confidence interval [CI] = 1.55–9.87) times more likely to experience 1-year mortality compared to those without IgG1 deficiency. Patients with IgG4 deficiency also had a higher risk of 1-year mortality compared to those without IgG4 deficiency (adjusted hazard ratio [HR] = 1.74; 95% CI = 1.02–2.98). In contrast, IgG2 (unadjusted HR = 1.36; 95% CI = 0.79–2.33) and IgG3 deficiency (adjusted HR = 1.27; 95% CI = 0.51–3.15) were not associated with increased risk of mortality in patients with COPD. The proportional hazards assumption was not met for the analysis of IgG2 deficiency and mortality. Survival analyses showed similar results (Fig. 2a–d).
Table 2.
Unadjusted and adjusted HRs related to 1-year mortality according to IgG subclass deficiency
| Type of IgG deficiency | Number at risk | 1-year mortality | Unadjusted model | Adjusted model* | ||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |||
| IgG1 deficiency | 9 | 55.6% (5/9) | 3.80 (1.54– 9.41) | 0.004 | 3.92 (1.55–9.87) | 0.004 |
| IgG2 deficiency† | 59 | 27.1% (16/59) | 1.36 (0.79–2.33) | 0.265 | NA† | NA† |
| IgG3 deficiency | 21 | 23.8% (5/21) | 1.29 (0.52–3.20) | 0.577 | 1.27 (0.51–3.15) | 0.612 |
| IgG4 deficiency | 55 | 30.9% (17/55) | 1.65 (0.97–2.79) | 0.063 | 1.74 (1.02–2.98) | 0.043 |
Data are presented as number, percentage, or ratios (95% CIs)
HR hazard ratio, IgG immunoglobulin G, CI confidence interval
*Adjusted for age, sex, ethnicity (white vs. other ethnicities), smoking status (current vs. non-current), asthma status, and cardiac comorbidity status
†The proportional hazards assumption was not met
Fig. 2.
Kaplan–Meier survival curves for mortality according to IgG subclass deficiency (a) IgG1 deficiency; (b) IgG2 deficiency; (c) IgG3 deficiency; (d) IgG4 deficiency. IgG immunoglobulin G
Besides IgG subclass deficiencies, there were other risk factors for 1-year mortality in COPD patients. As shown in Additional file 2: Table S2, whereas age (except for IgG4 subclass deficiency) and male sex increased the risk of 1-year mortality, there were no significant associations between ethnicity, smoking status, asthma, and cardiac comorbidities and 1-year mortality.
Impact of the number of IgG subclass deficiency on mortality
The 1-year mortality according to the number of IgG subclass deficiency is as follows: 19.7% [74/375] in those without any IgG subclass deficiencies, 17.1% [15/88] in those with 1 subclass deficiency, 39.1% [9/23] in those with 2 subclass deficiencies, 100% [2/2] in those with 3 deficiencies, and 100% [1/1] in one individual with deficiencies in all 4 subclasses. Patients with 2 or more deficiencies had a significantly higher 1-year mortality than those without any deficiencies or those with 1 deficiency (46.2% vs. 19.7% vs. 17.1%; p < 0.001), and there was a significant difference in the survival rate between the former and the two latter groups (p for log-rank test = 0.006) (Fig. 3). In multivariable Cox-regression analyses, patients with 2 or more deficiencies were 2.22 (95% CI = 1.18–4.17) and 2.44 (95% CI = 1.11–5.33) times more likely to experience 1-year mortality than those without any IgG subclass deficiencies or those with 1 deficiency, respectively (Table 3).
Fig. 3.
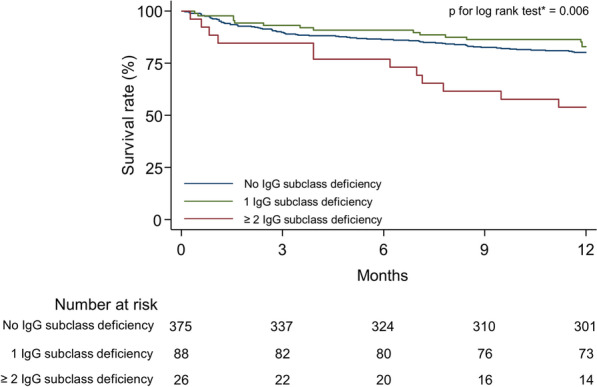
Kaplan–Meier survival curves for mortality according to the number of IgG subclass deficiencies. Bonferroni adjustment was performed for multiple comparisons. IgG immunoglobulin G
Table 3.
Unadjusted and adjusted HRs related to 1-year mortality according to the number of IgG subclass deficiency
| Number of IgG deficiency | Number at risk | 1-year mortality | Unadjusted model | Adjusted model* | ||
|---|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | p value | Adjusted HR (95% CI) | p value | |||
| 0 | 375 | 19.7% (74/375) | Reference | Reference | ||
| 1 | 88 | 17.1% (15/88) | 0.84 (0.48–1.46) | 0.528 | 0.91 (0.52–1.59) | 0.736 |
| 2 or more | 26 | 46.2% (12/26) | 2.70 (1.47–4.98) | 0.001 | 2.22 (1.18–4.17) | 0.014 |
Data are presented as numbers, percentages, or ratios (95% CIs)
HR hazard ratio, IgG immunoglobulin G, CI confidence interval
*Adjusted for age, sex, ethnicity (white vs. other ethnicities), smoking status (current vs. non-current), asthma status, and cardiac comorbidity status
Discussion
Hypogammaglobulinemia affects approximately 1 in 5 patients with moderate to severe COPD. However, the occurrence of individual IgG subclass deficiency in hospitalized COPD patients at a high risk of morbidity and mortality has not been well studied. Here, we showed that the probability of individual subclass deficiency in a high-risk group of patients was 1.8% for IgG1, 12.1% for IgG2, 4.3% for IgG3, and 11.2% for IgG4. Importantly, we found that 1-year mortality was highest in patients with IgG1 deficiency (50%), followed by IgG4 deficiency (27.1%), IgG2 deficiency (25.7%), IgG3 deficiency (19.4%), and lowest in those without any IgG subclass deficiencies (16.7%). Independent of age, sex, ethnicity, smoking status, asthma history, and cardiac comorbidities, IgG1 and IgG4 deficiency elevated the risk of mortality by 3.9-fold and 1.7-fold, respectively.
The overall occurrence of any IgG subclass deficiency in this study was 23.3%, which is similar to the findings reported previously (17.7–25.9%) [9, 15, 19]. However, the distribution of the IgG subclass deficiencies in this study was different from those of previous studies. We showed that the most common deficiencies were related to IgG2 and IgG4. In contrast, previous studies have shown IgG3 deficiency to be the most common [6, 9, 15] except for one notable study where IgG2 deficiency was the most prevalent [19]. Although the reasons for this discordance are not clear, it has been previously suggested that the distribution of IgG subclass deficiency varies according to the severity of COPD with the risk of IgG2 deficiency rising with increasing frequencies of AECOPDs [9]. The relatively high rates of IgG2 deficiency in the present study, which contained patients who were hospitalized with AECOPD, are consistent with this previous observation.
We previously showed that hypogammaglobulinemia is associated with an increased risk of mortality in patients with COPD [11]. We extend these findings by demonstrating increased 1-year mortality with IgG1 or IgG4 but not IgG2 or IgG3 subclass deficiency. The exact mechanisms by which IgG1 and IgG4 deficiencies confer increased mortality in patients with COPD are obscure and were beyond the purview of the present study. However, since most deaths occur during periods of severe lower respiratory tract infection, we posit that IgG1 deficiency increases the risk for severe AECOPDs [9]. This raises the possibility that Ig replacement therapy might improve survival in these patients. However, this hypothesis will require validation in a large randomized controlled trial before it can be implemented routinely in clinical practice.
Interestingly, we also found that IgG4 deficiency was associated with increased mortality. However, in our previous study which evaluated different cohorts of patients with stable COPD, we did not find that IgG4 deficiency was associated with increased risk of AECOPD or AECOPD-related hospitalization [9]. This raises the possibility that the impact of IgG4 deficiency on mortality may be indirect. Consistent with this notion, recent studies have shown that low serum IgG4 increases the risk of not only respiratory infections but also non-respiratory conditions including allergic, autoimmune, and cardiovascular diseases [20–26]. For these latter conditions, serum IgG levels appear to be inversely related to the severity of disease [23, 25, 26]. Thus, IgG4 deficiency might be a biomarker of increased burden of co-morbidity or may reflect the severity of the underlying COPD. Future studies should evaluate the potential causal role, if any, that IgG4 deficiency plays in the pathogenesis of COPD. We also found that the risk of mortality increases monotonically with the number of subclass deficiencies.
Unexpectedly, IgG2 deficiency, a predictor of AECOPD or hospitalization in the previous study of patients with stable COPD [9], was not associated with an increased risk of mortality. Although the reasons for this phenomenon are not clear, it should be noted that there are important differences in the severity of COPD between the current cohort and the one previously described [9]. In the current cohort, hospitalized patients with an AECOPD were enrolled; in contrast, all of the patients in the previous study [9] were stable at the time of recruitment. Furthermore, 12.1% of the patients in the present demonstrated IgG2 deficiency; whereas only 5.7% demonstrated IgG2 deficiency in the previous study.
In the era of personalized medicine, identification of Ig subclass deficient patients is attractive as it may be a modifiable and a treatable trait. Whereas total IgG deficiency is relatively common in COPD patients (~ 20%), subclass deficiency is rare. Indeed, we found that only 1.6% of our patients were IgG1 deficient and 5% were deficient in two or more subclasses. Given the cost and inconvenience of Ig replacement therapy, it may be more reasonable to target this therapy for those with subclass deficiencies rather than those with hypogammaglobulinemia based on total serum IgG levels. To date, only a few small observational studies have evaluated the possible salutary effects of replacement therapy in patients with COPD who also demonstrated hypogammaglobulinemia. One study of 14 patients showed that the administration of intravenous Ig was associated with a 90% reduction in the occurrence of AECOPD in predominantly hypogammaglobulinemic COPD patients [8]. Another study of 22 patients with hypogammaglobulinemia demonstrated that prophylactic antibiotics or Ig therapy might be beneficial in reducing the frequency of AECOPDs [27]. Because these study results were limited by study design and the small sample sizes, well-designed randomized controlled trials targeting IgG subclass deficiencies are needed to validate the promise of these early reports.
There were several limitations to the current study. First, clinically, it is generally recommended to measure IgG levels twice at least 1 month apart to determine IgG subclass deficiency [28, 29]. However, in the present study, IgG levels were measured only once at baseline. Moreover, for hospitalized patients, we were able to measure IgG levels only during the acute setting. It should be noted, however, that serum IgG levels are relatively stable and not affected by acute illness. The half-life of IgG subclasses is ~ 20–30 days [30]. Second, we could not determine the causes for the IgG subclass deficiencies. However, this was beyond the purpose of our study. Third, since this study included a small number of stable COPD patients, studies evaluating the impact of IgG subclass deficiencies on mortality in patients with stable COPD are needed. Although there was no significant association between IgG subclass deficiencies and mortality in stable COPD patients (Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5), these results are likely to be underpowered due to the small number of patients. Fourth, we did not collect data on causes of mortality as many died outside of the hospital. However, ascertaining and attributing causes to mortality are extremely challenging in COPD, who often harbor multiple co-morbidities. A previous study suggests that for hospitalized or recently hospitalized patients, respiratory tract infections, pulmonary embolism and cardiac failure are the leading causes of death [31]. How IgG subclass deficiencies modulate these terminal endpoints in COPD is uncertain. Future studies are needed to determine the mechanisms by which IgG subclass deficiencies lead to mortality in COPD patients.
Conclusions
IgG1 deficiency, through rare (1.8%), was associated with a 3.9-fold increased risk of 1-year mortality in patients with COPD. One in 9 patients with COPD had IgG4 deficiency, which increased mortality by 1.7-fold. Our study suggests that IgG subclass measurements can be useful to classify hospitalized patients with COPD at a high risk of mortality. These patients may be targets for future Ig replacement studies.
Supplementary Information
Additional file 1. Table S1. Baseline characteristics of study participants with each IgG subclass deficiency.
Additional file 2. Table S2. Adjusted HRs† of clinical risk factors for 1-year mortality.
Additional file 3. Table S3. Baseline characteristics of subjects with stable COPD.
Additional file 4. Table S4. Unadjusted and adjusted HRs related to 1-year mortality according to IgG subclass deficiency in stable COPD patients.
Additional file 5. Table S5. Unadjusted and adjusted HRs related to 1-year mortality according to the number of IgG subclass deficiency in stable COPD patients.
Acknowledgements
None.
Abbreviations
- AECOPD
Acute exacerbation of COPD
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- HR
Hazard ratio
- Ig
Immunoglobulin
Author contributions
Conceptualization: DDS; methodology: HL, CK, AM and DDS; software: HL, CK and AM; validation: HL, AM and DDS; formal analysis: HL and CK; investigation: HL and CK; resources: DDS; data curation: CK; original draft preparation: HL, CK and DDS; review and editing: AM, ZH, VC, RN and JML; visualization: HL and CK; supervision, DDS; project administration; DDS; funding acquisition, D.D.S. All authors read and approved the final manuscript.
Funding
The study was funded by Genome Canada, Genome British Columbia, Genome Quebec, Cana-dian Institutes of Health Research, Providence Health Care, and St Paul’s Foundation. DDS is a Tier 1 Canada Research Chair in COPD and holds the de Lazzari Family Chair at HLI; JL is a Tier 2 Canada Research Chair in Translational Airway Biology and a Michael Smith Foundation for Health/Providence Research Health Professional Investigator.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by Ethics board of each institution (certificate number H11-00786 for St. Paul’s Hospital and certificate number H13-00790 for Vancouver General Hospital).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hyun Lee and Cara Kovacs are co-first authors.
References
- 1.World Health Organization. 2019. Leading Causes of Death Globally. Available at https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- 2.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, Fabbri LM, Johnston SL. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 4.Bafadhel M, McKenna S, Terry S, Mistry V, Reid C, Haldar P, McCormick M, Haldar K, Kebadze T, Duvoix A, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184:662–671. doi: 10.1164/rccm.201104-0597OC. [DOI] [PubMed] [Google Scholar]
- 5.Holm AM, Andreassen SL, Christensen VL, Kongerud J, Almås Ø, Auråen H, Henriksen AH, Aaberge IS, Klingenberg O, Rustøen T. Hypogammaglobulinemia and risk of exacerbation and mortality in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:799–807. doi: 10.2147/COPD.S236656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J-H, Park S, Hwang YI, Jang SH, Jung K-S, Sim YS, Kim C-H, Kim C, Kim D-G. Immunoglobulin G subclass deficiencies in adult patients with chronic airway diseases. JKMS. 2016;31:1560–1565. doi: 10.3346/jkms.2016.31.10.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leitao Filho FS, Won Ra S, Mattman A, Schellenberg RS, Fishbane N, Criner GJ, Woodruff PG, Lazarus SC, Albert R, Connett JE, et al. Serum IgG and risk of exacerbations and hospitalizations in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2017;140:1164–1167. doi: 10.1016/j.jaci.2017.01.046. [DOI] [PubMed] [Google Scholar]
- 8.Cowan J, Gaudet L, Mulpuru S, Corrales-Medina V, Hawken S, Cameron C, Aaron SD, Cameron DW. A retrospective longitudinal within-subject risk interval analysis of immunoglobulin treatment for recurrent acute exacerbation of chronic obstructive pulmonary disease. PLoS ONE. 2015;10:e0142205. doi: 10.1371/journal.pone.0142205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leitao Filho FS, Ra SW, Mattman A, Schellenberg RS, Criner GJ, Woodruff PG, Lazarus SC, Albert R, Connett JE, Han MK, et al. Serum IgG subclass levels and risk of exacerbations and hospitalizations in patients with COPD. Respir Res. 2018;19:30. doi: 10.1186/s12931-018-0733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leitao Filho FS, Mattman A, Schellenberg R, Criner GJ, Woodruff P, Lazarus SC, Albert RK, Connett J, Han MK, Gay SE, et al. Serum IgG levels and risk of COPD hospitalization: a pooled meta-analysis. Chest. 2020;158:1420–1430. doi: 10.1016/j.chest.2020.04.058. [DOI] [PubMed] [Google Scholar]
- 11.Alotaibi NM, Filho FSL, Mattman A, Hollander Z, Chen V, Ng R, Leung JM, Sin DD. IgG levels and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204:362–365. doi: 10.1164/rccm.202102-0382LE. [DOI] [PubMed] [Google Scholar]
- 12.Schroeder HW, Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125:S41–S52. doi: 10.1016/j.jaci.2009.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol 2014, 5. [DOI] [PMC free article] [PubMed]
- 14.Keeffe S, Gzel A, Drury R, Cullina M, Greally J, Finnegan P. Immunoglobulin G subclasses and spirometry in patients with chronic obstructive pulmonary disease. Eur Respir J. 1991;4:932. [PubMed] [Google Scholar]
- 15.Qvarfordt I, Riise GC, Andersson BA, Larsson S. IgG subclasses in smokers with chronic bronchitis and recurrent exacerbations. Thorax. 2001;56:445. doi: 10.1136/thx.56.6.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alotaibi NM, Chen V, Hollander Z, Leipsic JA, Hague CJ, Murphy DT, DeMarco ML, FitzGerald JM, McManus BM, Ng RT, Sin DD. Phenotyping and outcomes of hospitalized COPD patients using rapid molecular diagnostics on sputum samples. Int J Chron Obstruct Pulmon Dis. 2019;14:311–319. doi: 10.2147/COPD.S188186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Gugten G, DeMarco ML, Chen LYC, Chin A, Carruthers M, Holmes DT, Mattman A. Resolution of spurious immunonephelometric IgG subclass measurement discrepancies by LC-MS/MS. Clin Chem. 2018;64:735–742. doi: 10.1373/clinchem.2017.282319. [DOI] [PubMed] [Google Scholar]
- 18.Schauer U, Stemberg F, Rieger CH, Borte M, Schubert S, Riedel F, Herz U, Renz H, Wick M, Carr-Smith HD, et al. IgG subclass concentrations in certified reference material 470 and reference values for children and adults determined with the binding site reagents. Clin Chem. 2003;49:1924–1929. doi: 10.1373/clinchem.2003.022350. [DOI] [PubMed] [Google Scholar]
- 19.O'Keeffe S, Gzel A, Drury R, Cullina M, Greally J, Finnegan P. Immunoglobulin G subclasses and spirometry in patients with chronic obstructive pulmonary disease. Eur Respir J. 1991;4:932–936. [PubMed] [Google Scholar]
- 20.Tian X, Deng Z, Wang S, Wang Y. Basic research and clinical reports associated with low serum IgG4 concentrations. Int Arch Allergy Immunol. 2020;181:149–158. doi: 10.1159/000503967. [DOI] [PubMed] [Google Scholar]
- 21.Beck CS, Heiner DC. Selective immunoglobulin G4 deficiency and recurrent infections of the respiratory tract. Am Rev Respir Dis. 1981;124:94–96. doi: 10.1164/arrd.1981.124.1.94. [DOI] [PubMed] [Google Scholar]
- 22.Moss RB, Carmack MA, Esrig S. Deficiency of IgG4 in children: association of isolated IgG4 deficiency with recurrent respiratory tract infection. J Pediatr. 1992;120:16–21. doi: 10.1016/S0022-3476(05)80590-6. [DOI] [PubMed] [Google Scholar]
- 23.van de Veen W, Stanic B, Yaman G, Wawrzyniak M, Söllner S, Akdis DG, Rückert B, Akdis CA, Akdis M. IgG4 production is confined to human IL-10-producing regulatory B cells that suppress antigen-specific immune responses. J Allergy Clin Immunol. 2013;131:1204–1212. doi: 10.1016/j.jaci.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Zhang H, Li P, Wu D, Xu D, Hou Y, Wang Q, Li M, Li Y, Zeng X, Zhang F, Shi Q. Serum IgG subclasses in autoimmune diseases. Medicine (Baltimore) 2015;94:e387. doi: 10.1097/MD.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakane K, Shibata K, Fujita S, Shimamoto S, Ito T, Kizawa S, Morita H, Sohmiya K, Hoshiga M, Ishizaka N. Association between serum immunoglobulin G4 concentration and cardiac function among elderly cardiology inpatients. Geriatr Gerontol Int. 2014;14:582–588. doi: 10.1111/ggi.12138. [DOI] [PubMed] [Google Scholar]
- 26.Burnett M, Wegienka G, Havstad S, Kim H, Johnson CC, Ownby D, Zoratti E. Relationship of dog- and cat-specific IgE and IgG4 levels to allergic symptoms on pet exposure. J Allergy Clin Immunol Pract. 2013;1:350–353. doi: 10.1016/j.jaip.2013.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCullagh BN, Comellas AP, Ballas ZK, Newell JD, Jr, Zimmerman MB, Azar AE. Antibody deficiency in patients with frequent exacerbations of Chronic Obstructive Pulmonary Disease (COPD) PLoS ONE. 2017;12:e0172437. doi: 10.1371/journal.pone.0172437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal S, Cunningham-Rundles C. Assessment and clinical interpretation of reduced IgG values. Ann Allergy, Asthma Immunol. 2007;99:281–283. doi: 10.1016/S1081-1206(10)60665-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonilla FA, Khan DA, Ballas ZK, Chinen J, Frank MM, Hsu JT, Keller M, Kobrynski LJ, Komarow HD, Mazer B, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol. 2015;136:1186–1205. doi: 10.1016/j.jaci.2015.04.049. [DOI] [PubMed] [Google Scholar]
- 30.Mankarious S, Lee M, Fischer S, Pyun KH, Ochs HD, Oxelius VA, Wedgwood RJ. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J Lab Clin Med. 1988;112:634–640. [PubMed] [Google Scholar]
- 31.Zvezdin B, Milutinov S, Kojicic M, Hadnadjev M, Hromis S, Markovic M, Gajic O. A postmortem analysis of major causes of early death in patients hospitalized with COPD exacerbation. Chest. 2009;136:376–380. doi: 10.1378/chest.08-2918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. Baseline characteristics of study participants with each IgG subclass deficiency.
Additional file 2. Table S2. Adjusted HRs† of clinical risk factors for 1-year mortality.
Additional file 3. Table S3. Baseline characteristics of subjects with stable COPD.
Additional file 4. Table S4. Unadjusted and adjusted HRs related to 1-year mortality according to IgG subclass deficiency in stable COPD patients.
Additional file 5. Table S5. Unadjusted and adjusted HRs related to 1-year mortality according to the number of IgG subclass deficiency in stable COPD patients.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.