Abstract
Background:
Shenling Baizhu San (SBS), a well-known Chinese medicine herbal formula, has been widely used for treating chronic diarrhea for thousands of years. However, the efficacy and safety of SBS in treating chronic diarrhea have not been fully assessed.
Objective:
This study evaluates the efficacy and safety of the herbal formula SBS in symptomatic relief of chronic diarrhea.
Methods:
English and Chinese language databases (PubMed, Cochrane Library, China National Knowledge Infrastructure, China Science and Technology Journal Database, Wanfang Data, and SinoMed electronic databases) were searched through April 2020 for relevant randomized controlled trials (RCTs). The outcomes in these RCTs included stool frequency, stool consistency, patient-reported satisfaction of chronic diarrhea treatment, quality of life and adverse events. Paired reviewers independently extracted data and conducted qualitative and quantitative analyses. The Cochrane revised risk of bias RoB-2 tool was applied to assess the risk of bias for each trial whereas the RevMan 5.3 software was used for outcomes data synthesis and meta-analysis. Mean difference (MD) and the 95% confidence interval (CI) were used to measure continuous data. The dichotomous data were analyzed via the relative risk (RR) with 95% CIs.
Results:
Fourteen RCTs including 1158 participants (54% males) with chronic diarrhea were included. Shenling Baizhu San combined with or without conventional medicine (CM) was associated with greater patient-reported satisfaction than CM alone. There was no increased risk of adverse events (AEs) during treatment.
Conclusion:
Treatment with SBS was associated with significant improvement in patient-reported satisfaction, irrespective of conventional medicine use. Rigorous and powered RCTs with objective outcome measures are needed to confirm the effects of SBS in specific gastrointestinal disease populations with chronic diarrhea symptoms.
Systematic review registration number (PROSPERO):
CRD42020178073
Keywords: Shenling Baizhu San, Samryungbaekchul-san, Jinryobyakujutsu-san, herbal formula, diarrhea, efficacy and safety
Introduction
Chronic diarrhea is defined as loose/watery stools with increased frequency (≥3 times/200 g per day) persisting longer than 4 weeks,1,2 and affects up to 5% of the global population.2,3 A key element in standard of care is the identification and treatment of any underlying etiology. In addition, symptomatic relief of diarrhea should be provided when clinically appropriate, as per clinical practice guidelines from the American Gastroenterological Association—for example, using opiates to slow down intestinal peristalsis and prolong the time of fluid absorption.2,4 However, opiates and adsorbents have limitations, including adverse effects5-7 and unsatisfactory treatment effect.7,8 In recent years, an increasing number of patients have sought complementary treatments, such as herbal formulas and dietary supplements, to alleviate chronic diarrhea.9,10
Herbal formulas have a long history of use for relieving gastrointestinal symptoms.11,12 One such formula, Shenling Baizhu San (SBS, Ginseng and Atractylodes Formula, Samryungbaekchul-san in Korean; Jinryobyakujutsu-san in Japanese), is frequently used for chronic diarrhea by clinicians in the Asia-Pacific region.13,14 The classic SBS formula is composed of 10 herbs (Table 1) but in clinical practice, minor modifications may be made based on a patient’s presentation. In addition to its clinical use, research shows that components of SBS may alleviate intestinal inflammation and alter the gut microbiome to improve water absorption and diarrhea.15-17
Table 1.
Constituent Herbs of Shenling Baizhu San.
| Scientific name | Latin pharmaceutical name | Chinese name | Part of herb used |
|---|---|---|---|
| Panax ginseng C. A. Mey. | Ginseng Radix | Ren Shen | Root |
| Poria cocos F. A. Wolf | Poria Sclerotium | Fu Ling | Sclerotium |
| Atractylodes macrocephala Koidz. | Atractylodis Rhizoma Alba | Bai Zhu | Rhizome |
| Glycyrrhiza uralensis Fisch. | Glycyrrhizae Radix | Gan Cao | Root |
| Dolichos lablab L. | Dolichorus Lablab Semen | Bai Bian Dou | Seed |
| Dioscorea opposita Thunb. | Dioscoreae Rhizoma | Shan Yao | Rhizome |
| Nelumbo nucifera Gaertn. | Nelumbinis Semen | Lian Zi | Seed |
| Platycodon grandifloras (Jacq.) A. DC. | Platycodi Radix | Jie Geng | Root |
| Amomum villosum Lour. | Amomi Fructus | Sha Ren | Fructus |
| Coix lacryma-jobi L. var. ma-yuen (Roman.) Stapf | Coicis Semen | Yi Yi Ren | Seed |
Shenling Baizhu San has been evaluated in clinical studies as a treatment for patients with chronic diarrhea.14,18 Evidence of efficacy, however, is mixed. A systematic review of currently available data, and a pooled analysis of efficacy and safety data from RCTs can help inform clinical practice. Here we summarize the current clinical evidence for SBS in the management of chronic diarrhea.
Methods
This study was registered under PROSPERO (CRD42020178073).
Eligibility Criteria
This review included RCTs published in any language. The interventions include SBS, with or without modifications, used alone or in combination with conventional medicine. Studies where SBS was combined with non-conventional therapies, such as acupuncture, massage, far infra-red physical therapy, thermotherapy, magnetic therapy, or pulse physical therapy were excluded. Studies that compared the effects of different modifications of SBS were also excluded as this is not the focus of this review. Our primary outcomes were stool frequency (measured by the exact number of defecations recorded per day) and stool consistency (changes from baseline assessed using the Bristol Stool Form Scale). Secondary outcomes were: (1) patient-reported satisfaction of chronic diarrhea treatment (percentage of patients who reported satisfaction of recovery from chronic diarrhea measured by either “cured cases” or “symptom relief rate” in the outcomes of included RCTs); (2) quality of life (score change from baseline); and (3) AEs.
Search Strategy, Study Selection, and Data Extraction
A literature search was conducted using PubMed, Cochrane Library, China National Knowledge Infrastructure (CNKI), China Science and Technology Journal Database (VIP Data), Wanfang Data, and SinoMed electronic databases through April 20th, 2020 with no language restrictions. Chinese translations of the search terms were used for Chinese databases. Two reviewers (HW and YNH) assessed the eligibility of each record. Initially, the title and abstract were screened. Studies that were not RCTs, did not include data on human subjects, chronic diarrhea, or orally administered pharmaceuticals, as well as those that did not refer to SBS or modified SBS were excluded at this stage. Further, the literature search has been updated to August 30th, 2021, and eligibility screening was assessed in reading the full text with the same criteria. Any disagreements over the selection of studies were resolved by a third reviewer (MXY). Detailed data were extracted from each study using a data-extraction form predefined by RevMan 5.3.
Risk-of-Bias Assessment
The risk of bias for each study was assessed independently by 2 reviewers (HW and YNH) using the Cochrane revised risk-of-bias RoB-2 tool. Before assessing, the reviewers were trained, and milestones and quality checks were reviewed by a senior researcher (MXY). Any disagreements were resolved by a third reviewer (MXY).
Statistical Analysis
The extracted efficacy data were entered in RevMan 5.3 for data synthesis and meta-analysis. Continuous data were analyzed using the mean difference (MD) and the 95% confidence interval (CI). Dichotomous data were analyzed using the relative risk (RR) with 95% CIs; and P < .05 indicated statistical significance according to the Cochrane Handbook. 19 For each pooled analysis, a heterogeneity test was performed using the chi-square statistic. The fixed-effect model was utilized to perform meta-analysis, except when I2 > 50%. In such a case, the random-effect model was used. When substantial heterogeneity was found, a subgroup or sensitivity analysis was carried out to identify the cause. 20 Possible publication bias was determined with a funnel plot test if 10 or more studies were included in 1 meta-analysis. A descriptive report was made for any undetermined sources of heterogeneity.
Results
Search Results
The initial database search yielded 5651 records. After screening the titles and abstracts, 157 full-text studies were further evaluated for eligibility criteria. In total, 14 trials met the inclusion criteria (Figure 1).
Figure 1.
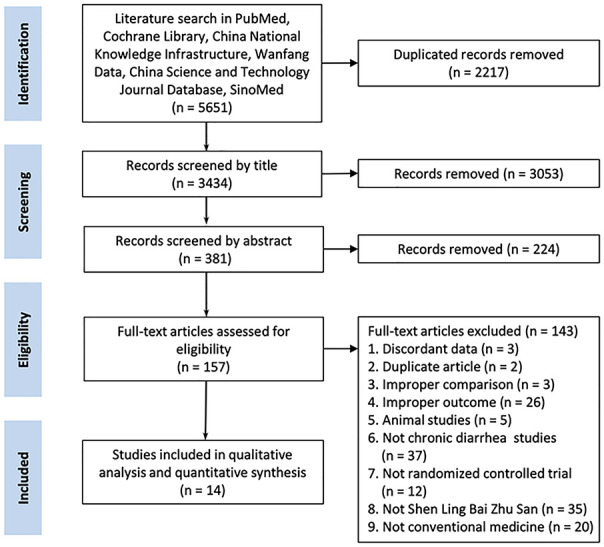
Flow diagram of systematic review.
Study process from the initial literature search to the final quantitative analysis. The number of studies included and excluded, and the reasons have been detailed.
Study Characteristics
This study included 14 RCTs with a total of 1158 participants (54% males) from South Korea 13 and China.21-33 The sample size of each trial was relatively small with the largest including 150 participants. 26 Among the 1158 adults who met the chronic diarrhea definition, 605 (52% males) were diagnosed with diarrhea-predominant irritable bowel syndrome according to ROME III guidelines 13,22,24,25,28,31-33; and 148 (59% males) were diagnosed with ulcerative colitis based on the Chinese Medical Association guidelines.21,27 The remaining 405 (59% males) participants had no specific diagnosis.23,26,29,30 The duration of diarrhea ranged from 4 weeks to 2 decades.
Shenling Baizhu San was administrated as an intervention in the form of concentrated granules,13,24,25,31,32 patented herbal medicine, 21 and herbal decoction.22,23,26-30,32 Only 1 trial used a standardized extract whose quality was ensured using a high-performance liquid chromatography array. 13 Four trials13,21,25,33 used the classic SBS formula while 10 trials22-24,26-32 used modified SBS formulas. The duration of treatment ranged from 10 days 29 to 24 weeks. 27 The comparators in the 14 included trials were pinaverium bromide,22,31,32 mesalazine,21,27 otilonium bromide, 13 paroxetine, 24 norfloxacin, 26 montmorillonite,25,29,30,33 sulfasalazine, 23 trimebutine maleate, 28 and placebo. 13 Patient-reported satisfaction, AEs, and quality of life were reported as clinical outcomes. All studies were conducted in a real-world clinical setting, including both outpatient and inpatient hospital departments. The main characteristics of the included studies are summarized in Table 2.
Table 2.
Characteristics of studies included.
| Study ID | Participants | Interventions b | Control b | Outcomes | Setting | Adverse events | |||
|---|---|---|---|---|---|---|---|---|---|
| Sample size | Age (years) a | Gender (M/F) | Disease Course (months) | ||||||
| He 21 | E: 24 C: 24 |
E: 39.36 ± 6.23 C: 37.76 ± 7.12 |
E: 16/8 C: 15/9 |
E: 56.10 ± 4.50 C: 48.30 ± 3.50 |
SBS, 90 d | Mesalazine, 90 d | AE | Outpatient, inpatient | No SAEs were found |
| Kang 22 | E: 25 C: 23 |
E: 28.62 ± 0.74 C: 30.01 ± 0.31 |
Not available | E: 4.97 ± 0.43 C: 3.84 ± 0.58 |
mSBS, 3 wk | Pinaverium bromide, 3 wk | PRS | Outpatient, inpatient | Not reported |
| Lee et al13 | E1: 20 E2: 20 Ca: 20 Cb: 20 |
E1: 42.90 ± 15.13 E2: 38.05 ± 15.27 Ca: 41.65 ± 14.26 Cb: 45.20 ± 13.56 |
E1: 17/3 E2: 16/4 Ca: 16/4 Cb: 11/9 |
E1: 150.96 ± 127.08 E2: 112.44 ± 84.6 Ca: 137.40 ± 109.92 Cb: 111.36 ± 138.84 |
E1: SBB + OB, 8 wk E2: SBS + P-OB, 8 wk |
Ca: P-SBS + OB, 8 wk Cb: P-SBS + P-OB, 8 wk |
SF SC PRS QOL AE |
Hospital | One ALT increase in Ca and two abdominal pain/fever in Cb |
| Lei 23 | E: 48 C: 48 |
Not available | E: 27/21 C: 26/22 |
Not available | mSBS, 3 mo | Sulfasalazine + Anisodamine Tablets + Codeine + Montmorillonite, 3 mo | PRS | Hospital | Not reported |
| Li et al 24 | E: 40 C: 40 |
E: 35.2 C: 34.6 |
E: 16/24 C: 17/23 |
Not available | mSBS + Paroxetine, 1 mo | Paroxetine, 1 mo | PRS | Outpatient, inpatient | Not reported |
| Li and Jiang 25 | E: 40 C: 40 |
E: 38.35 ± 11.85 C: 40.13 ± 11.75 |
E: 19/21 C: 17/23 |
E: 35.76 ± 21.36 C: 41.88 ± 24.36 |
SBS, 4 wk | Montmorillonite, 4 wk | PRS AE |
Hospital | Constipation |
| Ma 26 | E: 75 C: 75 |
E: 39.6 C: 38.9 |
E: 45/30 C: 47/28 |
E: 12–84 C: 12–72 |
mSBS, 30 d | Norfloxacin + fluid therapy + correction of electrolyte disorder + symptomatic support therapy, 30 d | PRS | Hospital | Not reported |
| Quan and Tan 27 | E: 50 C: 50 |
E: 44.02 ± 10.35 C: 43.51 ± 10.29 |
E: 27/23 C: 29/21 |
E: 2.4-228 C: 1.2-240 |
mSBS, 24 wk | Mesalazine, 24 wk | AE | In hospital | Nausea, vomiting, rash, allergy |
| Tian 28 | E: 30 C: 30 |
E: 40.92 ± 11.04 C: 40.13 ± 11.59 |
E: 16/14 C: 18/12 |
E: 25.92 ± 15.24 C: 34.44 ± 23.16 |
mSBS + Trimebutine maleate, 6 wk | Trimebutine maleate, 6 wk | PRS AE |
Outpatient | No SAEs or ADRs were found |
| Wang 29 | E: 47 C: 47 |
E: 45.40 ± 4.82 C: 45.35 ± 4.74 |
E: 23/24 C: 21/26 |
E: 14.16 ± 3.6 C: 13.32 ± 2.88 |
mSBS, 10 d | Montmorillonite, 10 d | PRS AE |
Hospital | Nausea, abdominal distention, constipation |
| Zhang 30 | E: 33 C: 32 |
E: 38.73 ± 12.64 C: 38.53 ± 12.69 |
E: 18/15 C: 19/13 |
E: 9.18 ± 3.92 C: 9.97 ± 4.39 |
mSBS, 4 wk | Montmorillonite, 4 wk | PRS AE |
Outpatient | No AEs were found |
| Zhang and Zhou 31 | E: 30 C: 30 |
Not available | E: 18/12 C: 14/16 |
E: 47.04 ± 29.88 C: 49.08 ± 27.60 |
mSBS, 4 wk | Pinaverium bromide, 4 wk | PRS AE |
Hospital | No AEs were found |
| Zhao and Cao 32 | E: 58 C: 59 |
E: 38.57 ± 6.94 C: 39.02 ± 6.95 |
E: 27/31 C: 30/29 |
E: 37.08 ± 28.92 C: 38.04 ± 29.28 |
mSBS + Pinaverium bromide, 1 mo | Pinaverium bromide, 1 mo | PRS | Hospital | Not reported |
| Zhong and Wu 33 | E: 40 C: 40 |
E: 46.1 C: 44.7 |
E: 19/21 C: 18/22 |
E: 4-108 C: 2-132 |
SBS + Montmorillonite, 4 wk | Montmorillonite, 4 wk | PRS | Outpatient | Not reported |
Abbreviations: E, experimental intervention; C, control intervention; OB, otilonium bromide; P-OB, placebo otilonium bromide; SBS, Shenling Baizhu San; P-SBS, placebo Shenling Baizhu San; mSBS, modified Shenling Baizhu San; SF, stool frequency; SC, stool consistency; PRS, patient-reported satisfaction; QOL, quality of life; ALT, alanine transaminase; AE, adverse event; SAE, serious adverse event; ADR, adverse drug reactions; SADE, serious adverse drug events.
Age of the participants is reported as mean ± standard deviation, or median (minimum-maximum), or mean, or (minimum-maximum), depending on the availability of data.
Both the name of the treatment and course length are reported in the column.
Randomized controlled trial with 4-arm, parallel-group design with 4 different intervention groups. According to the allocation principle of this study, 2 experimental groups (E1 and E2) and 2 control groups (Ca and Cb) were defined.
Quality Assessment
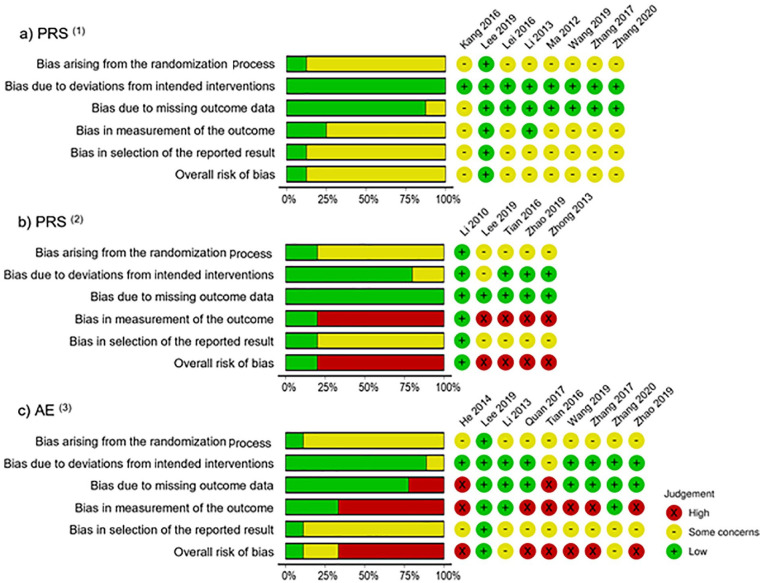
Based on the RoB-2 tool, the risk of bias associated with each outcome is reported individually (Figure 2a–c). One study reported stool frequency and stool consistency but was associated with concerns of risk of bias. The bias was mainly caused by an inadequate randomization process and/or improper outcome measurement, or improper reporting of results. For quality of life, the overall risk of bias was low, although only 1 study evaluated quality of life. 13 Both patient-reported satisfaction and AEs were associated with high risk of bias, especially in the missing data, blinding assessment, and outcome reporting domains. Considerable bias also originated from the randomization process.
Figure 2.
Risk-of-bias assessments using the revised Cochrane Risk-of-Bias 2 tool.
“Traffic light” plots of the domain-level judgments for each individual outcome, and weighted bar plots of the distribution of risk-of-bias judgments within each bias domain. Judgments ranged mostly in the yellow and red colors, reflecting “some concerns” and “high” risk of bias, respectively; “low” risk is represented by the green color. Figure 2a shows the risk-of-bias diagrams of included studies with patient-reported satisfaction as the outcome, comparing SBS alone versus conventional medicine; Figure 2b shows the risk-of-bias diagrams of included studies with patient-reported satisfaction as the outcome, comparing SBS with conventional medicine versus conventional medicine alone; and Figure 2c shows the risk-of-bias diagrams of included studies with adverse events; (1)Included studies with patient-reported satisfaction (PRS) comparing SBS alone versus conventional medicine; (2)Included studies with patient-reported satisfaction (PRS) comparing SBS with conventional medicine versus conventional medicine alone; (3)Included studies with adverse events (AE).
Outcome Measures
Stool frequency and stool consistency
We chose stool frequency and stool consistency as our primary outcomes because they are objective measurements. Only one of the 14 studies included in this analysis reported these outcomes.
Lee et al, 13 using a 2 × 2 design of SBS or otilonium bromide (OB) versus placebo SBS or placebo OB, did not find statistically significant improvement in stool frequency between the SBS and placebo SBS group but reported a significant difference in stool consistency during week 12 (SBS + OB: −1.33 ± 0.59; SBS + placebo OB: −1.41 ± 0.94; placebo SBS + OB: −0.65 ± 0.61; placebo SBS + placebo OB: −0.80 ± 0.68; P = .003) using the Bristol Stool Form Chart scale.
Patient-reported satisfaction with chronic diarrhea treatment
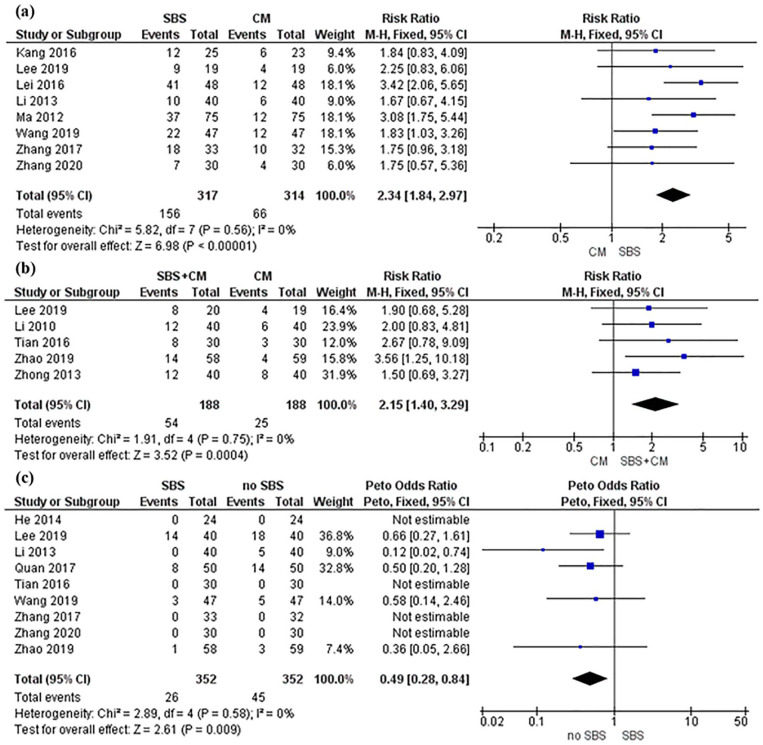
Pooled analysis from 8 trials13,22,23,25,26,29-31 showed SBS alone is associated with improved patient-reported satisfaction in chronic diarrhea treatment compared to conventional medicine (RR, 2.34; 95% CI, 1.84-2.97; P < .00001; I2 = 0%) (Figure 3a). Further analysis from 5 trials13,24,28,32,33 indicated that SBS in combination with conventional medicine is associated with improved patient-reported satisfaction in chronic diarrhea treatment compared to conventional medicine alone (RR, 2.15; 95% CI, 1.40-3.29; P = .0004; I2 = 0%) (Figure 3b). Heterogeneity test findings are consistent across all trials showing no significant heterogeneity. Of the 14 included studies, only Lee et al 13 used placebo control. The study found that SBS was more likely to positively affect patient-reported satisfaction compared with placebo at week 4 follow-up (P = 0.049); however, the effect was not statistically significant at the end of the 8-week study period. Publication bias with funnel plot test was not conducted because fewer than 10 studies were included in each meta-analysis.
Figure 3.
Forest plot of patient-reported satisfaction and adverse events.
Forest plot of patient-reported satisfaction comparing SBS with conventional medicine. In both Figure 3a and b, the black diamond is to the right of the vertical line of null effect, suggesting that SBS use, regardless of conventional medicine, results in significantly better patient-reported satisfaction than conventional medicine by itself. Figure 3b is the forest plot of patient-reported satisfaction comparing SBS with conventional medicine versus conventional medicine alone. Figure 3c is the forest plot of AEs of SBS versus no SBS. The black diamond is to the left side of the vertical line of null effect suggesting that SBS does not increase the risk of AEs.
Abbreviations: CM, conventional medicine; SBS, Shenling Baizhu San.
Quality of life
Lee et al 13 also reported quality of life as 1 of 8 items in secondary outcomes. The study did not find statistically significant difference when comparing SBS without otilonium bromide versus SBS with otilonium bromide versus placebo (MD, −2.71 ± 2.37, −3.17 ± 2.28, −2.44 ± 2.03, respectively P > .05).
Adverse events (AEs)
Pooled analysis from 9 trials13,21,25,27-32 indicated that SBS is not associated with a higher risk of AEs compared with no SBS (OR, 0.49; 95% CI, 0.28-0.84; P = .009; I2 = 0%) (Figure 3c). However, Lee et al 13 reported serious AEs with 1 case of elevated alanine transaminase in the otilonium bromide plus placebo SBS group, and 2 cases of abdominal pain or fever in the placebo otilonium plus placebo SBS group although the differences between the groups were statistically insignificant (P > .05).
Discussion
Management of chronic diarrhea remains a challenge for clinicians due to patients’ incomplete clinical response to treatment and adverse effects of long-term conventional medicine use. This systematic review analyzed data from 14 RCTs with 1158 participants who had experienced chronic diarrhea for more than 4 weeks. The results show that compared to conventional medicines, treatment with SBS alone or SBS combined with conventional medicines significantly improved patient-reported satisfaction. Furthermore, pooled analysis of safety data showed that SBS did not significantly increase AEs compared with no SBS. However, only 1 trial included our predefined major outcomes—stool frequency and stool consistency— indicating insufficient evidence for determining the effects of SBS on the above outcomes in patients with chronic diarrhea. Although the secondary outcomes of quality of life, satisfaction of symptom recovery, and AEs were collectively evaluated across the included trials, qualitative assessment revealed that these outcomes were associated with at least moderate risk of bias due to methodological limitations.
Clinical Implications
Although no current clinical practice guidelines recommend herbal medicines as therapeutics for chronic diarrhea, several studies provide evidence of chronic diarrhea symptom improvement with herbal medicine use. One systematic review suggests that single herb preparations (curcumin, desert Indian wheat, and wormwood) may improve diarrhea-related symptoms such as chronic diarrhea in patients with gastrointestinal disease. 9 An RCT reported that when compared to placebo, the herbal formula Tong Xie Yao Fang can reduce stool frequency and improve stool consistency in patients with diarrhea-predominant irritable bowel syndrome. 34 In the first systematic review to focus on the herbal formula SBS, we report here that available data favor SBS in significantly improving patient-reported satisfaction of chronic diarrhea treatment—with no increased occurrence of AEs regardless of concurrent use of conventional medicine. We also found insufficient direct evidence linking SBS to improvement in the objective outcome of stool frequency. SBS may improve stool consistency, although there was only 1 study that reported this endpoint.
Mechanistic studies have shown that SBS can modulate the composition of gut microbiota 35 and intestinal absorption as well as the mucosal ultrastructure. 36 Components of SBS, such as Panax ginseng and Atractylodes macrocephala, exhibit numerous biologic effects: Polysaccharides in Panax ginseng can regulate immune cells 37 and promote recovery of mucosa. 38 Atractylenolide III helps attenuate inflammation associated with 2,4,6-trinitrobenzenesulfonic acid-induced colitis. 39 In addition, Lv et al16,40 reported that SBS can enhance the richness and diversity of intestinal microbiota, increase acid metabolism, and reduce diarrhea-related intestinal, immune and infectious diseases.
Based on previous clinical evidence, pathophysiological findings, and evidence synthesized by the current study, SBS appears to be a promising option in the overall management of chronic diarrhea, especially when patient satisfaction is concerned.
Research Implications
This study also revealed methodological issues which should be addressed in future SBS clinical research to obtain more generalizable evidence for the use of SBS in patients with chronic diarrhea. First, most studies are underpowered, and thus, further validation of the effect in an adequately powered sample is needed; and RCT guidelines on randomization and allocation concealment should be followed. Second, diarrhea caused by either functional or organic etiologies is a symptom that is seen in various gastrointestinal disorders. In order to generate generalizable clinical evidence, clinical trials must use the global diagnostic code of gastrointestinal disease under which chronic diarrhea presents in order to reduce heterogeneity and ambiguity of evidence. 2 Third, a standardized SBS intervention with uniform ingredients and dosing regimen is needed to eliminate intervention inconsistencies. 41 Fourth, a valid placebo for SBS should be developed and used consistently. 41 Only 1 trial included in this analysis used a placebo SBS. However, there were validity concerns due to the use of lactose, an ingredient which can affect the digestive system of chronic diarrhea patients. 13 Finally, more targeted and specific outcome measures, such as abdominal pain intensity and stool consistency, should be used as primary endpoints, as per the United States Food and Drug Administration guidance to industry for treating irritable bowel syndrome with diarrhea. 42
Limitations and Strengths
There are several limitations in this study. First, the inclusion of different disease populations with chronic diarrhea symptoms increased heterogeneity, which may hinder the interpretation of data and inhibit the translation of evidence into clinical practice. Further subgroup analysis based on disease category may help address this issue, but it is methodologically limited due to the inadequate number of studies included. Consequently, further in-depth analyses of efficacy data pertinent to clinical practice, such as the optimal treatment dose, administration approaches, and the effectiveness of SBS in comparison with different subclasses of conventional medications, have not been systematically performed, reiterating the need to produce more quality data in the future.
The strength of this study is that it synthesized data from clinical trials to provide efficacy and safety evidence of 1 herbal treatment, SBS, for symptom management of chronic diarrhea rather than investigating several single herbs done in previous studies. This study also focuses on the clinical effectiveness of SBS for symptom relief of chronic diarrhea in real-world practice rather than in an experimental setting.
Conclusion
Shenling Baizhu San is a promising option in the overall management of chronic diarrhea. Current evidence suggests that it may substantially improve patient satisfaction with chronic diarrhea treatment irrespective of conventional medication use. However, the methodological limitations of studies included in this review do not allow for a definitive conclusion on SBS’s effects in reducing stool frequency and consistency in patients experiencing chronic diarrhea. More high-quality RCTs are warranted to evaluate the efficacy of SBS in specific gastrointestinal disease populations with chronic diarrhea symptoms.
Footnotes
Author Contributions: Gary Deng and Jun J. Mao designed the study. Ye Feng, Yi Lily Zhang, Yen-Nien Hou, and Hui Wang collected the data. Hui Wang and Yen-Nien Hou performed statistical analyses. Hui Wang and Yen-Nien Hou wrote the paper. Mingxiao Yang, Colleen M. Smith, Wei Hou and Jun J. Mao critically revised the paper. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Jun J. Mao reports grants from Tibet CheeZheng Tibetan Medicine Co. Ltd. and from Zhongke Health International LLC outside the submitted work. Hui Wang, Yen-Nien Hou, Mingxiao Yang, Ye Feng, Yi Lily Zhang, Colleen M. Smith, Wei Hou, and Gary Deng declare no conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This manuscript is supported in part by a grant from the National Institutes of Health/National Cancer Institute (P30 CA008748); by the Laurance S. Rockefeller Foundation (supports the MSK Herbal Research and Education in Oncology (HERO) program); and the Translational and Integrative Medicine Research Fund, the latter two at Memorial Sloan Kettering Cancer Center. Hui Wang is supported by a grant from the China Academy of Chinese Medical Sciences in China that supports the program of Evidence-based Research Implementation Scheme Design of Chinese Medicine for Cancer (ZZ13-024-6).
ORCID iD: Yen-Nien Hou  https://orcid.org/0000-0002-7975-5427
https://orcid.org/0000-0002-7975-5427
References
- 1. Fine KD, Schiller LR. AGA technical review on the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116:1464-1486. doi: 10.1016/s0016-5085(99)70513-5 [DOI] [PubMed] [Google Scholar]
- 2. Schiller LR, Pardi DS, Sellin JH. Chronic diarrhea: diagnosis and management. Clin Gastroenterol Hepatol. 2017;15:182-193.e3. doi: 10.1016/j.cgh.2016.07.028 [DOI] [PubMed] [Google Scholar]
- 3. Talley NJ, O’Keefe EA, Zinsmeister AR, Melton LJ, 3rd. Prevalence of gastrointestinal symptoms in the elderly: a population-based study. Gastroenterology. 1992;102:895-901. doi: 10.1016/0016-5085(92)90175-x [DOI] [PubMed] [Google Scholar]
- 4. American Gastroenterological Association medical position statement: guidelines for the evaluation and management of chronic diarrhea. Gastroenterology. 1999;116:1461-1463. doi: 10.1016/s0016-5085(99)70512-3 [DOI] [PubMed] [Google Scholar]
- 5. Palmer KR, Corbett CL, Holdsworth CD. Double-blind cross-over study comparing loperamide, codeine and diphenoxylate in the treatment of chronic diarrhea. Gastroenterology. 1980;79:1272-1275. [PubMed] [Google Scholar]
- 6. Schiller LR. Chronic diarrhea. Curr Treat Options Gastroenterol. 2005;8:259-266. doi: 10.1007/s11938-005-0018-8 [DOI] [PubMed] [Google Scholar]
- 7. Schiller LR, Hogan RB, Morawski SG, et al. Studies of the prevalence and significance of radiolabeled bile acid malabsorption in a group of patients with idiopathic chronic diarrhea. Gastroenterology. 1987;92:151-160. doi: 10.1016/0016-5085(87)90852-3 [DOI] [PubMed] [Google Scholar]
- 8. Dadu R, Hu MI, Cleeland C, et al. Efficacy of the natural clay, calcium aluminosilicate anti-diarrheal, in reducing medullary thyroid cancer-related diarrhea and its effects on quality of life: a pilot study. Thyroid. 2015;25:1085-1090. doi: 10.1089/thy.2015.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Langhorst J, Wulfert H, Lauche R, et al. Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis. 2015;9:86-106. doi: 10.1093/ecco-jcc/jju007 [DOI] [PubMed] [Google Scholar]
- 10. Lu WI, Lu DP. Impact of chinese herbal medicine on american society and health care system: perspective and concern. Evid Based Complement Alternat Med. 2014;2014:251891. doi: 10.1155/2014/251891. Evid-Based Complement Alternat Med. 2014;2014:251891-251891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim YS, Kim JW, Ha NY, Kim J, Ryu HS. Herbal therapies in functional gastrointestinal disorders: a narrative review and clinical implication. Front Psychiatry. 2020;11:601. doi: 10.3389/fpsyt.2020.00601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ried K, Travica N, Dorairaj R, Sali A. Herbal formula improves upper and lower gastrointestinal symptoms and gut health in Australian adults with digestive disorders. Nutr Res. 2020;76:37-51. doi: 10.1016/j.nutres.2020.02.008 [DOI] [PubMed] [Google Scholar]
- 13. Lee JH, Kim JI, Baeg MK, et al. Effect of samryungbaekchul-san combined with otilonium bromide on diarrhea-predominant irritable bowel syndrome: a pilot randomized controlled trial. J Clin Med. 2019;8:27. doi: 10.3390/jcm8101558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan J, Miao ZW, Lu J, et al. Acupuncture plus Chinese herbal medicine for irritable bowel syndrome with diarrhea: a systematic review and meta-analysis. Evid Based Complement Alternat Med. 2019;2019:7680963. doi: 10.1155/2019/7680963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. An X, Bao Q, Di S, et al. The interaction between the gut microbiota and herbal medicines. Biomed Pharmacother. 2019;118:109252. doi: 10.1016/j.biopha.2019.109252 [DOI] [PubMed] [Google Scholar]
- 16. Lv WJ, Liu C, Li YF, et al. Systems pharmacology and microbiome dissection of Shen Ling Bai Zhu San reveal multiscale treatment strategy for IBD. Oxid Med Cell Longev. 2019;2019:8194804-8194804. doi: 10.1155/2019/8194804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shi K, Qu L, Lin X, et al. Deep-fried atractylodis rhizoma protects against spleen deficiency-induced diarrhea through regulating intestinal inflammatory response and gut microbiota. Int J Mol Sci. 2020;21:2019. doi: 10.3390/ijms21010124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang L, Song Y, Jin P, et al. Shen-Ling-Bai-Zhu-San for ulcerative colitis: protocol for a systematic review and meta-analysis. Medicine. 2018;97:e12337-e12337. doi: 10.1097/MD.0000000000012337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. Oct 3 2019;10:Ed000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang M, Sun M, Du T, et al. The efficacy of acupuncture for stable angina pectoris: a systematic review and meta-analysis. Eur J Prev Cardiol. Published online September 17, 2019. doi: 10.1177/2047487319876761. [DOI] [PubMed] [Google Scholar]
- 21. He K. Ulcerative colitis spleen qi deficiency parallel randomized controlled study Shenlingbaizhu casual treatment. J Pract Tradit Chin Intern Med. 2014;28:58-59. [Google Scholar]
- 22. Kang H. Clinical observation on 25 cases of irritable bowel syndrome treated with modified Shen Ling Bai Zhu San. Guide China Med. 2016;14:201-202. [Google Scholar]
- 23. Lei C. Clinical study of Shenlingbaizhu powder in the treatment of chronic diarrhea. China Health Care Nutr. 2016;26:335. [Google Scholar]
- 24. Li C, Wu M, Wang L, Zhang J, Wu F, Pan Z. Clinical observation of Shen Ling Bai Zhu San combined with paroxetine in treatment of diarrhea predominant irritable bowel syndrome. Chin J Tradit Med Sci Technol. 2010;17:154-155. [Google Scholar]
- 25. Li Q, Jiang J. Clinical observation on 80 cases of diarrhea predominant irritable bowel syndrome treated with Shen Ling Bai Zhu granule. Guiding J Tradit Chin Med Phar. 2013;19:41-42. [Google Scholar]
- 26. Ma Z. Clinical observation of Shen Ling Bai Zhu san in treatment of chronic diarrhea. Contemp Med. 2012;18:148. [Google Scholar]
- 27. Quan L, Tan J. Clinical study of Shenling Baizhu San for ulcerative colitis. J New Chin Med. 2017;49:42-44. [Google Scholar]
- 28. Tian H. The Clinical Observation of Irritable Bowel Syndrome-Diarrhea Type of Spleen and Stomach Weakness Syndrome With the Treatment of ShenlingBaizhu Decoction Joint Trimebutine Maleate. Master’s thesis. Hubei University of Chinese Medicine, ; 2016. [Google Scholar]
- 29. Wang M. Clinical effect of Shenling Baizhu San in treating diarrhea with spleen deficiency. Shenzhen J Integr Trad Chin West Med. 2019;29:59-60. [Google Scholar]
- 30. Zhang T. Clinical effect of modified Shenling Baizhu power in treatment of chronic diarrhea with spleen deficiency. J Anhui Trad Chin Med Coll. 2017;36:45-47. [Google Scholar]
- 31. Zhang T, Zhou W. Clinical observation and mechanism study of modified Shen Ling Bai Zhu San in treatment of diarrhea predominant irritable bowel syndrome. Chin Med Mod Dist Edu China. 2020;18:74-76. [Google Scholar]
- 32. Zhao Y, Cao Z. Clinical efficacy and safety evaluation of Shen Ling Bai Zhu powder combined with pinaverium bromide tablets in the treatment of diarrhea predominant irritable bowel syndrome. World Chin Med. 2019;14:1278-1281. [Google Scholar]
- 33. Zhong Y, Wu Y. The observation of Shen Ling Bai Zhu granule combined with montmorillonite powder in treatment of 40 cases of diarrhea predominant irritable bowel syndrome. Jiang xi J Trad Chin Med. 2013;44:45-46. [Google Scholar]
- 34. Chen M, Tang TC, Wang Y, et al. Randomised clinical trial: Tong-Xie-Yao-Fang granules versus placebo for patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48:160-168. doi: 10.1111/apt.14817 [DOI] [PubMed] [Google Scholar]
- 35. Ma Q, Ouyang Y, Meng F, et al. A review of pharmacological and clinical studies on the application of Shenling Baizhu San in treatment of Ulcerative colitis. J Ethnopharmacol. 2019;244:112105. doi: 10.1016/j.jep.2019.112105 [DOI] [PubMed] [Google Scholar]
- 36. Ji HJ, Kang N, Chen T, et al. Shen-ling-bai-zhu-san, a spleen-tonifying Chinese herbal formula, alleviates lactose-induced chronic diarrhea in rats. J Ethnopharmacol. 2019;231:355-362. doi: 10.1016/j.jep.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 37. Kang S, Min H. Ginseng, the ‘immunity boost’: the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354-368. doi: 10.5142/jgr.2012.36.4.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li S, Qi Y, Chen L, et al. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic-associated diarrhea. Int J Biol Macromol. 2019;124:931-937. doi: 10.1016/j.ijbiomac.2018.11.271 [DOI] [PubMed] [Google Scholar]
- 39. Ren Y, Jiang W, Luo C, Zhang X, Huang M. Atractylenolide III ameliorates TNBS-induced intestinal inflammation in mice by reducing oxidative stress and regulating intestinal flora. Chem Biodivers. 2021;18:e2001001. doi: 10.1002/cbdv.202001001 [DOI] [PubMed] [Google Scholar]
- 40. Lv W, Liu C, Ye C, et al. Structural modulation of gut microbiota during alleviation of antibiotic-associated diarrhea with herbal formula. Int J Biol Macromol. 2017;105:1622-1629. doi: 10.1016/j.ijbiomac.2017.02.060 [DOI] [PubMed] [Google Scholar]
- 41. Liu JP, Yang M, Liu YX, Wei M, Grimsgaard S. Herbal medicines for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2006;1:CD004116. doi: 10.1002/14651858.CD004116.pub2 [DOI] [PubMed] [Google Scholar]
- 42. FDA. Guidance for industry: Irritable bowel syndrome—clinical evaluation of products for treatment. U.S. Department of Health and Human Service; 2012. [Google Scholar]