Abstract
In vitro assays of washed, excised roots revealed maximum potential ferric iron reduction rates of >100 μmol g (dry weight)−1 day−1 for three freshwater macrophytes and rates between 15 and 83 μmol (dry weight)−1 day−1 for two marine species. The rates varied with root morphology but not consistently (fine root activity exceeded smooth root activity in some but not all cases). Sodium molybdate added at final concentrations of 0.2 to 20 mM did not inhibit iron reduction by roots of marine macrophytes (Spartina alterniflora and Zostera marina). Roots of a freshwater macrophyte, Sparganium eurycarpum, that were incubated with an analog of humic acid precursors, anthroquinone disulfate (AQDS), reduced freshly precipitated iron oxyhydroxide contained in dialysis bags that excluded solutes with molecular weights of >1,000; no reduction occurred in the absence of AQDS. Bacterial enrichment cultures and isolates from freshwater and marine roots used a variety of carbon and energy sources (e.g., acetate, ethanol, succinate, toluene, and yeast extract) and ferric oxyhydroxide, ferric citrate, uranate, and AQDS as terminal electron acceptors. The temperature optima for a freshwater isolate and a marine isolate were equivalent (approximately 32°C). However, iron reduction by the freshwater isolate decreased with increasing salinity, while reduction by the marine isolate displayed a relatively broad optimum salinity between 20 and 35 ppt. Our results suggest that by participating in an active iron cycle and perhaps by reducing humic acids, iron reducers in the rhizoplane of aquatic macrophytes limit organic availability to other heterotrophs (including methanogens) in the rhizosphere and bulk sediments.
Dissimilatory iron reduction accounts for a significant fraction of anaerobic metabolism in certain freshwater and marine ecosystems (1, 23). The extent to which iron reduction contributes to mineralization depends in part on the rates of organic matter and ferric iron supply (22). The supply of ferric iron often requires regeneration from ferrous iron rather than exogenous oxidized iron input. In wetlands, iron regeneration occurs at oxic sediment-water (or sediment-air) interfaces, in animal burrows, and in the rhizosphere and rhizoplane of macrophyte roots (3, 17, 19, 34, 39). Several lines of evidence have suggested that root-associated iron oxidation occurs by both abiological and biological processes (16). Plaques of ferric oxyhydroxide have long been observed on aquatic plant roots and have been considered evidence that there is diffusive loss of molecular oxygen that reacts with ferrous iron chemically (4). Iron oxidation by plant enzymes has also been proposed (3), and recently described novel iron oxidizers (15, 16) might also be involved. Geochemical evidence based on fluctuating pools of several iron and sulfur species has provided further support for the hypothesis that significant, belowground, plant-mediated iron oxidation occurs (18, 28).
The ferric iron readily available on or near aquatic plant roots likely supports populations of iron reducers that play an important role in carbon flow. Rodin and Wetzel (34) have documented that high rates of iron reduction by rhizosphere sediments occur in a weakly acidic southeastern wetland and have shown that iron reducers limit the extent of methanogenesis and methane emission in such systems. We show here that significant rates of iron reduction can also occur on macrophyte roots growing in acidic peat. In addition, we show that microbially mediated iron reduction occurs on marine macrophyte roots under conditions that inhibit bacterial sulfate reduction. Our results extend earlier observations indicating that aquatic macrophyte roots support functionally (and phylogenetically) diverse microbial communities that are engaged in the full range of biogeochemical cycling known more typically from sediments.
MATERIALS AND METHODS
Roots were obtained from intact freshwater macrophytes, including Sparganium eurycarpum, Typha latifolia, and Pontederia cordata, collected during July through September 1997 from a marsh located near Route 130 in Bristol, Maine. Details concerning the acidic peats (pH 4 to 5) at the site and the collection and processing methods used have been described previously (21, 32). Roots were obtained from two marine macrophytes, Zostera marina and Spartina alterniflora, by using similar procedures. These plants were collected intact during August from a depth of about 1 m in the Damariscotta River in Maine and from Cod Cove in Maine, respectively. The freshwater macrophyte roots were washed quickly but extensively with tap water to remove all nonadhering sediment. In some cases the roots were sorted into two morphologically distinct categories, smooth and fine, as described previously (32). Approximately 1-g (fresh weight) portions of live roots were transferred to triplicate 40-cm3 Hungate tubes (Bellco, Inc.). The tubes contained 35 cm3 of deoxygenated deionized water with or without a pH buffer (0.01 M morpholineethanesulfonic acid [MES] [pH 5.5] or 0.01 M HEPES [pH 7.3]) or a substrate (25 mM glucose, 25 mM acetate, or 10% [headspace concentration] hydrogen). The tubes were flushed with nitrogen, sealed with neoprene stoppers, and then amended with about 50 μmol of ferric iron added as a freshly precipitated, neutralized suspension of the oxyhydroxide. The tubes were incubated at 30°C horizontally with reciprocal shaking at 100 rpm. Subsamples of the tube contents were obtained over time with a needle and syringe and were analyzed to determine their total ferrous iron contents by using a modification of the ferrozine method (18) that involved sample acidification to dissolve any solid phases and addition of ferrozine but not a reductant. The absorbance at 562 nm of the ferrozine-ferrous iron complex was assayed by using a spectrophotometer (Beckman model DU-640). Roots obtained from marine plants were processed similarly, except that seawater was used for the initial rinses and the roots were suspended in a solution of sulfate-free artificial seawater.
The ability of 1 mM anthroquinone disulfate (AQDS) to promote iron reduction was examined by incubating S. eurycarpum roots in 125-ml Erlenmeyer flasks that had anaerobic headspaces and contained 75 ml of deoxygenated deionized water supplemented with 0.01 M HEPES (pH 7.3). Ferric oxyhydroxide was added as a suspension to the flasks or in dialysis bags (1,000-molecular-weight cutoff) to parallel root samples; untreated root samples were used as controls. Ferrous iron accumulation was assayed as described above.
Enrichment cultures and isolation preparations were initiated after maximal iron reduction in the root incubation preparations. Subsamples of roots and medium were transferred to screw-cap test tubes containing a ferric citrate medium at pH 6.8 (12, 25). Several carbon sources, including acetate, glucose, succinate, and toluene (all at a concentration of 30 mM) as well as yeast extract (0.5%), were used for freshwater enrichment cultures; acetate, ethanol, glucose, and lactate were used for marine enrichment cultures. After multiple liquid phase transfers, culture dilutions (10−7 to 10−9) were plated onto solid ferric citrate medium containing 1.5% agar. Individual colonies were examined for purity and were subjected to additional characterization procedures. The salinity tolerance characteristics of a freshwater isolate and a marine isolate obtained from the plates were assayed by using a ferric citrate medium containing 25 mM acetate and modified with a 10× sulfate-free artificial seawater solution so that the salinities were two-fold greater than the salinity of seawater. The temperature responses of the same isolates were measured by using the acetate-ferric citrate medium and temperatures ranging from 0 to 51°C.
RESULTS
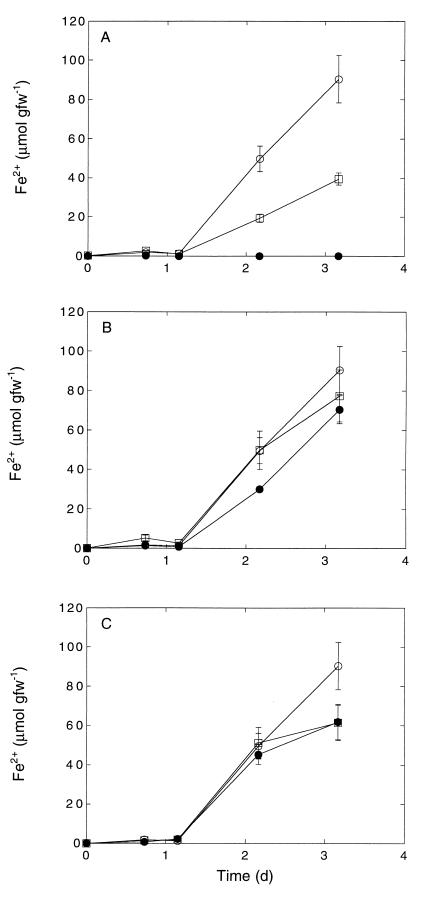
The roots of the plants sampled reduced added ferric oxyhydroxide at rates between about 16 and 83 μmol of Fe3+ g (dry weight)−1 day−1 for marine taxa and 134 to 531 μmol of Fe3+ g (dry weight)−1 day−1 for freshwater species (Table 1), typically with a short lag period of 24 to 48 h (Fig. 1 and 2). Neither exogenous glucose nor exogenous acetate stimulated iron reduction by S. eurycarpum during short-term assays (3 days) compared to roots incubated without added substrates. Ferrous iron from ferric oxyhydroxide plaques on the roots also accumulated in the medium of samples incubated without added ferric iron, although the levels were considerably lower than the levels in treatments with ferric iron (Fig. 1A). We observed little difference in iron reduction between roots incubated with an unbuffered medium and roots incubated with buffer at pH 5.5 (MES) or pH 7.3 (HEPES) (data not shown). No ferrous iron accumulation was observed during incubation with autoclaved roots (Fig. 1 and 3).
TABLE 1.
Estimated maximum potential iron reduction rates by roots of various aquatic macrophytes incubated anaerobically with freshly precipitated ferric oxyhydroxide at 30°C
| Roots | Iron reduction rate (μmol g [dry wt]−1 day−1 |
|---|---|
| Sparganium eurycarpum | 463 ± 20a |
| Pontederia cordata | |
| Smooth | 430 ± 86 |
| Fine | 134 ± 45 |
| Typha latifolia | |
| Smooth | 531 ± 72 |
| Fine | 372 ± 67 |
| Spartina alterniflora | 16 ± 1 |
| Zostera marina | 83 ± 15 |
Values are means ± standard errors based on three determinations.
FIG. 1.
S. eurycarpum Fe3+ reduction. (A) Symbols: ○, roots plus Fe3+; □, roots alone; ●, autoclaved roots. (B) Symbols: ○, roots plus Fe3+; □, roots plus glucose (25 mM); ●, roots plus acetate (25 mM). (C) Symbols: ○, roots plus Fe3+; □, deionized water medium; ●, HEPES (pH 7.3). Data are means ± standard errors based on three determinations. d, day; gfw−1, grams (fresh weight)−1.
FIG. 2.
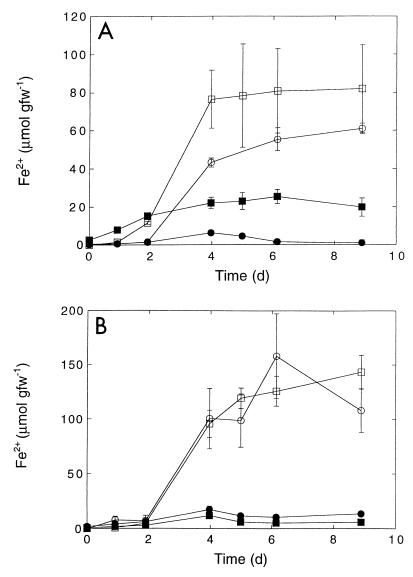
(A) Iron reduction by P. cordata. (B) Iron reduction by T. latifolia roots. Symbols: ○ and □, roots plus Fe3+; ● and ■, roots alone; □ and ■, smooth roots; ○ and ●, fine roots. Data are means ± standard errors based on three determinations. d, day; gfw−1, grams (fresh weight)−1.
FIG. 3.
(A) Iron reduction by Z. marina roots. Symbols: ○, roots plus Fe3+ and 2.2 mM molybdate; ●, roots plus Fe3+; □, roots alone; ■, autoclaved roots. (B) Iron reduction by S. alterniflora roots. Symbols: ○, roots plus Fe3+; □, roots alone; ●, autoclaved roots. Data are means ± standard errors based on three determinations. d, day; gfw−1, grams (fresh weight)−1.
The rates of iron reduction varied as a function of root type for P. cordata, and the activity on smooth roots was greater than the activity on fine roots (Fig. 2 and Table 1). A similar but statistically insignificant pattern was observed for T. latifolia, while the rates were equivalent for the two types of S. eurycarpum roots (Fig. 2 and Table 1; data for S. eurycarpum not shown). During all assays ferrous iron accumulation slowed after approximately 4 days, and there were only modest changes thereafter (Fig. 2 and 3). The maximal amount of iron reduced was usually less than 20% of the amount of ferric iron added. The rates of iron reduction estimated from the maximum slopes for ferrous iron accumulation varied for the taxa assayed, with the highest rates observed for T. latifolia and the lowest rates observed for S. alterniflora (Table 1).
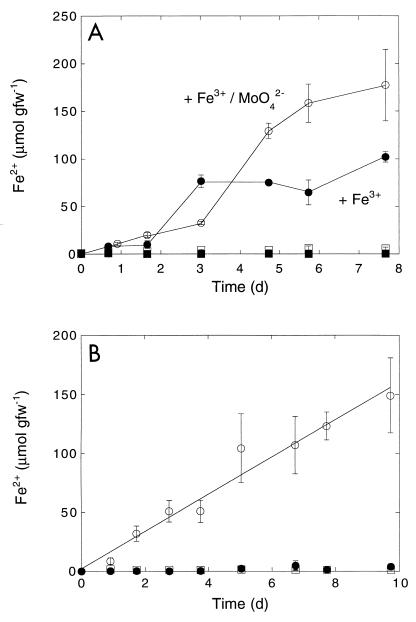
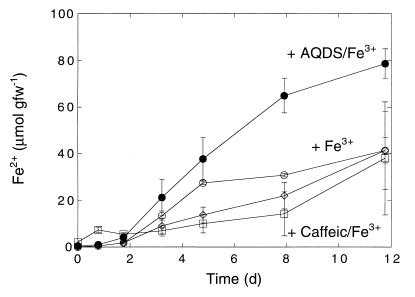
The ferrous iron accumulation during incubation with ferric oxyhydroxide in dialysis bags was limited and essentially equivalent to the ferrous iron accumulation by control roots to which no iron was added (Fig. 4). Addition of 1 mM AQDS stimulated iron reduction significantly, resulting in final ferrous iron concentrations comparable to the concentrations for roots incubated directly with iron (Fig. 1 and 4). However, the reduction rates during incubation with AQDS and iron in dialysis bags were somewhat lower. In contrast, a naturally occurring redox agent, caffeic acid (3,4-dihydroxycinnamic acid), added at a final concentration of 1 mM, did not support iron reduction at levels different than the control levels and inhibited reduction compared to AQDS.
FIG. 4.
Reduction by S. eurycarpum roots of Fe3+ in dialysis bags. Symbols: ●, roots plus AQDS and Fe3+; ○, roots plus Fe3+; □, roots plus caffeic acid and Fe3+; ◊, roots alone. Data are means ± standard errors based on three determinations. d, day; gfw−1, grams (fresh weight)−1.
Iron reduction by S. alterniflora and Z. marina roots occurred after a short lag period similar to the lag period observed with roots from freshwater plants (Fig. 3). The activity of Z. marina roots did not depend on exogenous sulfate and was not affected or slightly stimulated by addition of 2.2 mM sodium molybdate, a sulfate reduction inhibitor (Fig. 3A). The responses of S. alterniflora roots to molybdate at final concentrations of 0.2 to 22 mM were similar to the results obtained for Z. marina (data not shown). Hydrogen did not significantly affect S. alterniflora iron reduction (data not shown).
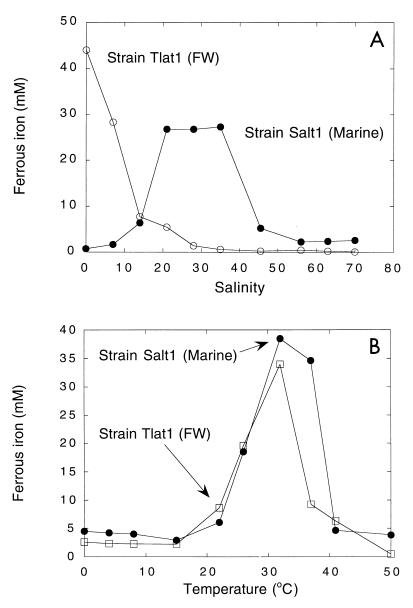
Root- and sediment-free enrichment cultures were obtained for all taxa. Most of the enrichment cultures were substantially pure, consisting of two or three morphotypes. In several cases pure cultures were obtained readily. Isolates obtained from roots of T. latifolia and S. alterniflora (isolates Tlat1 and Salt1, respectively) were morphologically similar to Geobacter species (i.e., short rods or slightly curved rods that were 1 to 2 by 0.5 μm and gram-negative and lacked spores). However, with strain Salt1, optimum iron reduction occurred at salinities between 20 and 35 ppt, while in contrast, iron reduction by Tlat1 decreased with increasing salinity (Fig. 5A). The temperature optima for Salt1 and Tlat1 were similar (about 32°C), although Salt1 exhibited slightly greater temperature tolerance between 33 and 40°C (Fig. 5B). A limited survey indicated that both strains could oxidize and grow on a similar range of substrates, including acetate, ethanol, succinate, and yeast extract; Tlat1 also utilized toluene, while Salt1 used lactate. Both strains used ferric citrate, amorphous iron oxyhydroxide, and AQDS as electron acceptors and were capable of reducing uranyl acetate; neither strain reduced sulfate by a dissimilatory mechanism.
FIG. 5.
(A) Iron reduction by a marine isolate (Salt1) and a freshwater isolate (Tlat1) as a function of medium salinity. (B) Iron reduction as a function of incubation temperature. Data are means of data from duplicate assays. FW, freshwater.
DISCUSSION
Freshwater and marine wetlands are well-known sites of intense biogeochemical cycling driven by high rates of primary production (29). Fermentation-methanogenesis accounts for a substantial fraction of the anaerobic carbon flow in the former environments, while sulfate reduction usually dominates the latter (17, 18). However, Roden and Wetzel (34) showed that iron reduction in unvegetated surface sediments of a weakly acidic, iron-rich southeastern wetland oxidizes levels of organic matter comparable to the levels oxidized by the combination of fermentation and methanogenesis. More importantly, these authors documented that relatively large pools of ferric iron and rapid rates of iron reduction occur in rhizosphere sediments of a common rush, Juncus effusus. Their observations also indicated that iron reduction inhibits rhizosphere methanogenesis. Since root turnover and exudation provide major sources of organic substrates for methanogenesis (41), processes that limit organic matter availability can have a substantial impact on wetland-atmosphere methane fluxes.
Data presented here (Table 1) indicate that the surfaces (rhizoplanes) of roots growing in acidic (pH ∼4) peat support active populations of iron reducers in addition to the populations found in anoxic rhizosphere sediments. After a brief lag period that probably reflected disturbance due to processing, iron was reduced by freshwater roots at rates that were typically >100 μmol g (dry weight)−1 day−1. In contrast, the rates of root-associated methanogenesis measured previously under comparable conditions with similar plants varied from 1 to 4 μmol of CH4 produced g (dry weight)−1 day−1 (20). The potential for root-associated iron reduction substantially exceeds the potential for methanogenesis even after allowing for the difference in reducing equivalents required for iron (1) and for methane (up to 8). Indeed, the potential iron reduction rates observed in this study roughly equal or exceed the rates of aerobic methane oxidation (24 to 240 μmol g [dry weight]−1 day−1 [20]). Thus, iron reduction may prove to be one of the most active heterotrophic processes in the freshwater rhizoplane.
Although a number of previous observations have documented that a variety of aerobic processes (e.g., ammonia, methane, CO, and iron oxidation) occur in the rhizoplane (8, 9, 16, 20, 31, 32), anaerobic processes (e.g., methanogenesis and denitrification) have also been reported (20, 31). The significance of these processes and iron reduction in particular likely results from the fact that roots are not uniformly permeable to oxygen; oxygen leakage or diffusion into the rhizoplane varies as a function of root age, morphology, and plant species (2–4, 8, 14, 35, 37). In addition, high densities of aerobic heterotrophs may create anoxic microzones that promote anaerobic activity.
The similarity in rates among the three freshwater taxa (Table 1) suggests that specific plant attributes, such as root ventilation mechanisms, gross root morphology, and phenology, have little impact on colonization by iron reducers. Root-associated iron reduction thus appears to be a general phenomenon, perhaps limited only by iron availability. Since iron oxyhydroxide plaques occur routinely due to chemical and plant iron oxidation (3, 4), aquatic macrophyte roots represent an important, common habitat for iron reducers that extends to depths well below the wetland surface.
The results of assays performed with AQDS and iron oxyhydroxide in dialysis bags indicate that humic acids or humic acid precursors may also promote root colonization by iron reducers (Fig. 4). Humic acid electron acceptors can substitute for ferric iron (27) and sustain metabolic activity to the extent that they are reoxidized. Reoxidation occurs via chemical coupling with ferric iron or other oxidants, perhaps including molecular oxygen leaking from roots. Humic acids can thereby function as a redox shuttle, allowing iron reducers to occupy anoxic regions of the rhizoplane adjacent to areas with oxygen leakage and ferric iron deposition. Obviously, such a redox shuttle can promote the activity of iron reducers in the rhizosphere as well, with the rhizoplane and its ferric oxyhydroxide plaques acting as an electron sink. However, use of humic acids as electron acceptors may be limited to specific humic acid (or precursor) structures (36). For example, caffeic acid, a plant metabolite related to humic acid precursors that is readily oxidized by ferric iron (38), does not appear to promote root-associated iron reduction (Fig. 4) and indeed may be inhibitory. This may be due in part to the fact that it does not form a quinone (36).
Based on patterns of ferrous iron accumulation, iron-reducing bacteria colonize the rhizoplanes of two marine macrophytes (Table 1 and Fig. 3). However, iron reduction by S. alterniflora and Z. marina roots proceeds at rates considerably less than the rates observed for freshwater taxa (Table 1). This may reflect smaller iron reducer populations on the former organisms. Although iron oxyhydroxide plaques occur on marine macrophyte roots, sulfide is abundant in marine rhizosphere sediments (10, 18) along with the sulfidogens that form it (17). Thus, chemical reduction by sulfides may limit the utilization for growth of iron oxyhydroxide plaques, just as sulfide dominates iron transformations in many organic compound-rich marine sediments (39).
Nonetheless, the fact that there is substantial ferrous iron accumulation in the absence of exogenous sulfate suggests that direct bacterial iron reduction contributes at least in part to root iron dynamics. Iron reduction by sulfate reducers has been previously invoked to explain siderite deposits in a salt marsh sediment (13), and various sulfidogens have been shown to reduce ferric iron but have not been grown on ferric iron in culture (24). Whether sulfate reducers, other dissimilatory iron reducers, or both account for iron reduction in the rhizoplane remains uncertain. Since molybdate inhibits sulfate reduction (30), it is tempting to interpret the lack of any inhibitory effect of molybdate on iron reduction (Fig. 3A) as evidence that dissimilatory iron reducers are important. However, molybdate does not inhibit iron reduction by Desulfovibrio desulfuricans (24) or some estuarine enrichments (40). Thus, partitioning iron transformations between sulfate reducers and dissimilatory iron reducers requires additional effort.
In addition to regulating iron availability, sulfur transformations might control the microbiology of iron reduction in the marine macrophyte rhizoplane. Although few marine dissimilatory iron reducers have been described to date, elemental sulfur reduction or thiosulfate reduction (but not sulfate reduction) appears to be a common attribute of the group (5, 11, 33). Elemental sulfur reducers capable of reducing iron (e.g., Desulfuromonas acetoxidans [33]) should be relatively insensitive to molybdate and could account for the Z. marina and S. alterniflora activity reported here. The rhizoplanes of these and other marine macrophytes are probably ideal habitats for a sulfur- and iron-reducing functional group due to the availability of various sulfur species resulting from chemical and microbiological sulfide oxidation. Sulfur and iron reducers (e.g., taxa similar to or including Geobacter sulfurreducens and Pelobacter carbinolicus [6, 26]) may also colonize freshwater plants, particularly in wetlands with elevated sulfate and sulfide concentrations.
It is not yet possible to develop generalizations about the physiological or phylogenetic attributes of root-associated iron reducers, either for marine plants or for freshwater plants. However, a limited preliminary characterization of freshwater strains revealed traits that are comparable to traits of the genus Geobacter (nonmotile, gram-negative rods that are capable of reducing ferric iron, uranate, and AQDS and of oxidizing acetate, ethanol, succinate, and toluene but not hydrogen [7, 12, 25]). A marine strain isolated from an enrichment culture containing molybdate exhibited characteristics similar to characteristics of freshwater isolates but was motile and oxidized lactate but not toluene. Although elemental sulfur reduction has not been examined yet with the marine isolates, motility is consistent with the characteristics of the genus Desulfuromonas. Marine and freshwater isolates are further distinguished by salinity tolerance characteristics; the marine strain examined exhibited a response characteristic of true marine bacteria, while the freshwater strain was strongly inhibited by increasing salinity and was clearly adapted for freshwater regimes (Fig. 5A). Both strains were mesophilic with an optimum temperature near 32°C (Fig. 5B), a value comparable to the values for other iron reducers (6, 7, 25) but notably lower than the optimum temperature for Desulfuromonas palmitatis (11). In spite of the mesophilic nature, significant activity at temperatures ranging from 0 to 20°C (the ecologically relevant range for the study site) indicated that in situ temperatures should not preclude iron reduction by the enrichment cultures and isolates described here.
In conclusion, the results presented here demonstrate that aquatic macrophyte roots harbor active populations of iron reducers that may contribute significantly to wetland biogeochemical cycling, including control of carbon flow through methanogenesis. Plant roots substantially increase the volume of sediment in which iron reduction may occur and probably provide niches that select for a variety of physiologically and perhaps phylogenetically novel strains.
ACKNOWLEDGMENTS
This work was supported in part by grant DEB-9528552 from the National Science Foundation. M.A.G. was the recipient of a National Science Foundation REU training award and a SURE internship from the Darling Marine Center.
We thank K. Hardy and P. Killorian for technical assistance.
Footnotes
Contribution 333 from the Darling Marine Center.
REFERENCES
- 1.Aller R C, Mackin J E, Cox R T., Jr Diagenesis of Fe and S in Amazon inner shelf muds: apparent dominance of Fe reduction and implications for the genesis of ironstones. Cont Shelf Res. 1986;6:263–289. [Google Scholar]
- 2.Andersen F Ø, Kristensen E. Oxygen microgradients in the rhizosphere of the mangrove Avicennia marina. Mar Ecol Prog Ser. 1988;44:201–204. [Google Scholar]
- 3.Armstrong W. The oxidising activity of roots in waterlogged soils. Physiol Plant. 1967;20:920–926. [Google Scholar]
- 4.Armstrong W. Root aeration in the wetland condition. In: Hook D D, Crawford R M M, editors. Plant life in anaerobic environments. Ann Arbor, Mich: Ann Arbor Science; 1978. pp. 269–297. [Google Scholar]
- 5.Caccavo F, Jr, Blakemore R P, Lovley D R. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay Estuary, New Hampshire. Appl Environ Microbiol. 1992;58:3211–3216. doi: 10.1128/aem.58.10.3211-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caccavo F, Jr, Lonergan D J, Lovley D R, Davis M, Stolz J, McInerney M J. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microbiol. 1994;60:3752–3759. doi: 10.1128/aem.60.10.3752-3759.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caccavo F, Jr, Coates J D, Rossello-Mora R A, Ludwig W, Schleifer K H, Lovley D R, McInerney M J. Geovibrio ferrireducens, a phylogenetically distinct dissimilatory Fe(III)-reducing bacterium. Arch Microbiol. 1996;165:370–376. doi: 10.1007/s002030050340. [DOI] [PubMed] [Google Scholar]
- 8.Calhoun A, King G M. Regulation of root-associated methanotrophy by oxygen availability in the rhizosphere of two aquatic macrophytes. Appl Environ Microbiol. 1997;63:3051–3058. doi: 10.1128/aem.63.8.3051-3058.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calhoun A, King G M. Characterization of root-associated methanotrophs from three freshwater macrophytes: Pontederia cordata, Sparganium eurycarpum, and Sagittaria latifolia. Appl Environ Microbiol. 1998;64:1099–1105. doi: 10.1128/aem.64.3.1099-1105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson P R, Jr, Forrest J. Uptake of dissolved sulfide by Spartina alterniflora: evidence from natural sulfur isotope abundance ratios. Science. 1982;216:633–635. doi: 10.1126/science.216.4546.633. [DOI] [PubMed] [Google Scholar]
- 11.Coates J D, Lonergran D J, Phillips E J P, Jenter H, Lovley D R. Desulfuromonas palmitatis sp. nov., a marine dissimilatory Fe(III) reducer that can oxidize long-chain fatty acids. Arch Microbiol. 1995;164:406–413. [PubMed] [Google Scholar]
- 12.Coates J D, Phillips E J P, Lonergan D J, Jenter H, Lovley D R. Isolation of Geobacter species from diverse sedimentary environments. Appl Environ Microbiol. 1996;62:1531–1536. doi: 10.1128/aem.62.5.1531-1536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman M L, Hedrick D B, Lovley D R, White D C, Pye K. Reduction of Fe(III) in sediments by sulphate-reducing bacteria. Nature (London) 1993;361:436–438. [Google Scholar]
- 14.Conlin T S S, Crowder A A. Location of radial oxygen loss and zones of potential iron uptake in a grass and two non-grass emergent species. Can J Bot. 1989;67:717–727. [Google Scholar]
- 15.Emerson D, Moyer C. Isolation and characterization of novel iron-oxidizing bacteria that grow at circumneutral pH. Appl Environ Microbiol. 1997;63:4784–4792. doi: 10.1128/aem.63.12.4784-4792.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emerson D, Weiss J V, Megonigal J P. Iron-oxidizing bacteria are associated with ferric hydroxide precipitates (Fe-plaque) on the roots of wetland plants. Appl Environ Microbiol. 1999;65:2758–2761. doi: 10.1128/aem.65.6.2758-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hines M E, Evans R S, Sharak Genthner B R, Willis S G, Friedman S, Rooney-Varga J N, Devereux R. Molecular phylogenetic and biogeochemical studies of sulfate-reducing bacteria in the rhizosphere of Spartina alterniflora. Appl Environ Microbiol. 1999;65:2209–2216. doi: 10.1128/aem.65.5.2209-2216.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.King G M. The dynamics of sulfur and sulfate reduction in a South Carolina salt marsh. Limnol Oceanogr. 1988;33:376–390. [Google Scholar]
- 19.King G M. Dehalogenation in marine sediments containing natural sources of halophenols. Appl Environ Microbiol. 1988;54:3049–3058. doi: 10.1128/aem.54.12.3079-3085.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King G M. Associations of methanotrophs with the roots and rhizomes of aquatic vegetation. Appl Environ Microbiol. 1994;60:3220–3227. doi: 10.1128/aem.60.9.3220-3227.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.King G M. In situ analyses of methane oxidation associated with the roots and rhizomes of a bur reed, Sparganium eurycarpum, in a Maine wetland. Appl Environ Microbiol. 1996;62:4548–4555. doi: 10.1128/aem.62.12.4548-4555.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lovley D R. Microbial reduction of iron, manganese and other metals. Adv Agron. 1995;54:175–231. [Google Scholar]
- 23.Lovley D R, Phillips E J P. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987;53:2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovley D R, Roden E E, Phillips E J P, Woodward J C. Enzymatic iron and uranium reduction by sulfate-reducing bacteria. Mar Geol. 1993;113:41–53. [Google Scholar]
- 25.Lovley D R, Giovannoni S J, White D C, Champine J E, Phillips E J P, Gorby Y A, Goodwin S. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol. 1993;159:336–344. doi: 10.1007/BF00290916. [DOI] [PubMed] [Google Scholar]
- 26.Lovley D R, Phillips E J P, Lonergan D J, Widman P K. Fe(III) and S° reduction by Pelobacter carbinolicus. Appl Environ Microbiol. 1995;61:2132–2138. doi: 10.1128/aem.61.6.2132-2138.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lovley D R, Coates J D, Blunt-Harris E L, Phillips E J P, Woodward J C. Humic substances as electron acceptors for microbial respiration. Nature (London) 1996;382:445–448. [Google Scholar]
- 28.Luther G W, III, Ferdelman T G, Kostka J E, Tsamakis E J, Church T M. Temporal and spatial variability of reduced sulfur species (FeS2, S2O2/3−) and porewater parameters in salt marsh sediments. Biogeochemistry. 1991;14:57–88. [Google Scholar]
- 29.Mitsch W J, Gosselink J G. Wetlands. New York, N.Y: Van Nostrand Reinhold; 1986. [Google Scholar]
- 30.Oremland R S, Capone D G. Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv Microb Ecol. 1988;10:285–383. [Google Scholar]
- 31.Reddy K R, Patrick W H, Jr, Lindau C W. Nitrification-denitrification at the plant root-sediment interface in wetlands. Limnol Oceanogr. 1989;34:1004–1013. [Google Scholar]
- 32.Rich J J, King G M. Carbon monoxide oxidation by bacteria associated with the roots of freshwater macrophytes. Appl Environ Microbiol. 1998;64:4939–4943. doi: 10.1128/aem.64.12.4939-4943.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roden E E, Lovley D R. Dissimilatory Fe(III) reduction by the marine microorganism Desulfuromonas acetoxidans. Appl Environ Microbiol. 1993;59:734–742. doi: 10.1128/aem.59.3.734-742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roden E E, Wetzel R G. Organic carbon oxidation and suppression of methane production by microbial Fe(III) oxide reduction in vegetated and unvegetated freshwater wetland sediments. Limnol Oceanogr. 1996;4:1733–1748. [Google Scholar]
- 35.Sand-Jensen K, Prahl C, Stokholm H. Oxygen release from roots of submerged aquatic macrophytes. Oikos. 1982;38:349–354. [Google Scholar]
- 36.Scott D T, McKnight D M, Blunt-Harris E L, Kolesar S E, Lovley D R. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ Sci Technol. 1998;32:2984–2989. [Google Scholar]
- 37.Smits A J M, Laan P, Thier R H. Root aerenchyma, oxygen leakage patterns and alcoholic fermentation ability of the roots of some nymphaeid and isoetid macrophytes in relation to the sediment type of their habitat. Aquat Bot. 1990;38:3–17. [Google Scholar]
- 38.Solinas V, Deiana S, Gessa C, Pistidda C, Rausa R. Reduction of the Fe(III)-desferrioxamine-B complexes by caffeic acid: a reduction mechanism of biochemical significance. Soil Biol Biochem. 1996;28:649–654. [Google Scholar]
- 39.Thamdrup B, Fossing H, Jørgensen B B. Manganese, iron, and sulfur cycling in a coastal marine sediment, Aarhus Bay, Denmark. Geochim Cosmochim Acta. 1994;58:5119–5129. [Google Scholar]
- 40.Tugel J B, Hines M E, Jones G E. Microbial iron reduction by enrichment cultures isolated from estuarine sediments. Appl Environ Microbiol. 1986;52:1167–1172. doi: 10.1128/aem.52.5.1167-1172.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whiting G J, Chanton J P. Primary production control of methane emission from wetlands. Nature (London) 1993;364:794–795. [Google Scholar]