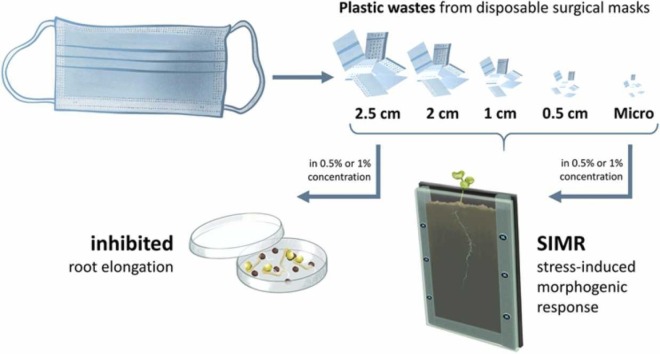
Abstract
Personal protective equipment, used extensively during the COVID-19 pandemic, heavily burdened the environment due to improper waste management. Owing to their fibrous structure, layered non-woven polypropylene (PP) disposable masks release secondary fragments at a much higher rate than other plastic waste types, thus, posing a barely understood new form of ecological hazard. Here we show that PP mask fragments of different sizes induce morphogenic responses in plants during their early development. Using in vitro systems and soil-filled rhizotrons, we found that several PP mask treatments modified the root growth of Brassica napus (L.) regardless of the experimental system. The environment around the root and mask fragments seemed to influence the effect of PP fabric fragment contamination on early root growth. In soil, primary root length was clearly inhibited by larger PP mask fragments at 1 % concentration, while the two smallest sizes of applied mask fragments caused distinct, concentration-dependent changes in the lateral root numbers. Our results indicate that PP can act as a stressor: contamination by PP surgical masks affects plant growth and hence, warrants attention. Further investigations regarding the effects of plastic pollution on plant-soil interactions involving various soil types are urgently needed.
Keywords: Polypropylene, Non-woven fabric, Brassica napus, Root system architecture
Graphical Abstract
1. Introduction
Environmental impacts of pollution with plastics, which are artificially produced materials, are among the biggest emerging threats to the Earth’s ecosystems (Kumar et al., 2021). Conventional, petroleum-based plastics are polymerized from hydrocarbon-derived monomers, and then improved with several, often toxic additives, including dyes, stabilizers, and plasticizers to achieve the desired texture and properties (Meng et al., 2021). Various types of plastic, such as PET (polyethylene), HDPE (high density polyethylene), PVC (polyvinylchloride), LDPE (low density polyethylene), PP (polypropylene), and PS (polystyrene) are used daily, which inevitably become waste at some point. Several scientifically based methods (e.g. recycling, combustion, pyrolysis, landfilling) have been established to deal with the rapidly produced plastic wastes, sustainable treatments, however, should be based on proper waste management (Evode et al., 2021). Plastic incineration not only emits many greenhouse gases (CO2, NOx), contributing to global climate change but, depending on the infrastructure, also exposes humans and natural ecosystems to toxic waste (Shen et al., 2020). Plastics found in the environment due to improper waste management majorly contribute to global warming due to its huge carbon footprint (Reid et al., 2019, Shen et al., 2020).
After the outbreak of the COVID-19 pandemic in 2020, people made significant lifestyle modifications that consequently led to environmental changes (Parashar and Hait, 2021). Several measures have been adopted to stop the pandemic; the most effective being the lockdown (staying at home), but additionally, social distancing, proper hygiene, and application of single use personal protective equipment (PPE), like medical masks, are also effective (Freedman, 2020; Lin et al., 2020).
Disposable masks are primarily used by healthcare workers to avoid infection. However, during the SARS outbreak in 2003 and H1N1 in 2009, people started using them more frequently (Elachola et al., 2020; Yang et al., 2011; Fadare and Okoffo, 2020).
In the current pandemic, approximately 129 billion single use face masks are discarded monthly worldwide (Prata et al., 2020) and if all are made of PP, it represents 645,000 tons of PP waste (Nghiem et al., 2021). According to Nghiem et al. (2021), at least 4 million tons of improperly treated PP waste from PPEs might have been released into the environment in the first two years of the pandemic where it poses a significant ecological hazard (Ardusso et al., 2021, Haddad et al., 2021) as a major source of microplastics (MPs) (Ma et al., 2021).
Disposable or single use face masks are usually made of polymers (primarily PP or occasionally polycarbonate, polyethylene, and polyester) (Potluri and Needham, 2005). As a thermoplastic polymer, PP is manufactured by chain-growth polymerization and can be processed thermally to fit the final product requirements (Nghiem et al., 2021). Single use PP masks are usually made of three fabric layers: (a) the outer water-repellent layer (spun bound, non-woven fabric) that provides mechanical strength and protection; (b) middle layer (also a non-woven, melt-blown fabric with high porosity for breathability) that intercepts water droplets; (c) the inner layer (soft fibers by filament spinning and thermal bonding) that is similar to the outer one. The edges are made of heat-wielded seams with two elastic ear loops (Dutton 2009; Spennemann, 2022; Fadare and Okoffo, 2020).
A recent study showed that PP masks released into the urban environment are relatively easily fragmented into smaller pieces due to various physical effects (e.g. lawn cutting equipment) (Spennemann, 2022). Since disposable PP masks are made of fibers (Aragaw, 2020), they release secondary MPs into the environment at a much higher rate than plastic boxes or bags due to heat or solar radiation (Ma et al., 2021, Shen et al., 2021). These fragmented PP micro-fibers are resistant to further degradation and persist in the environment for up to 450 years (Nghiem et al., 2021). Although PP (like most plastic polymers) is often considered as a chemically inert material, a recent study demonstrated that the photo-oxidation of disposable face masks might occur under direct sunlight exposure, inducing the generation of O-containing groups (e.g. hydroxyl, carbonyl, vinyl) and several changes in the polymer crystallinity, which could ultimately alter their thermal and mechanical behaviour (De-la-Torre et al., 2022). These processes might be associated with the adherence of other environmental contaminants to the rougher, cracked plastic surface, as well as with the release of PP micro-fibers and toxic substances (e.g. plasticizers or heavy metals), further enhancing their adverse effects (Sullivan et al., 2021, Delgado-Gallardo et al., 2022). Moreover, microbes or invasive pathogens find refuge and spread rapidly via the plastic particles from disposable face masks, which is particularly concerning as PPEs are currently used daily (Reid et al., 2019, Fadare and Okoffo, 2020, Zhou et al., 2022).
In addition to the aforementioned types, plastic pollutants also have various forms (e.g. beads, fragments, fibers, films) (Xu et al., 2020), and these affect environmental matrices and living organisms in multiple ways (e.g. damaged soil structure and nutrient cycling, increased toxicity, disrupted microbial community functions, retarded plant growth, accumulation of MP and nano-plastic structures in human food production chain, etc.) (Bouwmeester et al., 2015, Gao et al., 2019, Wu et al., 2021). In the environment, plastics are fragmented into macro (>2.5 cm), micro (<5 mm)- and nano-plastics (<100 nm or <1000 nm) via various physical, chemical, and biological processes (Gigault et al., 2018, Ng et al., 2018). Recently, the environmental hazards of these fragments (especially MPs) has been further amplified by the COVID-19 pandemic due to excessive usage of disposable surgical masks, which inevitably turns into an environmental pollutant (Shen et al., 2021, Spennemann, 2022). Investigating the microfiber emission from single-use tri-layer masks, Rathinamoorthy and Balasaraswathi (2022) revealed that (a) dry masks released a higher amount of microfibers than wet ones, (b) seawater degraded the masks at a higher rate than freshwater, and (c) weathered masks seem to shed more microfibers than new masks. Moreover, the examination of weathered masks further confirmed previous findings (Ma et al., 2021) that the middle layer produced the most fibers of all the layers. Ma et al. (2021), however, also characterized the released particles, which were found be mostly nano-sized. Currently, as plastics and MPs are ubiquitous in all environments, they are of increasing scientific interest. Although the issue of plastic pollution arose from marine ecosystems, research focus is shifting toward polluted pedosphere and effects of small plastic fragments on human health (Boots et al., 2019, Browne et al., 2007; Galloway et al., 2017; Gao et al., 2019; Mahon et al., 2017; Nizzetto et al., 2016; Rist et al., 2018; Wright et al., 2013; Kumar et al., 2022; Kyriakopoulos et al., 2022).
Previous literature on plastic-induced changes in terrestrial organisms, though limited, has clearly shown that the presence of plastics in soil can not only inhibit the growth and reproduction of soil invertebrates (i.e. micro-sized mask effects on springtails and earthworms) (Kwak and An, 2021) but can also influence plant growth and development (recently reviewed by Kumari et al., 2022). Nonetheless, our knowledge on this topic is scarce. So far, only a few ecotoxicological studies have been conducted (e.g. on germination, biomass production, or root elongation) to explore the interactions between plants and plastic contaminants (Allium fistulosum: de Souza Machado et al., 2019; Daucus carota: Lozano et al., 2021; Lepidium sativum: Balestri et al., 2019; Triticum aestivum: Qi et al., 2018; response of plant communities to drought: Lozano and Rillig, 2020; Lumbricus terrestris and Triticum aestivum: Huerta-Lwanga et al., 2021; Sinapis alba and Lepidium sativum: Liwarska-Bizukojc, 2022). Most of these studies examined the effects of PE (in its high- or low- density forms) on plants (de Souza Machado et al., 2019, Lozano et al., 2021, Balestri et al., 2019, Qi et al., 2018, Judy et al., 2019), while only a few investigated the phytotoxicological responses induced by PP, and so far, none of them have focused on PP in a non-woven fabric form.
Humanity is facing a novel environmental challenge due to extensive pollution with plastic wastes and MPs resulting from single use PPEs, particularly disposable masks (Ray et al., 2022, Lee and Kim, 2022). After use, they mostly end up in the environment (landfills, freshwaters or oceans), where the MP fibers continue to disintegrate into smaller pieces. Although several studies confirmed that PPEs were already present to varying degrees in the coastal zones from South America (Thiel et al., 2021; De-la-Torre et al., 2021; Ardusso et al., 2021) to Africa (Okuku et al., 2021), river outlets (Cordova et al., 2021), and urban environments (Ryan et al., 2020, Ammendolia et al., 2021, Abedin et al., 2022); based on the fate of degrading surgical masks, it can be anticipated that excessive PPE use due to COVID-19 can significantly increase the amount of related MPs in the environment within a short period of time (Fadare and Okoffo, 2020, Pizarro-Ortega et al., 2022).
While scientists agree that the burden of PPE waste, especially disposable PP masks, is inevitably of global concern due to environmental pollution, its exact effects on plant growth and development has not yet been examined.
The aim of this study was to examine the possible effects of disposable PP mask fragments of different sizes on the early growth and development of oilseed rape (Brassica napus L.) using in vitro and soil-filled rhizotron systems to model how PP PPE waste affects plants in a controlled and a realistic experimental setup. Since roots are the first to encounter soil contaminants, changes in the root system architecture at an early developmental stage influences the later development and possible tolerance of the plant. According to the literature, particulate PP seems to have varying effects on plant growth and development, thus, we hypothesized that PP in a non-woven form might also have an effect on the early growth on Brassica napus. To our knowledge, there are no other similar studies on this subject so far, thus, this work may help assess the impact of this current and large-scale pollution on plants.
2. Materials and methods
2.1. Preparation of surgical mask fragments
IIR type, 3-ply medical face masks (model reference: YLEN104) made of non-woven fabric [25 gsm spun bound PP (outside), 25 gsm melt-blown PP (middle), 25 gsm spun bound PP (inside)] and manufactured by Xiantiao Yongly Medical Products Co. Ltd. (Xiantao Hubei, China) were purchased from VWR International GmbH (cat. no. 113–9929). According to the technical datasheet and certificate of conformity, the masks comply with EN 14683:2019 +AC:2019 Type IIR requirements and ISO 9001:2015 & ISO 13485:2016 standards.
According to literature data, the characterization of different surgical masks from various sources concluded that commercially available masks are made of PP based on their Fourier transform infrared spectroscopy (FTIR) spectra, moreover, the fibers forming their layers are intact in their unused state (Saliu et al., 2021, Szefer et al., 2021, Benson et al., 2021).
After removing the metal nose bridge and elastic loops, the masks (thickness: 0.64 ± 0.12 mm) were cut manually with surface sterilized scissors into squares with edge lengths of 2.5, 2, 1, and 0.5 cm. In case of MP range, surface sterilized herb scissors were used and the edge lengths of the obtained irregular shaped pieces were below 5 mm.
2.2. Plant material, growth conditions, and rhizotron system
Brassica napus L. (oilseed rape; cv. GK Gabriella, obtained from the Cereal Research Non-profit Ltd, Szeged, Hungary) was used as a model plant. B. napus ranks second in world crop production among oil crops and have a very high economical and agricultural importance. Similar to other crops, B. napus is prone to abiotic stresses, and a huge amount of research investigated its responses to those (reviewed by Lohani et al., 2020).
For the in vitro systems, 10 surface sterilized [70 % (v/v) ethanol and 5 % (v/v) sodium hypochlorite] seeds were placed on 2 layers of filter paper in Petri dishes (diameter: 9 cm). Each filter paper was moistened with 5 mL of sterile distilled water. For mask treatment, fabric pieces of different sizes (based on the respective treatment) were added at 0.5 or 1 % (w/v) before placing the seeds. Previously, it was indicated that, even in industrial areas, MP pollution can be up to 7 % in soil (Fuller and Gautam, 2016). Therefore, plastic concentrations below this level (most commonly 1 %) can be considered as environmentally relevant for soils, heavily burdened by anthropogenic activities (Fei et al., 2020, Qi et al., 2018, Sun et al., 2022, Zhang et al., 2018, Zhang et al., 2022). In this study, the applied concentrations of plastic fragments were chosen accordingly: 1 % and 0.5 % treatments were used to model an environmentally realistic and a less severe plastic contamination, respectively. Petri dishes were then sealed with foil, and placed in a greenhouse under controlled conditions with 250 μmol m−2 s−1 photosynthetic photon flux density (white LED 5700 K) with far red supplementation (PSI, Drásov, Czech Republic), 12/12-hour light/day cycle, 24/22 °C day/night temperature, 55–60 % relative humidity, for 5 days (Molnár et al., 2020).
For the rhizotron experiments, 10 cm wide, 30 cm tall, and 1.6 cm thick (thickness of inner soil layer: 1 cm) rhizotrons were used as previously described in Feigl et al. (2019). These were filled with Klasmann Potgrond P blocking substrate (100 % frozen through black peat with a fine structure of maximum 8 mm size, pH 6.0; 210 mg N/l; 240 mg P2O5/l, 60.21 mg/kg Zn; Klasmann-Deilmann GmbH, Geeste, Germany) mixed with 20 % sand, with an initial water content of 70 %. In mask-treated rhizotrons, different sized fabric pieces (based on the respective treatment) were added at 0.5 or 1 % (w/w) to the soil/sand mixture and distributed by manual stirring. Prior to seeding, B. napus seeds were pre-germinated for 24 h at 26 °C in darkness, then a single germinated seed was transferred to the soil surface of the pre-filled rhizotrons each. Seedlings were covered with transparent plastic foil during the first 48 h to provide optimal humidity. The plants were watered with 10 mL distilled water every other day during the 14-day-long growth period. Rhizotrons were placed in a greenhouse under controlled conditions with 250 μmol m−2 s−1 photosynthetic photon flux density (white LED 5700 K) with far red supplementation (PSI, Drásov, Czech Republic), 12/12-hour light/day cycle, 24/22 °C day/night temperature, 55–60 % relative humidity, and scanned daily from the second day (Czur Shine 800 Pro, Czur Tech Co. Ltd., Dalian, China). At the end of the growth period, the rhizotrons were disassembled and the soil mixtures were used to determine soil enzyme activities and microbial cell counts.
2.3. Morphological measurements
In the in vitro systems, primary root lengths (PR length; mm) of the seedlings were measured and the visible lateral roots were counted (LR; pieces/root) after the 5-day-long growth period.
In the rhizotron systems, the scanned images were analyzed with Fiji software (http://fiji.sc/Fiji; Schindelin et al., 2012). In addition to measuring the PR lengths and LR numbers throughout the whole growth period, leaf numbers were also counted and both leaf area (LA; cm2) and shoot length (cm) values of the 14-days-old plants were enumerated.
Lateral root density (LRD) was calculated from the obtained PR lengths and LR numbers, and used to indicate changes in the root architecture.
2.4. Determination of soil enzyme activities
Soil catalase (CAT) and dehydrogenase (DH) activities were determined by titrimetric (Stpniewska et al., 2009) and colorimetric (Wolińska et al., 2016) procedures, respectively.
For CAT activity, 2 g of soil from a rhizotron was mixed with 40 mL distilled water and 5 mL of 0.3 % (w/w) H2O2 solution. After 20 min of incubation (25 °C, 160 rpm), the reaction was stopped by adding 5 mL of 1.5 M H2SO4. The suspension was filtrated and titrated with 0.02 M KMnO4 to eliminate residual H2O2. Finally, CAT activity was calculated using the reacting amount of permanganate and expressed as µmol H2O2/g dry soil weight/min. Soil samples without H2O2 addition were used as blanks.
Soil DH activity measurement was based on the microbial reduction of water-soluble 2,3,5-triphenyltetrazolium chloride (TTC) to a red formazan precipitate. A mixture composed of the rhizotron-soil sample (6 g), CaCO3 (0.12 g), distilled water (4 mL), and 3 % (w/v) TTC solution (1 mL) was incubated for 20 h at 30 °C, then extracted with ethanol (25 mL) for 1 h in the dark and the absorption of the filtered extract was determined at 485 nm. Micrograms of the produced triphenylformazan (TPF) per dry soil weight were used to express DH activity.
2.5. Microbial enumeration
Aerobic heterotrophic bacteria were enumerated according to Bodor et al. (2021) with minor modifications. Homogenized rhizotron-soil samples (2.5 g) were suspended in 5 mL of 0.9 % (w/v) sterile NaCl solution and incubated for 30 min (25 °C, 160 rpm). Subsequent serial dilutions of each suspension were plated on nutritionally rich LB media (10 g/L tryptone, 10 g/L NaCl, 5 g/L yeast extract, and 15 g/L bacto agar). Colony-forming units (CFUs) were counted 3 days after incubation at 25 °C. Microbial cell counts were expressed as logCFU per g of dry soil weight.
2.6. Statistical analysis
Data presented in the manuscript are parametric, their evaluation was conducted by Shapiro-Wilk normality test. Results are expressed as the mean ± s.d. Multiple comparison analyses were performed using SigmaStat 12 software using analysis of variance (ANOVA; p < 0.05) and Duncan's test; Student's t-test was performed using Microsoft Excel 2016 (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
We fitted simple linear models on the primary root length and lateral root number scores of each plant individual using time in days as the predictor. We used the slope of the model lines as a measure of growth rate and prepared multiple linear models for them using particle size, concentration, and their interaction as predictors. Particle size was handled as a continuous variable, while concentration as a categorical variable with three levels (0.5 % and 1 % for treatment groups and 0 % for the control). We carried out all calculations in R environment (Core Team, 2021), using built-in packages. For the PR length and LR number data, the means, s.d. values, and results of multiple comparison analyses are provided in the supplementary materials.
3. Results and discussion
3.1. Root growth of Brassica seedlings in vitro and in the rhizotron system
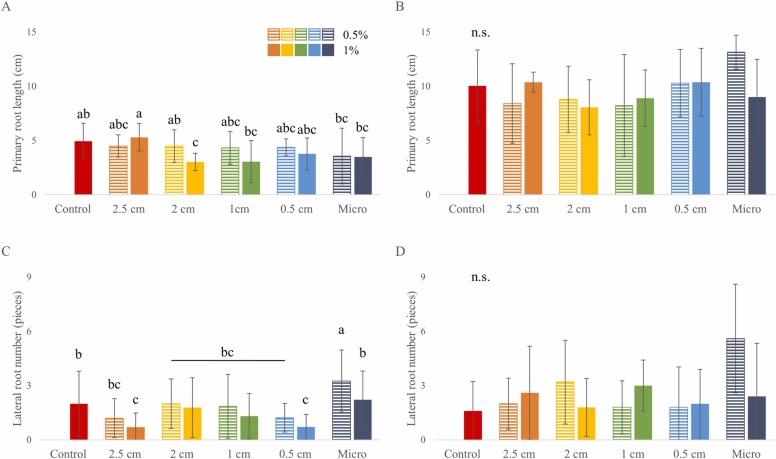
To study the effect of PP surgical mask fragments on the early development of rapeseed, a semi hydroponic in vitro system was used. After five days, all applied mask treatment except fragments with side lengths of 2.5 cm inhibited the PR elongation to an extent and this inhibition was significant upon the addition of 1 and 2 cm-long mask pieces at 1 % concentration and in the presence of micro-sized fragments at both concentrations ( Fig. 1A). Lateral root numbers of the seedlings were significantly decreased when treated with 0.5 and 2.5 cm-long mask fragments at 1 %. Addition of micro-sized mask pieces at 0.5 %, however, was able to increase the number of lateral roots significantly compared to the control (Fig. 1C).
Fig. 1.
Root growth parameters of 5-days-old B. napus seedlings grown in two experimental setups supplemented with PP surgical mask fragments of different sizes at 0.5 % and 1 % concentrations: (A) primary root lengths in the in vitro and (B) in the rhizotron systems; (C) lateral root numbers in the in vitro and (D) in the rhizotron systems. Different letters indicate significant differences according to Duncan-test (p ≤ 0.05), n.s.: no significant difference.
A soil-filled rhizotron system was also used to reveal the responses of rapeseed to PP mask fragments.
After five days of growth in the rhizotrons, only slight changes were detected in the PR length induced by mask pieces compared to the control values (Fig. 1B), while these responses were more pronounced in the in vitro system (Fig. 1A). Moreover, the root growth of the seedlings in the rhizotrons differed under some treatments than those in vitro: when the soil was polluted with fragments of 2.5, 2, and 0.5 cm, root lengths tended to be similar to the in vitro system. However, the mask pieces did not decrease the root lengths significantly. When micro-sized mask fragments were added at 0.5 %, a noticeable but statistically insignificant increase was observed in the PR length, contrary to that seen in vitro (Fig. 1B). It is noteworthy that the average primary roots were approximately twice as long in the rhizotrons compared to the plants grown in vitro, probably due to the optimal amount of soil nutrients available.
Comparison of the 5th-day LR numbers from the two systems revealed that the significant inhibition observed in vitro after treatment with 2.5 and 1 cm fragments at 1 %, could not be observed in the rhizotron system. However, a positive effect was exerted on the LR numbers in both experimental setups by the micro-sized particles at 0.5 % concentration (Fig. 1D). These results indicate that the soil system might ameliorate certain negative effects of PP pollution.
3.2. The effects of surgical mask fragments on the root growth dynamics and root system architecture
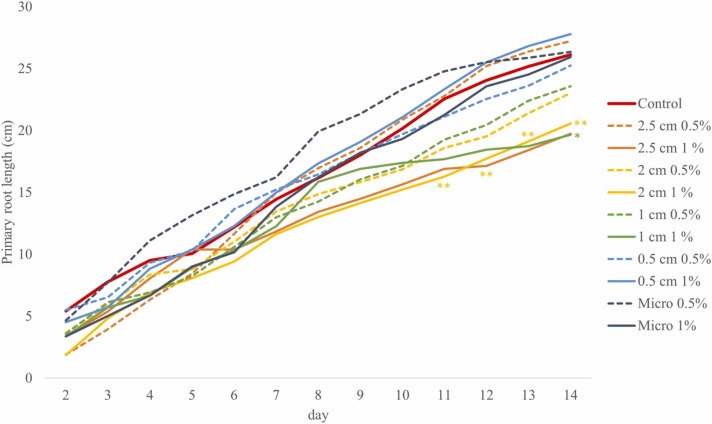
Here, the root development was followed throughout the 14-days-long cultivation period. The rhizotrons were scanned daily to study the dynamics of root growth in the presence of PP mask fragments of different sizes added at varying concentrations throughout the growth period ( Fig. 2). Compared to the control, PRs were visibly inhibited by larger PP mask fragments during the 14-day period, especially by fragments sized 2.5 cm and 2 cm, or even 1 cm at 1 % (with the exceptions of the 5th day for 2.5 cm PP and the 8th day for 1 cm PP, both at 1 %). Fragments sized 2.5 cm at 0.5 % inhibited PR growth in the first 7 days, and then, had no apparent effect during the second half of the growth period compared to the control. After the 6th day of the experiment, the soil pollution caused by PP mask fragments of 2 and 1 cm fragments at 0.5 % resulted in a moderate but visible growth inhibition than those at 1 %. Positive growth responses were induced only by the 0.5 cm fragments at 1 % (after the 5th day) and especially by the micro-sized particles at 0.5 % concentration. The growth promotion of the later, however, was equalized with the control by day 14 (Fig. 2).
Fig. 2.
Primary root lengths of B. napus seedlings grown in soil-filled rhizotron systems supplemented with PP surgical mask fragments of different sizes at 0.5 % and 1 % concentrations. Significant differences compared to the control are marked according to Student’s t-test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
The multiple linear model prepared for the growth rate of the primary root explained a significant proportion of the variation of data (F=4.07, p = 0.011). Among the predictors, size had a significant negative effect (t = −2.45, p = 0.017), but concentration did not (t = 1.21, p = 0.232). The interaction of the two variables also significantly affected growth rate (t = −3.13, p = 0.003); therefore, we split the model according to concentration levels and tested the effect of size again. We found that, at a concentration level of 0.5 %, particle size had no detectable effect (t = 1.29, p = 0.208), increasing particle size decreased the growth rate of the primary root when 1 % of plastic was applied (t = −3.28, p = 0.003).
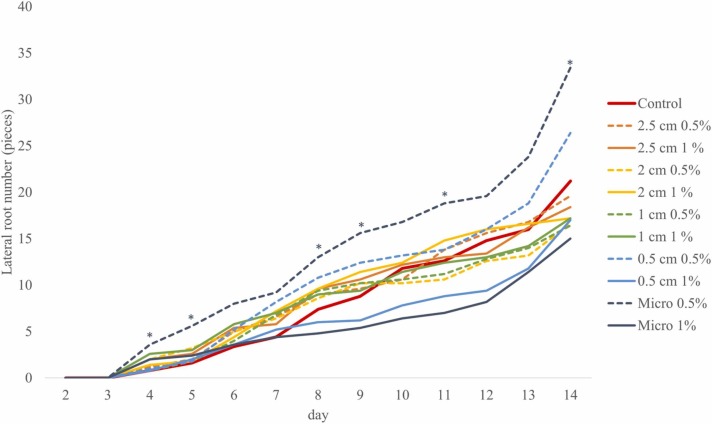
LR numbers fluctuated at near control values for most of the treatments, except the two smallest PP mask fragments, where a distinct and concentration-dependent response was observed: PP fragments of 0.5 cm and micro-sized particles at 0.5 % concentration positively influenced the LR numbers, resulting in a more branched root system; while both fragment sizes visibly decreased the LR numbers at a higher concentration (1 %) ( Fig. 3).
Fig. 3.
Lateral root numbers of B. napus seedlings grown in soil-filled rhizotron systems supplemented with PP surgical mask fragments of different sizes at 0.5 % and 1 % concentrations. Significant differences compared to the control are marked according to Student’s t-test (*p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001).
The multiple linear model for lateral root formation explained only an overall marginally significant proportion of the variation of the data (F=2.55, t = 0.066). Despite this, we tested the individual effects of the predictors and found that size had indeed no effect (t = −1.41, p = 0.165) but a higher concentration can negatively affect the growth rate (t = −2.61, p = 0.012). Since the interaction of the two variables was not significant (t = 1.42, p = 0.163), we did not test partial models.
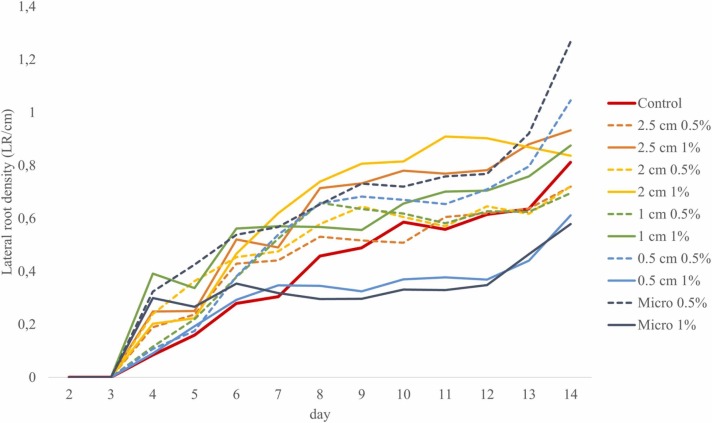
LRD, which is calculated by dividing the LR number by the PR length, shows the number of lateral roots within 1 cm of the primary root, and thus, can be used as an indicator for the changes in root architecture. Larger PP mask fragments (2.5, 2, and 1 cm) at 1 % concentration increased the LRD throughout the experiment due to decreased PR length as mentioned above. Smaller particles (0.5 cm and micro-sized) at 0.5 % concentration also increased the LRD, which, in this case, was due to increased LR numbers. In contrast, LRD considerably decreased in the second half of the experiment in response to the presence of 0.5 cm and micro-sized particles at 1 % concentration, as they inhibited LR formation as discussed above ( Fig. 4).
Fig. 4.
Lateral root density of B. napus throughout the 14 days of growing period in the soil-filled rhizotron system, supplemented with PP surgical mask fragments of different sizes at 0.5 % and 1 % concentrations.
Changes in the root system architecture due to different, especially non-lethal stressors, often trigger the adaptation of the root system and is known as stress-induced morphogenic response (SIMR). SIMR is a special and well-balanced combination of inhibited PR elongation and increased LR formation, which possibly enhances stress tolerance in plants (Potters et al., 2007). According to our results, SIMR-like alterations in the root system can be induced by PP pollution.
3.3. The effects of surgical mask fragments on the shoot growth of Brassica seedlings
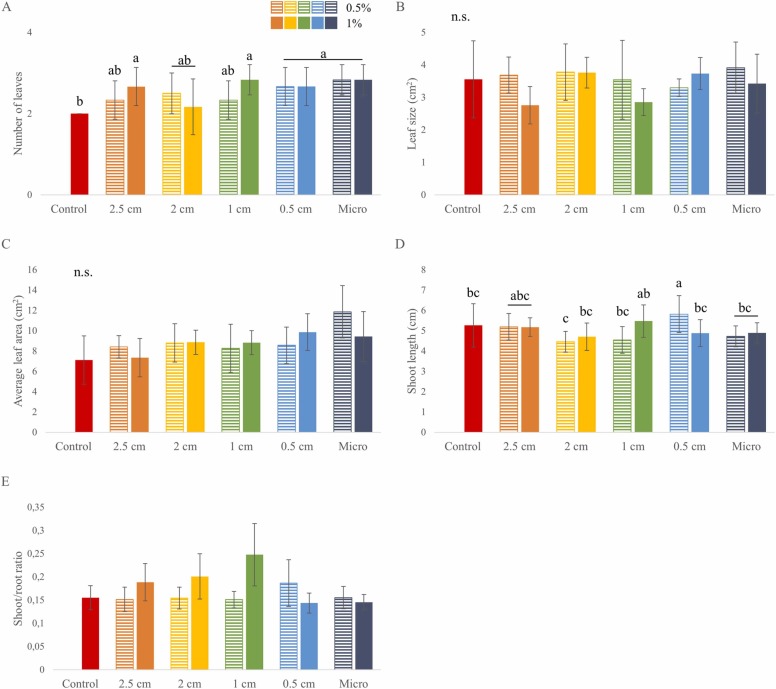
At the end of the rhizotron experiments, morphological characteristics of the shoots were also examined ( Fig. 5). The average leaf number per plant was significantly increased by most PP mask treatments compared to the control (Fig. 5A), while the average leaf size remained similar to the control (except when the soil was supplemented with 2.5 and 1 cm fragments at 1 % concentration, where the leaf sizes decreased noticeably) (Fig. 5B). For each treatment, the average LA value per plant was slightly above the control, which was considerably increased by pollution with micro-sized PP mask particles at 0.5 % (Fig. 5C).
Fig. 5.
Shoot growth parameters of the 14-days-old B. napus seedlings grown in the rhizotron system supplemented with PP surgical mask fragments of different sizes at 0.5 or 1 % concentrations: (A) number of leaves per plant; (B) average leaf size; (C) average leaf area per plant; (D) shoot length; and (E) shoot/root ratio. Different letters indicate significant differences according to Duncan-test (p ≤ 0.05), n.s.: no significant difference.
While the number, size, and area of the leaves were mostly positively affected by PP mask fragments, shoot length was only increased significantly when treated with 0.5 cm PP mask fragments at 0.5 % (Fig. 5D). The shoot/root ratio was increased notably by the presence of 2.5, 2, and 1 cm mask fragments at 1 % because of the root growth inhibition mentioned above (Fig. 5E). Furthermore, shoot/root ratio was also increased by the artificial soil pollution with 0.5 cm mask fragments at 0.5 %, which can be due to a slight increase in the shoot length instead of inhibition of PR elongation. Aerial parts of the plants were either not or positively influenced by PP mask treatments. Leaf numbers were significantly increased, especially by smaller PP fragments. Furthermore, at lower concentrations, micro-sized particles could noticeably increase the average LA of rapeseed.
Similar to our results, the available literature on the effects of PP on plant growth also tends to be inconclusive as the results depend on the plant species, the applied form and concentration , exposure time, or even on the experimental system (Supplementary table 3). Colzi et al. (2022) studied the phytotoxicological effects of PP (among other types of MPs from PET, PVC, and PE) on Cucurbita pepo and found that while 0.01% (w/w) of MPs did not influence plant growth, both 0.1 % and 0.2 % PP treatment led to the loss of fresh and dry weights of both roots and shoots. Of all the plastics tested, PP also proved to be second most detrimental to the growth of plant aerial parts. However, none of the applied PP treatments had any significant effect on the LA. Contrastingly, investigations on the effects of micro-sized PP on Daucus carota by Lozano et al. (2021) showed that PP in the forms of film, fiber, fragment, and foam increased the shoot mass while root mass was positively affected exclusively by PP fragments. Another study, assessing the acute and chronic toxicities of PP, PE, and PVC on Lepidium sativum, reported that PP MP treatment at 0.02 % (w/w) significantly inhibited germination after 6 days. Although plant height was also decreased, biomass production was higher than the control. After exposure to PP MPs for 21 days, no considerable changes were observed in the shoot height and leaf number of L. sativum, while the shoot biomass was significantly decreased (Pignattelli et al., 2020). Similarly, PP MPs significantly increased the total root length of Allium fistulosum, but reduced its total root diameter and root/leaf ratio (de Souza Machado et al., 2019).
While plastics do not induce direct acute toxic effects (Lian et al., 2020), they can alter plant growth presumably due to substances in plastic leachate (e.g. plasticizers, stabilizers, dyes, or additives) (Sullivan et al., 2021, Delgado-Gallardo et al., 2022). The toxicity mechanisms behind these responses are only studied in a few plant species and mostly investigated in case of nanoplastics as stressor (reviewed by Matthews et al., 2021). Nevertheless, it can be assumed that compared to the effects of nanoplastics, contamination with micro- and macro-sized plastics may have different effects on the mechanisms behind the growth responses, since the uptake of micro- and especially macro-plastics is unlikely.
3.4. The effects of surgical mask fragments on the microbial activity and cell counts in soil
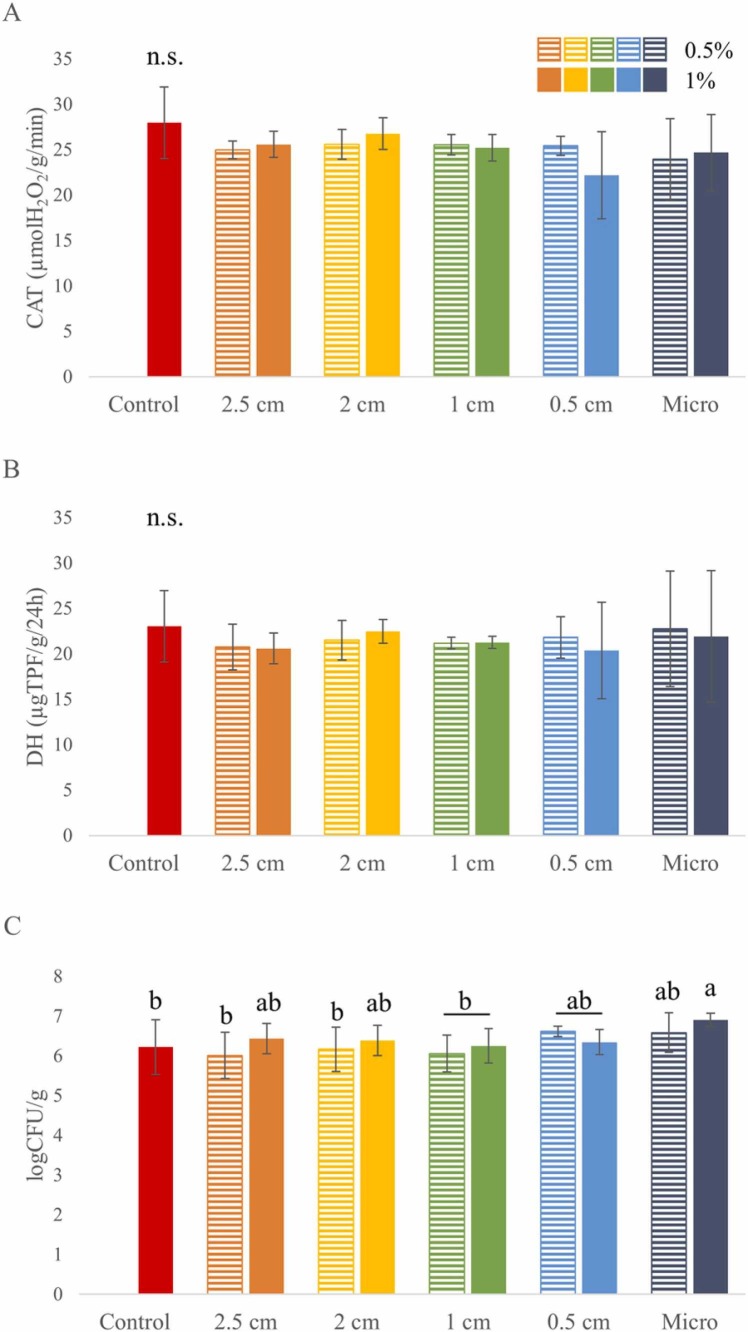
Plastic pollution can affect the soil microbiota and its activity by altering the hydrological and physicochemical characteristics (Yu et al., 2021). Due to their sensitivity to different stressors or contaminants, enzymes produced by soil-inhabiting microorganisms are often used as indirect ecotoxicological indicators for soil quality and health (Wolińska et al., 2016). CAT and DH activities, that are associated with the presence of aerobic microorganisms and decomposition of organic compounds, respectively, were determined in each rhizotron sample after the 14-days-period. According to our results, none of the applied PP mask treatments significantly changed their levels ( Fig. 6AB), implying that PP pollution might not have unfavourable effects on the microbial activity in this model soil system.
Fig. 6.
The activity of (A) catalase (CAT) and (B) dehydrogenase (DH) enzymes, and (C) the number of aerobic heterotrophic bacteria number measured in the rhizotron soils on the14th day. Different letters indicate significant differences according to Duncan-test (p ≤ 0.05), n.s.: no significant difference.
According to literature, changes in soil enzymatic activities induced by plastic pollution are as inconclusive as the aforementioned phytotoxicological responses and can be also dependent on several factors. Using soil mesocosms to model a natural wetland ecosystem, Yu et al. (2021) observed significantly decreased CAT activities induced by PP MPs (1 % of soil dry weight) after 20, 40, and 80 days, and found that the fresh weight and height of Bacopa sp. was also inhibited. Contrastingly, other studies reported enhanced soil enzyme activities in response to MPs (Liu et al., 2017, Huang et al., 2019), although, these studies applied different exposure time, plastic concentration, and soil type. MPs from PS inhibited the soil DH activity (Dong et al., 2021). Fibrous and microsphere forms of PP decreased the DH activity after 14 days, then promoted it after 29 days. Moreover, microsphere PP caused more changes in DH activity than fibers (Yi et al., 2021).
Although soil contamination can result in microbial biomass loss (Chodak et al., 2013, Romano-Armada et al., 2019), we detected significantly higher CFUs in PP-supplemented rhizotrons than in the non-contaminated control (Fig. 6C). Moreover, microbial cell counts increased with decrease in size of PP mask fragments, indicating that the porous structure of the smaller mask particles might provide a suitable microhabitat for microorganisms (possible due to increase in specific surface area owing to surgical mask fragmentation). This observation is consistent with the report by Gao et al. (2021), where small plastic particles promoted the growth of soil microorganisms more efficiently than larger fragments.
Taking into consideration that microbial CFUs increased due to PP pollution of the rhizotron soils without significant change in enzyme activities, it is possible that although more microbial cells were detected in the PP-polluted systems, they might not as active as those in the control system; which may be indicative of deteriorated soil quality as a stress marker. Notably, simplified artificial model systems, such as the rhizotron-soil system used in this study, might not have a well-established microbial community, and thus, any ecotoxicological conclusions cannot be directly applied to real soil environments. Therefore, further investigations regarding the effects of plastic pollution on plant-soil and microbe-soil interactions involving various soil types are urgently needed.
4. Conclusions
This study is the first to confirm that pollution with PP surgical mask fragments can alter the root growth and development of B. napus. The in vitro inhibition effects of PP mask wastes were less pronounced in the rhizotron system, suggesting that the environment, surrounding the root and mask pieces, can also influence the extent to which PP fabric fragment contamination affects early root growth of plants. Alterations in the two main root architecture parameters indicated intriguing stress-induced morphogenic responses, dependent on both the fragment size and the concentration of PP: (a) higher concentrations of the larger fragments and lower concentrations of the smaller particles increased LRD; (b) higher concentrations of the smaller particles decreased the LRD. The overall neutral or positive effect of PP mask fragments on the shoot parameters implies that the main organs of rapeseed seedlings respond differentially to PP pollutants with the root system being more sensitive. Although the results from soil enzyme and microbial cell count measurements in soil-filled rhizotron models cannot be directly applied to real soil environments, these parameters might be used as stress indicators for deteriorated soil quality. Our work highlights that the effects of excessive PPE use, such as surgical masks, during the COVID-19 pandemic and subsequent environmental contamination demands more attention also from the point of view of plants. Therefore, further investigations regarding the effects of PP waste pollution on plant-soil and microbe-soil interactions involving various soil types are urgently needed.
Statement of environmental implication
While scientists agree that the burden of plastic waste, especially disposable polypropylene masks due to COVID-19 pandemic, is inevitably of global concern, its exact effects on plants has not yet been examined. Our aim was to examine the effects of mask fragments of different sizes on the early growth of rapeseed. Although conventional plastics are chemically inert, PP mask treatment might alter root growth and development of rapeseed regardless of the experimental system. To our knowledge, there are no other studies on this subject so far, thus, this work may help assess the impact of this current pollution on plants.
CRediT authorship contribution statement
Enikő Mészáros: Data curation, Investigation, Writing – original draft, Writing – review & editing. Attila Bodor: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Ádám Szierer: Data curation, Investigation. Kovacs Etelka Kovács: Formal analysis. Katalin Perei: Resources, Validation, Writing – review & editing. Csaba Tölgyesi: Formal analysis, Methodology, Software, Validation, Writing – review & editing. Zoltán Bátori: Formal analysis, Methodology, Software, Validation, Writing – review & editing. Gábor Feigl: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences, Hungary (Grant no. BO/00181/21/4). G. F. was supported by the New National Excellence Program of the Ministry of Human Capacities, Hungary (UNKP-21-5-SZTE-567). The authors would like to express their gratitude toward Sarolta Papp for the excellent technical assistance.
Editor: Teresa A.P. Rocha-Santos
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2022.129255.
Appendix A. Supplementary material
Supplementary material
.
Supplementary material
.
Supplementary material
.
Supplementary material
.
References
- Abedin M., Khandaker M.U., Uddin M., Karim M., Ahamad M., Islam M., Idris A.M. PPE pollution in the terrestrial and aquatic environment of the Chittagong city area associated with the COVID-19 pandemic and concomitant health implications. Environ. Sci. Pollut. Res. 2022:1–13. doi: 10.1007/s11356-021-17859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammendolia J., Saturno J., Brooks A.L., Jacobs S., Jambeck J.R. An emerging source of plastic pollution: environmental presence of plastic personal protective equipment (PPE) debris related to COVID-19 in a metropolitan city. Environ. Pollut. 2021;269 doi: 10.1016/j.envpol.2020.116160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragaw T.A. Surgical face masks as a potential source for microplastic pollution in the COVID-19 scenario. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardusso M., Forero-López A.D., Buzzi N.S., Spetter C.V., Fernández-Severini M.D. COVID-19 pandemic repercussions on plastic and antiviral polymeric textile causing pollution on beaches and coasts of South America. Sci. Total Environ. 2021;763 doi: 10.1016/j.scitotenv.2020.144365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestri E., Menicagli V., Ligorini V., Fulignati S., Galletti A.M.R., Lardicci C. Phytotoxicity assessment of conventional and biodegradable plastic bags using seed germination test. Ecol. Indic. 2019;102:569–580. [Google Scholar]
- Benson N.U., Fred-Ahmadu O.H., Bassey D.E., Atayero A.A. COVID-19 pandemic and emerging plastic-based personal protective equipment waste pollution and management in Africa. J. Environ. Chem. Eng. 2021;9(3) doi: 10.1016/j.jece.2021.105222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodor A., Bounedjoum N., Feigl G., Duzs Á., Laczi K., Szilágyi Á., Perei K. Exploitation of extracellular organic matter from Micrococcus luteus to enhance ex situ bioremediation of soils polluted with used lubricants. J. Hazard. Mater. 2021;417 doi: 10.1016/j.jhazmat.2021.125996. [DOI] [PubMed] [Google Scholar]
- Boots B., Russell C.W., Green D.S. Effects of microplastics in soil ecosystems: above and below ground. Environ. Sci. Technol. 2019;53(19):11496–11506. doi: 10.1021/acs.est.9b03304. [DOI] [PubMed] [Google Scholar]
- Bouwmeester H., Hollman P.C., Peters R.J. Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: experiences from nanotoxicology. Environ. Sci. Technol. 2015;49(15):8932–8947. doi: 10.1021/acs.est.5b01090. [DOI] [PubMed] [Google Scholar]
- Browne M.A., Galloway T., Thompson R. Microplastic--an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007;3(4):559–561. doi: 10.1002/ieam.5630030412. [DOI] [PubMed] [Google Scholar]
- Chodak M., Gołębiewski M., Morawska-Płoskonka J., Kuduk K., Niklińska M. Diversity of microorganisms from forest soils differently polluted with heavy metals. Appl. Soil Ecol. 2013;64:7–14. [Google Scholar]
- Colzi I., Renna L., Bianchi E., Castellani M.B., Coppi A., Pignattelli S., Gonnelli C. Impact of microplastics on growth, photosynthesis and essential elements in Cucurbita pepo L. J. Hazard. Mater. 2022;423 doi: 10.1016/j.jhazmat.2021.127238. [DOI] [PubMed] [Google Scholar]
- Cordova M.R., Nurhati I.S., Riani E., Iswari M.Y. Unprecedented plastic-made personal protective equipment (PPE) debris in river outlets into Jakarta Bay during COVID-19 pandemic. Chemosphere. 2021;268 doi: 10.1016/j.chemosphere.2020.129360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza Machado A.A., Lau C.W., Kloas W., Bergmann J., Bachelier J.B., Faltin E., Rillig M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019;53(10):6044–6052. doi: 10.1021/acs.est.9b01339. [DOI] [PubMed] [Google Scholar]
- De-la-Torre G.E., Dioses-Salinas D.C., Dobaradaran S., Spitz J., Keshtkar M., Akhbarizadeh R., Tavakolian A. Physical and chemical degradation of littered personal protective equipment (PPE) under simulated environmental conditions. Mar. Pollut. Bull. 2022;178 doi: 10.1016/j.marpolbul.2022.113587. [DOI] [PubMed] [Google Scholar]
- De-la-Torre G.E., Rakib M.R.J., Pizarro-Ortega C.I., Dioses-Salinas D.C. Occurrence of personal protective equipment (PPE) associated with the COVID-19 pandemic along the coast of Lima, Peru. Sci. Total Environ. 2021;774 doi: 10.1016/j.scitotenv.2021.145774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado-Gallardo J., Sullivan G.L., Tokaryk M., Russell J.E., Davies G.R., Johns K.V., Sarp S. Disposable FFP2 and type IIR medical-grade face masks: an exhaustive analysis into the leaching of micro-and nanoparticles and chemical pollutants linked to the COVID-19 Pandemic. ACS Est. Water. 2022;2(4):527–538. doi: 10.1021/acsestwater.1c00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y., Gao M., Qiu W., Song Z. Effect of microplastics and arsenic on nutrients and microorganisms in rice rhizosphere soil. Ecotoxicol. Environ. Saf. 2021;211 doi: 10.1016/j.ecoenv.2021.111899. [DOI] [PubMed] [Google Scholar]
- Dutton K.C. Overview and analysis of the meltblown process and parameters. J. Textile Apparel Technol. Manag. 2009;6(1) [Google Scholar]
- Elachola H., Ebrahim S.H., Gozzer E. COVID-19: Facemask use prevalence in international airports in Asia, Europe and the Americas, March 2020. Travel Med. Infect. Dis. 2020;35 doi: 10.1016/j.tmaid.2020.101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evode N., Qamar S.A., Bilal M., Barceló D., Iqbal H.M. Plastic waste and its management strategies for environmental sustainability. Case Stud. Chem. Environ. Eng. 2021;4 [Google Scholar]
- Fadare O.O., Okoffo E.D. Covid-19 face masks: a potential source of microplastic fibers in the environment. Sci. Total Environ. 2020;737 doi: 10.1016/j.scitotenv.2020.140279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Y., Huang S., Zhang H., Tong Y., Wen D., Xia X., Barceló D. Response of soil enzyme activities and bacterial communities to the accumulation of microplastics in an acid cropped soil. Sci. Total Environ. 2020;707 doi: 10.1016/j.scitotenv.2019.135634. [DOI] [PubMed] [Google Scholar]
- Feigl G., Molnár Á., Szőllősi R., Ördög A., Törőcsik K., Oláh D., Kolbert Z. Zinc-induced root architectural changes of rhizotron-grown B. napus correlate with a differential nitro-oxidative response. Nitric Oxide. 2019;90:55–65. doi: 10.1016/j.niox.2019.06.003. [DOI] [PubMed] [Google Scholar]
- Freedman L. Strategy for a Pandemic: The UK and COVID-19. Survival. 2020;62(3):25–76. [Google Scholar]
- Fuller S., Gautam A. A procedure for measuring microplastics using pressurized fluid extraction. Environ. Sci. Technol. 2016;50(11):5774–5780. doi: 10.1021/acs.est.6b00816. [DOI] [PubMed] [Google Scholar]
- Galloway T.S., Cole M., Lewis C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017;1(5):1–8. doi: 10.1038/s41559-017-0116. [DOI] [PubMed] [Google Scholar]
- Gao B., Yao H., Li Y., Zhu Y. Microplastic addition alters the microbial community structure and stimulates soil carbon dioxide emissions in vegetable‐growing soil. Environ. Toxicol. Chem. 2021;40(2):352–365. doi: 10.1002/etc.4916. [DOI] [PubMed] [Google Scholar]
- Gao H., Yan C., Liu Q., Ding W., Chen B., Li Z. Effects of plastic mulching and plastic residue on agricultural production: a meta-analysis. Sci. Total Environ. 2019;651:484–492. doi: 10.1016/j.scitotenv.2018.09.105. [DOI] [PubMed] [Google Scholar]
- Gigault J., Ter Halle A., Baudrimont M., Pascal P.Y., Gauffre F., Phi T.L., Reynaud S. Current opinion: what is a nanoplastic? Environ. Pollut. 2018;235:1030–1034. doi: 10.1016/j.envpol.2018.01.024. [DOI] [PubMed] [Google Scholar]
- Haddad M.B., De-la-Torre G.E., Abelouah M.R., Hajji S., Alla A.A. Personal protective equipment (PPE) pollution associated with the COVID-19 pandemic along the coastline of Agadir, Morocco. Sci. Total Environ. 2021;798 doi: 10.1016/j.scitotenv.2021.149282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Zhao Y., Wang J., Zhang M., Jia W., Qin X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019;254 doi: 10.1016/j.envpol.2019.112983. [DOI] [PubMed] [Google Scholar]
- Huerta-Lwanga E., Mendoza-Vega J., Ribeiro O., Gertsen H., Peters P., Geissen V. Is the polylactic acid fiber in Green compost a risk for Llumbricus terrestris and TTriticum aestivum? Polymers. 2021;13(5):703. doi: 10.3390/polym13050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judy J.D., Williams M., Gregg A., Oliver D., Kumar A., Kookana R., Kirby J.K. Microplastics in municipal mixed-waste organic outputs induce minimal short to long-term toxicity in key terrestrial biota. Environ. Pollut. 2019;252:522–531. doi: 10.1016/j.envpol.2019.05.027. [DOI] [PubMed] [Google Scholar]
- Kumar R., Manna C., Padha S., Verma A., Sharma P., Dhar A., Bhattacharya P. Micro (nano) plastics pollution and human health: how plastics can induce carcinogenesis to humans? Chemosphere. 2022;298 doi: 10.1016/j.chemosphere.2022.134267. [DOI] [PubMed] [Google Scholar]
- Kumar R., Verma A., Shome A., Sinha R., Sinha S., Jha P.K., Vara Prasad P.V. Impacts of plastic pollution on ecosystem services, sustainable development goals, and need to focus on circular economy and policy interventions. Sustainability. 2021;13(17):9963. [Google Scholar]
- Kumari A., Rajput V.D., Mandzhieva S.S., Rajput S., Minkina T., Kaur R., Glinushkin A.P. Microplastic pollution: an emerging threat to terrestrial plants and insights into its remediation strategies. Plants. 2022;11(3):340. doi: 10.3390/plants11030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J.I., An Y.J. Post COVID-19 pandemic: Biofragmentation and soil ecotoxicological effects of microplastics derived from face masks. J. Hazard. Mater. 2021;416 doi: 10.1016/j.jhazmat.2021.126169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulos G.L., Zamparas M.G., Kapsalis V.C. Investigating the human impacts and the environmental consequences of microplastics disposal into water resources. Sustainability. 2022;14(2):828. [Google Scholar]
- Lee M., Kim H. COVID-19 pandemic and microplastic pollution. Nanomaterials. 2022;12(5):851. doi: 10.3390/nano12050851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian J., Wu J., Xiong H., Zeb A., Yang T., Su X., Liu W. Impact of polystyrene nanoplastics (PSNPs) on seed germination and seedling growth of wheat (Triticum aestivum L.) J. Hazard. Mater. 2020;385 doi: 10.1016/j.jhazmat.2019.121620. [DOI] [PubMed] [Google Scholar]
- Lin Y.H., Liu C.H., Chiu Y.C. Google searches for the keywords of “wash hands” predict the speed of national spread of COVID-19 outbreak among 21 countries. Brain Behav. Immun. 2020;87:30–32. doi: 10.1016/j.bbi.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Yang X., Liu G., Liang C., Xue S., Chen H., Geissen V. Response of soil dissolved organic matter to microplastic addition in Chinese loess soil. Chemosphere. 2017;185:907–917. doi: 10.1016/j.chemosphere.2017.07.064. [DOI] [PubMed] [Google Scholar]
- Liwarska-Bizukojc E. Phytotoxicity assessment of biodegradable and non-biodegradable plastics using seed germination and early growth tests. Chemosphere. 2022;289 doi: 10.1016/j.chemosphere.2021.133132. [DOI] [PubMed] [Google Scholar]
- Lohani N., Jain D., Singh M.B., Bhalla P.L. Engineering multiple abiotic stress tolerance in canola, Brassica napus. Front. Plant Sci. 2020:3. doi: 10.3389/fpls.2020.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano Y.M., Rillig M.C. Effects of microplastic fibers and drought on plant communities. Environ. Sci. Technol. 2020;54(10):6166–6173. doi: 10.1021/acs.est.0c01051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano Y.M., Lehnert T., Linck L.T., Lehmann A., Rillig M.C. Microplastic shape, polymer type, and concentration affect soil properties and plant biomass. Front. Plant Sci. 2021;12:169. doi: 10.3389/fpls.2021.616645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Chen F., Xu H., Jiang H., Liu J., Li P., Pan K. Face masks as a source of nanoplastics and microplastics in the environment: quantification, characterization, and potential for bioaccumulation. Environ. Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117748. [DOI] [PubMed] [Google Scholar]
- Mahon A.M., O’Connell B., Healy M.G., O’Connor I., Officer R., Nash R., Morrison L. Microplastics in sewage sludge: effects of treatment. Environ. Sci. Technol. 2017;51(2):810–818. doi: 10.1021/acs.est.6b04048. [DOI] [PubMed] [Google Scholar]
- Matthews S., Mai L., Jeong C.B., Lee J.S., Zeng E.Y., Xu E.G. Key mechanisms of micro-and nanoplastic (MNP) toxicity across taxonomic groups. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021;247 doi: 10.1016/j.cbpc.2021.109056. [DOI] [PubMed] [Google Scholar]
- Meng F., Yang X., Riksen M., Xu M., Geissen V. Response of common bean (Phaseolus vulgaris L.) growth to soil contaminated with microplastics. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142516. [DOI] [PubMed] [Google Scholar]
- Molnár Á., Papp M., Kovács D.Z., Bélteky P., Oláh D., Feigl G., Kolbert Z. Nitro-oxidative signalling induced by chemically synthetized zinc oxide nanoparticles (ZnO NPs) in Brassica species. Chemosphere. 2020;251 doi: 10.1016/j.chemosphere.2020.126419. [DOI] [PubMed] [Google Scholar]
- Ng E.L., Lwanga E.H., Eldridge S.M., Johnston P., Hu H.W., Geissen V., Chen D. An overview of microplastic and nanoplastic pollution in agroecosystems. Sci. Total Environ. 2018;627:1377–1388. doi: 10.1016/j.scitotenv.2018.01.341. [DOI] [PubMed] [Google Scholar]
- Nghiem L.D., Iqbal H.M., Zdarta J. The shadow pandemic of single use personal protective equipment plastic waste: a blue print for suppression and eradication. Case Stud. Chem. Environ. Eng. 2021;4 doi: 10.1016/j.cscee.2021.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizzetto L., Futter M., Langaas S. Are agricultural soils dumps for microplastics of urban origin? Environ. Sci. Technol. 2016;50(20):10777–10779. doi: 10.1021/acs.est.6b04140. [DOI] [PubMed] [Google Scholar]
- Okuku E., Kiteresi L., Owato G., Otieno K., Mwalugha C., Mbuche M., Omire J. The impacts of COVID-19 pandemic on marine litter pollution along the Kenyan Coast: a synthesis after 100 days following the first reported case in Kenya. Mar. Pollut. Bull. 2021;162 doi: 10.1016/j.marpolbul.2020.111840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar N., Hait S. Plastics in the time of COVID-19 pandemic: protector or polluter? Sci. Total Environ. 2021;759 doi: 10.1016/j.scitotenv.2020.144274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignattelli S., Broccoli A., Renzi M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020;727 doi: 10.1016/j.scitotenv.2020.138609. [DOI] [PubMed] [Google Scholar]
- Pizarro-Ortega C.I., Dioses-Salinas D.C., Severini M.D.F., López A.F., Rimondino G.N., Benson N.U., De-la-Torre G.E. Degradation of plastics associated with the COVID-19 pandemic. Mar. Pollut. Bull. 2022 doi: 10.1016/j.marpolbul.2022.113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potluri P., Needham P. Textiles Protection Text Prot. Elsevier BV,; 2005. Technical textiles for protection; pp. 151–175. [Google Scholar]
- Potters G., Pasternak T.P., Guisez Y., Palme K.J., Jansen M.A. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007;12(3):98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Prata J.C., Silva A.L., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020;54(13):7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Qi Y., Yang X., Pelaez A.M., Lwanga E.H., Beriot N., Gertsen H., Geissen V. Macro-and micro-plastics in soil-plant system: effects of plastic mulch film residues on wheat (Triticum aestivum) growth. Sci. Total Environ. 2018;645:1048–1056. doi: 10.1016/j.scitotenv.2018.07.229. [DOI] [PubMed] [Google Scholar]
- R Core Team, 2021: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: 〈https://www.R-project.org/〉.
- Rathinamoorthy R., Balasaraswathi S.R. Disposable tri-layer masks and microfiber pollution–an experimental analysis on dry and wet state emission. Sci. Total Environ. 2022;816 doi: 10.1016/j.scitotenv.2021.151562. [DOI] [PubMed] [Google Scholar]
- Ray S.S., Lee H.K., Huyen D.T.T., Chen S.S., Kwon Y.N. Microplastics waste in environment: a perspective on recycling issues from PPE kits and face masks during the COVID-19 pandemic. Environ. Technol. Innov. 2022 doi: 10.1016/j.eti.2022.102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid A.J., Carlson A.K., Creed I.F., Eliason E.J., Gell P.A., Johnson P.T., Cooke S.J. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019;94(3):849–873. doi: 10.1111/brv.12480. [DOI] [PubMed] [Google Scholar]
- Rist S., Almroth B.C., Hartmann N.B., Karlsson T.M. A critical perspective on early communications concerning human health aspects of microplastics. Sci. Total Environ. 2018;626:720–726. doi: 10.1016/j.scitotenv.2018.01.092. [DOI] [PubMed] [Google Scholar]
- Romano-Armada N., Amoroso M.J., Rajal V.B. Construction of a combined soil quality indicator to assess the effect of glyphosate application. Sci. Total Environ. 2019;682:639–649. doi: 10.1016/j.scitotenv.2019.05.079. [DOI] [PubMed] [Google Scholar]
- Ryan P.G., Maclean K., Weideman E.A. The impact of the COVID-19 lockdown on urban street litter in South Africa. Environ. Process. 2020;7(4):1303–1312. [Google Scholar]
- Saliu F., Veronelli M., Raguso C., Barana D., Galli P., Lasagni M. The release process of microfibers: from surgical face masks into the marine environment. Environ. Adv. 2021;4 [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Tinevez J.Y. Fiji: an open-source platform for biological-image analysis. Nat. Methods. 2012;9(7):676. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Huang W., Chen M., Song B., Zeng G., Zhang Y. Micro) plastic crisis: un-ignorable contribution to global greenhouse gas emissions and climate change. J. Clean. Prod. 2020;254 [Google Scholar]
- Shen M., Zeng Z., Song B., Yi H., Hu T., Zhang Y., Xiao R. Neglected microplastics pollution in global COVID-19: disposable surgical masks. Sci. Total Environ. 2021;790 doi: 10.1016/j.scitotenv.2021.148130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spennemann D.H. COVID-19 face masks as a long-term source of microplastics in recycled urban green waste. Sustainability. 2022;14(1):207. [Google Scholar]
- Stpniewska Z., Wolińska A., Ziomek J. Response of soil catalase activity to chromium contamination. J. Environ. Sci. 2009;21(8):1142–1147. doi: 10.1016/s1001-0742(08)62394-3. [DOI] [PubMed] [Google Scholar]
- Sullivan G.L., Delgado-Gallardo J., Watson T.M., Sarp S. An investigation into the leaching of micro and nano particles and chemical pollutants from disposable face masks-linked to the COVID-19 pandemic. Water Res. 2021;196 doi: 10.1016/j.watres.2021.117033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Duan C., Cao N., Ding C., Huang Y., Wang J. Biodegradable and conventional microplastics exhibit distinct microbiome, functionality, and metabolome changes in soil. J. Hazard. Mater. 2022;424 doi: 10.1016/j.jhazmat.2021.127282. [DOI] [PubMed] [Google Scholar]
- Szefer E.M., Majka T.M., Pielichowski K. Characterization and combustion behavior of single-use masks used during COVID-19 pandemic. Materials. 2021;14(13):3501. doi: 10.3390/ma14133501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel M., de Veer D., Espinoza-Fuenzalida N.L., Espinoza C., Gallardo C., Hinojosa I.A., Villablanca R. COVID lessons from the global south–face masks invading tourist beaches and recommendations for the outdoor seasons. Sci. Total Environ. 2021;786 [Google Scholar]
- Wolińska A., Kuźniar A., Szafranek-Nakonieczna A., Jastrzębska N., Roguska E., Stępniewska Z. Biological activity of autochthonic bacterial community in oil-contaminated soil. Water, Air, Soil Pollut. 2016;227(5):1–12. doi: 10.1007/s11270-016-2825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S.L., Thompson R.C., Galloway T.S. The physical impacts of microplastics on marine organisms: a review. Environ. Pollut. 2013;178:483–492. doi: 10.1016/j.envpol.2013.02.031. [DOI] [PubMed] [Google Scholar]
- Wu X., Lu J., Du M., Xu X., Beiyuan J., Sarkar B., Wang H. Particulate plastics-plant interaction in soil and its implications: a review. Sci. Total Environ. 2021;792 doi: 10.1016/j.scitotenv.2021.148337. [DOI] [PubMed] [Google Scholar]
- Xu B., Liu F., Cryder Z., Huang D., Lu Z., He Y., Xu J. Microplastics in the soil environment: occurrence, risks, interactions and fate–a review. Crit. Rev. Environ. Sci. Technol. 2020;50(21):2175–2222. [Google Scholar]
- Yang P., Seale H., MacIntyre C.R., Zhang H., Zhang Z., Zhang Y., Wang Q. Mask-wearing and respiratory infection in healthcare workers in Beijing, China. Braz. J. Infect. Dis. 2011;15(2):102–108. doi: 10.1016/S1413-8670(11)70153-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M., Zhou S., Zhang L., Ding S. The effects of three different microplastics on enzyme activities and microbial communities in soil. Water Environ. Res. 2021;93(1):24–32. doi: 10.1002/wer.1327. [DOI] [PubMed] [Google Scholar]
- Yu H., Qi W., Cao X., Hu J., Li Y., Peng J., Qu J. Microplastic residues in wetland ecosystems: do they truly threaten the plant-microbe-soil system? Environ. Int. 2021;156 doi: 10.1016/j.envint.2021.106708. [DOI] [PubMed] [Google Scholar]
- Zhang S., Yang X., Gertsen H., Peters P., Salánki T., Geissen V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2018;616:1056–1065. doi: 10.1016/j.scitotenv.2017.10.213. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Li X., Xiao M., Feng Z., Yu Y., Yao H. Effects of microplastics on soil carbon dioxide emissions and the microbial functional genes involved in organic carbon decomposition in agricultural soil. Sci. Total Environ. 2022;806 doi: 10.1016/j.scitotenv.2021.150714. [DOI] [PubMed] [Google Scholar]
- Zhou S.Y.D., Lin C., Yang K., Yang L.Y., Yang X.R., Huang F.Y., Neilson R., Zhu Y.G. Discarded masks as hotspots of antibiotic resistance genes during COVID-19 pandemic. J. Hazard. Mater. 2022;425 doi: 10.1016/j.jhazmat.2021.127774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material
Supplementary material