Abstract
Background:
HF is a heterogeneous disease characterized by significant metabolic disturbances, however the breadth of metabolic dysfunction prior to the onset of overt disease is not well understood. The purpose of this study was to determine the association of circulating metabolites with incident heart failure (HF) to uncover novel metabolic pathways to disease.
Methods:
We performed targeted plasma metabolomic profiling in a deeply phenotyped group of African Americans (AA) from the Jackson Heart Study (JHS; n=2,199). We related metabolites associated with incident HF to established etiological mechanisms including increased left ventricular mass index (LVMi) and incident coronary heart disease (CHD). Further, we evaluated differential associations of metabolites with heart failure with preserved ejection fraction (HFpEF) versus heart failure with reduced ejection fraction (HFrEF).
Results:
Metabolites associated with incident HF included products of post-transcriptional modifications of RNA, as well as polyamine and nitric oxide metabolism. A subset of metabolite-HF associations were independent of well-established HF pathways such as increased LVMi and incident CHD and included homoarginine (per 1 SD increase in metabolite level, HR 0.77, p value 1.2 X 10−3), diacetylspermine (HR 1.34. p value 3.4 X 10−3) and uridine (0.79, p value 3 X 10−4). Further, metabolites involved in pyrimidine metabolism (orotic acid) and collagen turnover (N-methylproline) among others were part of a distinct metabolic signature that differentiated individuals with HFpEF versus HFrEF.
Conclusion:
The integration of clinical phenotyping with plasma metabolomic profiling uncovered novel metabolic processes in non-traditional disease pathways underlying the heterogeneity of HF development in AAs.
Journal Subject Terms: Biomarkers, Metabolism, Translational Studies, Pathophysiology
Keywords: metabolomics, heart failure, HFrEF, HFpEF, personalized medicine
Introduction
Heart Failure (HF) is increasingly prevalent worldwide and a significant burden on healthcare resources1. The etiology of HF is heterogeneous and mechanisms of disease development are poorly understood. Metabolomics, the study of small molecules including sugars and amino acids, provides the most proximal molecular fingerprint to clinical phenotype and offers the ability to systematically interrogate alterations in metabolism that may contribute to the pathophysiology of disease states, prior to disease onset2, 3. This allows for the identification of at-risk individuals and provides avenues of study for potential therapeutic targets. Metabolomic profiling of large epidemiological cohorts has identified important markers and potential causal mechanisms in cardiometabolic diseases such as diabetes mellitus and CHD3–5. The application of metabolomic profiling in HF is of particular importance given the marked metabolic dysfunction associated with disease6, 7. However, few studies have examined the association of metabolites with incident HF3, and the potential role that disturbed metabolic processes play in the development of HF, prior to disease onset, is thus unclear.
African Americans (AA’s) are disproportionately affected by HF and have worse outcomes compared to other racial groups8. In addition to socioeconomic factors, key biological differences may play a role in the pathophysiology of disease in the AA population. To gain novel insights into these processes, we performed metabolomic profiling to investigate the association of circulating metabolites and incident HF in individuals from the Jackson Heart Study (JHS), a deeply phenotyped AA cohort from Jackson, Mississippi9. We specifically hypothesized that the integration of detailed clinical data with metabolomic profiling would identify metabolic processes associated with HF independent of established mechanisms of HF development in AAs.
Methods
Study Participants
The JHS is a prospective population based observational study of individuals residing in the Jackson, Mississippi metropolitan area, designed to investigate risk factors for cardiovascular disease (CVD) in AA’s. The JHS study design, recruitment and data collection have been described previously. In brief, 5306 AA’s from the Jackson tri-county area (Hinds, Rankin and Madison) were recruited for a baseline examination (2000–2004) and two follow-up examinations (2005–2008, and 2009–2012). Additional surveillance includes annual telephone follow-up and medical records review for adjudication of selected events10. The JHS was approved by the institutional review boards of Jackson State University, Tougaloo College, and the University of Mississippi Medical Center in Jackson, Mississippi. All study participants provided written informed consent. The current analysis was also approved by the institutional review board of Beth Israel Deaconess Medical Center. JHS metabolomics data have been deposited to the JHS Data Coordinating Center and will also be deposited in dbGAP; until posted in dbGAP, all JHS data are available from the JHS Data Coordinating Center on request (JHSccdc@umc.edu).
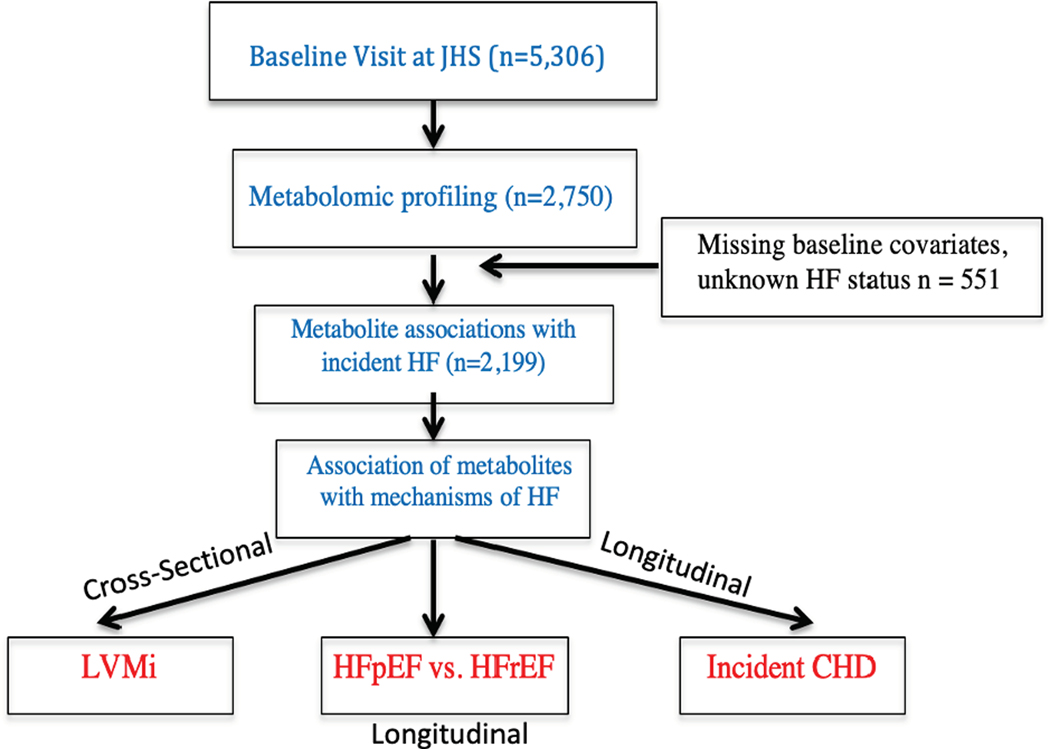
Metabolomic profiling was performed on plasma samples (n=2750) collected during baseline examination (Figure 1). Individuals in JHS with a history of HF or unknown HF status, as well as individuals with missing covariates were excluded from analysis (n=551; Figure 1). The samples that were profiled included nested case control studies for CHD (n=400) and chronic kidney disease (CKD; n= 759). The remaining samples were randomly selected (n=1591).
Figure 1:
Analysis plan for metabolomic profiling of incident heart failure in the Jackson Heart Study LVMi: left ventricular mass index; HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with reduced ejection fraction; CHD: coronary heart disease; HF: heart failure; JHS: Jackson Heart Study
Metabolite Profiling
Metabolite profiling was performed using liquid chromatography tandem mass spectrometry (LC-MS). Water soluble metabolites were profiled in the positive ion mode using a LC-MS system comprised of a Nexera X2 U-HPLC (Shimadzu Corp.; Marlborough, MA) coupled to a Q Exactive mass spectrometer (Thermo Fisher Scientific; Waltham, MA) and equipped with a hydrophilic interaction liquid chromatography (HILIC) column (Atlantis HILIC; Waters; Mildford, MA) as described in detail previously11. Raw data were processed using TraceFinder 3.1 (Thermo Fisher Scientific; Waltham, MA) and Progenesis QI (Nonlinear Dynamics; Newcastle upon Tyne, UK).
To measure organic acids and other intermediary metabolites in negative ionization or amide mode, chromatography was performed using an Agilent 1290 infinity LC system equipped with a Waters XBridge Amide column, coupled to an Agilent 6490 triple quadrupole mass spectrometer. Metabolite transitions were assayed using a dynamic multiple reaction monitoring system. LC-MS data were analyzed with Agilent Masshunter QQQ Quantitative analysis software.
Isotope labeled internal standards were monitored in each sample to ensure proper MS sensitivity for quality control. Pooled plasma samples were interspersed at intervals of 10 participant samples for standardization of drift over time and between batches. Additionally, separate pooled plasma was interspersed at every 20 injections to determine coefficient of variation for each metabolite over the run. Peaks were manually reviewed in a blinded fashion to assess quality. For each method, metabolite identities were confirmed using authentic reference standards or reference samples. Metabolites with poor peak quality and coefficient of variation of greater than 30% averaged across batches were removed from analysis. In total, 312 metabolites were included in the analysis. To determine whether relative concentrations for metabolites follow absolute quantification trends, stable isotopes of representative metabolites associated with incident heart failure (13C7,14N4-homoarginine, Choline-d4, 2-aminoadipic acid-d3, Uridine 15N2) were used to determine whether measured metabolite concentrations fall within the linear range of stable isotope calibration curves (Supplementalary Methods).
Clinical Co-variates
Current smoking status was obtained by questionnaire. Blood pressure was calculated by averaging two resting measurements. Hypertension was defined as systolic blood pressure of >140, diastolic blood pressure >90 or use of anti-hypertensive medication. Body mass index (BMI) was calculated using body weight indexed to height (kg/ m2). Diabetes mellitus status was determined by fasting glucose of >126, hemoglobin A1c >6.5% or use of anti-diabetic medication. High-density lipoprotein (HDL-C), total cholesterol, and creatinine were assayed directly using standard techniques12. Glomerular filtration rate was estimated by the chronic kidney disease epidemiologic collaboration (CKD-EPI) equation. Prevalent coronary heart disease status was assessed by questionnaire for history of myocardial infarction (MI), or evidence of MI on electrocardiogram.
Echocardiography
Two-dimensional transthoracic echocardiograms were performed by certified technicians (Sono4500; Phillips Medical System, Amsterdam, Netherlands) using standardized protocols. Interpretation and analysis were performed by experienced cardiologists on networked imaged workstations (Vericis; Camtronics Medical Systems) in accordance with American Society of Echocardiography recommendations. Left ventricular dimensions included left ventricular internal diameter at end diastole (LVEDD, mm), interventricular septal thickness in diastole (IVSd, mm), and posterior wall thickness in diastole (PWTd, mm). Left ventricular mass (LVM) was assessed using 2-D echocardiographic methods, and left ventricular mass index (LVMi) was calculated as LVM indexed to body surface area.
Brain Natriuretic Peptide Measurements
Plasma Brain Natriuretic Peptide (BNP), measured by a chemiluminescent immunoassay on a Siemens Advia Centaur with a limit of detection of 2.0 pg/mL, was available for 2,100 individuals who had undergone metabolic profiling.
Long Term Outcomes
HF adjudication has been described previously13. Time to first HF hospitalization was the primary outcome. HF adjudication began for individuals alive starting January 1, 2005 and extended through December 31, 2015. Medical records having discharge diagnoses including International classification of diseases, ninth revision, clinical modification codes (ICD-9) for primary diagnoses of heart failure were reviewed and adjudicated with examination of clinical documentation including presenting signs and symptoms, laboratory tests and imaging including echocardiography and magnetic resonance imaging. Adjudication of HFpEF vs. HFrEF was made by review for the subset of individuals with available hospitalization echocardiograms and other cardiac imaging data (i.e., cardiac magnetic resonance imaging). HFrEF was defined as an LVEF <50%. Incident CHD was defined as fatal or non-fatal myocardial infarction or coronary revascularization and was ascertained and adjudicated starting in 2000 through 2014.
Metabolomic Profiling and Heart Failure Outcomes in the Framingham Heart Study
To relate the associations of metabolites in an AA cohort from JHS to a Caucasian population, we tested the association of metabolites with incident HF in the Framingham Study (FHS) Offspring cohort14. Methods for LC-MS profiling in FHS have been described previously15, 16. HF adjudication was defined according to FHS criteria17. Data was reviewed by study committee including 2 cardiologists, and included hospitalization records as well as FHS clinic visits.
Statistical analysis
Quantitative clinical characteristics were calculated for HF cases and controls separately. Normally distributed continuous variables were presented as mean with standard deviations and non-normally distributed continuous variables were presented as median with interquartile ranges. Categorical values were presented as percentages. Comparison of clinical values between HF cases and controls was done using t-tests, chi-square, Mann Whitney and ANOVA as appropriate.
Metabolite LC-MS peak areas were log transformed and scaled to a mean of zero and standard deviation of 1. Cox proportional hazards regression was used to calculate hazard ratios (HR) and confidence intervals (95%) to estimate the association of individual metabolites with incident HF hospitalization, adjusting for age, sex, body mass index, hypertension diagnosis or treatment with hypertension medication, systolic blood pressure, diabetes mellitus, current smoking status, total cholesterol, high-density lipoprotein cholesterol, CHD history, eGFR and batch. To control for multiple hypothesis testing, a false discovery rate (FDR) < 5% was applied for statistical significance.
Metabolites associated with incident HF in JHS and measured in FHS were tested for association with incident HF in FHS in 2 models: model 1 was adjusted for age, sex and batch while model 2 was additionally adjusted for body mass index, hypertension diagnosis or treatment with hypertension medication, systolic blood pressure, diabetes mellitus, current smoking status, total cholesterol, high-density lipoprotein cholesterol, CHD history and eGFR.
Metabolites associated with incident HF hospitalization in JHS were tested for their association with left ventricular mass index (LVMi) using linear regression modeling adjusting for age, sex, body mass index, hypertension diagnosis or treatment with hypertension medication, systolic blood pressure, diabetes mellitus, current smoking status, history of CHD, eGFR and batch.
To assess the impact of the development of CHD on the association between metabolite levels and incident HF, incident CHD was modeled as a time dependent covariate in the cox regression analysis. Effect estimates were calculated for the metabolites in a multivariate model with and without incident CHD. Effect size change of incident CHD on metabolite association with incident HF was modeled as percent change in hazard ratio.
To assess discriminatory ability of metabolites in the prediction of incident HF, c-statistics were calculated for the following models: BNP (model 1), metabolites associated with incident HF in multivariate model (model 2), clinical risk factors including body mass index, hypertension diagnosis or treatment with hypertension medication, systolic blood pressure, diabetes mellitus, current smoking status, history of CHD, eGFR and BNP (model 3), BNP, clinical risk factors and metabolites associated with incident HF (model 4). The standard errors of the difference in C statistics between two models were estimated using a bootstrap method, accounting for the paired nature of the data. A Z test was then performed using the observed difference in C statistics and the standard error to examine whether or not there is statistical evidence of difference in the C statistics between the two models.
HF is a heterogeneous condition, and can be subdivided into HF with preserved ejection fraction (HFpEF) and HF with reduced ejection fraction (HfrEF). Standard analyses to estimate association between metabolites and one HF type do not address competing risks of the other. To account for this, all profiled metabolites associated with either incident HfpEF and/or HfrEF with nominal significance (p value < 0.05) were assessed for differential associations between the HF types using competing risk analyses using the Fine-Gray method18. The model was adjusted for age, sex, body mass index, hypertension diagnosis or treatment with hypertension medication, systolic blood pressure, diabetes mellitus, current smoking status, total cholesterol, high-density lipoprotein cholesterol, CHD history, eGFR and batch. A bootstrap method was applied to determine significant differences between the associations with HFpEF vs. HFrEF to assess the true marginal probability of cause-specific events. Due to the use of bootstrapped association analysis in refining confidence intervals, a p value for the difference in effect sizes between HFrEF and HFpEF of <0.05 was considered significant. All analyses were conducted using R 3.6.1 software and associated packages.
Results
Baseline Characteristics
Among the study participants with metabolomic profiling performed at baseline and eligible for analysis (Figure 1; n=2,199), 188 developed heart failure with a mean time for follow-up of 9.6 years. At baseline, HF cases were older and had higher rates of hypertension, obesity, diabetes mellitus, and prevalent CHD, and lower eGFR compared to controls (Table 1). Baseline clinical characteristics of the entire JHS Cohort (n=5,306) and of individuals with metabolomic profiling were similar (Supplementary Table I).
Table 1:
Baseline Characteristics for Cases and Controls of Incident Heart Failure in the Jackson Heart Study
| Variable | Cohort (n=2199) | Cases (n=188) | Controls (n=2011) |
|---|---|---|---|
|
| |||
| Age, y | 55.85 ± 12.49 | 65.17 ± 11.28 | 54.98 ± 12.24 |
| Female sex, n (%) | 1370 (62) | 123 (65) | 1247 (62) |
| BMI, kg/m2 | 31.58 ± 7.04 | 32.80 ± 7.47 | 31.47 ± 6.99 |
| SBP, mm Hg | 126.90 ± 17.89 | 135.43 ± 19.50 | 126.10 ± 17.52 |
| DBP, mm Hg | 79.01 ± 10.37 | 77.23 ± 11.55 | 79.18 ± 10.23 |
| Diabetes n (%), | 424 (19.28) | 77 (40.96) | 347 (17.25) |
| HbA1c (%) | 5.94 ± 1.24 | 6.43 ± 1.64 | 5.89 ± 1.19 |
| TC (mg/dL) | 200.61 ± 40.55 | 202.48 ± 45.88 | 200.44 ± 40.03 |
| HDL (mg/dL) | 51.87 ± 14.51 | 50.99 ± 15.05 | 51.95 ± 14.46 |
| eGFR(mL/min/1.73 m2) | 83.78 ± 18.66 | 72.80 ± 23.79 | 84.81 ± 17.77 |
| Current smoker n (%) | 244 (11.09) | 31 (16.49) | 213 (10.59) |
| CHD n (%) | 92 (4.18) | 20 (10.64) | 72 (3.58) |
| HTN | 1325 (60.25) | 155 (82.44) | 1170 (58.18) |
JHS: Jackson Heart Study; y: years; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; TC: total cholesterol; HDL: high density lipoprotein; eGFR: estimated glomerular filtration rate; CHD: coronary heart disease; HTN: hypertension
Metabolite Associations with Incident Heart Failure
The relationship between metabolites and incident HF hospitalization was assessed using cox proportional hazard regression. In a multivariate model, 14 metabolites were associated with the development of incident HF hospitalization (Table 2). Of these metabolites, 3 were associated with decreased risk: homoarginine (HR 0.77 per 1 SD increase in metabolite level, p value 1.2 X 10−3), 2-aminoadipic acid (HR 0.78. p value 1.2 X 10−3) and uridine (0.79, p value 3 X 10−4). Eleven metabolites were associated with an increased risk of incident HF, including pseudouridine, 2-deoxyuridine, methylhistidine, 4-acetamidobutanoate, choline, ectoine, N-acetylspermidine, diacetylspermine, dimethylguanidino valeric acid (DMGV), N-acetyl-l-alanine and N2-N2 dimethylguanosine (HR 1.29–1.73, p value 1.2 X 10−3 to 4 X 10−8). Analytic coefficients of variation for metabolites associated with incident HF are provided in Supplementary Table III.
Table 2:
Metabolites Associated with Incident Heart Failure in the Jackson Heart Study
| Metabolites | Hazard ratio (95% CI) | P value | Q value |
|---|---|---|---|
| pseudouridine | 1.73 (1.42–2.1) | 3.93E-08 | 1.31E-05 |
| 2-deoxyuridine | 1.56 (1.3–1.86) | 9.42E-07 | 1.57E-04 |
| 4-acetamidobutanoate | 1.49 (1.26–1.76) | 2.88E-06 | 3.20E-04 |
| methylhistidine | 1.42 (1.19–1.7) | 7.91E-05 | 6.59E-03 |
| N1-acetylspermidine | 1.32 (1.15–1.53) | 1.29E-04 | 8.60E-03 |
| DMGV | 1.43 (1.17–1.74) | 3.96E-04 | 0.019 |
| diacetylspermine | 1.34 (1.14–1.58) | 4.58E-04 | 0.019 |
| Uridine | 0.79 (0.70–0.90) | 3.73E-04 | 0.020 |
| Choline | 1.33 (1.13–1.58) | 6.79E-04 | 0.025 |
| N-Acetyl-L-Alanine | 1.36 (1.14–1.63) | 7.96E-04 | 0.027 |
| N2-N2-dimethylguanosine | 1.3 (1.11–1.53) | 1.32E-03 | 0.033 |
| Ectoine | 1.29 (1.1–1.51) | 1.44E-03 | 0.034 |
| 2-aminoadipic acid | 0.77 (0.66–0.90) | 1.29E-03 | 0.036 |
| homoarginine | 0.78 (0.66–0.90) | 1.27E-03 | 0.039 |
Model adjusted for age, sex, batch, body mass index, hypertension, systolic blood pressure, diabetes, eGFR, total cholesterol, high density lipoprotein, smoking status, history of coronary heart disease
Metabolite Associations with Both Left Ventricular Mass Index and Incident Heart Failure
Given the well-established relationship between LVMi and HF, we assessed the associations of metabolites implicated in HF development (Table 2) with baseline LVMi. After adjustment for clinical risk factors, of the metabolites associated with incident HF in the multivariate model, the majority of the metabolites (DMGV, homoarginine, diacetylspermine, uridine, ectoine, 2-aminoadipic acid, methylhistidine and N-acetylspermidine) were not significantly associated with LVMi. The remaining 6 metabolites were positively associated with LVMi: pseudouridine, 2-deoxyuridine, N-acetyl-l-alanine, N2-N2-dimethylguanosine, choline and 4-acetamidobutanoate (β coefficients 0.074 to 0.125, p value 0.02 to 6.96E−5; Table 3).
Table 3:
Metabolites Associated with Incident Heart Failure and Left Ventricular Mass Index
| Metabolites | β-coefficient | P value | Q value |
|---|---|---|---|
| pseudouridine | 0.125 (0.093–0.156) | 6.96E-05 | 9E-04 |
| 2-deoxyuridine | 0.078 (0.052–0.105) | 2.9E-03 | 0.020 |
| choline | 0.061 (0.038–0.085) | 7.9E-03 | 0.028 |
| N2-N2-dimethylguanosine | 0.070 (0.044–0.096) | 7E-03 | 0.032 |
| N-Acetyl-L-Alanine | 0.075 (0.045–0.105) | 0.012 | 0.033 |
| 4-acetamidobutanoate | 0.075(0.044–0.105) | 0.016 | 0.037 |
Model adjusted for age, sex, batch, body mass index, hypertension, systolic blood pressure, eGFR, diabetes, smoking status and history of coronary heart disease
Effect Modification of Incident Coronary Heart Disease
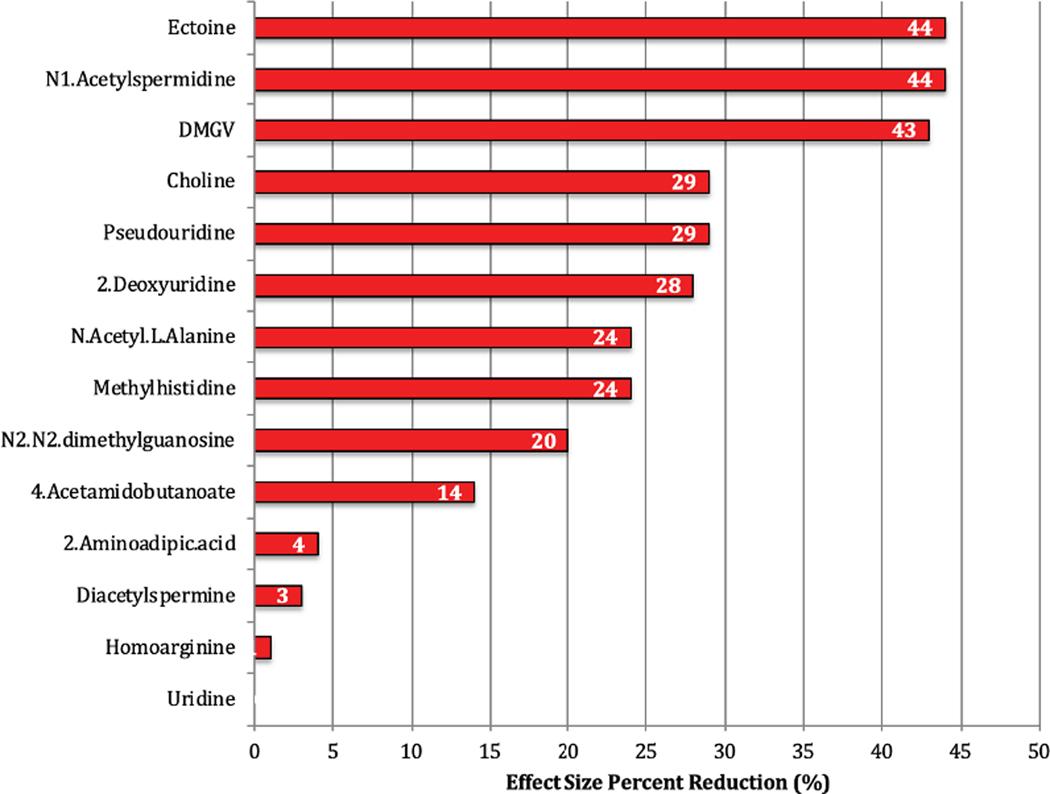
Metabolites associated with incident HF were also tested in a model accounting for incident CHD as a time dependent variable. Of the metabolites associated with incident HF in a multi-variable model, eight metabolites (N-acetyl-l-alanine, methylhistidine, 4-acetamidobutanoate, N2-N2-dimethylguanosine, homoarginine, uridine, 2-aminodadipic acid and diacetylspermine) had only a modest reduction in effect size (<25%), with uridine showing no attenuation when accounting for incident CHD (Figure 2). The remaining 6 metabolites had >25% reduction in effect size for risk of incident HF with ectoine and N-acetylspermidine showing the greatest reductions (delta HR −43% for each metabolite).
Figure 2:
Effect Size Modification of Incident Coronary Heart Disease on the Association of Metabolites with Incident Heart Failure The percent reduction in effect size (hazard ratio) for metabolites in a cox proportional hazards regression for incident heart failure with the addition of incident coronary heart disease as a time dependent variable. Baseline model was adjusted for age, sex, body mass index, hypertension, systolic blood pressure, total cholesterol, high density lipoprotein, Diabetes mellitus, eGFR, smoking status and history of coronary heart disease. Incident coronary heart disease was added as a time dependent covariate to determine change in effect size.
Risk Discrimination for Metabolites and Incident Heart Failure
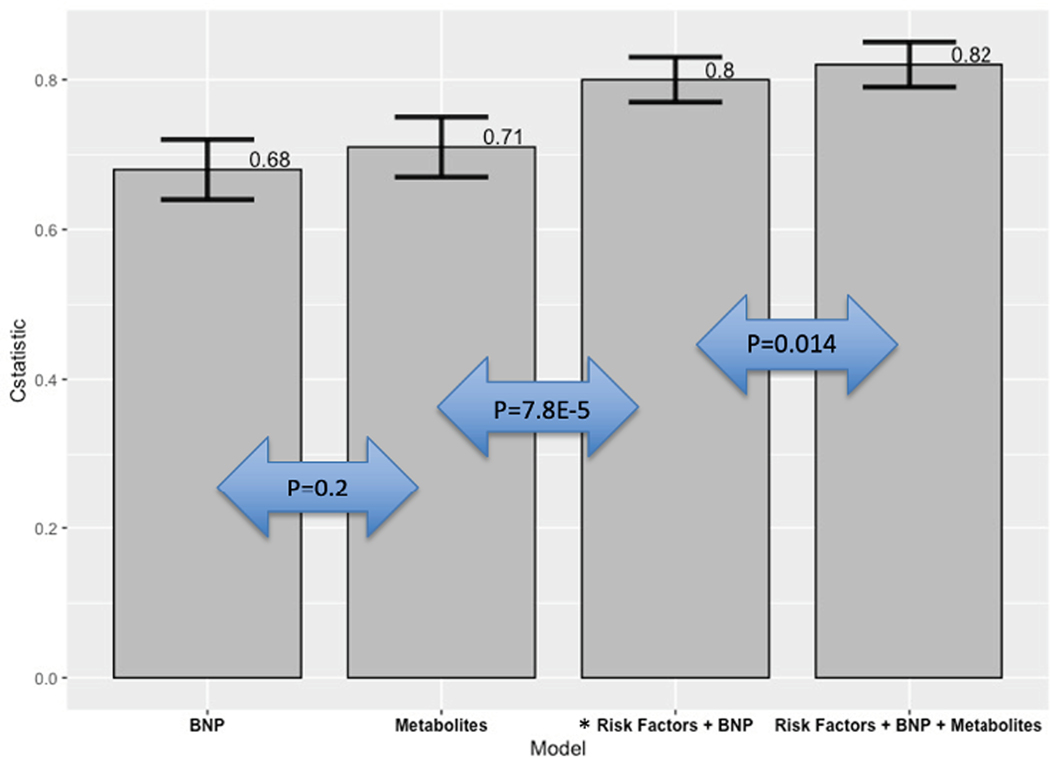
The additive discriminatory ability of metabolites in predicting HF in addition to known clinical risk factors including BNP was assessed using concordance statistics. Model 1 consisted of BNP alone in the association with incident HF, achieving a c-statistic of 0.68 (95% CI 0.65–0.71). Model 2 consisted of metabolites associated with incident HF (Table 2) in a multi-variable model (FDR <0.05) and had a c-statistic of 0.71 (95% CI 0.67–0.75; delta c-statistic between model 1 and model 2: 0.04, p value of 0.2). Model 3 consisted of clinical risk factors and BNP levels, with a c-statistic of 0.80 (95% CI 0.77–0.83; delta c-statistic between model 2 and model 3: 0.09, p value of 7.8E−5). Model 4 included clinical risk factors, BNP and the addition of metabolites from model 2 and had a c statistic of 0.82 (95% CI 0.79–0.85; delta c-statistic between models 3 and 4: + 0.02, p value 0.014 Figure 3).
Figure 3:
Risk discrimination for incident heart failure for metabolites vs. clinical risk factors. The C-statistic along with confidence intervals are displayed for each model in its association with incident heart failure.
BNP: Brain natreutic peptide
*Clinical risk factors: age, sex, body mass index, hypertension, systolic blood pressure, eGFR, diabetes, smoking status, total cholesterol, high density lipoprotein and history of coronary heart disease
Association of Metabolites with Incident Heart Failure in the Framingham Heart Study
Baseline demographics of participants with metabolomic profiling in FHS are provided Of the participants in FHS with available metabolomic profiling (n=2,372), 216 developed HF with mean time to HF of 12.9 years. At baseline, similar to JHS, HF cases were older and had higher rates of hypertension, obesity, diabetes mellitus, CHD, and lower eGFR compared to controls (Supplementary table 4). Metabolites associated with incident HF in JHS and measured in the FHS Offspring Cohort (choline, 2-aminoadipic acid and uridine) were tested for their association with incident HF in FHS. In age, sex and batch adjusted models using FDR <5%, choline (HR 1.24; 95% CI 1.08–1.44; p value 0.003) and 2-aminoadipic acid (HR 1.2; 95% CI 1.03–1.41; p value 0.02) were significantly associated with incident HF. In a model adjusting for additional risk factors for HF, none of these metabolites were associated with incident HF (HR 0.94–1.16; p value 0.08–0.49; Table 4).
Table 4:
Association of metabolites with incident heart failure in the Framingham Heart Study
| Model 1* | Model 2† | |||||||
|---|---|---|---|---|---|---|---|---|
| Metabolite | Hazard Ratio | 95% CI | P value | Q value | Hazard Ratio | 95% CI | P value | Q value |
| Choline | 1.24 | 1.08−1.44 | 0.003 | 0.01 | 1.16 | 0.99−1.37 | 0.08 | 0.24 |
| 2-aminoadipic acid | 1.2 | 1.03−1.41 | 0.02 | 0.03 | 1.03 | 0.86−1.24 | 0.73 | 0.74 |
| Uridine | 0.91 | 0.79−1.01 | 0.23 | 0.2 | 0.94 | 0.80−1.11 | 0.49 | 0.73 |
Model 1: Adjusted for age, sex and batch
Model 2: Model 1+ body mass index, eGFR, total cholesterol, high density lipoprotein, coronary heart disease, diabetes, hypertension, systolic blood pressure and smoking status.
Metabolite associations with HFpEF vs. HFrEF
Of the 188 individuals who developed HF, 164 individuals were able to be classified as HFpEF or HFrEF based on cardiac imaging data. Metabolites associated with incident HF were analyzed in competing risk models for the HF subtypes. None of the 14 metabolites associated with incident HF had significant differences in their associations between HFpEF versus HFrEF (Supplementary Table 6). In an exploratory analysis, all metabolites associated with either incident HFpEF or HFrEF with nominal significance were entered into competing risk models to assess differential relationships between the development of HFpEF vs. HFrEF. Of the additional metabolites tested, eight metabolites were differentially associated with HFpEF as compared to HFrEF (Orotic acid, C18–0-LPE_A, ATP, C16–1-LPC-plasmologen, C18–1-LPC, N-methylproline, C18–1-LPC-plasmologen and C16–0-LPE) and 3 metabolites were differentially associated with HFrEF (C22–5-LPC, 1-methyladenosine and C22–6-LPC; bootstrap p <0.05 for comparison between HFpEF and HFrEF; Table 5).
Table 5:
Competing risk model for differential association of metabolites with HFpEF vs. HFrEF
| HFrEF (n=77) | HFpEF (n=87) | Difference* | |||
|---|---|---|---|---|---|
| Metabolites | Hazard ratio | P value | Hazard ratio | P value | P value |
| HFpEF | |||||
| Orotic acid | 0.86 (0.76−0.98) | 0.25 | 1.37 (1.28−1.46) | 4.10E-06 | 4.4E-03 |
| C18.0.LPE_A | 0.88 (0.78−0.98) | 0.28 | 1.35 (1.21−1.49) | 4.8E-03 | 7.8E-03 |
| ATP | 0.924(0.83−1.03) | 0.46 | 1.46 (1.29−1.65) | 2.9E-03 | 9.1E-03 |
| C18.1.LPC.plasmalogen_minor | 0.83 (0.74−0.93) | 0.098 | 1.32 (1.18−1.49) | 0.018 | 9.6E-03 |
| C16.1.LPC.plasmalogen | 0.808 (0.71−0.90) | 0.069 | 1.28 (1.14−1.44) | 0.033 | 9.9E-03 |
| C18.1.LPC | 0.873 (0.77−0.98) | 0.24 | 1.33 (1.18−1.48) | 0.014 | 0.012 |
| N.methylproline | 1.05 (0.93−1.17) | 0.68 | 0.69 (0.616−0.773) | 0.001 | 0.020 |
| C18.1.LPC.plasmalogen | 0.885 (0.80−0.97) | 0.22 | 1.29 (1.15−1.46) | 0.033 | 0.021 |
| C16.0.LPE | 0.876 (0.77−0.98) | 0.27 | 1.3 (1.17−1.44) | 0.011 | 0.024 |
| HFrEF | |||||
| C22.5.LPC | 0.768 (0.685−0.861) | 0.02 | 1.22 (1.06−1.39) | 0.15 | 0.0188 |
| 1.methyladenosine | 1.59 (1.38−1.84) | 0.001 | 0.984 (0.863−1.12) | 0.9 | 0.0242 |
| C22.6.LPC | 0.759 (0.675−0.853) | 0.018 | 1.09 (0.972−1.23) | 0.45 | 0.0358 |
Model adjusted for age, sex, batch, body mass index, hypertension, systolic blood pressure, diabetes, eGFR, total cholesterol, high density lipoprotein, smoking status, history of coronary heart disease
P value for differential association in risk model based on bootstrap sampling
HFpEF: heart failure with preserved ejection fraction; HFrEF: heart failure with preserved ejection fraction
Discussion
In these analyses, we identified metabolites associated with the development of HF that take part in key metabolic processes including post-transcriptional modifications of RNA, polyamine metabolism, and others. While a subset of these associations are entirely novel, others have been identified previously in patients with existing HF, indicating a continuum of metabolic dysregulation in the progression of at risk individuals to overt disease. We show metabolites that are associated with increased LVMi and incident CHD, however, a majority of our findings are enriched for metabolomic pathways that are independent of these traditional mechanisms of disease development. We further delineate the heterogeneity in HF by highlighting metabolic differences between the development of HFpEF versus HFrEF.
Polyamine Metabolism
Products of polyamine synthesis and catabolism, N-acetylspermidine, diacetylspermine and 4-acetamidobutannoate, are associated with incident HF. Polyamines (spermine, spermidine and putrescine) are naturally occurring metabolites present in all living cells. These metabolites bind to and interact with negatively charged molecules including DNA, RNA and proteins and are thus implicated in several essential processes such as cellular growth and survival19. Intracellular concentrations of polyamines are tightly controlled and association of their catabolites with HF represents possible dysregulation of polyamine catabolism. Further, diacetylspermine, a metabolite previously associated with active cell proliferation in malignancy20, was not associated with LVMi and its association with HF was minimally attenuated by the adjustment for incident CHD, suggesting a novel association beyond traditional disease pathways in the development of HF.
Nitric Oxide Metabolism
Reactive oxygen species contribute to cardiac remodeling and progression to HF and antioxidants including endothelium derived nitric oxide (NO) help reduce accumulation of these molecules. AA’s have reduced basal levels of NO and therapies that augment levels in individuals with HF have proven to be beneficial in this population21. Several metabolites associated with the development of HF interact within the NO pathway. DMGV is a product of asymmetric dimethylarginine (ADMA), a naturally occurring metabolite that competes with L-arginine for the active site of NO synthase, thereby inhibiting NO production. While reduced global arginine availability and NO biosynthesis define a possible mechanism for increased cardiovascular risk, DMGV’s association with incident HF was independent of clinical risk factors, unlike other metabolites of arginine metabolism. This suggests possible mechanisms of HF development in addition to the NO pathway. We have previously shown that DMGV is a top metabolite marker of liver fat and is associated with incident diabetes in multiple epidemiological cohorts, including JHS 22. Further, elevated levels of DMGV have been associated with reduced cardiometabolic benefits of exercise23. DMGV’s association with incident HF in JHS was more pronounced in individuals with diabetes (Supplementary table VII), suggesting a possible mechanistic link between diabetes and HF, and highlighting the need for further studies of the role of DMGV in cardiometabolic disease and HF.
Post-Transcriptional Modifications: Shared Metabolomic Profiles between LVMi and Heart Failure
Metabolites involved in post-transcriptional modifications were among the top associations with both LVMi and incident HF. Uridine, a pyrimidine analogue, is associated with reduced LVMi (age and sex adjusted; Supplementary table VIII) and reduced risk for HF (multivariate model), while pseudouridine, an isomer of uridine, is associated with increased risk for both processes (multivariate model). The differing associations between uridine and pseudouridine may highlight the importance of pyrimidine flux in cellular processes that lead to the development of LVH and HF. Further, N2-N2 dimethylguanosine, a methylated product of the guanine nucleoside, is a common tRNA modification, previously found to be elevated in individuals with pulmonary arterial hypertension24. Post-transcriptional modifications are increased in periods of cellular stress such as hemodynamic overload. Further, post-transcriptional changes have been shown to play an important role in the development of cardiac hypertrophy and remodeling25. As LVH is a potent risk factor for HF, increased post-transcriptional modifications in both may offer insight into the transition from at-risk cardiac structure to overt disease, suggesting opportunities to investigate the regulation and modulation of these processes.
Metabolic Signatures of HFpEF vs. HFrEF
While HF is a heterogeneous condition with several endophenotypes as well as distinct physiology between its two main subtypes, HFrEF and HFpEF, there is overlap in both risk factors and disturbed metabolic processes26. As such, metabolites associated with incident HF were not differentially associated with either HF subtype in this study. A more comprehensive analysis of the metabolomic profiles revealed potentially distinct associations. Orotic acid, the top metabolite differentially associated with HFpEF as compared to HFrEF, is involved in pyrimidine metabolism, where pathway deficiencies are associated with renal impairment, hematologic disorders and immune deficiencies27. N-methylproline was differentially associated with the development of HFpEF as compared to HFrEF. Its precursor, proline is a significant component of collagen and collagen dependent myocardial stiffness is increased in patients with HFpEF28. The association of this proline derivative with HFpEF implicates a distinct pathophysiological process that may differentiate HFpEF from HFrEF and offer a novel pathway for investigation toward precision therapies in HFpEF.
Multi-Ethnic Comparisons in Metabolomic Profiling of Incident Heart Failure
Our study provides one of the few analyses of targeted metabolomics and all cause incident heart failure and includes an expanded platform of metabolites measured compared to previous studies. While this does limit comparisons between AA and whites in metabolomic profiles prior to developing HF, key insight can be gleaned from metabolomics studies in related phenotypes. N-acetylspermidine, a polyamine, was found in a predominately white cohort to be associated with increased mortality in patients with ischemic cardiomyopathy as well as with incident HF in patients with coronary artery disease but without HF at baseline29. Pseudouridine was associated with increased LVMI with consistent direction of effect and significance across AA and Caucasians, consistent with our findings30. Further, we analyzed the metabolites associated with incident HF in JHS in existing data from white individuals of European-ancestry participants in the Framingham Heart Study to assess possible biological similarities and differences in circulating metabolomic profiles prior to disease development. Of the metabolites associated with incident HF in JHS, three were also profiled in FHS (choline, 2-aminoadipic acid and uridine). Similar to JHS, choline was positively associated with HF in an age and sex adjusted analysis. However, 2- aminoadipic acid had an inverse association with incident HF, differing in its relationship to HF compared with AA’s. Further, these associations in FHS were significantly attenuated when adjusting for additional HF risk factors. These analyses and prior studies highlight both the potential overlap of metabolomic profiles in individuals prior to HF development and potential differences between populations of predominantly African descent versus white individuals of European ancestry.
Previously, metabolomic associations with HF have predominately been assessed in patients who have established disease, which precludes the assessment of temporal relationships between metabolites and disease onset. To our knowledge our study provides the most comprehensive targeted analysis of incident HF to date, suggesting potential mechanisms of disease development, and offering markers of risk and potential therapeutic targets for investigation. The detailed phenotyping of JHS allows robust adjustments for clinical factors including renal function, that may confound associations between metabolites and incident HF, and provides the ability to interrogate different phenotypes and mechanisms of the heterogeneous processes of HF development. Further, given that AA’s are disproportionately affected by HF, this study provides important findings regarding a process with relatively high morbidity in a population that is underrepresented in biomedical research.
The main limitation in our findings is the lack of external validation for most metabolites. Given our novel profiling techniques, data for a significant proportion of metabolites we have measured are unavailable for analysis in other epidemiological and metabolomic cohorts. Further, while HF disproportionately affects AAs, this population is underrepresented in epidemiological studies. Efforts are needed to expand metabolomic profiling to additional epidemiological cohorts and to adequately sample the African American population, to assess similarities and differences in metabolomic dysregulation between AA’s and other groups, prior to the onset of HF. Secondly, given the risk discrimination analysis was performed and validated in the same cohort, overfitting of data may be of concern. However bootstrapping of c-statistics should help mitigate this concern14. While we acknowledge, the limited improvement in risk discrimination for HF with the addition metabolites, our models for comparison are comprehensive in both clinical characteristics and biomarkers, and most importantly, our findings provide novel avenues towards biological mechanisms of HF. Finally, the adjudication of HF subtypes was limited given that it was based on entirely on left ventricular ejection fraction, and 29 out of the 164 individuals adjudicated for HF subtypes did not have discrete LVEF data available (i.e qualitative, broad ranges etc..). This inclusive definition of HFpEF likely limits ability to characterize specific HF pathways in AA’s, such as the contribution of hereditary Transthyretin cardiomyopathy, which results from a common polymorphism leading to HF in this population. However, it does allow for a heterogeneous group of patients included in our study, broadly reflecting HF mechanisms in the community.
Conclusions
Metabolomic profiling in the JHS provides novel associations between circulating metabolites and the development of HF in African Americans. Several metabolites are linked to previously uncharacterized metabolic processes in the development of HF, suggesting potential novel biomarkers, mechanisms and therapeutic targets for investigation. Further research is needed to test these findings in additional multi-ethnic populations and to determine biological mechanisms underlaying these metabolite associations with incident HF.
Supplementary Material
What’s new:
Heart failure is a heterogenous process marked by significant metabolic dysfunction.
In this study of African Americans in the Jackson Heart Study, we find novel metabolites and disturbed metabolic pathways including polyamine and nitric oxide metabolism associated with heart failure prior to disease onset
What are the clinical implications?
Given the increasing burden of heart failure, these findings offer novel biomarkers and potential targets for investigation for this heterogenous disease process.
Acknowledgements:
The authors wish to thank the staffs and participants of the JHS and FHS.
Sources of Funding:
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I/HHSN26800001) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute for Minority Health and Health Disparities (NIMHD). Dr. Tahir is supported by the John S. LaDue Memorial Fellowship in Cardiology. Dr. Katz is supported by NHLBI T32 postdoctoral training grant (T32HL007374–40). Dr. Cruz is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst (NIH/NCATS Award TR002542). Dr. Robbins is supported by the John S. LaDue Memorial Fellowship in Cardiology at Harvard Medical School. Dr. Benson is supported by NHLBI K08HL145095 award. Drs. Gerszten, Wang and Wilson are supported by NIH R01 DK081572.
The Framingham Heart Study (FHS) was supported by grants NO1-HC-25195, HHSN268201500001I and 75N92019D00031from the National Heart, Lung and Blood Institute. The present work was also supported by the NIH R01-DK-HL081572 grant. Dr. Vasan is supported in part by Evans Medical Foundation, Department of Medicine, and the Jay and Louis Coffman’s endowment, both at the Boston University School of Medicine
Nonstandard Abbreviations and Acronyms
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- CHD
Coronary heart disease
- CVD
Cardiovascular disease
- JHS
Jackson Heart Study
- AA
African American
- LVMi
Left ventricular mass index
Footnotes
Disclosures: Thomas J. Wang is co-inventor on patent applications related to biomarkers of heart failure. Dr. Wang has received consulting fees related to a data science advisory board for Novartis, unrelated to the topic of the manuscript. All others have none.
Disclaimer: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services
References
- 1.Savarese G and Lund LH. Global Public Health Burden of Heart Failure. Card Fail Rev. 2017;3:7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hunter WG, Kelly JP, McGarrah RW 3rd, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, Newgard CB, Felker GM, Hernandez AF, Velazquez EJ, Kraus WE and Shah SH. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. Journal of the American Heart Association. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SH, Kraus WE and Newgard CB. Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation. 2012;126:1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ussher JR, Elmariah S, Gerszten RE and Dyck JR. The Emerging Role of Metabolomics in the Diagnosis and Prognosis of Cardiovascular Disease. Journal of the American College of Cardiology. 2016;68:2850–2870. [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung Y-M, Wu Y, Schauer P, Smith JD, Allayee H, Tang WHW, DiDonato JA, Lusis AJ and Hazen SL. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharov VG, Todor AV, Silverman N, Goldstein S and Sabbah HN. Abnormal mitochondrial respiration in failed human myocardium. Journal of molecular and cellular cardiology. 2000;32:2361–7. [DOI] [PubMed] [Google Scholar]
- 7.Tang WH, Tong W, Shrestha K, Wang Z, Levison BS, Delfraino B, Hu B, Troughton RW, Klein AL and Hazen SL. Differential effects of arginine methylation on diastolic dysfunction and disease progression in patients with chronic systolic heart failure. European heart journal. 2008;29:2506–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL and Lima JAC. Differences in the Incidence of Congestive Heart Failure by Ethnicity: The Multi-Ethnic Study of Atherosclerosis. Archives of Internal Medicine. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor HA Jr. The Jackson Heart Study: an overview. Ethnicity & disease. 2005;15:S6-1–3. [PubMed] [Google Scholar]
- 10.Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C and Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethnicity & disease. 2005;15:S6-4–17. [PubMed] [Google Scholar]
- 11.Paynter NP, Balasubramanian R, Giulianini F, Wang DD, Tinker LF, Gopal S, Deik AA, Bullock K, Pierce KA, Scott J, Martinez-Gonzalez MA, Estruch R, Manson JE, Cook NR, Albert CM, Clish CB and Rexrode KM. Metabolic Predictors of Incident Coronary Heart Disease in Women. Circulation. 2018;137:841–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R and Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. The American journal of the medical sciences. 2004;328:131–44. [DOI] [PubMed] [Google Scholar]
- 13.Keku E, Rosamond W, Taylor HA Jr, Garrison R, Wyatt SB, Richard M, Jenkins B, Reeves L and Sarpong D. Cardiovascular disease event classification in the Jackson Heart Study: methods and procedures. Ethnicity & disease. 2005;15:S6-62–70. [PubMed] [Google Scholar]
- 14.Feinleib M, Kannel WB, Garrison RJ, McNamara PM and Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. [DOI] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O’Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB and Gerszten RE. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersson C, Liu C, Cheng S, Wang TJ, Gerszten RE, Larson MG and Vasan RS. Metabolomic signatures of cardiac remodelling and heart failure risk in the community. Advance Online Publication. ESC Heart Fail. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmood SS and Wang TJ. The epidemiology of congestive heart failure: the Framingham Heart Study perspective. Glob Heart. 2013;8:77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP and Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 19.Larque E, Sabater-Molina M and Zamora S. Biological significance of dietary polyamines. Nutrition (Burbank, Los Angeles County, Calif). 2007;23:87–95. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi Y, Sakaguchi K, Horio H, Hiramatsu K, Moriya S, Takahashi K and Kawakita M. Urinary N1, N12-diacetylspermine is a non-invasive marker for the diagnosis and prognosis of non-small-cell lung cancer. Br J Cancer. 2015;113:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor AL, Ziesche S, Yancy C, Carson P, D’Agostino R, Ferdinand K, Taylor M, Adams K, Sabolinski M, Worcel M and Cohn JN. Combination of Isosorbide Dinitrate and Hydralazine in Blacks with Heart Failure. New England Journal of Medicine. 2004;351:2049–2057. [DOI] [PubMed] [Google Scholar]
- 22.O’Sullivan JF, Morningstar JE, Yang Q, Zheng B, Gao Y, Jeanfavre S, Scott J, Fernandez C, Zheng H, O’Connor S, Cohen P, Vasan RS, Long MT, Wilson JG, Melander O, Wang TJ, Fox C, Peterson RT, Clish CB, Corey KE and Gerszten RE. Dimethylguanidino valeric acid is a marker of liver fat and predicts diabetes. The Journal of clinical investigation. 2017;127:4394–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbins JM, Herzig M, Morningstar J, Sarzynski MA, Cruz DE, Wang TJ, Gao Y, Wilson JG, Bouchard C, Rankinen T and Gerszten RE. Association of Dimethylguanidino Valeric Acid With Partial Resistance to Metabolic Health Benefits of Regular Exercise. JAMA cardiology. 2019;4:636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhodes CJ, Ghataorhe P, Wharton J, Rue-Albrecht KC, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris PA, Howard LS, Kiely DG, Peacock AJ, Pepke-Zaba J, Toshner MR, Wort SJ, Gibbs JSR, Lawrie A, Gräf S, Morrell NW and Wilkins MR. Plasma Metabolomics Implicates Modified Transfer RNAs and Altered Bioenergetics in the Outcomes of Pulmonary Arterial Hypertension. Circulation. 2017;135:460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ojamaa K, Petrie JF, Balkman C, Hong C and Klein I. Posttranscriptional modification of myosin heavy-chain gene expression in the hypertrophied rat myocardium. Proc Natl Acad Sci U S A. 1994;91:3468–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hunter WG, Kelly JP, McGarrah RW 3rd, Khouri MG, Craig D, Haynes C, Ilkayeva O, Stevens RD, Bain JR, Muehlbauer MJ, Newgard CB, Felker GM, Hernandez AF, Velazquez EJ, Kraus WE and Shah SH. Metabolomic Profiling Identifies Novel Circulating Biomarkers of Mitochondrial Dysfunction Differentially Elevated in Heart Failure With Preserved Versus Reduced Ejection Fraction: Evidence for Shared Metabolic Impairments in Clinical Heart Failure. Journal of the American Heart Association. 2016;5:e003190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Löffler M, Fairbanks LD, Zameitat E, Marinaki AM and Simmonds HA. Pyrimidine pathways in health and disease. Trends in Molecular Medicine. 2005;11:430–437. [DOI] [PubMed] [Google Scholar]
- 28.Zile MR, Baicu CF, Ikonomidis JS, Stroud RE, Nietert PJ, Bradshaw AD, Slater R, Palmer BM, Van Buren P, Meyer M, Redfield MM, Bull DA, Granzier HL and LeWinter MM. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: contributions of collagen and titin. Circulation. 2015;131:1247–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayak A, Liu C, Mehta A, Ko YA, Tahhan AS, Dhindsa DS, Uppal K, Jones DP, Butler J, Morris AA and Quyyumi AA. N8-Acetylspermidine: A Polyamine Biomarker in Ischemic Cardiomyopathy With Reduced Ejection Fraction. Journal of the American Heart Association. 2020;9:e016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razavi AC, Bazzano LA, He J, Li S, Fernandez C, Whelton SP, Krousel-Wood M, Nierenberg JL, Shi M, Li C, Mi X, Kinchen J and Kelly TN. Pseudouridine and N-formylmethionine associate with left ventricular mass index: Metabolome-wide association analysis of cardiac remodeling. Journal of molecular and cellular cardiology. 2020;140:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.