Abstract
Purpose
Terminated clinical trials are an inefficient use of financial, patient, and administrative resources. We reviewed ClinicalTrials.gov for completed and terminated clinical trials for glioblastoma multiforme (GBM) and compared reported characteristics of completed and terminated trials to identify factors associated with early trial termination.
Methods
ClinicalTrials.gov was queried to identify all completed and terminated GBM-related clinical trials. Trial characteristics were examined and the reason for trial termination was determined. Univariate analysis by Pearson’s chi-square and a multivariate logistic regression were performed to identify independent predictors of early trial termination.
Results
We identified 886 completed and terminated GBM-related trials between 2003 and 2020. Of these, 175 (19.8%) were terminated prior to completion. The most common reason for termination was participant accrual difficulties, accounting for 63 (36.0%) terminated trials. Trial termination was associated with trials that reported a primary purpose of diagnosis relative to treatment (OR = 2.952, p = 0.001).
Conclusion
Early termination of clinical trials investigating interventions for the treatment of GBM is associated with diagnostic trials relative to therapeutic trials. Patient accrual difficulties are the most commonly identified reason for early trial termination. Predictors of trial termination should be considered when designing GBM-related clinical trials to minimize the odds of early trial termination.
Keywords: Clinical trials, Clinical trial termination, Glioblastoma, Glioma, Glioblastoma trials
Introduction
Glioblastoma multiforme (GBM) is the most common primary central nervous system tumor [1]. With an incidence of 3.2 per 100,000 population, median survival of 15 months, and 5.5% 5-year survival rate, it is a morbid and poorly survived condition [1, 2]. Although GBM prognosis has not changed dramatically in the past decades, successful clinical trials help augment overall survival and are necessary to improve outcomes [2, 3].
Unfortunately, GBM trials commonly suffer from design inefficiencies during trial design and execution resulting in trial termination. Poor patient accrual remains the main reason associated with trial failure with only 8–11% of newly diagnosed GBM patients enrolling in clinical trials [4]. Additional reasons for early trial termination can vary from logistic to scientific issues. While patient accrual issues remain the most common, changes in sponsor decisions, determination of interventional futility, or safety issues are also commonly identified as other causes of early termination [5]. Terminated studies place an ethical burden on study subjects that can vary in scope with termination cause [6]. Whereas trials terminated for reasons such as futility and safety provide information that can be used as guidance for the diagnosis, treatment and outcomes for GBM patients, trials terminated for accrual issues and business decisions, among other reasons, serve little scientific purpose and fruitlessly expose patients to the risk of experimental treatment. In addition, clinical trials involve a substantial financial, administrative, and physical investment and prematurely terminated trials create financial and administrative burdens while failing to fulfill their scientific objectives [5]. Given the importance of improving clinical trial participation and completion, a 2020 Think Tank held by the Society for Neuro-Oncology intended to highlight areas for improvement in GBM clinical trials to address several recent clinical trials that failed to meet primary endpoints and suggested optimizing eligibility criteria and efficacy assessments prior to trial completion [3]. This is particularly important in later phase trial designs, such as Phase III and IV.
Section 801 of the Food and Drug Administration Amendments Act of 2007 (FDAAA 801) established the clinical trial registry, ClinicalTrials.gov, containing data on the recruitment status and characteristics of clinical trials [7]. ClinicalTrials.gov defines completed trials as those that have “ended normally, and participants are no longer being examined or treated” whereas those that are designated as terminated “[have] stopped early and will not start again” [8].
Prior studies have characterized the landscape of GBM-related clinical trials and assessed reasons for poor accrual [3, 4, 9–11]. However, to date studies assessing additional trial design characteristics associated with early trial termination are limited [3, 4, 9–11]. Here we review completed and terminated GBM-related clinical trials and assess causes of early trial termination. We further compare reported characteristics of completed and terminated GBM-related clinical trials to determine factors associated with early trial termination.
Materials and methods
Study sample
ClinicalTrials.gov was queried on March 31, 2022 by filtering by condition or disease “glioblastoma” to identify all GBM-related clinical trials. The recruitment status was then filtered to group all studies classified as either complete or terminated, and interventional studies were selected to identify clinical trials. Observational studies were excluded. A total of 886 trials were identified and included in further analysis, with the earliest interventional trial starting in 2003 and the latest ending in 2020.
Categorization of reasons for termination
Reason for trial termination was derived from recent analyses of cardiovascular trials [12]. The reason for study termination was grouped into one of the following categories: (1) participant accrual difficulties, (2) administrative or financial reasons, (3) futility, or changes in risk/ benefit profile, (4) sponsor decisions, (5) treatment recall, (6) study replacement or changes in standard of care, (7) COVID-19 pandemic–related or (8) other or unspecified reasons. Although the Why Study Stopped data element was only mandated for trials with a study start date on or after January 18, 2017, we found that 140 of 158 terminated trials (88.6%) identified with a start date prior to this date still reported a reason for termination [13].
Trial characteristics
Trial characteristics were extracted from ClinicalTrials.gov and categorically grouped to characterize the design of each trial. Variables analyzed were adapted from recent analyses of terminated cardiovascular clinical trials [12].
Study design was characterized by the trial phase, allocation model, employment of blinding, intervention model, and primary treatment type. Those studies designated as early phase I or phase I were categorized as phase I. Phase II and phase I/II studies were considered phase II studies. Phase III encompassed phase II/III and phase III studies, and phase IV studies were only comprised of phase IV studies. Study allocation was categorized as randomized, non-randomized, not applicable, or not reported. Blinding was abstracted dichotomously, with a positive result indicating those studies in which participants, investigators, care providers, and/ or outcomes assessors were blinded, and a negative result indicating no blinding or an open-label trial. Intervention model was used to characterize group assignment, and categorized as either cross-over, factorial, single-group, parallel, or not reported. The primary treatment type was abstracted and categorized as either behavioral, biological, drug, device, dietary supplement, drug, genetic, procedure, radiation, or other. Treatments designated as “other” included laboratory biomarker analyses and palliative therapy, amongst others.
Additional variables analyzed were funding sources, gender selectivity, primary study purpose, age eligibility, and number of study sites. Funding sources for each trial were categorized based on whether studies received funding from any combination of industry sources, the National Institutes of Health (NIH) or United States Federal agencies, or other sources including: academic institutions, such as universities and hospitals, individuals, or local organizations. Gender selectivity was characterized dichotomously, as either gender selective or nonselective. The primary study purpose was categorized as one of either basic science, device feasibility, diagnostic, health services research, screening, supportive care, prevention, treatment, or other. Age eligibility followed categories established by ClinicalTrials.gov, grouped by whether trials recruited any combination of the following groups: child (birth–17), adult (18–64), or older adult (65+) [8]. The number of study sites was abstracted and studies were categorized as either single-or multi-center if the number of study locations was equal to 1 or greater than 1, respectively.
Data analyses
The distribution of trial characteristics was compared by a univariate chi-square analysis for preliminary analysis. Significance level was set to p < 0.05. The results are found in Table 2.
Table 2.
Trial characteristics of completed and terminated GBM-related clinical trials
| Completed trials | Terminated trials | Total | p value | |
|---|---|---|---|---|
| n = 711 (80.2%) | n = 175 (19.8%) | n = 886 (100%) | ||
| Primary treatment | ||||
| Behavioral | 4 (100%) | 0 (0%) | 4 | 0.632 |
| Biological | 97 (80.8%) | 23 (19.2%) | 120 | |
| Device | 16 (66.7%) | 8 (33.3%) | 24 | |
| Dietary supplement | 7 (70.0%) | 3 (30.0%) | 10 | |
| Drug | 509 (80.7%) | 122 (19.3%) | 631 | |
| Genetic | 1 (50.0%) | 1 (50.0%) | 2 | |
| Procedure | 24 (80.0%) | 6 (20.0%) | 30 | |
| Radiation | 40 (83.3%) | 8 (16.7%) | 48 | |
| Other | 13 (76.5%) | 4 (23.5%) | 17 | |
| Gender | ||||
| Nonselective | 710 (80.3%) | 174 (19.7%) | 884 | 0.282 |
| Selective | 1 (50.0%) | 1 (50.0%) | 2 | |
| Phase | ||||
| 1 | 251 (80.4%) | 61 (19.6%) | 312 | 0.818 |
| 2 | 375 (80.6%) | 90 (19.4%) | 465 | |
| 3 | 40 (80.0%) | 10 (20.0%) | 50 | |
| 4 | 1 (50.0%) | 1 (50.0%) | 2 | |
| N/A | 44 (77.2%) | 13 (22.8%) | 57 | |
| Funding source | ||||
| Industry only | 151 (80.3%) | 37 (19.7%) | 188 | 0.266 |
| NIH only | 84 (84.0%) | 16 (16.0%) | 100 | |
| Other only | 178 (76.1%) | 56 (23.9%) | 234 | |
| Multi | 298 (81.9%) | 66 (18.1%) | 364 | |
| Randomization | ||||
| Randomized | 123 (79.4%) | 32 (20.6%) | 155 | < 0.0001 |
| Non-randomized | 171 (81.8%) | 38 (18.2%) | 209 | |
| Not applicable | 311 (75.7%) | 100 (24.3%) | 411 | |
| Not reported | 106 (95.5%) | 5 (4.5%) | 111 | |
| Intervention model | ||||
| Crossover | 5 (83.3%) | 1 (16.7%) | 6 | 0.0001 |
| Factorial | 1 (100%) | 0 (0.0%) | 1 | |
| Single-group | 416 (77.5%) | 121 (22.5%) | 537 | |
| Sequential | 16 (84.2%) | 3 (15.8%) | 19 | |
| Parallel | 154 (77.0%) | 46 (23.0%) | 200 | |
| Not reported | 119 (96.7%) | 4 (3.3%) | 123 | |
| Blinding | ||||
| Blinded | 36 (81.8%) | 8 (18.2%) | 44 | < 0.0001 |
| Unblinded | 578 (77.8%) | 165 (22.2%) | 743 | |
| N/A | 97 (98.0%) | 2 (2.0%) | 99 | |
| Primary purpose | ||||
| Basic science | 6 (66.7%) | 3 (33.3%) | 9 | 0.033 |
| Device feasibility | 1 (100%) | 0 (0%) | 1 | |
| Diagnostic | 24 (60.0%) | 16 (40.0%) | 40 | |
| Health services research | 3 (100%) | 0 (0%) | 3 | |
| Screening | 1 (100%) | 0 (0%) | 1 | |
| Supportive care | 14 (73.7%) | 5 (26.3%) | 19 | |
| Prevention | 0 (0%) | 1 (100%) | 1 | |
| Treatment | 651 (81.6%) | 147 (18.4%) | 798 | |
| Other | 8 (80.0%) | 2 (20.0%) | 10 | |
| Not reported | 3 (75.0%) | 1 (25.0%) | 4 | |
| Number of study centers | ||||
| Single center | 337 (76.2%) | 105 (23.6%) | 442 | 0.011 |
| Multicenter | 343 (84.5%) | 63 (15.5%) | 406 | |
| Not reported | 31 (81.6%) | 7 (18.4%) | 38 | |
| Age | ||||
| Child | 2 (66.7%) | 1 (33.3%) | 3 | 0.748 |
| Child, adult | 51 (76.1%) | 16 (23.9%) | 67 | |
| Child, adult | 35 (85.4%) | 6 (14.6%) | 41 | |
| Older adult | ||||
| Adult | 1 (100%) | 0 (0%) | 1 | |
| Adult, older adult | 615 (80.5%) | 149 (19.5%) | 764 | |
| Older adult | 7 (70.0%) | 3 (30.0%) | 10 | |
Significant values are identified in bold
A multivariate logistic regression was implemented to determine independent factors associated with trial termination as a function of several trial characteristics. Any factor that occurred in less than 1% of the trials was removed, and any predictor that occurred in more than 99% of trials was also removed. Furthermore, any predictors where the results would not be meaningful (e.g., the “other” or “unreported” categories) were also removed. A significance level of p < 0.05 was used, and the Bonferroni correction was utilized to correct for multiple comparisons, providing an adjusted significance level of p < 0.01. All inferential testing performed is intended to be exploratory.
All statistical analysis was performed in MATLAB R2020a (Mathworks Inc., Natick, MA) using custom scripts and functions. All tables were created in Microsoft Excel (Microsoft Inc., Redmond, WA) and figures were created with Adobe Illustrator (Adobe Inc., San Jose, CA). All data and scripts can be made available upon reasonable request.
Results
Reasons for trial termination
Participant accrual difficulties were cited as the most common cause of study termination and was identified in 63 of 175 terminated studies (36.0%). Less common causes of study termination included sponsor decisions in 34 trials (19.4%), followed by futility or changes in risk/ benefit profile because of study findings in 26 trials (14.9%). Administrative or financial reasons included events such as the principal investigator changing institution amongst others and accounted for 18 (10.3%) terminated trials. Study replacement or changes in standard of care accounted for 7 (4.0%) terminated studies and included those studies which were terminated because of external information, such as results of other trials. Reason for trial termination was either not provided or ambiguous for 19 (10.9%) studies. Reasons for trial termination are further detailed in Table 1.
Table 1.
Reasons for early termination of GBM-related clinical trials
| Total terminated trials | n = 175 | % |
|---|---|---|
| Reasons for termination | ||
| Participant accrual difficulties | 63 | 36.0 |
| Administrative or financial reasons | 18 | 10.3 |
| Futility or changes in risk/ benefit profile | 26 | 14.9 |
| Sponsor decision | 34 | 19.4 |
| Treatment recall | 7 | 4.0 |
| Study replacement or changes in standard of care | 7 | 4.0 |
| COVID-19 related | 1 | 0.6 |
| Other reason or reason not provided | 19 | 10.9 |
Univariate analysis of clinical trial characteristics
A total of 886 completed or terminated GBM-related clinical trials were identified between 2003 and 2022, of which 711 (80.2%) were completed and 175 (19.8%) were terminated. Trial characteristics are reported in Table 2.
The most common intervention type was drug-related (n = 631; 71.2%). The most common primary study purpose reported was treatment (n = 798; 90.1%). Univariate analysis by Pearson’s chi-square revealed statistically significant differences between completed and terminated trials in randomization (p < 0.0001), intervention model (p = 0.001), blinding (p < 0.0001), primary purpose (p = 0.033), and number of study centers (p = 0.011).
Multivariate assessment of predictors for trial termination
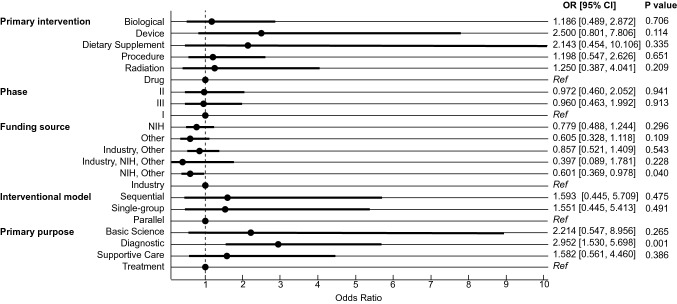
Multinomial logistic regression analysis was executed to analyze the relationship between trial characteristics and trial termination to determine independent predictors of trial termination. Most notably, trial termination was associated with trials that reported a primary purpose of diagnosis relative to treatment [OR = 2.952, 95% CI (1.530, 5.698), p = 0.001]. The results are summarized in Fig. 1.
Fig. 1.
Forest plot demonstrating results of multinomial logistic regression for terminated GBM-related clinical trials. “Other” funding sources include academic institutions such as universities, hospitals, individuals, or community-based organizations. Reference values for primary intervention, phase, funding source, interventional model, and primary purpose were drug, phase I, industry, parallel assignment, and treatment, respectively. OR odds ratio, CI confidence interval, NIH National Institutes of Health, Ref reference
Discussion
Considering the high mortality and poor prognosis associated with GBM, clinical trials remain of considerable importance to advance diagnostics and therapeutics for patients. Clinical trials involve a substantial financial, administrative, and physical investment and prematurely terminated trials create financial and administrative burdens while failing to fulfill their scientific objectives [5]. We reviewed completed and terminated clinical trials to identify trial characteristics that may be associated with early trial terminations.
In this study, documented reasons for trial termination were analyzed. Early trial termination was encountered in 19.8% of trials monitored on ClinicalTrials.gov dating back to 2003. Trials were most commonly terminated for participant accrual difficulties, constituting 36.0% of all terminated trials. Low participant accrual has previously been acknowledged as a problem in GBM-related trials and previous reports have suggested that only 8–11% of newly diagnosed GBM patients are enrolled in clinical trials [3, 4, 9–11]. While analyses of clinical trials in other fields most commonly cite participant accrual as the primary cause of trial termination, GBM-related trials are comparatively terminated at a higher rate than seen in analyses of terminated trials investigating other conditions [4, 5, 12]. A study in 2013 by Williams et al. analyzing all clinical trials reported on ClinicalTrials.gov concluded that approximately 12% of trials resulted in termination, 57% of which were due to participant accrual difficulties, 21% due to findings of the trial, and 10% for unspecified reasons [5].
GBM-related trials remain plagued by lengthy trial development times, inefficiencies in compiling and disseminating trial results, and a lack of easily accessible results, leading to poor patient understanding of the state of GBM therapies [4, 14]. Survey studies of brain tumor patients and prior studies have identified that clinicians fail to adequately educate brain tumor patients on clinical trials [10, 14]. Additionally, narrow inclusion criteria have been repeatedly cited as a barrier to meeting recruitment goals in clinical trials, and have been shown to particularly impair trial access in older adults [9, 15, 16]. Broadening eligibility criteria when possible and engaging referring providers such as primary care providers can help increase accrual [10, 11, 14, 16]. GBM is a disease primarily of older age, and therefore GBM-related clinical trials may particularly benefit from taking these principles into account during study design to minimize accrual difficulties [1, 11].
Furthermore, low patient enrollment in cancer-related clinical trials has been shown to disproportionately affect racial and ethnic minorities, women, and the elderly [15]. Socioeconomic barriers, distrust of the healthcare system, stringent inclusion criteria, language barriers, and race-related disparities in the diagnosis of conditions may offer an explanation for these disparities, as well as for low patient recruitment overall [15]. Problems with patient recruitment and accrual are further underscored by “Lasagna’s Law” which describes investigators’ tendency to overestimate participant goals for clinical trials [17]. In addition to overestimating predicting accrual, a recent study demonstrated that principal investigators tend to overestimate favorable trial outcomes [18]. Our results show that combined, accrual issues and trial outcome related issues (i.e., termination due to futility or safety) are responsible for 50.9% of terminations in GBM-related trials supporting previous hypotheses [15, 17, 18].
Our findings further support the ethical burdens placed on patients by failed GBM-related trials. Accrual issues, financial reasons, and sponsor decisions represent reasons for premature termination that fail to provide useful insights, and constitute 65.7% of terminated GBM trials. Patients enrolled in these trials undergo the risk of experimental treatment and bear an undue ethical burden when trials are terminated for reasons that do not provide practical information [6]. These findings underscore the need for investigators to set achievable recruitment targets for trials, with appropriately designed recruitment strategies and inclusion criteria to increase odds of trial completion.
Multivariate analysis identified independent predictors of early trial termination. Interestingly, logistic regression demonstrated increased odds of trial termination for trials with a primary purpose of diagnosis (OR = 2.952). The greater odds of termination in trials with a primary purpose of diagnosis relative to those that targeted treatment may reflect the motivations of patients enrolling in trials. Trials with a primary purpose of diagnosis focus on optimizing detection of disease [19]. Alternatively, a perception of personal benefit from treatment and an altruistic hope for better treatments were found to be motivators of participation [20]. As trials with a primary purpose of diagnosis focus on detection of disease, they may suffer lower patient enthusiasm for enrollment compared to treatment-focused trials due to decreased perceived personal therapeutic benefit and decreased ability to result in curative treatments. We believe study sponsors engaging in diagnostic clinical trials should be wary of the increased odds of termination and particularly take into account suggestions to minimize patient accrual issues, as suggested by prior literature [3, 4, 9–11]. Accrual issues were the most common cause of early termination in diagnostic studies in our analysis, responsible for 5 of 16 terminated diagnostic studies (31.3%). Based on these data, we suggest that investigators seeking to conduct diagnostic clinical trials on GBM take particular care to engage referring providers and broaden inclusion criteria when possible to mitigate patient accrual difficulties [3, 4, 9–11, 14].
This study has several limitations. The present study is intended as a qualitative analysis of trial characteristics and was not intended to comment on the scientific characteristics of GBM-related clinical trials. Data were collected at a single time point from ClinicalTrials.gov, which although considered a relatively comprehensive registry, does not contain all clinical trials. Notably, legal requirements for trial registration can vary by intervention type and trial phase. Therefore, these results should be interpreted in the context that this registry and these data are not comprehensive, and may even be biased towards including trials of certain intervention types and study designs [7]. Trial information reported on ClinicalTrials.gov is also further subject to the interpretation and errors of study sponsors or principal investigators reporting the information, and therefore may vary between studies and over time. Trials reported prior to 2007 were retroactively registered and these data may be less comprehensive. In particular, the retroactive nature of registration of these earlier trials may underestimate the rate of terminated trials in this period as we presume terminated trials would be less likely to retroactively register than completed trials. Additionally, the Why Study Stopped data element was introduced in February 2007, but only mandatory for those studies with a Study Start Date on or after January 18, 2017. Interestingly, we found that 88.6% of trials prior to this date still contained these data [13]. While we believe these data can be generalized to studies moving forward, they do not incorporate the reasons for trial termination for earlier clinical trials and those not registered on ClinicalTrials.gov.
Conclusion
Early termination was encountered in 19.8% of GBM-related clinical trials, most commonly due to participant accrual difficulties. Trials with a primary purpose of studying GBM diagnostics had increased odds of early termination compared with treatment-focused trials. The determined predictors of clinical trial termination should be taken into consideration during design of GBM-related studies when feasible, to increase odds of trial completion.
Author contributions
All authors contributed to study conception and design. Data collection and analysis were performed by HS, AM, and MG. Figures and tables were prepared by HS and AM. The first draft of the manuscript was written by HS and AM. All authors commented on previous versions of the manuscript, and read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Declarations
Conflict of interest
The authors have no relevant competing interests to disclose.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers. 2014;23(10):1985–1996. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013. Neuro-oncology. 2016 doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley SJ, Kothari S, Rahman R, Lee EQ, Dunn GP, Galanis E, Chang SM, Nabors LB, Ahluwalia MS, Stupp R, Mehta MP, Reardon DA, Grossman SA, Sulman EP, Sampson JH, Khagi S, Weller M, Cloughesy TF, Wen PY, Khasraw M. Glioblastoma clinical trials: current landscape and opportunities for improvement. Clin Cancer Res. 2022;28(4):594–602. doi: 10.1158/1078-0432.CCR-21-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanderbeek AM, Rahman R, Fell G, Ventz S, Chen T, Redd R, Parmigiani G, Cloughesy TF, Wen PY, Trippa L, Alexander BM. The clinical trials landscape for glioblastoma: is it adequate to develop new treatments? Neuro-Oncology. 2018;20(8):1034–1043. doi: 10.1093/neuonc/noy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams RJ, Tse T, DiPiazza K, Zarin DA. Terminated trials in the clinicaltrials. gov results database: evaluation of availability of primary outcome data and reasons for termination. PloS one. 2015 doi: 10.1371/journal.pone.0127242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlisle B, Kimmelman J, Ramsay T, MacKinnon N. Unsuccessful trial accrual and human subjects protections: an empirical analysis of recently closed trials. Clin trials. 2015;12(1):77–83. doi: 10.1177/1740774514558307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Food and Drug Administration Amendments Act of (Sept 27, 2007) Public Law. 110–85, Title VIII—clinical trial databases, 121 STAT. 904
- 8.ClinicalTrials.gov glossary of common site terms. [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biomedical Information. Available: https://clinicaltrials.gov/ct2/about-studies/glossary. Accessed 2 April 2022.
- 9.Lee EQ, Weller M, Sul J, Bagley SJ, Sahebjam S, van den Bent M, Ahluwalia M, Campian JL, Galanis E, Gilbert MR, Holdhoff M, Lesser GJ, Lieberman FS, Mehta MP, Penas-Prado M, Schreck KC, Strowd RE, Vogelbaum MA, Walbert T, Chang SM, Wen PY. Optimizing eligibility criteria and clinical trial conduct to enhance clinical trial participation for primary brain tumor patients. Neuro-Oncology. 2020;22(5):601–612. doi: 10.1093/neuonc/noaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee EQ, Chukwueke UN, Hervey-Jumper SL, de Groot JF, Leone JP, Armstrong TS, Chang SM, Arons D, Oliver K, Verble K, Musella A, Willmarth N, Alexander BM, Bates A, Doherty L, Galanis E, Gaffey S, Halkin T, Friday BE, Fouladi M, Wen PY. Barriers to accrual and enrollment in brain tumor trials. Neuro-Oncology. 2019;21(9):1100–1117. doi: 10.1093/neuonc/noz104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison RA, Anderson MD, Cachia D, Kamiya-Matsuoka C, Weathers SS, O'Brien BJ, Penas-Prado M, Yung W, Wu J, Yuan Y, de Groot JF. Clinical trial participation of patients with glioblastoma at the university of Texas MD Anderson cancer center. Eur J Cancer. 2019;112:83–93. doi: 10.1016/j.ejca.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 12.Bernardez-Pereira S, Lopes RD, Carrion MJ, Santucci EV, Soares RM, de Oliveira Abreu M, Laranjeira LN, Ikeoka DT, Zazula AD, Moreira FR, Cavalcanti AB, Mesquita ET, Peterson ED, Califf RM, Berwanger O. 2014. Prevalence characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: insights from the clinicaltrials gov registry. Am Heart J. [DOI] [PubMed]
- 13.ClinicalTrials.gov protocol registration data element definitions for interventional and observational studies. [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biomedical Information. [Updated 2020 Oct]. Available: http://prsinfo.clinicaltrials.gov/definitions.html. Accessed 2 April 2022.
- 14.Bates AJ, Couillard SA, Arons DF, Yung W, Vogelbaum M, Wen PY, Kingston AE. HOUT-15 brain tumor patient and caregiver survey on clinical trials: identifying attitudes and barriers to patient participation. Neuro-Oncology. 2017 doi: 10.1093/neuonc/nox168.446. [DOI] [Google Scholar]
- 15.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 16.Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, Wildes TM, Le-Rademacher JG, Kimmick GG, Tew WP, George K, Padam S, Liu J, Wong AR, Lynch A, Djulbegovic B, Mohile SG, Dale W, Cancer and Aging Research Group Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. Cancer J Clin. 2021;71(1):78–92. doi: 10.3322/caac.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogin V. Lasagna's law: a dish best served early. Contemp Clin Trials Commun. 2022;26:100900. doi: 10.1016/j.conctc.2022.100900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benjamin DM, Hey SP, MacPherson A, Hachem Y, Smith KS, Zhang SX, Wong S, Dolter S, Mandel DR, Kimmelman J. Principal investigators over-optimistically forecast scientific and operational outcomes for clinical trials. PLoS ONE. 2022;17(2):e0262862. doi: 10.1371/journal.pone.0262862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fares J, Kanojia D, Rashidi A, Ahmed AU, Balyasnikova IV, Lesniak MS. Diagnostic clinical trials in breast cancer brain metastases: barriers and innovations. Clin Breast Cancer. 2019;19(6):383–391. doi: 10.1016/j.clbc.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Institute of Medicine US Committee on Cancer Clinical Trials and the NCI Cooperative Group Program, Nass, S. J., Moses, H. L., Mendelsohn, J., editor. A national cancer clinical trials system for the 21st century: reinvigorating the NCI cooperative group program. US: National Academies Press; 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bernardez-Pereira S, Lopes RD, Carrion MJ, Santucci EV, Soares RM, de Oliveira Abreu M, Laranjeira LN, Ikeoka DT, Zazula AD, Moreira FR, Cavalcanti AB, Mesquita ET, Peterson ED, Califf RM, Berwanger O. 2014. Prevalence characteristics, and predictors of early termination of cardiovascular clinical trials due to low recruitment: insights from the clinicaltrials gov registry. Am Heart J. [DOI] [PubMed]