Abstract
Background
Effective smoking cessation programs exist but are underutilized by smokers, especially by disadvantaged smokers. Cessation interventions in dental settings have been shown to be effective, but are not consistently delivered due to provider burden and lack of training, especially on how to counsel smokers who are not motivated to quit.
Methods
This study is a 2-arm, phase III longitudinal randomized controlled efficacy trial to motivate utilization of evidenced based treatments (EBTs) for smoking cessation (e.g., state quitline, clinic-based counseling, the National Cancer Institute’s text message program, and pharmacotherapy). Patients attending an urban dental clinic (n = 376) will be randomized to an intervention group (INT; smoking cessation induction video delivered via VR headset during their teeth cleaning, brochure about EBTs, and a 4-week text message program) or control group (CTRL; relaxation video delivered via VR headset during teeth cleaning, the same brochure as INT, and assessment-only text messages). Assessments will occur at baseline, immediately after the clinic appointment, one-month post-appointment and 3-and 6 months later. We hypothesize INT will be more likely to contact EBTs vs CTRL and have greater utilization rates of EBTs. Secondary objectives are to test the efficacy of INT on point-prevalence smoking abstinence, quit smoking attempts, and motivation to quit vs. CTRL.
Discussion
Incorporating smoking cessation into a dental clinic visit and targeting all smokers, regardless of motivation to quit, provides proactive reach to cigarette smokers who otherwise may not seek treatment for smoking.
Trial registration
NCT04524533 Registered August 24, 2020.
Keywords: Smoking, Virtual reality headsets, Dentist
Introduction
Smoking remains the top cause of preventable, premature death in the US [1]. The prevalence of smoking is 14% in the general population, but the prevalence is much greater among those with low income and low education (21.4%) [2]. Only 42.2% of ever smokers with incomes below poverty level have quit compared to 64.5% with incomes at or above poverty level [1]. Despite evidence that these smokers have similar motivation to quit as the general population, cessation interventions such as medications, quitlines, and clinic-based programs are underutilized by underserved smokers [3–5]. These smokers are less likely to proactively seek smoking cessation services, so it is critical to find naturalistic settings to reach them, enhance their motivation to quit, and guide them towards evidenced-based treatments (EBTs).
One naturalistic setting to deliver smoking cessation is the dental clinic. Smoking has numerous detrimental effects on oral health, including oral squamous cell carcinoma and pre-cancers, impaired post-procedure healing, periodontal disease, mucosal lesions, gingival recession, dental implant failure [6, 7]. One meta-analysis of 14 smoking cessation studies implemented in dental settings has shown that patients who received brief behavioral counseling are 1.7 times more likely to quit than those who did not receive counseling [8]. However, there are many barriers to consistent implementation of smoking cessation counseling in dental settings, including provider lack of time and training, and lack of training in how to motivate smokers who are unmotivated to quit. Using technology could provide a cost-effective and time-efficient way of delivering evidenced-based smoking cessation in dental settings with a high degree of treatment fidelity.
Our pilot study developed and tested a video-based smoking cessation induction intervention delivered through a Virtual Reality (VR) headset, while patients were undergoing teeth cleaning. Smokers who were patients in an urban dental clinic (n = 23) wore the VR headset to watch a 10-minute smoking cessation induction video during their teeth cleaning. We demonstrated patient satisfaction, feasibility, impact on mediators, and no interference with clinical care. One month later, 5/23 patients reported smoking cessation, and 14/23 reported quit attempts [9].
The next step in this research is to conduct a clinical trial in which dental patients who smoke are randomized to watch either the above cessation induction video or a control video, both of which are viewed on VR headsets during teeth cleaning, in order to test whether the intervention video increases utilization of EBTs (Quitline, Clinic-based programs, NCI text message program, nicotine replacement therapy or other approved smoking cessation medications). We also added a text messaging program to be administered for 4 weeks after participants’ dental appointment, to supplement the video. In this paper, we describe the trial design, intervention content, measures, steps taken to integrate the study into the clinic workflow, and decisions made to maximize patient and provider comfort. Social Cognitive Theory (SCT) [10] was used to provide a foundation for the intervention, such that intervention content targeted SCT constructs (motivation, self-efficacy and outcome expectations) in order to affect behavior change. Previous studies have shown that changes in SCT constructs predict smoking cessation [11, 12].
Our study has potential for clinical public health significance, because although effective and low cost EBTs exist, innovations in treatment delivery are needed to drive smokers to engage with them.
Design
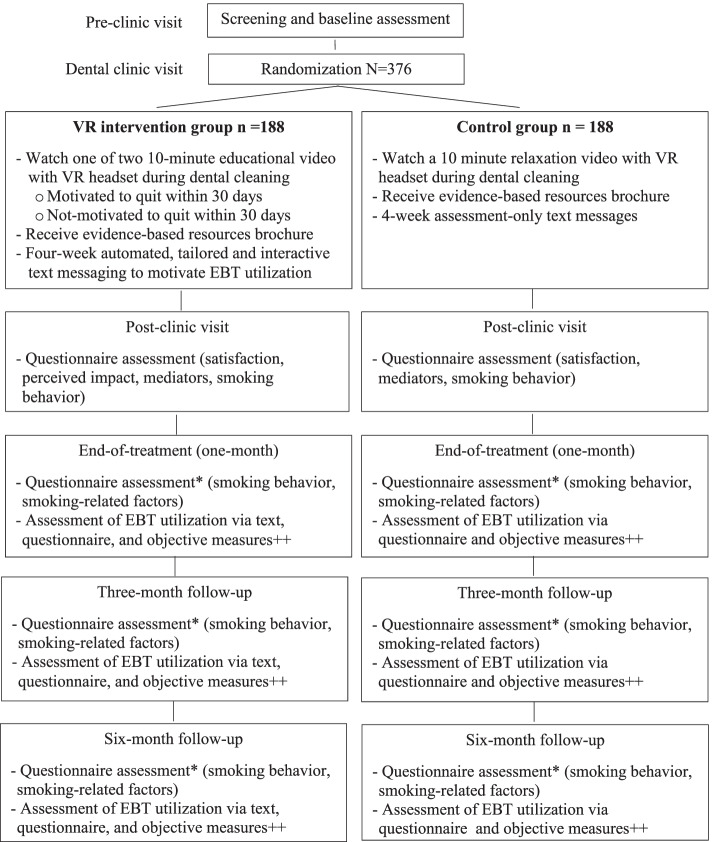
This study is a 2-arm, phase III longitudinal randomized controlled efficacy trial. Dental patients who smoke (n = 376) will be randomized to either intervention (INT) or control (CTRL; Fig. 1). The INT group will receive a 10-minute smoking cessation induction video delivered via VR headset during their teeth cleaning and a tailored, interactive, and automated text message program for four-weeks post-dental appointment to motivate utilization of EBTs. The CTRL group will receive a 10-minute relaxation video delivered via VR headset during teeth cleaning (to maintain masking of dental providers) and assessment-only text messages for 4 weeks post-appointment. Both groups will receive a brochure about EBTs. Assessments will occur at baseline, immediately after the clinic appointment, weekly for 1 month after the appointment (via text), and one-month post-appointment (end-of-treatment), and 3-and 6-months later.
Fig. 1.
CONSORT diagram of participant flow. *Self-report 7-day point-prevalence abstinence will be biochemically validated using cotinine test. ++EBT utilization of the Quitline and NCI text messages are objectively verified. Use of medication to quit and attending clinic programs are self-reported. Smoking status is objectively verified
The primary objective of this trial is to test the efficacy of INT in increasing contact with, and utilization of, EBTs for smoking cessation. We hypothesize that participants randomized to INT, relative to CTRL, will be more likely to contact any EBTs (e.g., the smoker’s quitline, clinic-based smoking cessation counseling, the NCI text message program ‘SmokefreeTXT,’ and pharmacotherapy) and have greater utilization of EBTs (e.g., greater number of counseling sessions and days using pharmacotherapy). The secondary objectives are to test the efficacy of the intervention on point-prevalence smoking abstinence, number of quit smoking attempts, and increasing motivation to quit smoking. As an exploratory aim, we will assess the role of moderators and mediators (SCT theory) of intervention effects. See Fig. 1 for participant flow.
Methods
Study setting
The study setting is the treatment center at Boston University Goldman School of Dental Medicine (BUGSDM). The patients receiving dental care at BUGSDM are local to the community and its surrounding areas and are therefore predominantly racial/ethnic minority and low-income. Of the total patients who received dental treatment in 2018 (prior to the study), 56% were female and the average age was 46 years old. Fifteen percent of patients reported being a current or past smoker; 58% were male, were 47 years old on average, and 59% relied on public dental insurance.
Recruitment and eligibility criteria
We will recruit patients through querying the clinic’s electronic dental record database (Salud) to identify current smokers who have an upcoming dental hygiene appointment (dental prophylaxis or scaling and root planning). These patients will be contacted to assess eligibility and receive a brochure and recruitment letter. Participants will be eligible if they (1) are at least 18 years old; (2) smoked 100 cigarettes or more in their lifetime and at least one cigarette in the past 7 days; (3) are fluent in English; (4) have the ability to see and hear an educational video using headphones that are inserted partially inside the ear; (5) have access to a mobile phone capable of receiving texts from our platform; (6) are willing to participate in a 4-week text messaging program and ‘opt into’ the program; (7) live in Massachusetts and not planning to move or travel for more than 1 month during the study; and (8) complete a baseline questionnaire prior to their scheduled appointment. Participants will be excluded if they: (1) have participated in the previous pilot study; (2) currently use medications for smoking cessation; (3) do not attend their scheduled dental appointment or do not have a rescheduled appointment within 45 days; (4) are currently participating in research involving smoking cessation or text messaging; (5) could not watch the video during the dental appointment. Participants do not have to quit or express a desire to quit smoking to participate in this study. The research assistant will review informed consent with patients who are eligible patients, and informed consent will be provided verbally. After informed consent, the patient’s contact information is stored in REDcap (Research Electronic Data Capture) and the patient is sent (email and text) the baseline questionnaire to complete prior to the visit.
Study visit
At the beginning of the dental appointment, participants will be randomized by the research assistant (RA) using the randomization module within the REDCap program, which then triggers the delivery of the video corresponding to the randomization group to the RA’s study phone. While in the dental chair, participants will be fit for VR headset and earbuds to ensure comfort and functionality. When the dental provider is ready to begin the procedure, the RA will insert the study phone in the participant’s VR headset and start the video. Participants are instructed to signal the RA when the video concludes, and if they are unable to see or hear the video at any time. Videos are not filmed in VR due to potential interference with clinical care. At the end of the video, participants receive a study brochure, compensation schedule, handout on EBTs, and a $30 gift card. Participants will be reminded that they will receive the ‘post-video questionnaire’ immediately after their appointment (via email and text links) and that their 4-week text messaging program will begin the following day.
Intervention group
The INT group will view one of two different 10-minute smoking cessation induction videos through the VR headset during the dental cleaning: one for smokers who are ready to quit in 30 days and one for smokers who are not yet ready to quit. Both videos feature current and former smokers, men and women, and people of different ages, races and ethnicities, and feature personal anecdotes from smokers and guidance from health professionals as well as information on EBTs. The “Ready to Quit” video emphasizes that the combination of behavioral strategies and medication is the most effective way to quit. Video content targets the constructs of our theoretical model (SCT; Table 1). For example, smokers talk about their reasons for quitting (motivation), how they have overcome cravings to smoke (promoting self-efficacy), and a physician discusses the benefits of using medications for quitting smoking (promoting outcome expectations). Former smokers discuss the medications that worked for them, as well as how they brought other activities in their lives to shift the focus away from cigarettes and cravings. The video for smokers ‘not ready to quit’ treads more lightly so as not to elicit denial and defensiveness regarding quitting smoking. Successful quitters admit that they had mixed feelings about quitting and discuss how they resolved their ambivalence. Issues common to unmotivated smokers are discussed (e.g., myths about stop smoking medications, getting stuck in ambivalence). Dr. William Miller, co-founder of Motivational Interviewing [13], discusses ways to resolve ambivalence, such as building a discrepancy between smoking behavior and goals/values. Taking small steps are emphasized and EBTs are discussed.
Table 1.
Text messages mapped onto Social Cognitive Theory constructs
| Social Cognitive Construct | Definition | Text message example |
|---|---|---|
| Behavioral capabilitya | Promote mastery through skills training and observational learning | “(First name), you watched this video when you were getting your teeth cleaned: (link)Feeling more motivated to quit? Here’s another one you might like: (link) |
| Goal settingb | Setting realistic, proximal and specific subgoals | It’s great that you’re trying NCI Texting! Adding Nicotine Replacement, Chantix, or Zyban can boost your chances even further! The MA quitline offers nicotine replacement for free & other low cost meds: 1-800-QUIT-NOW (1-800-784-8669) or https: (link) |
| Motivationa | Cognitions involved in proximal goal setting and activities to initiate change | “(First name) quitting smoking is a difficult decision. Every difficult decision has pros and cons. It’s helpful to make two lists: List the good things about smoking. Then, list the good things about quitting. How do they balance out?” |
| Outcome expectanciesc | Beliefs that changing the behavior will lead to the desired outcomes | Myth: Stop smoking medications don’t work. Truth: Research shows they double your chance of quitting! Chances are greater when adding behavioral support, like quitline or txt messages |
| Self-efficacyb | Confidence in one’s ability to take the necessary actions to achieve the desired outcome, and overcome barriers | “(First Name) Build your confidence to quit smoking by taking small steps! For example, practice the Four D’s before quitting: Delay your first cigarette, Deep breathing practice, Drink water, Do an activity to take your mind off smoking.” |
aExample of text message sent to participants who are not motivated to quit smoking within 30 days
bExample of text message sent to participants who are motivated to quit smoking within 30 days
cExample of text message sent to all participants
After the clinic visit, INT participants will receive 1-2 text messages per day for 1 month, focusing on building motivation to contact one or more EBTs (Table 1). Message features include interactive features (text message questions that allow tailoring of subsequent messages), quizzes, tailoring (e.g., readiness to contact an EBT vs. not ready; ready to quit vs. not ready, participant characteristics), and clickable links to EBTs and to the video they watched during their dental cleaning. Participants will also be given a clickable link to re-watch the video they viewed during the appointment, and a link to watch the other INT video not shown during their appointment.
For those who are not ready to quit, we based our text messages on what we have learned to be appealing to unmotivated smokers from our prior focus groups, interviews, and quantitative studies [14, 15]. Text message content focuses on addressing myths that surround nicotine replacement therapy and other stop smoking medications (e.g., concerns about addiction), taking small steps towards decision-making, providing motivational strategies, reinforcing messages in the video, providing tips on not getting stuck in ambivalence, and enhancing outcome expectations (e.g., that they have a better chance of quitting and having fewer cravings if they use EBTs, particularly a combination of counseling and medication). For those who are motivated to quit, information about EBTs, advantages of EBTs, preparing for cessation, and directions on how to connect with EBTs will be provided. If a participant is ready to quit within 30 days and wants to quit with SmokefreeTXT (the publicly available NCI program), they will be seamlessly transferred into that program by typing an ‘on demand’ keyword “NCI text.” The reason that we are administering the NCI program vs. have participants sign up for the external NCI program is so that we can more closely track usage and add assessments of mediators. Our team made some changes to the program to stay current with standard practice.
Control group
Participants randomized to CTRL will watch a relaxation video that is the same length as the video viewed by the INT (10 minutes), through the VR headset, while getting their teeth cleaned. The video uses guided imagery and depicts a nature setting. A voice-over narrates the details of the context, such as the sounds, tastes, smells, movements, texture, temperature, and pressure. The scene slowly changes (e.g., more flowers are grown, a bird flies by) to keep viewer’s attention. Guided imagery has been extensively documented in the literature [16]. CTRLs will not receive any content of the intervention text messages but rather only the assessment texts.
Randomization procedures
We will conduct a stratified block randomization procedure with small, random size blocks [17]. The randomization sequence was created using SAS 9.4 software (and integrated into REDCap) and is stratified by motivation to quit smoking (ready to quit within 30 days vs. not ready to quit within 30 days) [18], with a 1:1 allocation using random block sizes of 2 and 4. Only the Principal Investigator, the project director, the statistician, and the RAs will be unmasked to treatment condition. These RAs will not be involved in the collection of any outcome data. Because both groups will be watching a video, all dental providers will be blinded to the participant’s group assignment.
Equipment and text message provider
We will use SPECTRE virtual reality headset, which is designed to be compatible with smartphones. It has optical axis sliders, adjustable head-band straps, and capacitive touch buttons. Wired earbuds with mic and volume control will provide sound. We partnered with Agile Health to develop and implement the text message program. Their platform is a rules-based engine that schedules and delivers texts designed to encourage interactions as well as provide automated responses and deliver a personalized user experience. Their platform includes a dashboard that can be viewed from any computer and allows our team to monitor incoming and outgoing messages in real-time, input personalization settings without the help from a programmer, and respond to participant inactivity. The platform is compliant with the Health Insurance Portability and Accountability Act (HIPAA), with encrypted data in transit and at rest, and messages are delivered through a secure gateway in an encrypted format. While messages are ultimately delivered to members’ phones in an unencrypted manner, HIPAA risk is mitigated because there is no disclosure of personally identifiable information or protected health within the messages for this study.
Dental clinic workflow and communication
Boston University GSDM student providers will attend a presentation about the study, and presentations will occur with each new class of student providers. GSDM providers, group practice leaders, and clinic coordinators will be informed of their patient’s participation and the appointment at which study activities will take place. The RA will follow BUGSDM protocols and create a note within the electronic dental record to indicate that the patient is participating in the study.
Measures
Short assessments are given weekly through text messages for 4 weeks after the dental visit, and questionnaires are delivered electronically (REDCap) through links sent via text message and email before the dental visit (baseline), immediately after the dental visit, at the end of the four-week text message program and 3-and 6- months later. REDCap is a secure, web-based app designed to support data capture. It also has features to support cleaning, storage and analysis. See Table 2 for measures.
Table 2.
SPIRIT diagram of assessments and measures
| Timepoint | Study period | |||||||
|---|---|---|---|---|---|---|---|---|
| Enrollment | Baseline Pre-Clinic | Baseline In-Clinic | Post-Clinic Assessment | Weekly (4 weeks) | End-of-treatment | 3-Month Follow-Up | 6-Month Follow-Up | |
| ENROLLMENT | ||||||||
| Patient Identification | x | |||||||
| Eligibility Screen | x | |||||||
| Informed Consent | x | |||||||
| Text Message Enrollment | x | |||||||
| Randomization | x | |||||||
| INTERVENTION | ||||||||
| Control Video | x | |||||||
| Intervention Video | x | |||||||
| Text Message Program | x | |||||||
| ASSESSMENTS | ||||||||
| Sociodemographics | x | |||||||
| Dental Anxiety and Fear [19] | x | |||||||
| Perceived Stress Scale [20] | x | x | x | x | ||||
| Patient Health Questionnaire [21] | x | x | x | x | ||||
| Smoking History | x | |||||||
| Smoking Behavior | x | x | x | x | x | |||
| Nicotine Dependence [22] | x | |||||||
| Motivation to Quit [23, 24] | x | x | x | x | x | x | ||
| Motivation to Use EBTs | x | x | x | x | x | x | ||
| Self-Efficacy For Quitting [25] | x | x | x | x | x | |||
| Self-Efficacy for EBTs | x | x | x | x | x | x | ||
| Outcome Expectancies For Quitting [26] | x | x | x | x | x | |||
| Outcome Expectancies for EBTs | x | x | x | x | x | x | ||
| Quit Smoking Attempts | x | x | x | x | x | |||
| Past Use of Quit Smoking Methods | x | |||||||
| Nicotine Replacement Use [27] | x | x | x | x | x | |||
| Non-Nicotine Medications Use [28] | x | x | x | x | x | |||
| Quitline Use [29] | x | x | x | x | x | |||
| NCI Text Message Use [30] | x | x | x | x | x | |||
| Other Quit Methods Use | x | x | x | x | ||||
| Smoking Status [31] | x | x | x | x | x | |||
| Clinic-Based Cessation Programs [32] | x | x | x | x | x | |||
| Cotinine Assessment [33] | x | x | x | |||||
| Satisfaction | x | |||||||
| Perceived Impact | x | |||||||
| Likeability | x | |||||||
Primary outcome measures
The primary outcome measure is contact with EBTs for smoking cessation which includes: the “Massachusetts Smoker’s Quitline” (MSQ; a free, phone-based counseling service) [29, 34, 35], the National Cancer Institute’s “SmokefreeTXT” text message service to support smoking cessation [30, 36], clinic-based smoking cessation programs [32], and pharmacotherapy (nicotine replacement products or non-nicotine medications) [27, 28, 37]. Participants’ self-report utilization of the MSQ will be objectively verified (yes/no, number of counseling calls completed, and participant’s eligibility for, and provision of, nicotine replacement therapy products). Use of the text messaging program will also be objectively verified, including length of utilization and engagement.
Secondary outcome measures
Smoking abstinence will be assessed via self-report of no smoking, not even a puff, in the preceding 7 days (7-day point prevalence abstinence) [31]. Abstinence will be biochemically verified through salivary cotinine analysis (Salimetrics, Inc.) using the recommended cut off level of 15 ng/mL [33]. Motivation to quit smoking within 30 days will be assessed with Yes/No. Mediators of intervention effects include self-efficacy to refrain from smoking (Smoking Self-Efficacy Questionnaire (SEQ-12)) [25], motivation and readiness to quit smoking (Contemplation Ladder [23, 38] and the Stage of Change [24]), and outcome expectancies using an adapted measure to assess the extent to which it is believed that utilization of cessation resources will facilitate quitting and reduce craving (Smoking Consequences Questionnaire (SCQ)) [26].
During the four-week text message program, both groups will receive single-item mediator assessments through text messages; each of the three mediators (motivation and self-efficacy to use EBTs, and outcome expectations regarding the use of EBTs) are assessed twice during the text message program, each on a 1-10 scale (1 = not at all and 10 = very much). The INT group is additionally asked to report whether they have engaged with one or more of the four EBTs: MSQ, clinic-based programs, SmokefreeTXT, and pharmacotherapy (Nicotine Replacement Therapy, Bupropion or Varenicline). These questions are asked weekly during the text message program and monthly during the follow-up period.
Satisfaction and engagement
At the post-clinic assessment only, we will measure satisfaction with the videos as well as the experience of using the headset to watch the video during their dental cleaning (e.g., overall experience, headset comfort, ability to hear the provider, quality and amount of information presented, quality of animations in the video, and overall production quality). Engagement with the four-week text message program will be assessed automatically through program interaction. Engagement indicators include response rate (number of participants who responded to assessment texts divided by the number of participants), and number of unsolicited texts sent by participants (e.g., emojis, thumbs up, ok, thank you) [39].
Retention strategies
Enrolled participants will receive $30 for completing the baseline assessment, and $20 for completing the post-video questionnaire within 10 days of their dental appointment. For the 1, 3, and 6-month surveys, participants will receive $40 for completing each within 2 weeks of receiving the survey or $30 if they complete it after that time. We will also conduct a monthly $50 raffle during the 4-week text message program. Participants in both groups will receive one raffle entry if they respond to text messages that require a response (such texts will be preceded by a dollar sign “$”). After the intervention period, we will implement various cohort maintenance procedures such as automated survey reminders (text and email), letters, phone calls, text messages, and post-cards to collect updated contact information.
Sample size and analysis plan
The sample size calculation focuses on the comparison of INT vs CTRL on our primary outcome measure utilization of any EBTs for smoking cessation. We based our power analyses on effect sizes on the previous literature on EBT utilization rates [40, 41], on video interventions for smoking cessation [42, 43] and on a meta-analysis on the effectiveness of smoking cessation in dental settings [8]. From these sources, we conservatively estimated an effect size of OR = 2.05 (EBT utilization rates in INT 37.6% and CTRL 22.7%). To achieve 80% power (with a two-sided alpha =0.05 and assumption of 20% attrition), we will need to recruit 188 subjects per group. For the outcome of amount (dose) of EBTs, to our knowledge there are no published trials on which to base power. To be conservative, we based our power analysis on an analysis of covariance model. Assuming a two-sided alpha = 0.05, a medium effect size (Cohen’s f = 0.25) and controlling for confounders, we will have 85% power to detect group differences in EBT use.
Primary analyses will be based on the intention-to-treat (ITT) principle including all randomized participants. In the case of missing data, we will collect reasons for dropout to inform our assumption about missing mechanism. Secondary analyses using multiple imputation or inverse probability weighting to account for missing data will be explored. Sensitivity analyses will be performed to explore the effects of departures from assumptions made in these missing-data analyses. The primary outcome of group differences in utilization of any EBTs over the 7-month study period will be analyzed through a longitudinal logistic regression model, with the intervention effect described through an odds ratio and 95% confidence interval.
Data safety and monitoring plan
Data collected from participants will be kept confidential in accordance with our institutions regulation. Participants will be identified by ID numbers only, with all data stored and managed in REDCap hosted by Boston University in a secure, HIPAA-compliant server. Any necessary transfer of data will only occur through secure encrypted mail system. Unanticipated problems, adverse events, and serious adverse events will be collected on electronic case report forms and reported to our institutional review board and the funder (NIDCR) by the Principal Investigator. A data safety and monitoring board was deemed not necessary because the trial is no more than minimal risk. Final de-identified data files will be maintained by the PI at Boston University.
Dissemination plan
Study findings will be disseminated to the scientific community through presentations at local, national and international meetings and through peer-reviewed publications.
Discussion
Although the prevalence of smoking is 14% in the general population, it exceeds 30% among subgroups, such as those with low income and low education [2]. Underserved smokers are not likely to proactively seek smoking cessation counseling and are less likely to have ever used EBTs (e.g., counseling and medications) for quitting smoking [14, 44, 45]. Therefore, it is important to proactively reach smokers in their natural environments to motivate cessation and contact with EBTs. Our study employs an innovative smoking cessation induction intervention that can be delivered during a dental cleaning, which reduces provider burden to obtain training and implement counseling and also reduces patient burden for treatment engagement, which is particularly important for those who are less motivated to quit smoking. Using technology such as a virtual reality headset could provide a cost-effective and time-efficient way of delivering evidenced-based smoking cessation in dental settings with a high degree of treatment fidelity. Our intervention also utilizes text messaging for 4 weeks after the dental visit, to build on the video shown in the visit and to provide additional motivation to engage with EBTs.
Our intervention has theoretical significance because the video and text message program target hypothesized mediators that are in line with Social Cognitive Theory. This will enable analyses on mechanisms of action and active ingredients for change, which will be critical for proper dissemination. Our study has a high level of clinical significance because it has the potential to be easily integrated into the workflow of dental clinics with no provider training and minimal provider or patient burden. Our study addresses the criticism that there is a poor coordination of care between dentistry and tobacco cessation services [46]. The proposed study has the potential to have public health impact because if successful, our intervention could be disseminated to other dental clinics nationwide, targeting all smokers, not only those who are motivated to quit. Previous studies of smoking cessation in dental settings have relied on provider-based counseling, which can be costly in terms of provider training time, time spent delivering the intervention, and monitoring ongoing treatment fidelity [47]. Text message and video-based interventions are delivered exactly as designed, resulting in a 100% reliable intervention, providing confidence in the obtained results.
Most participants in our pilot study indicated that the length of the video was satisfactory. Smokers who are unmotivated to quit are not likely to participate in intensive interventions that have a high burden. Therefore, a video-based intervention that does not require additional time (shown during their dental cleaning) and receipt of 1 month of text messages (a low effort activity that is already a part of people’s everyday lives) is a nice fit for unmotivated smokers. The length of the one-month text message program is appropriate for the goal: providing information about EBTs and motivating smokers to connect with them. A longer program would be warranted if the text messages were focused on smoking cessation. We view the video as having a ‘priming effect’ to motivate smokers to contact EBTs. We chose to show a ‘relaxation video’ as the CTRL group because this topic is at least somewhat connected to the dental cleaning experience (e.g., to ease dental anxiety and discomfort), rather than watching a video on general health and wellness, for example.
Prior studies of smoking cessation in dental settings suffer from dissipation of treatment effects over time, lack of objective verification of major outcome variables, recruitment of only those smokers who are ready to quit, and lack of “Arranging follow-up” as recommended by the National Cancer Institute’s (NCI) 5 As [18]. Furthermore, few studies have specifically targeted settings that serve urban and low-income smokers. The current study will fill in the gaps by: 1) testing the intervention in a fully powered, longitudinal trial, 2) using objective measurement for primary outcomes and for smoking cessation, 3) delivering an intervention that has minimal provider and patient burden, increasing the likelihood that it will be disseminated, 4) using a novel method of delivering an intervention while clinical care is being provided and without a loss of treatment fidelity, 5) targeting all smokers—not only those who are ready to quit, and 6) testing mechanisms of change. Interventions that target underserved smokers in naturalistic settings are needed to connect people with EBTs, including those that are publicly funded, and target populations who are traditionally not adequately served by tobacco treatment programs. Reaching and providing underserved smokers with effective treatments may ultimately decrease smoking prevalence and improve morbidity from related health conditions.
Acknowledgements
The authors would like to express their gratitude to the Boston University Goldman School of Dental Medicine for their support and specifically to Dr. John Guarante, Associate Dean for Clinical Affairs, for his assistance with helping to integrate the study into the clinic, and also to Dr. Larry Dunham, for all of his time, effort and enthusiasm narrating parts of the video. The authors would also like to thank Dean Emeritus Jeffery Hutter for all his support and guidance.
Abbreviations
- BUGSDM
Boston University Goldman School of Dental Medicine
- CONSORT
Consolidated Standards of Reporting Trials
- CTRL
Control group
- EBT
Evidence based treatment
- INT
Intervention group
- ITT
Intention-to-treat
- MSQ
Massachusetts Smoker’s Quitline
- NCI
National Cancer Institute
- RA
Research assistant
- REDCap
Research Electronic Data Capture
- SCQ
Smoking Consequences Questionnaire
- SCT
Social Cognitive Theory
- SEQ-12
Smoking Self-Efficacy Questionnaire
- SPIRIT
Standard Protocol Items: Recommendations for Intervention Trials
- VR
Virtual reality
Authors’ contributions
BB: Responsible for the conception, overall design and conduct of the trial, principally responsible for creating the video, and writing the text messages, and wrote the introduction, design, methods, measures, sample size, Table 1, and discussion. Reviewed and edited all sections of the manuscript. RE: Provided feedback on the video, assisted with text message design, assisted with the design and measurement plan, and writing the measures, analytic plan and Tables 1 and 2; wrote Fig. 1. MJ: Provided feedback on video and responsible for integration into the dental clinic workflow. Wrote sections on study section and workflow. HH: Research assistant who helped develop study protocols and assisted with writing the methods section. EJ: Assisted with literature review and writing the introduction. JO: Research assistant who assisted with writing the methods section. HC: Assisted with writing the randomization section as well as analytic plan. LQ: Provided feedback on video and text messages and assisted writing various sections of the manuscript. SW: Assisted with writing the text messages, the section on intervention text messages and the section about the text message provider. All authors contributed to the current manuscript through review and editing and have approved the final manuscript.
Funding
This research is supported an award from by National Institute for Dental and Craniofacial Research (NIDCR) at the National Institutes of Health (grant number 4UH3DE028866-02; Belinda Borrelli, Principal Investigator). The trial sponsors address is: The National Institute for Dental and Craniofacial Research, Building 31, Room 2C39, 31 Center Drive, MSC 2290, Bethesda, MD 20892 USA. This funding agency had no role in the design of this study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit the results. The authors are solely responsible for the design of the research and for the preparation of the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study will be available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was reviewed and approved by the Boston University Institutional Review board on November 4, 2020 (IRB # 40368). Informed consent will be obtained from participants. We have a waiver of written documentation of informed consent because participants have to complete study activities prior to their appointment in the clinic. Therefore, verbal consent will be obtained from participants consenting via phone, with the research assistant. All data will be kept confidential and stored in a secured location on an institutional server, which is backed up daily. Only certain members of the study staff or others designated by the PI will have access to the study data. The study is currently recruiting. Protocol modifications will be reviewed and approved by our Institutional Review Board and communicated to our sponsor, NIDCR.
Consent for publication
Not applicable because we will not collect or videos of participants in this study. Additionally, no details on individuals are reported within the manuscript. We do not intend to use professional writers.
Competing interests
This work is supported by National Institute for Dental and Craniofacial Research at the National Institutes of Health (grant number 4UH3DE028866-02; Belinda Borrelli, Principal Investigator). The funder did not have a role in the study design, collection, management, analysis or interpretation of data, writing of this report, or decision to submit the report. SW is the President of Agile Health, the company that deployed the text messages described in this study. All other authors declare that they have no other competing interests
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.United States Department of Health and Human Services. Smoking Cessation . A report of the surgeon general. Atlanta: U.S. Department of Health and Human Services, Center for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2020. [Google Scholar]
- 2.Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults - United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736–1742. doi: 10.15585/mmwr.mm6946a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning KK, Ferketich AK, Salsberry PJ, Wewers ME. Socioeconomic disparity in provider-delivered assistance to quit smoking. Nicotine Tob Res. 2008;10(1):55–61. doi: 10.1080/14622200701704905. [DOI] [PubMed] [Google Scholar]
- 4.Orleans CT. Increasing the demand for and use of effective smoking-cessation treatments reaping the full health benefits of tobacco-control science and policy gains--in our lifetime. Am J Prev Med. 2007;33(Suppl):S340–S348. doi: 10.1016/j.amepre.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Zhuang YL, Gamst AC, Cummins SE, Wolfson T, Zhu SH. Comparison of smoking cessation between education groups: findings from 2 US National Surveys over 2 decades. Am J Public Health. 2015;105(2):373–379. doi: 10.2105/AJPH.2014.302222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couch ET, Chaffee BW, Gansky SA, Walsh MM. The changing tobacco landscape: what dental professionals need to know. J Am Dent Assoc. 2016;147(7):561–569. doi: 10.1016/j.adaj.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winn DM. Tobacco use and oral disease. J Dent Educ. 2001;65(4):306–312. doi: 10.1002/j.0022-0337.2001.65.4.tb03400.x. [DOI] [PubMed] [Google Scholar]
- 8.Carr AB, Ebbert J. Interventions for tobacco cessation in the dental setting. Cochrane Database Syst Rev. 2012;6:Cd005084. doi: 10.1002/14651858.CD005084.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrelli B, Rueras N, Jurasic M. Delivery of a smoking cessation induction intervention via virtual reality headset during a dental cleaning. Transl Behav Med. 2021;11(1):182–188. doi: 10.1093/tbm/ibz144. [DOI] [PubMed] [Google Scholar]
- 10.Bandura A. Social foundations of thought and action: a social cognitive theory: Prentice-Hall, Inc; 1986.
- 11.Borrelli B, Mermelstein R. Goal setting and behavior change in a smoking cessation program. Cognit Ther Res. 1994;18(1):69–83. doi: 10.1007/BF02359396. [DOI] [Google Scholar]
- 12.Borrelli B, Mermelstein R. The role of weight concern and self-efficacy in smoking cessation and weight gain among smokers in a clinic-based cessation program. Addict Behav. 1998;23(5):609–622. doi: 10.1016/S0306-4603(98)00014-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller WR, Rollnick S. Motivational interviewing : preparing people for change. 2. New York: Guilford Press; 2002. [Google Scholar]
- 14.Bartlett YK, Gartland N, Wearden A, Armitage CJ, Borrelli B. "It's my business, it's my body, it's my money": experiences of smokers who are not planning to quit in the next 30 days and their views about treatment options. BMC Public Health. 2016;15:716. doi: 10.1186/s12889-016-3395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Borrelli B, Gaynor S, Tooley E, Armitage CJ, Wearden A, Bartlett YK. Identification of three different types of smokers who are not motivated to quit: results from a latent class analysis. Health Psychol. 2018;37(2):179–187. doi: 10.1037/hea0000561. [DOI] [PubMed] [Google Scholar]
- 16.Kosslyn SM, Thompson WL, Ganis G. The case for mental imagery. New York: Oxford University Press; 2006.
- 17.Broglio K. Randomization in clinical trials: permuted blocks and stratification. JAMA. 2018;319(21):2223–2224. doi: 10.1001/jama.2018.6360. [DOI] [PubMed] [Google Scholar]
- 18.Fiore M, Jaén C, Baker T, Bailey W, Benowitz N, Curry S, et al. Treating tobacco use and dependence: 2008 update. Clinical practice guideline. Rockville: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 19.Armfield JM. Australian population norms for the index of dental anxiety and fear (IDAF-4C) Aust Dent J. 2011;56:16–22. doi: 10.1111/j.1834-7819.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The patient health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 22.Fagerstrom KO, Schneider NG. Measuring nicotine dependence: a review of the Fagerstrom tolerance questionnaire. J Behav Med. 1989;12(2):159–182. doi: 10.1007/BF00846549. [DOI] [PubMed] [Google Scholar]
- 23.Biener L, Abrams DB. The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol. 1991;10(5):360–365. doi: 10.1037/0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- 24.DiClemente CC, Prochaska JO, Fairhurst SK, Velicer WF, Velasquez MM, Rossi JS. The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol. 1991;59(2):295–304. doi: 10.1037/0022-006X.59.2.295. [DOI] [PubMed] [Google Scholar]
- 25.Etter JF, Bergman MM, Humair JP, Perneger TV. Development and validation of a scale measuring self-efficacy of current and former smokers. Addiction. 2000;95(6):901–913. doi: 10.1046/j.1360-0443.2000.9569017.x. [DOI] [PubMed] [Google Scholar]
- 26.Juliano LM, Brandon TH. Smokers’ Expectancies for nicotine replacement therapy vs. cigarettes. Nicotine Tob Res. 2004;6(3):569–574. doi: 10.1080/14622200410001696574. [DOI] [PubMed] [Google Scholar]
- 27.Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;2016(5):Cd006103. doi: 10.1002/14651858.CD006103.pub7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;2013(5):Cd009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matkin W, Ordóñez-Mena JM, Hartmann-Boyce J. Telephone counselling for smoking cessation. Cochrane Database Syst Rev. 2019;5(5):Cd002850. doi: 10.1002/14651858.CD002850.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cole-Lewis H, Augustson E, Sanders A, Schwarz M, Geng Y, Coa K, et al. Analysing user-reported data for enhancement of SmokefreeTXT: a national text message smoking cessation intervention. Tob Control. 2017;26(6):683–689. doi: 10.1136/tobaccocontrol-2016-052945. [DOI] [PubMed] [Google Scholar]
- 31.Piper ME, Bullen C, Krishnan-Sarin S, Rigotti NA, Steinberg ML, Streck JM, et al. Defining and measuring abstinence in clinical trials of smoking cessation interventions: an updated review. Nicotine Tob Res. 2020;22(7):1098–1106. doi: 10.1093/ntr/ntz110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patnode CD, Henderson JT, Coppola EL, Melnikow J, Durbin S, Thomas RG. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325(3):280–298. doi: 10.1001/jama.2020.23541. [DOI] [PubMed] [Google Scholar]
- 33.Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, et al. Biochemical verification of tobacco use and abstinence: 2019 update. Nicotine Tob Res. 2020;22(7):1086–1097. doi: 10.1093/ntr/ntz132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prout MN, Martinez O, Ballas J, Geller AC, Lash TL, Brooks D, et al. Who uses the Smoker's Quitline in Massachusetts? Tob Control. 2002;11(Suppl 2):ii74–ii75. doi: 10.1136/tc.11.suppl_2.ii74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stead LF, Hartmann-Boyce J, Perera R, Lancaster T. Telephone counselling for smoking cessation. Cochrane Database of Syst Rev. 2013;(8):Cd002850. [DOI] [PubMed]
- 36.Prutzman YM, Wiseman KP, Grady MA, Budenz A, Grenen EG, Vercammen LK, et al. Using digital technologies to reach tobacco users who want to quit: evidence from the National Cancer Institute’s Smokefree.gov initiative. Am J Prev Med. 2021;60(Suppl):S172–s184. doi: 10.1016/j.amepre.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter MJ, Hughes JR, Gray KM, Wahlquist AE, Saladin ME, Alberg AJ. Nicotine therapy sampling to induce quit attempts among smokers unmotivated to quit: a randomized clinical trial. Arch Intern Med. 2011;171(21):1901–1907. doi: 10.1001/archinternmed.2011.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrams DB, Biener L. Motivational characteristics of smokers at the workplace: a public health challenge. Prev Med. 1992;21(6):679–687. doi: 10.1016/0091-7435(92)90075-S. [DOI] [PubMed] [Google Scholar]
- 39.Borrelli B, Henshaw M, Endrighi R, Adams WG, Heeren T, Rosen RK, et al. An interactive parent-targeted text messaging intervention to improve Oral health in children attending urban pediatric clinics: feasibility randomized controlled trial. JMIR mHealth uHealth. 2019;7(11):e14247. doi: 10.2196/14247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brooks DR, Burtner JL, Borrelli B, Heeren TC, Evans T, Davine JA, et al. Twelve-month outcomes of a group-randomized community health advocate-led smoking cessation intervention in public housing. Nicotine Tob Res. 2018;20(12):1434–1441. doi: 10.1093/ntr/ntx193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu SS, van Ryn M, Nelson D, Burgess DJ, Thomas JL, Saul J, et al. Proactive tobacco treatment offering free nicotine replacement therapy and telephone counselling for socioeconomically disadvantaged smokers: a randomised clinical trial. Thorax. 2016;71(5):446–453. doi: 10.1136/thoraxjnl-2015-207904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hollis JF, Lichtenstein E, Vogt TM, Stevens VJ, Biglan A. Nurse-assisted counseling for smokers in primary care. Ann Intern Med. 1993;118(7):521–525. doi: 10.7326/0003-4819-118-7-199304010-00006. [DOI] [PubMed] [Google Scholar]
- 43.Tsoh JY, Kohn MA, Gerbert B. Promoting smoking cessation in pregnancy with video doctor plus provider cueing: a randomized trial. Acta Obstet Gynecol Scand. 2010;89(4):515–523. doi: 10.3109/00016341003678419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ku L, Bruen BK, Steinmetz E, Bysshe T. Medicaid tobacco cessation: big gaps remain in efforts to get smokers to quit. Health Aff. 2016;35(1):62–70. doi: 10.1377/hlthaff.2015.0756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy JM, Mahoney MC, Hyland AJ, Higbee C, Cummings KM. Disparity in the use of smoking cessation pharmacotherapy among Medicaid and general population smokers. J Public Health Manag Pract. 2005;11(4):341–345. doi: 10.1097/00124784-200507000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Tomar SL. Dentistry's role in tobacco control. J Am Dent Assoc. 2001;132:30S–35S. doi: 10.14219/jada.archive.2001.0386. [DOI] [PubMed] [Google Scholar]
- 47.Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71(Suppl 1):S52–S63. doi: 10.1111/j.1752-7325.2011.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study will be available from the corresponding author on reasonable request.