Abstract
BACKGROUND & AIMS:
Sorafenib has been the standard of care for patients with advanced hepatocellular carcinoma and although immunotherapeutic approaches are now challenging this position, it retains an advantage in HCV seropositive patients. We aimed to quantify the rate of tumour progression in patients receiving sorafenib and relate this figure to survival, both overall, and according to viral status.
METHODS:
Using serial data from an international clinical trial we applied a joint model to combine survival and progression over time so as to estimate the rate of tumour growth as assessed by tumour burden and serum AFP, and the impact of treatment on liver function.
RESULTS:
High tumour burden at baseline was associated with an increased risk of death. In patients still alive at the end of the study, the progression in relation to tumour burden was very low compared to those who died within the study. Overall, the change in mean tumour burden was 0.12 mm per day or an absolute growth rate of 3.6mm/month. Median doubling time (DT) was 665 days. For those who progressed above 0.12mm per day or 12% rate, median survival was 234 days compared to 384 days if the rate was below 12%. Tumour growth rate and serum AFP rise were significantly lower in those who were HCV seropositive as was the rate of decline in liver function. These results were replicated in two independent patient groups.
CONCLUSIONS:
Our analysis suggests that sorafenib treatment is associated with improved survival in patients with advanced hepatocellular carcinoma mainly by decreasing the rate of tumour growth and liver function deterioration among patients with HCV infection.
Keywords: hepatitis C virus, tumour progression rate, joint modelling
Lay summary:
Among patients receiving sorafenib for advanced hepatocellular carcinoma the rate of tumour growth (as assessed by changes in tumour size and the biomarker AFP) and the deterioration of liver function is less in those who have the Hepatitis C virus, than those who do not.
Introduction
Hepatocellular carcinoma (HCC) is the most prevalent type of primary liver cancer and the third leading cause of cancer death worldwide with over 500,000 people affected each year(1). The development of HCC has well-established causal links to chronic viral hepatitis, types B (HBV) and C (HCV) and other types of chronic liver disease. In the absence of a rigorous surveillance program, most patients with HCC are not suitable for potentially curative treatments, such as surgical resection, due to the advanced stage of the disease at presentation(2). The standard of care for advanced, unresectable HCC (aHCC), has been sorafenib. The SHARP trial which involved this antiangiogenic, multikinase inhibitor was the first prospective randomised placebo-controlled trial to show survival benefit for any systemic therapy in patients with aHCC although the absolute benefit was modest (median survival: 10.7 (sorafenib) vs 7.9 (placebo) months)(3) and the overall response rate is < 2%. Similar results were subsequently reported from the analogous Asia-Pacific (AP) study (6.5 vs 4.2 months)(4). Subsequent trials in which sorafenib was compared to other antiangiogenic agents all failed to achieve their primary endpoints or, in the case of Lenvatinib, showed only non-inferiority(5). We note there have been some promising results with immunotherapy (6, 7) and, recently the combination of atezolizumab and bevacizumab has shown significantly improved survival over sorafenib at one year (8).
Using a Bayesian hierarchical approach for individual patient data meta-analysis of three large prospective randomised controlled trials (RCTs), we showed that survival benefit attributable to sorafenib was largely confined to those who were HCV positive (9). Furthermore, a meta-analysis involving an entirely different methodological approach (reconstructed individual participant survival data from phase III RCTs) also identified HCV as the key variable indicating better survival(10). Such results were entirely consistent with findings in the head to head trial of sorafenib and sunitinib where the relative survival of HCV positive vs. HCV negative in the sorafenib arm was 17.6 vs 9.2 months (11). Similarly, the initial sub-group analysis of the SHARP trial(12) and the more recent analysis of the combined output of the SHARP and AP trials reported analogous figures of 14 vs 7.5 months and 14 vs 7.8 months respectively both of being placebo-controlled studies(13). This latter analysis led Bruix et al to conclude that that HCV positivity was of ‘paramount value’ in clinical trials and that trials in which patients were not stratified according to etiology were ‘vulnerable’.
Indeed since the survival curves of HCV negative patients receiving sorafenib or placebo were superimposable, and among the HCV positive patients sorafenib resulted in a near doubling of survival it seems likely that sorafenib will, despite current changes in the landscape of systemic therapy for aHCC, remain a valuable agent in this clearly defined subgroup.
In the light of this now compelling evidence that sorafenib is beneficial in HCV positive patients group, the question of just how sorafenib impacts on overall survival in this subgroup becomes relevant as will be the molecular mechanism. Here we test the hypothesis that sorafenib selectively increases survival in HCV seropositive patients by decreasing the rate of tumour growth and decline in liver function. We apply a statistical methodology known as joint modelling(14), that can jointly model (often censored) time to event data (in this case, time to death) and additional time-dependent covariates (in this case tumour burden, serum AFP levels and a measure of liver function, ALBI). Using this statistical methodology, we can correlate the rate of change of time-dependent covariates with outcome overall, and among patients with different aetiologies.
Patients and methods
The primary dataset for the model in this study comprised patients in the sorafenib-treated (control) arm of a randomised phase III clinical trial of linifanib vs. sorafenib in aHCC (15). Details of the patient groups are given in the relevant publication but, in brief, this was an international study involving 502 patients in whom the maximum diameter of identified target lesions was measured at approximately 6 weekly intervals. Patients were tested for HCV-Ab at a central laboratory. Reasons for study drug withdrawal and post-trial treatment are listed in Supplementary Tables 1 & 2 (Supplementary data).
Two further patient groups were used to test the generalisability of our analyses. The second patient group came from the sorafenib control arm of a study in which sorafenib was compared, in a randomised phase III trial, to brivanib(16). The comparability of the two clinical trial patient groups has been formally confirmed elsewhere(9). The third patient group represents real-world clinical practice and includes 59 sequential patients commenced on sorafenib therapy for aHCC at Addenbrooke’s Hospital in Cambridge between December 2013 and August 2017. Of the 11 patients classified as ‘HCV+ve’ 9 were HCV RNA+ve and 2 had cleared HCV with anti-viral therapy; 8 were histologically confirmed to be cirrhotic. Overall survival was based on censoring at the time of death or when the patient was last seen alive. A summary of the clinical features of the patient groups is given in Table 1.
Table 1.
Patient characteristics
| Variable | Linifanib study control arm | Brivanib study control arm | Cambridge study | |||
|---|---|---|---|---|---|---|
| Alive, N=164 (32.67%) | Died, N=338 (67.33%) | All, N=502 | Alive vs Died, p-value | N=588 | N=59 | |
| Age (years) (mean (SD)) | 60.79 (12.49) | 59.77 (11.56) | 60.10 (11.87) | 0.3696 | 59.54 (12.19) | 66.17 (10.19) |
| Male, n(%) | 140 (85.37) | 282 (83.43) | 422 (84.06) | 0.5787 | 492 (83.67) | 40 (67.8%) |
| Race, n(%) | ||||||
| Asian | 98 (59.76) | 240 (71.01) | 338 (67.33) | 0.0407 | 396 (67.35) | 2 (3.4) |
| White | 64 (39.02) | 91 (26.92) | 155 (30.88) | 176 (29.93) | 54 (91.5) | |
| Other | 2 (1.22) | 7 (2.07) | 9 (1.80) | 16 (2.72) | 3 (5.1) | |
| ECOG, n(%) | ||||||
| 0 | 115 (70.12) | 216 (63.91) | 331 (65.94) | 0.1681 | 361 (61.39) | 19 (32.2) |
| 1 | 49 (29.88) | 122 (36.09) | 171 (34.06) | 227 (38.61) | 37 (62.7) | |
| 2 | 3 (5.1) | |||||
| Vascular invasion, n(%) | 49 (29.88) | 153 (45.27) | 202 (40.24) | 0.001 | 170 (28.91) | 19 (32.2) |
| Regional lymph node metastasis, n(%) | 35 (21.34) | 106 (31.36) | 141 (28.09) | 0.0191 | 170 (28.91) | 4 (6.8) |
| Distant metastasis, n(%) | 72 (43.90) | 168 (49.70) | 240 (47.81) | 0.2223 | 299 (50.85) | 10 (16.9) |
| Extra-hepatic spread, n(%) | 87 (53.05) | 197 (58.28) | 284 (56.57) | 0.2670 | 372 (63.27) | 16 (27.1) |
| Ascites (slight), n(%) | 12 (7.32) | 64 (18.93) | 76 (15.14) | 0.0005 | 40 (6.80) | 9 (15.3) |
| Encephalopathy (grade 1–2), n(%) | 0 (0) | 1 (0.30) | 1 (0.20) | N/A | 0 (0) | 4 (6.8) |
| Portal vein thrombosis, n(%) | 41 (25.00) | 144 (42.60) | 185 (36.85) | 0.0001 | N/A | 23 (38.9) |
| Tumour burden (mm) (median (IQR)) | 63.00 (40.00, 111.00) | 99.00 (61.00, 153.00) | 87.00 (52.00, 137.00) | <0.0001 | 104.00 (55.50, 163.50) | 87.00 (49.50, 134.00) |
| Liver tumour morphology at diagnosis, n(%) | n=162 | n=337 | n=499 | n=588 | ||
| Uninodular and extent <=50% of liver | 54 (33.33) | 68 (20.18) | 122 (24.45) | 0.0043 | 108 (18.37) | 23 (38.9) |
| Multinodular and extent <=50% of liver | 78 (48.15) | 169 (50.15) | 247 (49.50) | 333 (56.63) | 24 (40.6) | |
| Massive or extent >50% of liver | 20 (12.35) | 72 (21.36) | 92 (18.44) | 112 (19.05) | 11 (18.6) | |
| Unknown | 10 (6.17) | 28 (8.31) | 38 (7.62) | 35 (5.95) | 1 (1.7) | |
| Aetiology | ||||||
| HBV | 74 (45.12) | 180 (53.25) | 254 (50.60) | 0.2042 | 230 (39.12) | 5 (8.5) |
| HCV | 37 (22.56) | 63 (18.64) | 100 (19.92) | 90 (15.31) | 6 (10.2) | |
| Alcohol | 14 (8.54) | 30 (8.88) | 44 (8.76) | 42 (7.14) | 11 (18.6) | |
| Haemochromatosis | 0 (0) | 4 (1.18) | 4 (0.80) | 3 (0.51) | 4 (6.8) | |
| Other | 34 (20.73) | 50 (14.79) | 84 (16.73) | 223 (37.92) [“none” constitutes 25.17% (n=148) of the data] |
28 (47.4) | |
| HBV + Alcohol | 0 (0) | 4 (1.18) | 4 (0.80) | 0 (0) | ||
| HCV + Alcohol | 5 (3.05) | 7 (2.07) | 12 (2.39) | 5 (8.5) | ||
| AFP (ng/ml) (median (IQR)) | 121.50 (8.41, 1568.80), n=164 | 876.00 (35.21, 12878.00), n=335 | 395.70 (19.60, 8101.00), n=499 | <0.0001 | 180.75 (8.5, 2984.3), n=572 | 81.00 (6.75, 897.30) |
| Albumin (g/l) (mean (SD)) | 41.40 (4.61), n=164 | 39.58 (4.70), n=337 | 40.18 (4.75), n=501 | <0.0001 | 38.65 (5.20), n=587 | 34 (4.64), n = 59 |
| Bilirubin (μmol/l) (median (IQR)) | 11.00 (8.00, 16.00), n=164 | 12.00 (9.00, 18.00), n=337 | 12.00 (8.00, 17.00), n=501 | 0.0192 | 13.68 (10.26, 20.52), n=586 | 12.00 (9.00, 36.00) |
| ALBI score (mean (SD)) | −2.82 (0.45), n=164 | −2.64 (0.46), n=337 | −2.70 (0.46), n=501 | <0.0001 | −2.51 (0.50), n=585 | −2.24 (0.45) n=59 |
| ALBI grade | n=164 | n=337 | n=501 | n=585 | n=59 | |
| 1 | 114 (69.51) | 186 (55.19) | 300 (59.88) | 0.0022 | 272 (46.50) | 14 (23.7) |
| 2 | 50 (30.49) | 151 (44.81) | 201 (40.12) | 304 (51.97) | 43 (72.9) | |
| 3 | 0 (0) | 0 (0) | 0 (0) | 9 (1.54) | 2 (3.4) | |
| Cause of death, n(%) | ||||||
| Disease progression | N/A | 291 (86.09) | N/A | N/A | NA | NA |
| Non-disease progression | N/A | 19 (5.62) | N/A | N/A | NA | NA |
| Other | N/A | 28 (8.28) | N/A | N.A | NA | NA |
| Death, n(%) | N/A | N/A | 338 (67.33) | N/A | 419 (71.26) | 52 (88.1) |
| Overall survival, months (95% C.I.) | N/A | N/A | 9.41 (8.16, 10.95) | N/A | 9.79 (8.51, 11.53) | 13.37 |
Abbreviations: AFP, alpha-fetoprotein; C.I., confidence intervals; ECOG, Eastern Cooperative Oncology Group; g/l, grams per litre; HBV, hepatitis B; HCV, hepatitis C; μmol/L, micromoles per litre; ng/ml, nanograms per millilitre; N/A, not applicable; NA, not available
Liver function was assessed by the ALBI score (17). This is a refinement of the conventional Child-Pugh Score (CPS) (18, 19) that was developed specifically for patients with HCC, with or without underlying liver disease. All the prognostic information of the CPS is gathered from just the serum albumin and serum bilirubin levels with appropriate statistical transformation. The model has been extensively validated at all disease stages, and shown to be at least as good as the CPS but with the advantage of being more objective (variables such as ascites and encephalopathy are not required) and more ‘granular’(19). The tumour-burden recorded represents the sum of the diameters of all target lesions on cross-sectional imaging. Figures (i.e. diagrams) representing change in tumour-burden over time refer to the net change in tumour burden i.e. combining progression and ‘regression’. To this extent, tumour response (meaning regression) was not relevant to our analysis; no patient had a net reduction in tumour burden over the time of the study.
Tumour Assessments
The diagnostic criteria were similar in all three patient groups. EASL/AASLD guidelines (as per date of study) were followed for initial tumour identification: a >1cm arterial phase hyper-enhancing lesion with portal phase washout in patients with HCV or chronic liver disease.
In all three datasets a CT scan of the full chest and abdomen (with imaging of liver and adrenal glands) was performed for all tumour assessments at screening, at the end of every 6 weeks following Study Day 1 until week 42, at the end of every 9 weeks thereafter, and at the final visit if not performed within the previous 4 weeks. If the subject discontinued the study prior to the end of the week 6 scan, then a CT scan was performed as close as possible to the end of week 6. Subjects were monitored, by local investigators by the same methodology until definite evidence of new metastasis. In the Cambridge series, all measurements were made by a single experienced radiologist (JT). Target lesions were defined according to criteria in place at the time of data collection(20–22). The tumour burden was defined by the sum of tumour diameters of all target lesions.(23)
Statistical methods
In this study, the disease progression is defined by worsening changes of tumour burden, AFP and ALBI score over time (longitudinal outcome measurements). The sequential measurements of all 3 outcomes were made on each patient, but the sequence was frequently terminated early through death of the patient during the intended follow-up period. This means that not all patients had their outcomes measured for the equal length of time and the patients who died had considerably shorter follow-up as compared to those who were still alive at the end of the study. Evidently, the missing values in outcome sequences due to death are nonignorable as death may be linked to the failure of treatment or intrinsically more aggressive disease(14). Therefore, in any study when the disease progression is monitored by variations of repeated measurements over time (tumour burden, AFP and ALBI score in our dataset, also called longitudinal outcome) which are terminated due to death, the treatment effect is an aggregated effect of both time-to-event (death in our dataset) and the longitudinal outcome process(24). The classical approaches such as the linear mixed models for longitudinal data and the Cox proportional hazards model for time-to-event data do not consider interdependencies between these two data types (longitudinal and time-to-event) simultaneously.
Incorporating the longitudinal measures directly into the Cox model as time-varying covariates is one of the modelling strategies; however, the longitudinal measures typically have a great deal of random error from individual to individual, and therefore this approach will lead to highly biased (typically attenuated) estimates of treatment effect. The joint model for longitudinal and time-to-event data is a powerful method that brings the two data types together (simultaneously) into a single model so that one can infer the dependence and association between the longitudinal data and time-to-event so as to better assess the effect of a treatment(25). We assume that survival is related to the covariate through a proportional hazards relationship with the underlying random effects and the parameter estimators for the survival models holding to proportional hazards.
Failing to take account of ‘informative missingness’ (missing longitudinal outcome measurements due to death) will lead to biased estimates of temporal variations(26). As a result, joint models are now increasingly used in clinical trial analysis to estimate the treatment and temporal effects, and often preferred over the Cox model or the linear mixed model alone(14, 26). Further details of the statistical methodology are provided under the Supplementary Data section.
We analysed tumour burden and AFP in natural log scale as longitudinal outcome required to be normally distributed for the joint model. Both submodels of the joint model included age and gender as covariates, and in the longitudinal submodel, HCV status (1 if present, 0 if absent) is included as an additional covariate. We used separate joint models to estimate the complete sequence of each longitudinal outcome for each patient over the intended follow-up period. The rates of increase in tumour burden, serum AFP and ALBI were computed from the slope of estimated complete profiles. As the estimates were based on joint model parameter estimates, to account for uncertainty due to both estimation processes, the 95% confidence interval (CI)s were derived from 500 bootstrap samples. The corresponding mean specific growth rate (SGR) and the median doubling time (DT) for tumour burden were also computed. To ensure that the rates of increase in all three outcomes predicted from corresponding longitudinal submodels are generalizable and therefore can be utilised in clinical practice, they were validated externally using completely independent data from two sources; the brivanib study(16) and ‘real-world’ overall rate with clinical data from Addenbrooke’s Hospital in Cambridge. The external predictive performance of the longitudinal submodel was assessed using predictive Rsquared(27, 28). R2 predict = 1 - SSE/SST where SSE (Sum of Squared Error) is the sum of the squared differences between predicted values and observed values of validation patient groups, and SST (Sum of Squared Total) is the sum of squared differences between observed values of validation patient group and the mean outcome value of the primary dataset at the baseline. The predicted values for validation patient groups were computed from the estimated coefficients β0, β1, and β2 of the longitudinal submodel of the primary dataset with corresponding random intercepts and slopes of patients from validation studies. R-squared value ranges from 0 to 1, and a perfect model has R-squared = 1. High R-squared values are thus preferable. Pvalues were derived at 5% significance level.
Results
Of the patients involved in the primary dataset, 422 (84%) were male. The mean age was 60.1 years (SD=11.9, range = 23–87) and 164 (32.7%) patients were still alive at the end of the study, while 338 (67.3%) had died. The median follow-up duration for those who were still alive was 123 days (maximum 725) and for those who died it was 42 days (maximum 357 days).
The sequential measurements of all 3 outcomes has been obtained intermittently for each patient until prior to corresponding terminating events. The length of each sequence was considerably shorter among those who died (median number of records = 2, IQR: 2, 4 over a maximum of 357 days). The patients who were still alive at the end of the study had a median of 5 (IQR: 2, 8) records, taken over a maximum of 725 days. The median survival was 286 days (95% CI 248, 333). The median survival for HCV-infected patients was 334 days (95% CI 259, 416), and for non-HCV-infected patients 259 days (95% CI 231, 317).
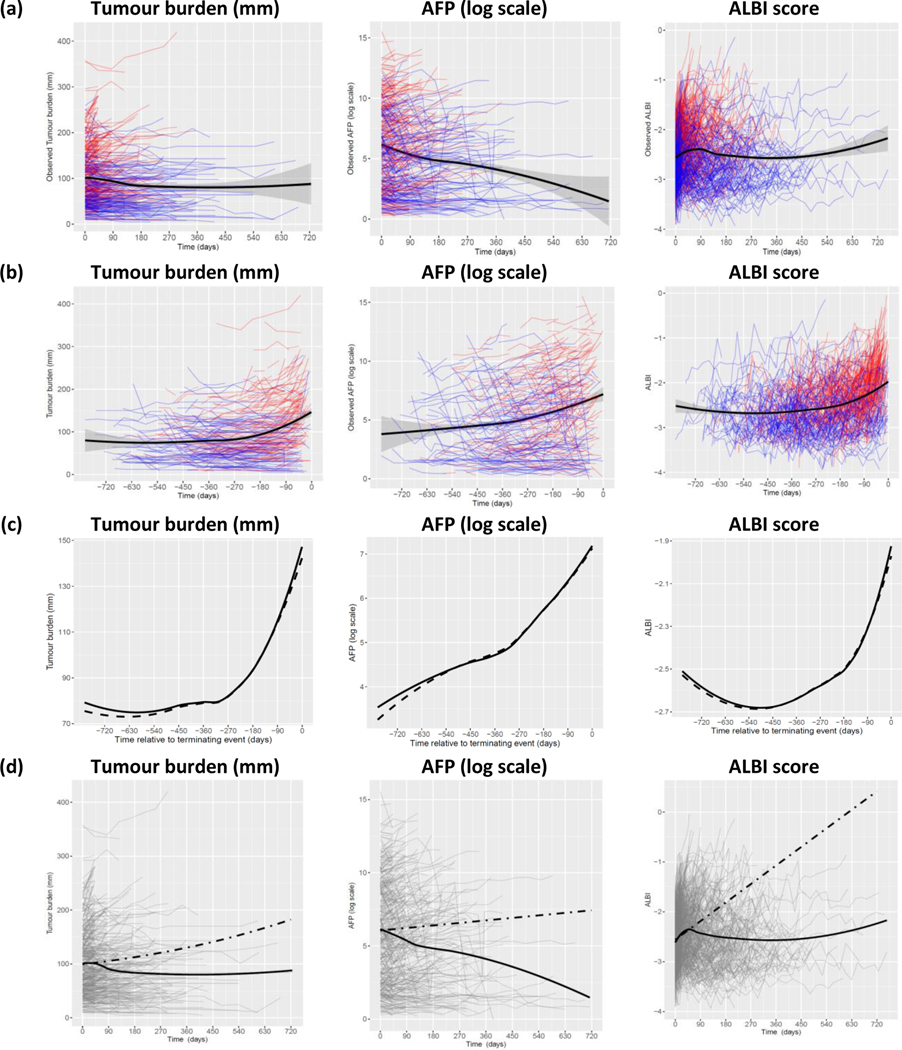
The observed individual profiles of the outcomes tumour burden, AFP and ALBI score, and the smooth estimate of mean profiles are shown in Figure 1(a). A smooth mean estimate was necessary because measurement times differed between patients. Many patients who died (thin red lines) had a short sequence with increasing profile in all 3 measures, while many who were still alive (thin blue lines) had a longer sequence with lower values. However, the mean profile (solid black line) may be misleading because death of patients with higher values would lead to a decrease over time in mean value among patients still alive. Therefore, the mean profile may not reflect an accurate population-level variation of tumour burden, AFP and ALBI score over time, and the decrease seen in the mean profile in Figure 1(a) is an artefact caused by those selective high values of those who died. To present the mean temporal change more accurately, we plotted observed values of each outcome against time relative to the terminating event (death or final follow-up time for those who were alive) as in Figure 1(b). As shown, overall, the tumour burden, AFP and ALBI score have begun to rise about a year prior to the terminating event.
Figure 1.
Observed individual and mean profiles of tumour burden, AFP & ALBI score against time.
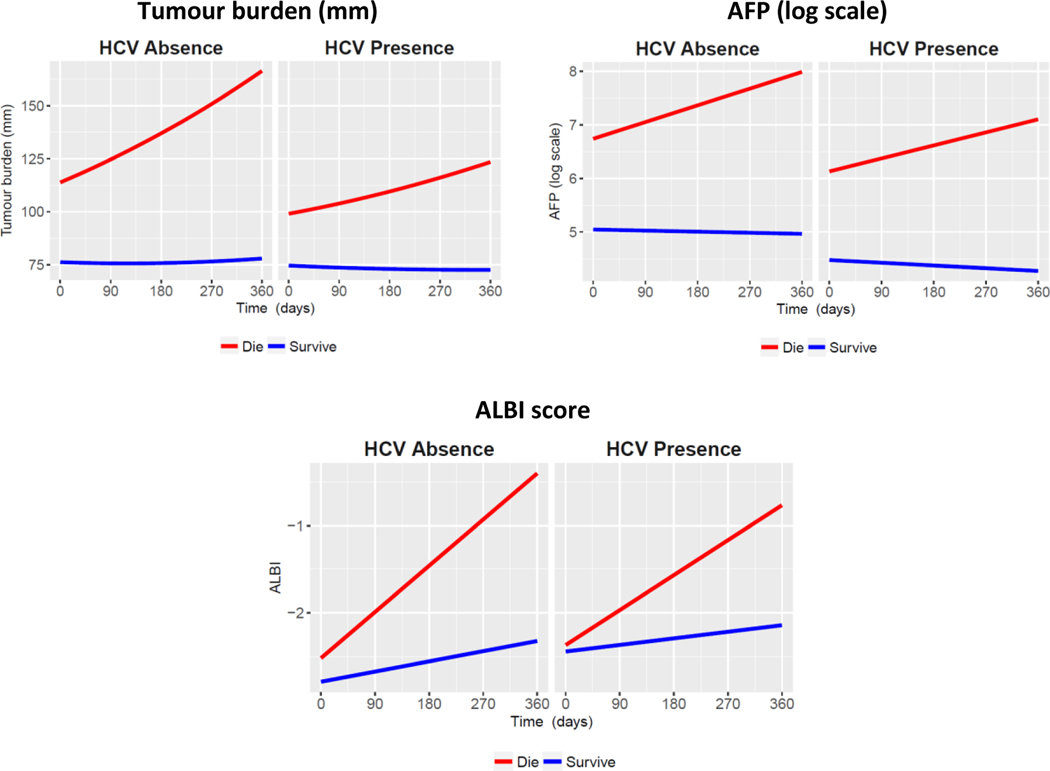
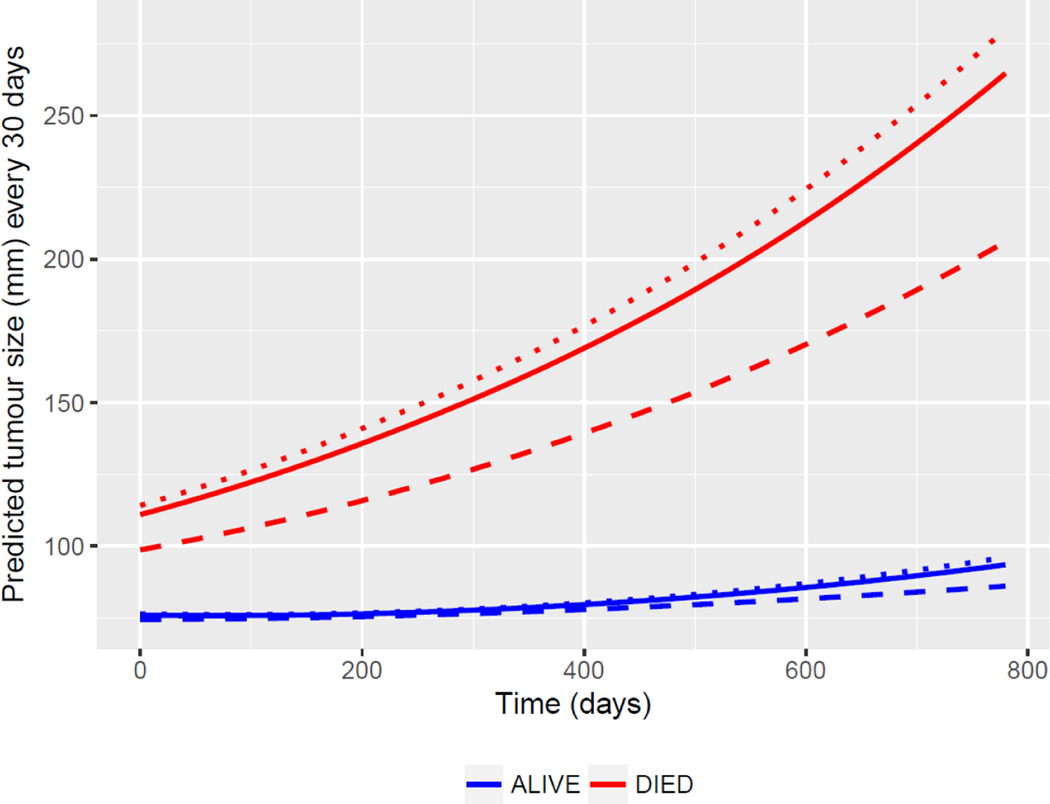
The estimated mean profile of tumour burden, AFP and ALBI score from the specified joint models and the observed mean profile against time relative to the terminating event are shown in Figure 1(c). We observed good agreement between the two mean profiles, and with all R-squared above 0.98, implying that the fitted model accurately represents the variability of all 3 outcome measurements over time. As estimated from the joint model, each outcome is associated with a significantly increased risk of death (p<0.0001 for estimated association parameter 𝛾, see joint model formulation in Supplementary Data section). To estimate progression during treatment with sorafenib more accurately, the “complete” (hypothetical) sequences of tumour burden, AFP and ALBI score for each patient until the maximum intended follow-up time of the study (about 2 years) were computed using the estimated longitudinal submodel coefficients and random effects. Figure 1(d) shows the predicted aggregated progression of the disease in term of changes of tumour burden, AFP and ALBI score over time. The predicted rates of tumour burden, AFP and ALBI score for HCV presence and absence are presented in Figure 2. Further, during the treatment with sorafenib, the rate of increase in tumour burden was significantly slower among patients with HCV infection compared to those without HCV, and rates of change in AFP and ALBI score were also significantly lower among the HCV-positive patients compared to the HCVnegative patients (see Table 2). Figure 3 shows the predicted mean profiles of tumour burden for those who died and those who were alive at the end of follow-up, as compared to the HCV absence (dotted line) and HCV presence (dashed line). The overall (population-level) estimate is shown by the solid line. From Figures 2 and 3, we have shown what would have been the mean profile of each biomarker for those who died if they were followed up to the 357 days. And how this is compared to those who were alive. During treatment with sorafenib, the overall rate of increase in tumour burden is estimated at 12% (95% CI 10, 14), however this rate drops to 5.6% (95% CI 3.6, 7.4 from Table 2) among those with HCV. The estimate 12% can be interpreted as a change in mean tumour burden of 0.12 mm per day or an absolute growth rate of 3.6mm/month. Tumour burden change can readily be calculated for the individual patient. In a follow-up study we will use a multivariate approach (tumour size, ALBI and AFP) to report the method for calculating the growth rate for individual patients.
Figure 2:
Predicted rates of tumour burden, AFP and ALBI score as compared to HCV absence and HCV presence
Table 2.
Estimated progression of the disease with respect to tumour burden, AFP and ALBI score during treatment with sorafenib
| Measure | Aggregated rate of change (95% CI) | Estimated rate of change among HCV presence compared to HCV absence (95% CI) | |
|---|---|---|---|
| HCV presence | HCV absence | ||
| Tumour burden | 0.0556 (0.0365, 0.0738) | 0.1373 (0.1202, 0.1550) | −0.0814 (−0.1019, −0.0610), p-val < 0.0001 |
| loge AFP | 0.0015 (0.0003, 0.0027) | 0.0023 (0.0018, 0.0028) | −0.0008 (−0.0016, −0.0001), p-val = 0.0445 |
| ALBI score | 0.0031 (0.0024, 0.0038) | 0.0045 (0.0041, 0.0049) | −0.0014 (−0.0017, −0.0010), p-val < 0.0001 |
Figure 3.
Predicted mean profiles of tumour burden. The dotted line represents HCV absence and the dashed line represents HCV presence.
It is apparent that among those patients still alive at the end of the study, the overall progression of the disease in relation to tumour burden, although marginally higher in the non-HCV group, was very low compared to those who died within the study (2%, 95% CI 0 to 4% vs. 19%, 95% CI 18 to 21%). Furthermore, it is noteworthy that although the progression was significantly slower (by 6%, 95% CI 3 to 8%, p-value <0.0001) in the patients who died in the HCV positive patient group their initial tumour burden was lower (by 8.76, 95% CI 3.05 to 14.48, p-value = 0.0026) suggesting that initial tumour burden, as well as viral aetiology influences progression of the disease. These observations may explain the previously reported relationship between survival and both tumour burden, and viral aetiology. The estimated mean specific growth rate (SGR) is 4.6 (95% CI 3.6, 5.5) x 10−4, and the median doubling time (DT) is 665 days (95% CI 616, 735) for tumour burden.
Applying the estimated longitudinal submodel of tumour burden to the independent validation patient groups gave predictive R-squared of 0.93 and 0.90 for BMS and Cambridge data respectively, indicating an excellent external validation. For AFP and ALBI, predictive R-squared were 0.92 and 0.82 for the brivanib trial group and 0.81 and 0.84 for the Cambridge data respectively. The model for HCV was validated from the BMS data only (due to the small number of HCV cases in the Cambridge series), and achieved a predictive R-squared of 0.93, 0.92, and 0.81 for tumour burden, AFP and ALBI respectively.
Discussion
The concept of disease progression is central to the management of patients with HCC and has become more so since the development of effective second-line systemic therapies to which patients may be changed when disease progression occurs on sorafenib(29). Currently, progression and regression are defined by percentage changes of apparent diameter on radiological assessment using CT scans, with progression being defined as an increase of 20% in target lesion longitudinal diameter, a threshold that is in part directed by the limitations of precision in radiological measurements. Scientific evidence for this precise threshold is limited (30). The analysis presented here deals with this concern. It is also notable that there is still controversy about the optimal criteria for response assessment and independent central review does not overcome all these issues, such as those surrounding contrast protocol (31). The importance of the pattern of recurrence is also important (30) and we note that development of a new metastasis was regarded as definitive evidence of progression this study. The relative benefits of blinded central versus local review of tumour response are also controversial(32). In the present study all tumour assessment was conducted locally, a situation which we believe more closely reflects ‘real-world’ practice.
Previously such analyses have been inhibited by the very wide difference in survival among patients treated with sorafenib, ranging from less than 2 months to more than 2 years(33), combined with the fact that the rate of net tumour burden increase is also very variable. Those that die early will have the least number of observations and are likely to be the same patients that have the fastest growing tumours. The latter observation has been reflected in terms such as ‘fast progressors’(34).
The statistical methodology of ‘joint’ modelling(14) overcomes these problems and allows us to model the change in tumour burden over time among patients receiving sorafenib. Most importantly we show for the first time, that it is possible to accurately and quantifiably measure the net population-level rate of tumour burden growth. Furthermore, we can objectively and quantifiably show that progression on therapy is associated with poorer survival. The estimated overall rate of 12% (95% CI 10, 14) in tumour burden was below the current (intuitive) RECIST guideline of 20% increase in tumour burden to indicate the progressive disease. In drug trials, an increase in the rate of tumour burden of 10% or more (the lower limit of the CI) seems a reasonable indication of treatment failure that might trigger a change in therapy.
While survival prediction is traditionally the focus of many clinical studies, a growing area in precision medicine is the forecasting of disease trajectories using the evolution of biomarkers over time. In this study, we have estimated the biomarker trajectories for the survival outcome to predict the change in disease progression. The Cambridge data were retrospective, hence limiting the availability and quality of data collected. The higher number of deaths could be potentially biased against rapid disease progressors.
Our analysis suggests that amongst patients receiving sorafenib for aHCC the rate of tumour growth is significantly slower amongst those who are HCV positive although we acknowledge that the aetiological classification of our cases is limited, a situation that we aim to address by the use of more modern and detailed datasets in future analyses. Furthermore, our results suggest that initial tumour burden, as well as viral aetiology influences progression and these observations may explain the previously reported relationship between survival and both tumour burden and viral aetiology(13). This selective effect of sorafenib in HCV could theoretically be an intrinsic attribute of HCV patients, and since there is no placebo group in our study we cannot definitively attribute the lower rate of tumour growth in the HCV positive group to sorafenib. However, the fact that in randomised placebo-controlled trials these sorafenib-treated HCV patients typically survived 14 months compared to 7 months in the placebo arm, makes this contention very unlikely(12, 13). Such contentions are also supported by the fact that the rate of AFP rise is significantly lower in sorafenib-treated HCV patients. AFP is a recognised tumour marker for HCC, changes in which have been linked to survival improvement(35). The third aspect of our analysis is that the rate of deterioration in liver function, as measured by ALBI, is significantly lower in the HCV positive patients. Again this is plausible since there is extensive animal work suggesting anti-angiogenic agents such as sorafenib are anti-fibrotic and decrease portal pressure and hence are likely to improve liver function(36, 37), but there has been no suggested reason why this should be specific for HCV infection. Finally, it was possible that the survival improvement attributable to sorafenib was solely related to improvement in the underlying liver function. The present study suggests that this may be partially the case but also provides strong evidence of a direct anti-tumour effect, at least in comparison to HCV negative patients. It therefore seems likely that even in the immune-therapeutic era sorafenib will maintain a role in this subset of patients with aHCC.
Although, the paramount importance of HCV as a marker of sorafenib sensitivity is now well documented, as is the vulnerability of trials involving sorafenib if aetiology is not taken into account at randomisation, the underlying molecular basis for this effect remains unknown. It is noteworthy that in the two trials that have met their primary endpoints (lenvatinib for non-inferiority) and the combination of atezolizumab and bevacizumab (for OS) have a lower percentage of HCV positive patients than those trials that did not met their primary endpoints. The implication is that in such studies the control arm (sorafenib|) was less competitive. No molecular biomarker of positive sorafenib response has been identified from the SHARP and AP studies, nor from adjuvant studies involving sorafenib(38). Sorafenib does not appear to have significant anti-viral activity(39). Further, there is little evidence that HCV promotes specific molecular subtypes of HCC that may be more susceptible to sorafenib(40). Nonetheless we acknowledge that our classification of aetiology as HCV vs. non-HCV has its limitations and in future we are aiming to broaden the aetiological classification to include HBV, NAFLD and other forms of chronic liver disease using more modern and detailed patient groups. Finally, it should be noted that in our primary dataset HCV seropositivity refers to the presence of HCV antibodies. Given that the clinical trials were conducted in the ‘pre-DDA’ era it is likely that most patients were, in fact, HCV RNA positive but without formal testing we cannot be definitive on this matter. The question of whether sorafenib will have a similar (selective) effect when the patient is non-viraemic is intriguing and worthy of further investigation.
Whilst we applied joint modelling here to aHCC, there seems no reason why such an approach might not be applied, with benefit, to other tumours involved in clinical trials in which serial measures of tumour burden are recorded. Thus, it should be feasible to compare the activity (in terms of impact on tumour progression rates) of two drugs within a randomised controlled trial without the influence of subsequent lines of therapy after the trial has been concluded. We are currently applying the same approach to other clinical variables (such as biomarkers and measures of liver function) within other clinical trials for aHCC.
Supplementary Material
Acknowledgments:
We are grateful to AbbVie Inc. and Bristol-Myers Squibb who generously gave us access to data from their studies (NCT 0200995930 and NCT 00858871 respectively) in which they acted as sponsor. T.J. was supported by Cancer Research UK funding (C42738/A24868), Cold Spring Harbor Laboratory (CSHL) and Northwell Health, the Pershing Square Foundation, the Mark Foundation for Cancer Research, and the US National Institute of Health through funding received as part of Cancer Center Support Development Funds granted to CSHL (5P30CA045508-31).
Financial Support:
The clinical trials data belongs to Bristol Myers Squibb and AbbVie Inc. The clinical data belongs to Cambridge University Hospitals NHS Foundation Trust
Footnotes
Declaration:
The clinical trial data came from a retrospective analysis of previously published clinical trials [NCT00858871and NCT01009593]. For the clinical practice dataset, anonymized patient data was collected from patients recruited at Addenbrooke’s Hospital, Cambridge after informed consent and with the approval of the Local Research Ethics Committee (16/NI/0196).
Data Availability: Further information about the Cambridge cohort is available, upon reasonable request from Dr Matthew Hoare (mwh20@cam.ac.uk). AbbVie remains committed to sharing clinical research data with researchers, as defined on Abbvie.com (https://www.abbvie.com/our-science/clinical-trials/clinical-trials-data-and-information-sharing.html) and Vivli (https://vivli.org/).
Conflicts of Interest: None
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182236. [Google Scholar]
- 3.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 4.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. [DOI] [PubMed] [Google Scholar]
- 5.Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. [DOI] [PubMed] [Google Scholar]
- 6.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: Primed to make a difference? Cancer. 2016;122(3):367–77. [DOI] [PubMed] [Google Scholar]
- 8.Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894–905. [DOI] [PubMed] [Google Scholar]
- 9.Jackson R, Psarelli EE, Berhane S, Khan H, Johnson P. Impact of Viral Status on Survival in Patients Receiving Sorafenib for Advanced Hepatocellular Cancer: A Meta-Analysis of Randomized Phase III Trials. J Clin Oncol. 2017;35(6):622–8. [DOI] [PubMed] [Google Scholar]
- 10.Cabibbo G, Cucchetti A, Camma C, Casadei-Gardini A, Celsa C, Emanuele Maria Rizzo G, et al. Outcomes of hepatocellular carcinoma patients treated with sorafenib: a meta-analysis of Phase III trials. Future Oncol. 2019;15(29):3411–22. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, Qin S, et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol. 2013;31(32):4067–75. [DOI] [PubMed] [Google Scholar]
- 12.Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57(4):821–9. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67(5):999–1008. [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim JG, Chu H, Chen LM. Basic concepts and methods for joint models of longitudinal and survival data. J Clin Oncol. 2010;28(16):2796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cainap C, Qin S, Huang WT, Chung IJ, Pan H, Cheng Y, et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: results of a randomized phase III trial. J Clin Oncol. 2015;33(2):172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson PJ, Qin S, Park JW, Poon RT, Raoul JL, Philip PA, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol. 2013;31(28):3517–24. [DOI] [PubMed] [Google Scholar]
- 17.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338–46. [DOI] [PubMed] [Google Scholar]
- 19.Lee SK, Song MJ, Kim SH, Park M. Comparing various scoring system for predicting overall survival according to treatment modalities in hepatocellular carcinoma focused on Platelet-albumin-bilirubin (PALBI) and albumin-bilirubin (ALBI) grade: A nationwide cohort study. PLoS One. 2019;14(5):e0216173. [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–47. [DOI] [PubMed] [Google Scholar]
- 21.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. [DOI] [PubMed] [Google Scholar]
- 22.Lencioni R. New data supporting modified RECIST (mRECIST) for Hepatocellular Carcinoma. Clin Cancer Res. 2013;19(6):1312–4. [DOI] [PubMed] [Google Scholar]
- 23.Tovoli F, Renzulli M, Granito A, Golfieri R, Bolondi L. Radiologic criteria of response to systemic treatments for hepatocellular carcinoma. Hepat Oncol. 2017;4(4):129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovanda LL, Kolamunnage-Dona R, Neely M, Maertens J, Lee M, Hope WW. Pharmacodynamics of Isavuconazole for Invasive Mold Disease: Role of Galactomannan for Real-Time Monitoring of Therapeutic Response. Clin Infect Dis. 2017;64(11):1557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell C, Kolamunnage-Dona R, Lowe J, Boland A, Petrou S, Doull I, et al. Magnesium sulphate in acute severe asthma in children (MAGNETIC): a randomised, placebo-controlled trial. Lancet Respir Med. 2013;1(4):301–8 [DOI] [PubMed] [Google Scholar]
- 26.Kolamunnage-Dona R, Williamson PR. Time-dependent efficacy of longitudinal biomarker for clinical endpoint. Stat Methods Med Res. 2018;27(6):1909–24. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa S, Schielzeth H. A general and simple method for obtaining R2 from generalized linear mixedeffects models. Methods in Ecology and Evolution. 2013;4(2):133–42. [Google Scholar]
- 28.Mendenhall WM, Sincich TL. Statistics for Engineering and the Sciences: CRC Press; 2016. [Google Scholar]
- 29.Regorafenib Bruix J. and the RESORCE trial: a new second-line option for hepatocellular carcinoma patients. Hepat Oncol. 2016;3(3):187–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reig M, Bruix J. Pattern of tumor progression in liver cancer: The missing partner in trial design. Hepatology. 2015;62(3):674–6. [DOI] [PubMed] [Google Scholar]
- 31.Yanaga Y, Awai K, Nakaura T, Namimoto T, Oda S, Funama Y, et al. Optimal contrast dose for depiction of hypervascular hepatocellular carcinoma at dynamic CT using 64-MDCT. AJR Am J Roentgenol. 2008;190(4):1003–9. [DOI] [PubMed] [Google Scholar]
- 32.Amit O, Mannino F, Stone AM, Bushnell W, Denne J, Helterbrand J, et al. Blinded independent central review of progression in cancer clinical trials: results from a meta-analysis. Eur J Cancer. 2011;47(12):1772–8. [DOI] [PubMed] [Google Scholar]
- 33.Berhane S, Fox R, Garcia-Finana M, Cucchetti A, Johnson P. Using prognostic and predictive clinical features to make personalised survival prediction in advanced hepatocellular carcinoma patients undergoing sorafenib treatment. Br J Cancer. 2019;121(2):117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruix J, Reig M, Sangro B. Assessment of treatment efficacy in hepatocellular carcinoma: Response rate, delay in progression or none of them. J Hepatol. 2017;66(6):1114–7. [DOI] [PubMed] [Google Scholar]
- 35.Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, et al. New utility of an old marker: serial alphafetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27(3):446–52. [DOI] [PubMed] [Google Scholar]
- 36.Pinter M, Sieghart W, Reiberger T, Rohr-Udilova N, Ferlitsch A, Peck-Radosavljevic M. The effects of sorafenib on the portal hypertensive syndrome in patients with liver cirrhosis and hepatocellular carcinoma--a pilot study. Aliment Pharmacol Ther. 2012;35(1):83–91. [DOI] [PubMed] [Google Scholar]
- 37.Coriat R, Gouya H, Mir O, Ropert S, Vignaux O, Chaussade S, et al. Reversible decrease of portal venous flow in cirrhotic patients: a positive side effect of sorafenib. PLoS One. 2011;6(2):e16978. [Google Scholar]
- 38.Pinyol R, Montal R, Bassaganyas L, Sia D, Takayama T, Chau GY, et al. Molecular predictors of prevention of recurrence in HCC with sorafenib as adjuvant treatment and prognostic factors in the phase 3 STORM trial. Gut. 2019;68(6):1065–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cabrera R, Limaye AR, Horne P, Mills R, Soldevila-Pico C, Clark V, et al. The anti-viral effect of sorafenib in hepatitis C-related hepatocellular carcinoma. Aliment Pharmacol Ther. 2013;37(1):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Himmelsbach K, Sauter D, Baumert TF, Ludwig L, Blum HE, Hildt E. New aspects of an anti-tumour drug: sorafenib efficiently inhibits HCV replication. Gut. 2009;58(12):1644–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.